Abstract
Digitization of healthcare will be a major innovation driver in the coming decade. Also, enabled by technological advancements and electronics miniaturization, wearable health device (WHD) applications are expected to grow exponentially. This, in turn, may make 4P medicine (predictive, precise, preventive and personalized) a more attainable goal within dialysis patient care. This article discusses different use cases where WHD could be of relevance for dialysis patient care, i.e. measurement of heart rate, arrhythmia detection, blood pressure, hyperkalaemia, fluid overload and physical activity. After adequate validation of the different WHD in this specific population, data obtained from WHD could form part of a body area network (BAN), which could serve different purposes such as feedback on actionable parameters like physical inactivity, fluid overload, danger signalling or event prediction. For a BAN to become clinical reality, not only must technical issues, cybersecurity and data privacy be addressed, but also adequate models based on artificial intelligence and mathematical analysis need to be developed for signal optimization, data representation, data reliability labelling and interpretation. Moreover, the potential of WHD and BAN can only be fulfilled if they are part of a transformative healthcare system with a shared responsibility between patients, healthcare providers and the payors, using a step-up approach that may include digital assistants and dedicated ‘digital clinics’. The coming decade will be critical in observing how these developments will impact and transform dialysis patient care and will undoubtedly ask for an increased ‘digital literacy’ for all those implicated in their care.
Keywords: blood pressure, dialysis, fluid overload, haemodialysis, physical activity
INTRODUCTION
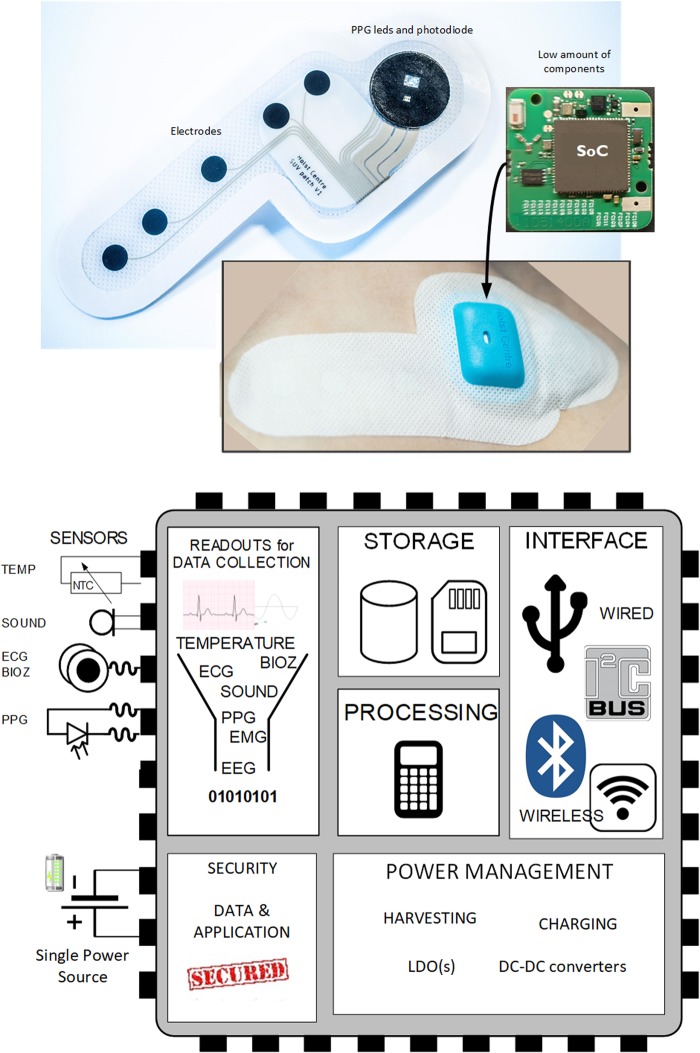
Artificial intelligence (AI), Big Data and technology-driven solutions will likely create the fourth phase of industrial revolution. This will encompass a fusion of rapidly evolving technologies across the physical, digital and biological domains. These advancements are likely to profoundly influence future healthcare. Next to developments in nanotechnology, omics, genome editing and tissue engineering, the digitization of healthcare is also expected to have a major impact in the next decade [1]. Regarding the last factor, it is likely that a major part of this revolution will not be driven by healthcare providers, but instead by manufacturers and consumers of commercially available wearable devices that monitor and report personal health-related data such as physical activity and vital signs in real-time. Electronics miniaturization is a key enabler for wearable health devices (WHD). For example, presently a 4.4 × 4.4 mm size chip can already contain a multiparameter monitor for electrocardiography (ECG), photoplethysmography (PPG), pulse oximetry (SpO2) and bioimpedance, including memory, processor and secured wireless communication (Figure 1) [2].
FIGURE 1.
Health patch using system-on-a-chip (SoC) and its high-level architecture [2].
Many of these developments will likely become relevant for the treatment of patients with advanced kidney failure, who display a unique challenge of comorbidities with a premature ageing process while undergoing invasive, expensive high-tech care [3]. A major challenge for the future of healthcare is to provide personalized care in view of a rapidly expanding healthcare system within financial limitations and an increasing demand for sustainability [4, 5].
Possibly, the digitization of healthcare provides the opportunity for enhancing the 4Ps (predictive, precise, preventive and personalized) of medicine [6]. However, the healthcare community needs to be fully aware of the potential benefits as well as the challenges with these developments in order to put them in context. Given the rapidly expanding availability of information through the Internet or other media, patients increasingly will expect a high degree of both medical knowledge and digital literacy of their healthcare providers [7], as well as an ability to meaningfully interpret these growing data streams to the patients’ benefit and to translate this information—where necessary—into appropriate action.
This short review aims to discuss potentially relevant developments for haemodialysis patients that may help dialysis caregivers of the future to achieve this goal.
MONITORING DURING DIALYSIS AND BEYOND: TOWARDS A CLINICAL INTELLIGENCE SYSTEM
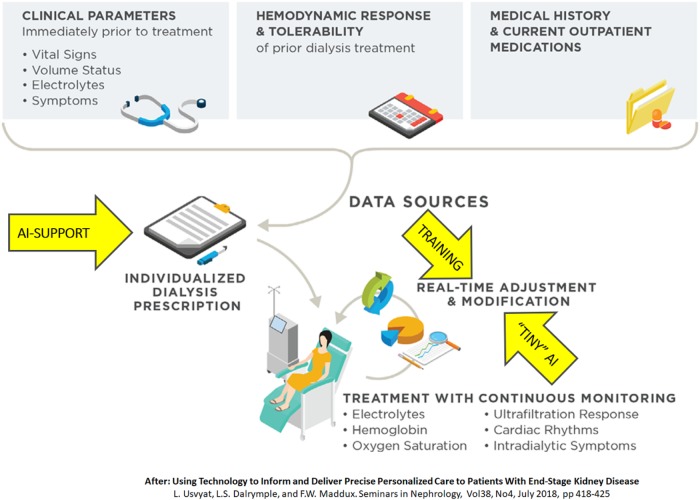
Patients on haemodialysis spend considerable time in different and often separated ecosystems, i.e. the dialysis clinic, hospital and their home environment. With haemodialysis, they undergo an intensive treatment that has profound pathophysiologic effects. There is substantial evidence suggesting that impaired organ perfusion during dialysis can result in irreversible organ damage [8]. Blood pressure (BP), which is at best an indirect marker of organ perfusion [9], is measured discontinuously during dialysis, whereas symptomatic drops in BP may suddenly occur when ventricular filling is critically reduced [10]. Recently, several studies addressed the relevance of changes in central venous oxygen saturation (scvO2), which can be measured continuously by an optical probe connected to the blood line in patients with a central venous catheter, as a surrogate marker of upper body perfusion and haemodynamic status. Changes in scvO2 were related to ultrafiltration (UF) rate but also independently to outcome [11, 12]. These are just examples of variables that could be monitored continuously with wearable and unobtrusive technologies (near-infrared spectroscopy, PPG or other optical methods). Non-invasive monitoring and interpretation of multiple physiological signals in combination with dialysis treatment parameters and patient characteristics can be aided by mathematical models and/or AI, which might result in a more precise prediction of pending organ ischaemia or sudden intradialytic hypotension and trigger alerts before a clinical event ensues. A preliminary example of such an approach was presented by Barbieri et al. [13], in which an artificial neural network predicted nadir systolic BP and heart rate with reasonable accuracy. In the future, such models could be combined with UF feedback control systems [10] that automatically adjust UF rate or treatment time when the algorithm would predict impending drops in tissue perfusion or BP [4]. These developments, which might also be of great relevance for home haemodialysis patients, may contribute to a clinical intelligence system surrounding dialysis treatments in the future (Figure 2) [4].
FIGURE 2.
Example of a ‘clinical intelligence system’ [4].
These developments logically would first build upon already existing telehealth systems for home dialysis patients and evolve from there [14]. Smart automated recognition of meaningful events, and automated data reliability indicators will increasingly become key to avoid ‘drowning’ clinicians in a flood of raw traces and false alarms [15].
REMOTE MONITORING
While patients are used to being monitored during a dialysis session, monitoring during daily life between dialysis treatments provides other challenges, in terms of patient acceptance, reliability of monitoring and liability [15]. Remote monitoring applies digital technologies to collect and transmit data to healthcare providers at a different location. This can be performed either in an online modus, where data are directly visible to the provider, as well as in an offline modus, where data can be stored and used for later analysis [16]. Whereas different technologies for remote monitoring are available, we will focus in this article on WHD applications.
Although many different applications for pervasive WHD have been described in the literature [17, 18], we will focus this review on a choice of monitoring systems for which a clinical rationale can be deduced. The common ground for these applications is that all are related to outcome and are potentially amenable for intervention (actionable).
PPG
One of the most frequently measured parameters by WHD is heart rate, usually by PPG, which detects the peripheral pulse by measuring variations in LED-light transmission through or reflection from the tissue. The ‘peak to peak’ interval is used to estimate heart rate [19] by means of a tachogram. In validation studies, PPG methods were generally reliable, at least in healthy subjects, while excluding episodes with vigorous physical activity [20, 21]. To the best of our knowledge, its reliability has not been systematically assessed in patients with abnormalities in forearm anatomy, such as the presence of an arteriovenous fistula. As will be discussed later, measuring abnormalities in heart rate or heart rate variability, especially when combined with assessment of physical activity, can be of relevance for the detection of arrhythmias. Moreover, in a case-based study changes in heart rate were found to be the first signal of inflammatory conditions before the onset of clinical presentation [22].
ECG
ECG measurement is relatively new in WHD (e.g. in patches or smartwatches). The newest generation Apple Watch, for example, offers iECG, measuring lead I between the wrist and the contralateral finger placed on the watch. Whereas continuous ECG is not practical in this way, tachyarrhythmias can be detected by the PPG-tachogram, which can alert the user to perform an iECG [19]. In healthy subjects, the ECG quality using this method was found to be acceptable [23].
Whereas automated detection of atrial fibrillation (AF) from PPG signals derived from a mobile phone is also possible [24], the largest trial so far was based on the detection of AF using smartwatches. In the Apple Heart study, tachogram-deduced possible AF was alerted to the wearer, who subsequently wore an ECG patch for 7 days [25]. In this study, the device detected an irregular pulse in 0.52% of subjects, whereas the algorithm showed a positive predictive value of 0.84 when the ECG was obtained during the time of irregular pulse notification. It should be noted that the Apple Watch study population was relatively young. Therefore, this method might even be more relevant for high-risk populations such as dialysis patients, where a 40% AF incidence has been observed [26]. However, it is uncertain whether improved AF detection, although clearly a risk factor for mortality, would result in a better outcome, certainly in light of the controversy regarding AF treatment with anticoagulation in dialysis patients [27]. Even in relatively healthy subjects, it is presently unclear whether subclinical AF should be followed by anticoagulation treatment or not, as results so far are equivocal [7].
Apart from morbidity directly associated with dialysis treatment, a major part of the mortality occurs during the inter-dialytic period, with sudden cardiac death as the most feared complication. A study of implantable loop recorders showed that bradycardias (defined as heart rate <40 beats/min during >6 s) occurred in 20% of patients [26], with a far higher incidence than ventricular tachycardia, which occurred in only 1% of the studied patients. These bradycardias occurred primarily at the end of the inter-dialytic interval, when the rate of cardiovascular mortality and sudden cardiac death is at its zenith [28, 29]. Recently, bradycardia in dialysis patients was linked to lower serum calcium levels [30].
It does not seem to be a remote possibility to modify smartwatch algorithms in such a way that bradycardias, e.g. detected by a smartwatch PPG-tachogram, would prompt the user to perform an ECG. Continuous ECG monitoring (e.g. by a wirelessly connected health patch) would not even need such a prompt. In tern, ‘red flag’ alerts could be immediately transferred to a monitoring unit from which appropriate action could be taken, or downloaded in case of a less severe alert (like Holters do presently). Even with present technologies this would appear to become achievable, as the ECG of an Apple Watch was able to detect ventricular tachycardias as well as heart block [31, 32], and health patches (some with multiple leads) are emerging as well.
BP
Although BP is routinely taken during dialysis sessions, pre- and post-dialytic BP values are only moderately representative for BP during the inter-dialytic period, whereas the relation between high ‘ambulatory’ BP levels and long-term outcome is far stronger as compared with ‘in-clinic’ BP measurements [33]. Nevertheless, in-clinic low BP levels were found to be strongly related to short-term mortality [34]. Yet, low inter-dialytic BP levels can also lead to impaired organ perfusion. Therefore, a rationale exists for BP measurements in the home setting. Whereas traditional periodic home BP measurements were shown to outperform in-clinic BP measurements on prediction of outcome [33], they still only represent a very short time period and may also fail to detect nadir BP levels that would be potentially modifiable by adjustment of dialysis treatment or medication. Cuffless BP measurements, provided they are reliable, would appear to be a major asset in the assessment of dialysis patients. Available cuffless BP measurements apply one of two principles: pulse transit time (PTT) or pulse arrival time (PAT). PAT measures the time between ECG R-wave (e.g. from a smartwatch or patch) and pulse wave arrival (from PPG). Variabilities in the heart pre-ejection period thus directly influence PAT. PTT compares two PPG signals with a known distance [15]. Both methods exploit pulse propagation velocity, for which BP is only one of many determinants, like vascular tree mechanical properties and others. Therefore, present cuffless measurements need frequent calibration against a standard (oscillometric) BP measurement, which to some extent would seem to limit its potential use. Moreover, the reliability of cuffless BP monitoring devices, which depend on various assumptions, has been questioned [35] and the method has not yet been validated in dialysis patients. Presently, the only AQ6Food and Drug Administration-approved smartwatch-based BP monitoring devices are based on wrist cuff measurements, which may still be too obtrusive for patients to wear all day. Such wrist cuff measurements also have not yet been validated in the dialysis population, that would provide additional challenges like abnormal vascular wall anatomy or mechanical arterial wall properties.
Hyperkalaemia
While the mechanisms of sudden cardiac death in haemodialysis patients have not been completely elucidated, abnormalities in electrolyte concentrations—most notably hyperkalaemia and fluid overload—are very likely to play a major role in its pathogenesis. Usually, however, potassium levels are measured only monthly and in the intermediate period hyperkalaemia can remain undetected. With the advance of ECG in WHD, detection of hyperkalaemia may become possible. A recent study showed that hyperkalaemia (serum level > 5.5 mmol/L) could be detected by a deep learning algorithm based on a two-lead ECG [36]. The authors, who validated this method in three different populations, reported sensitivities around 90% and specificities around 55%. Should an appropriate WHD algorithm become available, one could envision that hyperkalaemia-prone patients could take daily measurements and receive advice (dietary modification, potassium binder intake or schedule extra dialysis session). However, the likelihood of false positives presently appears high, so preferably ECG-based ‘suggestion’ of hyperkalaemia should be verified by point-of-care potassium testing. An interesting approach is derivation from sweat, which has a relatively strong (r = 0.78) relation to plasma concentration when stimulated by pilocarpine iontophoresis [37]. Ion-selective sensing in sweat patches or other body fluids seems feasible [38]. Future studies are needed to validate all these approaches in the dialysis population.
Fluid overload
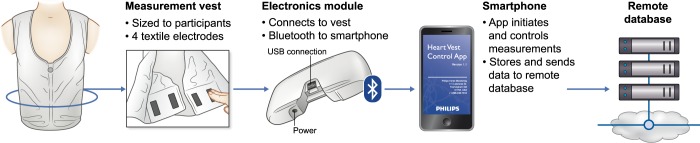
It has been convincingly shown in many observational studies that even minor degrees of fluid overload, assessed by bioimpedance spectroscopy (BIS) but also fluid depletion, are related to mortality in dialysis patients [39]. Although the evidence is somewhat less strong, technique-assisted adjustments of dry weight resulted in an improvement of surrogate outcome parameters in dialysis patients as well as in BP control [40]. Providing patients with feedback on the degree of fluid overload during non-dialysis days by easily interpretable methods might enhance patient participation. While especially new generation BIS devices are easily interpretable as they present fluid overload as an actual number, these are too expensive for home monitoring and require some technical expertise. Developments explored in the context of heart failure could, however, also be of relevance for dialysis patients, e.g. in the form of ‘smart’ T-shirts based on thoracic bioimpedance (Figure 3). In a recent study, 106 patients wore a ‘fluid accumulation vest’ for 45 days, with daily wireless data transfer to a mobile phone. Patient acceptability was good and an accompanying algorithm showed 72% accuracy for identifying recurrent heart failure events [41].
FIGURE 3.
Example of a ‘fluid accumulation vest’ based on thoracic bioimpedance for the detection of cardiac congestion, with the accompanying protocol [41].
Physical activity and sleep
It is well known that physical activity is severely reduced in patients with end-stage kidney disease (ESKD) even before the start of dialysis [42], and is compatible with a sedentary lifestyle [43]. A low physical activity is related to mortality and low quality of life in dialysis patients. Measuring physical activity by activity trackers is readily achieved by commercially available wearable devices including smartwatches and smartphones. In general, the accuracy of these devices regarding basic functions such as step count appears to be relatively accurate [44, 45], whereas the method is relatively unobtrusive. The most relevant question is whether tracking of physical activity by WHD with appropriate feedback could induce a behavioural change and improve outcomes. A recent systematic review in elderly subjects showed that interventions based on activity trackers resulted in a mean increase of 1558 steps/day [46]. In a randomized study of 3-month duration, the use of pedometers in combination with weekly counselling by researchers increased their average daily steps by 2256 above those of controls, but the effect was not sustained after the end of the intervention period [47]. Possibly, the use of digital assistants in combination with data from WHD could prolong the motivation. However, for patients with ESKD, no studies on this subject have so far been reported, and also in other chronic diseases, such as heart failure, the role of WHD in inducing behavioural change, e.g. in combination with app-based feedback, is still under investigation [48, 49]. Most consumer-grade physical activity monitoring devices also claim to be able to assess sleep. A majority of these devices collect signals from position, motion and heart rate sensors to input into proprietary algorithms that claim to characterize sleep and sleep stages accurately. Devices that have oxygen saturation sensors also claim to accurately assess sleep breathing disorders, e.g. apnoea. Their accuracies, when tested against gold standard polysomnography, range from poor to fair [44, 50]. However, given the cost and discomfort associated with in-centre polysomnography, such more affordable and less intrusive devices could present themselves as attractive alternatives. While sleep measurements may not be accurate every night, they could be useful in detecting longitudinal trends in sleep patterns.
PREREQUISITES FOR WHD
Several technical prerequisites for WHD have been discussed in our previous paper as well as by others [15]. These include technical safety, unobtrusiveness, ruggedness and user-friendliness. Alongside these, Al-Alusi et al. [51] pointed out three lines of evidence a new technology should provide in order to be clinically useful: validation (does the device accomplish what it aims to do), improving outcomes and cost-effectiveness. In order to successfully proceed to the next two stages, data derived from the wearable sensors must be accurate and robust, in order to prevent false-positive findings that may induce anxiety and carry the risk of unnecessary diagnostic procedures or overtreatment, as well as false-negative findings that would give an inappropriate sense of security. As for application in dialysis patients, we clearly are still in the first stage and for a meaningful application of WHD or other forms of telemonitoring in this population, strategies for patient-specific feedback should be developed to provide personalized value-based healthcare. However, we can learn from other fields such as cardiology, where the combination of telemonitoring and personalized feedback has already been tested in various studies and, in some cases, was shown to be associated with improved outcomes [52].
BODY AREA NETWORKS AND INTERNET-OF-THINGS
When combined, data from WHD, or even implantable devices, could be part of an integrated network, known as body area networks (BAN), or, in a more extensive configuration, synergistic personal area networks (SPAN) [53, 54]. These form part of the so-called digital phenotype of the patient, which in future developments should be interpreted in the context of the comorbidity of the patient and information derived from other sources, varying from genomics to social structure, in order to provide information for a real ‘personalized medicine’ [4].
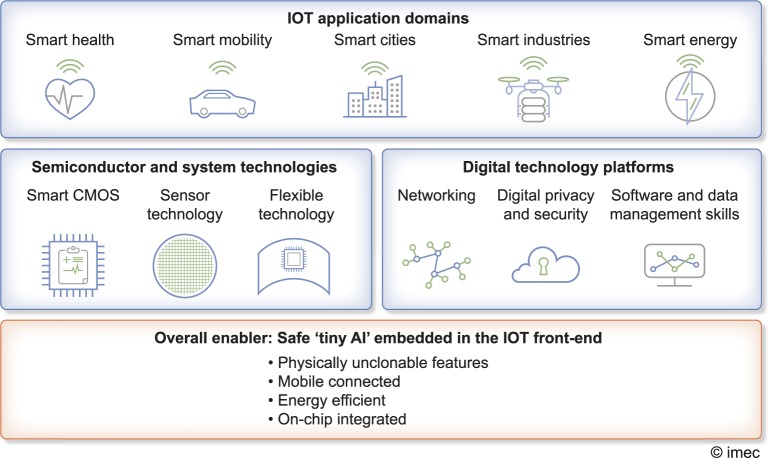
In a SPAN, sensors are connected to actuators in a so-called ‘smart environment’, being part of the so-called ‘Internet-of-Things’ (IoT) (Figure 4) [54, 56, 57].
FIGURE 4.
The IoT spans many application domains, including healthcare. Innovative hardware (semiconductor and system technologies) and software (digital technology platforms) drive the overall IoT expansion. For healthcare ‘tiny AI’, embedded in WHS will be a key enabler, completed by uncrackable cyber-safety from physically unclonable features and energy efficient safe wireless connection, all integrated on-chip. Figure adapted (with permission) from [55].
Such a system includes not only sensors, but also signal processors, data loggers and communication protocols [53]. The use of medical devices connected via the IoT is growing exponentially. In April 2019, it was anticipated ‘that by 2020 there will be over 161 million of them connected worldwide’ [58]. All these medical IoT devices produce a quickly growing, heterogeneous, frequently noisy and often incomplete set of records. Integration, interpreting and analysing all these data accordingly will become increasingly difficult, especially if all this needs to be done in (near) real time using centralized computing power [59].
Given the multitude of available data, which also need to be integrated to display a comprehensive summary of health status, AI likely will play an important role in both signal processing [60], which can already be embedded on a sensor chip (‘tiny AI’), as well as data processing. It may be wise to distribute intelligence by a pipeline of reasoning components (Cascade Reasoning Frameworks) that could be hosted partly within wearables, partly in the edge of the network and/or partly in the cloud.
Advanced predictive analytics, likely at least partly mediated through machine learning techniques, will also play a role in the contextual interpretation of data, as a ‘combination’ of multiple parameters and their trends may be more meaningful than a single parameter value [61]. As an example, we previously observed that systolic BP and inter-dialytic weight gain declined while inflammatory parameters went up before hospitalization or death [61, 62]. If factors such as inter-dialytic weight gain were to be assessed in isolation, completely different predictions could have arisen. Moreover, using in-clinic BP measurements in combination with clinical features, Lacson et al. [63] constructed a machine-learning programme that predicted features associated with BP variability but also specific BP profiles that were strongly related to outcome. In the future, predictive analytics could also profit from the readiness of massive data from wearable sensors using AI algorithms, but could also identify potential contributing factors to high-risk profiles.
BAN and SPAN could thus play a major role both in improving prediction and anomaly detection, as well as diagnosis and treatment support [53], if data are reliably obtained and available in a structured and easily interpretable manner. Moreover, where data derived from the BAN are actionable, it should be clear for the patient and healthcare provider which steps should be followed.
CHALLENGES FOR THE HEALTHCARE OF THE FUTURE
Whereas data derived from a combination of WHD could undoubtedly contribute to the improvement of 4P medicine and value-based healthcare, several challenges are apparent. Alongside the issues of validation, outcome improvement and cost-effectiveness, an important challenge is the issue of cybersecurity and data privacy. Threats can come from unexpected sources, as shown in a recent paper discussing the possibility of cyber attacks on healthcare devices using Unmanned Aerial Vehicles [55]. Physically unclonable features (PUFs) form an inherently unique signature method for individual digital chips (although mass-produced). PUFs are embedded in the hardware itself, forming a key that cannot be hacked by software. Other challenges are data ownership, liability, reimbursement and definition of responsibilities [16]. It would seem impossible that the interpretation of the data derived from the monitoring systems and the subsequent action would be the sole responsibility of healthcare professionals, as this would lead to an unmanageable workload and liability issues. In line with the 4P principle, society will have to define the responsibilities of patients, healthcare providers and other stakeholders. Widespread ethical and successful introduction of BAN can, in our opinion, only be achieved in a situation with shared responsibilities and a transformative and adaptive healthcare system. Importantly, society may also have to accept the possibilities of false-negative and false-positive data to some extent. It is also highly important to define responsibilities for critical and non-critical conditions. For non-critical conditions, personal digital assistants [16, 54] may become an intermediate between patients and healthcare provider, with also a potential intermediate in a ‘digital clinic’, where patients can have encounters with trained employees. Under most circumstances, continuous monitoring will neither be necessary or desirable, as adequate information, as in the example of a fluid accumulation vest, can also be obtained during single daily measurements. If we enter the era wherre patients are really monitored on a more or less continuous basis by unobtrusive techniques, then only highly clinically relevant and immediately actionable data should be transferred and all others stored for analysis and interpretation at a later stage [51]. In our opinion, real-time remote monitoring is likely only indicated for patients with a high risk for immediate life-threatening conditions that are also actionable.
CONCLUSION
It is very likely that, also driven by rapid technical and societal changes, WHD will enter clinical practice in the near future, including for dialysis patients. Whereas healthcare professionals may initiate some of these developments, patient preferences and a technology push from device manufacturers will also likely play a major role. Whereas the introduction of WHD, certainly when accompanied by adequate feedback and educational modules, could play a major role in the 4P healthcare of the future, a major challenge is to balance societal expectation with the workload and liability of healthcare professionals. If WHD and BAN are really applied on a large scale, then the organization of healthcare will have to transform to a structure with shared responsibility and a different structure in which digital assistants and ‘wireless clinics’ are involved. In order to handle the massive amount of complex data, AI will likely play a major role to aid in their structuring, presentation and interpretation. The ultimate goal would be reliable smart automated recognition of meaningful events, but if and how the developments in digital medicine will impact the care for dialysis patients in the next decade remains to be seen. Anyhow, both patients and their healthcare providers should be ready for these likely transformative changes in order to fulfil their full promise: enhancing 4P medicine in partnership with the patient.
CONFLICT OF INTEREST STATEMENT
F.W. is employee of IMEC, whereas M.H. and P.K. are employees of the Renal Research Institute, a wholly owned subsidiary of Fresenius Medicale Care (FMC). L.U. and S.C. are employees of FMC. J.K. and F.S. received reserach grant support from FMC in the past. There are no conflicts of interst with regards to the contents of the paper.
REFERENCES
- 1. Jung M. Digital health care and the fourth Industrial Revolution. Health Care Manag (Frederick) 2019; 38: 253–257 [DOI] [PubMed] [Google Scholar]
- 2. Song S, Konijnenburg M, Van Wegberg R. et al. A 769 muW battery-powered single-chip SoC with BLE for multi-modal vital sign monitoring health patches. IEEE Trans Biomed Circuits Syst 2019; 13: 1506–1517 [DOI] [PubMed] [Google Scholar]
- 3. Kooman JP, Kotanko P, Schols AM. et al. Chronic kidney disease and premature ageing. Nat Rev Nephrol 2014; 10: 732–742 [DOI] [PubMed] [Google Scholar]
- 4. Usvyat L, Dalrymple LS, Maddux FW.. Using technology to inform and deliver precise personalized care to patients with end-stage kidney disease. Semin Nephrol 2018; 38: 418–425 [DOI] [PubMed] [Google Scholar]
- 5. Califf RM. Future of personalized cardiovascular medicine: JACC state-of-the-art review. J Am Coll Cardiol 2018; 72: 3301–3309 [DOI] [PubMed] [Google Scholar]
- 6. Alonso SG, de la Torre Diez I, Zapirain BG.. Predictive, personalized, preventive and participatory (4P) medicine applied to telemedicine and eHealth in the literature. J Med Syst 2019; 43: 140. [DOI] [PubMed] [Google Scholar]
- 7. Ip JE. Wearable devices for cardiac rhythm diagnosis and management. JAMA 2019; 321: 337–338 [DOI] [PubMed] [Google Scholar]
- 8. McIntyre CW, Rosansky SJ.. Starting dialysis is dangerous: How do we balance the risk? Kidney Int 2012; 82: 382–387 [DOI] [PubMed] [Google Scholar]
- 9. Kooman JP, Katzarski K, van der Sande FM. et al. Hemodialysis: A model for extreme physiology in a vulnerable patient population. Semin Dial 2018; 31: 500–506 [DOI] [PubMed] [Google Scholar]
- 10. Koda Y, Aoike I.. Prevention of intradialytic hypotension with intermittent back-filtrate infusion haemodiafiltration: Insights into the underlying mechanism. Blood Purif 2019; 48 (Suppl 1): 1–6 [DOI] [PubMed] [Google Scholar]
- 11. Zhang H, Chan L, Meyring-Wosten A. et al. Association between intradialytic central venous oxygen saturation and ultrafiltration volume in chronic hemodialysis patients. Nephrol Dial Transplant 2018; 33: 1636–1642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chan L, Zhang H, Meyring-Wosten A. et al. Intradialytic central venous oxygen saturation is associated with clinical outcomes in hemodialysis patients. Sci Rep 2017; 7: 8581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Barbieri C, Cattinelli I, Neri L. et al. Development of an artificial intelligence model to guide the management of blood pressure, fluid volume, and dialysis dose in end-stage kidney disease patients: Proof of concept and first clinical assessment. Kidney Dis 2019; 5: 28–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lew SQ, Sikka N.. Operationalizing telehealth for home dialysis patients in the United States. Am J Kidney Dis 2019; 74: 95–100 [DOI] [PubMed] [Google Scholar]
- 15. Wieringa FP, Broers N, Kooman JP. et al. Wearable sensors: can they benefit patients with chronic kidney disease? Expert Rev Med Devices 2017; 14: 505–519 [DOI] [PubMed] [Google Scholar]
- 16. Atreja A, Francis S, Kurra S. et al. Digital medicine and evolution of remote patient monitoring in cardiac electrophysiology: A state-of-the-art perspective. Curr Treat Options Cardiovasc Med 2019; 21: 92. [DOI] [PubMed] [Google Scholar]
- 17. Witt D, Kellogg R, Snyder M. et al. Windows into human health through wearables data analytics. Curr Opin Biomed Eng 2019; 9: 28–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jeong IC, Bychkov D, Searson PC.. Wearable devices for precision medicine and health state monitoring. IEEE Trans Biomed Eng 2019; 66: 1242–1258 [DOI] [PubMed] [Google Scholar]
- 19. Isakadze N, Martin SS.. How useful is the smartwatch ECG? Trends Cardiovasc Med 2019; pii: S1050–1738(19)30149–5 [DOI] [PubMed] [Google Scholar]
- 20. Thomson EA, Nuss K, Comstock A. et al. Heart rate measures from the Apple Watch, Fitbit Charge HR 2, and electrocardiogram across different exercise intensities. J Sports Sci 2019; 37: 1411–1419 [DOI] [PubMed] [Google Scholar]
- 21. Khushhal A, Nichols S, Evans W. et al. Validity and reliability of the Apple Watch for measuring heart rate during exercise. Sports Med Int Open 2017; 1: E206–E211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li X, Dunn J, Salins D. et al. Digital health: Tracking physiomes and activity using wearable biosensors reveals useful health-related information. PLoS Biol 2017; 15: e2001402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Samol A, Bischof K, Luani B. et al. Single-lead ECG recordings including Einthoven and Wilson Leads by a smartwatch: A new era of patient directed early ECG differential diagnosis of cardiac diseases? Sensors (Basel) 2019; 19: 4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Proesmans T, Mortelmans C, Van Haelst R. et al. Mobile phone-based use of the photoplethysmography technique to detect atrial fibrillation in primary care: Diagnostic accuracy study of the FibriCheck app. JMIR Mhealth Uhealth 2019; 7: e12284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Perez MV, Mahaffey KW, Hedlin H. et al. Large-scale assessment of a smartwatch to identify atrial fibrillation. N Engl J Med 2019; 381: 1909–1917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Roy-Chaudhury P, Tumlin JA, Koplan BA. et al. Primary outcomes of the monitoring in dialysis study indicate that clinically significant arrhythmias are common in hemodialysis patients and related to dialytic cycle. Kidney Int 2018; 93: 941–951 [DOI] [PubMed] [Google Scholar]
- 27. Kruger T, Brandenburg V, Schlieper G. et al. Sailing between Scylla and Charybdis: Oral long-term anticoagulation in dialysis patients. Nephrol Dial Transplant 2013; 28: 534–541 [DOI] [PubMed] [Google Scholar]
- 28. Foley RN, Gilbertson DT, Murray T. et al. Long interdialytic interval and mortality among patients receiving hemodialysis. N Engl J Med 2011; 365: 1099–1107 [DOI] [PubMed] [Google Scholar]
- 29. Bleyer AJ, Hartman J, Brannon PC. et al. Characteristics of sudden death in hemodialysis patients. Kidney Int 2006; 69: 2268–2273 [DOI] [PubMed] [Google Scholar]
- 30. Loewe A, Lutz Y, Nairn D. et al. Hypocalcemia-induced slowing of human sinus node pacemaking. Biophys J 2019; 117: 2244–2254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yerasi C, O’Donoghue S, Satler LF. et al. Apple Watch detecting high-grade block after transcatheter aortic valve implantation. Eur Heart J 2019. doi: 10.1093/eurheartj/ehz854 [DOI] [PubMed] [Google Scholar]
- 32. Ringwald M, Crich A, Beysard N.. Smart watch recording of ventricular tachycardia: Case study. Am J Emerg Med 2019; pii: S0735–6757(19)30710–7 [DOI] [PubMed] [Google Scholar]
- 33. Agarwal R. Home and ambulatory blood pressure monitoring in chronic kidney disease. Curr Opin Nephrol Hypertens 2009; 18: 507–512 [DOI] [PubMed] [Google Scholar]
- 34. Maddux D, Usvyat LA, Xu D. et al. The association of weekly pre-hemodialysis systolic blood pressure and following week mortality. Kidney Blood Press Res 2018; 43: 88–97 [DOI] [PubMed] [Google Scholar]
- 35. van Helmond N, Freeman CG, Hahnen C. et al. The accuracy of blood pressure measurement by a smartwatch and a portable health device. Hosp Pract (1995) 2019; 47: 211–215 [DOI] [PubMed] [Google Scholar]
- 36. Galloway CD, Valys AV, Shreibati JB. et al. Development and validation of a deep-learning model to screen for hyperkalemia from the electrocardiogram. JAMA Cardiol 2019; 4: 428–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vairo D, Bruzzese L, Marlinge M. et al. Towards addressing the body electrolyte environment via sweat analysis: Pilocarpine iontophoresis supports assessment of plasma potassium concentration. Sci Rep 2017; 7: 11801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cuartero M, Parrilla M, Crespo GA.. Wearable potentiometric sensors for medical spplications. Sensors (Basel) 2019; 19: 363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dekker MJ, Marcelli D, Canaud BJ. et al. Impact of fluid status and inflammation and their interaction on survival: A study in an international hemodialysis patient cohort. Kidney Int 2017; 91: 1214–1223 [DOI] [PubMed] [Google Scholar]
- 40. Hur E, Usta M, Toz H. et al. Effect of fluid management guided by bioimpedance spectroscopy on cardiovascular parameters in hemodialysis patients: A randomized controlled trial. Am J Kidney Dis 2013; 61: 957–965 [DOI] [PubMed] [Google Scholar]
- 41. Darling CE, Dovancescu S, Saczynski JS. et al. Bioimpedance-based heart failure deterioration prediction using a prototype fluid accumulation vest-mobile phone dyad: An observational study. JMIR Cardio 2017; 1: e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Broers NJH, Martens RJH, Cornelis T. et al. Physical activity in end-stage renal disease patients: The effects of starting dialysis in the first 6 months after the transition period. Nephron 2017; 137: 47–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Avesani CM, Trolonge S, Deleaval P. et al. Physical activity and energy expenditure in haemodialysis patients: An international survey. Nephrol Dial Transplant 2012; 27: 2430–2434 [DOI] [PubMed] [Google Scholar]
- 44. Evenson KR, Goto MM, Furberg RD.. Systematic review of the validity and reliability of consumer-wearable activity trackers. Int J Behav Nutr Phys Act 2015; 12: 159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dorn D, Gorzelitz J, Gangnon R. et al. Automatic identification of physical activity type and duration by wearable activity trackers: A validation study. JMIR Mhealth Uhealth 2019; 7: e13547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Oliveira JS, Sherrington C, Zheng ERY. et al. Effect of interventions using physical activity trackers on physical activity in people aged 60 years and over: A systematic review and meta-analysis. Br J Sports Med 2019. doi: 10.1136/bjsports-2018-100324 [DOI] [PubMed] [Google Scholar]
- 47. Sheshadri A, Kittiskulnam P, Lazar AA. et al. A walking intervention to increase weekly steps in dialysis patients: A pilot randomized controlled trial. Am J Kidney Dis 2019; pii: S0272–6386(19)31006–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Alharbi M, Straiton N, Gallagher R.. Harnessing the potential of wearable activity trackers for heart failure self-care. Curr Heart Fail Rep 2017; 14: 23–29 [DOI] [PubMed] [Google Scholar]
- 49. Beatty AL, Magnusson SL, Fortney JC. et al. VA FitHeart, a mobile app for cardiac rehabilitation: Usability study. JMIR Hum Factors 2018; 5: e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. de Zambotti M, Goldstone A, Claudatos S. et al. A validation study of Fitbit Charge 2 compared with polysomnography in adults. Chronobiol Int 2018; 35: 465–476 [DOI] [PubMed] [Google Scholar]
- 51. Al-Alusi MA, Ding E, McManus DD. et al. Wearing your heart on your sleeve: The future of cardiac rhythm monitoring. Curr Cardiol Rep 2019; 21: 158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Eurlings C, Boyne JJ, de Boer RA. et al. Telemedicine in heart failure-more than nice to have? Neth Heart J 2019; 27: 5–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Dias D, Paulo Silva Cunha J.. Wearable health devices-vital sign monitoring, systems and technologies. Sensors (Basel) 2018; 18: 2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Jovanov E. Wearables meet IoT: Synergistic personal area networks (SPANs). Sensors (Basel) 2019; 19: 4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sethuraman SC, Vijayakumar V, Walczak S.. Cyber attacks on healthcare devices using unmanned aerial vehicles. J Med Syst 2020; 44: 29. [DOI] [PubMed] [Google Scholar]
- 56. Bayo-Monton JL, Martinez-Millana A, Han W. et al. Wearable sensors integrated with Internet of Things for advancing eHealth care. Sensors (Basel) 2018; 18: 1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wieringa FP, Van Hoof C. Connected health and diagnostics – Healthcare within everybody’s reach. In: Workshop on Reconfigurable Sensor Systems Integrated with Artificial Intelligence & Data Harnessing to Enable Personalized Medicine, US National Science Foundation, Alexandria VA, March 7–8 2019F.
- 58. Mavrogiorgou A, Kiourtis A, Perakis K. et al. IoT in healthcare: Achieving interoperability of high-quality data acquired by IoT medical devices. Sensors (Basel) 2019; 19: 1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. De Brouwer M, Ongenae F, Bonte P. et al. Towards a cascading reasoning framework to support responsive ambient-intelligent healthcare interventions. Sensors (Basel) 2018; 18: 3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Banaee H, Ahmed MU, Loutfi A.. Data mining for wearable sensors in health monitoring systems: a review of recent trends and challenges. Sensors (Basel) 2013; 13: 17472–17500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kooman JP, Usvyat LA, Dekker MJE. et al. Cycles, arrows and turbulence: Time patterns in renal disease, a path from epidemiology to personalized medicine? Blood Purif 2019; 47: 171–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Usvyat LA, Barth C, Bayh I. et al. Interdialytic weight gain, systolic blood pressure, serum albumin, and C-reactive protein levels change in chronic dialysis patients prior to death. Kidney Int 2013; 84: 149–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lacson RC, Baker B, Suresh H. et al. Use of machine-learning algorithms to determine features of systolic blood pressure variability that predict poor outcomes in hypertensive patients. Clin Kidney J 2019; 12: 206–212 [DOI] [PMC free article] [PubMed] [Google Scholar]