Abstract
Chronic kidney disease (CKD) is a clinical model of premature ageing characterized by cardiovascular disease, persistent uraemic inflammation, osteoporosis muscle wasting and frailty. The accelerated early vascular ageing (EVA) process mediated by medial vascular calcification (VC) is a hallmark of senescence as well as a strong predictor of cardiovascular morbidity and mortality in the CKD population. Current clinical therapeutic strategies and novel treatments for VC have not yet been proven to prevent or reverse VC progression in patients with CKD. Knowledge of the fundamental mechanism underlying EVA is urgently needed to identify and develop novel and efficient therapeutic targets for VC and EVA. An accumulating body of evidence indicates that deoxyribonucleic acid (DNA) damage–induced cellular senescence and ‘inflammaging’ may largely contribute to such pathological conditions characterized by accelerated EVA. Growing evidence shows that nuclear factor erythroid 2–related factor 2 (NRF2) signalling and vitamin K play a crucial role in counteracting oxidative stress, DNA damage, senescence and inflammaging, whereby NRF2 activation and vitamin K supplementation may provide a novel treatment target for EVA. In this review we discuss the link between senescence and EVA in the context of CKD, with a focus on the role of NRF2 and vitamin K in DNA damage signalling, senescence and inflammaging.
Keywords: age, cardiovascular, CKD, inflammation, oxidative stress
Patients with chronic kidney disease (CKD) are characterized by an accelerated ageing process, including cardiovascular complications, persistent uraemic inflammation, muscle wasting, osteoporosis and frailty, preceding initiation of renal replacement therapy with dialysis or kidney transplantation [1, 2]. Early vascular ageing (EVA) is an evolving construct elaborating the concept of premature unsuccessful vascular ageing with arterial stiffness as an intermediate endpoint and independent predictor of cardiovascular disease (CVD) and mortality [3]. In CKD, EVA is reflected by extensive media vascular calcification (VC), a cell-mediated process primarily driven by vascular smooth muscle cells (VSMCs) [4]. The extent of VC may be seen as a measure of biological vascular age. In patients with CKD, the arterial wall appears older than the chronological vascular age of the patients. This premature ageing comes with concomitant chronic inflammation, persistent oxidative stress, deoxyribonucleic acid (DNA) damage, repeated cellular insults and imbalanced pro-ageing and anti-ageing systems [2]. As EVA is a hallmark of ageing, as well as a strong predictor of cardiovascular morbidity and mortality in CKD, prevention and treatment of EVA is crucial. Current clinical strategies focus on the management of CKD–mineral bone disorders and inhibition of calcium phosphate deposition, including phosphate binders, calcimimetics, vitamin K, magnesium and crystallization inhibitors (e.g. pyrophosphate, sodium thiosulphate and fetuin). Such established treatments have been evaluated in clinical studies, yet the results remain inconclusive and it remains unclear whether a comparable treatment is effective in mitigating VC progression in patients with CKD [5].
Recently the unravelling of the molecular targets of VC has brought potential novel treatment for EVA. A recent randomized Phase 2b study by Raggi et al. [6] showed that intravenous myo-inositol hexaphosphate attenuated progression of vascular aortic valve calcification in HD patients. Moreover, serum- and glucocorticoid-inducible kinase 1 (SGK1) has been identified as a key regulator of VC, partly through nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κΒ) signalling, and genetic depletion or pharmacological inhibition of SGK1 substantially dissipated osteogenesis of VSMCs in vitro and blunted VC progression in apolipoprotein E–deficient mice in vivo [7]. Chemerin/ChemR23 signalling and pro-inflammatory cytokines interleukin (IL)-1β, tumour necrosis factor have also been identified as key regulators in the regulation of VC in vitro [8]. Aside from this, both preclinical and clinical evidence has suggested apabetalone, an inhibitor of bromodomain and extra-terminal proteins targeting bromodomain 2, exerts a direct effect in downregulating cellular responses to VC in vitro and thus improving cardiovascular outcomes observed in clinical trials [9].
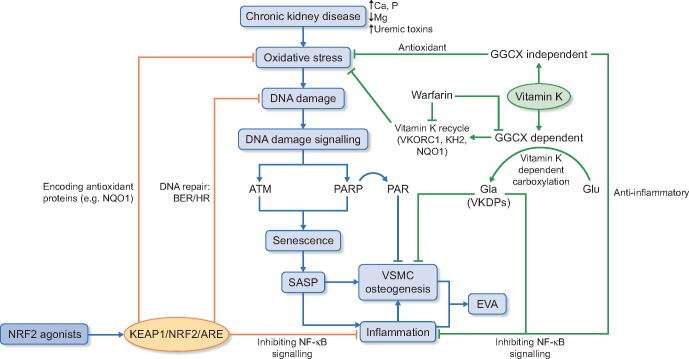
Despite the ongoing exploration of therapeutic strategies in VC, no treatments have so far proven to prevent or thoroughly reverse VC progression in CKD, thus fundamental solution to EVA is needed. Although the exact underlying mechanisms of EVA in CKD have not been fully elucidated, DNA damage and cellular senescence appear to play a fundamental role in its initiation and progression. Of note, concomitant with cellular senescence, another common feature of ageing-related conditions is persistent low-grade sterile inflammation, ‘inflammaging’ [10]. The source(s) of chronic inflammation in EVA remains to be determined, but one possible origin could be derived from the senescence-mediated senescence-associated secretory phenotype (SASP), characterized by a secretion profile with pro-inflammatory cytokines, growth factors and soluble receptors that poison the surrounding tissues and affect their function [11]. An emerging intriguing putative cause of EVA and ageing that need studies in CKD is clonal haematopoiesis [12]. In this review we discuss the pathophysiological links between senescence and EVA in the context of CKD, with a focus on the potential role of nuclear factor erythroid 2–related factor 2 (NRF2) and vitamin K in driving DNA damage, senescence and inflammaging in EVA (Figure 1).
FIGURE 1.
EVA in CKD, the role of NRF2 signalling and vitamin K in mediating DNA damage, senescence and inflammaging. BER, base excision repair; HR, homologous recombination repair.
DNA DAMAGE AND CELLULAR SENESCENCE IN EVA
The evidence of EVA manifested as media VC and artery stiffness in CKD and the role of cellular senescence in VSMC osteogenesis and calcification was recently reviewed [13]. The key component leading to cellular senescence is the accumulation and persistence of DNA damage. During normal cell proliferation, the DNA damage response (DDR) is activated to enable repair of DNA strand breaks as a consequence of exposure to genotoxic stressors and in response to telomeric DNA erosion [14]. However, if DNA damage exceeds critical levels, the cell will undergo cell death processes. At near-critical levels, the cell will undergo growth arrest and senescence [15]. In the uraemic milieu, DDR pathways become functionally deficient as a result of excessive allostatic overload (including excessive oxidative stress) and thus promote the generation of cellular senescence, and its consequential apoptosis-resistant phenotype [16]. Moreover, accumulating evidence has supported the essential role of DNA damage signalling in calcification. VSMCs cultured by serial passaging acquire DNA damage features, with cumulative γH2AX, 53BP1 foci and p16 expression and, strikingly, upregulated alkaline phosphatase activity and Runx2 expression in parallel [17, 18], suggesting a direct link between senescence and calcification. In addition, co-culture experiments with mesenchymal progenitor cells have shown that the activation of osteogenic differentiation was possibly mediated by secretory profiles of ageing VSMCs, i.e. SASP, including IL-6, bone morphogenetic protein 2 (BMP-2) and osteoprotegerin (OPG), which are crucial molecules in modulating calcification processes [18]. Such findings further imply that the existence of senescent VSMCs may not only drive osteogenic differentiation and calcification locally, but also induce VSMC calcification at remote sites, triggering both local and systemic stem cells to undergo osteogenic differentiation.
One of the major transducers in DNA damage signalling is ataxia telangiectasia-mutated (ATM) protein kinase, which upregulates cell cycle inhibitors and leads to cell cycle arrest and cellular senescence [14]. A recent study reported that VSMCs from children on dialysis showed increased DNA damage signalling with elevated γH2AX and phosphorylated ATM/ATM and Rad3-related levels compared with that from control subjects. In parallel, such increased expression was accompanied with increased p16 levels, suggesting VSMC senescence was mediated by ATM transduction in vitro [19]. Aside from this, ATM-mediated DNA damage signalling also plays an important role in osteogenic differentiation and calcification of VSMCs. Inhibition of this pathway prevents osteogenesis and calcification observed in VSMCs from dialysis patients [19]. More importantly, such a diminished calcification process was accompanied by a downregulated pattern of SASP expression, indicated by IL-6, BMP2 and OPG [19]. In vivo, ATM knockout mice displayed increased susceptibility to infections due to immunodeficiency and a premature ageing phenotype [20].
Another important transducer in DNA damage signalling—poly adenosine diphosphate (ADP) ribose (PAR)—exemplifies new insight into the role of DNA damage in VC. Biologically, PAR is synthesized by PAR polymerase (PARP) enzymes in response to oxidative stress and DNA damage and previous observations have indicated that PARP inhibition could block cell necrosis and osteogenic differentiation [21]. Recent data show that PAR was present in the extracellular matrix of both physiological bone formation and pathological VSMC calcification in vitro and in vivo and that the inhibition of PARP enzymatic activity reduced mineralization [22]. Such a finding has further promoted the notion that PARP-mediated DNA damage signalling can regulate osteoblast and VSMC osteogenic differentiation and that the generation of PAR plays a key role in both bone and vasculature mineralization. A better understanding of PARP in VC is needed and it is tempting to speculate that PARP inhibitors may provide new therapeutic target for EVA.
NRF2 SIGNALLING AND EVA
Strong evidence indicates that EVA is associated with increased production of reactive oxygen species (ROS) and diminished capacity to repair oxidative DNA damage in the vascular wall. Numerous endogenous antioxidant systems are in place to counteract oxidative stress, among which, NRF2/Kelch-like ECH-associated protein 1 (KEAP1)-dependent antioxidant defence pathway is of particular importance. We have previously discussed lessons from evolution and the animal kingdom, as well as clinical models of accelerated ageing processes, illustrating the key role of the evolutionary NRF2/KEAP1 system in premature ageing in detail [23].
The NRF2/KEAP1 element pathway
NRF2 is an evolutionary transcription factor regulating a broad array of cytoprotective genes involved in antioxidant and anti-inflammatory defences, detoxification, protein homoeostasis and anti-ageing process. In basal circumstances, NRF is bound by its negative modulator KEAP1 in the cytosol. KEAP is a substrate adapter protein targeting NRF2 for proteasomal degradation. ROS or electrophiles-induced oxidation of critical cysteine residues on KEAP1 causes modification in its tertiary structure and leads to detachment of NRF2 from KEAP1, allowing the subsequent translocation of NRF2 from cytosol to the nucleus, where it binds to conserved antioxidant response element (ARE) sequences in the promotor region of the NRF2 targeting genes. While the activation NRF2 further requires the binding of small Maf proteins, negative regulators of NRF2 activation such as Bach1 bind to Maf proteins as an antagonist. Classic NRF2 target genes encode hundreds of antioxidant proteins, such as catalase, haeme oxygenase 1, glutathione peroxidases, NAD(P)H quinone dehydrogenase (NQO1), glutathione S-transferases, peroxiredoxins, thioredoxins, thioredoxin reductases and glutamate-cysteine ligase.
NRF2 signalling in senescence and EVA
It has been suggested that NRF2 transcriptional activity declines with ageing in the vasculature, resulting in a broad range of premature vascular ageing phenotypes, including EVA [24, 25]. Preclinical studies have shown that ageing impairs the efficiency of NRF2-dependent antioxidant defences, resulting in more oxidative stress and damage in aged cells than in young cells [24, 25]. Critical for the scope of the present discussion, it is important to note that the protective impact of NRF2 on cellular senescence and ageing is not only dependent on its antioxidant function, as mentioned above, but protection can be immediate, as reflected by induction of genes regulated by AREs, secondary through induction macromolecular damage repair or tertiary through activation of regeneration/tissue repair pathways. There is emerging evidence that NRF2 also modulates cellular DNA repair signalling and counteracts DNA damage induced by oxidative stressors. In fact, NRF2 signalling is directly involved in regulating genes related to DNA damage repair, including homologous recombination repair and base excision repair mechanisms [26]. Ribonucleic acid (RNA) interference experiments have shown that NRF2 dysfunction associates with downregulated DNA damage repair and primary epithelial cultures from mice genetically depleted of NRF2 presented with persistent DNA damage [27, 28].
The mechanisms underlying dysregulation of NRF2 signalling in cellular senescence and ageing are multifaceted. First, expression of NRF2 is modulated by microRNAs [29]. Ageing-induced dysfunction of microRNA expression in endothelial cells from aged rats has been shown to correlate with downregulation of NRF2 [27]. Second, the activation and nuclear translocation of NRF2 may also be diminished in the context of ageing. For instance, age-related alterations in post-translational protein modifications in cysteine residues in KEAP1 and phosphorylation/acetylation of NRF2 can disrupt the activation and release of NRF2. Third, ageing may also interfere with NRF2 binding cofactors (e.g. Maf proteins), thereby impairing the transcription of NRF2 target genes. Indeed, cells from patients with Hutchinson–Gilford progeria syndrome demonstrate that progerin sequesters NRF2, resulting in nuclear mislocalization and impaired programming of NRF2 transcriptional activity [30]. Another example of competing Maf binding is NRF2/NF-κΒ signalling. It has been shown that NRF2 dysfunction in VSMCs is linked with NF-κΒ activation and persistent sterile vascular inflammation Moreover, circulating molecules such as insulin-like growth factor 1 (IGF-1) and klotho also contribute to NRF2 signalling in EVA. For instance, decreased circulating IGF-1 associates with NRF2 dysfunction in arteries from mice, and klotho, a potent anti-ageing factor, can magnify its protective effects on vascular cells via activating NRF2 [31]. In addition, recent data have demonstrated the direct role of NRF2 in cellular senescence since Fulop et al. [32] reported that genetic depletion of NRF2 exacerbated age-related induction of senescence markers and SASP factors in elevated inflammatory status of the hippocampus, which elegantly illustrated the role of NRF2 in senescence and inflammaging. Rodent models have also shown that obesity-induced oxidative stress was exacerbated in genetically NRF2-deficient mice, partly mediated by accelerated cellular senescence [33].
NRF2 signalling and VC
Aside from the role of NRF2 signalling in senescence and ageing, adaptive activation of NRF2 has been demonstrated in response to various inducers in phenotypic remodelling of VSMCs and numerous agonists of NRF2 signalling have proven to be effective in alleviating the pathological VSMC osteogenesis in VC. A recent study reported that butylhydroquinone, a classic NRF2 agonist, significantly improves high phosphate–induced calcification of VSMC via activation of KEAP1/NRF2/P62 signalling and repressing ROS production [34]. Dimethyl fumarate also attenuates hyperphosphataemia-induced VSMC calcification in vitro and vitamin D3–induced VC in vivo in a manner of stimulating NRF2 activity [35]. Resveratrol, a scavenger for free radicals, can also ameliorate oxidative injury in rat VSMCs undergoing calcification, by activating sirtuin-1 and NRF2 [36]. Interestingly, another NRF2 agonist, hydrogen sulphide, has now been re-recognized for its beneficial role in mediating trans-sulphuration of KEAP1 and stimulating nuclear translocation of NRF2. Indeed, hydrogen sulphide attenuates circulating calciprotein particle (CPP)-induced VSMC calcification in vitro via the NRF2/KEAP1 signalling system, by enhancing NQO1 expression [37]. Taken together, these emerging data have implied that stimulating NRF2/KEAP1 signalling can attenuate VSMC calcification in response to different inducers, which could potentially provide new insights for VC treatment strategies.
VITAMIN K AND AGEING
Vitamin K is a group name for substances that share the ability to serve as cofactors for the microsomal enzyme γ-glutamyl carboxylase (GGCX). Natural forms of vitamin K are vitamin K1 (phylloquinone) and vitamin K2 [menaquinones (MKs)]. Phylloquinone is obtained exclusively via the diet (e.g. leafy green vegetables and dairy produce), whereas menaquinones are synthesized by anaerobic bacteria. The beneficial role of vitamin K in health has far exceeded its function as a GGCX-dependent hepatic clotting factor in coagulation. Post-translational protein modification or vitamin K–dependent carboxylation is crucial in converting glutamic acid residues (Glu) into γ-carboxyglutamate (Gla), also known as vitamin K–dependent proteins (VKDPs). The conversion and activation of extrahepatic Gla proteins play an important role in multiple physiopathological processes of energy metabolism, inflammation, bone minimization and ectopic VC. More importantly, emerging evidence has revealed the multifunctional capacity of vitamin K with its antioxidant and anti-inflammatory effects, independent from being a cofactor for GGCX. Given the growing data illustrating the key role of vitamin K in GGCX-dependent carboxylation of VKDPs and GGCX-independent effects of being anti-inflammatory and antioxidant, it is not surprising that vitamin K might be beneficial to cardiovascular health and ageing. Indeed, the clinical role of vitamin K in several age-related chronic diseases has been well illustrated [38]. Since vitamin K1 lowered vascular inflammation by regulating NF-κB/NRF transcription factors via activation of vitamin K–dependent Gla proteins [39], the effects of vitamin K antagonists of NRF2 expression need to be studied.
Accelerated ageing processes often manifest with CVD complications, muscle wasting, osteoporosis and frailty, concomitant with a chronic, low-grade inflammation. A large body of epidemiological studies has shown the possible involvement of vitamin K in ageing-related disease phenotypes. Data from the Health ABC study indicate that a lower vitamin K status measured by vitamin K1 and desphospho-uncarboxylated matrix Gla-protein (dp-ucMGP) was associated with reduced lower extremity function during 4–5 years of follow-up [40]. The prospective data analysis from the Longitudinal Aging Study Amsterdam, a cohort study with 13-year follow-up, found that poor vitamin K status indicated by high dp-ucMGP levels at baseline was associated with a higher frailty index score in ageing subjects [41]. With regard to the effect of vitamin K in osteoporosis, the results from different clinical studies remain equivocal. While some studies conducted in the Japanese population have shown that high doses of vitamin K2 are effective in the prevention and treatment of osteoporosis, data from a systemic review and meta-analysis including 36 randomized controlled trials concluded that vitamin K had no effect on vertebral fracture incidences among postmenopausal and osteoporotic patients [42]. Given the variability and heterogeneity of vitamin K supplementation involved in different studies, it can be speculated that different forms and dosages of vitamin K may have different efficacy and that a higher dose of vitamin K supplement is most likely to ensure a better carboxylation of osteocalcin that is crucial for bone formation. Such an assumption has indeed been validated in a recent prospective cohort study evaluating the dose effect of MK-4 on osteocalcin carboxylation among postmenopausal women with osteoporotic fractures. The researchers concluded that high MK-4 was more effective in osteocalcin carboxylation with no major side effects [43]. Although the underlying mechanism behind the association between vitamin K and an ageing phenotype has not been clearly illustrated, such epidemiological evidence at least highlights the beneficial effect of vitamin K over and above its role as a nutritional remedy or supplement.
Potential role of vitamin K in senescence
As previously mentioned, excessive levels of ROS results in DNA damage, mitochondrial dysfunction and cellular senescence in the context of CKD, coincident with a chronic and low-grade inflammatory status (i.e. inflammaging), largely mediated by SASP factors. Although no direct effect of vitamin K in cellular senescence processes has been identified, it is tempting to speculate that vitamin K exerts an important role in cellular senescence given its antioxidant and anti-inflammatory effects. Here the vitamin K cycle is of particular interest. In vertebrates, the paralogous enzyme of vitamin K epoxide reductase complex subunit1 (VKORC1), VKORC1-like 1, has been found to mitigate intracellular oxidative damage [44]. Moreover, vitamin K1-hydroquinone (KH2), aside from being a cofactor for GGCX and vitamin K–dependent carboxylation, also acts as a strong radical scavenger, exerting 10–100 times more potent antioxidant capacity compared with other agents, including vitamin E [45]. Additionally, NQO1, one of the enzymes catalyzing the reduction of vitamin K to KH2, competes with other enzymes in the vitamin K redox cycle via skipping formation of semiquinone and ROS, thereby protecting cells against oxidative stress and cytotoxicity [46]. Intriguingly, it is worth noting that NQO1, one of the classic antioxidant proteins encoded by an NRF2 target gene, could serve as a link between NRF2 signalling and the vitamin K cycle, although a role in this context remains to be defined. Apart from being an antioxidant, the role of vitamin K in mitochondrial function also deserves attention since vitamin K2 acts as a mitochondrial carrier in Drosophila, where mitochondrial dysfunction was rescued by vitamin K2 addition via restoration of adenosine triphosphate production [47].
The anti-inflammatory activity of vitamin K is likely to have a role in cellular senescence. Based on our previously reported inverse association between low levels of carboxylated active osteocalcin and high vascular CDKN2A/p16INK4a expression [48], it can be theorized that deficiency of vitamin K promotes vascular senescence. Preclinical studies have shown that MK-4 substantially reduced lipopolysaccharide (LPS)-induced activation of NF-κΒ in cultured macrophage-like THP-1 cells via inhibiting IκB kinase α/β phosphorylation in a warfarin-independent manner. Addition of vitamin K1 improved the inflammatory status induced by LPS in rodent models [49]. Moreover, substantial clinical data show an inverse association between vitamin K status and circulating inflammatory markers. For instance, in the Framingham Offspring Study, vitamin K status evaluated by plasma phylloquinone concentration and phylloquinone intake, was negatively correlated with inflammatory markers, suggesting the potential role of vitamin K in inflammation [50]. Data from the Multi-Ethnic Study of Atherosclerosis also showed that serum K1 was inversely associated with several circulating inflammatory markers, such as IL-6, C-reactive protein and intercellular adhesion molecule 1 [51]. Taken together, these data support the anti-inflammatory effect of vitamin K both at the molecular and systemic levels, and more importantly, its potential and protective role in inflammaging.
Vitamin K, warfarin and VC
Evidence from preclinical studies has established a role for vitamin K in VC, mainly through the carboxylation of VKDP MGP. Initial work on the biology of MGP was conducted in a knockout mouse model, where MGP-deficient mice developed extensive calcification both in arteries and cartilage within 2 months [52]. With the knowledge that MGP is vitamin K dependent, further work evaluating the effect of vitamin K antagonist, primarily warfarin, was continued. Warfarin-treated mice developed coronary artery disease in vivo with vulnerable plaque phenotype and vitamin K administration prevented and reversed VC progression, further suggesting vitamin K–dependent MGP was inhibited by warfarin [53]. Such a relationship between vitamin K, warfarin and VC has also been noted in clinical observations. Clinical and epidemiological studies have also indicated that long-term warfarin treatment increases the risk of VC in certain individuals, possibly as a result of poor vitamin K status [54]. Moreover, a recent cross-sectional observational study revealed that while use of vitamin K antagonists contributes to the presence of VC, such an effect was not observed in non-vitamin K antagonist oral anticoagulant users, implying the exclusive role of vitamin K metabolism in VC development [55].
In CKD, vitamin K deficiency is highly prevalent and associated with VC and cardiovascular mortality [56]. A prospective randomized clinical trial performed among 200 prevalent haemodialysis patients showed a dose-dependent decrease of dp-ucMGP by MK7 supplementation [57]. Nevertheless, the recent Valkyrie Trail showed that whereas withdrawal of vitamin K antagonists and high-dose vitamin K2 supplement did improve vitamin K status in patients on haemodialysis with atrial fibrillation, no significant effect was observed in preventing VC progression evaluated by thoracic aorta calcium score and pulse wave velocity [58]. Given the multifaceted aetiology of VC, it is likely that a single intervention cannot redeem the VC process and the exact role of vitamin K (K1 versus K2) in the complexity of the VC process remains to be delineated. More solid perspectives and results are to be expected from ongoing randomized clinical trials (e.g. VItaK-CAC trial, VitaVask, the Aortic Valve DECalcification trial) evaluating the effect of vitamin K supplementation in VC progression among CKD population [59, 60].
CONCLUSION
To better develop a novel therapeutic strategy targeting EVA in patients with CKD, it is imperative to clarify the fundamental mechanisms underlying the premature ageing process and associated inflammaging. Here we have discussed ATM/PARP signalling–mediated DNA damage and subsequent cellular senescence, which may elucidate such pathological conditions characterized by accelerated EVA. Growing evidence suggests that NRF2 signalling and vitamin K play a crucial role in counteracting oxidative stress, DNA damage, cellular senescence and inflammaging, whereby NRF2 activation and vitamin K supplementation may provide novel treatment targets for EVA. Future studies testing the long-term protective effects of NRF2 and vitamin K in EVA and senescence-related endpoints are warranted and a combinative treatment with senotherapeutic agents could also be informative.
FUNDING
The study benefited from generous support from the Strategic Research Program in Diabetes at Karolinska Institutet (Swedish Research Council grant 2009-1068), the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement 722609), International Network for Training on Risks of Vascular Intimal Calcification and roads to Regression of Cardiovascular Disease (INTRICARE; www.intricare.eu), the Heart and Lung Foundation (P.S.), CIMED (P.S.), Njurfonden (P.S.) and Westmans Foundation (P.S.).
CONFLICT OF INTEREST STATEMENT
P.S. is on scientific advisory boards of REATA, Baxter and AstraZeneca. L.J.S. is a consultant for Nattopharma ASA and IDS. The other authors do not report any conflicts of interest.
REFERENCES
- 1. Stenvinkel P, Larsson TE.. Chronic kidney disease: a clinical model of premature aging. Am J Kidney Dis 2013; 62: 339–351 [DOI] [PubMed] [Google Scholar]
- 2. Kooman JP, Kotanko P, Schols A. et al. Chronic kidney disease and premature ageing. Nat Rev Nephrol 2014; 10: 732–742 [DOI] [PubMed] [Google Scholar]
- 3. Cunha PG, Boutouyrie P, Nilsson PM. et al. Early vascular ageing (EVA): definitions and clinical applicability. Curr Hypertens Rev 2017; 13: 8–15 [DOI] [PubMed] [Google Scholar]
- 4. Shanahan CM. Mechanisms of vascular calcification in CKD—evidence for premature ageing? Nat Rev Nephrol 2013; 9: 661–670 [DOI] [PubMed] [Google Scholar]
- 5. Wu M, Rementer C, Giachelli CM.. Vascular calcification: an update on mechanisms and challenges in treatment. Calcif Tissue Int 2013; 93: 365–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Raggi P, Bellasi A, Bushinsky D. et al. Slowing progression of cardiovascular calcification with SNF472 in patients on hemodialysis: results of a randomized, phase 2b study. Circulation 2019; doi: 10.1161/CIRCULATIONAHA.119.044195 [DOI] [PubMed] [Google Scholar]
- 7. Voelkl J, Luong TTD, Tuffaha R. et al. SGK1 induces vascular smooth muscle cell calcification through NF-κB signaling. J Clin Invest 2018; 128: 3024–3040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Carracedo M, Witasp A, Qureshi AR. et al. Chemerin inhibits vascular calcification through ChemR23 and is associated with lower coronary calcium in chronic kidney disease. J Intern Med 2019; 286: 449–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gilham D, Tsujikawa LM, Sarsons CD. et al. Apabetalone downregulates factors and pathways associated with vascular calcification. Atherosclerosis 2019; 280: 75–84 [DOI] [PubMed] [Google Scholar]
- 10. Franceschi C, Garagnani P, Parini P. et al. Inflammaging: a new immune–metabolic viewpoint for age-related diseases. Nat Rev Endocrinol 2018; 14: 576–590 [DOI] [PubMed] [Google Scholar]
- 11. Muñoz-Espín D, Serrano M.. Cellular senescence: from physiology to pathology. Nat Rev Mol Cell Biol 2014; 15: 482–496 [DOI] [PubMed] [Google Scholar]
- 12. Jaiswal S, Ebert BL.. Clonal hematopoiesis in human aging and disease. Science 2019; 366: eaan4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dai L, Qureshi AR, Witasp A. et al. Early vascular ageing and cellular senescence in chronic kidney disease. Comput Struct Biotechnol J 2019; 17: 721–729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shiloh Y, Ziv Y.. The ATM protein kinase: regulating the cellular response to genotoxic stress, and more. Nat Rev Mol Cell Biol 2013; 14: 197–210 [PubMed] [Google Scholar]
- 15. Shiels PG, Davies RW.. Ageing and the death of neurones In: Davies FR, Morris BJ (eds). Molecular Biology of the Neuron. New York: Oxford University Press, 2004: 439–468 [Google Scholar]
- 16. Vaziri ND. Oxidative stress in uremia: nature, mechanisms, and potential consequences. Semin Nephrol 2004; 24: 469–473 [DOI] [PubMed] [Google Scholar]
- 17. Ragnauth CD, Warren DT, Liu Y. et al. Prelamin A acts to accelerate smooth muscle cell senescence and is a novel biomarker of human vascular aging. Circulation 2010; 121: 2200–2210 [DOI] [PubMed] [Google Scholar]
- 18. Liu Y, Drozdov I, Shroff R. et al. Prelamin A accelerates vascular calcification via activation of the DNA damage response and senescence-associated secretory phenotype in vascular smooth muscle cells. Circ Res 2013; 112: e99–e109 [DOI] [PubMed] [Google Scholar]
- 19. Sanchis P, Ho CY, Liu Y. et al. Arterial ‘inflammaging’ drives vascular calcification in children on dialysis. Kidney Int 2019; 95: 958–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Barlow C, Hirotsune S, Paylor R. et al. Atm-deficient mice: a paradigm of ataxia telangiectasia. Cell 1996; 86: 159–171 [DOI] [PubMed] [Google Scholar]
- 21. Robaszkiewicz A, Erdélyi K, Kovács K. et al. Hydrogen peroxide-induced poly(ADP-ribosyl)ation regulates osteogenic differentiation-associated cell death. Free Radic Biol Med 2012; 53: 1552–1564 [DOI] [PubMed] [Google Scholar]
- 22. Müller KH, Hayward R, Rajan R. et al. Poly(ADP-ribose) links the DNA damage response and biomineralization. Cell Rep 2019; 27: 3124–3138.e13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stenvinkel P, Meyer CJ, Block GA. et al. Understanding the role of the cytoprotective transcription factor nuclear factor erythroid 2–related factor 2—lessons from evolution, the animal kingdom and rare progeroid syndromes. Nephrol Dial Transplant 2019; doi: 10.1093/ndt/gfz120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ungvari Z, Bailey-Downs L, Gautam T. et al. Age-associated vascular oxidative stress, Nrf2 dysfunction, and NF-κB activation in the nonhuman primate Macaca mulatta. J Gerontol A Biol Sci Med Sci 2011; 66A: 866–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ungvari Z, Bailey-Downs L, Sosnowska D. et al. Vascular oxidative stress in aging: a homeostatic failure due to dysregulation of NRF2-mediated antioxidant response. Am J Physiol Heart Circ Physiol 2011; 301: H363–H372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jayakumar S, Pal D, Sandur SK.. Nrf2 facilitates repair of radiation induced DNA damage through homologous recombination repair pathway in a ROS independent manner in cancer cells. Mutat Res 2015; 779: 33–45 [DOI] [PubMed] [Google Scholar]
- 27. Csiszar A, Gautam T, Sosnowska D. et al. Caloric restriction confers persistent anti-oxidative, pro-angiogenic, and anti-inflammatory effects and promotes anti-aging miRNA expression profile in cerebromicrovascular endothelial cells of aged rats. Am J Physiol Heart Circ Physiol 2014; 307: H292–H306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Reddy NM, Kleeberger SR, Bream JH. et al. Genetic disruption of the Nrf2 compromises cell-cycle progression by impairing GSH-induced redox signaling. Oncogene 2008; 27: 5821–5832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cheng X, Ku C-H, Siow R.. Regulation of the Nrf2 antioxidant pathway by microRNAs: new players in micromanaging redox homeostasis. Free Radic Biol Med 2013; 64: 4–11 [DOI] [PubMed] [Google Scholar]
- 30. Kubben N, Zhang W, Wang L. et al. Repression of the antioxidant NRF2 pathway in premature aging. Cell 2016; 165: 1361–1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bailey-Downs LC, Mitschelen M, Sosnowska D. et al. Liver-specific knockdown of IGF-1 decreases vascular oxidative stress resistance by impairing the Nrf2-dependent antioxidant response: a novel model of vascular aging. J Gerontol A Biol Sci Med Sci 2012; 67A: 313–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fulop GA, Kiss T, Tarantini S. et al. Nrf2 deficiency in aged mice exacerbates cellular senescence promoting cerebrovascular inflammation. GeroScience 2018; 40: 513–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tarantini S, Valcarcel-Ares MN, Yabluchanskiy A. et al. Nrf2 deficiency exacerbates obesity-induced oxidative stress, neurovascular dysfunction, blood-brain barrier disruption, neuroinflammation, amyloidogenic gene expression, and cognitive decline in mice, mimicking the aging phenotype. J Gerontol A Biol Sci Med Sci 2018; 73: 853–863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wei R, Enaka M, Muragaki Y.. Activation of KEAP1/NRF2/P62 signaling alleviates high phosphate-induced calcification of vascular smooth muscle cells by suppressing reactive oxygen species production. Sci Rep 2019; 9: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ha CM, Park S, Choi YK. et al. Activation of Nrf2 by dimethyl fumarate improves vascular calcification. Vascul Pharmacol 2014; 63: 29–36 [DOI] [PubMed] [Google Scholar]
- 36. Zhang P, Li Y, Du Y. et al. Resveratrol ameliorated vascular calcification by regulating Sirt-1 and Nrf2. Transplant Proc 2016; 48: 3378–3386 [DOI] [PubMed] [Google Scholar]
- 37. Aghagolzadeh P, Radpour R, Bachtler M. et al. Hydrogen sulfide attenuates calcification of vascular smooth muscle cells via KEAP1/NRF2/NQO1 activation. Atherosclerosis 2017; 265: 78–86 [DOI] [PubMed] [Google Scholar]
- 38. Shea MK, Kritchevsky SB, Loeser RF. et al. Vitamin K status and mobility limitation and disability in older adults: the health, aging, and body composition study. J Gerontol A Biol Sci Med Sci 2019; doi: 10.1093/gerona/glz108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dihingia A, Ozah D, Baruah PK. et al. Prophylactic role of vitamin K supplementation on vascular inflammation in type 2 diabetes by regulating the NF-κB/Nrf2 pathway via activating Gla proteins. Food Funct 2018; 9: 450–462 [DOI] [PubMed] [Google Scholar]
- 40. Shea MK, Loeser RF, Hsu F-C. et al. Vitamin K status and lower extremity function in older adults: the health aging and body composition study. Gerona 2016; 71: 1348–1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Machado-Fragua MD, Hoogendijk EO, Struijk EA. et al. High dephospho-uncarboxylated matrix Gla protein concentrations, a plasma biomarker of vitamin K, in relation to frailty: the Longitudinal Aging Study Amsterdam. Eur J Nutr 2019; doi: 10.1007/s00394-019-01984-9 [DOI] [PubMed] [Google Scholar]
- 42. Mott A, Bradley T, Wright K. et al. Effect of vitamin K on bone mineral density and fractures in adults: an updated systematic review and meta-analysis of randomised controlled trials. Osteoporos Int 2019; 30: 1543–1559 [DOI] [PubMed] [Google Scholar]
- 43. K Giri T, Newton D, Chaudhary O. et al. Maximal dose-response of vitamin-K2 (menaquinone-4) on undercarboxylated osteocalcin in women with osteoporosis. Int J Vitam Nutr Res 2019; doi: 10.1024/0300-9831/a000554 [DOI] [PubMed] [Google Scholar]
- 44. Westhofen P, Watzka M, Marinova M. et al. Human vitamin K 2, 3-epoxide reductase complex subunit 1-like 1 (VKORC1L1) mediates vitamin K-dependent intracellular antioxidant function. J Biol Chem 2011; 286: 15085–15094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mukai K, Itoh S, Morimoto H.. Stopped-flow kinetic study of vitamin E regeneration reaction with biological hydroquinones (reduced forms of ubiquinone, vitamin K, and tocopherolquinone) in solution. J Biol Chem 1992; 267: 22277–22281 [PubMed] [Google Scholar]
- 46. Gong X, Gutala R, Jaiswal AK.. Quinone oxidoreductases and vitamin K metabolism. Vitam Horm 2008; 78: 85–101 [DOI] [PubMed] [Google Scholar]
- 47. Vos M, Esposito G, Edirisinghe JN. et al. Vitamin K2 is a mitochondrial electron carrier that rescues Pink1 deficiency. Science 2012; 336: 1306–1310 [DOI] [PubMed] [Google Scholar]
- 48. Stenvinkel P, Luttropp K, McGuinness D. et al. CDKN2A/p16INK4a expression is associated with vascular progeria in chronic kidney disease. Aging 2017; 9: 494–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ohsaki Y, Shirakawa H, Miura A. et al. Vitamin K suppresses the lipopolysaccharide-induced expression of inflammatory cytokines in cultured macrophage-like cells via the inhibition of the activation of nuclear factor κB through the repression of IKKα/β phosphorylation. J Nutr Biochem 2010; 21: 1120–1126 [DOI] [PubMed] [Google Scholar]
- 50. Shea MK, Booth SL, Massaro JM. et al. Vitamin K and vitamin D status: associations with inflammatory markers in the Framingham offspring study. Am J Epidemiol 2007; 167: 313–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Shea K, Cushman M, Booth S. et al. Associations between vitamin K status and haemostatic and inflammatory biomarkers in community-dwelling adults. Thromb Haemost 2014; 112: 438–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Luo G, Ducy P, McKee MD. et al. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature 1997; 386: 78–81 [DOI] [PubMed] [Google Scholar]
- 53. Schurgers LJ, Spronk HMH, Soute BAM. et al. Regression of warfarin-induced medial elastocalcinosis by high intake of vitamin K in rats. Blood 2007; 109: 2823–2831 [DOI] [PubMed] [Google Scholar]
- 54. Siltari A, Vapaatalo H.. Vascular calcification, vitamin K and warfarin therapy – possible or plausible connection? Basic Clin Pharmacol Toxicol 2018; 122: 19–24 [DOI] [PubMed] [Google Scholar]
- 55. Peeters F, Dudink E, Kimenai D. et al. Vitamin K antagonists, non–vitamin K antagonist oral anticoagulants, and vascular calcification in patients with atrial fibrillation. TH Open 2018; 2: e391–e398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Krueger T, Westenfeld R, Ketteler M. et al. Vitamin K deficiency in CKD patients: a modifiable risk factor for vascular calcification? Kidney Int 2009; 76: 18–22 [DOI] [PubMed] [Google Scholar]
- 57. Caluwe R, Vandecasteele S, Van Vlem B. et al. Vitamin K2 supplementation in haemodialysis patients: a randomized dose-finding study. Nephrol Dial Transplant 2014; 29: 1385–1390 [DOI] [PubMed] [Google Scholar]
- 58. De Vriese AS, Caluwé R, Pyfferoen L. et al. Multicenter randomized controlled trial of vitamin K antagonist replacement by rivaroxaban with or without vitamin K2 in hemodialysis patients with atrial fibrillation: the Valkyrie Study. J Am Soc Nephrol 2020; 31: 186–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Vossen LM, Schurgers LJ, van Varik BJ. et al. Menaquinone-7 supplementation to reduce vascular calcification in patients with coronary artery disease: rationale and study protocol (VitaK-CAC Trial). Nutrients 2015; 7: 8905–8915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lindholt JS, Frandsen NE, Fredgart MH. et al. Effects of menaquinone-7 supplementation in patients with aortic valve calcification: study protocol for a randomised controlled trial. BMJ Open 2018; 8: e022019. [DOI] [PMC free article] [PubMed] [Google Scholar]