Abstract
Diagnostic stewardship is an increasingly recognized means to reduce unnecessary tests and diagnostic errors. As a leading cause of healthcare-associated infection for which accurate laboratory diagnosis remains a challenge, Clostridium difficile offers an ideal opportunity to apply the principles of diagnostic stewardship. The recently updated 2017 Infectious Diseases Society of America (IDSA)-Society for Healthcare Epidemiology of America (SHEA) Clinical Practice Guidelines for C. difficile infection now recommend separate diagnostic strategies depending on whether an institution has adopted diagnostic stewardship in test decision making. IDSA-SHEA endorsement of diagnostic stewardship for C. difficile highlights the increasing role of diagnostic stewardship in hospitals. In this opinion piece, we introduce the concept of diagnostic stewardship by discussing the new IDSA-SHEA diagnostic recommendations for laboratory diagnosis of C. difficile. We outline recent examples of diagnostic stewardship, challenges to implementation, potential downsides and propose future areas of study.
Keywords: Clostridium difficile, diagnostic error, diagnostic stewardship
Introduction
Clostridium difficile is the leading pathogen causing healthcare-associated infections (HAIs) [1]. However, overdiagnosis of C. difficile infection (CDI) is also suspected to be common and up to half of hospitalized patients with a positive C. difficile nucleic acid amplification test (NAAT) may represent colonization as opposed to infection [2]. Unnecessary treatment of false-positive C. difficile tests may lead to additional cost, prolonged hospitalization and increased risk for treatment side effects.
Unfortunately, no prospectively validated diagnostic criteria for CDI exists [3, 4]. Distinguishing true disease from colonization with toxin-positive C. difficile strains is often challenging and education alone is simply not enough to limit inappropriate test utilization [5]. As many hospitals have adopted highly-sensitive NAAT for C. difficile instead of less-sensitive tests detecting antigen, many have implemented coordinated user or systems-based interventions to avoid unnecessary test utilization, known as diagnostic stewardship [6].
The 2010 Infectious Diseases Society of America (IDSA)-Society for Healthcare Epidemiology of America (SHEA) Clinical Practice Guidelines for Management of CDI outlined testing methodology options for CDI [NAAT or two-step enzyme immunoassay (EIA) for glutamate dehydrogenase (GDH) with confirmatory toxin A and B EIA] and indications for testing (presence of diarrhea, symptoms and suspicion for CDI) [3]. However, neither specific recommendations regarding the preferred testing method nor discussion of appropriate test utilization were included in the guidance. Newly updated IDSA-SHEA Clinical Practice Guidelines, published in February 2018, recommend different approaches to C. difficile testing (using single or multistep diagnostic methods), depending on whether “there are preagreed institutional criteria for patient stool submission” [7]. If preagreed institutional criteria are present, NAAT alone may be used, and if not, the guideline recommends a multi-step algorithm incorporating toxin EIA (i.e. NAAT plus toxin EIA; GDH plus toxin EIA; or GDH plus toxin EIA, with discordant results adjudicated by NAAT) to improve positive predictive value [7]. Preagreed institutional criteria specifically refers to limiting C. difficile testing on patients receiving laxatives within 48 h and those without diarrhea (defined as unexplained, new-onset, diarrhea with ≥3 unformed stools within 24 h) [7]. These guidelines also recommend against repeated testing during the same episode of diarrhea within 7 days. To our knowledge, this is the first IDSA or SHEA guideline to recommend different testing strategies, depending on whether diagnostic stewardship strategies are practiced.
Diagnostic stewardship for C. difficile
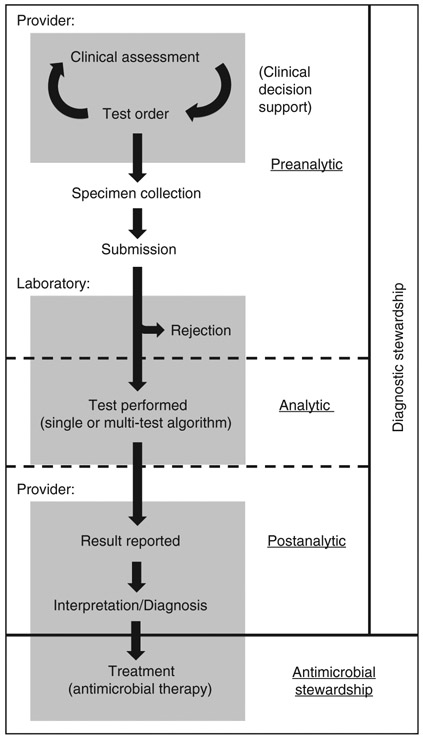
Diagnostic stewardship is considered to occur during three diagnostic stages: pre-analytic (test decision-making and specimen collection), analytic (test methodology) and post-analytic (result interpretation and reporting) (Figure 1) [8]. Common diagnostic stewardship practices used to improve C. difficile test utilization include, for example, clinical decision support or laboratory refusal of formed stool specimens (pre-analytic), multi-test algorithms to optimize performance (analytic), and suppression of C. difficile results on multiplex molecular panels for gastrointestinal infections (post-analytic).
Figure 1:
Stages of stewardship along the diagnostic pathway.
Preanalytic stage
Many laboratory-related errors can be traced to the preanalytic stage [9], which is a logical point of intervention for C. difficile testing. A variety of approaches have been tried, with variable success (Table 1). In perhaps the largest published study of diagnostic stewardship for C. difficile testing to date, White and colleagues at the University of Pennsylvania implemented a computerized clinical decision support (CCDS) tool, which was triggered in patients who had received laxatives within the previous 36 h and displayed a message to consider stopping laxatives and reassessing in 24 h prior to testing [12]. This led to a small but statistically significant reduction in inappropriate tests; however, the overall proportion of patients who received testing was unchanged [12].
Table 1:
Examples of pre-analytic diagnostic stewardship interventions published for C. difficile.
| Institution | Test methodology | Provider or lab-based [primary intervention(s)] |
Provider education | Hard stopa | Reduction in: | Patient safety systematically examined? |
|
|---|---|---|---|---|---|---|---|
| Testing | CDI events | ||||||
| University of Virginia [10] | NAAT | Provider (CCDS focusing on duplicate tests and indications for testing) [3] | Yes (email, video, in-person education, electronic dashboard) | No | 41% | 31% ↓ HO-CDI | No |
| University of California, Irvine [11] | NAAT | Provider (CCDS requiring indications and notified if laxative within 24 h) | None described | Yes (ID/GI specialist approval) | 56% | 54% ↓ HO-CDI | No |
| University of Pennsylvania [12] | EIA for GDH and toxin A/B then NAAT for discordant toxin results | Provider (integrated order set triggered for patients who had received laxatives within 36 h) | Yes (email, screensaver) | No | Not statistically significant (proportion inappropriate tests significantly reduced) | Not reported | Yes (no significant increase in CDI-related complications among patients with HO-CDI) |
| Cambridge Health Alliance [13] | NAAT (switched to GDH and toxin A/B EIA for > hospital day 3 during study) | Provider (CCDS, testing protocol triggered on hospital days 1-3 by diarrhea documentation to facilitate early testing) | Yes | No | Not reported | Statistically significant reduction in standardized infection ratio for C. difficile | No |
| Royal Victoria Hospital, UK [14] | EIA for toxin A/B | Provider (permanent decision-making algorithm visual aid checklist disseminated to staff) | Yes (memorandum) | No | 4.3% (proportion inappropriate tests significantly reduced) | 50% ↓ All positive tests | No |
| Christiana Hospital [15] | NAAT | Provider (CCDS, laxative alert) | None described | Yes (telephone laboratory approval) | 30% | 45% ↓ HO-CDI (not statistically significant) | No |
| Children’s Mercy Hospital [16] | EIA for GDH and toxin A/B then NAAT for discordant toxin results | Provider (CCDS-based ordering algorithm) and lab (stolid stool specimen refusal) | Yes (lecture, newsletter article) | No | No sustained changes ordering practices observed | Not reported | No |
| University of Southern California [17] | NAAT | Lab (specimen refusal based on time to collection or solid stool) | Yes (memo, grand rounds, screensaver) | N/A | 43% | 60% ↓HO-CDI | Yes (no increase in CDI-related complications) |
| Stanford University [18] | NAAT | Lab (specimen refusal based on absence of clinical criteria) | None described | N/A | 31% | 25% ↓HO-CDI | Yes (no significant increase in leukocytosis, ICU admission, or 30 day mortality) |
Refers to a requirement for the provider to perform an action outside of the CCDS tool to order a test, such as calling the laboratory for permission. CCDS, Computerized clinical decision support; HO-CDI, National Healthcare Safety Network-defined Hospital-Onset Clostridium difficile infection events; h, hours; NAAT, nucleic acid amplification test; GDH, glutamate dehydrogenase; EIA, enzyme immunoassay; N/A, not applicable.
Quan and colleagues at the University of California Irvine recently described a more punitive strategy that guided clinicians on appropriate CDI testing based on the following criteria: (1) ≥3 liquid or watery stools in 24 h, (2) no alternate cause for diarrhea, (3) no laxative use within 24 h, (4) no previous C. difficile NAAT result within 7 days and (5) age > 1 year. While the first two criteria only required an affirmative attestation from the ordering provider, the latter three were automatically pulled by the computer physician order entry (CPOE) system. If there were any contraindications to testing (e.g. laxative use within 24 h, a previous C. difficile test result within 7 days), a “hard stop” to the order would be reached, requiring either exiting the order or test approval through an infectious diseases or gastrointestinal specialist. Orders with infectious diseases or gastrointestinal specialist approval were reviewed, and in those cases where approval was recorded in the order but not actually given by the appropriate specialist, the ordering provider received an email stating that C. difficile orders were being monitored and repeated failure to obtain appropriate approval for testing when required would be reported to the Chief Medical Officer and/or Departmental/Divisional leadership. Pre- vs. post-intervention analysis demonstrated a 56% drop in C. difficile testing and a 54% reduction in National Healthcare Safety Network (NSHN)-reported hospital-onset CDI (HO-CDI) laboratory-identified (LabID) events [11], surveillance metric for hospitals that likely over-captures true rates of CDI [2].
Yen and colleagues shifted focus away from the ordering provider and authorized laboratory personnel to cancel orders if a stool sample was not received within 24 h of placement and at the same time implemented a “stick test” whereas stools were rejected in accordance with the commonly used “Brecher criteria” [19]. As a result, they observed 43% fewer tests and a 60% reduction in CDI events.
We recently reported a 41% reduction in overall C. difficile testing as well as significant reductions in inappropriate duplicate negative (within 3 days following a previous negative) and duplicate positive (within 14 days following a previous positive) tests following introduction of a provider-based bundled CCDS tool at the University of Virginia Health System [10]. We also observed a significant reduction in HO-CDI events over the 10-month post-intervention period compared to the 18-month pre-intervention period [10].
An essential question regarding pre-analytic diagnostic stewardship strategies for CDI and other HAIs is whether the interventions are associated with harm due to delayed or missed diagnoses. White et al. observed a somewhat troubling, although not statistically significant, increase in CDI-related complications associated with the CCDS (ICU transfer for CDI complication, surgery for CDI complication, or CDI contributing to death) (5% baseline vs. 8.9% post-intervention, p = 0.11) [12]. The delay in diagnosis was also examined among those with complications and only one case was observed in which testing was delayed more than 24 h (although the authors did not quantify delays less than 24 h). Although adverse patient outcomes were not systematically addressed in our study, increased CDI-related complications or mortality were not observed.
Truong et al. at Stanford University circumvented provider behavior by authorizing laboratory staff to enforce testing criteria (presence of >2 unformed stools in 24 h and absence of laxatives in 48 h) by canceling tests based on nurse reporting of stool occurrences and “real time clinical data tracking” [18] leading to significantly (32%) reduced tests and LabID HO-CDI events (rate decreased from 13.0 to 9.7 cases per 10,000 patient days). Among 375 episodes of canceled orders, rates of increasing leukocytosis (within 7 days), ICU admission, and 30 day all-cause mortality were not significantly different from patients with accepted orders who were C. difficile negative. However, there were trends towards increased mortality and ICU admission amongst patients in the canceled group.
Earlier testing during hospitalization (before hospital day 4) may reduce LabID cases of HO-CDI at least in part by identifying more cases as community-onset CDI (CO-CDI) [20], and some hospitals have honed in on this aspect of testing. For example, a laxative alert requiring a hard stop (requiring providers to call the laboratory to order a C. difficile test if the patient had received laxatives) only fired >36 h after admission [15]. Another study implemented a strategy to “optimally identify” patients matching the NHSN surveillance definition for CO-CDI [21], by encouraging early testing through a nurse-driven protocol [13]. While a diagnostic stewardship approach motivated primarily to shift quality metric classification (in this case from HO-CDI to community-onset CDI) certainly would (and did) reduce surveillance detection of HO-CDI, it does not appear to accomplish the primary goal of diagnostic stewardship, avoiding unnecessary test utilization. Stewardship that focuses on earlier C. difficile testing could be seen as “gaming the system” to reduce surveillance detection of HO-CDI in settings where asymptomatic carriage rates can reach 15% [22]. Furthermore, testing too early could promote misdiagnosis and mistreatment of non-C. difficile associated diarrhea and the 2017 IDSA-SHEA C. difficile Guidelines state that there is insufficient evidence to support screening for asymptomatic C. difficile carriage [7].
Analytic stage
Various diagnostic methodologies are used to detect CDI. The tissue culture cytotoxicity assay performed on fresh stool is regarded as the reference test of choice for epidemiologic studies; however, cost, slow turnaround time, expertise required to perform this method and lack of standardization make the use of this method unattractive in clinical settings [3]. Clinical options include tests for C. difficile organism (GDH EIA), toxin antigen or gene (EIA, NAAT), or algorithmized combinations of these tests; however, debate exists regarding the optimal diagnostic approach [23-25]. While some studies suggest that EIA for C. difficile toxin may more accurately identify patients with true infection, others argue that toxin EIA does not have sufficient negative predictive value to be clinically reliable on its own and a NAAT-only approach is often preferred despite increased potential for over-diagnosis [2, 26, 27]. Diagnostic stewardship offers the potential for reducing NAAT false positives by preventing testing in the presence of low pretest probability while preserving the benefits of increased NAAT sensitivity. Novel diagnostic methodologies such as ultrasensitive quantitative toxin assays [28] and NAAT polymerase chain reaction cycle threshold analysis [29] are also under investigation.
Postanalytics stage
Result interpretation by providers is an important component to consider as the final opportunity for stewardship along the diagnostic pathway. A binary positive or negative result does not take into account nuances in test methodology, testing algorithm or population risk factors affecting CDI diagnosis and treatment, any of which may be unknown or unrecognized by the provider.
Unfortunately, scant evidence exists examining postanalytic stewardship for C. difficile, let alone a specific approach. Likewise, the 2017 IDSA-SHEA guidelines provide little guidance to support postanalytic stewardship other than to point out observational data that suggest clinical symptoms of CDI may help to more accurately interpret C. difficile tests [30]. Regardless, clear evidence-based postanalytic stewardship could conceivably reduce diagnostic error for CDI. For example, Kamboj et al. found that 51 out of 118 patients (43%) at a tertiary care cancer center (presumably at high risk for CDI and CDI-related complications) with a positive C. difficile NAAT but negative toxin EIA had a positive reference cell cytotoxicity assay, three of which met clinical criteria for severe CDI [31]. This alarming rate of nearly half potential false positives, if a NAAT with confirmatory toxin EIA multistep approach is used, suggests that at least some toxin EIA-negative patients may warrant treatment if clinical suspicion for CDI is high, despite a “negative” result. One approach could be to report relevant posttest probability results, similar to offering a local antibiogram to guide antibiotic treatment; however, lack of a “gold standard” for CDI would be an issue.
Conclusions
Diagnostic stewardship begins with specific and high-quality consensus recommendations to guide a testing approach. While the 2017 IDSA-SHEA guidelines provide a preliminary framework recommendation for preagreed institutional criteria to improve utilization of C. difficile NAATs, we are in the early stages of honing preanalytic diagnostic stewardship for C. difficile as well as other tests for infectious or non-infectious conditions.
Diagnostic stewardship practices for C. difficile require validation and standardization. Previous studies show wide variations in the efficacy of reducing “inappropriate” testing and surveillance CDI events. Some strategies fail to change provider behaviors while others succeed, potentially owing to differences in diagnostic stage of intervention, testing methodology, implementation strategy, specific recommendation(s) and local provider or patient population. For example, introduction of a decision-making algorithm for C. difficile testing was associated with significantly reduced testing post-intervention at an acute care hospital in the UK, while a similar intervention used at a US pediatric hospital had no sustained effect on ordering practices [14, 16]. Other features such as “hard stops” (e.g. requiring specialist consultation or laboratory approval to order C. difficile tests deemed unnecessary) [11] or using time to specimen collection (e.g. canceling orders if a sample is not received within 24 h) [17] may generate frustration among providers without clear benefit.
Existing studies examining diagnostic stewardship for C. difficile have significant limitations including small sample sizes, limited follow-up and combined endpoints. Study reproducibility and external validity are also limited across institutions with varying levels of resources and with distinct issues surrounding test utilization. Most importantly, potential harm associated with reduced testing is often not captured prospectively, and at the patient level.
The 2017 IDSA-SHEA guidelines suggest that institutions using an NAAT alone approach without preagreed institutional criteria should adopt a multistep testing algorithm in order to improve test performance (positive and negative predictive value) or else implement preagreed institutional criteria for testing [7]. The guidelines also mention that preagreed institutional criteria may be used in combination with multistep testing but no qualification is made to indicate the superior approach among these three options (preagreed institutional criteria + NAAT alone, multistep testing alone, or preagreed institutional criteria + multistep testing) and their equivalence has not been studied.
The updated guidelines for C. difficile demonstrate progress towards improving test utilization for C. difficile by incorporating recommendations for diagnostic stewardship; however, this is just a starting point. Much work remains to be done to standardize effective stewardship practices. Which specific set(s) of questions are required for an effective stewardship approach? The success of several preanalytic diagnostic stewardship interventions that targeted inappropriate testing for patients receiving laxatives demonstrated that this can be an effective countermeasure. The success of our non-laxative based CCDS intervention suggests focusing on laxatives may not be essential. Determining whether sequential addition of more efforts leads to stepwise improvements in efficacy is important for streamlining interventions.
Regarding preanalytic and postanalytic stewardship, where stewards interface directly with providers, provider satisfaction and emergence of workarounds (e.g. oral vancomycin treatment without testing to justify treatment) are particularly vital process measures to determine feasibility and detect unintended consequences. At the analytic stage, special considerations must be made regarding cost and technical capabilities. Postanalytic stewardship strategies must be carefully implemented to ensure results are not misinterpreted. As with all stages of the analytic process, it is essential to consider patient safety.
The purpose of diagnostic stewardship is to achieve a state of optimal test use. While reducing unnecessary tests can have many potential benefits, test underuse risks missed diagnoses and potential harm related to untreated conditions, for which the stakes are particularly high for infections such as CDI. Despite evidence that diagnostic stewardship strategies can effectively reduce inappropriate C. difficile testing, the impacts on patient safety are largely unknown. Thus, future studies examining diagnostic stewardship for CDI and other HAIs should include patient level outcomes measures to detect potential harm, particularly among the subset of patients for whom tests are prevented by stewardship.
Summary
We wonder whether the diagnostic options recommended by the 2017 IDSA-SHEA guidelines are truly equivalent and hypothesize that the optimal diagnostic approach to C. difficile testing involves a multifaceted approach, with stewardship introduced across the diagnostic pathway including pre-analytic (e.g. CCDS tools, laboratory refusal of inappropriate specimens), analytic (e.g. enhancing test performance using innovative or multi-step testing) and post-analytic stages (e.g. encouraging appropriate result interpretation) [28, 29].
As is the case with many quality improvement efforts, bundled interventions and quasi-experimental study designs often entangle the interpretation of individual diagnostic stewardship strategies. Further research is required to determine the optimal combination of strategies that guide providers to order the right test, on the right patient, at the right time, and with the right interpretation.
Acknowledgments
Research Funding: This research was supported by the National Institutes of Health Infectious Diseases Training Grant (no. 5T-32AI007046-41, Funder Id: 10.13039/100000060).
Footnotes
Employment or leadership: None declared.
Honorarium: None declared.
Competing interests: The funding organization(s) played no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the report for publication.
Contributor Information
Gregory R. Madden, Division of Infectious Diseases and International Health, Department of Medicine, University of Virginia Health System, Charlottesville, VA, USA..
Melinda D. Poulter, Clinical Microbiology Laboratory, Department of Pathology, University of Virginia Health System, Charlottesville, VA, USA
Costi D. Sifri, Division of Infectious Diseases and International Health, Department of Medicine, University of Virginia Health System, P.O. Box 800473, Charlottesville, VA 22908-0473, USA; Office of Hospital Epidemiology/ Infection Prevention and Control, University of Virginia Health System, Charlottesville, VA, USA.
References
- 1.Dubberke ER, Wertheimer AI. Review of current literature on the economic burden of Clostridium difficile infection. Infect Control Hosp Epidemiol 2015;30:57–66. [DOI] [PubMed] [Google Scholar]
- 2.Polage CR, Gyorke CE, Kennedy MA, Leslie JL, Chin DL, Wang S, et al. Overdiagnosis of Clostridium difficile infection in the molecular test era. JAMA Intern Med 2015;175:1792–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen SH, Gerding DN, Johnson S, Kelly CP, Loo VG, McDonald LC, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA). Infect Cont Hosp Epidemiol 2010;31:431–55. [DOI] [PubMed] [Google Scholar]
- 4.Curtin BF, Zarbalian Y, Flasar MH, Rosenvinge von E. Clostridium difficile-associated disease: adherence with current guidelines at a tertiary medical center. World J Gastroenterol 2013;19:8647–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buckel WR, Avdic E, Carroll KC, Gunaseelan V, Hadhazy E, Cosgrove SE. Gut check: Clostridium difficile testing and treatment in the molecular testing era. Infect Control Hosp Epidemiol 2015;36:217–21. [DOI] [PubMed] [Google Scholar]
- 6.Madden GR, Weinstein RA, Sifri CD. Diagnostic stewardship for healthcare-associated infections: opportunities and challenges to safely reduce test use. Infect Control Hosp Epidemiol 2018;80:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McDonald LC, Gerding DN, Johnson S, Bakken JS, Carroll KC, Coffin SE, et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis 2018;336:1049. [DOI] [PubMed] [Google Scholar]
- 8.Morgan DJ, Malani P, Diekema DJ. Diagnostic stewardshipleveraging the laboratory to improve antimicrobial use. JAMA 2017;318:607–8. [DOI] [PubMed] [Google Scholar]
- 9.Plebani M, Sciacovelli L, Aita A, Padoan A, Chiozza ML. Quality indicators to detect pre-analytical errors in laboratory testing. Clin Chim Acta 2014;432:44–8. [DOI] [PubMed] [Google Scholar]
- 10.Madden GR, German Mesner I, Cox HL, Mathers AJ, Lyman JA, Sifri CD, et al. Reduced Clostridium difficile tests and laboratory-identified events with a computerized clinical decision support tool and financial incentive. Infect Control Hosp Epidemiol 2018;4:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Quan KA, Yim J, Merrill D, Khusbu U, Madey K, Dickey L, et al. Reductions in Clostridium difficile infection (CDI) rates using realtime automated clinical criteria verification to enforce appropriate testing. Infect Control Hosp Epidemiol 2018;39:625–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.White DR, Hamilton KW, Pegues DA, Hanish A, Umscheid CA. The impact of a computerized clinical decision support tool on inappropriate Clostridium difficile testing. Infect Control Hosp Epidemiol 2017;38:1204–8. [DOI] [PubMed] [Google Scholar]
- 13.Bruno-Murtha LA, Osgood RA, Alexandre CE. A successful strategy to decrease hospital-onset Clostridium difficile. Infect Control Hosp Epidemiol 2018;39:234–6. [DOI] [PubMed] [Google Scholar]
- 14.Thompson I, Lavelle C, Leonard L. An evaluation of the effectiveness of an algorithm intervention in reducing inappropriate faecal samples sent for Clostridium difficile testing. J Infect Prevent 2016;17:278–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drees M, Dressler R, Taylor K, Ayala J, Kahigian G, Briody C, et al. Testing stewardship: a “Hard Stop” to reduce inappropriate C. diff testing. Open Forum Infect Dis 2017;4:S1–2. [Google Scholar]
- 16.Klatte JM, Selvarangan R, Jackson MA, Myers AL. Reducing overutilization of testing for Clostridium difficile infection in a pediatric hospital system: a quality improvement initiative. Hosp Pediatr 2016;6:9–14. [DOI] [PubMed] [Google Scholar]
- 17.Yen C, Holtom P, Butler-Wu SM, Wald-Dickler N, Shulman I, Spellberg B. Reducing Clostridium difficile colitis rates via cost-saving diagnostic stewardship. Infect Control Hosp Epidemiol 2018;4:1–3. [DOI] [PubMed] [Google Scholar]
- 18.Truong CY, Gombar S, Wilson R, Sundararajan G, Tekic N, Holubar M, et al. Real-time electronic tracking of diarrheal episodes and laxative therapy enables verification of Clostridium difficile clinical testing criteria and reduction of Clostridium difficile infection rates. J Clin Microbiol 2017;55:1276–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brecher SM, Novak-Weekley SM, Nagy E. Laboratory diagnosis of Clostridium difficile infections: there is light at the end of the colon. Clin Infect Dis 2013;57:1175–81. [DOI] [PubMed] [Google Scholar]
- 20.Gase KA, Haley VB, Xiong K, Van Antwerpen C, Stricof RL. Comparison of 2 Clostridium difficile surveillance methods: National Healthcare Safety Network’s laboratory-identified event reporting module versus clinical infection surveillance. Infect Control Hosp Epidemiol 2013;34:284–90. [DOI] [PubMed] [Google Scholar]
- 21.CDC/NHSN Surveillance Definitions for Specific Types of Infections [Internet]. Centers for Disease Control and Prevention Website. 2017. [cited 2017 Apr 26]. pp. 1–29. Available from: https://www.cdc.gov/nhsn/pdfs/pscmanual/17pscnosinfdef_current.pdf.
- 22.Alasmari F, Seiler SM, Hink T, Burnham CA, Dubberke ER. Prevalence and risk factors for asymptomatic Clostridium difficile carriage. Clin Infect Dis 2014;59:216–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crobach MJ, Planche T, Eckert C, Barbut F, Terveer EM, Dekkers OM, et al. European society of clinical microbiology and infectious diseases: update of the diagnostic guidance document for Clostridium difficile infection. Clin Microbiol Infect 2016;22:S63–81. [DOI] [PubMed] [Google Scholar]
- 24.Leffler DA, Lamont JT. Clostridium difficile infection. N Engl J Med 2015;372:1539–48. [DOI] [PubMed] [Google Scholar]
- 25.Bartsch SM, Umscheid CA, Nachamkin I, Hamilton K, Lee BY. Comparing the economic and health benefits of different approaches to diagnosing Clostridium difficile infection. Clin Microbiol Infect 2015;21:77.e1–9. [DOI] [PubMed] [Google Scholar]
- 26.Planche TD, Davies KA, Coen PG, Finney JM, Monahan IM, Morris KA, et al. Differences in outcome according to Clostridium difficile testing method: a prospective multicentre diagnostic validation study of C. difficile infection. Lancet Infect Dis 2013;13:936–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fang FC, Polage CR, Wilcox MH. Point-counterpoint: what is the optimal approach for detection of Clostridium difficile infection? J Clin Microbiol 2017;55:670–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pollock NR. Ultrasensitive detection and quantification of toxins for optimized diagnosis of Clostridium difficile infection. J Clin Microbiol 2016;54:259–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Senchyna F, Gaur RL, Gombar S, Truong CY, Schroeder LF, Banaei N. Clostridium difficile PCR cycle threshold predicts free toxin. J Clin Microbiol 2017;55:2651–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dubberke ER, Han Z, Bobo L, Hink T, Lawrence B, Copper S, et al. Impact of clinical symptoms on interpretation of diagnostic assays for Clostridium difficile infections. J Clin Microbiol 2011;49:2887–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kamboj M, Brite J, McMillen T, Robilotti E, Herrera A, Sepkowitz K, et al. Potential of real-time PCR threshold cycle (CT) to predict presence of free toxin and clinically relevant C. difficile infection (CDI) in patients with cancer. J Infect 2018;76:369–75. [DOI] [PMC free article] [PubMed] [Google Scholar]