Abstract
BACKGROUND:
We projected the clinical outcomes, cost-effectiveness, and budget impact of ibalizumab plus an optimized background regimen (OBR) for people with multidrug-resistant HIV in the US.
METHODS:
Using the Cost-Effectiveness of Preventing AIDS Complications (CEPAC) microsimulation model and a health care sector perspective, we compared two treatment strategies for multidrug-resistant HIV: 1) IBA+OBR—ibalizumab plus OBR, and 2) OBR—OBR alone. Ibalizumab efficacy and cohort characteristics were from trial data: mean age 49y, 85% male, mean CD4 150/μl. Six-month viral suppression was 50% with IBA+OBR and 0% with OBR. The ibalizumab loading dose cost $10,500 and subsequent ibalizumab injections cost $8,400/month; OBR cost $4,500/month. Incremental cost-effectiveness ratios (ICERs) were calculated using discounted (3%/year) quality-adjusted life years (QALYs) and costs. ICERs≤$100,000/QALY were considered cost-effective. We performed sensitivity analysis on key parameters and examined budget impact.
RESULTS:
In the base case, five-year survival increased from 38% with OBR to 47% with IBA+OBR. Lifetime costs were $301,700/person with OBR and $661,800/person with IBA+OBR; the ICER for IBA+OBR compared to OBR was $260,900/QALY. IBA+OBR was not cost-effective even with 100% efficacy. IBA+OBR became cost-effective at base case efficacy if ibalizumab cost was reduced by ≥88%. For an estimated 12,000 people with multidrug-resistant HIV in the US, IBA+OBR increased care costs by $1.8 billion (1.5% of total treatment budget) over five years.
CONCLUSIONS:
For people with multidrug-resistant HIV lacking other treatment options, ibalizumab will substantially increase survival when effective. While adding ibalizumab to OBR is not cost-effective, the low number of eligible patients in the US makes the budget impact relatively small.
Keywords: HIV, cost-effectiveness, ibalizumab, multidrug-resistant HIV, budget impact
INTRODUCTION
Most people with HIV can achieve virologic suppression with currently available antiretroviral therapy (ART).1 A small subset of patients (estimated at 12,000 in the US), however, have multidrug-resistant (MDR) HIV infection for which standard antiretroviral treatments are ineffective.1–5 Until recently, the only therapeutic option for individuals who had MDR HIV and multiple failed regimens was treatment with a best-possible or “optimized” regimen consisting of a combination of the most potent antiretroviral drugs specific to their resistance profiles. With severe MDR HIV, this strategy does not lead to virologic suppression but can slow CD4 count decline6–8 due to the impaired viral fitness of some resistant strains.
Approved by the U.S. Food and Drug Administration (FDA) under the Orphan Drug Act in March 2018, ibalizumab is the first monoclonal antibody licensed to treat MDR HIV.9,10 This drug, when combined with an optimized background regimen (OBR), showed promise in its first phase 3 trial, TMB-301, with 50% of patients achieving virologic suppression at 6 months.11 All patients suppressed at 6 months who completed the 12 month trial (TMB-311) showed sustained suppression at 12 months.12 These results suggest that ibalizumab combined with OBR is an effective treatment and may improve outcomes for people with MDR HIV.
Biologics, including monoclonal antibodies (mAbs), are among the most expensive drugs available in the US.13,14 Pharmaceutical companies attribute the high cost of mAbs to dose requirements and manufacturing complexity.13 Ibalizumab is no exception—with treatment administered intravenously every 2 weeks at an infusion center or at home with homecare service assistance, ibalizumab treatment alone costs ~$111,000/year in 2018 US dollars.15–17 Salvage regimens typically cost ~$54,000/year, thus total treatment costs for someone on ibalizumab and a salvage OBR are ~$164,000/year, three times as high as treatment with a salvage regimen alone.15 Our objective was to project the clinical impact, cost, and cost-effectiveness of ibalizumab over the short and long term for people with MDR HIV in the US.
METHODS
Analytic overview and model structure
We used the Cost-effectiveness of Preventing AIDS Complications (CEPAC) model, a validated Monte Carlo microsimulation model of HIV disease, to project and compare the clinical and economic impact of two strategies for people with MDR HIV: 1) IBA+OBR (ibalizumab and OBR treatment) and 2) OBR (OBR alone).18–20 We modeled a population of patients with MDR HIV using cohort characteristics and efficacy results from the TMB-301 (25-week results) and TMB-311 (48-week results) trials to model IBA+OBR.11,12 There was no control group in either trial, and both reported efficacies for IBA with OBR. Lacking efficacy data for OBR alone, we assumed that patients taking only OBR would not achieve virologic suppression or CD4 increase, but would remain on OBR.1,8
CEPAC is a health-state transition model that tracks simulated individual patients through their lifetimes and projects quality-adjusted life expectancy and HIV-associated medical costs. ART efficacy and retention in care depend on the patient’s model-specified adherence level. Patients who are more adherent have a higher probability of virologic suppression on treatment and a lower probability of disengaging from care. Patients who leave care have a monthly probability of returning, which increases in the event of an acute opportunistic infection (OI). Additional details about the CEPAC model are available online: https://www.massgeneral.org/mpec/cepac/.
Model outcomes included 5-year survival, life expectancy expressed in quality-adjusted life years (QALYs), and direct medical costs. QALYs and costs were discounted 3%/year.21 Using the difference in life expectancy and cost between strategies, we calculated the incremental cost-effectiveness ratio (ICER) for IBA+OBR compared to OBR. We examined model outcomes over 5-year and lifetime horizons. We used a health sector perspective and considered an ICER ≤ $100,000/QALY to be cost-effective.22,23 The number of people with MDR HIV in the US is uncertain; we assessed 5-year budget impact of IBA+OBR compared to OBR for an estimated 12,000 people with MDR HIV.5
Strategies
In the IBA+OBR strategy, simulated patients start ibalizumab treatment in addition to OBR. Ibalizumab treatment is initiated with a 2,000mg intravenous loading dose, followed by an 800mg dose every two weeks for as long as HIV RNA remains <50copies/mL.1 We used a common optimized background regimen—ritonavir-boosted darunavir (DRV/r) (twice daily) + tenofovir alafenamide/emtricitabine (TAF/FTC). While on ibalizumab treatment, patients incur ibalizumab costs, its infusion costs, and OBR drug costs. For patients in the IBA+OBR strategy, those who do not suppress (within 3 months; 6 doses) or who experience a major toxicity (within 4 months) on ibalizumab are switched to OBR alone and continue this regimen indefinitely.1 We presume that patients attaining virologic suppression on ibalizumab remain suppressed unless they are lost to follow-up (LTFU). Patients who return to care after being LTFU resuppress on ibalizumab if they had virologic suppression initially.
In the OBR strategy, individuals do not initiate ibalizumab and instead take a virologically non-suppressive OBR for the duration of the simulation and incur only the costs of that regimen. This OBR regimen is the same as that for patients in the IBA+OBR strategy: DRV/r (twice daily) + TAF/FTC.
In both strategies, OBR alone does not lead to virologic suppression and patients do not experience an increase in CD4 cells. Despite virologic failure, however, they experience slowed CD4 decline compared to those not on ART.24 In both strategies, patients incur medical treatment costs for routine care and acute OI events in addition to regimen-specific costs.
Input Parameters
Cohort characteristics
Modeled cohort characteristics reflected the population of patients in the TMB-301 trial, assumed to be representative of people with MDR HIV in the US. Mean initial age was 49y, 85% were male, and mean initial CD4 count was 150/μl (Table 1).11
Table 1.
Base case input parameters for a modeling analysis of IBA+OBR compared to OBR for people with MDR HIV in the US
| Parameter | Base Case Value | Reference | |
|---|---|---|---|
| Cohort characteristics | TMB-301 triala (base case) | TMB-202 trialb | |
| Age, mean years (SD) | 49 (11) | 48 (7) | 11,32 |
| Sex, male/female, % | 85/15 | 89/11 | |
| Initial CD4 cell count, mean cells/μL (SD) | 150 (182) | 109 (105) | |
| HIV RNA distribution, %c | |||
| >100,000 copies/mL | 17 | 52 | |
| 30,001 – 100,000 copies/mL | 33 | 27 | |
| 10,001 – 30,000 copies/mL | 2 | 14 | |
| 3,001 – 10,000 copies/mL | 28 | 6 | |
| ≤3,000 copies/mL | 20 | 1 | |
| Regimen characteristics | IBA+OBR | OBR | |
| Suppression, mean <50 copies/mL at 6 m, % | 50 | 0 | 11 |
| CD4 count increase/month mean cells/μL (SD) | 11,32 | ||
| First 2 months | 27 (14) | 0 | |
| After 2 months | 2 (1) | 0 | |
| Serious adverse events requiring regimen change, % | 20 | 0 | 11 |
| Time to serious adverse event, mean months (SD) | 3 (1) | 0 | |
| CD4 count decline/month on failing ART, rate multiplierd |
0.45 | 0.45 | 24 |
| Costs of care (2018 USD) | IBA+OBR | OBR | |
| CD4-dependent monthly routine care costs | 300 – 1,300 | 300 – 1,300 | 65 |
| Medication costs | 15 | ||
| Ibalizumab loading dose (2000mg) | $10,500 | -- | |
| Ibalizumab, $/month (800mg doses) | $8,400 | -- | |
| OBR, $/month (DRV/r (bid) + TAF/FTC) | $4,500 | $4,500 | |
| Infusion costs, $/monthe | $840 | -- | 16,17 |
IBA+OBR: ibalizumab and optimized background regimen treatment strategy. OBR: optimized background regimen only strategy. MDR: multidrug-resistant. SD: standard deviation. DRV/r: ritonavir-boosted darunavir. TAF: tenofovir alafenamide. FTC: emtricitabine. USD: US dollars. bid: twice a day.
The TMB-301 trial is the phase-3 trial of ibalizumab (n = 40) and we use cohort characteristics from this trial in our base case analysis.11
The TMB-202 trial is a phase-2b trial of ibalizumab (n = 113); we use cohort characteristics from this trial in a scenario analysis.32
For the TMB-301 trial (base case), the HIV RNA distribution was derived from the mean, standard deviation, median, minimum, and maximum HIV RNA levels and % with HIV RNA > 100,000 copies/mL provided by Emu.11 With these inputs, we used SAS to group individuals into strata using minimum, median, and maximum and forcing a prescribed % to have HIV RNA > 100,000 copies/mL, to calculate the mean and standard deviation within each group assuming 3-sigma rule, and then to simulate the three strata using truncated normal distributions to force individuals within each stratum to lie within the minimum and maximum of the stratum. For the TMB-202 trial, the HIV RNA distribution was derived from the mean, minimum and maximum HIV RNA levels reported. With these inputs, we used SAS to group individuals into strata, calculate the mean and standard deviation within each group using the 4-sigma rule, and then simulate the two strata using truncated normal distribution to force individuals within each stratum to lie within the minimum and maximum of the stratum.
This multiplier is applied to a natural history rate of CD4 decline experienced by people with HIV who are not on any treatment and accounts for the slowed CD4 decline experienced by people with HIV who are on treatment but virologically failing.
Infusion costs were estimated using two recent studies of infusion costs of monoclonal antibodies in hospital-based infusion centers, including drug acquisition, labor, overhead, and laboratory costs. The cost per infusion was similar in both studies and thus we averaged their reported costs. Since ibalizumab is administered twice per month, we then multiplied by two to derive the monthly infusion costs of ibalizumab.
Treatment efficacy
In IBA+OBR, simulated patients add ibalizumab to their OBR and undergo monitoring for failure, with HIV RNA testing monthly and CD4 testing every three months.1 The likelihood of attaining virologic suppression on ibalizumab at 6 months is 50%.11 Among all people initiating ibalizumab, there is a 20% probability of discontinuing ibalizumab within the first 4 months.11 All patients with virologic suppression at 6 months in TMB-301 remained suppressed at 12 months in TMB-311 if they stayed in the trial through 12 months. Lacking longer-term follow-up data, we assumed in the base case that patients who initially suppress on ibalizumab remain suppressed long-term unless LTFU; i.e., patients only experience failure after initial suppression if they leave care. This demonstrates the maximum potential clinical benefit of ibalizumab.
We modeled treatment with OBR alone as virologically ineffective, both for those who fail or discontinue ibalizumab and for those in the OBR strategy. These patients still experience some ART benefit: they have a 45% lower decline in CD4 cells/month compared to those not on ART.8,24
Adherence to medication and engagement in care
We assumed that patients with MDR HIV have similar adherence to the general population of people with HIV in the US, with a mean adherence of 89%. We tested this assumption in sensitivity analysis through examining the impact of ibalizumab efficacy on our results.19,25,26 Once suppressed, people have an adherence-dependent likelihood of becoming LTFU: from 0.01% monthly probability for those who are most adherent to 6.8% for those who are least adherent, resulting in 13% of people being LTFU within the first 2 years.19,27–29 Those who are LTFU do not receive treatment and experience the natural progression of HIV disease. After patients are LTFU, they return to care if they experience an OI; otherwise, 6 months after becoming LTFU, patients return to care at a monthly probability of 1.5%.30
Costs
A standard OBR regimen that includes DRV/r (twice daily) + TAF/FTC costs $4,500/month,15 based on average wholesale price (AWP) discounted by 23% for branded drugs.31 The ibalizumab 2,000mg loading dose costs $10,500, and an 800mg maintenance dose costs $4,200/infusion.15 Thus, ibalizumab costs $14,700 for the first month of treatment, which includes the loading dose, and $8,400/month thereafter. In addition to drug costs, each infusion costs $420.16,17
HIV transmissions
We used viral load - dependent monthly transmission probabilities to estimate the number of transmissions in each strategy. A detailed description of the methods and results of this analysis can be found in the Supplementary Material.
Scenario analysis and sensitivity analysis
Base case cohort characteristics were from patients in the only published phase 3 trial of ibalizumab.11 Given that the characteristics of patients with MDR HIV in the US are largely unknown and the TMB-301 cohort was small (n = 40), we also considered a second scenario using cohort characteristics reported in TMB-202, a phase 2b trial for ibalizumab (n = 113).32 In that scenario, the mean initial age of the population was 48y, 89% were male, the mean initial CD4 count was 109/μl, and the mean viral load was higher than the base case, with 52% having HIV viral load >100,000 copies/mL versus 17% in the base case (Table 1).
We examined several parameters in one-way sensitivity analyses, including: ibalizumab efficacy and costs, mean initial CD4 count, rate of CD4 decline on OBR treatment, probability of serious adverse events, and probability of failing ibalizumab after initial viral suppression. We varied each parameter from 0.5x-2.0x its base case value. Ibalizumab efficacy was used as a proxy measure to examine varying levels of adherence to ibalizumab treatment specifically and not to the entire treatment regimen, which included OBR. To address the possibility that patients who initially respond to ibalizumab may lose virologic suppression over time, we investigated the possibility that those initially attaining virologic suppression on ibalizumab could subsequently experience failure. Given that the long-term efficacy of ibalizumab is unknown, we varied this parameter from 0–0.5% monthly probability of failing the regimen after 12 months of virologic suppression. In a two-way sensitivity analysis stratified by time horizon, we examined the influences of ibalizumab efficacy and cost when varied simultaneously.
We also conducted a probabilistic sensitivity analysis (PSA), in which we simultaneously varied IBA efficacy (42.8–64.2%), CD4 decline multiplier on failed ART (.25-.65), time between experiencing toxicity and discontinuing IBA (1–6 months), and quality of life decrement due to toxicity on IBA (0–0.2). Values for each of these parameters were randomly drawn from uniform distributions, and values for other parameters were taken from the base case. The analysis included 5,000 random sets of draws, and 1,000,000 individuals were simulated for each set. From the model results for the OBR and IBA strategies, we calculated net monetary benefit, defined as: (Quality-adjusted life-years)*(Willingness-to-pay)* - (Lifetime costs). We generated cost-effectiveness acceptability curves, showing the probability that a treatment regimen would be preferred, in terms of net monetary benefit, at different levels of willingness-to-pay (WTP).
Budget impact
We used model outcomes to predict the budget impact of treating the estimated 12,000 individuals with MDR HIV in the US with ibalizumab as compared to OBR alone from a payer perspective. This analysis included direct medical costs for medications, outpatient visits, and hospitalizations. By convention, budget impact results were not discounted.33 Given that the number of patients with MDR HIV who would need ibalizumab is uncertain, we examined the budget impact across a range of 5,000–15,000 patients.
RESULTS
Base case
In the base case, 5-year survival increased from 38% with OBR to 47% with IBA+OBR (Table 2). When limited to a 5-year horizon, discounted life expectancy was 2.61 QALYs with OBR vs. 2.84 QALYs with IBR+OBR and costs increased from $212,800 to $354,100. IBA+OBR had an ICER of $598,600/QALY gained at five years.
Table 2.
Clinical impact and cost-effectiveness of IBA+OBR compared with OBR for people with MDR HIV in the US
| Cohort | 5-yr survival (% alive) |
Lifetime QALE (y) | Total lifetime cost (USD)a |
ICER (USD/QALY)a | |
|---|---|---|---|---|---|
| Base case | OBR | 38 | 3.74 | $301,700 | -- |
| IBA+OBR | 47 | 5.12 | $661,800 | $260,900 | |
| Scenario analysis | |||||
| TMB-202 trialb | OBR | 33 | 3.21 | $276,100 | -- |
| IBA+OBR | 44 | 4.64 | $637,100 | $250,900 | |
IBA+OBR: ibalizumab and optimized background regimen treatment strategy. OBR: optimized background regimen only strategy. MDR: multidrug-resistant. QALE: quality-adjusted life expectancy. QALY: quality-adjusted life year. USD: 2018 US dollars. ICER: incremental cost-effectiveness ratio. PY: person-year.
Discounted 3% annually.
The TMB-202 trial is a phase-2b trial of ibalizumab (n = 113); we use cohort characteristics from this trial in a scenario analysis.32
Over a lifetime, life expectancy increased substantially with ibalizumab, from 3.74 QALYs with OBR to 5.12 QALYs with IBA+OBR. Costs also increased, from $301,700/person with OBR to $661,800/person with IBA+OBR. IBA+OBR was not cost-effective compared to OBR, with an ICER of $260,900/QALY gained.
Sensitivity analysis
Scenario analyses
In a scenario analysis using cohort characteristics from the TMB-202 ibalizumab trial, IBA+OBR had an ICER of $250,900/QALY over a lifetime compared to OBR (Table 2). Life expectancy was lower than the base case in both the IBA+OBR and OBR strategies (4.64 QALYs and 3.21 QALYs). The difference in life expectancy between the strategies was marginally changed: 1.38 QALYs in the base case vs. 1.44 QALYs in this scenario.
One-way sensitivity analysis
The probability of virologic suppression on an IBA regimen was the most influential parameter on clinical outcomes. For example, at 75% suppression probability on an IBA regimen, those in the IBA+OBR strategy had an undiscounted life expectancy of 7.61 QALYs (compared to 6.47 in the base case). However, this strategy was still not cost-effective, ICER = $252,800/QALY.
The cost of ibalizumab was the most influential parameter in determining the cost-effectiveness of IBA+OBR compared with OBR. Even when ibalizumab cost decreased by 50%, IBA+OBR had an ICER of $168,600/QALY gained over a lifetime; the ibalizumab medication cost would have to decrease by at least 88% (costing $1,000/month) to make the IBA+OBR strategy cost-effective with an ICER of $98,500/QALY. Other parameters, including mean initial CD4 count, for those on OBR, probability of suppression on an IBA regimen, probability of serious adverse events on ibalizumab, and probability of failure after initial suppression did not have substantial impact on the ICER when varied across wide ranges (Figure 1).
Figure 1. One-way sensitivity analysis on the cost-effectiveness of IBA+OBR compared to OBR for people with MDR HIV in the US.
This tornado diagram shows the ICERs (x-axis) for IBA+OBR compared to OBR for multiple input parameters (y-axis). The base case value for each input parameter is listed in parentheses before the semi-colon. The range across which we varied each parameter is listed after the semi-colon, with the value resulting in the lowest ICER before the hyphen and the value resulting in the highest ICER after the hyphen. The dotted grey line shows the $100,000/QALY cost-effectiveness threshold. Since people were switched from ibalizumab combined with OBR to OBR alone if they developed a serious adverse event, an increasing probability of serious adverse events modestly decreased the ICER; this is because of the substantial cost difference between OBR alone and ibalizumab combined with OBR. In addition, when including the probability of failing ibalizumab after initial suppression, the cost savings associated with a shorter time on ibalizumab for those in the IBA+OBR strategy outweighed the life expectancy decrement, making the ICER somewhat lower than the base case ICER.
IBA: ibalizumab. ART: antiretroviral therapy. IBA+OBR: ibalizumab and optimized background regimen treatment strategy. OBR: optimized background regimen only treatment strategy. ICER: incremental cost-effectiveness ratio. QALY: quality-adjusted life year.
aThese results reflect variation in the cost of both the loading dose and subsequent monthly doses, but only the monthly costs are listed.
When we included the possibility of losing susceptibility to ibalizumab over time, we found that while life expectancy on ibalizumab decreased from the base case value (from 5.12 QALYs to 4.85 QALYs), the cost savings associated with a shorter time on ibalizumab for those in the IBA+OBR strategy outweighed the life expectancy decrement, making the ICER somewhat lower ($237,200/QALY) than the base case ICER (Table 2).
Two-way and probabilistic sensitivity analysis
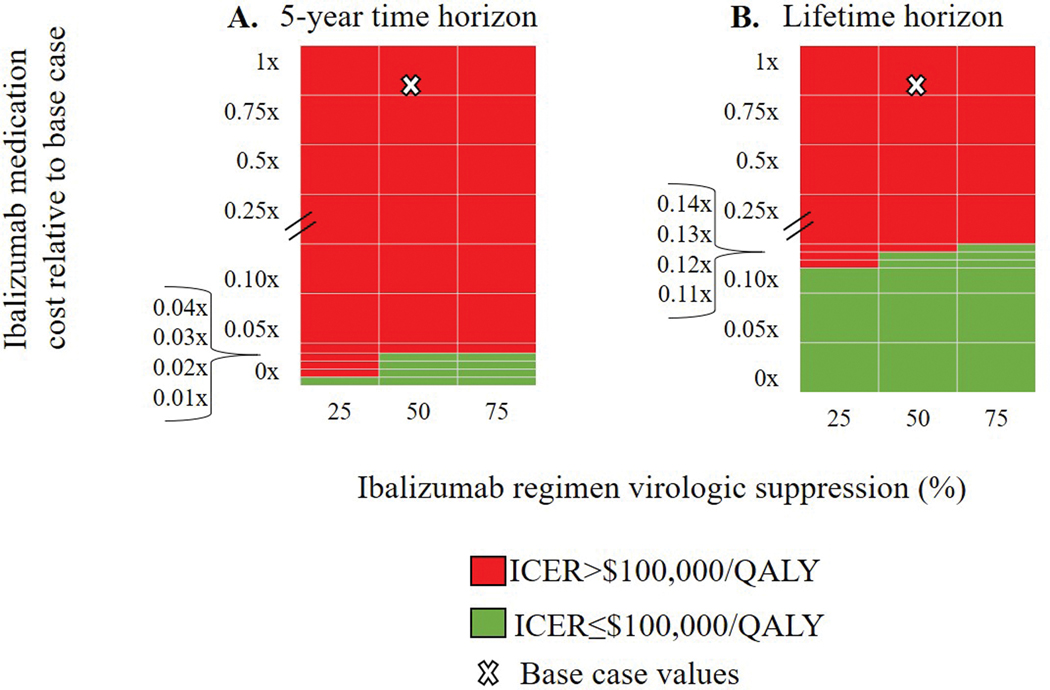
When we varied both ibalizumab medication cost and IBA regimen efficacy in two-way sensitivity analysis over both 5-year and lifetime horizons, cost-effectiveness was driven by cost (Figure 2). Over a 5-year horizon, IBA+OBR became cost-effective only when ibalizumab cost was reduced by greater than 97–99%, for ibalizumab efficacies ranging from 75–25%. Over a lifetime, IBA+OBR was cost-effective for the same range of ibalizumab efficacies when ibalizumab cost was reduced by greater than 87–90%. In the PSA, when the WTP threshold was below $250,000/QALY, OBR was always the preferred strategy from the cost-effectiveness point. When the WTP threshold was $263,000/QALY, OBR and IBA+OBR had equivalent likelihoods of being preferred. When the WTP threshold was above $282,000/QALY, IBA was always the preferred strategy.
Figure 2. The cost-effectiveness of IBA+OBR compared to OBR at different time horizons when varying ibalizumab suppression probabilities and ibalizumab costs.
On the vertical axis, we varied the cost of ibalizumab relative to the base case, i.e., 0.5x indicates that the cost was half the base case value (0x-1x base case cost). On the horizontal axis, we varied the probability of suppression on ibalizumab from 25%−75%. Each panel shows a different time horizon: 5 years (left) and lifetime (right). The white X indicates the base case result for each time horizon. IBA+OBR became cost-effective compared to OBR only when ibalizumab drug cost was <0.04x (5-year horizon) or <0.14x (lifetime) the base case cost.
ICER: incremental cost-effectiveness ratio. QALY: quality-adjusted life year. IBA+OBR: ibalizumab and optimized background regimen treatment strategy. OBR: optimized background regimen only treatment strategy.
Budget impact
From a payer perspective, IBA+OBR would cost an additional $1.8 billion for 12,000 patients treated over five years compared to OBR. The additional cost of ibalizumab treatment for patients with MDR HIV (~1.7% of the 700,000 people with HIV in care in the US) would comprise ~1.5% of the $119 billion total budget for HIV care in the US over five years.34,35 In sensitivity analysis, the budget impact ranged from $750 million to $2.2 billion for a population between 5,000 and 15,000 patients with MDR HIV in the US.
DISCUSSION
We modeled the clinical impact and cost-effectiveness of ibalizumab, a new salvage therapy for people with MDR HIV. Adding ibalizumab to an OBR increased 5-year survival by 9% compared to OBR alone. The incremental cost-effectiveness ratio was $260,900/QALY, far higher than a commonly cited $100,000/QALY threshold for cost-effectiveness in the US.22,23 When examined against a broader range of potential US cost-effectiveness thresholds—from $55,000/QALY (the ICER for center hemodialysis, a common lower bound willingness-to-pay threshold) to $180,000/QALY (3x annual per capita Gross Domestic Product)—adding ibalizumab to an OBR is still well outside the range of cost-effectiveness.36,37 The high ICER for adding ibalizumab was driven by the medication’s high cost; given that ibalizumab treatment alone costs $111,000/year and is administered for as long as it remains effective, it cannot be cost-effective at a threshold of $100,000 per each quality-adjusted life year gained. Therefore, there was no ibalizumab efficacy at which IBA+OBR became cost-effective compared to OBR without substantial reduction in ibalizumab cost. Despite ibalizumab’s high cost, the budget impact in the US is projected to be relatively modest given the small number of people eligible for this treatment. These costs would be distributed across a variety of payers: government and private insurers and/or patients.
Antiretroviral medications with novel mechanisms of action can play an important role for a very small proportion of people with HIV. Most of the individuals who might benefit from such agents were first treated in the early days of ART with less potent regimens that had low resistance barriers; ongoing treatment in the face of viral replication selected for ever increasing degrees of drug resistance to multiple different classes of drugs.38 The addition of a single active drug to a failing regimen, which was done due to the lack of treatment options and the sequential approval of new drugs, further contributed to drug resistance.38–40 Given that current treatments are much more effective and less likely to select for resistance, new cases of MDR HIV are increasingly rare.41 The precise scope of the MDR HIV problem across the US is unclear because there is limited national surveillance of HIV drug resistance, but estimates put the number of people with MDR HIV at fewer than 12,000 nationwide and this is unlikely to grow appreciably.5 Thus, while expensive, the use of ibalizumab will necessarily be limited. In addition, other drugs are in development that do not have cross-resistance with existing antiretrovirals;42,43 these are not monoclonal antibodies, and hence if approved may replace ibalizumab by providing the necessary antiviral activity at a lower cost.
Monoclonal antibodies like ibalizumab represent the fastest growing group of pharmaceutical molecules approved for use in the US and globally, with biologic therapies being used for a variety of diseases, including cancer and autoimmune disorders.44 Biologics are promising in their specificity and potential for rapid development and commercialization.45 Biologics also represent a growing fraction of global pharmaceutical sales.14 The cost of biologics varies widely, but they are typically expensive compared to other medication types, ranging from $10,000-$700,000/year.46,47 With biologics constituting an increasing proportion of overall healthcare spending in the US, efforts to reduce the cost of these therapies will be important for containing healthcare costs.14
Given the high cost, treatments with some monoclonal antibodies have been shown to become cost-effective by generally accepted US standards only with >50% decreases in cost.48–51 In treating head and neck squamous cell carcinoma, treatment with nivolumab became cost-effective when the cost of therapy was reduced by 70%.48 PSK9 inhibitors used to treat cardiovascular disease would require a cost reduction of 71% to meet a cost-effectiveness threshold of $100,000/QALY.49–51 Our analysis, like others, highlights the need to reduce production and other costs of biologic therapies while maintaining incentives to continue research and orphan drug development.52 Biosimilars, biologic medical products that are highly similar to and have no clinically meaningful difference from corresponding FDA-approved reference biologics, have some promise for reducing the budget impact of biologic therapy, but biosimilars are unlikely to have as much impact on costs as generics, given the high costs of production and the rigorous regulatory process for biosimilars.53–58 Changing costs with biosimilars might alter our cost-effectiveness conclusions, but with orphan drug exclusivity, ibalizumab’s manufacturers will have exclusive marketing rights through 2025.59 In other instances, treatment with mAbs has been shown to be cost-effective without a decrease in cost; this can occur when mAbs are found to increase life expectancy when compared to a similarly expensive alternative like standard chemotherapy.60,61
As in all modeling analyses, this study is limited by incomplete data. We based the analysis on data from the TMB-301 and TMB-311 trials and are subject to their limitations: only 48-week data are available, the number of participants in each trial was small (n=40 and n=113), and there were no control groups. Without control data or additional literature on patients with MDR HIV to inform OBR parameters in this population, we made assumptions about the average efficacy of OBR in the context of MDR HIV. In the trials, each participant had at least one active drug in their OBR. Our assumption that OBR was not suppressive means that we attributed all successful treatment observed in the trials to ibalizumab. This potentially overestimated the projected effectiveness and cost-effectiveness of ibalizumab-based therapy. In addition, there are limited clinical data about people with MDR HIV in the US. We modeled a population with the characteristics of those in the trial, but this may not be representative of the MDR HIV population at large. We used ibalizumab efficacy as reported in the TMB-301 trial. However, trial-reported efficacy may differ substantially from effectiveness in clinical settings. In the base case, we assumed that this population had similar adherence to the US general HIV population. However, decreasing adherence to ibalizumab infusions by decreasing ibalizumab efficacy did not substantially affect the results.
We did not model individualized OBR regimens because: 1) we assumed that all OBR regimens would be non-suppressive in patients who are candidates for ibalizumab; 2) varying the cost of OBR would not affect the cost-effectiveness results, given that patients in both OBR and IBA+OBR strategies are prescribed OBR. If we were to base medication costs off of the Federal Supply Schedule instead of the discounted REDBOOK costs, ibalizumab would be more expensive than in the base case and the ICER would increase.62 If ibalizumab were to be effective without an OBR or with fewer medications in the OBR, the ICER would decrease. In addition, we assumed that patients who return to care after being LTFU resuppress on ibalizumab if they had initially achieved virologic suppression. This is an optimistic assumption given that being LTFU may be associated with acquired drug resistance. A lower probability of resuppression after returning to care would make the IBA+OBR both less effective and less cost-effective. Finally, it is possible that infusion-based therapy impacts quality of life differently than oral regimens, if there were quality of life decrements to ibalizumab infusions then it would be even less cost-effective than in the base case results.
Because we focused on salvage therapy for advanced disease, we did not incorporate the impact of transmissions into our analysis. The difference in the number of transmissions in each strategy is small (see Supplementary Material). However, transmission rates will vary depending on population characteristics. For example, the TMB-202 trial population examined in a scenario analysis had higher baseline viral loads than in our base case cohort; this led to a marginally higher transmission rate (Supplementary Table 1). In addition, a formal discussion of the Rule of Rescue—justifiably spending more on individuals whose lives are in immediate peril—was beyond the scope of this paper.63,64 However, the Rule of Rescue could be considered and would support our budget impact conclusions that even for an expensive drug, the small population size renders the financial burden across payers relatively minimal.
We project that ibalizumab will substantially improve survival for people with MDR HIV. As with most biologics, the cost of ibalizumab is high, and thus ibalizumab is not cost-effective by general US criteria despite its clinical benefit. Nevertheless, for highly treatment-experienced people with MDR HIV who have limited treatment options and virologic failure, ibalizumab is currently the only available treatment that will improve survival. Given the small size of the US population that will benefit from ibalizumab, the total budget impact to payers will be low despite the high cost of the drug.
Supplementary Material
ACKNOWLEDGEMENTS
The authors acknowledge Pamela Pei for technical expertise as well as Nicole McCann and Mylinh Le for assistance with the analysis and manuscript.
Sources of Funding:
PES is a Scientific Advisory Board member for Gilead, GlaxoSmithKline/ViiV Healthcare, Merck, and Janssen; he has received grant support to his institution from Gilead, Merck, and GSK/ViiV.
This publication was made possible by funding from the National Institutes of Health [R01 AI042006, R37 AI093269, K01 DA042687], and the Steve and Deborah Gorlin MGH Research Scholars Award (RPW). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH or the Massachusetts General Hospital Executive Committee on Research.
Footnotes
Conflicts of Interest
LRIM, JAS, FMS, KPR, EL, RPW, and KAF have no conflicts of interest to declare.
REFERENCES
- 1.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. October 2018. https://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf. Accessed March 28, 2018.
- 2.Paquet AC, Solberg OD, Napolitano LA, et al. A decade of HIV-1 drug resistance in the United States: trends and characteristics in a large protease/reverse transcriptase and co-receptor tropism database from 2003 to 2012. Antivir Ther. 2014;19(4):435–441. [DOI] [PubMed] [Google Scholar]
- 3.De Luca A, Dunn D, Zazzi M, et al. Declining prevalence of HIV-1 drug resistance in antiretroviral treatment-exposed individuals in Western Europe. J Infect Dis. 2013;207(8):1216–1220. [DOI] [PubMed] [Google Scholar]
- 4.Lima VD, Harrigan PR, Sénécal M, et al. Epidemiology of antiretroviral multiclass resistance. Am J Epidemiol. 2010;172(4):460–468. [DOI] [PubMed] [Google Scholar]
- 5.Theratechnologies Inc. Theratechnologies announces FDA approval of breakthrough therapy, trogarzoTM (ibalizumab-uiyk) injection, the first HIV-1 inhibitor and long-acting monoclonal antibody for multidrug resistant HIV-1. https://www.prnewswire.com/news-releases/theratechnologies-announces-fda-approval-of-breakthrough-therapy-trogarzo-ibalizumab-uiyk-injection-the-first-hiv-1-inhibitor-and-long-acting-monoclonal-antibody-for-multidrug-resistant-hiv-1-300609280.html. Accessed March 28, 2019.
- 6.Steigbigel RT, Cooper DA, Teppler H, et al. Long-term efficacy and safety of raltegravir combined with optimized background therapy in treatment-experienced patients with drug-resistant HIV infection: week 96 results of the BENCHMRK 1 and 2 phase III trials. Clin Infect Dis. 2010;50(4):605–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morand‐Joubert L, Ghosn J, Delaugerre C, et al. Lack of benefit of 3-month intensification with enfuvirtide plus optimized background regimen (OBR) versus OBR alone in patients with multiple therapeutic failures: The INNOVE study. J Med Virol. 2012;84(11):1710–1718. [DOI] [PubMed] [Google Scholar]
- 8.Deeks SG, Barbour JD, Martin JN, et al. Sustained CD4+ T cell response after virologic failure of protease inhibitor-based regimens in patients with human immunodeficiency virus infection. J Infect Dis. 2000;181(3):946–953. [DOI] [PubMed] [Google Scholar]
- 9.Office of the Commissioner. Press announcements - FDA approves new HIV treatment for patients who have limited treatment options. U.S. Food and Drug Administration; https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm599657.htm? Published March 6, 2018 Accessed March 28, 2019. [Google Scholar]
- 10.Markham A Ibalizumab: first global approval. Drugs. 2018;78(7):781–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Emu B, Fessel J, Schrader S, et al. Phase 3 study of ibalizumab for multidrug-resistant HIV-1. N Engl J Med. 2018;379(7):645–654. [DOI] [PubMed] [Google Scholar]
- 12.Emu B, Weinheimer S, Cohen Z, et al. Analysis of patients completing the ibalizumab Phase III trial and expanded access program. Presented at HIV Glasgow 2018, Glasgow, 28–31 October 2018 J Int AIDS Soc. 2018;21:18. [Google Scholar]
- 13.Shaughnessy AF. Monoclonal antibodies: magic bullets with a hefty price tag. BMJ. 2012;345:e8346. [DOI] [PubMed] [Google Scholar]
- 14.Mulcahy AW, Hlavka JP, Case SR. Biosimilar cost savings in the United States. https://www.rand.org/pubs/perspectives/PE264.html. Published 2017 Accessed March 28, 2019. [PMC free article] [PubMed]
- 15.RED BOOK search results - MICROMEDEX. https://www-micromedexsolutions-com.ezp-prod1.hul.harvard.edu/micromedex2/librarian/CS/9631AA/ND_PR/evidencexpert/ND_P/evidencexpert/DUPLICATIONSHIELDSYNC/DD1613/ND_PG/evidencexpert/ND_B/evidencexpert/ND_AppProduct/evidencexpert/ND_T/evidencexpert/PFActionId/redbook.ShowProductSearchResults?SearchTerm=ibalizumab-uiyk&searchType=redbookGenericName&searchTermId=932434&searchContent=REDBOOK&searchFilterAD=filterADActive&searchFilterRepackager=filterExcludeRepackager&searchPattern=%5Eibalizumab. Accessed March 28, 2019.
- 16.Afzali A, Ogden K, Friedman ML, et al. Costs of providing infusion therapy for patients with inflammatory bowel disease in a hospital-based infusion center setting. J Med Econ. 2017;20(4):409–422. [DOI] [PubMed] [Google Scholar]
- 17.Schmier J, Ogden K, Nickman N, et al. Costs of providing infusion therapy for rheumatoid arthritis in a hospital-based infusion center setting. Clin Ther. 2017;39(8):1600–1617. [DOI] [PubMed] [Google Scholar]
- 18.Walensky RP, Sax PE, Nakamura YM, et al. Economic savings versus health losses: the cost-effectiveness of generic antiretroviral therapy in the United States. Ann Intern Med. 2013;158(2):84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ross EL, Weinstein MC, Schackman BR, et al. The clinical role and cost-effectiveness of long-acting antiretroviral therapy. Clin Infect Dis. 2015;60(7):1102–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Borre ED, Hyle EP, Paltiel AD, et al. The clinical and economic impact of attaining national HIV/AIDS strategy treatment targets in the United States. J Infect Dis. 2017;216(7):798–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanders GD, Neumann PJ, Basu A, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. JAMA. 2016;316(10):1093–1103. [DOI] [PubMed] [Google Scholar]
- 22.Anderson Jeffrey L, Heidenreich Paul A, Barnett Paul G, et al. ACC/AHA statement on cost/value methodology in clinical practice guidelines and performance measures. Circulation. 2014;129(22):2329–2345. [DOI] [PubMed] [Google Scholar]
- 23.Cameron D, Ubels J, Norström F. On what basis are medical cost-effectiveness thresholds set? Clashing opinions and an absence of data: a systematic review. Glob Health Action. 2018;11(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Calmy A, Balestre E, Bonnet F, et al. Mean CD4 cell count changes in patients failing a first-line antiretroviral therapy in resource-limited settings. BMC Infect Dis. 2012;12:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sax PE, Meyers JL, Mugavero M, et al. Adherence to antiretroviral treatment and correlation with risk of hospitalization among commercially insured HIV patients in the United States. PLoS One. 2012;7(2):e31591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hirsch JD, Gonzales M, Rosenquist A, et al. Antiretroviral therapy adherence, medication use, and health care costs during 3 years of a community pharmacy medication therapy management program for Medi-Cal beneficiaries with HIV/AIDS. J Manag Care Pharm JMCP. 2011;17(3):213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rebeiro PF, Gange SJ, Horberg MA, et al. Geographic variations in retention in care among HIV-infected adults in the united states. PLoS ONE. 2016;11(1):e0146119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fleishman JA, Yehia BR, Moore RD, et al. Establishment, retention, and loss to follow-up in outpatient HIV care. J Acquir Immune Defic Syndr. 2012;60(3):249–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lubelchek RJ, Finnegan KJ, Hotton AL, et al. Assessing the use of HIV surveillance data to help gauge patient retention-in-care. J Acquir Immune Defic Syndr 1999. 2015;69 Suppl 1:S25–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Helleberg M, Engsig FN, Kronborg G, et al. Retention in a public healthcare system with free access to treatment: a Danish nationwide HIV cohort study. AIDS. 2012;26(6):741–748. [DOI] [PubMed] [Google Scholar]
- 31.Levinson DR. Medicaid drug price comparisons: average manufacturer price to published prices. Dep Health Hum Serv Off Insp Gen. June 2005:34. [Google Scholar]
- 32.Khanlou H, Gathe J, Schrader S, et al. Safety, efficacy, and pharmacokinetics of ibalizumab in treatment-experienced HIV-1 infected patients: a phase 2b study. Presented at: 51st Interscience Conference on Antimicrobial Agents and Chemotherapy; 2011; Chicago, Illinois. [Google Scholar]
- 33.Sullivan SD, Mauskopf JA, Augustovski F, et al. Budget impact analysis—principles of good practice: report of the ISPOR 2012 budget impact analysis good practice II task force. Value Health. 2014;17(1):5–14. [DOI] [PubMed] [Google Scholar]
- 34.Budget. HIV.gov. https://www.hiv.gov/federal-response/funding/budget. Published January 15, 2018 Accessed January 3, 2019.
- 35.HIV in the United States | statistics overview | Statistics Center | HIV/AIDS | CDC. https://www.cdc.gov/hiv/statistics/overview/ataglance.html. Published December 10, 2018 Accessed March 28, 2019.
- 36.Winkelmayer WC, Weinstein MC, Mittleman MA, et al. Health economic evaluations: the special case of end-stage renal disease treatment. Med Decis Mak Int J Soc Med Decis Mak. 2002;22(5):417–430. [DOI] [PubMed] [Google Scholar]
- 37.The World Bank. GDP per capita (current US$). World Bank national accounts data and OECD national accounts data files. https://data.worldbank.org/indicator/NY.GDP.PCAP.CD?locations=US. Accessed March 28, 2019.
- 38.Struble K, Murray J, Cheng B, et al. Antiretroviral therapies for treatment-experienced patients: current status and research challenges. AIDS Lond Engl. 2005;19(8):747–756. [DOI] [PubMed] [Google Scholar]
- 39.Richman DD, Morton SC, Wrin T, et al. The prevalence of antiretroviral drug resistance in the United States. AIDS Lond Engl. 2004;18(10):1393–1401. [DOI] [PubMed] [Google Scholar]
- 40.Weinstock HS, Zaidi I, Heneine W, et al. The epidemiology of antiretroviral drug resistance among drug-naive HIV-1-infected persons in 10 US cities. J Infect Dis. 2004;189(12):2174–2180. [DOI] [PubMed] [Google Scholar]
- 41.Yazdanpanah Y, Fagard C, Descamps D, et al. High rate of virologic suppression with raltegravir plus etravirine and darunavir/ritonavir among treatment-experienced patients infected with multidrug-resistant HIV: results of the ANRS. Clin Infect Dis. 2009;49(9):1441–1449. [DOI] [PubMed] [Google Scholar]
- 42.Aberg J, Molina J-M, Kozal M, et al. Week 48 safety and efficacy of the HIV-1 attachement inhibitor prodrug fostemsavir in heavily treatment-experienced participants (BRIGHTE Study). Abstract 0334A. Presented at: HIV Drug Therapy; 2018; Glasgow. [DOI] [PubMed] [Google Scholar]
- 43.DeJesus E, Harward S, Jewell R, et al. A phase IIA study of novel maturation inhibitor GSK2838232 in HIV patients. Abstract 142. Presented at: Conference on Retroviruses and Opportunistic Infections; 2019; Seattle, Washington. [Google Scholar]
- 44.Liu JKH. The history of monoclonal antibody development – Progress, remaining challenges and future innovations. Ann Med Surg. 2014;3(4):113–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ecker DM, Jones SD, Levine HL. The therapeutic monoclonal antibody market. mAbs. 2014;7(1):9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.BioMarin prices orphan drug at $702,000, promises big discounts. https://www.bloomberg.com/news/articles/2017-04-27/biomarin-prices-orphan-drug-at-702-000-promises-big-discounts. Published April 27, 2017 Accessed March 28, 2019.
- 47.The value of biologics. July 2010. http://www.ahdbonline.com/issues/2008/march-2008-vol-1-no-2/371-feature-371. Accessed March 28, 2019.
- 48.Tringale KR, Carroll KT, Zakeri K, et al. Cost-effectiveness analysis of nivolumab for treatment of platinum-resistant recurrent or metastatic squamous cell carcinoma of the head and neck. J Natl Cancer Inst. 2018;110(5):479–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fonarow GC, Keech AC, Pedersen TR, et al. Cost-effectiveness of evolocumab therapy for reducing cardiovascular events in patients with atherosclerotic cardiovascular disease. JAMA Cardiol. 2017;2(10):1069–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arrieta A, Hong JC, Khera R, et al. Updated cost-effectiveness assessments of PCSK9 inhibitors from the perspectives of the health system and private payers: insights derived from the FOURIER trial. JAMA Cardiol. 2017;2(12):1369–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kazi DS, Penko J, Coxson PG, et al. Updated cost-effectiveness analysis of PCSK9 inhibitors based on the results of the FOURIER trial. JAMA. 2017;318(8):748–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Coyle D, Cheung MC, Evans GA. Opportunity cost of funding drugs for rare diseases: the cost-effectiveness of eculizumab in paroxysmal nocturnal hemoglobinuria. Med Decis Mak Int J Soc Med Decis Mak. 2014;34(8):1016–1029. [DOI] [PubMed] [Google Scholar]
- 53.Gulácsi L, Brodszky V, Baji P, et al. The rituximab siosimilar CT-P10 in rheumatology and cancer: a budget impact analysis in 28 european countries. Adv Ther. 2017;34(5):1128–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van der Gronde T, Uyl-de Groot CA, Pieters T. Addressing the challenge of high-priced prescription drugs in the era of precision medicine: A systematic review of drug life cycles, therapeutic drug markets and regulatory frameworks. PLoS ONE. 2017;12(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Manova M, Savova A, Vasileva M, et al. Comparative price analysis of biological products for treatment of rheumatoid arthritis. Front Pharmacol. 2018;9:1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Blackstone EA, Joseph PF. The economics of biosimilars. Am Health Drug Benefits. 2013;6(8):469–478. [PMC free article] [PubMed] [Google Scholar]
- 57.Dolan C Opportunities and challenges in biosimilar uptake in oncology. Am J Manag Care. 2018;24(11 Suppl):S237–S243. [PubMed] [Google Scholar]
- 58.Center for Drug Evaluation and Research. Biosimilars - biosimilar development, review, and approval. https://www.fda.gov/drugs/developmentapprovalprocess/howdrugsaredevelopedandapproved/approvalapplications/therapeuticbiologicapplications/biosimilars/ucm580429.htm#data. Accessed March 28, 2019.
- 59.Search orphan drug designations and approvals. https://www.accessdata.fda.gov/scripts/opdlisting/oopd/detailedIndex.cfm?cfgridkey=321910. Accessed March 28, 2019.
- 60.Chouaid C, Bensimon L, Clay E, et al. Cost-effectiveness analysis of pembrolizumab versus standard-of-care chemotherapy for first-line treatment of PD-L1 positive (>50%) metastatic squamous and non-squamous non-small cell lung cancer in France. Lung Cancer. 2019;127:44–52. [DOI] [PubMed] [Google Scholar]
- 61.Verma V, Sprave T, Haque W, et al. A systematic review of the cost and cost-effectiveness studies of immune checkpoint inhibitors. J Immunother Cancer. 2018;6(1):128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Office of Procurement, Acquisition and Logistics. Pharmaceutical prices - Federal Supply Schedule (FSS). https://www.va.gov/opal/nac/fss/pharmPrices.asp. Accessed March 28, 2019.
- 63.McKie J, Richardson J. The rule of rescue. Soc Sci Med 1982. 2003;56(12):2407–2419. [DOI] [PubMed] [Google Scholar]
- 64.Orr S, Wolff J. Reconciling cost-effectiveness with the rule of rescue: the institutional division of moral labour. Theory Decis. 2015;78(4):525–538. [Google Scholar]
- 65.Centers for Medicare and Medicaid Services. Medicare physician fee schedule 2017. http://www.cms.gov/apps/physician-fee-schedule/overview.aspx. Accessed March 28, 2018.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.