Abstract
The adaptive response characterized by a biphasic curve is known as hormesis. In a hormesis framework, exposure to low doses leads to protective and beneficial responses while exposures to high doses are damaging and detrimental. Comparative physiologists have studied hormesis for over a century, but our understanding of hormesis is fragmented due to rifts in consensus and taxonomic-specific terminology. Hormesis has been and is currently known by multiple names; preconditioning, conditioning, pretreatment, cross tolerance, adaptive homeostasis, and rapid stress hardening (mostly low temperature: rapid cold hardening). These are the most common names used to describe adaptive stress responses in animals. These responses are mechanistically similar, while having stress-specific responses, but they all can fall under the umbrella of hormesis. Here we review how hormesis studies have revealed animal performance benefits in response to changes in oxygen, temperature, ionizing radiation, heavy metals, pesticides, dehydration, gravity, and crowding. And how almost universally, hormetic responses are characterized by increases in performance that include either increases in reproduction, longevity, or both. And while the field can benefit from additional mechanistic work, we know that many of these responses are rooted in increases of antioxidants and oxidative stress protective mechanisms; including heat shock proteins. There is a clear, yet not fully elucidated, overlap between hormesis and the preparation for oxidative stress theory; which predicts part of the responses associated with hormesis. We discuss this, and the need for additional work into animal hormetic effects particularly focusing on the cost of hormesis.
Keywords: dose response, antioxidants, life history, trade-offs, POS hypothesis
INTRODUCTION
The notion in the field of toxicology that the dose makes the poison, referencing dose responses and lethal effects, dates back to Paracelsus more than 500 years ago (Mattson and Calabrese 2010), and it is at the heart of the concept known as hormesis. The idea behind the term hormesis dates back to the 1800s when Hugo Schulz first described a low dose stimulatory response associated with a toxic agent; sodium hypochlorite (Calabrese and Baldwin 2000). But the term itself was first used to describe a low dose excitatory response of fungi to red cedar tree extracts (Southam and Ehrlich 1943, Calabrese 2014). As we use the term today, hormesis is defined as an adaptive biphasic dose response where low doses result in protective effects that can lead to improved organismal performance while high doses result in detrimental effects that lead to negative performance and fitness consequences (Calabrese and Baldwin 2001, Calabrese et al. 2007, Mattson 2008, Calabrese 2016a,b). In this framework, mild exposure to chemical, biotic, or abiotic stressors elicits an adaptive response that elevates cellular defenses and protects the organism. This protection is accompanied by a boost in performance that goes beyond that seen in untreated individuals as seen in our own hormetic dose response curve of Drosophila melanogaster exposed to x-ray irradiation (Fig. 1). Beyond toxicology and stress physiology, interest in this protective mechanism is growing in regard to human aging, the treatment of disease (Calabrese and Blain 2005, Calabrese et al. 2012), and exercise physiology (Ji et al. 2010, Ji et al. 2016). In this review, we will focus on how comparative animal physiologists have studied hormesis in non-human animals, and why there is additional need for mechanistic work elucidating how hormesis affects animal performance, as well as, the cost associated with this adaptive protective response.
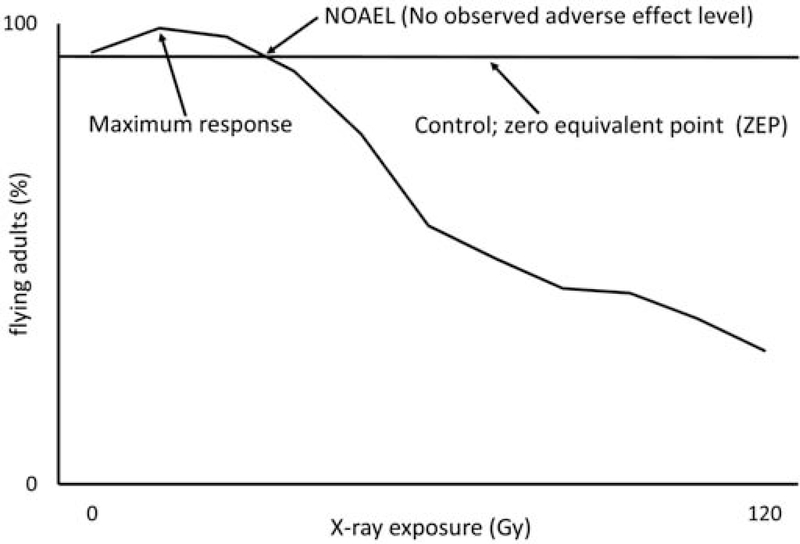
Figure 1.
X-ray irradiation dose response curve showing the effect of increasing doses on the flight ability of Drosophila melanogaster. The increased performance as a result of hormesis is seen at the lowest doses as a hormesis model would predict. Three quantitative effects of hormesis (zero equivalent point, maximum response, and no observed adverse effect level) are labelled based on Calabrese et al. 2012, and show the control effect, the hormetic effect, and the point where negative effects being, respectively.
In the comparative physiology literature, hormesis is known by different names and one of the biggest disconnects in stress physiology is the challenge that biological stress response terminology represents (Calabrese et al. 2007). Different subdisciplines focus on their own terminology to the disadvantage of an overall and universal term such as hormesis. Adaptive response and cross tolerance are the most common terms that displace hormesis in the literature. The term adaptive response refers to a plastic response, that occurs after exposure to mild doses of a toxic agent, aimed at the restoration of homeostasis (Samson and Cairns 1977). Similarly, adaptive homeostasis is used to explain the increased or decreased seen in the homeostatic range of an animal exposed to sub-lethal conditions (Davies 2016). While the term cross tolerance refers to the animal’s ability to defend against damage caused by stressor B after a brief exposure to stressor A (Gruber and Keyser 1945). All four terms fall under the banner of hormesis, as hormesis is an adaptive response to stress that elevates cellular defenses targeting the restoration of homeostasis; those elevated defenses can then provide additional protection from a second, often more challenging stressor (Mattson 2008). The terms preconditioning or pretreatment are also occasionally encountered in the comparative physiology literature. Under pretreatment, preconditioning, or conditioning a mild/brief stressor is applied to the animal followed by a stronger stressor or cellular damaging event. The pretreatment is meant to elevate the cellular defenses to offer protection from the stronger stressor. The way we define and use pretreatment and preconditioning is functionally no different than the usage of cross tolerance. Catfish pretreated with hypoxia (low oxygen) being able to survive higher temperatures (Burleson and Silva 2011) and anoxia protecting flies from gamma radiation (Fig. 2; López-Martínez and Hahn 2012, 2014) are just a couple of examples of this type of hormesis. What clusters these approaches together is that they rely on the adaptive response to low doses that is hormesis.
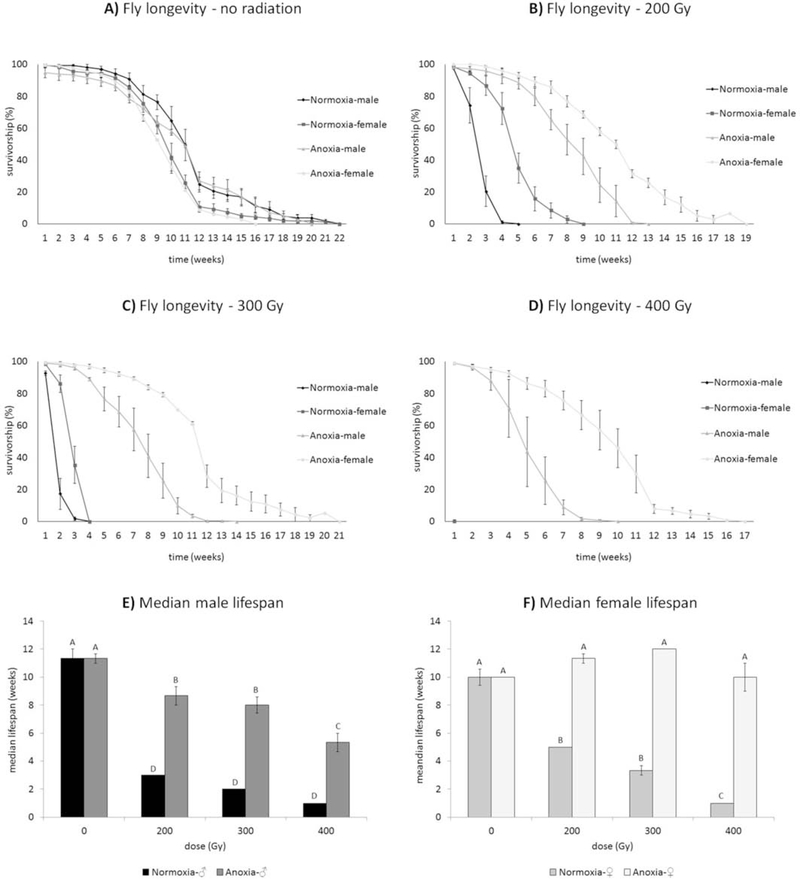
Figure 2.
Anoxia hormesis protects from irradiation-induced oxidative damage in a cross tolerance hormetic framework. The strong protective effect of anoxia was evident at all doses (B, C, & D) tested but was more dramatic at 400 Gy (D), where the irradiated control flies die within 24 hrs. but anoxia hormesis flies live more than two months. Sex differences can be seen at all doses and females tend to live longer at higher doses (F; Figure 2 from López-Martínez and Hahn 2014).
One of the central tenets of hormesis is that it allows for cellular protection to build up during the mild dose exposure, and these defenses are present for some time following the end of the exposure (López-Martínez and Hahn 2012). It is these defenses that prevent the accumulation of stress-induced damage and therefore improve organismal performance over time. At the core of this mechanism of cellular protection is the mitochondria. It is hypothesized that reactive oxygen species (ROS) are disproportionately generated during bouts of stress (Halliwell and Gutteridge 1993), and during mild stress, these ROS serve as signaling molecules that promote normal functioning (Ristow and Schmeisser 2014, Sies 2017). In fact, it may be small amounts of ROS production that are a crucial part of the adaptive response we call hormesis (Huang et al. 2019). These ROS are thought to signal that the resumption of normal metabolism will be accompanied by higher and damaging levels of ROS production, and the ensuing oxidative damage, thus activating cellular defenses. It is this mechanism that is described as the Preparation for Oxidative Stress (POS) hypothesis (Hermes-Lima et al. 1998, Hermes-Lima and Zenteno-Savin 2002, Giraud-Billoud et al. 2019). And because there is ample evidence that animals prepare for oxidative stress when exposed to mild stress (Storey 1996, Giraud-Billoud et al. 2019), there is an overlap between the POS hypothesis and hormesis that is not yet currently fully understood.
OXYGEN HORMESIS
Low oxygen has been one of the leading stressors known to confer hormetic effects dating back at least 45 years (Robinson 1975). The benefits of low oxygen are mechanistically rooted in the POS hypothesis (Giraud-Billoud et al. 2019), where the mitochondria upon experiencing a decrease in oxygen, prepares for the ensuing oxygen reperfusion by elevating cellular defenses; primarily oxidative stress defenses (Hermes-Lima et al. 1998, 2001). These defenses often exceed what is required to compensate for oxygen reperfusion damage and the animal benefits from the additional protective boost. In addition to the multiple scenarios where an animal may encounter hypoxia in their habitat (under water, underground, inside hosts, high altitudes, etc.), many animals possess varying degrees of tolerance to a total lack of oxygen; anoxia (Storey 1996). The bulk of the low oxygen hormesis work has focused in anoxia and it is connected to the remarkable tolerance that some animals have to an oxygen free environment. Vertebrates do not have the robust tolerance for long periods in anoxia at physiological-relevant temperatures (above 20ºC) that invertebrates have (Storey 1996), still we find that poikilothermic vertebrates are able to survive prolonged periods of anoxia (i.e. more than 24hrs), if the exposure occurs at low temperatures normally associated with overwintering responses. When temperatures range from 3 to 5ºC, red-sided garter snakes, Thamnophis sirtalis parietalis, and leopard frogs, Rana pipiens, can survive several days of anoxia (Churchill and Storey 1992, Pinder et al., 1992), while red-eared slider turtles, Trachemys scripta elegans, experience anoxia for three to four months during their overwintering period (Hermes-Lima and Zenteno-Savín 2002). This tolerance to an oxygen free environment for such a long period is connected to increased activities of various antioxidant enzymes (Hermes-Lima and Zenteno-Savín 2002). An impressive anoxic response is seen in the goldfish, Carassius auratus, where individuals can survive an eight-hour exposure at physiologically relevant temperatures (20ºC; Lushchak et al. 2001). On the other hand, invertebrates encounter anoxia at higher degrees due to their soildwelling stages and their semi-aquatic ecologies, and they have evolved adaptations to prevent damage from oxygen deprivation (Storey 1996, Harrison et al. 2006). Because of this, invertebrates can tolerate longer periods of anoxia at physiological temperatures. The Caribbean fruit fly, Anastrepha suspensa, can survive upwards of 50 hours at 25ºC without negatively impacting flight performance (López-Martínez and Hahn 2012). On the more extreme side, larvae tiger beetles in the genus Cicindela can survive more than six days in anoxia, which is usually accompanied by being submerged in water (Hoback et al. 1998). For both invertebrates and vertebrates, survival of prolonged periods of anoxia is a remarkable physiological feat, nevertheless, most of this work has focused on extended anoxia tolerance and not on hormesis. While these animals may gain survival, longevity, and/or higher reproductive outputs from their anoxia exposure; whether these exposures lead to hormesis remains largely unexplored. In a hormetic framework, it is short bouts of anoxia exposure (minutes to a few hours) that will trigger protective mechanisms that confer defense and boost performance.
Most of what we know about anoxia hormesis comes from cross tolerance experiments where anoxia is used as a preconditioning treatment prior to exposure to sub-lethal or lethal doses of stress. In the locust, Locusta migratoria, thermotolerance increases after a short (1 hr.) exposure to anoxia (Wu et al. 2002). Locust that experienced anoxia can survive up to 1.5 hours at 53ºC; a benefit that is connected to the long flights experienced during migration that are accompanied by increased oxygen demand and high temperatures. On the other temperature extreme we have house flies, Musca domestica, that are able to survive at −7ºC after a short exposure (40 minutes) to anoxia (Coulson and Bale 1992). In this context, anoxia acts as the mild temperature pretreatment that triggers the adaptive hormetic response known as rapid stress hardening (RSH; described in the next section). Anoxia hormesis can also protect from additional exposure to anoxia. In Anastrepha suspensa, anoxia experienced during development triggers the reallocation of stored lipids and changes the dynamics of recovery by reducing the oxygen debt, without decreasing adult fecundity and longevity (Visser et al. 2018).
When it comes to anoxia hormesis, the most understood protective effects that boost animal performance come from work that examines the effects of anoxia when combined with a strong oxidizing event; ionizing radiation. Whether using gamma radiation or X-rays, short exposure (1 hr.) of anoxia prior to irradiation leads to significant improvements in organismal performance. The first recorded evidence of this was in codling moths, Cydia pomonella, where an oxygen free environment led to survival of normally lethal gamma radiation doses (Robinson 1975). This prompted an interest in exploring whether hypoxia could have similar protective effects and in fact low oxygen is results in improved treatment survivorship, flight performance, and mating competitiveness in Mediterranean fruit flies, Ceratitis capitata (Hooper 1971, Ohinata et al. 1977, Nestel 2007). In another fruit fly, A. suspensa, the mechanism of anoxia hormesis involves the upregulation of various antioxidant enzymes (mitochondrial and cytosolic SODs and glutathione peroxidase), which help lower radiation-induced oxidative stress and increase flight ability, starvation resistance, mating success, and longevity (López-Martínez and Hahn 2012, 2014). The increases in longevity were significant, allowing flies that would die in a matter of hours to live for weeks (Fig. 2). This mechanistic finding of how anoxia confers its hormetic benefits also provides additional support to the preparation for oxygen stress hypothesis as the same mitochondrial protection mechanisms are involved in both responses, providing further evidence for the link between hormesis and the POS hypothesis (Giraud-Billoud et al. 2019, Geihs et al. 2020). While most of these anoxia hormetic effects are male-specific, females survive higher doses of gamma radiation when combined with anoxia conditioning (López-Martínez and Hahn 2012). The benefits extend into mating, where male mating success is higher in anoxia-irradiated males at the peak of sexual maturity (10 days after treatment), but the males remain sexually competitive into old age (30 days); mating at a higher rate (19:1 at 30 days vs 3:1 at 10 days) than there non-hormetic counterparts (López-Martínez and Hahn 2014). Similar effects were recorded in the cactus moth, Cactoblastis cactorum, where anoxia prior to X-ray irradiation improves flight performance (López-Martínez et al. 2014). These male moths were more likely to fly, flew for longer periods of time, and for further distances. Additionally, the male moths had increased antioxidant capacity, which was linked with increased mating success, living longer, and higher F1 progeny hatching. In Trichoplusia ni moths, anoxia hormesis rescues gamma radiation induced mortality (López-Martínez et al. 2016b).
Most of these anoxia hormetic effects were male-specific and the likely reason is that females bear a strong cost of hormesis that has been harder to quantify in males. Dating back to Robinson’s work, he found that the anoxia-mediated survival of lethal gamma radiation doses experienced by the moths came with a reduction of F1 offspring (~10% less; Robinson 1975). Even though he did not present his findings in a hormesis context, this represents the first recorded cost of anoxia hormesis and one of the first ever recorded costs associated with any type of hormesis. Since then, other groups have found that the cost of anoxia hormesis is connected to reproduction. In the cabbage looper moth T. ni, females receiving anoxia hormesis (in the absence of cross tolerance), experienced a significant decreased in the number of eggs laid (~60%), and an additional decrease in the number of laid eggs that hatched (~70%; López-Martínez et al. 2016b). This reduction in fecundity and fertility associated with anoxia hormesis was also recently found in the mealworm beetle, Tenebrio molitor. Female beetles that lived longer and were more active during old age as a result of anoxia hormesis, experience a total reproduction output decline of 40% (De La Torre and López-Martínez unpublished). This suggests that the anoxia hormesis protective response operates under a classic life history tradeoff response (Stearns 1989). Quantifying the costs of hormesis is crucial given the increased performance experienced by the animals that received it, and these benefits extend beyond the parental generation and provide transgenerational protection. In cactus moths, there was higher pupation and adult emergence of F1 offspring (López-Martínez et al. 2014). And the offspring that experienced higher rates of survival were more readily able to build protective webs upon hatching, in the absence of food (López-Martínez et al. 2016a). An additional and usual protective effect of anoxia was the rescue of X-ray and gamma radiation-induced sterility in cactus and cabbage looper moths (López-Martínez et al. 2016a,b); indicating that at least a fraction of the radiation-induced sterility normally associated with ionizing radiation sterilization is related to oxidative damage to DNA that can be prevented with anoxia hormesis.
TEMPERATURE HORMESIS
Given the connection between seasonality and temperature, we find a lot of the work on temperature hormesis primarily focusing on low temperature as it relates to overwintering survival strategies (Storey and Storey 1988, Denlinger and Lee 2010). A common type of animal temperature hormesis is rapid cold hardening (RCH). First studied in flies (Lee et al. 1987), under RCH a brief (1 to 2hr) exposure to non-freezing low temperatures (2 to 5ºC) triggers an adaptive response that provides protection from lower temperatures and freezing injury (Chen et al. 1987). The literature on RCH is extensive with at least 120 papers that carry rapid cold hardening in the title and at least an additional 100 that deal, in part, with the phenomena. The known short-term effects of RCH include increased survival of sub-zero exposures (Denlinger and Lee 2010), decreased chill-coma inducing temperature (Kelty and Lee 1999), decreased lower freeze tolerance limit (Lee et al. 2006), decreased water loss rates (Yoder et al. 2006, Wada and Matsukura 2011), and increased cell viability protecting against apoptosis (Yi et al. 2007). Investigations into RCH have mostly focused on the short-term survival benefits of this type of hormesis, but studies into the long-term benefits have revealed that this type of hormesis is not just about short-term stress survival. Some of the long-term effects are connected to fitness as RCH preserves courtship and mating performance in flies (Shreve et al. 2004), increases fecundity and longevity in aphids (Powell and Bale 2005), prevents the disruption of learning/conditioning in flies (Kim et al. 2005), and preserves flight ability in butterflies (Larsen and Lee 1994). Mechanistically, sugar alcohols and trehalose are involved in this type of hormesis, alongside heat shock proteins and membrane restructuring genes. Transcriptomic analysis have revealed that redox signaling is also a part of this type of hormesis response (MacMillan et al. 2016).
As in most types of hormesis, very little work exists quantifying the cost of RCH. It has been found that in certain species, like Musca domestica, the increased cold tolerance associated with RCH can lead to shorter lifespan, reduced oviposition, and lower F1 emergence rates (Coulson and Bale 1992), but this was not the case in Drosophila melanogaster (Kelty and Lee 1999) or Sarcophaga crassipalpis were RCH rescues cold-shock induced losses in fecundity in both sexes (Rinehart et al. 2000). It is conceivable that the reduction in reproduction could be masked by the increased performance in the short term, but it would be made apparent in the long-term. It stands to reason that a certain tradeoff between performance and reproduction must be present in this hormetic scenario (Stearns 1989), and previous studies have focused more on the strong and broad short-term response than the less visible long-term effects, including the cost of RCH. Rapid stress hardening (RSH) responses are mostly studied in low temperature (RCH), but there is some work indicating that rapid heat hardening (RHH) also confers protection from lethal high temperatures. A brief (1 hr.) pretreatment at 37ºC showed improved survival at 43ºC in the codling moth, Cydia pomonella (Chidawanyika and Terblanche 2011) but this was not the case in hematophagous bed bugs, Cimex lectularius (Benoit et al. 2009a). In other systems, like the channel catfish Ictalurus punctatus, a preconditioning low oxygen treatment triggers an RHH response that increases their CTmax (Burleson and Silva 2011). This indicates that hormetic responses to temperature, just like those to anoxia, are widespread among animals and may be triggered by short/sublethal temperature exposures or other stressors such as variations in oxygen concentration.
IONIZING RADIATION HORMESIS
Ionizing radiation (ultraviolet, gamma rays, and X-rays) may be one of the first nonchemical stressors used to investigate low dose protective effects. This work dates back over one hundred years when low dose X-ray experiments first showed lifespan extension in animals (Davey 1917, 1919). At a time that predates much of our understanding of oxidative stress, antioxidant biology, and hormesis; it was clear that X-rays had a protective effect in flour beetles, Tribolium confusum, with potential to stimulate cell proliferation and immune function (Calabrese 2013). Much of what we know about X-ray hormesis comes from insects, and a large proportion of the work relates to radiation-based control strategies like the sterile insect technique (SIT; Klassen and Curtis 2005) and phytosanitary irradiation (IPT; Hallman 2011); where high doses are used to achieve pest control through sterility or death. However, in a hormesis context, we are interested in the effect that low doses have at providing protection and ionizing radiation-induced lifespan extensions have been observed in flies, mosquitoes, moths, crickets, beetles, and wasps (Calabrese 2013). In addition to finding the appropriate hormetic dose that can induce protection and boost performance, the age of the individual at the time of treatment can have dramatic effects on hormesis. A few days can strongly influence response outcomes between a treatment being hormetic, and extending lifespan, or becoming lethal in beetles (Ducoff 1975) and caterpillars (López-Martínez et al. 2016b). Exposure to low dose gamma radiation shortened embryogenesis time and led to heavier larvae that produced more silk in their pupal cocoons in the silkworm, Bombyx mori (Yusifov et al. 1990, Shibamoto et al. 2017). This represents a strong hormetic effect that improved multiple life history traits in these animals, but without lifespan information we do not know the full extent of the benefit to the silkworms. These hormetic effects of low dose ionizing radiation are not limited to invertebrates, and low dose gamma radiation is known to extend the lifespan of laboratory mice by about 20% (Caratero et al. 1998).
While the mechanism behind low dose X-rays life extension has not been completely elucidated (see Table 1), there seems to be a connection to the mitochondria and energetics as seen in Drosophila where starvation plays a role in their increase in longevity (Lamb 1964). These flies live longer when low dose radiation is combined with starvation. The redox signaling connection is deepened when Drosophila mutants for antioxidant genes, heat shock proteins, and DNA repair genes had reduced activity and shorter lifespans when exposed to low dose X-rays (Moskalev et al. 2006, Moskalev et al. 2009). Whether looking at males or females, low dose gamma radiation extends lifespan in Drosophila, and that hormetic effect is connected with the elevated expression of stress genes including heat shock proteins (Zhikrevetskaya et al. 2015). Additionally, expression of antioxidant enzymes and oxidative stress response genes seem to be a crucial component of a proposed mechanism for lifespan extension in response to X-ray hormesis (Seong et al. 2011). This mechanistic explanation has gathered support from a vertebrate model where low-dose X-rays trigger increases in glutathione, catalase, and glutathione-S-transferase in the tissues and blood of female Wistar rats (Sharma et al. 2019). This increase in antioxidants led to a decrease of oxidative damage to the liver, kidney, and brain in these animals. And while oxidative damage in the blood was not decreased as it was in tissues, there was an increase of immune cells (lymphocytes and eosinophils) in response to low dose radiation indicating a level of protection being activated in the blood (Sharma et al. 2019).
Table 1.
Redox, stress, immune, and other proteins are involved in the adaptive response known as hormesis. Certain metabolites have also been implicated in hormesis.
| Animal Model | Stress | Mechanism | Literature | |
|---|---|---|---|---|
 |
Common garter snake (Thamnophis sirtalis parietalis) | Anoxia | Increases in CAT, total SOD, & GST | Hermes-Lima & Zenteno-Savín 2002 |
 |
Goldfish (Carassius auratus) | Anoxia | Increase in liver CAT activity | Lushchak et al. 2001 |
 |
Northern leopard frog (Rana pipiens) | Anoxia | Increases in CAT & GPX activity | Hermes-Lima & Storey 1996 |
 |
Red-eared slider (Trachemys scripta elegans) | Anoxia | Increases in AHR, GR, & GSH-synthetase activity | Willmore & Storey 1997 |
 |
Caribbean fruit fly (Anastrepha suspensa) | Anoxia | Increases in SOD-Mn, & GPx activity. Decreases in LPO & PC | López-Martínez & Hahn 2012 |
 |
Cactus Moth (Cactoblastis cactorum) | Anoxia | Increase in antioxidant capacity. Decreases in LPO & PC | López-Martínez et al., 2014 |
 |
Nematode (C. elegans) | Arsenite | Upregulation of mitochondrial proteins, including SKN-1 & DAF-16. | Schmeisser et al. 2013 |
 |
McCoy mouse cells (Mus musculus) | Cadmium | Upregulation in HSP70 & metallothionein | Damelin et al. 2000 |
 |
Vinegar Fly (Drosophila melanogaster) | Crowding | No effect on expression of SOD but increase in CAT. Upregulation of HSP70, dTOR, dFOXO, InR, & dSir2. | Youn et al. 2018 |
 |
Antarctic midge (Belgica Antarctica) | Dehydration | Upregulation of SOD, CAT, Mtn, & smHSPs. Increases in concentration of glycerol & trehalose. | Michaud et al. 2008, Benoit et al. 2009b, López-Martínez et al 2009. |
 |
Cichlid fish (Oreochromis mossambicus) | Hyper gravity | Increase in protein matrix deposition | Anken et al. 2001 |
 |
Vinegar Fly (Drosophila melanogaster) | Hypergravity | Decreases in SOD & CAT activity | Le Bourg & Fournier 2004 |
 |
Multiple plant species | Ionizing radiation (gamma) | Oxidative stress reduction, increased enzymatic activity,& increased nucleic acid & protein synthesis. | Brito et al. 2018 |
 |
Vinegar Fly (Drosophila melanogaster) | Ionizing radiation (gamma) | Upregulation of foxo, tefu, Cyp6a20, CG13323, Fer3, Hsp70Aa, & per, p53, Brca2, Hus1-like, spn-B mei-9, RAD54, mus309, & wrinkled (DNA repair), Clk, DJNK, hpo, Sod, GstE3, CG9360, & Cyp4e2, & PCNA. Upregulation of DNA repair, HSPs, antioxidant defense, & apoptosis genes. Upregulation of homeostasis, metabolism, DNA repair, immunity genes in the Toll pathway & fungal infection genes. |
Zhikrevetskaya et al. 2015 Moskalev et al. 2006, 2009. Seong et al. 2011 Seong et al. 2012 |
 |
Wistar Rats (Rattus norvegicus) | Ionizing radiation (X-rays) | Decrease in LPO, & increased activity of CAT & GST. | Sharma et al. 2019 |
 |
Copepods (Tigriopus californicus) | Ionizing radiation (ultraviolet) | Upregulation of GST, Mn-SOD, GR, GPX, & HSPs. | Heine et al. 2019 |
 |
Flesh Fly (Sarcophaga crassipalpis) | Temperature (RCH) | Increase in glycerol, glucose, sorbitol, pyruvate, alanine, trehalose & glutamate levels. Protein upregulation of smHSPs & ATP synthase. Increase in oleic acid (membrane restructuring). | Chen et al. 1998, Overgaard et al. 2007, Michaud & Denlinger 2006, 2007, Li & Denlinger 2008 |
 |
Bed Bug (Cimex lectularius) | Temperature & Dehydration | Upregulation of HSP70 & HSP90 | Benoit et al. 2009 |
Abbreviations: RCH = rapid cold hardening, SOD = Superoxide dismutase, MN-SOD = manganese-SOD, CAT = catalase, GST = glutathione S transferase, GSH = reduced glutathione, GPx = glutathione peroxidase, GR = glutathione reductase, TG = total glutathione, Mtn = Metallothionein, HSP = heat shock protein, HSF = heat shock factor, LPO = lipid peroxidation, PC = protein carbonyls.
Another type of ionizing radiation, ultraviolet radiation (UVR), is more pervasive across animal taxa because it reaches the earth’s surface and penetrates deeply into the waters (UVA320–400nm; Misra et al. 2002), some of it penetrates through regions with thinner ozone layers (UVB-(290–320nm; McKenzie et al. 2011), but fortunately its most energetic and damaging form is absorbed by the atmosphere (UVC-200 −290nm; Schuch et al. 2017). UVA incidence is naturally much higher than UVB in any given latitude in the northern and southern hemispheres (Schuch et al. 2017), but UVB represents a challenge for polar animals (López-Martínez et al. 2008). Increases in ROS production and the ensuing oxidative stress are associated with increases in the activity of various antioxidant enzymes (Hermes-Lima et al. 1998, Agnez-Lima et al. 2012, Won et al. 2014). This response is mediated in part by transcription factors that stimulate antioxidant gene expression (Cadet et al. 2005, Agnez-Lima et al. 2012). The combination of the production of ROS and the increase in antioxidant enzymes suggests that UVR hormesis would likely occur given the appropriate level of exposure, nonetheless, little experimental data exists. Most data on UVR hormesis comes from plants, where UVR promotes growth (Tezuka et al. 1993). There is recent data showing that marine copepods exposed to nonlethal UVR doses, will have larger first clutches of eggs (Heine et al. 2019) and we have data indicating that UVR exposure in Drosophila flies improves performance (Berry III and López-Martínez unpublished data). These are the first steps in trying to quantify UVR hormetic effects, and if these effects follow the same pattern as other types of ionizing radiation then they would also be rooted in redox signaling and the POS hypothesis.
CHEMICAL HORMESIS
Although comparative physiology hormesis work largely focuses on environmental abiotic factors and how they lead to hormetic effects on animals, studies featuring environmental toxins and pesticides continue to increase in prevalence because of the potential exposure of animals to low doses of these chemicals and the effect of those exposures can have on their performance and survival. Heavy metals like arsenic and cadmium are dangerous soil contaminants but low doses of arsenite prolongs lifespan in nematodes (Schmeisser et al. 2013). Arsenite triggers the production of ROS which in turn activates antioxidant defenses. These defenses are linked to the longer life experienced by the worms, similarly to other types of hormesis (Fig. 3). Low doses of cadmium increase growth rate in American toads (Bufo americanus, James and Little 2003) and leopard frogs (Rana pipiens, Gross et al. 2007), and result in body size gains and faster metamorphic rates in Chinese toads (Bufo gargarizans, Ya et al. 2019). Additionally, low doses of cadmium elicit a protective heat shock protein response in mouse cells (Damelin 2000).
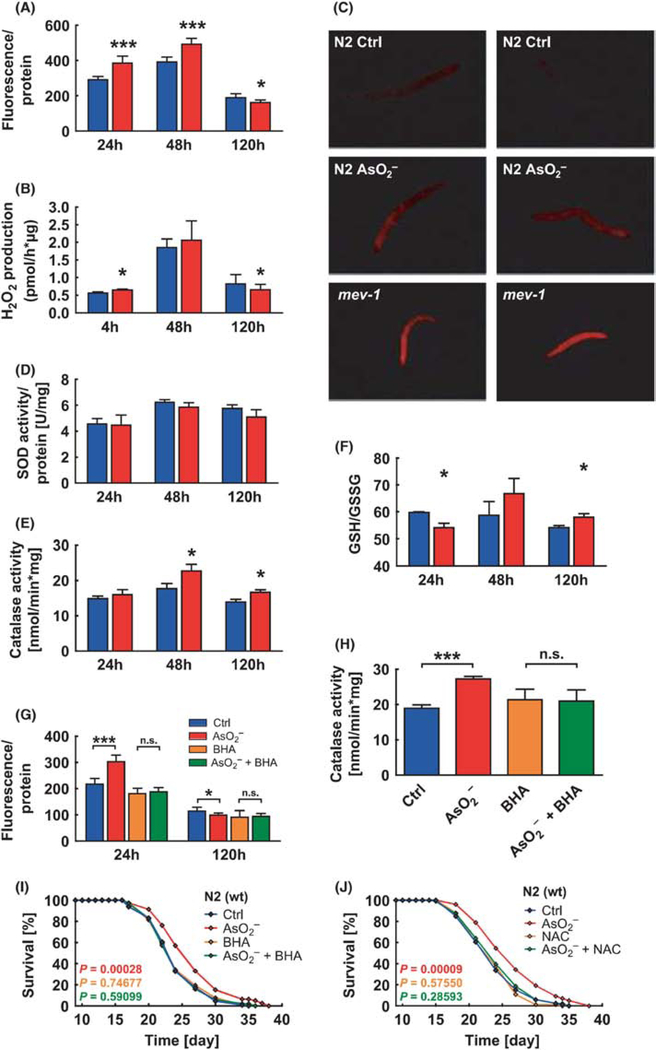
Figure 3.
Chemical hormesis (arsenite) triggers an increase in the production of reactive oxygen species (ROS; A & B). The presence of ROS triggers an increase in antioxidants, both enzymes (D & E) and non-enzymatic ones (F). That strong protective response leads to an increase in longevity (I & J) for the arsenite treated worms (Figure 3 from Schmeisser et al. 2013).
Another big class of environmental contaminants where hormesis has been found is pesticides. At low doses, pesticides can increase fecundity and reproductive output by increasing the net reproductive rate (NRR); a measure of how well females are being replaced in subsequent generations. Neonicotinoids, like clothianidin, increase fecundity and NRR in black cutworm moths (Agrotis ipsilon, Ding et al. 2018). Imidacloprid, another neonicotinoid, leads to faster development and higher NRR in thrips (Frankliniella occidentalis, Cao et al. 2019), increased fecundity in Paederus fuscipes beetles (Feng et al. 2019), increased reproductive output and survival under stress in green peach aphids (Myzus persicae, Rix et al. 2016), and increased survival when challenged with higher doses in M. persicae (Rix and Cutler 2018). The pyrethroid Lambda-cyhalothrin increases fecundity and NRR in Mythimna separata moths (Li et at. 2019), and the pyrethroid deltamethrin increases NRR in maize weevils (Sitophilus zeamais, Guedes et al. 2010), while cyantraniliprole, a ryanoid insecticide, increases fecundity in Drosophila suzukii (Shaw et al. 2019). Even glyphosate, known to have hormetic effects in plants (Brito et al. 2018), was found to make earthworms grow heavier and live longer when exposure was combined with warmer soil temperatures; possibly a type of cross tolerance hormesis (Pochron et al. 2019). Because of concerns over off-target effects of insecticides and colony collapse disorder, Cutler and Rix (2015) reviewed whether pesticide hormesis was recorded in honeybees. Caffeine and nicotine were shown to positively impact long-term memory and retention (Wright et al. 2013), and improved olfactory learning (Thany and Gauthier 2005). The effects of chemical hormesis in honeybees were not originally reported as hormesis but it was the efforts of additional investigation that revealed them as such (Cutler and Rix 2015). It is likely that there are more pesticide hormetic effects in the literature, as these effects are not reported as hormetic but rather as failures of pesticide efficacy. Pesticide hormesis is not just limited to invertebrates, wood frogs survive exposure from certain insecticides after experiencing prior exposure to different insecticides (Hua et al. 2013). Thus, it is foreseeable that pesticides trigger a type of rapid stress hardening hormetic response that allows for additional protection.
OTHER TYPES OF HORMESIS
Hormesis has also been investigated in a variety of systems and in response to stressors that are far less understood than oxygen, temperature, and chemicals. Mild (small amounts of water loss) or slow (water loss over a longer period) dehydration is associated with hormetic effects in a cross-tolerance framework. Dehydration (mild or slow) leads to increased pupariation, increased cell viability, and faster recovery from low temperature exposure in flesh flies, Sarcophaga bullata (Yi et al. 2017). In a polar insect, Belgica antarctica, slow mild dehydration leads to greater survival when exposed to −14ºC (Benoit et al. 2009b, Kawarasaki et al. 2019); and that slow rate of dehydration also increases survival to lethal high temperatures (30 ºC; Benoit et al. 2009b). There seems to be a type of pathway cross talk between low doses of anoxia, temperature, and dehydration which allows these stressors to induce rapid stress hardening responses and protect against lethal temperatures. Additionally, these three stressors are linked through shared metabolomic profile responses (Michaud et al. 2008). Slow dehydration is associated with increased expression of heat shock proteins, antioxidant enzymes, and membrane remodeling genes (López-Martínez et al. 2009). Even when that dehydration comes about from exposure to hyperosmotic sea water, it results in higher freezing tolerance survival in B. antarctica (Elnitsky et al. 2009). This effect of dehydration extends to D. melanogaster flies that can recover faster from chill coma if they were previously selected for dehydration resistance, although there is evidence that this hormetic effect might not be entirely related to the selection event (Sinclair et al. 2007).
A stressor that is far less understood than most is gravity and there is strong evidence that hypergravity (3 or 5 g) has hormetic effects in Drosophila flies (Le Bourg et al. 2004). Two weeks at hypergravity at a young age increased longevity in males (Le Bourg et al. 2004). Hypergravity also had additional protective effects as it triggered a rapid stress hardening response that increased survival time at 37ºC. But these improvements in longevity and survival are not related to the antioxidants enzymes (Le Bourg and Fournier 2004), which indicates that this type of hormesis does not align with the POS hypothesis and likely results from a completely different mechanism. Hypergravity also has an effect in cichlid fish, Oreochromis mossambicus, were otoliths growth was decreased but overall size was not (Anken et al. 2001). It is unclear whether this otolith growth response represents a hormetic effect and if so what the magnitude of that effect might be, but responses to hypergravity are not restricted to invertebrates and lower vertebrates and have also been recorded in pregnant Sprague-Dawley rats (Plaut et al. 2003). In the mammary tissues of these rats, the metabolic rate was decreased as a response of gravity manipulation as gravitational load increased; an effect similar to the response of flies to repeated anoxia (Visser et al. 2018).
A recent hormetic effect has been associated with crowding during development. In the larvae of Drosophila, crowding increases survival time at low (−3ºC) and high (38ºC) temperatures (Youn et al. 2018). Higher density crowded larvae emerged as adults faster and lived longer (Lushchak et al. 2019). It is clear from these two studies that certain stress genes (i.e. HSP70) are involved in this adaptive stress response but the role of antioxidant enzymes and oxidative stress protection genes is not as clear. Larval crowing leads to competition and the reduction of high-quality food, and in addition to the stresses associated from a larger group of animals present (i.e. temperature and water balance), starvation leads to mitochondrial efficiency differences. These mitochondrial differences are linked to ROS production and likely play a role in this rather unique type of hormesis.
THE MECHANISM OF HORMESIS
In the last ten years, we have seen nearly a doubling in the number of hormesis publications that aim at understanding the mechanisms of these adaptive responses (Calabrese et al. 2016A). The mechanistic underpinnings for the extensive performance effects seen in animals as a result of hormesis range from the quantification of specific polyols to genome-wide analysis highlighting specific pathways (Table 1). There are specific key players that are consistently linked to hormetic responses throughout the animal kingdom. Genes related to redox signaling, such as antioxidant enzymes and non-enzymatic antioxidants, whose role is the prevention/reduction of oxidative damage are involved nearly everywhere they are investigated (Table 1). Whether it is gene expression (Moskalev et al. 2009, Seong et al. 2012), protein expression (Yi et al. 2007), or enzyme activity (Hermes-Lima et al. 1998, Hermes-Lima and Zenteno-Savín 2002, López-Martínez and Hahn 2012), antioxidant mechanisms that reduce oxidative damage play a pervasive role in hormesis. Stress genes, like heat shock proteins, are also involved along with genes involved in DNA repair and apoptosis. To date, the data published indicates that redox and stress signaling play a central role in most hormetic responses, indicating a potential universal mechanism for hormesis. However, given the wide array of stressors that animals endure, there are stress-specific responses that widen the mechanistic targets of hormesis. Such are the cases of RCH and hypergravity. Under rapid cold hardening, multiple low temperature mechanisms (homeoviscous adaptation, increase polyol concentration, and freezing resistance) are activated in this response; in addition to the expected hormesis genes. Hypergravity exposure seems to be independent of the involvement of the main antioxidant enzymes, which challenges the notion of a universal hormetic mechanism. Our own ongoing Drosophila transcriptomic work comparing different hormesis treatments (low oxygen, low temperature, x-ray irradiation, UV irradiation) points toward general and unique responses for each condition. We are emboldened by recent research in this area and we encourage more mechanism studies that compare contextual factors in stress response to further our understanding of the role hormesis plays in animals.
FUTURE DIRECTIONS
We highlighted studies that show some of the remarkable protective effects that hormesis can have in animals across the spectrum from tiny invertebrates to mammals. These effects can be short and long-term, and whenever a broad time scale is used, hormesis is found to even have protective transgenerational effects. Short-term effects include treatment survival, improved performance, and increased mating success while long-term effects range from increasing longevity and performance at old age to improved offspring performance and starvation resistance. While we are hopeful and encouraged by the recent uptake in the study of hormesis in animals, we want to highlight the need for studies that deal with the mechanism of different types of hormesis, as well as, the cost of this adaptive response. Hormesis is rooted in the preparation for oxygen stress hypothesis, but there is evidence that hormetic benefits extend beyond antioxidant and oxidative stress, and into membrane remodeling and other aspects of animal physiology. The cost of hormesis to the parental and subsequent generations remains elusive with just a handful of studies aiming to quantify it. Understanding the cost of hormesis will allow us to elucidate how hormetic mechanisms evolved, which selection pressures drove and continue to drive these responses, and what might be the full range of these adaptive responses. There is a strong link between hormetic effects and the age of application, and a clear connection exists when these hormetic treatments are applied early in life leading to long-lasting effects present at advanced age (Le Bourg 2005, López-Martínez and Hahn 2014, López-Martínez et al. 2014, López-Martínez et al. 2016a,b, Visser et al. 2018). The studies outlined in this review reinforce the need for additional hormesis investigation into how small doses of chemical, biotic, and abiotic stressors can dramatically improve organismal performance in non-human animals.
Highlights.
Hormesis is an adaptive response that is also known as preconditioning, pretreatment, cross tolerance, and rapid stress hardening.
The vast majority of hormesis work in non-human animals has investigated changes in oxygen or temperature which are connected to overwintering strategies.
The mechanism of hormesis involves antioxidant enzymes and oxidative stress protective gene responses much like the preparation for oxidative stress hypothesis.
Little is known about the cost of hormesis, but decreased reproduction output has been implicated multiple times.
ACKNOWLEDGMENTS
RBIII and GLM conceived the idea for the review, carried out the literature search, and wrote the manuscript. Support for RBIII came from NMSU RISE (2R25GM 061222-18) and for GLM from the National Science Foundation Office of Integrative Actives RII Track-2 #1826834. The authors wish to thank Alyssa De La Torre, Nubia Rivas, Angel Padilla, and Michael Balogh for their assistance in building the hormesis database for the Comparative Stress Physiology Laboratory.
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Agnez-Lima LF, Melo JT, Silva AE, Oliveira AHS, Timoteo ARS, LimaBessa KM, … & Menck CF (2012). DNA damage by singlet oxygen and cellular protective mechanisms. Mutation Research/Reviews in Mutation Research, 751(1), 15–28, 10.1016/j.mrrev.2011.12.005 [DOI] [PubMed] [Google Scholar]
- 2.Anken RH, Ibsch M, Breuer J, & Rahmann H. (2001). Effect of hypergravity on the Ca/Sr composition of developing otoliths of larval cichlid fish (Oreochromis mossambicus). Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology, 128(2), 369–377, 10.1016/S1095-6433(00)00316-0 [DOI] [PubMed] [Google Scholar]
- 3.Benoit JB, Lopez-Martinez G, Elnitsky MA, Lee RE Jr, & Denlinger DL (2009a). Dehydration-induced cross tolerance of Belgica antarctica larvae to cold and heat is facilitated by trehalose accumulation. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology, 152(4), 518–523, 10.1016/j.cbpa.2008.12.009 [DOI] [PubMed] [Google Scholar]
- 4.Benoit JB, Lopez-Martinez G, Elnitsky MA, Lee RE Jr, & Denlinger DL (2009b). Dehydration-induced cross tolerance of Belgica antarctica larvae to cold and heat is facilitated by trehalose accumulation. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology, 152(4), 518–523, 10.1016/j.cbpa.2008.12.009 [DOI] [PubMed] [Google Scholar]
- 5.Benoit JB, López-Martínez G, Teets NM, Phillips SA, & Denlinger DL (2009). Responses of the bed bug, Cimex lectularius, to temperature extremes and dehydration: levels of tolerance, rapid cold hardening, and expression of heat shock proteins. Medical and Veterinary Entomology, 23(4), 418–425, 10.1111/j.1365-2915.2009.00832.x [DOI] [PubMed] [Google Scholar]
- 6.Brito IP, Tropaldi L, Carbonari CA, & Velini ED (2018). Hormetic effects of glyphosate on plants. Pest management science, 74(5), 1064–1070, 10.1002/ps.4523 [DOI] [PubMed] [Google Scholar]
- 7.Burleson ML, & Silva PE (2011). Cross tolerance to environmental stressors: effects of hypoxic acclimation on cardiovascular responses of channel catfish (Ictalurus punctatus) to a thermal challenge. Journal of thermal biology, 36(4), 250–254, 10.1016/j.jtherbio.2011.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cadet J, Sage E, & Douki T, (2005). Ultraviolet radiation-mediated damage to cellular DNA. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis, 571(1), 3–17. doi.org/10.1016/j.mrfmmm.2004.09.012 [DOI] [PubMed] [Google Scholar]
- 9.Calabrese EJ, & Baldwin LA (2000). Radiation hormesis: its historical foundations as a biological hypothesis. Human & experimental toxicology, 19(1), 41–75, https://doi.org/10.1191%2F096032700678815602 [DOI] [PubMed] [Google Scholar]
- 10.Calabrese EJ, & Baldwin LA (2001). Hormesis: U-shaped dose responses and their centrality in toxicology. Trends in pharmacological sciences, 22(6), 285–291, 10.1016/S0165-6147(00)01719-3 [DOI] [PubMed] [Google Scholar]
- 11.Calabrese EJ, & Blain R. (2005). The occurrence of hormetic dose responses in the toxicological literature, the hormesis database: an overview. Toxicology and applied pharmacology, 202(3), 289–301, 10.1016/j.taap.2004.06.023 [DOI] [PubMed] [Google Scholar]
- 12.Calabrese EJ, Bachmann KA, Bailer AJ, Bolger PM, Borak J, Cai L, … & Cook RR (2007). Biological stress response terminology: integrating the concepts of adaptive response and preconditioning stress within a hormetic dose–response framework. Toxicology and applied pharmacology, 222(1), 122–128, 10.1016/j.taap.2007.02.015 [DOI] [PubMed] [Google Scholar]
- 13.Calabrese EJ, Iavicoli I, & Calabrese V. (2012). Hormesis: why it is important to biogerontologists. Biogerontology, 13(3), 215–235. 10.1007/s10522-012-93747 [DOI] [PubMed] [Google Scholar]
- 14.Calabrese EJ (2013). Low doses of radiation can enhance insect lifespans. Biogerontology, 14(4), 365–381, 10.1007/s10522-013-9436-5 [DOI] [PubMed] [Google Scholar]
- 15.Calabrese EJ (2014). Hormesis: a fundamental concept in biology. Microbial cell, 1(5), 145–149. DOI: 10.15698/mic2014.05.145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calabrese EJ (2016a). Preconditioning is hormesis part I: Documentation, dose-response features and mechanistic foundations. Pharmacological Research, 110, 242–264, 10.1016/j.phrs.2015.12.021 [DOI] [PubMed] [Google Scholar]
- 17.Calabrese EJ (2016b). Preconditioning is hormesis part II: How the conditioning dose mediates protection: Dose optimization within temporal and mechanistic frameworks. Pharmacological Research, 110, 265–275. DOI: 10.1016/j.phrs.2015.12.020 [DOI] [PubMed] [Google Scholar]
- 18.Cao Y, Yang H, Li J, Wang C, Li C, & Gao Y. (2019). Sublethal Effects of Imidacloprid on the Population Development of Western Flower Thrips Frankliniella occidentalis (Thysanoptera: Thripidae). Insects, 10(1), 3, 10.3390/insects10010003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caratero A, Courtade M, Bonnet L, Planel H, & Caratero C. (1998). Effect of a continuous gamma irradiation at a very low dose on the life span of mice. Gerontology, 44(5), 272–276, 10.1159/000022024 [DOI] [PubMed] [Google Scholar]
- 20.Chen CP, Denlinger DL, & Lee RE Jr (1987). Cold-shock injury and rapid cold hardening in the flesh fly Sarcophaga crassipalpis. Physiological Zoology, 60(3), 297–304, 10.1086/physzool.60.3.30162282 [DOI] [Google Scholar]
- 21.Chidawanyika F, & Terblanche JS (2011). Rapid thermal responses and thermal tolerance in adult codling moth Cydia pomonella (Lepidoptera: Tortricidae). Journal of Insect Physiology, 57(1), 108–117, 10.1016/j.jinsphys.2010.09.013 [DOI] [PubMed] [Google Scholar]
- 22.Churchill TA, & Storey KB (1992). Freezing survival of the garter snake Thamnophis sirtalis parietalis. Canadian Journal of Zoology, 70, 99–105. 10.1139/z92-015 [DOI] [Google Scholar]
- 23.Coulson SC, & Bale JS (1992). Effect of rapid cold hardening on reproduction and survival of offspring in the housefly Musca domestica. Journal of Insect Physiology, 38(6), 421–424, 10.1016/0022-1910(92)90118-W [DOI] [Google Scholar]
- 24.Cutler GC, & Rix RR (2015). Can poisons stimulate bees? Appreciating the potential of hormesis in bee–pesticide research. Pest management science, 71(10), 1368–1370, 10.1002/ps.4042 [DOI] [PubMed] [Google Scholar]
- 25.Damelin LH, Vokes S, Whitcutt JM, Damelin SB, & Alexander JJ (2000). Hormesis: a stress response in cells exposed to low levels of heavy metals. Human & experimental toxicology, 19(7), 420–430, https://doi.org/10.1191%2F096032700678816133 [DOI] [PubMed] [Google Scholar]
- 26.Davey WP (1917). The effect of X-rays on the length of life of Tribolium confusum. Journal of Experimental Zoology, 22(3), 573–592, 10.1002/jez.1400220305 [DOI] [Google Scholar]
- 27.Davey WP (1919). Prolongation of life of Tribolium confusum apparently due to small doses of X-rays. Journal of Experimental Zoology, 28(3), 447–458, 10.1002/jez.1400280305 [DOI] [Google Scholar]
- 28.Davies KJA (2016). Adaptive homeostasis. Molecular Aspects of Medicine, 49, 1–7, doi: 10.1016/j.mam.2016.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Denlinger DL, & Lee RE Jr (Eds.). (2010). Low temperature biology of insects. Cambridge University Press. [Google Scholar]
- 30.Ding J, Zhao Y, Zhang Z, Xu C, & Mu W. (2018). Sublethal and hormesis effects of clothianidin on the black cutworm (Lepidoptera: Noctuidae). Journal of economic entomology, 111(6), 2809–2816, 10.1093/jee/toy254 [DOI] [PubMed] [Google Scholar]
- 31.Ducoff HS (1975). Form of the increased longevity of Tribolium after Xirradiation. Experimental gerontology, 10(3–4), 189–193, 10.1016/05315565(75)90031-5 [DOI] [PubMed] [Google Scholar]
- 32.Elnitsky MA, Benoit JB, Lopez-Martinez G, Denlinger DL, & Lee RE (2009). Osmoregulation and salinity tolerance in the Antarctic midge, Belgica antarctica: seawater exposure confers enhanced tolerance to freezing and dehydration. Journal of Experimental Biology, 212(17), 2864–2871, https://jeb.biologists.org/content/212/17/2864.short [DOI] [PubMed] [Google Scholar]
- 33.Feng WB, Bong LJ, Dai SM, & Neoh KB (2019). Effect of imidacloprid exposure on life history traits in the agricultural generalist predator Paederus beetle: Lack of fitness cost but strong hormetic effect and skewed sex ratio. Ecotoxicology and environmental safety, 174, 390–400, 10.1016/j.ecoenv.2019.03.003 [DOI] [PubMed] [Google Scholar]
- 34.Geihs MA, Moreira DC, López-Martínez G, Minari M, Ferreira-Cravo M, Carvajalino-Fernández JM, & Hermes-Lima M. (2020). Commentary: Ultraviolet radiation triggers “Preparation for Oxdative Stress” antioxidant response in animals: similarities and interplay with other stressors. Comparative Biochemistry and Physiology, Part A, 239 10.1016/j.cbpa.2019.110585 [DOI] [PubMed] [Google Scholar]
- 35.Giraud-Billoud M, Rivera-Ingraham GA, Moreira DC, Burmester T, Castro-Vazquez A, Carvajalino-Fernández JM, Dafre A, Niu C, Tremblay N, Paital B, Rosa R, Storey JM, Vega IA, Zhang W, Yepiz-Plascencia G, Zenteno-Savin T, Storey KB, & Hermes-Lima M. (2019). Twenty years of the ‘Preparation for Oxidative Stress’ (POS) theory: Ecophysiological advantages and molecular strategies. Comparative Biochemistry and Physiology A, 234, 36–49. 10.1016/j.cbpa.2019.04.004 [DOI] [PubMed] [Google Scholar]
- 36.Gross JA, Chen TH, & Karasov WH (2007). Lethal and sublethal effects of chronic cadmium exposure on northern leopard frog (Rana pipiens) tadpoles. Environmental Toxicology and Chemistry: An International Journal, 26(6), 1192–1197, 10.1897/06-479R.1 [DOI] [PubMed] [Google Scholar]
- 37.Gruber CM, & Keyser GF (1946). A study on the development of tolerance and cross tolerance to barbiturates in experimental animals. Journal of Pharmacology and Experimental Therapeutics, 86(2), 186–196, http://www.worldcat.org/issn/1521-0103 [PubMed] [Google Scholar]
- 38.Guedes NMP, Tolledo J, Corrêa AS, & Guedes RNC (2010). Insecticide-induced hormesis in an insecticide-resistant strain of the maize weevil, Sitophilus zeamais. Journal of Applied Entomology, 134(2), 142–148, 10.1111/j.1439-0418.2009.01462.x [DOI] [Google Scholar]
- 39.Halliwell B, & Gutteridge JM (2015). Free radicals in biology and medicine. USA, Oxford University Press. [Google Scholar]
- 40.Hallman GJ (2011). Phytosanitary applications of irradiation. Comprehensive Reviews in Food Science and Food Safety, 10(2), 143–151, 10.1111/j.15414337.2010.00144.x [DOI] [Google Scholar]
- 41.Harrison J, Frazier MR, Henry JR, Kaiser A, Klok CJ, & Rascón B. (2006). Responses of terrestrial insects to hypoxia or hyperoxia. Respiratory Physiology & Neurobiology, 154, 4–17. 10.1016/j.resp.2006.02.008 [DOI] [PubMed] [Google Scholar]
- 42.Heine KB, Powers MJ, Kallenberg C, Tucker VL, & Hood WR (2019). Ultraviolet irradiation increases size of the first clutch but decreases longevity in a marine copepod. Ecology and evolution, 9(17), 9759–9767, 10.1002/ece3.5510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hermes-Lima M, Storey JM, & Storey KB, (1998). Antioxidant defenses and metabolic depression. The hypothesis of preparation for oxidative stress in land snails. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology, 120(3), 437–448. doi.org/10.1016/S0305-0491(98)10053-6 [DOI] [PubMed] [Google Scholar]
- 44.Hermes-Lima M, Storey JM, & Storey KB (2001). Antioxidant defenses and animal adaptation to oxygen availability during environmental stress In Cell and Molecular Response to Stress (2)263–287, Elsevier, 10.1016/S1568-1254(01)80022-X [DOI] [Google Scholar]
- 45.Hermes-Lima M, & Zenteno-Savín T. (2002). Animal response to drastic changes in oxygen availability and physiological oxidative stress. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology, 133(4), 537–556, 10.1016/S1532-0456(02)00080-7 [DOI] [PubMed] [Google Scholar]
- 46.Hoback WW, Stanley DW, Higley LG, & Barnhart MC (1998). Survival of immersion and anoxia by larval tiger beetles, Cicindela togata. American Midland Naturalist Journal, 140, 27–33. [Google Scholar]
- 47.Hooper GHS (1971). Competitiveness of gamma-sterilized males of the Mediterranean fruit fly: effect of irradiating pupal or adult stage and or irradiating pupae in nitrogen. Journal of Economic Entomology, 64, 1364–1368. 10.1093/jee/64.6.1364 [DOI] [PubMed] [Google Scholar]
- 48.Hua J, Cothran R, Stoler A, & Relyea R. (2013). Cross-tolerance in amphibians: Wood frog mortality when exposed to three insecticides with a common mode of action. Environmental toxicology and chemistry, 32(4), 932–936, 10.1002/etc.2121 [DOI] [PubMed] [Google Scholar]
- 49.Huang X, Li J, Song S, Wang L, Lin Z, Ouyang Z, & Yu R. (2019). Hormesis effect of hydrogen peroxide on the promoter activity of neuropeptide receptor PAC1-R. Journal of Food Biochemistry, e12877, 10.1111/jfbc.12877 [DOI] [PubMed] [Google Scholar]
- 50.James SM, Little EE (2003). The effects of chronic cadmium exposure on American toad (Bufo americanus) tadpoles. Environmental Toxicology and Chemistry: An International Journal, 22(2), 377–380, 10.1002/etc.5620220219 [DOI] [PubMed] [Google Scholar]
- 51.Ji LL, Kang C, & Zhang Y. (2010). Exercise-induced hormesis and skeletal muscle health. Free Radical Biology and Medicine, 98, 113–122, doi: 10.1016/j.freeradbiomed.2016.02.025 [DOI] [PubMed] [Google Scholar]
- 52.Ji LL, Dickman JR, Kang C, & Koenig R. (2016). Exercise-induced hormesis mal help healthy aging. Dose-Response, 8, 73−79, 10.2203/dose-response.09048.Ji [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kawarasaki Y, Teets NM, Philip BN, Potts LJ, Gantz JD, Denlinger DL, & Lee RE (2019). Characterization of drought-induced rapid cold-hardening in the Antarctic midge, Belgica antarctica. Polar Biology, 1–10, 10.1007/s00300-019-02503-6 [DOI] [Google Scholar]
- 54.Kelty JD, & Lee RE Jr (1999). Induction of rapid cold hardening by cooling at ecologically relevant rates in Drosophila melanogaster. Journal of insect physiology, 45(8), 719–726, 10.1016/S0022-1910(99)00040-2 [DOI] [PubMed] [Google Scholar]
- 55.Kim YS, Denlinger DL, & Smith B. (2005). Spatial Conditioning in the Flesh Fly, Sarcophaga crassipalpis: Disruption of Learning by Cold Shock and Protection by Rapid Cold Hardening. Journal of Asia-Pacific Entomology, 8(4), 345–351, 10.1016/S1226-8615(08)60256-3 [DOI] [Google Scholar]
- 56.Klassen W, & Curtis CF (2005). History of the sterile insect technique. Sterile insect technique, (3–36), 10.1007/1-4020-4051-2_1 [DOI] [Google Scholar]
- 57.Lamb MJ (1964). The effects of radiation on the longevity of female Drosophila subobscura. Journal of Insect Physiology, 10, 487–497. doi: 10.1016/00221910(64)90072-1 [DOI] [Google Scholar]
- 58.Larsen KJ, & Lee RE Jr. (1994). Cold tolerance including rapid cold-hardening and inoculative freezing of fall migrant monarch butterflies in Ohio. Journal of Insect Physiology, 40(10), 859–864, 10.1016/0022-1910(94)90019-1 [DOI] [Google Scholar]
- 59.Le Bourg É, Toffin É, & Massé A. (2004). Male Drosophila melanogaster flies exposed to hypergravity at young age are protected against a non-lethal heat shock at middle age but not against behavioral impairments due to this shock. Biogerontology, 5(6), 431–443, 10.1007/s10522-004-3200-9 [DOI] [PubMed] [Google Scholar]
- 60.Le Bourg E. & Fournier D. (2004). Is lifespan extension accompanied by improved antioxidant defences? A study of superoxide dismutase and catalase in Drosophila melanogaster flies that lived in hypergravity at a young age. Biogerontology, 5, 261–266. doi: 10.1023/B:BGEN.0000038046.37590.03 [DOI] [PubMed] [Google Scholar]
- 61.Le Bourg É (2005). Hormetic protection of Drosophila melanogaster middle-aged male flies from heat stress by mildly stressing them at young age. Naturwissenschaften, 92(6), 293–296, 10.1007/s00114-005-0627-z [DOI] [PubMed] [Google Scholar]
- 62.Lee RE, Chen CP, & Denlinger DL (1987). A rapid cold-hardening process in insects. Science, 238(4832), 1415–1417, doi: 10.1126/science.238.4832.1415 [DOI] [PubMed] [Google Scholar]
- 63.Lee RE, Elnitsky MA, Rinehart JP, Hayward SA, Sandro LH, & Denlinger D. (2006). Rapid cold-hardening increases the freezing tolerance of the Antarctic midge Belgica antarctica. Journal of Experimental Biology, 209(3), 399–406, https://jeb.biologists.org/content/209/3/399.short [DOI] [PubMed] [Google Scholar]
- 64.Li XR, Li Y, Wang W, He N, Tan XL, & Yang XQ (2019). LC50 of lambdacyhalothrin stimulates reproduction on the moth Mythimna separata (Walker). Pesticide biochemistry and physiology, 153, 47–54, 10.1016/j.pestbp.2018.11.001 [DOI] [PubMed] [Google Scholar]
- 65.López-Martínez G, Elnitsky MA, Benoit JB, Lee RE Jr, & Denlinger DL (2008). High resistance to oxidative damage in the Antarctic midge Belgica antarctica, and developmentally linked expression of genes encoding superoxide dismutase, catalase and heat shock proteins. Insect biochemistry and molecular biology, 38(8), 796–804, 10.1016/j.ibmb.2008.05.006 [DOI] [PubMed] [Google Scholar]
- 66.López-Martínez G, Benoit JB, Rinehart JP, Elnitsky MA, Lee RE Jr., & Denlinger DL (2009). Dehydration, rehydration, and overhydration alter patterns of gene expression in the Antarctic midge, Belgica antarctica. Journal of Comparative Physiology B, 179, 481–491. doi: 10.1007/s00360-008-0334-0 [DOI] [PubMed] [Google Scholar]
- 67.López-Martínez G, & Hahn DA (2012). Short-term anoxic conditioning hormesis boosts antioxidant defenses, lowers oxidative damage following irradiation and enhances male sexual performance in the Caribbean fruit fly, Anastrepha suspensa. Journal of Experimental Biology, 215(12), 2150–2161. doi: 10.1242/jeb.065631 [DOI] [PubMed] [Google Scholar]
- 68.López-Martínez G, & Hahn DA (2014). Early life hormetic treatments decrease irradiation-induced oxidative damage, increase longevity, and enhance sexual performance during old age in the Caribbean fruit fly. PLoS ONE, 9(1), e88128, 10.1371/journal.pone.0088128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.López-Martínez G, Carpenter JE, Hight SD, & Hahn DA (2014). Low-oxygen atmospheric treatment improves the performance of irradiation-sterilized male cactus moths used in SIT. Journal of Economic Entomology, 107(1), 185–197, 10.1603/EC13370 [DOI] [PubMed] [Google Scholar]
- 70.López-Martínez G, Carpenter JE, Hight SD, & Hahn DA (2016a). Anoxiaconditioning hormesis alters the relationship between irradiation doses for survival and sterility in the cactus moth, Cactoblastis cactorum (Lepidoptera:Pyralidae). Florida Entomologist, 99(sp1),95–104, 10.1653/024.099.sp113 [DOI] [Google Scholar]
- 71.López-Martínez G, Meagher RL, Jeffers LA, Bailey WD, & Hahn DA (2016b). Low oxygen atmosphere enhances post-irradiation survival of Trichoplusia Ni (Lepidoptera: Noctuidae). Florida Entomologist, 99(2), 24–33, https://journals.flvc.org/flaent/article/view/88670 [Google Scholar]
- 72.Lushchak VI, Lushchak LP, Mota AA, & Hermes-Lima M. (2001). Oxidative stress and antioxidant defenses in goldfish Carassius auratus during anoxia and reoxygenation. American Journal of Physiology, 280, R100–R107. doi: 10.1152/ajpregu.2001.280.1.R100 [DOI] [PubMed] [Google Scholar]
- 73.Lushchak OV, Karaman HS, Kozeretska IA, Koliada AK, Zabuga OG, Pisaruk AV, & Vaiserman AM (2019). Larval crowding results in hormesis-like effects on longevity in Drosophila: timing of eclosion as a model. Biogerontology, 20(2), 191–201, 10.1007/s10522-018-9786-0 [DOI] [PubMed] [Google Scholar]
- 74.MacMillan HA, Knee JM, Dennis AB, Udaka H, Marshall KE, Merritt TJS, & Sinclair B. (2016). Cold acclimation wholly reorganizes the Drosophila melanogaster transcriptome and metabolome. Scientific Reports, 30/ doi: 10.1038/srep28999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mattson MP (2008). Hormesis defined. Ageing Research Reviews, 7(1), 1–7. doi: 10.1016/j.arr.2007.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mattson MP, & Calabrese EJ (2010). Hormesis. Totowa, NJ: Humana Press. [Google Scholar]
- 77.McKenzie RL, Aucamp PJ, Bais AF, Björn LO, Ilyas M, & Madronich S. (2011). Ozone depletion and climate change: impacts on UV radiation. Photochemical & Photobiological Sciences, 10(2), 182–198, doi: 10.1039/C0PP90034F [DOI] [PubMed] [Google Scholar]
- 78.Michaud MR, Benoit JB, López-Martínez G, Elnitsky MA, Lee RE Jr., & Denlinger DL (2008). Metabolomics reveals unique and shared metabolic changes in response to heat shock, freezing, and desiccation in the Antarctic midge, Belgica antarctica. Journal of Insect Physiology, 54, 645–655. doi: 10.1016/j.jinsphys.2008.01.003 [DOI] [PubMed] [Google Scholar]
- 79.Misra RB, Babu GS, Ray RS, & Hans RK (2002). Tubifex: a sensitive model for UV-B-induced phototoxicity. Ecotoxicology and environmental safety, 52(3), 288–295, 10.1006/eesa.2002.2184 [DOI] [PubMed] [Google Scholar]
- 80.Moskalev AA, Yazkiv AS, & Zainullin VG (2006). Effect of low-dose irradiation on the lifespan in various strains of Drosophila melanogaster. Russian Journal of Genetics, 42(6), 628–635. doi: 10.1134/S102279540606007X [DOI] [PubMed] [Google Scholar]
- 81.Moskalev A, Shaposhnikov M, & Turysheva E. (2009). Life span alteration after irradiation in Drosophila melanogaster strains with mutation of Hsf and Hsps. Biogerontology, 10, 3–11. doi: 10.1007/s10522-008-9147-5 [DOI] [PubMed] [Google Scholar]
- 82.Nestel D, Nemny-Lavy E, Islam SM, Wornoayporn V, & Cáceres C. (2007). Effects of pre-irradiation conditioning of medfly pupae (Diptera: Tephritidae): hypoxia and quality of sterile males. Florida Entomologist, 90, 80–87. [Google Scholar]
- 83.Ohinata K, Ashraf M, & Harris EJ (1977). Mediterranean fruit flies: sterility and sexual competitiveness in the laboratory after treatment with gamma irradiation in air, carbon dioxide, helium, nitrogen or partial vacuum. Journal of Economic Entomology, 70, 165–168. doi: 10.1093/jee/70.2.165 [DOI] [PubMed] [Google Scholar]
- 84.Pinder AW, Storey KB, & Ultsch GR (1992). Estivation and hibernation In: Feder ME, Burggren WW (Eds.), Environmental Biology of the Amphibia. University of Chicago Press, Chicago, pp. 250–274. [Google Scholar]
- 85.Plaut K, Maple RL, Wade CE, Baer LA, & Ronca AE (2003). Effects of hypergravity on mammary metabolic function: gravity acts as a continuum. Journal of Applied Physiology, 95(6), 2350–2354, 10.1152/japplphysiol.00287.2003 [DOI] [PubMed] [Google Scholar]
- 86.Pochron S, Choudhury M, Gomez R, Hussaini S, Illuzzi K, Mann M, Mezic M, Nikakis J, & Tucker C. (2019). Temperature and body mass drive earthworm (Eisenia fetida) sensitivity to a popular glyphosate-based herbicide. Applied Soil Ecology, 139, 32–39, 10.1016/j.apsoil.2019.03.015 [DOI] [Google Scholar]
- 87.Powell SJ, & Bale JS (2005). Low temperature acclimated populations of the grain aphid Sitobion avenae retain ability to rapidly cold harden with enhanced fitness. Journal of Experimental Biology, 208(13), 2615–2620, doi: 10.1242/jeb.01685 [DOI] [PubMed] [Google Scholar]
- 88.Rinehart JP, Yocum GD, & Denlinger DL (2000). Thermotolerance and rapid cold hardening ameliorate the negative effects of brief exposures to high or low temperatures on fecundity in the flesh fly, Sarcophaga crassipalpis. Physiological Entomology, 25(4), 330336, 10.1111/j.1365-3032.2000.00201.x [DOI] [Google Scholar]
- 89.Ristow M, & Schmeisser K. (2014). Mitohormesis: promoting health and lifespan by increased levels of reactive oxygen species (ROS). Dose-Response, 12(2), dose-response, https://doi.org/10.2203%2Fdose-response.13-035.Ristow [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rix RR, Ayyanath MM, & Cutler GC (2016). Sublethal concentrations of imidacloprid increase reproduction, alter expression of detoxification genes, and prime Myzus persicae for subsequent stress. Journal of pest science, 89(2), 581–589, 10.1007/s10340-015-0716-5 [DOI] [Google Scholar]
- 91.Rix RR, & Cutler GC (2018). Does multigenerational exposure to hormetic concentrations of imidacloprid precondition aphids for increased insecticide tolerance? Pest management science, 74(2), 314–322, 10.1002/ps.473 [DOI] [PubMed] [Google Scholar]
- 92.Robinson AS (1975). Influence of anoxia during gamma irradiation on the fertility and competitiveness of the adult male codling moth, Laspeyresia pomonella (L.). Radiation Research, 61, 526–534. 10.2307/3574127 [DOI] [PubMed] [Google Scholar]
- 93.Samson L, & Cairns J. (1977). A new pathway for DNA repair in Escherichia coli. Nature, 267(5608), 281, 10.1038/267281a0 [DOI] [PubMed] [Google Scholar]
- 94.Schmeisser K, Mansfeld J, Kuhlow D, Weimer S, Priebe S, Heiland I, & Price NL (2013). Role of sirtuins in lifespan regulation is linked to methylation of nicotinamide. Nature chemical biology, 9(11), 693, 10.1038/nchembio.1352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Schmeisser S, Schmeisser K, Weimer S, Groth M, Priebe S, Fazius E, Kuhlow D, Denis P, Jürgen EW, Reinhard G, Platzer M, Zarse K, Michael R. (2013). Mitochondrial hormesis links low-dose arsenite exposure to lifespan extension. Aging cell, 12(3), 508–517, 10.1111/acel.12076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schuch AP, Moreno NC, Schuch NJ, Menck CFM, & Garcia CCM (2017). Sunlight damage to cellular DNA: Focus on oxidatively generated lesions. Free Radical Biology and Medicine, 107, 110–124, 10.1016/j.freeradbiomed.2017.01.029 [DOI] [PubMed] [Google Scholar]
- 97.Seong KM, Kim CS, Seo SW, Jeon HY, Lee BS, Nam SY, … & Jin YW(2011). Genome-wide analysis of low-dose irradiated male Drosophila melanogaster with extended longevity. Biogerontology, 12(2), 93–107, 10.1007/s10522-0109295-2 [DOI] [PubMed] [Google Scholar]
- 98.Seong KM, Kim CS, Lee BS, Nam SY, Yang KH, Kim JY, Park JJ, Min KJ, & Jin YW (2012). Low-dose radiation induces Drosophila innate immunity through Toll pathway activation. Journal of Radiation Research, 53, 242–249, 10.1269/jrr.11170 [DOI] [PubMed] [Google Scholar]
- 99.Sharma S, Singla N, Chadha VD, & Dhawan DK (2019). A concept of radiation hormesis: stimulation of antioxidant machinery in rats by low dose ionizing radiation. Hellenic journal of nuclear medicine, 22(1), 43–48, 10.1967/s002449910958 [DOI] [PubMed] [Google Scholar]
- 100.Shaw B, Brain P, Wijnen H, Fountain MT (2019). Implications of sub-lethal rates of insecticides and daily time of application on Drosophila suzukii lifecycle. Crop Protection, 121, 182–194, 10.1016/j.cropro.2019.04.006 [DOI] [Google Scholar]
- 101.Shibamoto Y, Kamei Y, Kamei K, Tsuchiya T, & Aoyama N. (2017). Continuous low-dose-rate irradiation promotes growth of silkworms. Dose Response,15(4), https://doi.org/10.1177%2F1559325817735252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shreve SM, Kelty JD, & Lee RE (2004). Preservation of reproductive behaviors during modest cooling: rapid cold-hardening fine-tunes organismal response. Journal of Experimental Biology, 207(11), 1797–1802, doi: 10.1242/jeb.00951 [DOI] [PubMed] [Google Scholar]
- 103.Sies H. (2017). Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: oxidative eustress. Redox biology, 11, 613–619, 10.1016/j.redox.2016.12.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sinclair BJ, Nelson S, Nilson TL, Roberts SP, Gibbs AG (2007). The effect of selection for desiccation resistance on cold tolerance of Drosophila melanogaster. Physiological Entomology, 32(4), 322–327, 10.1111/j.13653032.2007.00585.x [DOI] [Google Scholar]
- 105.Southam CM, & Ehrlich J. (1943). Effects of extracts of western red-cedar heartwood on certain wood-decaying fungi in culture. Phytopathology, 33, 517–524. doi: 10.3390/ijms140713109 [DOI] [Google Scholar]
- 106.Stearns SC 1989. Trade-offs in life-history evolution. Functional Ecology, 3, 259–268. doi: 10.2307/2389364 [DOI] [Google Scholar]
- 107.Storey KB, & Storey JM (1988). Freeze tolerance in animals. Physiological Reviews, 68(1), 27–84, 10.1152/physrev.1988.68.1.27 [DOI] [PubMed] [Google Scholar]
- 108.Storey KB (1996). Oxidative stress: animal adaptations in nature. Brazilian Journal of Medical and Biological Research, 29, 1715–1733. [PubMed] [Google Scholar]
- 109.Tezuka T, Hotta T, & Watanabe I. (1993). Growth promotion of tomato and radish plants by solar UV radiation reaching the Earth’s surface. Journal of Photochemistry and Photobiology B: Biology, 19(1), 61–66, 10.1016/1011-1344(93)80094-P [DOI] [Google Scholar]
- 110.Thany SH, & Gauthier M. (2005). Nicotine injected into the antennal lobes induces a rapid modulation of sucrose threshold and improves short-term memory in the honeybee Apis mellifera. Brain research, 1039(1–2), 216–219, 10.1016/j.brainres.2005.01.056 [DOI] [PubMed] [Google Scholar]
- 111.Visser B, Williams C,M, Hahn DA, Short CA, López-Martínez G. (2018). Hormetic benefits of prior anoxia exposure in buffering anoxia stress in a soil-pupating insect. Journal of Experimental Biology, 221(6), doi: 10.1242/jeb.167825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wada T, Matsukura K. (2011). Linkage of cold hardiness with desiccation tolerance in the invasive freshwater apple snail, Pomacea canaliculata (Caenogastropoda: Ampullariidae). Journal of Molluscan Studies, 77(2), 149–153, 10.1093/mollus/eyq049 [DOI] [Google Scholar]
- 113.Won EJ, Lee Y, Han J, Hwang UK, Shin KH, Park HG, & Lee JS (2014). Effects of UV radiation on hatching, lipid peroxidation, and fatty acid composition in the copepod Paracyclopina nana. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology, 165, 60–66, 10.1016/j.cbpc.2014.06.001 [DOI] [PubMed] [Google Scholar]
- 114.Wright GA, Baker DD, Palmer MJ, Stabler D, Mustard JA, Power EF, … & Stevenson PC (2013). Caffeine in floral nectar enhances a pollinator’s memory of reward. Science, 339(6124), 1202–1204, doi: 10.1126/science.1228806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wu BS, Lee JK, Thompson KM, Walker VK, Moyes CD, & Robertson RM (2002). Anoxia induces thermotolerance in the locust flight system. Journal of Experimental Biology, 205(6), 815–827, http://www.worldcat.org/issn/1477-9145 [DOI] [PubMed] [Google Scholar]
- 116.Ya J, Ju Z, Wang H, Zhao H. (2019). Exposure to cadmium induced gut histopathological damages and microbiota alterations of Chinese toad (Bufo gargarizans) larvae. Ecotoxicology and environmental safety, 180, 449–456, 10.1016/j.ecoenv.2019.05.038 [DOI] [PubMed] [Google Scholar]
- 117.Yi SX, Moore CW, & Lee RE (2007). Rapid cold-hardening protects Drosophila melanogaster from cold-induced apoptosis. Apoptosis, 12(7), 1183–1193, 10.1007/s10495-006-0048-2 [DOI] [PubMed] [Google Scholar]
- 118.Yi SX, Gantz JD, Lee RE (2017). Desiccation enhances rapid cold-hardening in the flesh fly Sarcophaga bullata: evidence for cross tolerance between rapid physiological responses. Journal of Comparative Physiology B, 187(1), 79–86, 10.1007/s00360-016-1030-0 [DOI] [PubMed] [Google Scholar]
- 119.Yoder JA, Benoit JB, Denlinger DL, & Rivers DB (2006). Stress-induced accumulation of glycerol in the flesh fly, Sarcophaga bullata: evidence indicating antidesiccant and cryoprotectant functions of this polyol and a role for the brain in coordinating the response. Journal of Insect Physiology, 52(2), 202–214. 10.1016/j.jinsphys.2005.10.005 [DOI] [PubMed] [Google Scholar]
- 120.Youn H, Renault D, & Colinet H. (2018). Hormesis-like effect of mild larval crowding on thermotolerance in Drosophila flies. Journal of Experimental Biology, 221(2), doi: 10.1242/jeb.169342 [DOI] [PubMed] [Google Scholar]
- 121.Yusifov NI, Kuzin AM, Agaev FA, & Alieva SG (1990). The effect of low level ionizing radiation on embryogenesis of silkworm, Bombyx mori L. Radiation and environmental biophysics, 29(4), 323–327. 10.1007/BF01210412 [DOI] [PubMed] [Google Scholar]
- 122.Zhikrevetskaya S, Peregudova D, Danilov A, Plyusnina E, Krasnov G, Dmitriev A, Kudryavtseva A, Shaposhnikov M, & Moskalev A. (2015). Effect of low doses (5–40 cGy) of gamma-irradiation on lifespan and stress-related genes expression profile in Drosophila melanogaster. PLoS One, 10(8), 10.1371/journal.pone.0133840 [DOI] [PMC free article] [PubMed] [Google Scholar]