Abstract
People with advanced chronic kidney disease and evidence of progression have a high risk of renal replacement therapy. Specialized transition clinics could offer a better option for preparing these patients for dialysis, transplantation or conservative care. This review focuses on the different aspects of such transition clinics. We discuss which patients should be referred to these units and when referral should take place. Patient involvement in the decision-making process is important and requires unbiased patient education. There are many themes, both patient-centred and within the healthcare structure, that will influence the process of shared decision-making and the modality choice. Aspects of placing an access for haemodialysis and peritoneal dialysis are reviewed. Finally, we discuss the importance of pre-emptive transplantation and a planned dialysis start, all with a focus on multidisciplinary collaboration at the transition clinic.
Keywords: haemodialysis, kidney transplantation, nutrition, peritoneal dialysis, pre-dialysis, vascular access
INTRODUCTION
Chronic kidney disease (CKD) is a complex disorder and one of the major public health problems affecting millions of people globally. Projections have demonstrated that the disease will continue to grow; estimates show that the prevalence of CKD increased by 87% between 1990 and 2014 [1]. The complexity of CKD can be illustrated by the large number of other comorbidities, the number of prescribed drugs, hospitalization rates and high mortality [2]. The frequency of polypharmacy is ~80% in those with CKD and increases with CKD severity [3].
Although ~6% of the prevalent European population is diagnosed with CKD Stage 3+, only a minority of those will progress [4]. Those who progress have a higher risk of developing end-stage kidney disease (ESKD) in need of dialysis or transplantation [renal replacement therapy (RRT)] [4, 5] and also have a higher risk of death and cardiovascular disease events [5, 6]. Thus those with a progressive disease make up an even more complex subset of the CKD population. The Kidney Disease: Improving Global Outcomes (KDIGO) guidelines state that the goals for CKD follow-up should be identification, prevention and treatment of CKD-related complications such as anaemia, acidosis and metabolic disorders; planning and preparation for RRT; psychosocial support and provision of palliative care [7]. Complex patients like those with advanced-stage CKD are difficult to fit into a traditional outpatient clinic. Instead, they are referred to specialized transition clinics, where the focus is to offer a more holistic approach and prepare the patient for RRT. Today there is also a shift in the attitude towards patient involvement and a greater understanding that ‘one-size-fits-all’ is not necessarily the best care [8]. This review will discuss the different aspects of such transition clinics and their role in offering more personalized CKD care.
WHEN AND WHOM TO REFER TO THE TRANSITION CLINIC
Early or timely referral has consistently been associated with better outcomes, such as improved survival, lower hospitalization rates, higher uptake in peritoneal dialysis (PD), better access to kidney transplant waiting lists and lower rates of RRT initiation with a temporary central venous catheter [9, 10]. Although the definition of early referral varies, most authors consider referral at least 3–6 months before RRT initiation to be timely [9]. To slow the progression of CKD, however, a referral to nephrology services 3–6 months before RRT could be regarded as late. Instead, KDIGO recommends that any patient with an estimated glomerular filtration rate (eGFR) <30 mL/min/1.73 m2, significant albuminuria (>30 mg/mmol) or CKD progression should be referred to a nephrologist [7]. For referrals to a transition clinic, where the focus is more to prepare the patient for RRT, a minimum of 12 months is reasonable, given the necessary services (patient education, ultrasonography and surgery) could be provided within this time frame. However, it may be difficult to assess who needs to start RRT within 12 months, and patients may require a longer period of time to reach their decision. Also, among those with advanced stage CKD (G4–G5) eligible for the transition clinic there are different risk of progressing [4]. In nephrology-referred cohorts, the estimated progression rate varies considerably across populations, and even in Europe the annual loss of eGFR has been estimated to range between 0.77 and 2.35 mL/min/1.73 m2 [11]. Non-linear eGFR decline also needs to be taken into account and makes projections of the progressive disease difficult. It has even been suggested that intermittent episodes of acute kidney injury are the main force driving CKD progression [12]. However, many older adults with CKD have a non-progressive disease and a higher risk of dying with CKD than starting RRT [13]. In an Italian cohort of referred patients with an eGFR <60 mL/min/1.73 m2, the RRT incidence was only 8.3/100 person-years [13], and in a Swedish referred cohort, the risk of RRT at a similar CKD stage was even lower [4]. In the latter study, ~35% of the patients experienced no overall decline in eGFR at all. Thus, to guide resources towards patients with the highest risk of CKD progression and the need for multidisciplinary intervention, referral to a transition clinic benefits from a risk-based approach instead of a static eGFR limit (e.g. <15 mL/min/1.73 m2). KDIGO recommends that patients with a risk of ESKD in the range of 10–20% within 1 year should be referred for RRT planning [7]. In Ontario, Canada, a risk-based referral system was adopted and only those with a 2-year RRT risk >10% were accepted at the multidisciplinary clinic. This led to cost savings of ~6 million Canadian dollars [14]. The RRT risk can be estimated using a validated prediction tool, of which the Kidney Failure Risk Equation is the most extensively validated [15]. So far there has been no direct comparison between different prediction tools and there may be local circumstances that favour the use of other validated equations.
PATIENT EDUCATION AND TREATMENT MODALITY CHOICE
There is a growing consensus that decisions regarding treatment choices should be shared between the patient and health professionals [8]. One of the most important questions for patients referred to the transition clinic is the choice of treatment modality. Previous studies have shown that not only do the patients feel uninformed, they also feel they do not actually have a choice [16, 17]; 61% of those starting dialysis regret the choice they made [18]. Shared decision-making differs by treatment modality, not only in terms of the values that are important, but also in how the patient experiences involvement in the decision. PD patients more often feel that they have been involved in the modality choice as compared with haemodialysis (HD) patients [19]. The counselling of a patient regarding dialysis modality and conservative care should be timely, patient-centred and incorporate the perspectives and values of the individual [20]. In that sense, an education programme at the transition clinic is important. Modality counselling should start at least 9–12 months before RRT is initiated [7]. CKD awareness is low in the public and many patients feel they have a poor understanding of their disease [17]. Emotions such as fear may impair and postpone the decision-making process and may ultimately lead to blame put on the healthcare team [21]. Conversely, early pre-dialysis education is associated with better uptake in home-based modalities [22]. Patient education should also include information about all treatment modalities to avoid biases; the doctor’s preference has an effect on the patients’ choice [16]. To create the optimal circumstances for shared decision-making, the sessions should be kept short, ideally ~15 min and focus only on three to five points at a time [23]. These should be explained simply and separately, using ‘teach-back’ techniques. Furthermore, repetition over time will help patients increase their knowledge, reach a decision and be able to amend it if circumstances change that could have an influence on their choice.
The barriers for shared decision-making may lie within the healthcare organization but they could also depend on several patient-focused reasons [20]. Those who choose PD are more likely to have higher education, less comorbidity burden, married and employed [17]. The patients will also integrate the experiences and values of people they know, for example, a family member, a neighbour or a friend [24]. Importantly, the personal values and beliefs of the patient will greatly influence the decision. The most vital factors to most patients when choosing a dialysis modality are independence, quality of life and flexibility [16]. It has been shown that those who choose PD value flexibility and autonomy, while patients who prefer HD value a planned schedule and having someone else taking care of them [17]. Generally patients strive to maintain their current quality of life and lifestyle and will favour a treatment that enables them to do so. There are also other patient-related factors that influence the decision. Patient comorbidity, such as cognitive disorders, may prevent someone from starting a home-based therapy such as PD or home HD. These factors are important to identify and screen for early in the process. Another important influencing factor in the decision-making process is health literacy, defined as the ability of a person to obtain, process and appreciate basic medical information and risks [25]. Patients with low health literacy have generally worse outcomes, and they also have a lower chance of being truly involved in their decision-making. This means that even in a standard educational pre-dialysis programme, it is important to identify individuals with low health literacy and use different educational techniques to guide their decisions [25].
An informed decision also means the decision not to choose dialysis. Evidence that dialysis treatment actually prolongs life or improves the quality of life in frail elderly patients is lacking. The life expectancy of older individuals (>75 years) on dialysis is about three to four times lower than that of the general population [26]. In nursing home residents newly started on dialysis, only 58% were alive after 1 year and 13% had maintained their functional status [27]. The patient and his/her family need to receive balanced information about conservative management as well as dialysis modalities. This further highlights the necessity of incorporating principles of supportive care into the transition clinic. Once a patient has opted for conservative care, he/she could either be referred to a palliative unit or be kept under the umbrella of the transition clinic with adequate support from a specialized team. However, the perspective of palliative medicine should always be present and aim to identify values and expectations that could have an impact on choices and possible treatments over time. Over the course of progressive CKD, uraemic symptoms are likely to be aggravated, and strategies must be identified to assess and manage these [28]. When the end of life nears, there should be a possibility to refer the patient to hospice care.
The multidisciplinary care model
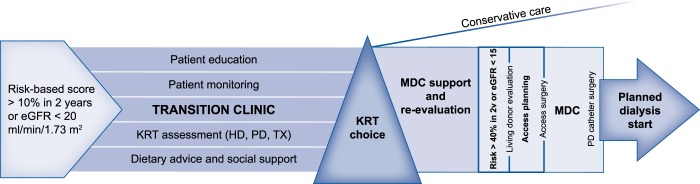
A way to enhance structured patient education and advanced care planning is by involving a multidisciplinary team at the transition clinic (Table 1). This means that a team consisting of, for example, specialized nurses, dieticians, social workers and pharmacists provides additional support to patients alongside the traditional nephrologist visits (Figure 1). There are many studies in favour of a multidisciplinary approach at the pre-dialysis clinic, most of which are observational studies [29]. A recent meta-analysis consisting of 18 cohorts showed that having access to a multidisciplinary team increased survival and decreased the risk of RRT initiation and starting dialysis with a central venous catheter [30]. Among the observational studies in favour of a multidisciplinary model is one that showed participation in nurse-led clinics improved the frequency of starting dialysis with a permanent vascular access and increased the number of patients choosing home-based therapies [31]. Recently a cluster randomized controlled trial demonstrated encouraging support for the model as well, although in earlier CKD stages. The Effectiveness of Integrated Care on Delaying Progression of Stage 3 and 4 Chronic Kidney Disease in Rural Communities of Thailand [32] study observed a 2.7 mL/min/1.73 m2 slower eGFR decline in those who were referred to a multidisciplinary team in addition to standard of care. In Canada, a training network for pharmacists incorporated in a previously working multidisciplinary setting decreased the number of drug-related problems [33]. Although the multidisciplinary team approach intuitively seems better, there is still no consensus about how this should be organized. So far, the studies have used different combinations of health workers, different referral criteria and different frequencies of follow-up. Ideally at the transition clinic, it should be possible to adapt the resources to the needs of the patients; some may need more social support, others may benefit from closer monitoring by the nephrologist or specialized nurse and others may need an extended educational programme.
Table 1.
Resources at the transition CKD clinic
| Resource | Task 1 | Task 2 | Task 3 | Task 4 | Task 5 |
|---|---|---|---|---|---|
| Nephrologist(s) including some with PD experience | Monitoring of CKD progression and patient health | Treatment of blood pressure, fluid retention, anaemia, acidosis, hyperphosphataemia, hyperkalaemia, etc. | MDC [modality decision, access planning (PD and HD)] | Kidney transplantation evaluation and referral for living donor investigation | Referral for planned dialysis initiation |
| Renal nurse | Patient education | MDC (modality decision) | Patient monitoring of symptoms and changes in decision | Access monitoring | |
| Dietician | General dietary advice and nutritional evaluation | Control of hyperphosphataemia, hyperkalaemia and salt intake | Low-protein diet and/or additional energy intake | ||
| Social worker | Evaluation of the general social situation | Support of patients and family | |||
| Radiologist | Vein mapping (duplex) | MDC (vascular access planning) | |||
| Surgeon | MDC (vascular access planning) | Vascular access surgery | PD catheter surgerya | ||
| Geriatrician | Provision of palliative care | Support of conservatively treated patient | Referral to hospice |
Could also be done by, e.g. a specialized nephrologist or radiologist. MDC: multidisciplinary conference.
FIGURE 1.
Schematic figure of an example transition clinic. MDC: multidisciplinary conference.
Protein-energy wasting is common in patients starting dialysis and is linked to higher mortality [34]. In pre-dialysis patients with Stages 4 and 5 CKD as many as 27% suffer from malnutrition [35]. CKD Stages 4 and 5 patients have a lower spontaneous energy intake than the general population and protein intake decreases spontaneously with lower renal function [36]. Maybe as a result of these alterations, patients start to lose weight from an eGFR of 35 mL/min/1.73 m2. An annualized weight loss >5% during the pre-dialysis period is associated with higher all-cause mortality [37]. Dietary support should be part of the multidisciplinary care offered at the transition clinic. In a US study, the involvement of a dietician at least 12 months prior to dialysis resulted in lower post-dialysis mortality [38]. Dieticians should also be involved when treating patients with a low-protein diet in the pre-dialysis period. A low-protein diet (which could consist of a low-normal diet of 0.8 g/kg/day, a low-protein diet of 0.6 g/kg/day or a very low-protein diet of 0.3 g/kg/day) could prolong the time to RRT and maybe also reduce the eGFR decline [39]. In the pre-dialysis transition clinic, dietary treatment could be used to reduce uraemic symptoms and prevent the development of hyperkalaemia and hyperphosphataemia alongside other medical treatments. The dietician then has an important role in maintaining adequate energy and nutrient intake and monitoring nutritional status to avoid the development of protein-energy wasting.
ACCESS PREPARATION FOR HD AND PD
One result of early education and an informed shared decision on dialysis modality is the possibility to better plan for a vascular access in those who have chosen HD as their first modality of treatment. For people on dialysis, the recommended vascular access is an arteriovenous fistula, followed by an arteriovenous graft. In 2003, the Fistula First Breakthrough Initiative was formed in the USA and greatly impacted guidelines on vascular access planning [40]. More recently, there has been an increased understanding that the optimal access could differ between individuals. There may be patient characteristics such as age, diabetes status, vascular anatomy, other comorbidities and patient preferences that may influence the vascular access choice [41].
It is recommended to refer the patient for a dialysis access assessment when eGFR is between 15 and 20 mL/min/1.73 m2 and there is the progressive loss of renal function [7]. It is a challenge to prepare a timely vascular access, and although the aim is for patients to start dialysis with a working access, only 27% of patients in a large US cohort [42] started dialysis with a central venous catheter. Conversely, in a study where nephrologists aimed to have a vascular access prepared 6 months before dialysis initiation, 50% had still not started dialysis 1 year later [43]. With the use of validated prediction tools, these numbers may improve. Tangri et al. [14] recently suggested that patients should be referred for vascular access surgery when the probability of RRT is 40% within 2 years, although this threshold remains to be validated. Using an interdisciplinary approach and a team consisting of a vascular surgeon, radiologist and nephrologist, the success rate of creating a working access may improve. The time between the decision to start dialysis and full access maturation could be shortened if the nephrologist refers the patient for a vein mapping examination once the decision is made [44]. Subsequently, a multidisciplinary conference including the vascular surgeon selects the primary access and a plan for surgery. After formation of the access, a specialized nurse follows up the maturation status and alerts the nephrologist about signs of delayed maturation that may require additional surgery or endovascular interventions.
PD is an alternate option for most patients approaching ESKD. Therefore a PD-experienced nephrologist as well as an experienced surgeon and a dedicated nursing team should be part of the transition clinic [45]. Absolute contraindications are rare and consist of previous extensive abdominal surgery (especially open implantation of a vascular prosthesis), recurrent diverticulitis episodes, chronic inflammatory bowel disease [46] and, in particular, the willingness of the patient (and sometimes his or her relatives) to undergo home treatment. Often mentioned relative contraindications, for example, obesity [47], polycystic kidney disease [48] or frailty, should not per se lead to an exclusion from PD but should cause the PD team how determine how to deal with these preconditions, for example, by using special catheter types, different implantation techniques and alternative catheter exit sites or assisted PD.
To help patients and family members with the decision in choosing a treatment modality, it may be helpful to introduce them to other patients undergoing HD and PD. Even better would be a renal replacement ‘workshop’. A structured interview, which could also involve the partner or close relatives, should lead to informed consent. After the patient has opted for PD, more specific planning should be commenced. PD treatment should be initiated when uraemic symptoms start to appear (e.g. physical weakness or inappetence) but well before critical (hyperkalaemia, pulmonary hypervolaemia) or long-lasting side effects (wasting and sarcopenia) develop. The time in between can be used to pre-train the patient, and in some cases family members, for the upcoming procedures and to make a home visit by PD staff to check for hygiene problems and storage capacity for PD materials and solutions.
Before concrete planning for the catheter insertion procedure, the abdominal wall of the patient must be inspected for scars and hernias. Together with the surgeon, the catheter insertion procedure (open, laparoscopic) and the kind of catheter are determined. In case of previous extensive intra-abdominal surgery or peritonitis, an advanced laparoscopic procedure with the possibility to place the catheter tip under the site and for additional adhesiolysis or omentopexy may be scheduled [49]. Together with the patient, the exit site must be established considering personal preferences, clothing habits and individual skin peculiarities (e.g. skin folds, obesity and eczema).
A break-in period of 2 weeks between catheter placement and dialysis initiation is recommended by the latest International Society for Peritoneal Dialysis (ISPD) guideline to prevent mechanical complications, especially leakage of dialysis fluid [46]. If additional surgical procedures, in most cases hernia repairs, are necessary, then a longer interval may be useful. If an urgent treatment start is indicated, then low-volume, high-frequency automated PD in the supine position can be initiated immediately after catheter insertion to avoid intermittent intravenous catheterization [50].
TRANSPLANTATION
The European Best Practice Guidelines state that every ESKD patient should be considered for kidney transplantation unless there is an absolute contraindication [51]. These include uncontrolled cancer, active systemic infections and any condition with a life expectancy <2 years. Kidney transplantation improves patient survival compared with both dialysis entities [52] and facilitates a better quality of life despite the need for long-term immunosuppression. Before a patient approaches ESKD, preparations for waiting list admittance should be started. These include a standardized comprehensive briefing about outcomes compared with those of dialysis methods, sources of grafts and possible complications. After informed consent, the evaluation procedures can be initiated. The intensity of the evaluation depends on the pre-existing medical conditions of the applicant. Special attention should be paid to cardiovascular function, former malignancies, chronic infections, obesity and metabolic diseases and the original kidney disease. Psychological aspects must be considered; in particular, adherence must be evaluated. Cardiovascular disease is the main cause of mortality after transplantation. The diagnosis of coronary heart disease in ESKD patients is not trivial due to often oligosymptomatic patients, impaired sensitivity and specificity of diagnostic procedures, impaired exercise capacity of CKD patients and low levels of evidence for testing this population. For asymptomatic low-risk patients (age <50 years, no diabetes and no former coronary artery disease) with a normal electrocardiogram, no further testing is recommended [53]. All other candidates should be tested by dobutamine stress echocardiography or myocardial stress scintigraphy. In case of suspicious results, coronary angiography is indicated. Evidence for this sequence is not high grade [54], and a randomized controlled trial is actually recruiting to clarify this part of the transplant evaluation [55].
The best outcome (patient and graft survival) is attained by transplanting a candidate before the initiation of dialysis (pre-emptive kidney transplantation) [56], the reasons for this are not clear and differences in immune reactivity with consecutively lower rejection rates are cited. Pre-emptive kidney transplantation is mostly achieved by living donation, which means that a potential donor should be selected and evaluated already early during the transplantation assessment to allow for careful donor evaluation without being pressed for time.
DIALYSIS INITIATION
RRT should be initiated if there are uraemic symptoms, electrolyte disturbances, fluid retention, hypertension and deterioration in nutritional status that is insufficiently treated with other medical treatments. This often occurs when GFR is in between 5 and 10 mL/min/1.73 m2. The Initiating Dialysis Early and Late study showed that there is no benefit from starting dialysis early in terms of eGFR; patient survival and quality of life were no better in the early group compared with the late start group [57]. Still, many patients started with more preserved renal function, when eGFR is >10 mL/min/1.73 m2. Furthermore, elderly patients with advanced stage CKD are more likely to initiate dialysis during an episode of acute kidney injury. By monitoring the patient closely through frequent laboratory testing and clinical examinations (fluid retention, blood pressure and patient-reported symptoms) when eGFR is dropping, it is possible to tailor dialysis initiation to when both the patient and nephrologist feel the timing is optimal. It is important to remember that although eGFR may be accurate on a population level, it could be biased for an individual, especially in those with poor functional capacity, low muscle mass, overhydration and low-protein intake [58]. Thus eGFR may remain constant while the true GFR is declining. If there is a discrepancy between patient-reported uraemic symptoms and eGFR, then it could be helpful to assess GFR through other estimating equations (e.g. cystatin C) or tracer methods (iohexol and Cr51-EDTA).
In summary, in patients with progressive and advanced stage CKD, referral to a transition clinic may help provide timely patient education, advance care planning and dialysis and kidney transplantation preparation. To help with limited resources, decision-making could benefit from a risk-based approach. The transition clinic could consist of nurse-led clinics to better monitor patient symptoms and tailor dialysis education and planning to the patients’ preferences in collaboration with specialists in both PD and HD. The overall aim for the transition clinic should be to start dialysis in the right patient at the right time with a working access and with the patient feeling involved and satisfied with the decision.
CONFLICT OF INTEREST STATEMENT
This article has not been published previously in whole or part, except in abstract format. The authors have no financial conflicts of interests in relation to the submitted work. K.L. reports no conflicts of interests. M.E. reports advisory board participation for Astellas and AstraZeneca, payment for lectures from Astellas and Vifor Pharma and institutional grants for work unrelated to this submission from AstraZeneca and Astellas.
REFERENCES
- 1. Xie Y, Bowe B, Mokdad AH. et al. Analysis of the Global Burden of Disease study highlights the global, regional, and national trends of chronic kidney disease epidemiology from 1990 to 2016. Kidney Int 2018; 94: 567–581 [DOI] [PubMed] [Google Scholar]
- 2. Tonelli M, Wiebe N, Manns BJ. et al. Comparison of the complexity of patients seen by different medical subspecialists in a universal health care system. JAMA Network Open 2018; 1: e184852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schmidt I, Hübner S, Nadal J. et al. Patterns of medication use and the burden of polypharmacy in patients with chronic kidney disease: the German Chronic Kidney Disease study. Clin Kidney J 2019; 12: 663–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lundström UH, Gasparini A, Bellocco R. et al. Low renal replacement therapy incidence among slowly progressing elderly chronic kidney disease patients referred to nephrology care: an observational study. BMC Nephrol 2017; 18: 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Coresh J, Turin TC, Matsushita K. et al. Decline in estimated glomerular filtration rate and subsequent risk of end-stage renal disease and mortality. JAMA 2014; 311: 2518–2531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Evans M, Grams ME, Sang Y. et al. Risk factors for prognosis in patients with severely decreased GFR. Kidney Int Rep 2018; 3: 625–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kidney Disease: Improving Global Outcomes CKD Work Group. KDIGO clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013; 3: 1–150 [Google Scholar]
- 8. Eckardt K-U, Bansal N, Coresh J. et al. Improving the prognosis of patients with severely decreased glomerular filtration rate (CKD G4+): conclusions from a KDIGO Controversies Conference. Kidney Int 2018; 93: 1281–1292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Smart NA, Dieberg G, Ladhani M. et al. Early referral to specialist nephrology services for preventing the progression to end-stage kidney disease. Cochrane Database Syst Rev 2014; 6: CD007333. [DOI] [PubMed] [Google Scholar]
- 10. Stack AG. Impact of timing of nephrology referral and pre-ESRD care on mortality risk among new ESRD patients in the United States. Am J Kidney Dis 2003; 41: 310–318 [DOI] [PubMed] [Google Scholar]
- 11. Brück K, Jager KJ, Zoccali C. et al. Different rates of progression and mortality in patients with chronic kidney disease at outpatient nephrology clinics across Europe. Kidney Int 2018; 93: 1432–1441 [DOI] [PubMed] [Google Scholar]
- 12. Johnson RJ, Rodriguez-Iturbe B.. Rethinking progression of CKD as a process of punctuated equilibrium. Nat Rev Nephrol 2018; 14: 411–412 [DOI] [PubMed] [Google Scholar]
- 13. De Nicola L,, Minutolo R, Chiodini P. et al. ; for the Italian Society of Nephrology Study Group ‘TArget Blood pressure LEvels (TABLE) in CKD. The effect of increasing age on the prognosis of non-dialysis patients with chronic kidney disease receiving stable nephrology care. Kidney Int 2012; 82: 482–488 [DOI] [PubMed] [Google Scholar]
- 14. Tangri N, Ferguson T, Komenda P.. Pro: risk scores for chronic kidney disease progression are robust, powerful and ready for implementation. Nephrol Dial Transplant 2017; 32: 748–751 [DOI] [PubMed] [Google Scholar]
- 15. Tangri N, Grams ME, Levey AS. et al. ; for the CKD Prognosis Consortium. Multinational assessment of accuracy of equations for predicting risk of kidney failure: a meta-analysis. JAMA 2016; 315: 164–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dahlerus C, Quinn M, Messersmith E. et al. Patient perspectives on the choice of dialysis modality: results from the Empowering Patients on Choices for Renal Replacement Therapy (EPOCH-RRT) study. Am J Kidney Dis 2016; 68: 901–910 [DOI] [PubMed] [Google Scholar]
- 17. Harwood L, Clark AM.. Understanding pre-dialysis modality decision-making: a meta-synthesis of qualitative studies. Int J Nurse Stud 2013; 50: 109–120 [DOI] [PubMed] [Google Scholar]
- 18. Davison SN. End-of-life care preferences and needs: perceptions of patients with chronic kidney disease. Clin J Am Soc Nephrol 2010; 5: 195–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zee J, Zhao J, Subramanian L. et al. Perceptions about the dialysis modality decision process among peritoneal dialysis and in-center hemodialysis patients. BMC Nephrol 2018; 19: 298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cassidy B, Getchell L, Harwood L. et al. Barriers to education and shared decision making in the chronic kidney disease population: a narrative review. Can J Kidney Health Dis 2018; 5: 2054358118803322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Murray MA, Brunier G, Chung JO. et al. A systematic review of factors influencing decision-making in adults living with chronic kidney disease. Patient Educ Couns 2009; 76: 149–158 [DOI] [PubMed] [Google Scholar]
- 22. Chanouzas D, Ng KP, Fallouh B. et al. What influences patient choice of treatment modality at the pre-dialysis stage? Nephrol Dial Transplant 2012; 27: 1542–1547 [DOI] [PubMed] [Google Scholar]
- 23. Collister D, Russell R, Verdon J. et al. Perspectives on optimizing care of patients in multidisciplinary chronic kidney disease clinics. Can J Kidney Health Dis 2016; 3: 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Winterbottom A, Bekker HL, Conner M. et al. Choosing dialysis modality: decision making in a chronic illness context. Health Expect 2014; 17: 710–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Coulter A, Ellins J.. Effectiveness of strategies for informing, educating, and involving patients. BMJ 2007; 335: 24–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.U.S. Renal Data System. USRDS Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Kidney Disease in the United States. Bethesda, MD: National Insititutes of Health, National Institutes of Diabetes and Digestive and Kidney Disease, 2013 [Google Scholar]
- 27. Kurella Tamura M, Covinsky KE, Chertow GM. et al. Functional status of elderly adults before and after initiation of dialysis. N Engl J Med 2009; 361: 1539–1547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Raghavan D, Holley JL.. Conservative care of the elderly ckd patient: a practical guide. Adv Chronic Kidney Dis 2016; 23: 51–56 [DOI] [PubMed] [Google Scholar]
- 29. Wang S-M, Hsiao L-C, Ting IW. et al. Multidisciplinary care in patients with chronic kidney disease: a systematic review and meta-analysis. Eur J Intern Med 2015; 26: 640–645 [DOI] [PubMed] [Google Scholar]
- 30. Shi Y, Xiong J, Chen Y. et al. The effectiveness of multidisciplinary care models for patients with chronic kidney disease: a systematic review and meta-analysis. Int Urol Nephrol 2018; 50: 301–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pagels AA, Wång M, Wengström Y.. The impact of a nurse-led clinic on self-care ability, disease-specific knowledge, and home dialysis modality. Nephrol Nurs J 2008; 35: 242–248 [PubMed] [Google Scholar]
- 32. Jiamjariyapon T, Ingsathit A, Pongpirul K. et al. Effectiveness of integrated care on delaying progression of stage 3-4 chronic kidney disease in rural communities of Thailand (ESCORT study): a cluster randomized controlled trial. BMC Nephrol 2017; 18: 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lalonde L, Quintana-Bárcena P, Lord A. et al. community pharmacist training-and-communication network and drug-related problems in patients with CKD: a multicenter, cluster-randomized, controlled trial. Am J Kidney Dis 2017; 70: 386–396 [DOI] [PubMed] [Google Scholar]
- 34. Fouque D, Kalantar-Zadeh K, Kopple J. et al. A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int 2008; 73: 391–398 [DOI] [PubMed] [Google Scholar]
- 35. Windahl K, Faxén Irving G, Almquist T. et al. Prevalence and risk of protein-energy wasting assessed by subjective global assessment in older adults with advanced chronic kidney disease: results from the EQUAL study. J Ren Nutr 2018; 28: 165–174 [DOI] [PubMed] [Google Scholar]
- 36. Kopple JD, Greene T, Chumlea WC. et al. Relationship between nutritional status and the glomerular filtration rate: results from the MDRD study. Kidney Int 2000; 57: 1688–1703 [DOI] [PubMed] [Google Scholar]
- 37. Ku E, Kopple JD, Johansen KL. et al. Longitudinal weight change during CKD progression and its association with subsequent mortality. Am J Kidney Dis 2018; 71: 657–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Appel LJ, Wright JT, Greene T. et al. Intensive blood-pressure control in hypertensive chronic kidney disease. N Engl J Med 2010; 363: 918–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hahn D, Hodson EM, Fouque D.. Low protein diets for non‐diabetic adults with chronic kidney disease. Cochrane Database Syst Rev 2018; 10: CD001892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lynch JR, Wasse H, Armistead NC. et al. Achieving the goal of the fistula first breakthrough initiative for prevalent maintenance hemodialysis patients. Am J Kidney Dis 2011; 57: 78–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Drew DA, Lok CE, Cohen JT. et al. Vascular access choice in incident hemodialysis patients: a decision analysis. J Am Soc Nephrol 2015; 26: 183–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Segal JH, Hirth RA.. The cost of putting fistula first. Am J Kidney Dis 2018; 72: 1–3 [DOI] [PubMed] [Google Scholar]
- 43. Cheung AK, Chang TI, Cushman WC. et al. Blood pressure in chronic kidney disease: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int 2019; 95: 1027–1036 [DOI] [PubMed] [Google Scholar]
- 44. Hedin U. Vascular access: a never-ending story. J Cardiovasc Surg (Torino) 2014; 55: 793–801 [PubMed] [Google Scholar]
- 45. Woodrow G, Fan SL, Reid C. et al. Renal association clinical practice guideline on peritoneal dialysis in adults and children. BMC Nephrol 2017; 18: 333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Crabtree JH, Shrestha BM, Chow K-M. et al. Creating and maintaining optimal peritoneal dialysis access in the adult patient: 2019 update. Perit Dial Int 2019; 39: 414–436 [DOI] [PubMed] [Google Scholar]
- 47. Kennedy C, Bargman J.. Peritoneal dialysis in the obese patient. Clin J Am Soc Nephrol 2019; CJN.10300819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Dupont V, Kanagaratnam L, Sigogne M. et al. Outcome of polycystic kidney disease patients on peritoneal dialysis: systematic review of literature and meta-analysis. PLoS One 2018; 13: e0196769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Crabtree JH, Burchette RJ.. Effective use of laparoscopy for long-term peritoneal dialysis access. Am J Surg 2009; 198: 135–141 [DOI] [PubMed] [Google Scholar]
- 50. Arramreddy R, Zheng S, Saxena AB. et al. Urgent-start peritoneal dialysis: a chance for a new beginning. Am J Kidney Dis 2014; 63: 390–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.European Expert Group on Renal Transplantation, European Renal Association, European Society for Organ Transplantation. European best practice guidelines for renal transplantation (part 1). Nephrol Dial Transplant 2000; 15: 1–85 [Google Scholar]
- 52. Wolfe RA, Ashby VB, Milford EL. et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med 1999; 341: 1725–1730 [DOI] [PubMed] [Google Scholar]
- 53. Campbell S, Pilmore H, Gracey D. et al. KHA-CARI guideline: recipient assessment for transplantation. Nephrology (Carlton) 2013; 18: 455–462 [DOI] [PubMed] [Google Scholar]
- 54. Lentine KL, Hurst FP, Jindal RM. et al. Cardiovascular risk assessment among potential kidney transplant candidates: approaches and controversies. Am J Kidney Dis 2010; 55: 152–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ying T, Gill J, Webster A. et al. Canadian-Australasian randomised trial of screening kidney transplant candidates for coronary artery disease—a trial protocol for the CARSK study. Am Heart J 2019; 214: 175–183 [DOI] [PubMed] [Google Scholar]
- 56. Gill J, Tonelli M, Johnson N. et al. Why do preemptive kidney transplant recipients have an allograft survival advantage? Transplant 2004; 78: 873–879 [DOI] [PubMed] [Google Scholar]
- 57. Cooper BA, Branley P, Bulfone L. et al. A randomized, controlled trial of early versus late initiation of dialysis. N Engl J Med 2010; 363: 609–619 [DOI] [PubMed] [Google Scholar]
- 58. Björk J, Grubb A, Larsson A. et al. Accuracy of GFR estimating equations combining standardized cystatin C and creatinine assays: a cross-sectional study in Sweden. Clin Chem Lab Med 2015; 53: 403–414 [DOI] [PubMed] [Google Scholar]