This cohort study investigates longitudinal patterns of cardiovascular health starting in childhood and their association with subclinical atherosclerosis in middle age.
Key Points
Question
Is there an association between long-term patterns in cardiovascular health starting in childhood and subclinical atherosclerosis in middle age?
Findings
In this cohort study of 9388 individuals, a significant number of children were classified as having an intermediate cardiovascular health score by 8 years of age. Many of these children experienced more rapid declines in cardiovascular health, had greater carotid intima-media thickness, and were more likely to have high carotid intima-media thickness by middle age.
Meaning
Increasing the proportion of children who reach adulthood in ideal cardiovascular health may be associated with reduced burden of cardiovascular disease at later age.
Abstract
Importance
Cross-sectional measures of cardiovascular health (CVH) have been associated with cardiovascular disease in older age, but little is known about longitudinal trajectories in CVH and their association with subclinical atherosclerosis in middle age.
Objectives
To model long-term patterns in CVH starting in childhood and to assess their association with subclinical atherosclerosis in middle age.
Design, Setting, and Participants
This cohort study used data from 5 prospective cardiovascular cohort studies from the United States and Finland from 1973 to 2015. A total of 9388 participants aged 8 to 55 years had at least 3 examinations and were eligible for this study. Statistical analysis was performed from December 1, 2015, to June 1, 2019.
Exposures
Clinical CVH factors (body mass index, total cholesterol level, blood pressure, and glucose level) were classified as ideal, intermediate, or poor, and were summed as a clinical CVH score. Group-based latent class modeling identified trajectories in this score over time.
Main Outcomes and Measures
Carotid intima-media thickness (cIMT) was measured for participants in 3 cohorts, and high cIMT was defined as a value at or above the 90th percentile. The association between CVH trajectory and cIMT was modeled using both linear and logistic regression adjusted for demographics, baseline health behaviors, and baseline (or proximal) CVH score.
Results
Among 9388 participants (5146 [55%] female; 6228 [66%] white; baseline mean [SD] age, 17.5 [7.5] years), 5 distinct trajectory groups were identified: high-late decline (1518 participants [16%]), high-moderate decline (2403 [26%]), high-early decline (3066 [32%]), intermediate-late decline (1475 [16%]), and intermediate-early decline (926 [10%]). The high-late decline group had significantly lower adjusted cIMT vs other trajectory groups (high-late decline: 0.64 mm [95% CI, 0.63-0.65 mm] vs intermediate-early decline: 0.72 mm [95% CI, 0.69-0.75 mm] when adjusted for demographics and baseline smoking, diet, and physical activity; P < .01). The intermediate-early declining group had higher odds of high cIMT (odds ratio, 2.4; 95% CI, 1.3-4.5) compared with the high-late decline group, even after adjustment for baseline or proximal CVH score.
Conclusions and Relevance
In this study, CVH declined from childhood into adulthood. Promoting and preserving ideal CVH from early life onward may be associated with reduced CVD risk later in life.
Introduction
In 2010, the American Heart Association (AHA) defined ideal cardiovascular health (CVH) and set its goal to improve CVH by 20% while reducing cardiovascular disease (CVD) mortality by 20% during the following decade.1 Significant decreases in CVD mortality have occurred since that time, and many risk factors among adults appear to be improving.2 However, poor CVH among children may derail these improvements as they grow into adults with a significant burden of cardiovascular risk.3
In the United States, fewer than 5% of adults have ideal CVH.4 According to serial, cross-sectional surveys,4,5,6 such as the National Health and Nutrition Examination Surveys (NHANES), the prevalence of ideal CVH is greater at successively earlier ages. Single measures of CVH during adolescence and young adulthood have been associated with the incidence of hypertension, metabolic syndrome, and hyperlipidemia and an increased burden of subclinical atherosclerosis.5,7,8,9,10,11,12 In particular, assessment of carotid intima-media thickness (cIMT) with ultrasonography is a noninvasive measure of CHD risk and has been shown to more accurately identify individuals at higher risk for CVD than using major risk factors alone.13,14 Among 4 cohorts of the International Childhood Cardiovascular Cohort consortium, a higher number of ideal CVH metrics in early adulthood (mean [SD] age, 36.6 [3.2] years) was cross-sectionally associated with thinner cIMT.7 The presence of cardiovascular risk factors at ages 9, 12, 15, and 18 years has been shown to be associated with elevated cIMT in adulthood.9 However, although these studies have examined cardiovascular risk at specific ages, little is known about longitudinal trajectories of CVH and their association with subclinical atherosclerosis in middle age.
This study had 2 distinct aims. We sought to describe CVH trajectories, for the first time from childhood through middle age (ages 8-55 years). We then sought to determine the association of these patterns with subclinical atherosclerosis as measured by cIMT in middle age. To address these aims we used pooled data from 5 childhood and young adult prospective cohorts including the Cardiovascular Risk in Young Finns Study (YFS), Bogalusa Heart Study (BHS), Project HeartBeat!, the Special Turku Coronary Risk Factor Intervention Project (STRIP), and the Coronary Artery Risk Development (CARDIA) study.15,16,17,18,19,20,21
Methods
Cohorts and Participants
This cohort study included participants from 5 cohorts. Each cohort collected clinical measurements at in-person examinations and used questionnaires to collect data on lifestyle variables over time. eTable 1 in the Supplement presents the demographics at baseline and follow-up for each cohort.
The BHS recruited a total of 11 796 children and adolescents, from whom data were used for 10 451 (approximately 33.3% African American and approximately 66.7% white) during 7 risk factor screenings between 1973 and 1993. More than 1300 children were subsequently followed-up during an adult examination between 20 and 37 years of age.15 The YFS included 3596 children aged 3 to 18 years at baseline in 1980; this study used data from 1986 onwards since this period was when glucose levels were first assessed. The subcohorts were followed up at years 3, 6, 9, 12, 21, 27, and 30.16 The CARDIA study recruited 5115 black and white young adults between 18 and 30 years of age at baseline from March 1985 to June 1986, from 4 sites across the United States.17 Since baseline, there have been 8 follow-up examinations with more than 30 years of follow-up.18 Project HeartBeat! data included 678 participants aged 8, 11, or 14 years at baseline in October 1991, and followed-up every 4 months for up to 4 years.19,20 These data provide an understanding of cardiovascular health patterns from ages 8 through 18 years.22 The STRIP study began in 1990 and was a randomized clinical trial of dietary intervention begun in infancy. The current study used the cohort of control children and incorporated only the data collected for children aged 15 to 19 years (n = 490).21 Aim 1 of this study was addressed using data from eligible participants from all 5 cohorts. Aim 2 included eligible participants from the 3 cohorts (YFS, BHS, and CARDIA) that measured cIMT during adult follow-up visits. Statistical analysis was performed from December 1, 2015, to June 1, 2019.
Cardiovascular Health Scoring
Body Mass Index
Body mass index was calculated as measured weight in kilograms divided by height in meters squared at each examination. Body mass index was converted to an age- and sex-specific percentile for those younger than 20 years using the Centers for Disease Control and Prevention calculation.23
Blood Pressure
Systolic and diastolic blood pressure were measured in all cohorts at every examination. Blood pressures were converted to a percentile for those younger than 20 years using the National Institutes of Health equations from the fourth report.24
Cholesterol Levels
Serum lipid levels were measured at each examination for each cohort. Given the different thresholds used for pediatric vs adult guidelines, we observed an artificial increase in mean cholesterol score when using the AHA-defined cut points.1 We used the midpoint of the pediatric and adult cut points for all ages to allow for analyses across childhood and adulthood (Table 1).
Table 1. Cardiovascular Health Score Components.
| Metric, age, y | Cardiovascular health type | ||
|---|---|---|---|
| Ideal | Intermediate | Poor | |
| Body mass indexa | |||
| ≥20 | <25.0 | 25.0-29.9 | ≥30.0 |
| <20 | <85th percentile | 85th-95th percentile | >95th percentile |
| Total cholesterol level, mg/dL | |||
| ≥20 | <185 (unmedicated) | 185-219; <185 (treated) | ≥220 |
| <20 | <185 (unmedicated) | 185-219; <185 (treated) | ≥220 |
| Blood pressure, mm Hg | |||
| ≥20 | <120/80 (unmedicated) | SBP of 120-139; DBP of 80-89; <120/80 (treated) | SBP≥140 or DBP≥90 |
| <20 | <90th percentile | 90th-95th percentile, SBP≥120, or DBP≥80 | >95th percentile |
| Blood glucose level, mg/dL | |||
| ≥20 | <100 (fasting, unmedicated) | 100-125; <100 (fasting or treated) | ≥126 (fasting) |
| <20 | <100 (fasting, unmedicated) | 100-125; <100 (fasting or treated) | ≥126 (fasting) |
| Physical activity | |||
| ≥20 | ≥150 min of moderate or ≥75 min of vigorous exercise per wk | 1-149 min of moderate or 1-74 min of vigorous exercise per wk | No exercise |
| <20 | ≥60 min of moderate- or vigorous-intensity activity daily | >0 and <60 min of moderate- or vigorous-intensity activity daily | No exercise |
| Components of healthy diet, No. | |||
| ≥20 | 4-5 | 2-3 | 0-1 |
| <20 | 4-5 | 2-3 | 0-1 |
| Smoker | |||
| ≥20 | Never or quit >12 mo ago | Former or quit ≤12 mo ago | Current |
| <20 | Never tried or smoked whole cigarette | NA | Tried before 30 d |
Abbreviation: NA, not applicable.
SI conversion factors: To convert cholesterol to millimoles per liter, multiply by 0.0259; glucose to millimoles per liter, multiply by 0.0555.
Calculated as weight in kilograms divided by height in meters squared.
Blood Glucose Level
Fasting blood (plasma or serum) glucose level was measured at multiple examinations across each cohort. Ideal, intermediate, and poor levels of glucose were defined according to AHA definitions.1
Diet
A summary for scoring the diet components is given in Table 1 (components of a healthy diet: ideal [4-5]; intermediate [2-3]; poor [0-1]); any cohort-specific changes to this scoring are described in the eMethods in the Supplement.
Physical Activity
A summary for scoring the minutes of physical activity is given in Table 1 (age <20 years: ideal [≥60 minutes of moderate- or vigorous-intensity activity daily], intermediate [>0 and <60 minutes of moderate-or vigorous-intensity activity daily], poor [no exercise]; age ≥20 years: ideal [≥150 minutes of moderate or >75 minutes of vigorous exercise per week], intermediate [1-149 minutes of moderate or 1-74 minutes of vigorous exercise per week], poor [no exercise]). The BHS physical activities were categorized as moderate or vigorous using metabolic equivalents and methods described previously.25 The YFS physical activity data varied among years; thus, the only variables included in score calculation were frequency and intensity to determine minutes per day of moderate and vigorous activity.8
The CARDIA physical activity data used a heavy, moderate, and total intensity score calculated using previously described methods.26 We calculated quintiles of that total intensity score at each examination and used methods similar to those for calculating the aforementioned diet score. The top 40% were scored as ideal, the middle 40% as intermediate, and the bottom 20% as poor physical activity for each examination separately.
Project HeartBeat! physical activity data were collected using a 24-hour recall of minutes of moderate and vigorous activities and included time for physical education class (none of the other cohorts included this). We assumed that the data for these 24 hours were typical of every day and subtracted a 30-minute penalty to correct for the physical education discrepancy.
The STRIP study collected data on habitual physical activity using self-report.27 We calculated minutes per day of moderate and vigorous activity by multiplying frequency by duration, assuming that data were typical of every day.
Smoking
Smoking data were captured differently in all 5 cohorts. In BHS, YFS, and Project HeartBeat!, there was no time component given to delineate between former smokers and current smokers; thus, individuals were categorized as either poor or ideal. The CARDIA participants could be categorized according to the criteria given in Table 1 (age <20 years: ideal [never tried or never smoked a whole cigarette, poor [tried smoking before 30 days]; age ≥20 years: ideal [never smoked or quit >12 months ago], intermediate [former smoker or quit ≤12 months ago], poor [current smoker]). The STRIP data included a yes or no variable for “never smoked a cigarette”; thus, participants were categorized as ideal or poor.
CVH Scores
Each body mass index, blood pressure, blood glucose level, total cholesterol level, smoking, diet, and physical activity observation was converted to a CVH score using cut points based on AHA criteria (Table 1).1 Scores ranged from 2 (most favorable) to 0 (least favorable), with 0 representing poor; 1, intermediate; and 2, ideal CVH.
Scores from 4 of the 7 CVH metrics—body mass index, blood pressure, total cholesterol level, and blood glucose level—were summed to create a clinical CVH score. This score ranged from 8 to 0, with 8 being ideal CVH and 0 being poor CVH. Scores from all 7 CVH metrics were summed to create a full CVH score ranging from 14 to 0, with 14 being ideal CVH and 0 being poor CVH. The clinical CVH score was used in the primary analyses because it was available for participants at 3 or more examinations in all 5 of the cohorts. The CVH score including all 7 metrics was only examined in an exploratory analysis because it was only available at 3 or more examinations in 3 cohorts (YFS, CARDIA, and Project HeartBeat!).
Carotid IMT
The cIMT data in adulthood were available from CARDIA, YFS, and BHS (eFigure in the Supplement gives a timeline of cIMT collection relative to study follow-up). The BHS conducted carotid artery ultrasonography at follow-up (ages 20-43 years). The B-mode ultrasonography examinations were performed according to protocols described previously and had high intraindividual reproducibility with similar mean differences.28 Among the YFS participants, carotid ultrasonography was conducted at adult follow-up examinations in 2001 and 2007 (ages 24-45 years). The standardized B-mode ultrasonography studies have been previously described.29 A second ultrasonography was performed for 60 participants at 3 months after their initial visit and showed high intraindividual reproducibility.29 High-resolution B-mode ultrasonography was conducted at year 20 in CARDIA (ages 38-50 years) among 92% of participants who were seen in the clinics. The CARDIA ultrasonography studies have been described by Polak et al30 and had correlation coefficients in replicate studies ranging from 0.72 for the bulb to 0.88 for the internal carotid artery. The cIMT was measured at more than 1 occasion in YFS and BHS; thus, the most recent measurement was used for analysis.
In addition to the continuous cIMT measurement, an age-, sex-, race-, and cohort-specific 90th percentile cut point determined high cIMT, a method used by Magnussen et al.31 The cut points for all analyses were calculated using the participants in the clinical CVH score group (eTable 2 in the Supplement).
Covariates
Demographic variables including the participants’ age, sex, and race were collected in all cohorts. The BHS, CARDIA, and YFS included a self-reported measure of years of education for the participants’ mother and father. In this study, parental educational level was categorized as seen in Table 2 to harmonize the data among cohorts.
Table 2. Baseline Characteristics and Examination Counts by Clinical CVH Score Trajectory Groupa.
| Characteristic | CVH score trajectory group | P value | |||||
|---|---|---|---|---|---|---|---|
| High-late decline (n = 1518) | High-moderate decline (n = 2403) | High-early decline (n = 3066) | Intermediate-late decline (n = 1475) | Intermediate-early decline (n = 926) | Overall (N = 9388) | ||
| Baseline characteristicb | |||||||
| Female | 1026 (67.6) | 1379 (57.4) | 1480 (48.3) | 860 (58.3) | 401 (43.3) | 5146 (54.8) | <.001 |
| Race | |||||||
| White | 1156 (76.2) | 1627 (67.7) | 1874 (61.1) | 1030 (69.8) | 541 (58.4) | 6228 (66.3) | <.001 |
| Black | 362 (23.8) | 768 (32.0) | 1191 (38.8) | 444 (30.1) | 384 (41.5) | 3149 (33.5) | |
| Other | 0 | 8 (0.3) | 1 (0.03) | 1 (0.1) | 1 (0.1) | 11 (0.1) | |
| Age, mean (SD), y | 19.26 (7.7) | 17.20 (7.4) | 17.10 (7.4) | 17.61 (7.6) | 16.05 (7.3) | 17.45 (7.5) | <.001 |
| Age group, y | |||||||
| 8-11 | 417 (27.5) | 882 (36.7) | 1079 (35.2) | 494 (33.5) | 387 (41.8) | 3259 (34.7) | <.001 |
| 12-19 | 318 (21.0) | 576 (24.0) | 820 (26.7) | 374 (25.4) | 239 (25.8) | 2327 (24.8) | |
| ≥20 | 783 (51.6) | 945 (39.3) | 1167 (38.1) | 607 (41.2) | 300 (32.4) | 3802 (40.5) | |
| Cohort | |||||||
| Young Finns | 259 (17.1) | 400 (16.6) | 658 (21.5) | 420 (28.5) | 225 (24.3) | 1962 (20.9) | <.001 |
| Project HeartBeat! | 0 | 61 (2.5) | 32 (1.0) | 10 (0.7) | 11 (1.2) | 114 (1.2) | |
| CARDIA | 861 (56.7) | 1042 (43.4) | 1301 (42.4) | 660 (44.8) | 336 (36.3) | 4200 (44.7) | |
| Bogalusa | 398 (26.2) | 822 (34.2) | 995 (32.4) | 358 (24.3) | 334 (36.1) | 2907 (31.0) | |
| STRIP | 0 | 78 (3.2) | 80 (2.6) | 27 (1.8) | 20 (2.2) | 205 (2.2) | |
| Clinical measures, mean (SD) | |||||||
| BMI | 19.2 (3.0) | 19.5 (3.6) | 21.8 (5.9) | 21.3 (4.6) | 25.0 (7.0) | 21.0 (5.2) | <.001 |
| Percentile BMI by age and sexc | 34.5 (24.1) | 38.3 (23.8) | 50.5 (27.8) | 53.4 (31.1) | 75.4 (25.9) | 48.4 (29.1) | <.001 |
| Blood pressure, mm Hg | |||||||
| Systolic | 103.7 (10.1) | 104.5 (10.4) | 109.2 (12.4) | 110.2 (11.9) | 115.0 (12.4) | 107.8 (12.0) | <.001 |
| Percentile systolicc | 31.6 (21.4) | 34.5 (21.9) | 42.0 (24.7) | 51.6 (27.5) | 61.0 (25.8) | 41.7 (25.6) | <.001 |
| Diastolic | 60.7 (12.4) | 58.8 (13.9) | 61.4 (15.3) | 63.1 (13.9) | 63.8 (16.0) | 61.1 (14.4) | <.001 |
| Percentile diastolicc | 20.6 (20.3) | 20.3 (19.9) | 22.4 (22.3) | 25.8 (24.8) | 28.0 (24.9) | 22.6 (22.2) | <.001 |
| Total cholesterol level, mg/dL | 162.9 (26.2) | 165.3 (27.3) | 182.3 (35.1) | 188.2 (36.7) | 199.8 (37.1) | 177.4 (34.6) | <.001 |
| Fasting glucose level, mg/dL | 80.7 (7.9) | 81.2 (7.7) | 83.2 (9.7) | 82.8 (9.5) | 89.00 (27.5) | 82.7 (12.1) | <.001 |
| Ideal behavior scoresd | |||||||
| Smoking | 1092 (71.9) | 1776 (73.9) | 2259 (73.7) | 1061 (71.9) | 685 (74.0) | 6873 (73.2) | .01 |
| Diet | 547 (41.6) | 571 (29.3) | 641 (24.9) | 416 (32.4) | 166 (21.4) | 2341 (29.7) | <.001 |
| Physical activity | 440 (33.7) | 592 (30.3) | 736 (28.9) | 350 (27.4) | 215 (27.8) | 2333 (29.7) | .02 |
| Maternal educational level, ye | |||||||
| <6 | 31 (2.4) | 72 (3.9) | 130 (5.4) | 65 (5.3) | 32 (4.3) | 330 (4.4) | <.001 |
| 6-9 | 154 (12.2) | 233 (12.6) | 436 (18.0) | 210 (17.0) | 166 (22.5) | 1199 (16.0) | |
| 9-12 | 510 (40.4) | 850 (46.1) | 1103 (45.5) | 508 (41.1) | 350 (47.5) | 3321 (44.3) | |
| 12-16 | 419 (33.2) | 513 (27.8) | 581 (24.0) | 311 (25.2) | 145 (19.7) | 1969 (26.2) | |
| >16 (graduate school) | 149 (11.8) | 175 (9.5) | 173 (7.1) | 141 (11.4) | 44 (6.0) | 682 (9.1) | |
| Paternal educational level, ye | |||||||
| <6 | 48 (3.9) | 100 (5.6) | 149 (6.4) | 74 (6.1) | 54 (7.6) | 425 (5.8) | <.001 |
| 6-9 | 159 (12.8) | 295 (16.6) | 513 (21.9) | 271 (22.5) | 164 (23.1) | 1402 (19.3) | |
| 9-12 | 454 (36.6) | 753 (42.3) | 1020 (43.6) | 449 (37.3) | 331 (46.7) | 3007 (41.3) | |
| 12-16 | 362 (29.2) | 442 (24.8) | 498 (21.3) | 281 (23.4) | 126 (17.8) | 1709 (23.5) | |
| >16 (graduate school) | 218 (17.6) | 192 (10.8) | 162 (6.9) | 128 (10.6) | 34 (4.8) | 734 (10.1) | |
| Examinations with clinical cardiovascular scores, mean (SD), No. | 4.8 (1.3) | 4.6 (1.4) | 4.6 (1.4) | 4.5 (1.3) | 4.5 (1.4) | 4.6 (1.4) | <.001 |
| cIMT measurements | 1088 (71.7) | 1490 (62.0) | 2035 (66.4) | 1058 (71.7) | 616 (66.5) | 6287 (67.0) | <.001 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); cIMT, carotid intima-media thickness; CVH, cardiovascular health.
A 5-group, cubic order trajectory model was used. Data are presented as number (percentage) of individuals unless otherwise indicated.
Baseline age group and clinical measures were taken from a participant’s first examination even if that examination had a missing clinical score.
For BMI (n = 3343), systolic blood pressure, and diastolic blood pressure (n = 1412), percentiles were only available for those younger than 20 years at baseline.
Behavior scores were not available for all participants. Imputed and observed values were used: smoking scores (9388 [100%]); diet (7894 [84%]); physical activity (7855 [84%]). Ideal behavior scores are described in the Methods section.
Maternal and paternal education data were not available for all individuals. Imputed and observed values were used: father (7277 [78%]); mother (7501 [80%]).
Statistical Analysis
In the primary analysis for aim 1, participants with clinical CVH scores at 3 or more distinct ages were included in latent class trajectory modeling using the SAS procedure Proc Traj.32,33 We used posterior predictive probabilities assigned by Proc Traj to impute group membership 20 times per individual to account for uncertainty in group assignment.
To address aim 2, the association between trajectory group assignment and cIMT was modeled using regression among 6287 participants who had cIMT measured in middle age. All models were sequentially adjusted for demographic characteristics (age, race, sex, cohort, and maternal and paternal educational level) and either baseline or proximal clinical score because of the correlation between baseline and proximal clinical scores.
Analyses of the clinical CVH score were additionally adjusted for baseline lifestyle factors, including baseline diet, smoking, and physical activity scores. To address missing covariates, parental educational level and baseline lifestyle variables (physical activity, diet) were imputed in R34 (The R Project for Statistical Computing) using the mice package.35 The 20 imputations were generated by Gibbs sampling, and the predictors for each imputed variable included all other covariates in the model. Regression models were run using the imputed data sets and results were pooled using Proc MiAnalyze (SAS Institute Inc). Statistical analysis was performed using SAS version 9.4. Statistical significance was set a priori at 2-sided P < .05.
Results
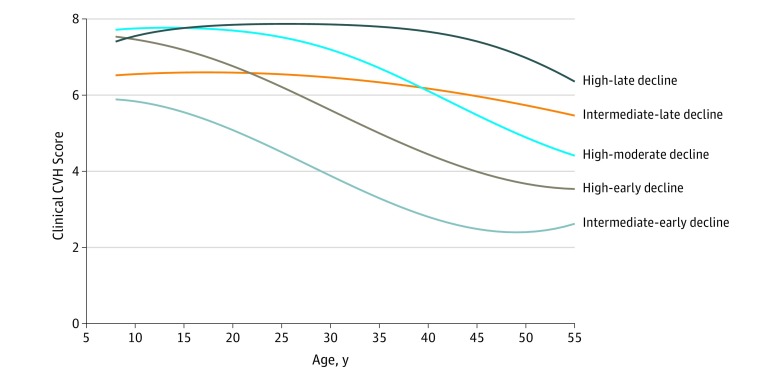
This study included 9388 children and young adults; 5146 (55%) were female, 6228 (66%) were white, the mean (SD) age at baseline was 17.5 (7.5) years, and the mean (SD) follow-up time was 23.0 (8.1) years. We identified 5 distinct trajectories in clinical CVH starting at 8 years of age and continuing through 55 years of age (Figure). These 5 trajectory groups included a high-late decline group that maintained high clinical CVH scores throughout childhood and early adulthood (1518 participants [16%]), a high-moderate decline group (2403 [26%]), a high-early decline group (3066 [32%]), an intermediate-late decline group (1475 [16%]), and an intermediate-early decline group (926 [10%]). Three of the 5 groups started at similarly high levels of clinical CVH at 8 years of age, whereas 2 started at an intermediate level of clinical CVH and including 25% of the population. However, the patterns of CVH in these groups diverged throughout childhood and adulthood.
Figure. Clinical Cardiovascular Health Score Trajectories by Age.
The clinical cardiovascular health score (range, 0-8) was the result of the sum of points (0, 1, or 2) for body mass index, total cholesterol level, blood pressure, and blood glucose level. The analysis included participants with clinical cardiovascular health scores at 3 or more distinct ages.
Groups with higher childhood clinical CVH scores were more likely to be female and to have more favorable levels of cardiovascular risk factors. For example, the high-late declining group was 67.6% female with percentile body mass index of 34.5 compared with 43.3% female with percentile body mass index of 75.4 among the intermediate-early decline group (Table 2). Groups that started at intermediate levels and/or experienced rapid declines were less likely to have an ideal diet (intermediate-early decline: 166 [21.4%]; high-late decline: 547 [41.6%]; P < .001) or meet guidelines for the recommended amount of physical activity (intermediate-early decline: 215 [27.8%]; high-late decline: 440 [33.7%]; P = .02) at baseline. Individuals in the high-late decline clinical CVH group had higher maternal and paternal educational levels (558 [45%] mothers and 580 [47%] of fathers had greater than a high school education compared with 2651 mothers [35%] and 2443 fathers [34%] overall; P < .001).
Among the 3763 participants who had 3 or more measures of the full CVH score, we identified 4 unique trajectories in full CVH score for those aged 8 to 60 years, including a high-stable group (656 [17%]), stable group (1445 [38%]), an intermediate-decline group (1287 [34%]), and a low-decline group (375 [10%]) (eFigure in the Supplement). The high stable group was more likely to be female (386 [59%], P < .001), to be white (531 [81%], P < .001), and to have higher parental educational level (high school and above: 393 maternal [61%] and 431 paternal [66%], P < .001) (eTable 3 in the Supplement). eTable 4 in the Supplement shows the cross-tabulation of trajectory group membership for the full CVH score and the clinical CVH score.
The clinical CVH score trajectory group was associated with continuous cIMT in adulthood (Table 3). After adjustment for demographics and baseline smoking, physical activity, and diet, the high-late decline CVH group had the thinnest cIMT (0.64 mm; 95% CI, 0.63-0.65 mm), whereas the group with the poorest health, the intermediate-early decline group, had the thickest cIMT (0.72 mm; 95% CI, 0.69-0.75 mm; P < .001). Trajectory groups that began with an intermediate CVH score and those who experienced more rapid decline over time had the thickest cIMT. These patterns remained consistent even after adjustment for the baseline or proximal CVH scores, indicating that clinical CVH trajectories, representing the pattern and timing of change in CVH, were associated with cIMT in middle age beyond cross-sectional CVH scores in childhood or concurrent with the cIMT. Clinical CVH trajectories were also associated higher odds of cIMT, based on age, race, and cohort-specific cut points in middle age (Table 4).
Table 3. Association Between Carotid Intima-Media Thickness and Clinical CVH Score Trajectory Group.
| Clinical CVH score trajectory groupa | Carotid intima-media thickness, mm | |||||
|---|---|---|---|---|---|---|
| Adjustedb | Adjusted and baseline scorec | Adjusted and proximal scored | ||||
| Point estimate, mean (95% CI) | P Value | Point estimate, mean (95% CI) | P Value | Point estimate, mean (95% CI) | P Value | |
| High-late decline | 0.64 (0.63-0.65) | NA | 0.72 (0.69-0.75) | NA | 0.71 (0.68-0.74) | NA |
| High-moderate decline | 0.67 (0.64-0.69) | <.001 | 0.75 (0.71-0.79) | <.001 | 0.72 (0.68-0.77) | .04 |
| High-early decline | 0.69 (0.67-0.71) | <.001 | 0.76 (0.72-0.80) | <.001 | 0.73 (0.69-0.78) | .003 |
| Intermediate-late decline | 0.67 (0.64-0.69) | <.001 | 0.74 (0.69-0.78) | .02 | 0.73 (0.68-0.77) | .02 |
| Intermediate-early decline | 0.72 (0.69-0.75) | <.001 | 0.78 (0.73-0.83) | <.001 | 0.75 (0.70-0.80) | <.001 |
Abbreviations: cIMT, carotid intima-media thickness; CVH, cardiovascular health; NA, not applicable.
Calculation of the CVH score is detailed in the CVH Scores subsection of the Methods section.
Adjusted for age, sex, race, cohort, maternal and paternal educational level, baseline smoking, physical activity, and diet scores.
Adjusted for age, sex, race, cohort, maternal and paternal educational level, baseline smoking, physical activity, diet scores, and baseline CVH score. Baseline CVH score was the earliest recorded CVH score per participant.
Adjusted for age, sex, race, cohort, maternal and paternal educational level, baseline smoking, physical activity, diet scores, and proximal CVH score. Proximal CVH score was the clinical CVH score from the examination at or before the cIMT measurement.
Table 4. Association Between Clinical CVH Score Trajectory and High cIMTa.
| Clinical CVH Score Trajectoryb | Adjusted modelc | Adjusted and baseline scored | Adjusted and proximal scoree | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | OR (95% CI) | P Value | |
| High-late decline | 1 [Reference] | NA | 1 [Reference] | NA | 1 [Reference] | NA |
| High-moderate decline | 1.58 (1.11-2.23) | .010 | 1.54 (1.09-2.18) | .01 | 1.32 (0.90-1.95) | .16 |
| High-early decline | 2.61 (1.89-3.61) | <.001 | 2.38 (1.68-3.36) | <.001 | 1.88 (1.20-2.95) | .01 |
| Intermediate-late decline | 1.67 (1.16-2.43) | .001 | 1.54 (1.05-2.26) | .03 | 1.44 (0.98-2.13) | .06 |
| Intermediate-early decline | 3.81 (2.60-5.59) | <.001 | 3.12 (1.92-5.05) | <.001 | 2.35 (1.26-4.37) | .01 |
Abbreviations: CVH, cardiovascular health; cIMT, carotid intima-media thickness; OR, odds ratio.
See eTable 2 in the Supplement for cohort, race, sex, and age-specific 90th percentile cIMT cut points. High cIMT was defined as a cIMT value at or above the given cut point.
Calculation of the CVH score is detailed in the CVH Scores subsection of the Methods section.
Adjusted for age, sex, race, cohort, maternal and paternal educational level, baseline smoking, physical activity, and diet scores.
Adjusted for age, sex, race, cohort, maternal and paternal educational levels, baseline smoking, physical activity, diet scores, and baseline CVH score. Baseline CVH score was the earliest recorded CVH score per participant.
Adjusted for age, sex, race, cohort, maternal and paternal educational level, baseline smoking, physical activity, diet scores, and proximal CVH score. Proximal CVH score was the clinical CVH score from the examination at or before the cIMT measurement.
In exploratory analyses, we found a significant association of full CVH score trajectory with cIMT as measured continuously or stratified as high cIMT (eTable 5 and eTable 6 in the Supplement). These results were generally consistent across cohorts (eTable 7 in the Supplement).
Discussion
Among this large, pooled cohort, we identified 5 novel and distinct trajectories in clinical CVH score among individuals aged 8 to 55 years. These CVH trajectories were significantly associated with the level of subclinical atherosclerosis in middle age independent of single cross-sectional measures of CVH at either baseline or the time of cIMT measurement. Groups beginning in childhood with poor CVH experienced more rapid declines in CVH, had greater cIMT, and were more likely to have high cIMT by middle age.
This study presented, to our knowledge, the first data on long-term trajectories of CVH starting in childhood. These findings showed that at an individual-level CVH score decreased with age and that these decreases started earlier than expected. Of importance, at 8 years of age, there was significant stratification in clinical CVH, with more than 25% of the population having intermediate CVH. Future studies should examine the timing and causes for these decreases in CVH score so that interventions can be designed to effectively promote and maintain ideal CVH throughout the lifetime.
Our findings are consistent with previous studies8,9 that reported that childhood CVH was associated with later risk for CVD. Previous work by the International Childhood Cardiovascular Cohort Consortium showed that the number of cardiovascular risk factors starting at 9 years of age was significantly associated with subclinical atherosclerosis in adulthood.9 In addition, numerous studies3,20,36,37 have found individual CVH metrics, including obesity, diabetes, and blood pressure, measured in childhood may be associated with the incidence of CVD later in life.
Studies are increasingly showing the detrimental outcomes associated with cumulative exposures to elevated CVD risk factors.38,39,40 Even when CVD risk factors, such as blood pressure, are treated to optimal levels, individuals remain at higher risk for future CVD compared with individuals who had maintained optimal blood pressure throughout their lives without medication.41 There is evidence that this residual risk may be attributable to a greater burden of CVD risk associated with cumulative elevations, even within the normal range before treatment.41 Although it remains important to provide treatment to individuals with elevated risk factor levels, the most effective way to reduce the burden of future CVD may be to prevent the development of those CVD risk factors, an approach termed primordial prevention. There is a large body of literature showing effective interventions that may help individuals maintain ideal CVH.42,43 Our findings suggest that these interventions are critical and should be implemented early in life to prevent the loss of CVH and future CVD development.
Strengths and Limitations
This study has several strengths, including a large harmonized data set with multiple examinations from childhood through middle age using individual participant data. In addition, we used sophisticated trajectory methods to examine long-term patterns in CVH, representing both the absolute level of CVH and the slopes of CVH change.
This study has limitations. First, the studies from which the cohort data were derived had variations in protocols and methods of CVH measurement, which may have resulted in some misclassification. However, this is likely to be nondifferential, therefore producing conservative estimates. In addition, creating a summary score for CVH rank-ordered people and lessened differences in absolute values that may occur across cohorts. Slightly less than half of all cohort participants attended 3 or more examinations and thus could be included in the trajectory analysis. However, participants included in this analysis had similar demographics and risk factor levels compared with those who had fewer than 3 examinations (eTable 8 in the Supplement). In addition, when analyses were performed using inverse probability weighting to account for differences in the likelihood of having cIMT measurements, we found that results were consistent and the association of CVH trajectories with cIMT remained positive and significant. As with all observational studies, there is the potential for additional confounding by unmeasured covariates.
Conclusions
The findings suggest that maintaining high levels of CVH from childhood onward may be associated with a reduced future burden of cardiovascular disease. All trajectory groups experienced a decline in CVH during childhood and adolescence through early adulthood. Future research appears to be needed to determine the effect of targeting interventions aimed at preserving and promoting CVH to those children at highest risk of experiencing a decline in CVH. Increasing the proportion of children who reach adulthood in ideal CVH may be associated with a reduced burden of cardiovascular disease at a later age and could help achieve the AHA’s 2020 goals of improving the CVH of all individuals in the United States.
eMethods. Diet Scoring
eReferences
eTable 1. Demographics at Baseline and at Follow-up by Cohort
eTable 2. Cohort, Age, Sex, and Race Specific Cut Points for Defining High cIMT
eTable 3. Baseline Characteristics and Exam Counts by CVH Score Trajectory Group
eTable 4. Cross-tabulation of Trajectory Group Membership, for Those With a Full CVH Score Trajectory Group Assignment (N = 3736)
eTable 5. Association Between Carotid IMT (mm) and CVH Score Trajectory Group
eTable 6. The Association Between High Carotid IMT and CVH Score Trajectory Group
eTable 7. The Association Between High Carotid IMT and CVH Score Cohort-specific Trajectory Group, Stratified by Cohort
eTable 8. Baseline Characteristics and Exam Counts by Analytic Sample
eFigure. CVH Score Trajectories by Age
References
- 1.Lloyd-Jones DM, Hong Y, Labarthe D, et al. ; American Heart Association Strategic Planning Task Force and Statistics Committee . Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation. 2010;121(4):586-613. doi: 10.1161/CIRCULATIONAHA.109.192703 [DOI] [PubMed] [Google Scholar]
- 2.Mozaffarian D, Benjamin EJ, Go AS, et al. ; Writing Group Members; American Heart Association Statistics Committee; Stroke Statistics Subcommittee . Heart disease and stroke statistics–2016 update: a report from the American Heart Association. Circulation. 2016;133(4):e38-e360. doi: 10.1161/CIR.0000000000000350 [DOI] [PubMed] [Google Scholar]
- 3.Steinberger J, Daniels SR, Hagberg N, et al. ; American Heart Association Atherosclerosis, Hypertension, and Obesity in the Young Committee of the Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; Council on Epidemiology and Prevention; Council on Functional Genomics and Translational Biology; and Stroke Council . Cardiovascular health promotion in children: challenges and opportunities for 2020 and beyond: a scientific statement from the American Heart Association. Circulation. 2016;134(12):e236-e255. doi: 10.1161/CIR.0000000000000441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shay CM, Ning H, Allen NB, et al. Status of cardiovascular health in US adults: prevalence estimates from the National Health and Nutrition Examination Surveys (NHANES) 2003-2008. Circulation. 2012;125(1):45-56. doi: 10.1161/CIRCULATIONAHA.111.035733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shay CM, Ning H, Daniels SR, Rooks CR, Gidding SS, Lloyd-Jones DM. Status of cardiovascular health in US adolescents: prevalence estimates from the National Health and Nutrition Examination Surveys (NHANES) 2005-2010. Circulation. 2013;127(13):1369-1376. doi: 10.1161/CIRCULATIONAHA.113.001559 [DOI] [PubMed] [Google Scholar]
- 6.Ning H, Labarthe DR, Shay CM, et al. Status of cardiovascular health in US children up to 11 years of age: the National Health and Nutrition Examination Surveys 2003-2010. Circ Cardiovasc Qual Outcomes. 2015;8(2):164-171. doi: 10.1161/CIRCOUTCOMES.114.001274 [DOI] [PubMed] [Google Scholar]
- 7.Oikonen M, Laitinen TT, Magnussen CG, et al. Ideal cardiovascular health in young adult populations from the United States, Finland, and Australia and its association with cIMT: the International Childhood Cardiovascular Cohort Consortium. J Am Heart Assoc. 2013;2(3):e000244. doi: 10.1161/JAHA.113.000244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laitinen TT, Pahkala K, Magnussen CG, et al. Ideal cardiovascular health in childhood and cardiometabolic outcomes in adulthood: the Cardiovascular Risk in Young Finns Study. Circulation. 2012;125(16):1971-1978. doi: 10.1161/CIRCULATIONAHA.111.073585 [DOI] [PubMed] [Google Scholar]
- 9.Juonala M, Magnussen CG, Venn A, et al. Influence of age on associations between childhood risk factors and carotid intima-media thickness in adulthood: the Cardiovascular Risk in Young Finns Study, the Childhood Determinants of Adult Health Study, the Bogalusa Heart Study, and the Muscatine Study for the International Childhood Cardiovascular Cohort (i3C) Consortium. Circulation. 2010;122(24):2514-2520. doi: 10.1161/CIRCULATIONAHA.110.966465 [DOI] [PubMed] [Google Scholar]
- 10.Laitinen TT, Ruohonen S, Juonala M, et al. Ideal cardiovascular health in childhood–longitudinal associations with cardiac structure and function: the Special Turku Coronary Risk Factor Intervention Project (STRIP) and the Cardiovascular Risk in Young Finns Study (YFS). Int J Cardiol. 2017;230:304-309. doi: 10.1016/j.ijcard.2016.12.117 [DOI] [PubMed] [Google Scholar]
- 11.Laitinen TT, Pahkala K, Magnussen CG, et al. Lifetime measures of ideal cardiovascular health and their association with subclinical atherosclerosis: the Cardiovascular Risk in Young Finns Study. Int J Cardiol. 2015;185:186-191. doi: 10.1016/j.ijcard.2015.03.051 [DOI] [PubMed] [Google Scholar]
- 12.Pahkala K, Hietalampi H, Laitinen TT, et al. Ideal cardiovascular health in adolescence: effect of lifestyle intervention and association with vascular intima-media thickness and elasticity (the Special Turku Coronary Risk Factor Intervention Project for Children [STRIP] study). Circulation. 2013;127(21):2088-2096. doi: 10.1161/CIRCULATIONAHA.112.000761 [DOI] [PubMed] [Google Scholar]
- 13.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143-3421. doi: 10.1161/circ.106.25.3143 [DOI] [PubMed] [Google Scholar]
- 14.Stein JH, Korcarz CE, Hurst RT, et al. ; American Society of Echocardiography Carotid Intima-Media Thickness Task Force.; Endorsed by the Society for Vascular Medicine . Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force [published correction appears in J Am Soc Echocardiogr. 2008;21(4):376]. J Am Soc Echocardiogr. 2008;21(2):93-111. doi: 10.1016/j.echo.2007.11.011 [DOI] [PubMed] [Google Scholar]
- 15.ClinicalTrials.gov. Bogalusa Heart Study. https://clinicaltrials.gov/ct2/show/record/NCT00005129?term=bogalusa&rank=1. Accessed Sept 21, 2016.
- 16.Raitakari OT, Juonala M, Rönnemaa T, et al. Cohort profile: the cardiovascular risk in Young Finns Study. Int J Epidemiol. 2008;37(6):1220-1226. doi: 10.1093/ije/dym225 [DOI] [PubMed] [Google Scholar]
- 17.Friedman GD, Cutter GR, Donahue RP, et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41(11):1105-1116. doi: 10.1016/0895-4356(88)90080-7 [DOI] [PubMed] [Google Scholar]
- 18.National Institutes of Health. National Heart. Lung, and Blood Institute The coronary artery risk development in young adults study (CARDIA). https://web.archive.org/web/20160729174049/https://www.nhlbi.nih.gov/research/resources/obesity/population/cardia.htm. Published 2014. Accessed September 21, 2016.
- 19.Labarthe DR, Dai S, Day RS, Fulton JE, Grunbaum JA; Project HeartBeat! Writing Group . Findings from Project HeartBeat!: their importance for CVD prevention. Am J Prev Med. 2009;37(1)(suppl):S105-S115. doi: 10.1016/j.amepre.2009.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Labarthe DR, Dai S, Day RS, et al. Project HeartBeat!: concept, development, and design. Am J Prev Med. 2009;37(1)(suppl):S9-S16. doi: 10.1016/j.amepre.2009.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simell O, Niinikoski H, Rönnemaa T, et al. ; STRIP Study Group . Cohort profile: the STRIP Study (Special Turku Coronary Risk Factor Intervention Project), an infancy-onset dietary and life-style intervention trial. Int J Epidemiol. 2009;38(3):650-655. doi: 10.1093/ije/dyn072 [DOI] [PubMed] [Google Scholar]
- 22.Labarthe DR, Dai S, Harrist RB. Blood lipids, blood pressure, and BMI in childhood and adolescence: background to Project HeartBeat! Am J Prev Med. 2009;37(1)(suppl):S3-S8. doi: 10.1016/j.amepre.2009.04.015 [DOI] [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention, Division of Nutrition, Physical Activity, and Obesity. A SAS program for the 2000 CDC Growth Charts (ages 0 to <20 years). 2016. https://web.archive.org/web/20151211001307/https://www.cdc.gov/nccdphp/dnpao/growthcharts/resources/sas.htm Accessed December 10, 2015.
- 24.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114(2)(Suppl 4th Report):555-576. doi: 10.1542/peds.114.2.S2.555 [DOI] [PubMed] [Google Scholar]
- 25.Myers L, Strikmiller PK, Webber LS, Berenson GS. Physical and sedentary activity in school children grades 5-8: the Bogalusa Heart Study. Med Sci Sports Exerc. 1996;28(7):852-859. doi: 10.1097/00005768-199607000-00012 [DOI] [PubMed] [Google Scholar]
- 26.Jacobs DR Jr, Hahn LP, Haskell WL, Pirie P, Sidney S. Validity and reliability of short physical activity history: CARDIA and the Minnesota Heart Health Program. J Cardiopulm Rehabil. 1989;9(11):448-459. doi: 10.1097/00008483-198911000-00003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pahkala K, Heinonen OJ, Simell O, et al. Association of physical activity with vascular endothelial function and intima-media thickness. Circulation. 2011;124(18):1956-1963. doi: 10.1161/CIRCULATIONAHA.111.043851 [DOI] [PubMed] [Google Scholar]
- 28.Li S, Chen W, Srinivasan SR, et al. Childhood cardiovascular risk factors and carotid vascular changes in adulthood: the Bogalusa Heart Study. JAMA. 2003;290(17):2271-2276. doi: 10.1001/jama.290.17.2271 [DOI] [PubMed] [Google Scholar]
- 29.Raitakari OT, Juonala M, Kähönen M, et al. Cardiovascular risk factors in childhood and carotid artery intima-media thickness in adulthood: the Cardiovascular Risk in Young Finns Study. JAMA. 2003;290(17):2277-2283. doi: 10.1001/jama.290.17.2277 [DOI] [PubMed] [Google Scholar]
- 30.Polak JF, Person SD, Wei GS, et al. Segment-specific associations of carotid intima-media thickness with cardiovascular risk factors: the Coronary Artery Risk Development in Young Adults (CARDIA) study. Stroke. 2010;41(1):9-15. doi: 10.1161/STROKEAHA.109.566596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Magnussen CG, Venn A, Thomson R, et al. The association of pediatric low- and high-density lipoprotein cholesterol dyslipidemia classifications and change in dyslipidemia status with carotid intima-media thickness in adulthood evidence from the cardiovascular risk in Young Finns study, the Bogalusa Heart study, and the CDAH (Childhood Determinants of Adult Health) study. J Am Coll Cardiol. 2009;53(10):860-869. doi: 10.1016/j.jacc.2008.09.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jones BL, Nagin DS. Advances in group-based trajectory modeling and an SAS procedure for estimating them. Sociol Methods Res. 2007;35(4):542-571. doi: 10.1177/0049124106292364 [DOI] [Google Scholar]
- 33.Jones BL, Nagin DS, Roeder K. A SAS procedure based on mixture models for estimating developmental trajectories. Sociol Methods Res. 2001;29(3):374-393. doi: 10.1177/0049124101029003005 [DOI] [Google Scholar]
- 34.RC Team. A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2017. [Google Scholar]
- 35.vanBuuren S, Groothuis-Oudshoor K. MICE: multivariate imputation by chained equations in R. J Stat Softw. 2011;45(3):1-67. [Google Scholar]
- 36.Petkeviciene J, Klumbiene J, Kriaucioniene V, Raskiliene A, Sakyte E, Ceponiene I. Anthropometric measurements in childhood and prediction of cardiovascular risk factors in adulthood: Kaunas cardiovascular risk cohort study. BMC Public Health. 2015;15:218. doi: 10.1186/s12889-015-1528-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Umer A, Kelley GA, Cottrell LE, Giacobbi P Jr, Innes KE, Lilly CL. Childhood obesity and adult cardiovascular disease risk factors: a systematic review with meta-analysis. BMC Public Health. 2017;17(1):683. doi: 10.1186/s12889-017-4691-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Allen NB, Siddique J, Wilkins JT, et al. Blood pressure trajectories in early adulthood and subclinical atherosclerosis in middle age. JAMA. 2014;311(5):490-497. doi: 10.1001/jama.2013.285122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reis JP, Loria CM, Lewis CE, et al. Association between duration of overall and abdominal obesity beginning in young adulthood and coronary artery calcification in middle age. JAMA. 2013;310(3):280-288. doi: 10.1001/jama.2013.7833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pletcher MJ, Bibbins-Domingo K, Lewis CE, et al. Prehypertension during young adulthood and coronary calcium later in life. Ann Intern Med. 2008;149(2):91-99. doi: 10.7326/0003-4819-149-2-200807150-00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu K, Colangelo LA, Daviglus ML, et al. Can antihypertensive treatment restore the risk of cardiovascular disease to ideal levels?: the Coronary Artery Risk Development in Young Adults (CARDIA) study and the Multi-Ethnic Study of Atherosclerosis (MESA). J Am Heart Assoc. 2015;4(9):e002275. doi: 10.1161/JAHA.115.002275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Riegel B, Moser DK, Buck HG, et al. ; American Heart Association Council on Cardiovascular and Stroke Nursing; Council on Peripheral Vascular Disease; and Council on Quality of Care and Outcomes Research . Self-care for the prevention and management of cardiovascular disease and stroke: a scientific statement for healthcare professionals from the American Heart Association. J Am Heart Assoc. 2017;6(9):e006997. doi: 10.1161/JAHA.117.006997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lloyd-Jones DM, Huffman MD, Karmali KN, et al. Estimating longitudinal risks and benefits from cardiovascular preventive therapies among Medicare patients: the Million Hearts Longitudinal ASCVD Risk Assessment Tool: a special report from the American Heart Association and American College of Cardiology. Circulation. 2017;135(13):e793-e813. doi: 10.1161/CIR.0000000000000467 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Diet Scoring
eReferences
eTable 1. Demographics at Baseline and at Follow-up by Cohort
eTable 2. Cohort, Age, Sex, and Race Specific Cut Points for Defining High cIMT
eTable 3. Baseline Characteristics and Exam Counts by CVH Score Trajectory Group
eTable 4. Cross-tabulation of Trajectory Group Membership, for Those With a Full CVH Score Trajectory Group Assignment (N = 3736)
eTable 5. Association Between Carotid IMT (mm) and CVH Score Trajectory Group
eTable 6. The Association Between High Carotid IMT and CVH Score Trajectory Group
eTable 7. The Association Between High Carotid IMT and CVH Score Cohort-specific Trajectory Group, Stratified by Cohort
eTable 8. Baseline Characteristics and Exam Counts by Analytic Sample
eFigure. CVH Score Trajectories by Age