Angiotensin-receptor blockers (ARBs) are one of four drug classes recommended for initial treatment of hypertension. These medications are commonly used not only for hypertension — a condition present in 45.6% of U.S. adults — but also for heart failure and chronic kidney disease.1,2 On January 25, 2019, Food and Drug Administration (FDA) Commissioner Scott Gottlieb and Director of the FDA Center for Drug Evaluation and Research Janet Woodcock released a statement updating the public on large-scale voluntary recalls of various ARB-containing products. Two probable carcinogens have been identified in active pharmaceutical ingredients used by some manufacturers of valsartan, irbesartan, and losartan. The impurities arose during manufacture of the ingredients in two factories located in China and India. The same day, the Wall Street Journal reported that as many as 2 million patients had probably been exposed to the impurities, N-Nitrosodimethylamine (NDMA) and N-Nitrosodiethylamine (NDEA). Most recently, a third impurity, N-Nitroso N-Methyl 4-amino butyric acid (NMBA), has been identified in an ARB product, causing a new recall. These recalls are of growing concern to patients, clinicians, and organizations delivering primary care or complex, multidisciplinary health care, and they highlight several issues related to the readiness of our health systems, trust between patients and providers, uncertain drug-dose equivalences, and the regulation of drug manufacturing in the global marketplace.
Although not all products containing valsartan, irbesartan, or losartan that are marketed in the United States have been recalled, the scope of the exposure, the scale of the 20 recalls, and their impact on patient care are staggering (see timeline). FDA officials believe that U.S. patients have been ingesting ARBs containing carcinogenic impurities for approximately 4 years; and they estimate that for every 8000 patients taking the highest dose of an affected product for the full 4 years, one new cancer beyond the background incidence would be expected. More than 61 million prescriptions were written for valsartan, irbesartan, or losartan in the United States in 2016.1 In addition to the recall of millions of pill bottles, at least 1.9 U.S. tons of active pharmaceutical ingredients were recalled by one manufacturer. One third of all FDA drug recalls issued since July 2018 have involved ARB-containing products, and together the recalls have affected one sixth of U.S. ARB manufacturers.
timeline.
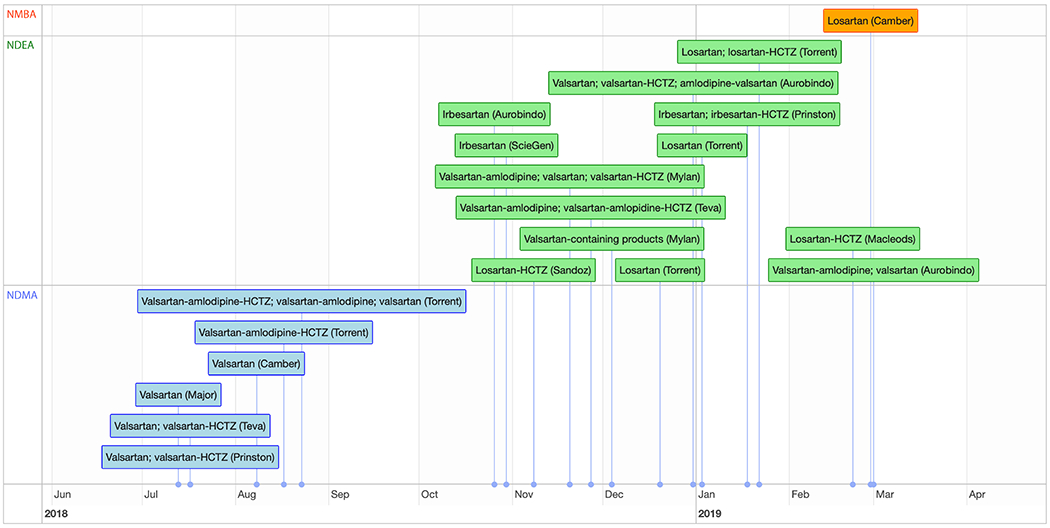
Recalls of Angiotensin-Receptor Blockers.
The first phase of recalls involved the genotoxic impurity NDMA (blue); the second involved NDEA (green); an additional recent recall involved NMBA (orange). Company names refer to the manufacturer and are not always the same as the distributor.
The compounds triggering these recalls are known as genotoxic impurities because they have the potential to damage DNA. The FDA found NDMA in affected valsartan products in concentrations ranging from 300 to 20,000 ng per tablet and in December 2018, FDA announced that it had set interim acceptable limits of NDMA (96 ng per day) and NDEA (26.5 ng per day), which they estimate would confer a 1 in 100,000 risk of causing cancer after 70 years of exposure (https://www.fda.gov/Drugs/DrugSafety/ucm613916.htm). FDA officials believe a change in the process used by the Chinese pharmaceutical company Zhejiang Huahai to manufacture active ingredients resulted in the inclusion of impurities.
Medicinal chemist and Science Translational Medicine blogger Derek Lowe has shed light on the recalls, highlighting that often a few manufacturers of active pharmaceutical ingredients are responsible for supplying all the companies that use them to make pills, which sometimes sell those pills to other companies for repackaging. Lowe also describes a technical process that may have caused the impurities.4 Zheijing Huahai has patented a method of manufacturing tetrazoles, a chemical group present in valsartan, irbesartan, and losartan and common to certain other ARBs and additional drug products. The European Medicines Agency and other experts have identified the reaction of dimethylamine and sodium nitrite under acidic conditions in synthesizing tetrazole groups as a potential source of NDMA.5 The finding of NMBA as an impurity or contaminant in an ARB product is very recent, and FDA’s investigation is ongoing.
Although some products containing valsartan, irbesartan, or losartan remain commercially available in the United States, patients and the health care system have paid a steep price beyond the health concerns and anxiety arising from exposure to recalled products. Some unaffected manufacturers have increased valsartan prices twofold, threefold, or even more.6 And the lack of selected ARB products has placed pressure on the supply chain for nonrecalled ARBs.
Though the FDA pinpoints a specific formulation and manufacturer in each recall, the dissemination of, and response to, FDA reports is uneven. Patients and clinicians may hear about recalls through the news media, social media, pharmacies, health care providers, or friends. Moreover, the public may hear about a recall of a “hypertension drug” but not know the specific product and manufacturer. Thus, recalls may trigger unnecessary concern among many people receiving antihypertensive therapy — and may be ignored by people who take ARBs for heart failure or chronic kidney disease. The burden of response has fallen to clinicians, pharmacies, and health care systems, most of which lack the infrastructure or resources to respond promptly to patients’ concerns.
Meanwhile, clinicians switching patients from one ARB to another are faced with the challenge of selecting an equipotent dose using a different formulation or active ingredient, often without adequate data from studies directly comparing drugs within the class. As a result, in order to avoid hyperkalemia, hypotension, undertreated hypertension, and harmful drug–drug interactions, additional laboratory tests and patient communications or visits have been required to assess safety and efficacy. As recalls have emerged slowly over this 6-month period, some clinicians have switched patients from a drug that was recalled early — valsartan — to one that was recalled later — irbesartan or losartan; the requisite additional switches have further undermined patients’ confidence in their clinician, their health care system, the drug supply chain, or all of the above.
FDA officials have stated that the agency began checking all ARB APIs and medicines marketed in the US for impurities. It is not yet clear whether manufacturers also employed an API synthesis process that risks introducing genotoxic impurities into products containing olmesartan or candesartan; the chemical structures of these ARBs also contain a tetrazole ring. The task of determining which products might be affected by unsafe tetrazole-synthesis methods is daunting, and the effort will have to be global in scale.
The ARB recalls demonstrate the FDA’s effectiveness in tracking the distribution of products within an increasingly complex supply chain of active pharmaceutical ingredients to their destinations throughout the United States. A larger challenge is inspection of the more than 80% of registered ingredient manufacturers that are located outside the United States. The FDA has taken steps to prepare for the ongoing globalization of drug manufacturing: it now has offices in China, India, Europe, and Latin America; and its Office of Regional and Country Affairs is responsible for additional Asian countries, as well as Canada, Australia, the Middle East, and African countries. In 2015, for the first time, the agency conducted more foreign than domestic inspections.
Nevertheless, the series of ARB recalls is a stress test for the FDA and for the capacity of health systems to respond to problems in the drug supply chain. Health systems would do well to assess their response to the recalls. Often, neither the prescriber nor the patient knows which manufacturer’s product will be available at the pharmacy, so physicians must rely on pharmacies to ensure that they are not distributing a recalled product. Health systems and physicians may therefore have limited insight into the number of their patients who are affected.
The ARB recalls provide an opportunity for the FDA, pharmacies, and health systems to evaluate all aspects of the response, from timely identification of potential impurities, to removal of recalled lots, to helping patients navigate the complexities of prescription changes, to monitoring them for adverse events after these changes are made. We can hope that efforts of the FDA and other regulatory agencies prevent the need for future recalls, but health systems and care providers should prepare themselves for what will happen if they don’t.
Footnotes
Disclosure forms provided by the authors are available at NEJM.org.
References
- 1.Clin Calc Drug Stats Database: Free U.S. outpatient drug usage statistics. (Accessed February 1, 2019, at https://clinicalc.com/DrugStats.)
- 2.Muntner P, Carey RM, Gidding S, et al. Potential U.S. population impact of the 2017 ACC/AHA high blood pressure guideline. J Am Coll Cardiol 2018;71:109–18.29146532 [Google Scholar]
- 3.Lowe D The sartan contamination story. In the pipeline. Science Translational Medicine January 4, 2019. https://blogs.sciencemag.org/pipeline/archives/2019/01/04/the-sartan-contamination-story
- 4.European Medicines Agency. CHMP List of questions to be addressed by the marketing authorisation holders for valsartan containing medicinal products. 2018.
- 5.Loftus P Drugmakers raise prices amid shortages, Recalls; patients and pharmacies grapple with sharply higher costs for certain generic drugs. Wall Street Journal, January 18, 2019. [Google Scholar]


