Abstract
Glycoderivatives are an important class of molecules with enormous relevance in numerous biological phenomena; therefore, they have a key role in the learning, understanding, and assessment of different diseases. Nanotechnology, and in particular the design of new nanomaterials, is one of the areas of greatest interest today. In this case, graphene nanomaterials represent very interesting platforms for studying glycosystems, glyconanomaterials that combine the biomolecular recognition and the characteristics of nanoscale objects in the development of early diagnosis systems, and efficient specific therapeutic modalities. In this mini-review, we discuss some results recently described in the literature on the conjugation of graphene materials and carbohydrates through the selective interaction of glycoenzymes in graphene to create new materials with biosensing applications, the development and application of sugar–graphene composites, and finally biosystems combining the properties of graphene with metallic nanoparticles and sugars for the creation of excellent glyconanomaterials as novel systems for the therapy or diagnosis of important diseases such as cancer or diabetes.
1. Introduction
Saccharides of cell surface glycoproteins and glycolipids contribute to many biological phenomena such as adhesion, cancer cell metastasis, cellular recognition, or infection of pathogens by protein–carbohydrate interactions.1,2 However, a single interaction is usually weak, but multivalent ligands can compensate for this deficiency by binding multiple binding sites to one biological entity simultaneously.
At this point, many different research laboratories have developed novel strategies for the synthesis of tailor-made glycoderivatives, such as oligosaccharides, glycopolymers, or glycopeptides, as carbohydrate-based multivalent ligands for tailored and structurally defined glycan-based probes for biomedical applications.1
Nanotechnology, and in particular nanomedicine, represents one of the most applied technologies in biomedicine for the prevention and treatment of diseases. This technology involves the use of nanoscale materials—such as biocompatible nanoparticles, nanostructures and nanorobots—for diagnosis, delivery, sensing, or actuation purposes in a living organism.2
In this term, graphene—sp2-hybridized carbon framework of one atom thickness—has emerged as a new alternative in biomedical applications.3 The two-dimensional (2D) planar structure of graphene provides a large surface area for loading drugs/biomolecules and the possibility of conjugating fluorescent dyes for bioimaging. The high near-infrared absorbance makes graphene ideal for photothermal therapy. Also, graphene and derivatives (graphene oxide, GO) showed excellent electrochemical properties better than other materials for biosensor fabrication. Henceforth, graphene turns out to be a reliable multifunctional material for use in diagnosis and treatment. It exhibits antibacterial property by directly interacting with the cell membrane. Potential application of graphene and derivatives (graphene oxide) as a scaffold for the attachment and proliferation of stem cells and neuronal cells is captivating in a tissue regeneration scenario.
Fabrication of 2D graphene into a 3D structure is made possible with the help of 3D printing, a revolutionary technology having promising applications in tissue and organ engineering. Indeed, graphene materials conjugated with different molecules such as quantum dots, PEG, biotin, etc., have been successfully described for biomedical applications.3
However, the efficient, sustainable, and economical production of graphene is an important issue for its industrial development. At this point several strategies have been recently developed using biomolecules for synthesizing few- or multilayers graphene from graphite.4 In particular, one strategy consists of the use of enzymes for selective exfoliation of graphite flakes to graphene, obtaining a functionalized graphene material at mild conditions in a high amount.4a
Glyconanomaterials have been recently described by different strategies involving metals, magnetism, fluorescence or liposome, silica glycomaterials, polymers, and dendrimers.5 Graphene nanomaterials represent very interesting platforms to study glycosystems. Glyconanomaterials which combine the biomolecular recognition and the features of nanoscale objects, where graphene has important roles in enhancing the relatively weak affinities of single carbohydrate ligands to the corresponding receptors, effectively amplifies the carbohydrate-mediated interactions.
Furthermore, the application of metal nanoparticles —particularly gold, silver or magnetic iron nanoparticles— has demonstrated to be an advantage in biomedicine,6 being the combination of these with glycans and graphene a new technology to create smart materials in biology.
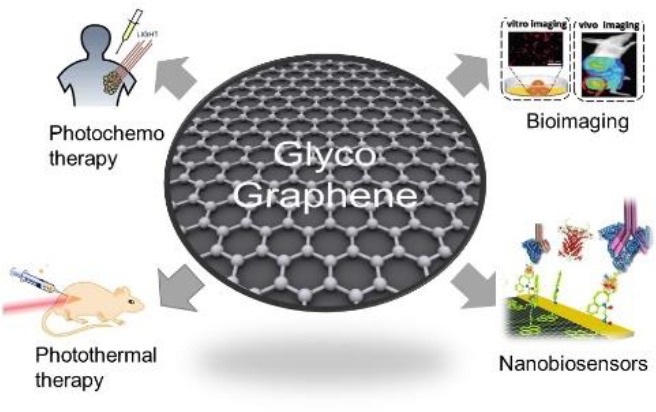
In this mini-review, we have focused on the main current results described in the literature on the development of graphene-based glyconanomaterials (glycographene) for applications in photochemo and photothermal therapy, bioimaging and nanobiosensor for different diseases (Figure 1).
Figure 1.
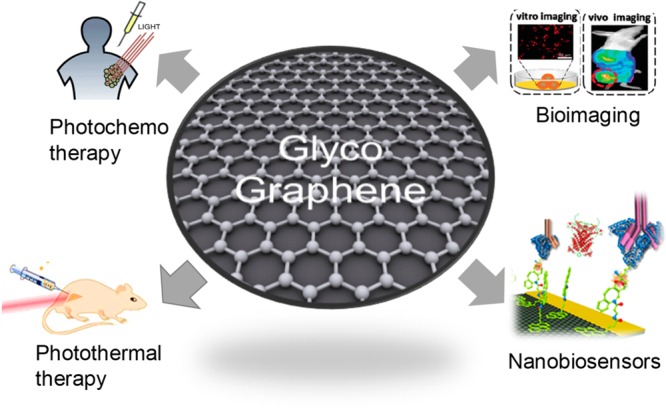
New graphene glyconanomaterial (glycographene) technology in biosensor and therapy applications.
2. Immobilization of Glycoenzymes on Graphene Materials
Graphene is becoming a very attractive material for protein immobilization to be used as biosensors for detection of carbohydrates.7 The most important and widely applied are glucose biosensors based on immobilization of glucose oxidase (GOX), but recently there has been an increase in interest for the preparation of efficient lactose biosensors, which is a serious challenge due to the necessity of immobilization of two enzymes, β-galactosidase and GOX. In a vast majority of publications, biosensors for sugar determination with immobilized enzymes are constructed to measure the electrochemical signal, but there are also important examples of enzyme-containing optical sugar biosensors. With respect to the biochemical part of the biosensor, enzyme-based biosensors are still the most frequently used biosensing platforms surpassing the immunosensors (using antibodies as the recognition element), the genosensors (using nucleic acids), the aptasensors (using aptamers), and the cell-based sensors.8
Nanomaterials are becoming increasingly important in this field of application because they have properties that can overcome the drawbacks of conventional biosensors (limited stability, biofouling, and matrix interferences) and improve the performance factors of electrochemical biosensors such as sensitivity, response time, and limit of detection (LOD). The large surface area increases the immobilized biomolecule loading, but additional requests are ease of preparation, chemical stability for the use as a suitable matrix in biosensor fabrication, and fast electron transfer kinetics, which enhance the electroactive surface area. Among them, electrochemical enzymatic biosensors based on graphene or graphene oxide have an important role in the pharmaceutical and biomedical fields due to their sensitivity, specificity, cost-effectiveness, simplicity, and possibility of miniaturization.
The modification of the graphene surface with metals has been demonstrated as an alternative to improve biosensor performance.9−11
In this way, Park and co-workers have recently developed a nanostructured metal–enzyme–graphene composite as a glucose biosensor.9
The nanomaterial was created on a gold chip, generating on it GO nanofibers by using poly(vinyl alcohol) (PVA) with GO, and then AuNPs were deposited on graphene material. The final step was deposition of the glucose oxidase (GOX)/peroxidase-Cu nanoflower nanohybrid on the AuNP–graphene. This enzyme–graphene composite was tested as a biosensor, obtaining highly linear electrochemical response to glucose concentration, with limit of detection as low as 0.018 μM glucose.9 Moreover, this novel biosensor showed very high selectivity and stability.9
Another interesting metal–graphene system as a glucose biosensor based on a very complex amine-terminated multiwall carbon nanotube (MWCNT)/polyaniline/reduced graphene oxide/gold nanoparticle modified screen-printed carbon electrode (SPCE) with immobilized GOX has been recently developed.10 The NH2-MWCNT/rGO hybrid composite was applied due to better electrochemical sensing ability, enhanced direct electron transfer, and more capacitance when compared with simple NH2-MWCNTs and rGO-modified electrodes. For improvement of electrical conductivity, polyaniline was included in the composite electrode, without any binder, in order to shorten the ion diffusion path and reduce interfacial resistance. Then citrate-stabilized gold nanoparticles were incorporated, and GOX was directly immobilized on the fucntionalized graphene electrode by adsorption.10 The biosensor was tested by amperometric analysis in a human blood serum sample with very high selectivity, stability with a low detection limit of 64 μM, and good sensitivity (246 μ Acm–2 mM–1).10
Among the most widely used mediators for the construction of amperometric biosensors are ferrocene derivatives. The modification of the reduced graphene oxide (rGO) surface with nanosized ferrocene moieties has been recently developed as new glucose biosensors.10 Two different ferrocene starting materials were used, and the graphene oxide doped with carboxylated ferrocene exhibited the best conductivity. Furthermore, these carboxylic groups were used for covalent immobilization of GOX on the graphene surface. The biosensor showed very high selectivity toward glucose with a low detection limit of 0.01–0.04 μM.11
Another direction of biosensor dosing is the application of site-specific interactions between enzymes and nanomaterials by bioaffinity or cofactor–apoenzyme interaction (Figure 2).
Figure 2.
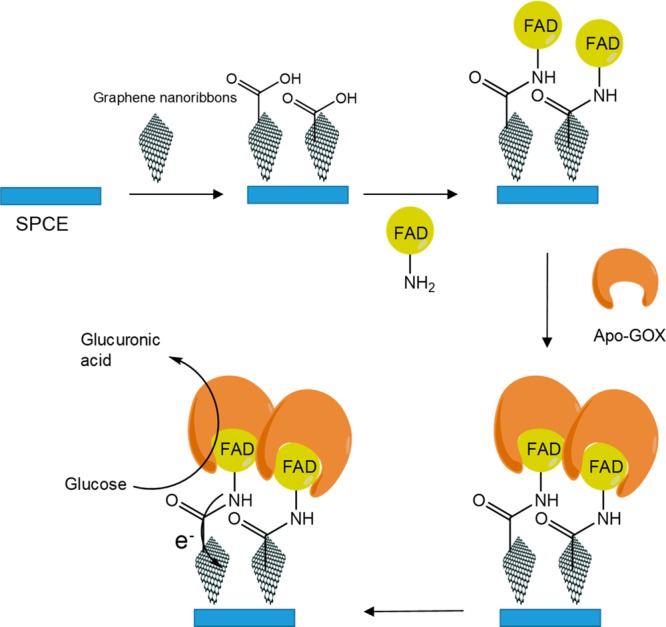
Immobilization of GOX based on the apoenzyme–cofactor interaction.
One example of the latter has been described by Kalcher and co-workers.12 The idea consisted of the fabrication of a glucose biosensor by a site-specific immobilization of GOX by FAD-functionalized graphene. FAD is a cofactor of the enzymes, and this was used as a biosensor with desired orientation of enzyme and improved electron transfer due to the direct electrical communication between the cofactor and electrode surface (Figure 2). FAD was covalently attached to graphene nanoribbons (previously adsorbed on a screen-printed carbon electrode), and in the next step the apoenzyme was added to recombine with the cofactor. Analytical performance of the biosensor was analyzed by amperometric measurements. Measured LOD was 0.1 mM glucose, which has lower sensitivity in comparison to other nanomaterial-based biosensors, but it has sufficient sensitivity for glucose determination in human serum.12
In another case, a very interesting example of bioaffinity immobilization of GOX was described by Zhou and co-workers.13 The immobilization was based on the fact that GOX is a glycoprotein with high-mannose-type carbohydrate content (10–16%, w/w), which can be used as an immobilization site by using a lectin–sugar interaction. Concanavalin A (ConA), being the most extensively studied lectin, was used in this immobilization. Hence, selectively oriented immobilization of GOX on GO was based on the lectin–sugar biospecific interaction between ConA and the glycoenzyme, which is important for the final efficiency of the biosensor.14 First, ConA was covalently immobilized on preactivated GO through diimide-activated amidation. Second, oriented immobilization of GOX was performed based on the strong biospecific affinity between sugar residues of GOX and ConA.13
Lactose biosensors are more complex because the transformation of lactose to an electrochemically detectable compound (hydrogen peroxide) comprises two consequetive reactions: hydrolysis of lactose to glucose and galactose catalyzed with β-galactosidase and subsequent GOX-catalyzed oxidation of glucose to gluconic acid with production of H2O2. An interesting example of graphene application for lactose biosensors has been recently reported by Nguyen et al.15 focused on enzyme-co-immobilization on the Pt microelectrode modified with graphene and 1,5-polydiaminonaphthalene (P(1,5-DAN)) film, where the combination enhanced the sensitivity of the electrode. Finally, the performance of the obtained electrode was tested as lactose biosensors and excellent linearity of the relationship between amperometric output, and lactose concentration was observed with LOD of 1.3 μg mL–1 and sensitivity of 1.33 μA ml μg–1.15
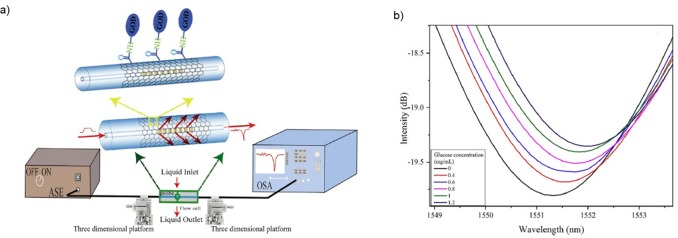
Although biosensors with immobilized enzymes for detection of sugars almost exclusively use electrochemical measurement as output correlated with sugar concentration, other types of detection methods are emerging. Recently, Cai and co-workers have developed an optical fiber-based in situ label-free glucose biosensor (Figure 3).16
Figure 3.
(a) Fiber optic biosensor with covalently immobilized GOX and experimental setup for an optic glucose sensor. (b) Transmission spectra of an optimized optic biosensor. Figure was reprinted from ref (16). Copyright © 2020, Elsevier.
The first step in optical biosensor construction was deposition of GO film on long-period fiber grating (LPFG), which is particularly appropriate for detection of biological molecules. Then, immobilization of GOX on GO was performed by mixing EDC and NHS as two cross-linking reagents. It must be emphasized that for adequate performance of an optical biosensor it is necessary to select a suitable sensitive material for improving grating sensitivity. The LPFG sensor system consists of a light source, LPFG probe, and an optical spectrum analyzer (Figure 3a). The reaction catalyzed by GOX creates gluconic acid and H2O2, which lead to an evident shift of the LPFG transmission spectrum due to the greater change of the surrounding refractive index (Figure 3b). The sensitivity of glucose measurement was 0.77 nm. Moreover, the selectivity of the LPFG biosensor was high in the presence of usual interferences in the determination of blood glucose concentration (ascorbic acid, Na+, K+, Cl–, ...). Additionally, another advantage of this system was a very short response time determined (2.16 s).16
3. Conjugation of Carbohydrates to Graphene Materials
Coating and functionalization of graphene derivative materials (GO and rGO) have been demonstrated to be efficient methods for the preparation of novel nanomaterials to be employed in cancer treatment, in particular for drug delivery, photothermal therapy (PTT), and combination cancer therapy.17−21 The simultaneous reduction and functionalization process of GO derivatives enables us to prepare and carry out direct surface modification to fulfill the requirements of combinatorial cancer therapy. In this sense, polysaccharides, such as hyaluronic acid, chitosan, and fucoidan, can be promising for functionalization and reduction of GO derivatives based on their biocompatibility, aqueous solubility, and potential biologically active characteristics due to there being plenty of available functional groups.19
Ligand-modified polymers, such as mannose-modified cyclodextrin or lactose-functionalized magnetoliposomes, have been demonstrated as efficient targets for liver cancer cells.21 In this way, a large amount of materials modified with galactose (i.e., galactosylated chitosan, galactosylated polyethylene glycol, galactose-modified polyethylene acetate, and galactose-modified polycaprolactone) have been used as a drug delivery system for hepatic carcinoma therapy. Thus, Yu and co-workers21 have developed a novel drug delivery system consisting of nanoparticles based on galactosylated chitosan/graphene oxide/doxorubicin (GC–GO–DOX). DOX is a widely used aromatic drug for cancer treatment. The use of chitosan (CS) glycopolymer was aimed at not only increasing the biocompatibility of the drug carrier for sustained and controlled drug release but also improving the stability of the nanocomposite in biological media.
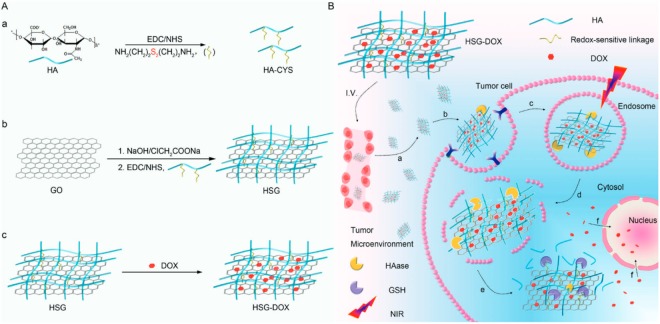
On the other hand, since the lack of active targeting (and subsequently a poor tumor cell internalization) has limited the therapeutic effect of GO-based drug delivery systems and paradoxically exacerbated collateral damage, hyaluronic acid (HA) has been employed in order to investigate the possibility of active targeting. HA is a well-understood biodegradable polymer which exhibits low immunogenicity and specific targeting to receptors such as cluster determinant 44 (CD44) overexpressed in many malignant tumors. Huo and co-workers18 developed a redox-sensitive, hyaluronic-acid-decorated graphene oxide nanosheet (HSG) for tumor cytoplasm-specific rapid delivery using near-infrared (NIR) irradiation-controlled endo-/lysosome disruption and redox-triggered cytoplasmic drug release (Figure 4). The prepared system consisted of GO conjugated via disulfide linkages with a synthesized thiol-glycopolymer (HA-CYS).
Figure 4.
(A) Scheme of HSG-DOX nanosheet preparation and (B) NIR irradiation-controlled endo-/lysosomal escape for tumor cytoplasm-selective delivery for enhanced cancer treatment. Reprinted from ref (18). Copyright © 2017, American Chemical Society.
After the glycopolymer modification of GO, DOX was added to the material, taking advantage of the high drug loading capacity of GO due to its π-conjugated system and large specific area, keeping on the graphene surface via π–π stacking interaction.
The modified sugar moiety improves the system compared to standard graphene oxide, including high biological stability, enhanced drug loading capacity for aromatic molecules, HA receptor mediated active tumor targeting, greater NIR absorption and thermal energy translation, and a sharp redox-dependent response for accelerated cargo release.18
Another recent strategy is based on the preparation of a new glycographene, based on the functionalization of reduced graphene oxide with a new glycopolymer, synthesized by grafting hyaluronic acid onto poly(maleic anhydride-alt-1-octadecene) (PMAO).20
This amphiphilic polymer permits us to obtain a graphene material with a hydrophilic surface with sugar molecules to be used in targeted cancer PTT.
Considering the sustainability and economical point of view in the fabrication of nanosystems applied in biomedicine, the use of a natural source could represent a good alternative. At this point, Fucoidan (Fu), a fucose-rich sulfated polysaccharide which is extracted from brown algae and brown seaweed, has been demonstrated to display advantageous properties such as antiviral, anticancer, antiproliferative, and anti-inflammatory effects,19 thereby indicating the convenience of its use for biological applications.
In this regard, Kim and co-workers19 have created a nanoglyco device using a Fu glycopolymer with a combinatorial approach for cancer therapy. Fu was previously conjugated on the graphene oxide by chemical reaction of OH of the sugar with the epoxide group of the graphene oxide. This enables us to obtain a hydrophilic water-soluble graphene material.19
After that, the DOX molecule was conjugated with the polymer on the graphene surface to obtain the nanoglycomaterial. Then, the photothermal therapeutic effect and cytotoxicity of Fu-rGO, photothermal intracellular distribution of DOX-Fu-rGO, and combinatorial therapeutic effect of DOX-Fu-rGO were studied using human cervical cancer line (HeLa) cells.19
Excellent cellular permeabilization was observed for the DOX-Fu-rGO nanomaterial with a sustained release of DOX inside the HeLa cells. Furthermore, the trimodal treatment of chemo-biothermo (DOX/Fu/PTT) treated cells showed only 10.99% of cell viability, considering 100% for the untreated HeLa cells.19 These results attest the efficiency of this combinatorial treatment.
4. Conjugation of Carbohydrate and Metal Nanoparticles to Graphene Materials
Graphene or graphene oxide (GO), as a two-dimensional carbon (2D) nanomaterial with unique physicochemical properties, has attracted much attention in the field of therapy and diagnosis. GO and graphene have good biocompatibility and near-infrared absorbance (NIR), thus creating an opportunity to prepare carriers of cancer photochemotherapy drugs. Recently, a widely prescribed strategy to achieve efficient external targeting is to integrate GO with metal nanoparticles (Fe, Au, Cu, etc.) to form a hybrid, which can lead to drug carriers through an external magnetic field in both separations as in the treatment of the tumor.22 In addition, if a biomolecule (DNA, enzyme, glucose, hyaluronic acid, etc.) is added to this hybrid, it can help to incorporate the medicine and offer greater biocompatibility.23−26
In particular, a simple carbohydrate molecule as glucose was applied for the preparation of a glyco-Fe-graphene material.23 This is an interesting and green strategy where Fe2+ can promote oxidation of the glucose in the presence of oxygen dissolved in the aqueous solution as shown in the following reaction:
This caused a chemical exfoliation of reduced graphene oxide sheets with suitable water solubility through functionalization of the reduced sheets by gluconate ions.
Thus, this new glyco-Fe material (GRGO-Fe) was utilized as a biocompatible nanomaterial suspension for a highly efficient NIR photothermal therapy of LNCaP prostate cancer cells in vitro. It was found that the GRGO-Fe with a concentration of 1 mg mL–1 required only 0.5 min for complete destruction of the cancer cells under irradiation of an 808 nm laser source with power density of 7.5 W cm–2. These results indicated that the GRGO-Fe can be one promising biocompatible nanomaterial for photothermal nanotherapy of cancer cells.
Recently, a novel glycosylated protein-Co nanosheet–graphene system has been developed as a nitrite electrochemical biosensor.24 A porous Co3O4 hexagonal nanosheet was conjugated with horseradish peroxidase (HRP), and then this was immobilized on reduced graphene oxide. Finally, this nanocomposite was supported on a glassy carbon electrode. Co3O4 nanosheets show excellent biocompatibility providing a protective microenvironment for HRP to retain its enzymatic stability and activity. Also, graphene material is an outstanding medium of efficient electrical communication between the enzyme and the electrode. For the entrapment of the target substrate on its high surface area, the resulting substrate gets close to a large quantity of enzymes immobilized in the porous Co3O4 nanosheets.24 This nanomaterial displays an extremely low limit of detection (LOD) of nitrite as 0.21 μM.24
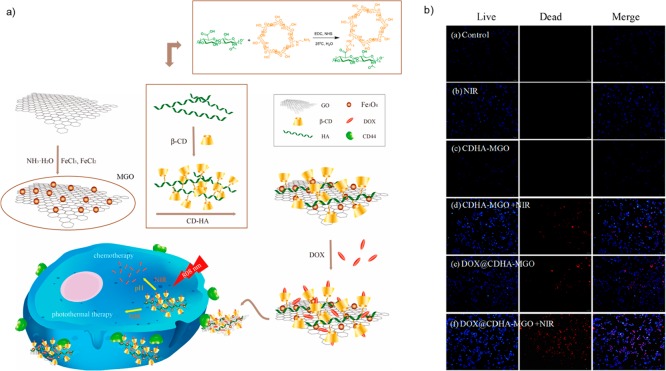
Another type of glyconanomaterial has been synthesized by Shuang and co-workers22 for chemotherapy (Figure 5) where a new biocompatible and hydrophilic β-cyclodextrin-hyaluronic acid (CDHA) glycopolymer was conjugated with GO grafted with Fe3O4 magnetic nanoparticles (MGO) (Figure 5a). GO nanosheets were synthesized from graphite powder and then combined with an iron salt to form magnetite nanoparticles on the graphene surface (Figure 5a).22 On the other hand, the CDHA polymer was synthesized by means of an amide condensation reaction between sodium salt mono-6-deoxy-6-ethylenediamino-β-CD and HA using carbodiimide (EDC)/N-hydroxysuccinimide (NHS) strategy (Figure 5a). The synthesized CDHA polymers improved the stability of MGO under physiological conditions for other sugar molecules which can help the biodistribution in living organisms27 and offered the ability to target cancer cells with HA receptors. Subsequently, a common anticancer drug, doxorubicin (DOX), was loaded into the CDHA-MGO by host–host interaction and hydrophobic interaction. The nanocomposite loaded with DOX (DOX@CDHA-MGO) exhibited sustainable releases activated by NIR in the acidic environment of the tumors (Figure 5a). The in vitro DOX@CDHA-MGO cell absorption experiment showed recognition of active targeting mediated by the CD44 receptor and chemo–photothermal synergistic therapy of hepatoma cells (Figure 5b). Therefore, this glycoiron nanomaterial possesses an important feature: (i) high loading efficiency of antitumor drug for chemotherapy, (ii) high NIR absorption and efficient heat transformation for photothermal therapy, (iii) CD44 targeting for accumulation within tumor cells via HA conjugation, and (iv) easy separation and magnetic targeting properties with an external magnetic field.
Figure 5.
(a) Synthesis and application of DOX@CDHA-MGO. (b) In vitro assay of photochemotherapy of tumor cells. Blue: live cells. Red: dead cells. Bar = 50 μm. Figure was adapted from ref (22) which is an open access article distributed under the Creative Commons Attribution License which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
In 2019, the measurement of glycated hemoglobin (known as HbA1c), the most important glycated protein according to the American Diabetes Association,28 has been identified as a standard diagnostic tool for detection, evaluation, and management of diabetes. This glycosylated variety of hemoglobin is formed in nature when this protein is exposed to plasma sugars (glucose, galactose, or fructose), and its N-terminal valine residues are chemically modified via a nonenzymatic pathway. The formation of the sugar–Hb linkage indicates the presence of excessive sugar in the bloodstream, often indicative of diabetes.29 Thus, Omidinia and co-workers25 have recently developed a disposable electrochemical nanobiosensor for HbA1c determination in real blood samples. In this case a gold–graphene nanocomposite was fabricated. Selective interaction of Au–thiol groups was used as a mechanism for HbA1c identification in blood samples.
This nanocomposite showed extraordinary properties to the electrode, providing a repeatable and reproducible measurement with exceptionally high sensitivity (269.2 μA cm–2) in comparison with other biosensors and wide linear range of 1 nM–13.83 μM with a low detection limit of 1 nM.25
5. Summary and Outlook
In this mini-review, we outlined a variety of novel nanomaterials based on the combination of graphene and glycoderivatives, exhibiting promising properties regarding its application in diagnosis and therapy of different diseases such as cancer or diabetes. Also, the development of graphene–glycoenzyme materials as sugar biosensors has been discussed.
Therefore, future aspects could include the creation of new systems of more simple production of functionalized graphene which permits a selective modification of glycomolecules, glycopeptides, and glycoproteins for biosensor preparation, where the selectivity of the molecule is crucial, or novel methods to control the transport and realese of a target molecule. Also, the combination of glycographene with metal nanoparticles which improved therapeutic strategies, such as thermotherapy system, will be crucial. Therefore, the application of a multidisciplinary collaborative approach will permit successful fabrication of new materials for future nanomedicine.
Acknowledgments
This work was supported by the Spanish Government, the Spanish National Research Council (CSIC), by Project 201980E081 and the Serbian Ministry of Education, Science, and Technological Development (Project III 46010). The Authors would like to acknowledge networking support by the COST Action CA18132 (GLYCONANOPROBES).
Biographies
Noelia Losada-Garcia was born in Estepona, Malaga (Spain) (1993). She obtained her Bachelor’s degree in Chemical Engineering in 2017 from Universidad de Málaga (UMA). Next, she obtained her Master’s degree in 2019 in Chemical Engineering from Universidad Autonoma de Madrid (UAM) – Universidad Rey Juan Carlos (URJC). She joined Prof. Palomo’s laboratory in 2017 as an internship student. She is currently a Ph.D. student in Palomo’s laboratory at the ICP-CSIC in Madrid. Her research focuses on the development of copper bionanohybrids for application in sustainable chemistry, nanobiomaterials, and biological chemistry.
Ivan Rodriguez-Oliva was born in Malaga, Spain (1998). He is currently finishing his degree in Organic Chemistry from Universidad Autónoma de Madrid (UAM). He completed internship work in J.M. Palomo’s group at the Institute of Catalysis (ICP, CSIC), and he is currently developing his Bachelor’s thesis in Palomo’s laboratory.
Milica Simovic was born in Gornji Milanovac, Serbia (1986). She received her Ph.D. degree in Biochemical Engineering and Biotechnology in 2016 from the University of Belgrade. Currently, she is an Associate Research Scientist at the Department of Biochemical Engineering and Biotechnology of the Faculty of Technology and Metallurgy, Belgrade. She has published more than 20 articles in international scientific journals. Her current research interests are focused on application of enzymes with transglycosylative activity in the synthesis of bioactive compounds and enzymatic synthesis in microaqueous media.
Dejan I. Bezbradica was born in Pakrac, Croatia (1974). He received his Ph.D. degree in Biochemical Engineering and Biotechnology in 2007 from the University of Belgrade. He is a Full Professor at the Department of Biochemical Engineering and Biotechnology of Faculty of Technology and Metallurgy, Belgrade. He has published more than 70 articles in international scientific journals. His current research interests are focused on immobilization of enzymes and their application in biosensors or synthesis of bioactive compounds, kinetic modelling of enzymatic reactions, and design of nanobiocatalysts.
Jose M. Palomo was born in Coin, Malaga (Spain) (1976). He received his Ph.D. degree (summa cum laude) in 2003 from Madrid Universidad Autonoma (Spain) working in the Institute of Catalysis (ICP, CSIC). Afterward, Jose moved to the Max Planck Institute in Dortmund, Germany (2004–2006), as an EMBO postdoctoral researcher. In 2006, he began his appointment as Associate Research Scientist at the Biocatalysis Department in ICP-CSIC. From 2009, he has been a Tenured Scientist (Associate Professor) in ICP-CSIC. Jose has published more than 150 articles in high-impact journals, with more than 7800 citations (H-index of 43). His current research interests are in glycochemistry, nanobiotechnology, nanobiomaterials, protein chemistry, nanocatalysis, chemical biology, and biocatalysis.
The authors declare no competing financial interest.
References
- a Filice M.; Guisan J. M.; Terreni M.; Palomo J. M. Regioselective monodeprotection of peracetylated carbohydrates. Nat. Protoc. 2012, 7, 1783–1796. 10.1038/nprot.2012.098. [DOI] [PubMed] [Google Scholar]; b Delbianco M.; Kononov A.; Poveda A.; Yu Y.; Diercks T.; Jiménez-Barbero J.; Seeberger P. H. Well-Defined Oligo- and Polysaccharides as Ideal Probes for Structural Studies. J. Am. Chem. Soc. 2018, 140, 5421–5426. 10.1021/jacs.8b00254. [DOI] [PubMed] [Google Scholar]
- Li J.; Pu K. Development of organic semiconducting materials for deep-tissue optical imaging, phototherapy and photoactivation. Chem. Soc. Rev. 2019, 48, 38–71. 10.1039/C8CS00001H. [DOI] [PubMed] [Google Scholar]
- a Black A.; Urbanos F. J.; Roberts J.; Acebrón M.; Bernardo-Gavito R.; Juárez B. H.; Robinson B. J.; Young R. J.; Vázquez de Parga A. L.; Granados D. Photodetecting Heterostructures from Graphene and Encapsulated Colloidal Quantum Dot Films. ACS Omega 2019, 4, 15824–15828. 10.1021/acsomega.9b01449. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Saifullah B.; Buskaran K.; Shaikh R. B.; Barahuie F.; Fakurazi S.; Moklas M.A. M.; Hussein M. Z. Grapheneoxide–PEG–protocatechuic acid nanocomposite formulationwith improved anticancer properties. Nanomaterials 2018, 8, 820. 10.3390/nano8100820. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Chen P.; Yue H.; Zhai X.; Huang Z.; Ma G.-H.; Wei W.; Yan L.-T. Transport of a graphene nanosheet sandwiched inside cell membranes. Sci. Adv. 2019, 5, eaaw3192 10.1126/sciadv.aaw3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Losada-Garcia N.; Rodriguez-Otero A.; Palomo J. M. Efficient production of multi-layer graphene from graphite flakes in water by lipase-graphene sheets conjugation. Nanomaterials 2020, 10, 7. 10.3390/nano10010007. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Zhao S.; Xie S.; Zhao Z.; Zhang J.; Li L.; Xin Z. Efficiency Production of Graphene by Tannic Acid-Assisted Exfoliation of Graphite in Water. ACS Sustainable Chem. Eng. 2018, 6, 7652–7661. 10.1021/acssuschemeng.8b00497. [DOI] [Google Scholar]
- Hao N.; Neranon K.; Ramström O.; Yan M. Glyconanomaterials for biosensing applications. Biosens. Bioelectron. 2016, 76, 113–130. 10.1016/j.bios.2015.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azharuddin M.; Zhu G. H.; Das D.; Ozgur E.; Uzun L.; Turner A. P. F.; Patra H. K. A repertoire of biomedical applications of noble metal nanoparticles. Chem. Commun. 2019, 55, 6964–6996. 10.1039/C9CC01741K. [DOI] [PubMed] [Google Scholar]
- Kucherenko I. S.; Soldatkin O. O.; Kucherenko D. Y.; Soldatkina O. V.; Dzyadevych S. V. Advances in nanomaterial application in enzyme-based electrochemical biosensors: a review. Nanoscale Adv. 2019, 1, 4560–4577. 10.1039/C9NA00491B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritea L.; Tertis M.; Sandulescu R.; Cristea C. Enzymes–Graphene Platforms for Electrochemical Biosensors Design With Biomedical Applications. Methods Enzymol. 2018, 609, 293–333. 10.1016/bs.mie.2018.05.010. [DOI] [PubMed] [Google Scholar]
- Baek S. H.; Roh J.; Park C. Y.; Kim M. W.; Shi R.; Kailasa S. K.; Park T. J. Cu-nanoflower decorated gold nanoparticles-graphene oxide nanofiber as electrochemical biosensor for glucose detection. Mater. Sci. Eng., C 2020, 107, 110273. 10.1016/j.msec.2019.110273. [DOI] [PubMed] [Google Scholar]
- Maity D.; Minitha C. R.; Rajendra Kumar R. T. Glucose oxidase immobilized amine terminated multiwall carbon nanotubes/reduced graphene oxide/polyaniline/gold nanoparticles modified screen-printed carbon electrode for highly sensitive amperometric glucose detection. Mater. Sci. Eng., C 2019, 105, 110075. 10.1016/j.msec.2019.110075. [DOI] [PubMed] [Google Scholar]
- Matysiak-Brynda E.; Sek J. P.; Kasprzak A.; Królikowska A.; Donten M.; Patrzalek M.; Poplawska M.; Nowicka A. M. Reduced graphene oxide doping with nanometer-sized ferrocene moieties – New active material for glucose redox sensors. Biosens. Bioelectron. 2019, 128, 23–31. 10.1016/j.bios.2018.12.037. [DOI] [PubMed] [Google Scholar]
- Mehmeti E.; Stanković D. M.; Chaiyo S.; Zavasnik J.; Žagar K.; Kalcher K. Wiring of glucose oxidase with graphene nanoribbons: an electrochemical third generation glucose biosensor. Microchim. Acta 2017, 184, 1127–1134. 10.1007/s00604-017-2115-5. [DOI] [Google Scholar]
- Zhou L.; Jiang Y.; Gao J.; Zhao X.; Ma L.; Zhou Q. Oriented immobilization of glucose oxidase on graphene oxide. Biochem. Eng. J. 2012, 69, 28–31. 10.1016/j.bej.2012.07.025. [DOI] [Google Scholar]
- Sumaryada T.; Gunawan M. S.; Perdana S.; Arjo S.; Maddu A. A Molecular Interaction Analysis Reveals the Possible Roles of Graphene Oxide in a Glucose Biosensor. Biosensors 2019, 9, 18. 10.3390/bios9010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen B. H.; Nguyen B. T.; Van Vu H.; Van Nguyen C.; Nguyen D. T.; Nguyen L. T.; Vu T. T.; Tran L. D. Development of label-free electrochemical lactose biosensor based on graphene/poly(1,5-diaminonaphthalene) film. Curr. Appl. Phys. 2016, 16, 135–140. 10.1016/j.cap.2015.11.004. [DOI] [Google Scholar]
- Xu B.; Huang J.; Ding L.; Cai J. Graphene oxide-functionalized long period fiber grating for ultrafast label free glucose biosensor. Mater. Sci. Eng., C 2020, 107, 110329. 10.1016/j.msec.2019.110329. [DOI] [PubMed] [Google Scholar]
- Fan Y.; Shi S.; Ma J.; Guo Y. A paper-based electrochemical immunosensor with reduced graphene oxide/thionine/gold nanoparticles nanocomposites modification for the detection of cancer antigen 125. Biosens. Bioelectron. 2019, 135, 1–7. 10.1016/j.bios.2019.03.063. [DOI] [PubMed] [Google Scholar]
- Yin T.; Liu J.; Zhao Z.; Zhao Y.; Dong L.; Yang M.; Zhou J.; Huo M. Redox Sensitive Hyaluronic Acid-Decorated Graphene Oxide for Photothermally Controlled Tumor-Cytoplasm-Selective Rapid Drug Delivery. Adv. Funct. Mater. 2017, 27, 1604620. 10.1002/adfm.201604620. [DOI] [Google Scholar]
- Kang S.; Hong Y. L.; Ku B. C.; Lee S.; Ryu S.; Min D. H.; Jang H.; Kim Y. K. Synthesis of biologically-active reduced graphene oxide by using fucoidan as a multifunctional agent for combination cancer therapy. Nanotechnology 2018, 29, 475604. 10.1088/1361-6528/aadfa5. [DOI] [PubMed] [Google Scholar]
- Lima-Sousa R.; de Melo-Diogo D.; Alves C. G.; Costa E. C.; Ferreira P.; Louro R. O.; Correia I. J. Hyaluronic acid functionalized green reduced graphene oxide for targeted cancer photothermal therapy. Carbohydr. Polym. 2018, 200, 93–99. 10.1016/j.carbpol.2018.07.066. [DOI] [PubMed] [Google Scholar]
- Wang C.; Zhang Z.; Chen B.; Gu L.; Li Y.; Yu S. Design and evaluation of galactosylated chitosan/graphene oxide nanoparticles as a drug delivery system. J. Colloid Interface Sci. 2018, 516, 332–341. 10.1016/j.jcis.2018.01.073. [DOI] [PubMed] [Google Scholar]
- Liang W.; Huang Y.; Lu D.; Ma X.; Gong T.; Cui X.; Yu B.; Yang C.; Dong C.; Shuang S. β-Cyclodextrin-Hyaluronic Acid Polymer Functionalized Magnetic Graphene Oxide Nanocomposites for Targeted Photo-Chemotherapy of Tumor Cells. Polymers 2019, 11, 133. 10.3390/polym11010133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhavan O.; Ghaderi E.; Aghayee S.; Fereydooni Y.; Talebi A. The use of a glucose-reduced graphene oxide suspension for photothermal cancer therapy. J. Mater. Chem. 2012, 22, 13773–13781. 10.1039/c2jm31396k. [DOI] [Google Scholar]
- Liu H.; Guo K.; Lv J.; Gao Y.; Duan C.; Deng L.; Zhu Z. A novel nitrite biosensor based on the direct electrochemistry of horseradish peroxidase immobilized on porous Co3O4 nanosheets and reduced graphene oxide composite modified electrode. Sens. Actuators, B 2017, 238, 249–256. 10.1016/j.snb.2016.07.073. [DOI] [Google Scholar]
- Shajaripour Jaberi S. Y.; Ghaffarinejad A.; Omidinia E. An electrochemical paperbased nano-genosensor modified with reduced graphene oxide-gold nanostructure for determination of glycated hemoglobin in blood. Anal. Chim. Acta 2019, 1078, 42–52. 10.1016/j.aca.2019.06.018. [DOI] [PubMed] [Google Scholar]
- Syama S.; Mohanan P. V. Comprehensive Application of Graphene: Emphasis on Biomedical Concerns. Nano-Micro Lett. 2019, 11, 6–31. 10.1007/s40820-019-0237-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J.; Li J.; Lyu Y.; Miao Q.; Pu K. Molecular optical imaging probes for early diagnosis of drug-induced acute kidney injury. Nat. Mater. 2019, 18, 1133–1143. 10.1038/s41563-019-0378-4. [DOI] [PubMed] [Google Scholar]
- Association A. D. 2. Classification and diagnosis of diabetes. Diabetes Care 2017, 40, S11–S24. 10.2337/dc17-S005. [DOI] [PubMed] [Google Scholar]
- Kaur J.; Jiang C.; Liu G. Different strategies for detection of HbA1c emphasizing on biosensors and point-of-care analyzers. Biosens. Bioelectron. 2019, 123, 85–100. 10.1016/j.bios.2018.06.018. [DOI] [PubMed] [Google Scholar]