Key Points
Question
Is the length of time lived in ideal cardiovascular health in midlife associated with cardiometabolic outcomes or with all-cause mortality later in life?
Findings
In this cohort study, using data from 1445 middle-aged participants from the Framingham Heart Study Offspring cohort, for each 5-year period that participants had intermediate or ideal cardiovascular health, they were 33% less likely to develop hypertension, 14% less likely to die, and approximately 25% less likely to develop diabetes, chronic kidney disease, or cardiovascular disease than individuals in poor cardiovascular health.
Meaning
Living longer in better cardiovascular health during midlife may be associated with lower risk of chronic disease or mortality later in life.
Abstract
Importance
The American Heart Association ideal cardiovascular health (CVH) score is associated with the risk of cardiovascular disease (CVD) and mortality. However, it is unclear whether the number of years spent in ideal CVH is associated with morbidity or with mortality.
Objective
To evaluate whether living longer with a higher CVH score in midlife is associated with lower risk of hypertension, diabetes, chronic kidney disease, CVD and its subtypes (coronary heart disease, stroke, congestive heart failure, and peripheral artery disease), or all-cause mortality in later life.
Design, Setting, and Participants
This prospective cohort study used data from 1445 participants from 1991 to 2015 who participated in the community-based Framingham Heart Study Offspring investigation conducted in Massachusetts. The CVH scores of participants were assessed at examination cycles 5, 6, and 7 (1991-1995; 1995-1998; and 1998-2001, respectively). Individuals were excluded from analyses of the association between duration of CVH score and outcomes if they had the outcome of interest at the seventh examination. The median follow-up was approximately 16 years. Data were analyzed from April 2018 to October 2019. The CVH score categories were poor for scores 0 to 7, intermediate for scores 8 to 11, and ideal for scores 12 to 14. A composite score was derived based on smoking status, diet, physical activity, resting blood pressure levels, body mass index, fasting blood glucose levels, and total serum cholesterol levels.
Main Outcomes and Measures
Number of events and number at risk for each main outcome, including incident hypertension, diabetes, chronic kidney disease, CVD, and all-cause mortality, after the seventh examination.
Results
Of 1445 eligible participants, the mean (SD) age was 60 (9) years, and 751 (52%) were women. Number of events/number at risk for each main outcome after the seventh examination were 348/795 for incident hypertension, 104/1304 for diabetes, 198/918 for chronic kidney disease, 210/1285 for CVD, and 300/1445 for all-cause mortality. At the seventh examination, participants mostly had poor (568 [39%]) or intermediate (782 [54%]) CVH scores. For each antecedent (before examination cycle 7) 5-year duration that participants had intermediate or ideal CVH, they were less likely to develop adverse outcomes (hazards ratios of 0.67 [95% CI, 0.56-0.80] for incident hypertension, 0.73 [95% CI, 0.57-0.93] for diabetes, 0.75 [95% CI, 0.63-0.89] for chronic kidney disease, 0.73 [95% CI, 0.63-0.85] for CVD, and 0.86 [95% CI, 0.76-0.97] for all-cause mortality) relative to living the same amount of time in poor CVH (referent group). No effect modification was observed by age or by sex.
Conclusions and Relevance
These results suggest that more time spent in better CVH in midlife may have salutary cardiometabolic benefits and may be associated with lower mortality later in life.
This cohort study using data from the Framingham Heart Study Offspring evaluates whether having good cardiovascular health for a longer time in midlife is associated with lower risk of hypertension, diabetes, chronic kidney disease, cardiovascular disease, or all-cause mortality later in life.
Introduction
The American Heart Association recommended a renewed focus on primordial prevention to reduce the development of risk factors for cardiovascular disease (CVD) as part of its 2020 Impact Goal to improve population cardiovascular health (CVH) by 20% and to reduce CVD mortality by 20%. For this reason, the American Heart Association developed a score describing CVH that incorporates measures of both lifestyle behaviors (smoking, diet, and physical activity) and risk factors (blood pressure level, body mass index [BMI, calculated as weight in kilograms divided by height in meters squared], and fasting total cholesterol and blood glucose concentrations) that are associated with higher risk of CVD.1 Community-based studies indicate that higher CVH scores are associated with lower risk of diabetes, CVD, and all-cause mortality.2,3,4,5,6,7,8 Higher CVH scores are also associated with lower levels of indicators of subclinical disease, greater telomere length, and better vascular function.9,10,11,12,13,14,15,16 Despite the significance of the CVH score and the modifiable nature of its components, little is known about how the maintenance of individuals’ low or high CVH scores over time (ie, duration of CVH score) is associated with morbidity or mortality risks.
Of the few studies that considered how changes in CVH scores are associated with health outcomes, most examined only differences in scores over time without accounting for the time spent by individuals in specific CVH score categories.17,18,19,20 Quantifying whether longer time spent in better or worse CVH throughout midlife is associated with risk of cardiometabolic outcomes or with all-cause mortality may contribute to public health knowledge and may give rise to conversations between individuals and their health care physicians during routine physical examinations regarding the benefits of reaching and maintaining ideal CVH. We hypothesized that individuals maintaining better CVH for a longer time would have lower risk of developing hypertension, diabetes, chronic kidney disease (CKD), CVD and its subtypes (coronary heart disease [CHD], stroke, congestive heart failure [CHF], and peripheral artery disease), or death. We tested this hypothesis using data from the community-based Framingham Offspring Study (FOS) sample.
Methods
Study Sample
The sampling methods and design of the FOS have been described elsewhere.21 For the present investigation, 3159 participants (contributing 9477 person-examinations) of the FOS who attended examination cycles 5 (1991-1995), 6 (1995-1998), and 7 (1998-2001) were eligible. We excluded people who had any of the following conditions at these examinations: 19 people with serum creatinine levels of 2 mg/dL or more (to convert to micromoles per liter, multiply by 88.4), which is indicative of potential renal impairment; 26 people with a BMI less than 18.5, and 1669 people missing CVH score components. These exclusions resulted in a base sample of 4335 person-examinations contributed by 1445 unique participants (sample 1; eFigure in the Supplement). Individuals were excluded from analyses of the association between duration of CVH score and a select outcome if they had that specific outcome prevalent at their seventh examination cycle (samples 2-5; eFigure in the Supplement). Data from examination cycles 5, 6, and 7 were included to calculate the duration spent by individuals in a given CVH score category. The study protocols were approved by the Boston University Medical Center Institutional Review Board, and all participants provided written informed consent in a manner consistent with the Common Rule requirements. No one received compensation or was offered any incentive for participating in the analysis of the present study.
CVH Score
At each examination cycle, participants underwent measurements of their resting blood pressure, height, weight, total cholesterol level, and fasting blood glucose level using standardized protocols and assays.22,23,24 Participants self-reported their smoking status (current smoker, former smoker, or never smoker), diet, and physical activity. Diet was assessed using a food frequency questionnaire that captured consumption of fruits and vegetables (>4.5 cups/d), fish (2 or more 3.5-oz servings/wk), sodium (<1500 mg/d), sugar-sweetened beverages (≤450 kcal/wk), and fiber-rich whole grains (3 or more 1-oz equivalent servings/d). Diet quality was considered poor, intermediate, or ideal if participants met 0, 1, or at least 2 dietary components, respectively (eTable 1 in the Supplement). For the fifth and seventh examination cycles, a participant’s physical activity was assessed using a questionnaire-based physical activity index, which was derived using the following formula: Hours Sleeping + (1.1 × Hours Engaged in Sedentary Activities) + (1.5 × Hours Engaged in Slight Physical Activity) + (2.4 × Hours Engaged in Moderate Physical Activity) + (5 × Hours Engaged in Heavy Physical Activity). At the sixth examination, a participant’s physical activity was assessed using the Paffenbarger Physical Activity Questionnaire. Participants with physical activity values in the lowest 2 quartiles were considered to have poor physical activity levels, whereas those in the third and highest quartiles were considered to have intermediate and ideal levels, respectively.
As described previously,19 a CVH score was calculated for each participant at each of the examinations they attended, and the score was based on 7 component metrics: smoking status, diet, physical activity, total serum cholesterol level, resting blood pressure, BMI, and fasting blood glucose level (eTable 1 in the Supplement). For each metric, participants received 0, 1, or 2 points, representing poor, intermediate, or ideal categories, respectively. Participants with overall scores of 0 to 7, 8 to 11, or 12 to 14 points were categorized as having poor, intermediate, or ideal CVH, respectively. Owing to the relatively low number of people with an ideal CVH score in this sample, the intermediate and ideal CVH categories were combined. The number of years lived in each CVH category was calculated for each participant as the number of examination cycles (0-3) in which the participant was in that CVH score category multiplied by 4 (the mean time interval in years between FOS visits). We treated this 4-category variable (0, 1, 2, or 3 examinations spent with intermediate or ideal CVH) as a continuous variable in which each examination represented 4 years. We divided the number of years spent with an intermediate or ideal CVH by 5 to model the effects of a 5-year increment of time.
Outcome Events
Our primary outcomes were the occurrence of the following events during follow-up after the seventh examination cycle: hypertension (defined as systolic blood pressure [SBP] ≥140 mm Hg or diastolic blood pressure [DBP] ≥90 mm Hg or the use of antihypertensive medication), diabetes (fasting blood glucose level ≥126 mg/dL or a nonfasting blood glucose level ≥200 mg/dL [to convert to millimoles per liter, multiply by 0.0555] or the use of hypoglycemic medication), CKD (estimated glomerular filtration rate <60 mL/min/1.73 m2 or urine albumin to creatinine ratio ≥25 mg/g in men or ≥35 mg/g in women; for an approximate conversion of urine albumin to creatinine ratio from milligrams per gram to milligrams per mole, divide by 10), first CVD event (composite of stroke, transient ischemic attack, myocardial infarction, acute coronary syndrome, angina pectoris, intermittent claudication, or CHF), and all-cause mortality. Incident hypertension, diabetes, and CKD were assessed at follow-up FOS examination cycles 8 (2005-2008) and 9 (2011-2014), whereas CVD and all-cause mortality were assessed continually throughout the end of 2015. Our secondary outcomes were risk of CVD subtypes: CHD (myocardial infarction, coronary insufficiency, or angina pectoris), stroke, CHF, peripheral artery disease, and other CVD (defined as transient ischemic attack or death due to a nonstroke, non-CHD CVD event). A panel of 3 physicians (including R.S.V.) adjudicated all CVD events for the FOS using a standardized protocol and outcome definitions.25
Statistical Analysis
The number of years spent by each participant in a specific CVH score category (in 5-year increments; independent variable) between the fifth and seventh examination cycles was associated with the time to hypertension, diabetes, CKD, CVD, and all-cause mortality on follow-up after the seventh examination cycle using Cox proportional hazards regression, after confirming that the assumptions for proportionality of hazards were met for each outcome. We used the number of years in the poor CVH score category as the reference condition. All models were adjusted for age and sex. Models evaluating individual cardiometabolic outcomes were additionally adjusted for the continuous level of the specific CVH score component at examination cycle 7 (ie, for hypertension as the outcome, we adjusted models for baseline [seventh examination] SBP and DBP; for diabetes as the outcome, we adjusted for baseline fasting blood glucose level; for CKD as the outcome, we adjusted for baseline estimated glomerular filtration rate and for the urine albumin to creatinine ratio). Cox proportional hazards regression models with discrete time intervals were used for the models evaluating time to hypertension, diabetes, and CKD because ascertainment of these outcomes was dependent on attendance of a subsequent FOS examination. In secondary analyses, the time spent in CVH score categories (in 5-year increments) was associated with the individual subtypes of cardiovascular disease (ie, CHD, stroke, CHF, peripheral artery disease, and other CVD). In each analysis, participants with prevalent CVD of the specific subtype evaluated were excluded, and models were adjusted for age, sex, and prevalence of other CVD subtypes.
We assessed whether age or sex modified the associations between the duration in a given CVH score category and the risk of each outcome (separate models for each modifier and outcome). Sensitivity analyses were performed for the primary outcomes, with additional adjustment for educational status and also, separately, including participants who had a missing CVH score at examination cycle 5 or 6. For the latter sensitivity analysis, we used all known examination cycles at intermediate or ideal CVH to contribute 4 years toward the number of years at intermediate or ideal CVH score; we did not carry back or forward any values, nor did we use imputation. A 2-sided P < .05 was considered statistically significant. We conducted all analyses in the present study from April 2018 to October 2019 using SAS, version 9.4 (SAS Institute Inc).
Results
The characteristics of the study sample at the seventh FOS examination cycle by time lived in an intermediate or ideal CVH score category are summarized in Table 1 and are given by sex in eTable 2 in the Supplement. Of the 1445 participants included in sample 1, 751 (52%) were women, and the mean (SD) age was 60 (9) years. Most participants (568 [39%]) had poor or intermediate (782 [54%]) CVH scores. In general, 1714 people excluded from sample 1 were older (mean [SD] age, (60 [9] years vs 63 [10] years; P < .001), had higher SBP (mean [SD], 128 [19] mm Hg vs 126 [18] mm Hg; P = .047), were less likely to have attained a high school degree (1226 [96%] vs 1394 [92%]; P < .001), were more likely to smoke (138 [10%] vs 256 (15%]; P < .001) and to take lipid-lowering medications (286 [20%] vs 393 [23%]; P = .03), and had a higher prevalence of most of the primary outcomes compared with the included individuals investigated in this analysis (eTable 3 in the Supplement).
Table 1. Characteristics of the Study Sample at Baseline (Seventh Examination, Sample 1) Based on Duration of Time Lived in Intermediate or Ideal CVH.
| Characteristic | Duration of CVH Score in Intermediate or Ideal CVH by Category, No. (%) of Participants | ||
|---|---|---|---|
| 1 (>10 y; n = 656) | 2 (5-10 y; n = 271) | 3 (<5 y; n = 518) | |
| Age, mean (SD), y | 59 (9) | 61 (9) | 62 (9) |
| Attained high school degreea | 557 (97) | 234 (97) | 435 (95) |
| BMI, mean (SD) | 25 (3) | 28 (4) | 31 (5) |
| Current smoker | 32 (5) | 22 (8) | 84 (16) |
| Diet score, mean (SD)b | 1.9 (0.9) | 1.6 (0.9) | 1.3 (0.9) |
| Physical activity score | |||
| Ideal | 193 (29) | 80 (30) | 81 (16) |
| Intermediate | 195 (30) | 64 (24) | 116 (22) |
| Poor | 268 (41) | 127 (47) | 321 (62) |
| Systolic blood pressure, mean (SD), mm Hg | 119 (16) | 128 (17) | 134 (17) |
| Diastolic blood pressure, mean (SD), mm Hg | 72 (9) | 75 (9) | 77 (10) |
| Hypertensive | 168 (26) | 125 (46) | 357 (67) |
| Antihypertension medication | 122 (19) | 94 (35) | 270 (52) |
| Total cholesterol level, mean (SD), mg/dL | 195 (31) | 203 (36) | 208 (40) |
| High-density lipoprotein cholesterol level, mean (SD) mg/dL | 58 (16) | 52 (15) | 49 (15) |
| Lipid lowering medication | 66 (10) | 70 (26) | 150 (29) |
| Fasting blood glucose level (median, Q1/Q3), mg/dL | 93 (88/99) | 99 (92/107) | 105 (98/117) |
| Diabetes | 11 (2) | 19 (7) | 111 (21) |
| Diabetes medication | 9 (1) | 12 (4) | 57 (11) |
| Prevalent CVD | 48 (7) | 33 (12) | 79 (15) |
| CHD | 37 (6) | 21 (8) | 50 (10) |
| Stroke | 7 (1) | 6 (2) | 10 (2) |
| CHF | 4 (1) | 3 (1) | 8 (2) |
| PAD | 7 (1) | 8 (3) | 22 (4) |
| Other CVD | 4 (1) | 7 (3) | 9 (2) |
| CKD or microalbuminuria | 50 (10) | 30 (15) | 73 (19) |
| CVH score, mean (SD) | 9.9 (1.4) | 8.0 (1.4) | 6.1 (1.3) |
| CVH score category | |||
| Ideal (12-14) | 92 (14) | 3 (1) | 0 (0) |
| Intermediate (8-11) | 564 (86) | 166 (61) | 52 (10) |
| Poor (0-7) | 0 (0) | 102 (38) | 466 (90) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CHD, coronary heart disease; CHF, congestive heart failure; CKD, chronic kidney disease; CVD, cardiovascular disease; CVH, cardiovascular health; PAD, peripheral artery disease; Q, quartile.
SI conversion factors: To convert total cholesterol and high-density lipoprotein cholesterol levels to millimoles per liter, multiply by 0.0259; blood glucose levels to millimoles per liter, by 0.0555.
Not all values sum to 100% because of missing data (eg, educational attainment data are for 573 participants in category 1, rather than 656 participants).
Healthy diet score: Values for each participant could be 0, 1, or 2 depending on the number of components satisfied (>4.5 cups per day of fruits and vegetables, 2 or more 3.5-oz servings per week of fish, <1500 mg/d of sodium, ≤450 kcal per week of sugar-sweetened beverages, and 3 or more 1-oz equivalent servings per day of fiber-rich whole grains).
Primary Outcomes
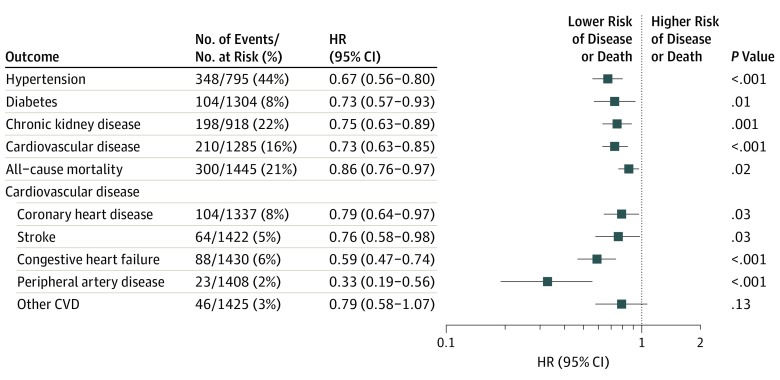
Adjusting for age and sex, each 5-year period that participants lived with an intermediate or ideal CVH score was associated with a lower risk of developing new-onset hypertension (hazard ratio [HR], 0.67; 95% CI, 0.56-0.80; P < .001), diabetes (HR, 0.73; 95% CI, 0.57-0.93; P = .01), or CKD (HR, 0.75; 95% CI, 0.63-0.89; P = .001) during approximately 12 years; of having an incident CVD event during a median of 15.7 years (HR, 0.73; 95% CI, 0.63-0.85; P < .001); and of dying during a median of 15.8 years (HR, 0.86; 95% CI, 0.76-0.97; P = .02) (Table 2 and Figure). Outcome-specific analyses excluded only those with the specific outcome prevalent at the seventh examination cycle. For example, for analyses using hypertension as an outcome, we excluded 650 individuals with hypertension at the seventh examination cycle. After further adjusting these models for educational level and, separately, including people with missing data at the fifth or sixth examination, our results remained robust (eTable 4 and eTable 5 in the Supplement, respectively). We did not observe statistically significant effect modification of the association between time in a given CVH score category and any outcome by either age or sex (P > .05 for all interaction terms). Nevertheless, the associations between time spent in intermediate or ideal CVH and the development of hypertension, diabetes, or CVD appeared stronger for younger individuals (eg, for an individual aged 50 years compared with an individual aged 60 or 70 years), although the associations were not statistically significant.
Table 2. Association of Maintaining Intermediate or Ideal CVH for 5 Years in Midlife With Risk of Developing Hypertension, Diabetes, CKD, CVD, or All-Cause Mortality on Follow-up.
| Outcomea | No. of Events/No. at Risk (%) | HR (95% CI) | P Value |
|---|---|---|---|
| Hypertensionb | 348/795 (44) | 0.67 (0.56-0.80) | <.001 |
| Diabetesc | 104/1304 (8) | 0.73 (0.57-0.93) | .01 |
| CKDd | 198/918 (22) | 0.75 (0.63-0.89) | .001 |
| CVDe | 210/1285 (16) | 0.73 (0.63-0.85) | <.001 |
| All-cause mortality | 300/1445 (21) | 0.86 (0.76-0.97) | .02 |
Abbreviations: CKD, chronic kidney disease; CVD, cardiovascular disease; CVH, cardiovascular health; HR, hazard ratio.
All models adjusted for age and sex.
Models also adjusted for baseline systolic blood pressure and diastolic blood pressure.
Models also adjusted for baseline fasting blood glucose level.
Models also adjusted for baseline estimated glomerular filtration rate and urine albumin to creatinine ratio.
First CVD event: stroke, transient ischemic attack, myocardial infarction, coronary insufficiency, angina pectoris, intermittent claudication, or heart failure.
Figure. Forest Plot for the Association Between Intermediate or Ideal Cardiovascular Health Duration (in 5-Year Increments) in Midlife and the Risk of Developing Outcomes on Follow-up.
Horizontal lines indicate the results of each subgroup analysis; error bars, 95% CI. CVD indicates cardiovascular disease; HR, hazard ratio.
In addition, the time spent in an intermediate or ideal status for individual components of the CVH score was associated with certain outcomes during follow-up in models adjusting for all other components. Specifically, we observed that time spent in intermediate or ideal blood pressure and smoking status was significantly associated with a lower risk of hypertension (HR, 0.31; 95% CI, 0.18-0.54; P < .001 for blood pressure; HR, 0.78; 95% CI, 0.65-0.94; P = .01 for smoking status) and a lower risk of CVD (HR, 0.78; 95% CI, 0.66-0.91; P = .003 for blood pressure; HR, 0.80; 95% CI, 0.66-0.97; P = .02 for smoking). Time spent in intermediate or ideal blood pressure (HR, 0.74; 95% CI, 0.56-0.97; P = .03) and BMI status (HR, 0.64; 95% CI, 0.52-0.78; P < .001) was significantly associated with a lower risk of diabetes. Time spent in intermediate or ideal BMI status (HR, 0.77; 95% CI, 0.65-0.91; P = .003) was also significantly associated with a lower risk of CKD. In addition, time spent in intermediate or ideal blood glucose level (HR, 0.66; 95% CI, 0.56-0.77; P < .001) and smoking status (HR, 0.67; 95% CI, 0.58-0.78; P < .001) was significantly associated with a lower risk of mortality (eTable 6 in the Supplement).
Secondary Outcomes
The duration of time lived in different CVH score categories was also associated with the risk of individual CVD subtypes (Table 3 and Figure). After adjustment for age, sex, and previous CVD event, each 5-year period that participants had intermediate or ideal CVH was associated with a lower risk of CHD (HR, 0.79; 95% CI, 0.64-0.97; P = .03), stroke (HR, 0.76; 95% CI, 0.58-0.98; P = .03), CHF (HR, 0.59; 95% CI, 0.47-0.74; P < .001), and peripheral artery disease (HR, 0.33; 95% CI, 0.19-0.56; P < .001) (Table 3 and Figure). After further adjustment for educational level, the results were similar. There was no effect modification by age or sex of the associations between duration in a given CVH score category and occurrence of any secondary outcome.
Table 3. Association of Maintaining Intermediate or Ideal Cardiovascular Health for 5 Years in Midlife With Risk of Individual Cardiovascular Disease Subtype on Follow-up.
| Outcomea | No. of Events/No. at Risk (%) | HR (95% CI) | P Value |
|---|---|---|---|
| Coronary heart diseaseb | 104/1337 (8) | 0.79 (0.64-0.97) | .03 |
| Stroke | 64/1422 (5) | 0.76 (0.58-0.98) | .03 |
| Congestive heart failure | 88/1430 (6) | 0.59 (0.47-0.74) | <.001 |
| Peripheral artery disease | 23/1408 (2) | 0.33 (0.19-0.56) | <.001 |
| Other cardiovascular disease | 46/1425 (3) | 0.79 (0.58-1.07) | .13 |
All models adjusted for age, sex, and previous cardiovascular disease event.
Includes myocardial infarction, coronary insufficiency, or angina pectoris.
Discussion
Our primary finding was that more time spent with a higher (better) CVH score in midlife was associated with a lower risk of developing hypertension, diabetes, CKD, CVD, CVD subtypes, or all-cause mortality later in life. The ability to quantify these associations is important for public health knowledge and, perhaps, of clinical importance. In addition, our results indicated that living longer in adulthood with better CVH may be potentially beneficial regardless of age because we did not observe statistically significant effect modification by age of the associations between duration in a given CVH score category and any outcome. Overall, our findings support the importance of promoting healthy behaviors throughout the life course.
Our results were largely consistent with studies that have used single time point CVH score assessments. Such studies have reported that CVH scores are inversely associated with the occurrence of diabetes during 5 years,26 CKD during 9.5 years,27 CVD during 18.7 years,28 and all-cause mortality during 18 years.29 Our findings are consistent with protective associations of a higher CVH score with several health outcomes. Our investigation extends prior observations by suggesting that the associations were maintained when the duration of the CVH score was modeled.
Few studies, to date, have considered how changes in CVH scores over time are associated with the occurrence of subclinical or clinical outcomes at follow-up. Individuals with consistently low CVH scores have a higher risk of chronic disease outcomes,20 people with consistently high CVH scores have a lower likelihood of subclinical and overt CVD and all-cause mortality,19 and individuals with CVH scores that improve over time have a lower pulse wave velocity.17 The previous studies suggest that at least part of the association between CVH score and certain cardiometabolic outcomes (eg, hypertension) may be due to mechanisms involving vascular aging, such as vascular structural remodeling, loss of vascular homeostasis, and atherogenesis.9,10,11,12,13,14,15,16,30,31 We extended those prior analyses to examine the duration individuals lived in poor, intermediate, or ideal CVH and also to consider a range of clinical outcomes, such as incident hypertension, diabetes, CKD, CVD subtypes, and all-cause mortality. Considering the duration of time lived in different CVH score categories helps us better understand whether the cumulative burden of factors such as smoking, poor diet, and high BMI may be associated with these clinical outcomes. Although previous work considered the cumulative burden of certain CVD risk factors or combinations of factors over time in association with clinical outcomes,32,33 we considered a composite measure of CVD risk factors as summarized by the CVH score.
Despite our observation that a longer duration of having at least intermediate CVH in midlife was associated with a lower likelihood of adverse clinical outcomes later in life, it can be challenging for individuals to change their CVH score category. Previous studies have reported that CVH scores tend to be relatively stable or decline over the life course.19,34,35 For example, for multiple examination cycles within the FOS cohort, 71% of people maintained their CVH score category (scores either ≥8 or ≤7), and 20% of the participants overall moved from high to low CVH scores.19 These prior observations, in combination with our present finding that each additional period lived in intermediate or ideal CVH was associated with a lower risk of clinical outcomes or all-cause mortality, support public health policies that foster behaviors that promote better CVH.
Strengths and Limitations
A strength of our investigation was the availability of serial data collected for nearly 25 years from a large community-based sample. We were able to examine whether time lived in a CVH category was associated with several health outcomes. Our approach also had several limitations. First, for consistency with previous Framingham Heart Study analyses, the definitions of the physical activity and diet components were slightly different from the precise ones advocated by the American Heart Association. Physical activity and diet were also self-reported, which might lead to an underestimation of the associations for maintaining ideal CVH. Second, we evaluated the associations between the composite CVH score (regardless of the contributions from each of the 7 components) and many outcomes at follow-up. Several of the outcomes evaluated were defined using variables that are themselves part of components of the CVH risk score (eg, blood pressure with hypertension; blood glucose levels with diabetes). Although we adjusted for the baseline levels of variables (at examination cycle 7) defining these CVH components, further adjustment for values at examination cycles 5 and 6 may have resulted in attenuated associations. However, we observed consistent directionality of the effect estimates. Third, although the results were robust when including participants missing a CVH score for at least 1 examination cycle, the individuals excluded from the analysis were slightly older and less healthy. Therefore, by excluding approximately half of the potential participants, we may have introduced bias. It is likely that our results are more generalizable to healthier individuals. Fourth, we did not focus on CVH score trajectories over time in this analysis, and therefore we did not evaluate the fluctuations in the CVH score for examination cycles 5, 6, and 7. Fifth, because we combined individuals in intermediate and ideal CVH into 1 score category owing to the small number of people in the ideal CVH category, we were unable to assess the risks associated with those in ideal CVH. Sixth, given that the mean age of the participants at the start of follow-up was 60 years, we cannot assess how the duration of time spent in CVH score categories earlier in life is associated with disease outcomes. Seventh, the FOS cohort was relatively homogeneous; most participants were white, middle-aged, and older individuals of European ancestry; therefore, our results may not be generalizable to other age groups and races/ethnicities.
Conclusions
Our observational findings from a large community-based sample indicated that longer time lived in intermediate or ideal CVH in midlife is associated with a lower risk of developing hypertension, diabetes, CKD, CVD, or all-cause mortality in later life. Our analysis is consistent with the concept that improving the CVH of a community through individual- and societal-level changes might result in lowering morbidity and mortality.
eFigure. Derivation of Study Samples
eTable 1. Definition of Each Level of the CVH Score Components
eTable 2. Characteristics of the Study Sample at Baseline (7th FOS Examination Cycle, Sample 1)
eTable 3. Comparison of Participant Characteristics Between Those Who Were Included in the Study (Sample 1) and Those Excluded
eTable 4. Association of Maintaining Intermediate or Ideal CVH for 5 Years in Midlife With the Risk of Developing Hypertension, Diabetes, CKD, CVD, or All-Cause Mortality on Follow-up: Sensitivity Analysis Adjusting for Educational Attainment
eTable 5. Association of Maintaining Intermediate or Ideal CVH for 5 Years in Midlife With the Risk of Developing Hypertension, Diabetes, CKD, CVD, and All-Cause Mortality on Follow-up: Sensitivity Analysis Including People With Missing Data at Either the 5th or 6th Examination
eTable 6. Association of Maintaining Intermediate or Ideal CVH Components for 5 Years in Midlife With the Risk of Developing Hypertension, Diabetes, CKD, CVD, and All-Cause Mortality on Follow-up
References
- 1.Lloyd-Jones DM, Hong Y, Labarthe D, et al. ; American Heart Association Strategic Planning Task Force and Statistics Committee . Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation. 2010;121(4):586-613. doi: 10.1161/CIRCULATIONAHA.109.192703 [DOI] [PubMed] [Google Scholar]
- 2.Dong C, Rundek T, Wright CB, Anwar Z, Elkind MSV, Sacco RL. Ideal cardiovascular health predicts lower risks of myocardial infarction, stroke, and vascular death across whites, blacks, and Hispanics: the northern Manhattan study. Circulation. 2012;125(24):2975-2984. doi: 10.1161/CIRCULATIONAHA.111.081083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang Q, Cogswell ME, Flanders WD, et al. Trends in cardiovascular health metrics and associations with all-cause and CVD mortality among US adults. JAMA. 2012;307(12):1273-1283. doi: 10.1001/jama.2012.339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang Q, Zhou Y, Gao X, et al. Ideal cardiovascular health metrics and the risks of ischemic and intracerebral hemorrhagic stroke. Stroke. 2013;44(9):2451-2456. doi: 10.1161/STROKEAHA.113.678839 [DOI] [PubMed] [Google Scholar]
- 5.Liu Y, Chi HJ, Cui LF, et al. The ideal cardiovascular health metrics associated inversely with mortality from all causes and from cardiovascular diseases among adults in a Northern Chinese industrial city. PLoS One. 2014;9(2):e89161. doi: 10.1371/journal.pone.0089161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lachman S, Peters RJ, Lentjes MA, et al. Ideal cardiovascular health and risk of cardiovascular events in the EPIC-Norfolk prospective population study. Eur J Prev Cardiol. 2016;23(9):986-994. doi: 10.1177/2047487315602015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nayor M, Enserro DM, Vasan RS, Xanthakis V. Cardiovascular health status and incidence of heart failure in the Framingham Offspring Study. Circ Heart Fail. 2016;9(1):e002416. doi: 10.1161/CIRCHEARTFAILURE.115.002416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Effoe VS, Carnethon MR, Echouffo-Tcheugui JB, et al. The American Heart Association ideal cardiovascular health and incident type 2 diabetes mellitus among blacks: the Jackson Heart Study. J Am Heart Assoc. 2017;6(6):e005008. doi: 10.1161/JAHA.116.005008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xanthakis V, Enserro DM, Murabito JM, et al. Ideal cardiovascular health: associations with biomarkers and subclinical disease and impact on incidence of cardiovascular disease in the Framingham Offspring Study. Circulation. 2014;130(19):1676-1683. doi: 10.1161/CIRCULATIONAHA.114.009273 [DOI] [PubMed] [Google Scholar]
- 10.Shah AM, Claggett B, Folsom AR, et al. Ideal cardiovascular health during adult life and cardiovascular structure and function among the elderly. Circulation. 2015;132(21):1979-1989. doi: 10.1161/CIRCULATIONAHA.115.017882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gaye B, Mustafic H, Laurent S, et al. Ideal cardiovascular health and subclinical markers of carotid structure and function: the Paris Prospective Study III. Arterioscler Thromb Vasc Biol. 2016;36(10):2115-2124. doi: 10.1161/ATVBAHA.116.307920 [DOI] [PubMed] [Google Scholar]
- 12.Robbins JM, Petrone AB, Carr JJ, et al. Association of ideal cardiovascular health and calcified atherosclerotic plaque in the coronary arteries: the National Heart, Lung, and Blood Institute Family Heart Study. Am Heart J. 2015;169(3):371-378.e1. doi: 10.1016/j.ahj.2014.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peng H, Mete M, Desale S, et al. Leukocyte telomere length and ideal cardiovascular health in American Indians: the Strong Heart Family Study. Eur J Epidemiol. 2017;32(1):67-75. doi: 10.1007/s10654-016-0199-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Santos IS, Goulart AC, Pereira AC, Lotufo PA, Benseñor IM. Association between cardiovascular health score and carotid intima-media thickness: cross-sectional analysis of the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil) baseline assessment. J Am Soc Echocardiogr. 2016;29(12):1207-1216.e4. doi: 10.1016/j.echo.2016.09.001 [DOI] [PubMed] [Google Scholar]
- 15.Zheng X, Zhang R, Liu X, et al. Association between cumulative exposure to ideal cardiovascular health and arterial stiffness. Atherosclerosis. 2017;260:56-62. doi: 10.1016/j.atherosclerosis.2017.03.018 [DOI] [PubMed] [Google Scholar]
- 16.Shpilsky D, Bambs C, Kip K, et al. Association between ideal cardiovascular health and markers of subclinical cardiovascular disease. Clin Cardiol. 2018;41(12):1593-1599. doi: 10.1002/clc.23096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aatola H, Hutri-Kähönen N, Juonala M, et al. Prospective relationship of change in ideal cardiovascular health status and arterial stiffness: the Cardiovascular Risk in Young Finns Study. J Am Heart Assoc. 2014;3(2):e000532. doi: 10.1161/JAHA.113.000532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hwang S-J, Onuma O, Massaro JM, et al. Maintenance of ideal cardiovascular health and coronary artery calcium progression in low-risk men and women in the Framingham Heart Study. Circ Cardiovasc Imaging. 2018;11(1):e006209. doi: 10.1161/CIRCIMAGING.117.006209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Enserro DM, Vasan RS, Xanthakis V. Twenty-year trends in the American Heart Association cardiovascular health score and impact on subclinical and clinical cardiovascular disease: the Framingham Offspring Study. J Am Heart Assoc. 2018;7(11):e008741. doi: 10.1161/JAHA.118.008741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Sloten TT, Tafflet M, Périer M-C, et al. Association of change in cardiovascular risk factors with incident cardiovascular events. JAMA. 2018;320(17):1793-1804. doi: 10.1001/jama.2018.16975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families: the Framingham offspring study. Am J Epidemiol. 1979;110(3):281-290. doi: 10.1093/oxfordjournals.aje.a112813 [DOI] [PubMed] [Google Scholar]
- 22.Vasan RS, Larson MG, Leip EP, Kannel WB, Levy D. Assessment of frequency of progression to hypertension in non-hypertensive participants in the Framingham Heart Study: a cohort study. Lancet. 2001;358(9294):1682-1686. doi: 10.1016/S0140-6736(01)06710-1 [DOI] [PubMed] [Google Scholar]
- 23.Meigs JB, Mittleman MA, Nathan DM, et al. Hyperinsulinemia, hyperglycemia, and impaired hemostasis: the Framingham Offspring Study. JAMA. 2000;283(2):221-228. doi: 10.1001/jama.283.2.221 [DOI] [PubMed] [Google Scholar]
- 24.Kathiresan S, Manning AK, Demissie S, et al. A genome-wide association study for blood lipid phenotypes in the Framingham Heart Study. BMC Med Genet. 2007;8(1)(suppl 1):S17. doi: 10.1186/1471-2350-8-S1-S17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kannel WB, Wolf PA, Garrison RJ. Some Risk Factors Related to the Annual Incidence of Cardiovascular Disease and Death in Pooled Repeated Biennial Measurements: Framingham Heart Study, 30 Year Follow-up. Bethesda, MD: US Dept of Health and Human Services; 1987. [Google Scholar]
- 26.Fretts AM, Howard BV, McKnight B, et al. Life’s Simple 7 and incidence of diabetes among American Indians: the Strong Heart Family Study. Diabetes Care. 2014;37(8):2240-2245. doi: 10.2337/dc13-2267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ogunmoroti O, Allen NB, Cushman M, et al. Association between life’s simple 7 and noncardiovascular disease: the Multi-Ethnic Study of Atherosclerosis. J Am Heart Assoc. 2016;5(10):e003954. doi: 10.1161/JAHA.116.003954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Folsom AR, Yatsuya H, Nettleton JA, Lutsey PL, Cushman M, Rosamond WD; ARIC Study Investigators . Community prevalence of ideal cardiovascular health, by the American Heart Association definition, and relationship with cardiovascular disease incidence. J Am Coll Cardiol. 2011;57(16):1690-1696. doi: 10.1016/j.jacc.2010.11.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim JY, Ko Y-J, Rhee CW, et al. Cardiovascular health metrics and all-cause and cardiovascular disease mortality among middle-aged men in Korea: the Seoul male cohort study. J Prev Med Public Health. 2013;46(6):319-328. doi: 10.3961/jpmph.2013.46.6.319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Widlansky ME, Gokce N, Keaney JF Jr, Vita JA. The clinical implications of endothelial dysfunction. J Am Coll Cardiol. 2003;42(7):1149-1160. doi: 10.1016/S0735-1097(03)00994-X [DOI] [PubMed] [Google Scholar]
- 31.Palombo C, Kozakova M. Arterial stiffness, atherosclerosis and cardiovascular risk: pathophysiologic mechanisms and emerging clinical indications. Vascul Pharmacol. 2016;77:1-7. doi: 10.1016/j.vph.2015.11.083 [DOI] [PubMed] [Google Scholar]
- 32.Hallan S, de Mutsert R, Carlsen S, Dekker FW, Aasarød K, Holmen J. Obesity, smoking, and physical inactivity as risk factors for CKD: are men more vulnerable? Am J Kidney Dis. 2006;47(3):396-405. doi: 10.1053/j.ajkd.2005.11.027 [DOI] [PubMed] [Google Scholar]
- 33.Brancati FL, Wang N-Y, Mead LA, Liang K-Y, Klag MJ. Body weight patterns from 20 to 49 years of age and subsequent risk for diabetes mellitus: the Johns Hopkins Precursors Study. Arch Intern Med. 1999;159(9):957-963. doi: 10.1001/archinte.159.9.957 [DOI] [PubMed] [Google Scholar]
- 34.Savelieva K, Pulkki-Råback L, Jokela M, et al. Intergenerational transmission of socioeconomic position and ideal cardiovascular health: 32-year follow-up study. Health Psychol. 2017;36(3):270-279. doi: 10.1037/hea0000441 [DOI] [PubMed] [Google Scholar]
- 35.Djoussé L, Petrone AB, Blackshear C, et al. Prevalence and changes over time of ideal cardiovascular health metrics among African-Americans: the Jackson Heart Study. Prev Med. 2015;74:111-116. doi: 10.1016/j.ypmed.2015.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. Derivation of Study Samples
eTable 1. Definition of Each Level of the CVH Score Components
eTable 2. Characteristics of the Study Sample at Baseline (7th FOS Examination Cycle, Sample 1)
eTable 3. Comparison of Participant Characteristics Between Those Who Were Included in the Study (Sample 1) and Those Excluded
eTable 4. Association of Maintaining Intermediate or Ideal CVH for 5 Years in Midlife With the Risk of Developing Hypertension, Diabetes, CKD, CVD, or All-Cause Mortality on Follow-up: Sensitivity Analysis Adjusting for Educational Attainment
eTable 5. Association of Maintaining Intermediate or Ideal CVH for 5 Years in Midlife With the Risk of Developing Hypertension, Diabetes, CKD, CVD, and All-Cause Mortality on Follow-up: Sensitivity Analysis Including People With Missing Data at Either the 5th or 6th Examination
eTable 6. Association of Maintaining Intermediate or Ideal CVH Components for 5 Years in Midlife With the Risk of Developing Hypertension, Diabetes, CKD, CVD, and All-Cause Mortality on Follow-up