Abstract
Cisplatin is a highly toxic material used clinically as a potent chemotherapeutic. While effective against some cancers, toxicity limits widespread use and low solubility confounds delivery. To formulate a better tolerated and more water-soluble form of cisplatin, we designed a rapid expansion of supercritical solutions (RESS) technique with supercritical carbon dioxide (sc-CO2) to collect nanoclusters of cisplatin embedded in dry ice, in a dual-stage collection vessel cooled to liquid nitrogen temperature. These nanoclusters were solubilized in deionized water and further concentrated (up to 51.3 mM) by a Rotovap process, yielding stable cisplatin solutions with solubility up to 15 × (w/w) greater than that of normal cisplatin. Extensive material characterizations of the solutions were carried out to determine any chemical and/or structural changes of the RESS-processed cisplatin. In vitro cytotoxicity studies of these aqueous solutions showed increased cell viability and early apoptosis compared to equivalent concentrations of standard cisplatin solutions. In vivo studies using zebrafish embryos revealed that standard cisplatin solutions were acutely toxic and caused death of rapidly proliferating cells compared to RESS-processed cisplatin, which were better tolerated with reduced general cell death. Increased water solubility and matched chemical identity of RESS-processed aqueous cisplatin solutions indicate the potential to open up novel drug-delivery routes, which is beneficial for new pharmaceutical design and development.
Introduction
The current trend in cancer research is to develop noninvasive, early-stage detection, diagnostics, and either an outright cure or reduction of tumor burden for advanced cases.1,2 In this context, the role of platinum-based drugs on cancer treatment as a standard-of-care chemotherapy has been transformative. Four platinum-based drugs, namely, cisplatin, carboplatin, oxaliplatin, and nedaplatin, are currently approved for the treatment of a range of solid tumors. Among these, cisplatin has been the most potent antitumor and chemotherapeutic agent used for the treatment of many solid malignancies including head, neck, bladder, ovarian, and small and nonsmall lung cancers.3−5 Therefore, cisplatin represents a broad-spectrum anticancer agent.
The anticancer activity of cisplatin is largely attributed to its potent effects on the genome of dividing cells. It causes inter- and intrastrand DNA cross-linking, which inhibits DNA synthesis, repair and replication, and gene transcription. Moreover, it also restricts protein synthesis and cell proliferation6−9 and therefore is only partially selective for tumors, as other rapidly proliferating cells are also affected. Thus, the clinical utility of cisplatin is hampered by its toxicological considerations. While the efficacy of cisplatin on certain types of cancers, namely, testicular and ovarian, is unmatched, the extreme systemic toxicity of the drug reduces compliance with standard treatment regimens.10 This is aggravated by the fact that treatments are in general target-unspecific requiring high dosage levels inflicting extended tissue toxicity. It has also been reported that cisplatin showed a variable cytotoxic response due to drug-handling methods and storage conditions as they potentially impact its chemical stability and water solubility.8−12
The mechanism of acquired cisplatin resistance is attributed to the increase in drug efflux, drug inactivation, alterations in DNA repair, and processing of drug-induced DNA damage.6,8 Because of this, cisplatin treatments have a tendency to induce severe side effects like cochlea damage and hearing loss that relies on various pharmacological parameters like the dosage type (single or cumulative) and administration (schedule and means), systemic and individual factors (skin pigmentation, age, blood pH, diet), and interaction with the therapies.8−11 In addition to the cytotoxic effects, high dosage levels result in a combination of side effects like nephrotoxicity, neurotoxicity, or ototoxicity.6−11
Several strategies to minimize cisplatin dosage without compromising its efficacy have been attempted.6,13 However, the poor water solubility of cisplatin (∼1 mg/mL),14−17 bioavailability, degradation in aqueous solutions, and intravenous mode of administration are the main limiting factors in clinical applications.15−19 Cisplatin is formulated either in a saline solution for clinical use or using a buffer (dimethyl formamide (DMF)/phosphate-buffered saline (PBS)) to prevent drug inactivation prior to administration and to promote drug stabilization. Though cisplatin is highly soluble in dimethylsulfoxide (DMSO), this vehicle results in decreased activity/deactivation due to ligand displacement by the sulfur group in DMSO.16 Neat higher water solubility has the advantage of enabling more effective and less toxic drug-delivery options of cisplatin for the patients. One of the approaches to increase its water solubility would be to exploit the Gibbs–Thomson effect, a well-documented phenomenon of increasing solubility with decreasing particle size.20,21 Among various available particle size reduction technologies, supercritical carbon dioxide (sc-CO2)-based processes are the most promising, as they are scalable, nontoxic, solvent-free, and environmentally compatible.20−22
In this study, we aimed to improve the solubility of cisplatin to increase the capacity for delivery as a chemotherapeutic agent. We describe a new process to generate nanoclusters of cisplatin using a custom-designed, constant (P,T) sc-CO2 process with a 15× higher water solubility. We investigate the efficacy of these aqueous nanocluster solutions from in vitro studies with HeLa cells and in vivo studies using early-stage zebrafish embryos as a whole animal model for toxicity and efficacy. We have recently shown that metal-based compounds delivered through a novel trefoil knot structure effectively induce cell death in the rapidly dividing cells of early zebrafish embryos and tissue culture cells,23 demonstrating the utility of this system for in vivo studies. This combined approach identified the unique properties of a novel cisplatin formulation that could be of importance for improving the delivery of cisplatin as a widely used anticancer drug.
Experimental Section
Rapid Expansion of Supercritical Solutions (RESS) Process
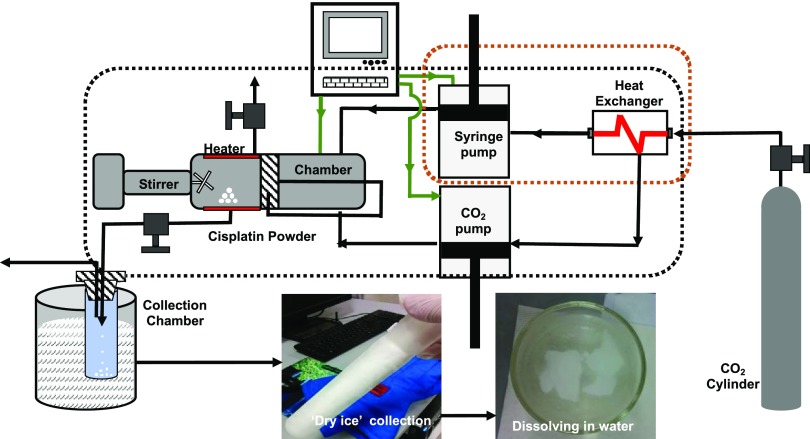
The schematic of the custom-designed RESS equipment is shown in Figure 1. In a RESS experiment, the process chamber is first loaded with 100 mg of standard cisplatin powder (Sigma-Aldrich) and sealed firmly. Liquid carbon dioxide (99.5% purity) is injected into the process chamber using a syringe pump. The total volume of liquid carbon dioxide used for each experiment is 265 mL. The operating pressure of the process chamber is increased to 300 bar by a CO2 pump and maintained constant throughout the experiment. In-built cartridge heaters are used to maintain a chamber temperature of 40 °C throughout the entire process. The above operating pressure and temperature are selected to convert the liquid carbon dioxide into its supercritical state, i.e., supercritical carbon dioxide (sc-CO2). A magnetically coupled mixer with a rotation speed of 120 rpm is used to dissolve the cisplatin powder in sc-CO2 and create a true solution. This mixing process is continued for 6 h when the mixer is turned off, and the system is allowed to equilibrate for 30 min. The outlet of the process chamber is connected to a stainless steel capillary tube (125 μm ID) via a manually operated needle valve.
Figure 1.
Schematic of RESS Process: schematic diagram illustrating the custom-designed supercritical carbon dioxide (sc-CO2)-based rapid expansion of supercritical solutions (RESS) technique used for the precipitation of cisplatin. Standard cisplatin powder (∼100 mg) was dissolved in a continuous stream of carbon dioxide under supercritical conditions (i.e., temperature and pressure of 40 °C and 300 bar, respectively) in a process chamber. This mixture was allowed to expand at atmospheric pressure through capillary in a liquid-nitrogen-cooled collection vessel.
Cisplatin nanoclusters are produced by depressurizing the process chamber at a constant (P,T) by manually opening the needle valve. The outlet of the capillary tube is directed into a dual-stage collection vessel cooled below the freezing point of solid carbon dioxide (T ≤ −78 °C, coolant medium: liquid nitrogen). The expansion process accompanied by a rapid reduction in density results in a turbulent, supersonic jet stream, further cooled by the Joules–Thomson effect. The combination of the sudden increase in super-saturation and turbulence-induced high shear in a cold supersonic jet produced nanoclusters of cisplatin, embedded in dry ice in the dual-stage collection vessel. These nanoclusters embedded in dry ice were further dissolved in deionized water under ambient conditions resulting in a true (i.e., clear and transparent) solution (Figure 1).
The unique feature of the RESS process is that it eliminates the Ostwald ripening phenomenon typically associated with any solution precipitation process.24 During the depressurization, extremely high super-saturation levels are reached in a short timeframe resulting in the precipitation of cisplatin nanoclusters from the sc-CO2 solution, in a cold supersonic jet stream of gaseous CO2. Cisplatin has zero solubility in gaseous CO2, and hence there is zero Ostwald ripening. Our patented process reported here25 describes the ability of our process to produce a highly monodisperse and stable neat cisplatin nanocluster dispersions in water without any surfactants or capping agents. The aqueous dispersions were further concentrated using a low-temperature vacuum evaporation system (i.e., Rotovap system). Cisplatin remained dispersed (“solubilized”) in water over a period of several months at room temperature without any notable visual turbidity or residue.
Material Characterizations
Microscopic Characterizations
RESS-processed cisplatin aqueous solutions were initially characterized to confirm that the drug retained its chemical and structural integrity. The solution was drop-cast and dried on transmission electron microscopy (TEM) grids, and high-resolution transmission electron microscopy (HRTEM) characterization was performed using a Talos F200X Field Emission Gun (FEG) TEM equipped with Ceta 16M camera, with a lattice fringe resolution of 0.14 nm at an accelerating voltage of 200 kV. High-resolution images of periodic structures were analyzed using Totally Integrated Automation (TIA) software. Surface morphology of the dried films was analyzed by a field emission scanning electron microscope (FE-SEM, Quanta FEG 450). Samples were examined at a 10 kV accelerating voltage under high-vacuum conditions. Atomic force microscopy (AFM) characterizations of drop-cast and dried samples were done (Agilent 5500, Keysight Technologies) in noncontact mode. A set point of 1.5 V was kept constant for these measurements. The topographic scans were collected with a speed of 0.30 (In/s) for 512 points/lines. These scans were postprocessed by Gwyddion 2.53 software, an SPM data visualization and analysis tool. X-ray diffraction (XRD) measurements of drop-cast and dried samples on silicon substrates were made using a PANalytical Empyrean X-ray diffractometer equipped with a Cu anode X-ray tube operated at 45 kV and 40 mA in Bragg–Brentano geometry.
Compositional Characterizations
The chemical composition of these drop-cast and dried samples was determined by energy-dispersive X-ray spectroscopy (EDS) attachment of FE-SEM (FE-SEM, Quanta FEG 450). The samples were examined at a 10 kV accelerating voltage and a working distance of 10 mm under high-vacuum condition. EDS spectra were collected over a period of 1 min. A confocal Raman microscope (WiTec Alpha300, WITec Gmbh) at 532 nm excitation was used for chemical composition identification. X-ray photoelectron spectroscopy (XPS, AXIS Ultra DLD, Kratos Analytical) was used to determine the chemical identification and core-level binding energies. The photoelectrons were excited with a monochromatic Al Kα (1486.6 eV) X-ray source operating at an operating power of 117 W. X-ray photoelectron spectroscopy survey spectra were collected using a pass energy of 160 eV. HR-XPS spectra of the elements (Pt 4f and Cl 2p) were collected using a pass energy of 20 eV with a step size of 0.1 eV. Charging effects of the sample were improved by adjusting the C 1s peak at 284.6 eV with a ±0.1 eV accuracy as reference for the surface as well as a local shift of the entire spectrum, respectively. The residual pressure inside the chamber was maintained at 10–8 bar during XPS measurements. High-resolution XPS was used to assess the exact binding energy location and oxidation states. High-resolution XPS data were fitted with the Gaussian peak fitting procedure.
In Vitro Analysis of Cytotoxic Effects
Cell Culture
Human cervical cancer HeLa cells (ATCC No. CCL2) were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Sigma-Aldrich) supplemented with 10% fetal bovine serum (FBS; GE Healthcare Life Sciences), 4500 mg/L dextrose, 4 mM l-glutamine, and 1% penicillin/streptomycin (all from Sigma-Aldrich), in 5% CO2 at 37 °C. Once the cells reached ∼90% confluence, they were split into fractions using 0.25% trypsin-ethylenediaminetetraacetic acid (EDTA) (Sigma-Aldrich) and propagated or used in experiments.
Cell Treatment Solution Preparation
A 5 mM stock solution of standard cisplatin was prepared in water, sonicated for 10 min, and then diluted in DMEM without FBS to obtain final concentrations of 10–500 μM. Likewise, two selected stock solutions, namely, NC-5 (NanoCluster-Cisp-5 of 51 mM concn) and NC-4 (NanoCluster-Cisp-4 of 31 mM concentration), were diluted in DMEM without FBS to obtain the same desired concentrations.
In Vitro Cell Viability Assay
Cell viability was measured using Cell Titer-Blue (CTB) assay (Promega, Mannheim, Germany), which measures the reduction of the nonfluorescent resazurin compound into a highly fluorescent resorufin product by dehydrogenase enzymes in living cells.26 Therefore, the quantity of resorufin product is directly proportional to the number of viable cells. HeLa cells were seeded at a density of 5 × 103 cells/well in 100 μL of complete DMEM in standard 96-well plates. After culturing for 24 h at 37 °C in 5% CO2, the medium was replaced with 100 μL of medium containing standard cisplatin, NC-5, or NC-4, at the desired concentration, and cells were incubated for 24 h at 37 °C. Thereafter, 20 μL of the CTB reagent was added to each well and incubated for 2 h at 37 °C. Cell viability was assessed by measuring the fluorescence of resorufin product (λex/λem = 560:590 nm) on a Synergy H1MF Multi-Mode microplate reader (BioTek, Winooski, Vermont). Control cells were treated with the carrier diluted in DMEM without FBS, while wells with medium (carrier diluted in DMEM without FBS) alone served as a blank. CTB reduction was determined from the ratio of fluorescence of treated wells to control wells.
In Vitro Apoptosis Assay
Apoptotic cells were monitored by using fluorescein isothiocyanate (FITC) Annexin V/Dead Cell Apoptosis Kit (Molecular Probes, Inc., Eugene, OR), in which FITC-conjugated annexin V binds to exposed phosphatidylserines in apoptotic cells,27 and propidium iodide (PI), a membrane-impermeant dye, binds to nucleic acids of necrotic and late apoptotic cells.28
HeLa cells were seeded at a density of 1 × 106 cells/well in complete DMEM in six-well plates and cultured for 24 h at 37 °C in 5% CO2. The cells were then treated with 25 μM standard cisplatin, NC-5, or NC-4, and incubated for an additional 24 h at 37 °C in 5% CO2. Subsequently, the cells were washed with ice-cold PBS, harvested using 0.25% trypsin-EDTA, centrifuged at 1100 rpm for 5 min, and then resuspended in 1× annexin-binding buffer (10 mM HEPES, 140 mM NaCl, 2.5 mM CaCl2, pH 7.4). To 100 μL of cell suspension, 5 μL of FITC-conjugated annexin V and 1 μL of PI (100 μg/mL) were added, followed by incubation at room temperature for 15 min. Immediately afterward, fluorescence was measured using fluorescence-activated cell sorting (FACS) on a BD FACSAria III cell sorter (BD Biosciences, San Jose, CA) and analysis was performed using the BD FACSDiva software. A total of 10 000 cells/sample were analyzed using the green channel FL-1 for FITC-conjugated annexin V and red channel FL-3 for PI to determine the fractions of live (annexin V–/PI–), early apoptotic (annexin V+/PI–), late apoptotic (annexin V+/PI+), and necrotic (annexin V–/PI+) cells.
In Vivo Studies
Zebrafish Husbandry and Treatment
Embryos derived from natural spawning of zebrafish from strains of AB, TAB5, or TAB14 were maintained in a 14:10 light:dark cycle in the NYUAD aquaculture facility. The NYUAD Institutional Animal Care and Use Committee approved all procedures. Embryos were collected within 1 h of spawning, unfertilized eggs and abnormal embryos were removed, and embryos were sorted into individual wells of a six-well plate prior to 6 h post fertilization (hpf). Each assay for embryo viability or cell death included 10–20 zebrafish embryos per exposure and was repeated for at least three clutches. Embryos were treated from 9 hpf to 5 days’ post fertilization (dpf) with 0, 100, and 200 μM of NC-5 and NC-4 in embryo water compared to standard cisplatin diluted from the same 5 mM stock used for in vitro studies. We identified the lethal dose 50 (LD50) to be 250 μM for standard cisplatin (not shown), and therefore chose concentrations just below this, namely, 100 and 200 μM as subtoxic concentrations to test for toxicity in this model. Embryos remained in the drug solution for the duration of the study and scored for mortality at 24, 48, 72, 96, and 120 hpf. To test the effects of chorion on drug accessibility to embryo, the chorion was removed using fine gauge needles prior to 8 hpf where indicated.
Acridine Orange Staining for Cell Death
Surviving embryos at 24 hpf were assessed as described23 using Acridine Orange incorporation to detect cell death. In brief, embryos were dechorionated and incubated for 30 min in 5 μg/mL acridine orange made from a 10 mg/mL stock diluted with egg water. Following exposure, embryos were washed five times with 10× volume of egg water and then mounted on glass slides with 3% methyl cellulose using the GFP filter on Nikon SMZ2500, equipped with a fluorescent attachment, an LED light source, and a Nikon DSQi2 camera.
Statistical Analysis
Data are expressed as mean ± standard deviation (SD). All statistical analyses were carried out using Prism 7.0 software (GraphPad Software, Inc., La Jolla, CA). For in vitro cytotoxicity data analysis, statistical significance between the different treatments was assessed using one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test. For flow cytometry data analysis, Student’s t-test was performed between the treatment and control groups. For zebrafish toxicity studies, Kaplan–Meier plots were used for survival and a log-rank test to assess statistical significance. For the acridine orange assay, larvae with at least five cells that stained with acidine orange cells were scored positive for cell death and one-way analysis of variance (ANOVA) was utilized to assess the statistically significant differences of the treatments. A value of P < 0.05 was considered statistically significant for all of these analyses.
Results
To overcome the issues of solubility and toxicity, we characterized the structural and chemical integrity of the aqueous cisplatin solution NC-0 (i.e., NanoCluster-Cisp-0) obtained by dissolving the nanoclusters embedded in dry ice in deionized water under ambient conditions. We used the Rotovap process in a stepwise fashion to create additional formulations with increasing cisplatin concentrations. Starting with NC-0, we created five formulations, namely, NC-1, NC-2, NC-3, NC-4, and NC-5, with increasing cisplatin concentrations [NC-0] < [NC-1] < [NC-2] < [NC-3] < [NC-4] < [NC-5]. These formulations were neat nanocluster dispersions of cisplatin that do not have any surfactants or other capping agents on their surface to stabilize the primary particles. The uniqueness (i.e., novel characteristic) of our process is that these dispersions are stable in water for over a year without the need of any (foreign) surface-stabilizing agents. In effect, the cisplatin has been solubilized in water. The thermogravimetric analysis technique was used to determine the cisplatin concentration of NC-0, NC-4, and NC-5 to be 8.36, 31.5, and 51.3 mM, respectively. The calculations were performed from the weight percentages of the material recovered at 100 ° C using thermogravimetric analysis (TGA) studies. We have been able to obtain stable nanocluster dispersions (“solutions”) with 15× times more cisplatin in solution than the standard cisplatin solubility.14−16,19 Among these, NC-0 and NC-5 were characterized for microstructural and chemical integrity. NC-4 and NC-5 were selected for in vitro and in vivo studies along with the starting material, referred to as standard cisplatin (RCref) (i.e., standard cisplatin powder dissolved in water).
Material Characterization/Analysis
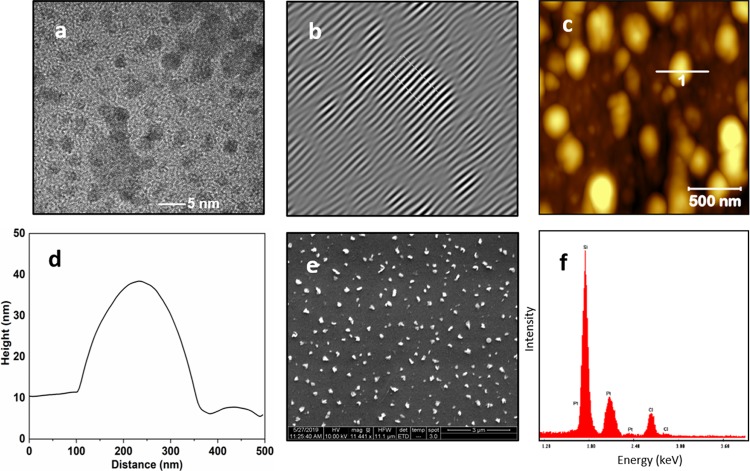
TEM, AFM, SEM, and EDS characterizations of NC-0 are shown in Figure 2a–f. TEM data (Figure 2a,b) revealed finer nanostructures in the size range of 3–5 nm. Lattice fringes evaluated from one of the fine structures by high-resolution TEM imaging showed a fringe width of 0.27 nm. AFM topographic data confirmed the presence of randomly ordered and irregular-shaped structures (Figure 2c), and the height profile extracted from the marked nanostructure is shown in Figure 2d. The size of the nanostructure measured from the height profile is around 200–300 nm. SEM data also showed irregular-shaped submicron-sized nanoparticles in the size range of 200–300 nm (Figure 2e). Compositional analysis using energy-dispersive spectroscopy (EDS) during SEM imaging (Figure 2f) showed Si, Pt, and Cl. The highest intensity peak observed in EDS spectrum was due to the silicon substrate, and the presence of Pt and Cl spectral peaks is attributed to cisplatin. Wide-angle X-ray diffraction (XRD) data for NC-0 films, drop-cast and dried on silicon substrate (Figure S1), showed some of peaks associated with triclinic crystalline (β-polymorph) cisplatin (PDF 00-050-0643).29
Figure 2.
Materials characterization of NC-0: (a, b) TEM imaging shows a cisplatin nanoparticle of typical size of 3–5 nm in diameter. Lattice fringes with a fringe width of 0.27 nm are observed. (c, d) AFM imaging of drop-cast and dried cisplatin reveals irregular-shaped submicron-sized cisplatin nanoparticles. (e) SEM imaging shows diverse shaped submicron-sized nanoparticle with a typical size of 200–300 nm. (f) EDS spectrum collected for NC-0 shows the presence of Pt and Cl in addition to silicon substrate indicative of cisplatin.
Confocal Raman spectrum of NC-0 (Figure S2a) showed characteristic cisplatin bands in the 100–600 cm–1 range, namely, a broad peak at 349 cm–1 (Pt–Cl) and a weak shoulder observed around 522 cm–1 (Pt–N stretching).30−33 Cisplatin is also reported to have Raman-active bands between 1200 and 1700 cm–1 due to NH3 deformation modes.32,34 The Raman bands from the RESS-processed sample (NC-0) compare favorably to the Fourier transform (FT)–Raman data reported by Amado et al. using Nd:YAG (yttrium aluminum garnet) laser with an excitation wavelength of 1064 nm. XPS spectra for NC-0 collected over the binding energy range of 0–300 eV are shown in Figure S2b. The spectra revealed highly resolved bands at 75, 100, 150, 197, and 285 eV for Pt (4f), Si (2p), Si (2s), Cl (2p), and C (1s), respectively. Pt, Cl, and C are attributed to cisplatin, while Si is due to the silicon substrate. High-resolution spectra collected for Pt (4f) in the binding energy range of 68–85 eV (Figure S2c) revealed the presence of three broad peaks that were deconvoluted into two doublets 4f7/2 (71.4, 72.5, 73.7 eV) and 4f 5/2 (75.3, 76.4, 77.8 eV) corresponding to Pt 2+ and Pt 4+ oxidations states.35 High-resolution spectra of Cl (2p) (Figure S2d) revealed Cl 2p3/2 and Cl 2p1/2.36 The precise binding energy locations were identified by deconvolution of the experimental curve at 197.4 and 198.3 eV, respectively. The presence of sharp bands in the high-resolution spectra at Cl 2p1/2 is attributed to chloride ion from solubilized cisplatin in water.37 Overall, it appears that the RESS-processed cisplatin seemed to (mostly) retain its chemical and structural identity. In principle, these characterizations are expected to serve as a benchmark for our future cisplatin optimization studies.
TEM characterization of NC-5 (Figure 3a) revealed a fine particle structure in the agglomerates that is much larger in size than NC-0. High-resolution TEM imaging (Figure 3b) confirmed that the lattice structure of NC-5 is identical to that of NC-0. AFM data (Figure 3c) showed a combination of a distribution of 100–300 nm and 1–5 μm sized particles. The height profile of a selected larger particle was around 700 nm (Figure 3d), about 3 times greater than that of NC-0. SEM data (Figure 3e) were consistent with the AFM observations and showed a distribution of 100–300 nm and 3–5 μm sized agglomerated particles that are much larger than those of NC-0. EDS data collected during SEM imaging (Figure 3f) showed identical elements (i.e., Si, Pt, and Cl) observed for NC-0. The presence of Pt and Cl peaks is ascribed to cisplatin, and the dominant peak is assigned to the silicon substrate. XRD data of the drop-cast and dried film from the concentrated NC-5 solution (Figure S3) was found to be similar to NC-0. XRD data showed the presence of more significant primarily triclinic crystalline (β-polymorph) of cisplatin peaks, consistent with the TEM data.29 In addition, NC-5 showed a distinct super-lattice structure based on observed low-angle peaks. However, the structure could not be elucidated as there was not a self-consistent ordered spacing within the three low-angle peaks. Confocal Raman spectroscopy and XPS data collected for NC-5 were found to be identical to those of NC-0. Therefore, the data collected for NC-0 are only reported and data for NC-5 are not included.
Figure 3.
Materials characterization of the highest cisplatin concentration NC-5: (a, b) TEM imaging shows a higher electron density and spherical-shaped cisplatin nanoparticles. Lattice fringes obtained for the highest cisplatin concentration hold an identical fringe width of 0.27 nm compared to NC-0. (c, d) AFM imaging of drop-cast and dried cisplatin NC-5 reveals irregularly shaped nanosized (100–300 nm) and micron-sized (1–5 μm) particles. (e) SEM imaging shows spherical-shaped (5–10 μm) agglomerated cisplatin particles. (f) EDS spectrum collected for NC-5 shows the presence of Pt and Cl.
Optical UV–vis spectroscopic data (Figure S4) of standard cisplatin (powder) dissolved in deionized water (RCref) as reference show that it is identical to NC-0 with a major absorption band at 200 nm. NC-3 displayed a highly resolved, red-shifted primary band at 230 nm and a shoulder at 285 nm. The dominant absorption band (∼200 nm) observed for RCref and NC-0 was absent in NC-3. The band located at 285 nm became more pronounced as the [Cisplatin] is stepwise increased in these formulations, namely, [NC-3] < [NC-4] < [NC-5]. Formulation NC-5 had two primary bands at 230 and 285 nm. The observed red shifts of the major band from 200 to 230 and 280 nm at higher [Cisplatin] concentrations are attributed to increased cluster sizes at higher [Cisplatin]38 consistent with our TEM observations.
In Vitro Cancer Cell Cytotoxicity and Apoptosis
The cytotoxic effects of standard cisplatin and aqueous cisplatin solutions prepared via RESS processing (NC-4 and NC-5) were assessed using Cell Titer-Blue (CTB) assay, which measures the reduction of nonfluorescent resazurin into highly fluorescent resorufin in living cells. We treated human cervical cancer HeLa cells with increasing concentrations of standard cisplatin and compared this to the aqueous formulations of cisplatin (Figure 4). As expected, cisplatin decreased the cell viability in a dose-dependent manner, with the lowest cisplatin concentration tested (10 μM) yielding a CTB response of 63 ± 1% of controls, while at 50 μM, the response was lowered to 9 ± 3% of controls. The IC50 value is highly dependent on the experimental conditions, including the incubation time and cell density, as well as the cell viability/toxicity assay used.39,40 Nevertheless, the IC50 value of cisplatin calculated here was 17 ± 4 μM, which is within the range reported in the literature.41−43 Similarly, NC-5 decreased the HeLa cell viability in a dose-dependent manner (82 ± 3 and 65 ± 8% viabilities at 10 and 50 μM, respectively; IC50 = 72 ± 7 μM). On the other hand, NC-4 did not adversely affect the HeLa cell viability up to a concentration of 50 μM, whereas at concentrations of 100 μM and higher, the CTB response decreased in a dose-dependent manner (IC50 = 162 ± 4 μM). In summary, both of the aqueous formulations of cisplatin are effective at killing cancer cells, with NC-5 exhibiting increased cytotoxicity relative to NC-4. However, both NC-5 and NC-4 were less toxic than standard cisplatin.
Figure 4.
Aqueous solutions of cisplatin exhibit less in vitro cytotoxicity than standard cisplatin. Dose-dependent inhibition of Cell Titer-Blue (CTB) reduction. HeLa cells were treated with the indicated concentrations of standard cisplatin (displayed as St. Cisplatin in figure), NC-5 or NC-4, for 24 h. Cells treated with vehicle alone were used as a control, while wells with medium alone served as a blank. CTB reduction was determined from the ratio of fluorescence of treated wells to control wells. The error bars represent the SD of at least four independent triplet-well trials. ns, nonsignificant and *P < 0.05 compared to other treatment groups.
FITC-conjugated annex V/PI staining was used to evaluate whether standard cisplatin and aqueous cisplatin formulations (i.e., NC-4 and NC-5) induced cancer cell death via apoptosis or necrosis (Figure 5). Treatment of HeLa cells with cisplatin resulted in a large number of cells undergoing early and late apoptosis (44 ± 4 and 54 ± 5%, respectively, of controls) (Figure 5a–d). Similarly, exposure to NC-5 caused the majority of cells to undergo early apoptosis (Figure 5d,e). Consistent with our findings of reduced cytotoxicity of NC-4, cells treated with this compound had a higher population of live cells (79 ± 6%), with much smaller populations of early and late apoptotic cells (16 ± 6 and 3%, respectively) (Figure 5c,e), compared to treatment with either standard cisplatin or NC-5. These studies verify the greater efficacy of NC-5 at inducing cancer cell death compared to NC-4. The higher prevalence of early apoptotic cells in NC-5-treated samples suggests that the effect of this compound at inducing apoptosis is slower acting compared to standard cisplatin.
Figure 5.
Aqueous cisplatin formulations have lower efficacy at inducing apoptosis compared to standard cisplatin. FACS analysis of annexin V/PI staining of HeLa cells that were either untreated (control, a) or treated with 25 μM cisplatin (b), NC-4 (c), or NC-5 (d) for 24 h. The bottom left quadrant (annexin V–/PI–) represents live cells; bottom right (annexin V+/PI–), early apoptotic cells; top right (annexin V+/PI+), late apoptotic cells; and top left (annexin V–/PI+), necrotic cells. (e) Summary of the incidence of early and late apoptosis and necrosis in HeLa cells that were treated with 25 μM standard cisplatin, NC-4, or NC-5. The error bars represent the SD of four independent trials. ns, nonsignificant and *P < 0.05 compared to the respective controls. The red line indicates statistical comparison between the early apoptotic cells following treatment with standard cisplatin and NC-5.
In Vivo Studies of Zebrafish Embryos
The early stages of zebrafish embryonic development are characterized by rapid cell proliferation. We used this as an in vivo system to test the effects of these novel compounds on cell division by exposing embryos to standard cisplatin and aqueous cisplatin formulations (NC-4 and NC-5) for a period of 5 dpf and assayed for survival, as shown in Figure 6a. Standard cisplatin and NC-4 caused larval death at different timepoints, while larvae exposed to equivalent concentrations of NC-5 survived the entire treatment protocol (Figure 6b). Of these compounds, standard cisplatin was found to be the most toxic, with all embryos dying as early as 48 hpf, whereas the mortality caused by NC-4 occurred much later (from 96 hpf). In NC-5, it was well tolerated and did not induce any death during the 24–120 hpf.
Figure 6.
Aqueous cisplatin is less toxic than standard cisplatin in zebrafish embryos. (a) Schematic of treatment timeline and assessment by mortality scoring. (b) Survival at 5 dpf with LC50 indicated for NC-5, NC-4, or standard cisplatin (Cis-Pt). (c) Kaplan–Meier plot showing survival trend during exposure to NC-5, NC-4, or standard cisplatin (Cis-Pt) at indicated timepoints (d) Kaplan–Meier plot showing survival trend after dechorionation at 24 hpf during exposure to varying concentrations of NC-5, NC-5, or standard cisplatin at indicated timepoints. (e) Representative images of acridine orange-stained zebrafish 24 hpf embryos treated with different concentrations of NC-5, NC-4, or standard cisplatin (Cis-Pt). (f) Analysis of cell death phenotype in larval trunk using acridine orange staining. (N = 2 (5–7 fish per clutch)) *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001 compared to the control.
Interestingly, larvae exposed to both NC-4 and NC-5 failed to hatch out of their chorion. Therefore, survival was assayed after dechorionation at 24 hpf to rule out any effect of this on drug access to the embryos. We observed a similar trend in survival for all compounds with and without the chorion, albeit the absence of the chorion did sensitize the embryos to standard cisplatin and NC-4 toxicity (Figure 6c), suggesting that the chorion may slightly impede access of these compounds to the embryos but that this was not a major contributor to the effects of these compounds.
Cisplatin induces apoptosis, and we therefore assessed the effects of cisplatin and its derivatives in embryos at a stage when we predicted the cells to be most sensitive to DNA damage due to the rapid cell division in the head and trunk (24 hpf). We used acridine orange staining for dead and dying cells on live embryos. All compounds induced some cell death in the head of 24 hpf embryos (Figure 6d). Notably, this was a time point when NC-5 did not cause any larval death but did induce significant cell death. This suggests that standard cisplatin and NC-4 cause widespread toxicity, whereas NC-5 is better tolerated and only causes death of rapidly proliferating cells (Figure 6d).
Discussion
We report, for the first time, an environmentally benign, scalable sc-CO2 RESS technique to produce an aqueous nanocluster dispersion of potent anticancer drug cisplatin. The custom-designed RESS process resulted in a significant (>15×) increase in the cisplatin water solubility. Increasing the cisplatin concentration resulted in an increase in the nanocluster size. Materials characterization showed that the process did not have any degradation effect on the drug and retained its chemical and structural integrity. Therefore, we conclude that this approach successfully generated a new formulation of cisplatin that could be beneficial for reducing the toxic side effects of this drug in cancer patients undergoing cisplatin-based therapy.
Given the importance of cisplatin as an anticancer agent, we first assessed the ability of these aqueous formulations on cancer cell viability in vitro. We found that HeLa cells treated with aqueous cisplatin formulations (NC-4 and NC-5) had decreased cell viability in a dose-dependent manner compared to controls; however, these compounds were less effective (toxic) than standard cisplatin. Our findings show that the cell death pattern induced by NC-4 and NC-5 differed from standard cisplatin in that NC-4-treated cells were largely viable at the time point investigated, whereas NC-5 treatment increased the number of cells in early apoptosis. We presume that those cells will eventually proceed to late apoptosis. Interestingly, most NC-4-treated cells remained viable throughout the treatment protocol, in contrast to cisplatin-treated cells, where most were apoptotic. This suggests either lower efficacy of NC-4 or that there is a delayed timeframe of action of these aqueous formulations.
Zebrafish are a well-established vertebrate system for toxicological studies and preclinical drug assessment.44,45 The rapid cell division of early embryos is an effective experimental model to evaluate chemotherapies and other compounds in the context of nontransformed cells in vivo.46−49 Cisplatin has been shown to effectively kill the hair cells of zebrafish lateral line,50−53 zebrafish have been used to examine cisplatin, and other metal-based chemotherapies cause cell death.54
Our studies using this model showed that NC-4 and NC-5 were better tolerated than standard cisplatin. A similar effect was observed in vitro. Thus, in both cancer and noncancer cells, standard cisplatin has greater cytotoxicity than the aqueous formulations. This could suggest that these new formulations reduce efficacy compared to cisplatin. Alternatively, this finding could reflect a delayed release of the cell-damaging agent (platinum), which would be beneficial to treating cancer cells. Our finding that the LC50 for normal cells was higher than that for cancer cells represents an optimal profile for a drug designed to specifically target cancer. However, this effect could be related to bioavailability, as some compounds may be more accessible to cells in vitro compared to the complex multicellular organisms. This feature is important for future studies to evaluate the potential of these compounds for clinical use.
We were surprised to find that in zebrafish embryos, NC-5 was better tolerated than NC-4, as the opposite pattern was found in studies with HeLa cells. This could be attributed to intrinsic differences in the cell cycle control and DNA repair mechanisms in cancer cells compared to normal cells or could reflect the differential ability of NC-4 compared to NC-5 to enter cells in vitro compared to cells in vivo. Finally, it is possible that in vivo, these solutions have different cellular targets. For instance, the lethality observed by NC-4 exposure could be due to preferential targeting of the rapidly dividing cells in the head and trunk, or a potent off-target effect that interferes with essential functions in the embryo. In contrast, NC-5 might only affect those cells that share features with cancer cells (poorly differentiated and rapidly dividing). This would suggest an exciting possibility that NC-5 could eliminate systemic toxicity of cisplatin but improves the cancer-killing effect, making it ideally suited as a chemotherapy treatment.
Summary
We describe an advance in the chemical formulation of cisplatin, an important and widely used cancer drug. By generating nanoclusters of cisplatin, we achieved a 15× higher water solubility of this compound. The targeted cancer cell-killing effects of these formulations offer the opportunity to alter the drug dosage levels or drug transmission routes. The higher aqueous solubility and selected organ toxicity could potentially offer to open up a new and viable drug administration route of cisplatin drug.
Acknowledgments
The authors acknowledge Dr. James Weston, Core Technology Platform, NYUAD, for his help in acquiring the XRD data and Thomas Blanton, International Centre for Diffraction Data, for discussion of the XRD data. They thank the NYUAD Core Technology Platform for equipment support.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.9b03917.
XRD characterization of NC-0: (Figure S1); compositional characterizations of NC-0: (Figure S2); XRD characterization of NC-5: (Figure S3); UV–vis spectroscopy data: (Figure S4) (PDF)
Author Contributions
S.K.S. conducted sc-CO2 experiments, did most of the material characterizations, and contributed to the writing of the manuscript. S.A.H. and M.K. performed in vitro cell viability assays. L.P. performed the FACS and apoptosis assay. A.R.N. participated in experimental design, execution, and data analysis for all in vivo experiments and contributed to writing the manuscript. R.P. carried out the TEM characterization and analysis. M.M. guided and supervised the in vitro experiments and participated in writing the manuscript. K.C.S. participated in experimental design, data analysis and interpretation, and writing the manuscript. R.J. supervised sc-CO2 experiments, analyzed the materials data, and participated in the writing of the manuscript.
Funding for this work was provided by NYU Abu Dhabi.
The authors declare no competing financial interest.
Supplementary Material
References
- Kalinich M.; Haber D. A. Cancer Detection: Seeking Signals in Blood. Science 2018, 359, 866–867. 10.1126/science.aas9102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couzin-Frankel J. Cancer Immunotherapy. Science 2013, 342, 1432–1433. 10.1126/science.342.6165.1432. [DOI] [PubMed] [Google Scholar]
- Vo C. L.-N.; Park C.; Lee B.-J. Current Trends and Future Perspectives of Solid Dispersions Containing Poorly Water-Soluble Drugs. Eur. J. Pharm. Biopharm. 2013, 85, 799–813. 10.1016/j.ejpb.2013.09.007. [DOI] [PubMed] [Google Scholar]
- Palazzo B.; Iafisco M.; Laforgia M.; Margiotta N.; Natile G.; Bianchi C. L.; Walsh D.; Mann S.; Roveri N. Biomimetic Hydroxyapatite-Drug Nanocrystals as Potential Bone Substitutes with Antitumor Drug Delivery Properties. Adv. Funct. Mater. 2007, 17, 2180–2188. 10.1002/adfm.200600361. [DOI] [Google Scholar]
- Frangioni J. V. New Technologies for Human Cancer Imaging. J. Clin. Oncol. 2008, 26, 4012–4021. 10.1200/JCO.2007.14.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin C. F.; Tian Q.; Setyawati M. I.; Fang W.; Tan E. S. Q.; Leong D. T.; Ang W. H. Tuning the Activity of Platinum(IV) Anticancer Complexes through Asymmetric Acylation. J. Med. Chem. 2012, 55, 7571–7582. 10.1021/jm300580y. [DOI] [PubMed] [Google Scholar]
- Takahara P. M.; Rosenzweig A. C.; Frederick C. A.; Lippard S. J. Crystal Structure of Double-Stranded DNA Containing the Major Adduct of the Anticancer Drug Cisplatin. Nature 1995, 377, 649–652. 10.1038/377649a0. [DOI] [PubMed] [Google Scholar]
- Shaloam D.; Tchounwou P. B. Cisplatin in Cancer Therapy: Molecular Mechanisms of Action. Eur. J. Pharmacol. 2014, 740, 364–378. 10.1016/j.ejphar.2014.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J.; Ruo S. T.; Wang C. H. Biodegradable Microparticles and Fiber Fabrics for Sustained Delivery of Cisplatin to Treat C6 Glioma in Vitro. J. Biomed. Mater. Res., Part A 2008, 85, 897–908. 10.1002/jbm.a.31499. [DOI] [PubMed] [Google Scholar]
- Sakhno L. A.; Yurchenko O. V.; Maslenniy V. N.; Bardakhivskaya K. I.; Nikolaeva V. V.; Ivanyuk A. A.; Shevchuk O. O.; Korotich V. G.; Nikolaev V. G. Enterosorption as a Method to Decrease the Systemic Toxicity of Cisplatin. Exp. Oncol. 2013, 35, 45–52. [PubMed] [Google Scholar]
- Peng J.; Qi T.; Liao J.; Chu B.; Yang Q.; Li W.; Qu Y.; Luo F.; Qian Z. Controlled Release of Cisplatin from PH-Thermal Dual Responsive Nanogels. Biomaterials 2013, 34, 8726–8740. 10.1016/j.biomaterials.2013.07.092. [DOI] [PubMed] [Google Scholar]
- Sievers R. E. Formation of Aqueous Small Droplet Aerosols Assisted by Supercritical Carbon Dioxide. Aerosol Sci. Technol. 1999, 30, 3–15. 10.1080/713834046. [DOI] [Google Scholar]
- Kumari A.; Yadav S. K.; Yadav S. C. Biodegradable Polymeric Nanoparticles Based Drug Delivery Systems. Colloids Surf., B 2010, 75, 1–18. 10.1016/j.colsurfb.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Long D. F.; Repta A. J. Cisplatin: Chemistry, Distribution and Biotransformation. Biopharm. Drug Dispos. 1981, 2, 1–16. 10.1002/bdd.2510020102. [DOI] [PubMed] [Google Scholar]
- Ting V. P.; Schmidtmann M.; Wilson C. C.; Weller M. T. Cisplatin: Polymorphism and Structural Insights into an Important Chemotherapeutic Drug. Angew. Chem., Int. Ed. 2010, 49, 9408–9411. 10.1002/anie.201003185. [DOI] [PubMed] [Google Scholar]
- Hall M. D.; Telma K. A.; Chang K.-E.; Lee T. D.; Madigan J. P.; Lloyd J. R.; Goldlust I. S.; Hoeschele J. D.; Gottesman M. M. Say No to DMSO: Dimethylsulfoxide Inactivates Cisplatin, Carboplatin and Other Platinum Complexes. Cancer Res. 2014, 74, 3913–3922. 10.1158/0008-5472.CAN-14-0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvao J.; Davis B.; Tilley M.; Normando E.; Duchen M. R.; Cordeiro M. F. Unexpected Low-Dose Toxicity of the Universal Solvent DMSO. FASEB J. 2014, 28, 1317–1330. 10.1096/fj.13-235440. [DOI] [PubMed] [Google Scholar]
- Zhang H.; Wang S.; Weber S. G. Nanocomposite Teflon AF 2400 Films as Tunable Platforms for Selective Transport. Anal. Chem. 2012, 84, 9920–9927. 10.1021/ac3022289. [DOI] [PubMed] [Google Scholar]
- Feng L.; De Dille A.; Jameson V. J.; Smith L.; Dernell W. S.; Manning M. C. Improved Potency of Cisplatin by Hydrophobic Ion Pairing. Cancer Chemother. Pharmacol. 2004, 54, 441–448. 10.1007/s00280-004-0840-z. [DOI] [PubMed] [Google Scholar]
- Rasenack N.; Müller B. W. Micron-Size Drug Particles: Common and Novel Micronization Techniques. Pharm. Dev. Technol. 2004, 9, 1–13. 10.1081/PDT-120027417. [DOI] [PubMed] [Google Scholar]
- Sharma S. K.; Jagannathan R. High Throughput RESS Processing of Sub-10 nm Ibuprofen Nanoparticles. J. Supercrit. Fluids 2016, 109, 74–79. 10.1016/j.supflu.2015.11.019. [DOI] [Google Scholar]
- Sharma S. K.; Woldetsadik A. D.; Blanton T.; O’Connor M. J.; Magzoub M.; Jagannathan R. Production of Nanostructured Molecular Liquids by Supercritical CO2 Processing. OpenNano 2017, 2, 9–18. 10.1016/j.onano.2016.11.001. [DOI] [Google Scholar]
- Benyettou F.; Prakasam T.; Ramdas Nair A.; Witzel I. I.; Alhashimi M.; Skorjanc T.; Olsen J. C.; Sadler K. C.; Trabolsi A. Potent and Selective in Vitro and in Vivo Antiproliferative Effects of Metal-Organic Trefoil Knots. Chem. Sci. 2019, 10, 5884–5892. 10.1039/C9SC01218D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen R. C.; Matson D. W.; Smith R. D. Rapid Precipitation of Low Vapor Pressure Solids from Supercritical Fluid Solutions: The Formation of Thin Films and Powders. J. Am. Chem. Soc. 1986, 108, 2100–2102. 10.1021/ja00268a066. [DOI] [Google Scholar]
- Jagannathan R.; Irvin G.; Blanton T.; Jagannathan S. Organic Nanoparticles: Preparation, Self-Assembly, and Properties. Adv. Funct. Mater. 2006, 16, 747–753. 10.1002/adfm.200600003. [DOI] [Google Scholar]
- Braz M. G.; Fávero Salvadori D. M. Influence of Endogenous and Synthetic Female Sex Hormones on Human Blood Cells in Vitro Studied with Comet Assay. Toxicol. In Vitro 2007, 21, 972–976. 10.1016/j.tiv.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Paulsson M.; Frey B.; Mielenz D.; Gaipl U.; Etich J.; Rosenbaum S.; Stermann J.; Kreft S.; Frie C.; Brachvogel B.; et al. Identification of Novel Binding Partners (Annexins) for the Cell Death Signal Phosphatidylserine and Definition of Their Recognition Motif. J. Biol. Chem. 2010, 286, 5708–5716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daehne S.; Resch-Genger U.; Wolfbeis O. S.. Near-Infrared Dyes for High Technology Applications; Springer Science+Business Media Dordrecht, 1997; pp 1–452. [Google Scholar]
- De Carvalho L. A. E. B.; Marques M. P. M.; Martin C.; Parker S. F.; Tomkinson J. Inelastic Neutron Scattering Study of Pt II Complexes Displaying Anticancer Properties. ChemPhysChem 2011, 12, 1334–1341. 10.1002/cphc.201001067. [DOI] [PubMed] [Google Scholar]
- Ciobotaru C. C.; Damian C. M.; Matei E.; Iovu H.; Xps X. P. S.; Tga T. Covalent Functionalization of Graphene Oxide with Cisplatin. Mater. Plast. 2014, 51, 75–80. [Google Scholar]
- Marques M. P. M.; Valero R.; Parker S. F.; Tomkinson J.; Batista De Carvalho L. A. E. Polymorphism in Cisplatin Anticancer Drug. J. Phys. Chem. B 2013, 117, 6421–6429. 10.1021/jp403486z. [DOI] [PubMed] [Google Scholar]
- Torres M.; Khan S.; Duplanty M.; Lozano H. C.; Morris T. J.; Nguyen T.; Rostovtsev Y. V.; Deyonker N. J.; Mirsaleh-Kohan N. Raman and Infrared Studies of Platinum-Based Drugs: Cisplatin, Carboplatin, Oxaliplatin, Nedaplatin, and Heptaplatin. J. Phys. Chem. A 2018, 122, 6934–6952. 10.1021/acs.jpca.8b04023. [DOI] [PubMed] [Google Scholar]
- De Carvalho A. L. M. B.; Pilling M.; Gardner P.; Doherty J.; Cinque G.; Wehbe K.; Kelley C.; De Carvalho L. A. E. B.; Marques M. P. M. Chemotherapeutic Response to Cisplatin-like Drugs in Human Breast Cancer Cells Probed by Vibrational Microspectroscopy. Faraday Discuss. 2016, 187, 273–298. 10.1039/C5FD00148J. [DOI] [PubMed] [Google Scholar]
- Amado A. M.; Fiuza S. M.; Marques M. P. M.; De Carvalho L. A. E. B. Conformational and Vibrational Study of Platinum(II) Anticancer Drugs: Cis-Diamminedichloroplatinum (II) as a Case Study. J. Chem. Phys. 2007, 127, 185104 10.1063/1.2787528. [DOI] [PubMed] [Google Scholar]
- Zhang G.; Yang D.; Sacher E. X-Ray Photoelectron Spectroscopic Analysis of Pt Nanoparticles on Highly Oriented Pyrolytic Graphite, Using Symmetric Component Line Shapes. J. Phys. Chem. C 2007, 111, 565–570. 10.1021/jp065606+. [DOI] [Google Scholar]
- Félix R.; Llobera-Vila N.; Hartmann C.; Klimm C.; Hartig M.; Wilks R. G.; Bär M. Preparation and In-System Study of SnCl2 Precursor Layers: Towards Vacuum-Based Synthesis of Pb-Free Perovskites. RSC Adv. 2018, 8, 67–73. 10.1039/C7RA12172E. [DOI] [Google Scholar]
- Xiao F.; Yao X.; Bao Q.; Li D.; Zheng Y. Sensitive Marker of the Cisplatin-DNA Interaction: X-Ray Photoelectron Spectroscopy of CL. Bioinorg. Chem. Appl. 2012, 2012, 1–10. 10.1155/2012/649640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gharibshahi E.; Saion E. Influence of Dose on Particle Size and Optical Properties of Colloidal Platinum Nanoparticles. Int. J. Mol. Sci. 2012, 13, 14723–14741. 10.3390/ijms131114723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magzoub M.; Miranker A. D. Concentration-Dependent Transitions Govern the Subcellular Localization of Islet Amyloid Polypeptide. FASEB J. 2012, 26, 1228–1238. 10.1096/fj.11-194613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y.; Zhu Q.; Chen M.; Huang Q.; Wang W.; Li Q.; Huang Y.; Di W. The Changing 50% Inhibitory Concentration (IC50) of Cisplatin: A Pilot Study on the Artifacts of the MTT Assay and the Precise Measurement of Density-Dependent Chemoresistance in Ovarian Cancer. Oncotarget 2016, 7, 70803–70821. 10.18632/oncotarget.12223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q.; Shu Y. Pharmacological Modulation of Cytotoxicity and Cellular Uptake of Anti-Cancer Drugs by PDE5 Inhibitors in Lung Cancer Cells. Pharm. Res. 2014, 31, 86–96. 10.1007/s11095-013-1134-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A.; Naaz A.; Prakasham A. P.; Gangwar M. K.; Butcher R. J.; Panda D.; Ghosh P. Potent Anticancer Activity with High Selectivity of a Chiral Palladium N-Heterocyclic Carbene Complex. ACS Omega 2017, 2, 4632–4646. 10.1021/acsomega.7b00688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardito S.; Isella C.; Medico E.; Marchiò L.; Bevilacqua E.; Hatzoglou M.; Bussolati O.; Franchi-Gazzola R. The Thioxotriazole Copper (II) Complex A0 Induces Endoplasmic Reticulum Stress and Paraptotic Death in Human Cancer Cells. J. Biol. Chem. 2009, 284, 24306–24319. 10.1074/jbc.M109.026583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bambino K.; Chu J. Zebrafish in Toxicology and Environmental Health. Curr. Top. Dev. Biol. 2017, 331–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horzmann K. A.; Freeman J. L. Making Waves: New Developments in Toxicology with the Zebrafish. Toxicol. Sci. 2018, 163, 5–12. 10.1093/toxsci/kfy044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustine-Rauch K.; Zhang C. X.; Panzica-Kelly J. M. In Vitro Developmental Toxicology Assays: A Review of the State of the Science of Rodent and Zebrafish Whole Embryo Culture and Embryonic Stem Cell Assays. Birth Defects Res., Part C 2010, 90, 87–98. 10.1002/bdrc.20175. [DOI] [PubMed] [Google Scholar]
- Tamplin O. J.; White R. M.; Jing L.; Kaufman C. K.; Lacadie S. A.; Li P.; Taylor A. M.; Zon L. I. Small Molecule Screening in Zebrafish: Swimming in Potential Drug Therapies. Wiley Interdiscip. Rev.: Dev. Biol. 2012, 1, 459–468. 10.1002/wdev.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Y. J.; Jia Y. F.; Chen N.; Bian W. P.; Li Q. K.; Ma Y. B.; Chen Y. L.; Pei D. S. Zebrafish as a Model System to Study Toxicology. Environ. Toxicol. Chem. 2014, 33, 11–17. 10.1002/etc.2406. [DOI] [PubMed] [Google Scholar]
- Wang Y. H.; Cheng C. C.; Lee E. J.; Chiou M. L.; Pai C. W.; Wen C. C.; C W. L.; C Y. H. A Novel Phenotype-Based Approach for Systematically Screening Antiproliferation Metallodrugs. Chem.-Biol. Interact. 2009, 182, 84–91. 10.1016/j.cbi.2009.08.005. [DOI] [PubMed] [Google Scholar]
- Stawicki T. M.; Esterberg R.; Hailey D. W.; Raible D. W.; Rubel E. W. Using the Zebrafish Lateral Line to Uncover Novel Mechanisms of Action and Prevention in Drug-Induced Hair Cell Death. Front. Cell. Neurosci. 2015, 9, e22347 10.3389/fncel.2015.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens K. N.; Santos F.; Roberts B.; Linbo T.; Coffin A. B.; Knisely A. J.; Simon J. A.; Rubel E. W.; Raible D. W. Identification of Genetic and Chemical Modulators of Zebrafish Mechanosensory Hair Cell Death. PLoS Genet. 2008, 4, e1000020 10.1371/journal.pgen.1000020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou H. C.; Raible D. W.; Rubel E. W. Cisplatin Induced Hair Cell Loss in Zebrafish (Danio Rerio) Lateral Line. Hear. Res. 2007, 233, 46–53. 10.1016/j.heares.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung G. Y.; Wu C. L.; Chou Y. L.; Chien C. T.; Horng J. L.; Lin L. Y. Cisplatin Exposure Impairs Ionocytes and Hair Cells in the Skin of Zebrafish Embryos. Aquat. Toxicol. 2019, 209, 168–177. 10.1016/j.aquatox.2019.02.006. [DOI] [PubMed] [Google Scholar]
- Karas B. F.; Côrte-Real L.; Doherty C. L.; Valente A.; Cooper K. R.; Buckley B. T. A Novel Screening Method for Transition Metal-Based Anticancer Compounds Using Zebrafish Embryo-Larval Assay and Inductively Coupled Plasma-Mass Spectrometry Analysis. J. Appl. Toxicol. 2019, 39, 1173–1180. 10.1002/jat.3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.