Abstract
A hydrothermal (HT) coating was applied to the biomedical Mg–Zn–Ca alloy surface by microarc oxidation (MAO) and heat treatment. Then, the corrosion resistance and biocompatibility of the coated alloy was evaluated in vitro and in vivo. The corrosion rate (CR) of HT-coated implants was significantly lower in experiment. In addition, this CR increased over time in vivo but was stable, albeit higher, in vitro. The proliferation, adhesion, and live activity of bone marrow stem cells (BMSCs) were significantly greater on the surface of the HT-coated Mg alloy in vitro. Serum Mg2+ was always within the normal range in rabbits with implants, although Ca2+ was higher than normal for both uncoated and coated scaffolds. There were no significant pathological effects on the main organs of alloy-implanted rabbits compared with healthy animals. Thus, the HT coating significantly improved the corrosion resistance and biocompatibility of the Mg–Zn–Ca alloy.
1. Introduction
Magnesium (Mg) and its alloys have attracted much attention in the production of metallic, biodegradable implants because of their excellent biocompatibility and mechanical properties.1−3 As well known, the magnesium ion is the fourth most abundant cation in the body. A healthy adult of 70 kg generally contains about 21–35 g physiological Mg,4 and over half of these are stored in bone tissue.5,6 Mg is also the second most abundant cation in cells and is known to be involved in about 300 enzymatic reactions.7,8 Moreover, as an orthopedic biomaterial, the Young’s coefficient of the Mg alloy is close to that of human bone, which could effectively reduce or avoid postoperative complications of stress concentrations and shielding.9 Mg ions could join in many physiological processes when Mg alloys were degraded to release it in the body.10,11 For example, these Mg ions may enhance bone regeneration by affecting extracellular matrix proteins and transcription factors, such as BMP-2.12,13 Meanwhile, excessive Mg2+ is excreted through urine or feces without toxic effects on the body.14 The most important is that the Mg alloy can be adjusted to resorb after the bone-healing process. This could further reduce the demand to perform a second surgery to remove it, as well as eradicate the related complication, including unpleasant infection and expense.15,16
A disadvantage of the Mg alloy is that it has poor corrosion resistance in the body because it will degrade to produce Mg(OH)2 and H2 in aqueous environments.17−19 Mg(OH)2 could be further converted into highly soluble magnesium chloride (MgCl2) when the Cl– concentration exceeds 30 mM in the surrounding environment.20 This characteristic can greatly reduce the ultimate strength and fatigue life of Mg-based implants in the body, which is a major obstacle to their clinical applications. In addition, rapid degradation of the Mg alloy can release hydrogen, which can accumulate around the implant and even lead to subcutaneous emphysema. The hydrogen can also lead to local alkalization around the implant, which can result in side effects.18,19 Thus, improvement of the corrosion resistance of the Mg alloy is necessary to adapt to the needs of clinical applications.
Some methods have been developed to improve the corrosion resistance of magnesium. For instance, surface coating efficiently increases the degradation resistance of the Mg alloy,21,22 which is carried out using methods such as microarc oxidation (MAO)23,24 and electrophoresis deposition (EPD).25 Dou et al.26 reported that MAO coatings adhere tightly to the substrate and result in higher corrosion and wear resistance, and, therefore, this approach has been widely used to improve the corrosion resistance of Mg alloys.27−30 Meanwhile, EPD is also an effective method for improving the degradation resistance of Mg alloys,31,32 as no evaporated gas is involved, and thus pores can be avoided during the coating process.33 The application of these technologies can ensure the mechanical integrity of Mg alloys at the initial stage of implantation. Thus, Mg-based orthopedic equipment is possible, given that the corrosion of the material can be controlled in vivo.34 However, both MAO and EPD methods have some limitations. For example, the major barrier to EPD coatings was poor adhesion, which to some extent, reduced the corrosion resistance of the coatings. In addition, our previous research found that micropores and microcracks exist on the surface of MAO coatings, and thus, over time, electrolytes can penetrate into the surface of the substrate, leading to the formation of corrosion cracks.29
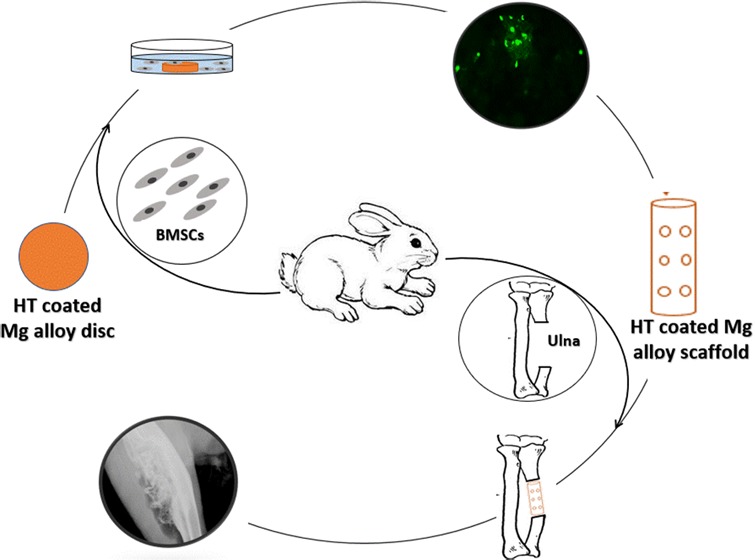
To improve the resistance of Mg alloy to corrosion, we have developed a hydrothermal (HT) coating that is applied by heat treatment and MAO methods, and its chemical composition and mechanical properties were evaluated in our previous study.35 Here, we apply the HT coating to the surface of a Mg–Zn–Ca alloy and evaluate the biocompatibility and corrosion resistance of this coated alloy in vitro and in vivo (Figure 1).
Figure 1.
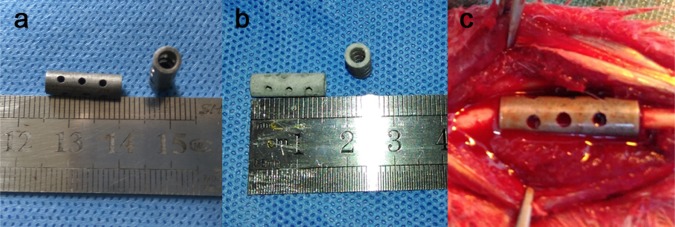
Uncoated and coated Mg–Zn–Ca alloy scaffolds used in this study. (a) Uncoated scaffolds are shown. (b) Hydrothermal (HT)-coated scaffolds are shown. (c) Positioning of a scaffold containing autogenous morselized bone at a bone defect in the rabbit ulna.
2. Results
2.1. Immersion Testing of the HT-Coated and Uncoated Mg–Zn–Ca Alloy
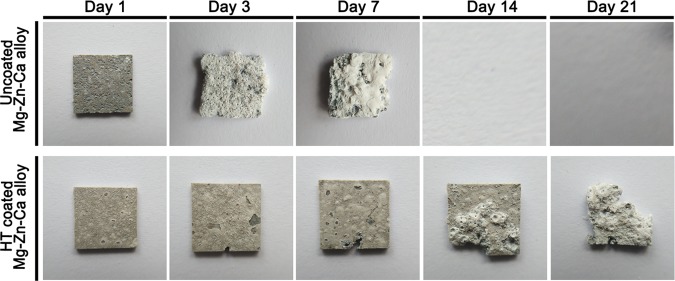
The corrosive production was rinsed to remove by chromic acid solution on the surface of uncoated and HT-coated Mg–Zn–Ca sample when it was soaked in the simulated body fluid (SBF) solution after 1, 3, 7, 14, and 21 days, respectively. The gross appearance of samples is shown in Figure 2. The uncoated samples were completely degraded upon immersion by day 14. Meanwhile, HT-coated samples remained almost intact on day 14 and had lost about half of their volume by day 21. The corrosion rate (CR) of samples was calculated based on their lost weight, as shown in Table 1. At 7 days, the CR of uncoated samples was higher than that of HT-coated samples. Between 14 and 21 days, the CR of the coated samples remained stable.
Figure 2.
Gross appearance of the uncoated and HT-coated Mg–Zn–Ca alloy after being immersed in SBF solution for 1, 3, 7, 14, and 21 days. The uncoated alloy had completely dissolved by day 14.
Table 1. CR of Uncoated and HT-Coated Mg–Zn–Ca Samples after Immersion in SBF Solutiona.
| CR (mm/y) for different
immersion times | |||||
|---|---|---|---|---|---|
| alloy sample | 1 day | 3 days | 7 days | 14 days | 21 days |
| uncoated | 76.79 ± 3.50 | 80.04 ± 2.65 | 76.86 ± 3.85 | ||
| HT coated | 23.95 ± 1.28* | 23.38 ± 1.47* | 21.34 ± 0.97* | 20.09 ± 1.18 | 20.15 ± 1.43 |
Compared to uncoated sample *p < 0.05.
2.2. Indirect Cytotoxicity Analysis of Mg Alloy Samples in Vitro
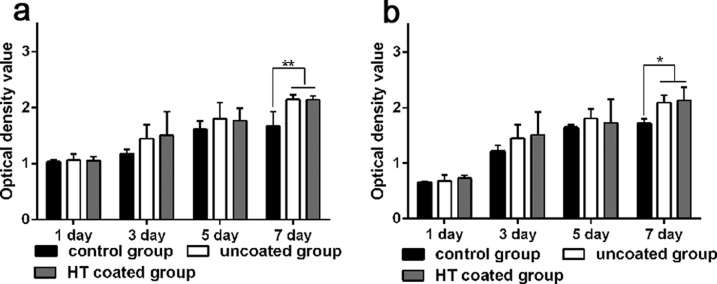
For the assessment of indirect cytotoxicity in vitro, the medium that had been incubated with Mg–Zn–Ca alloy samples for 3 and 7 days was collected and then used as the culture medium for bone marrow stem cells (BMSCs) for an additional 1, 3, 5, and 7 days. Cell proliferation in the culture medium that had been preincubated with the coated or uncoated Mg alloy was similar to that in the control medium (Figure 3). Only after 7 days in culture was there a significant difference between the control medium and the two preincubated media, although there was no significant difference in proliferation between the uncoated and coated groups.
Figure 3.
Cell proliferation of BMSCs in medium preincubated with the Mg alloy. (a, b) BMSCs were cultured in the control medium or in the medium collected after a HT-coated or uncoated Mg–Zn–Ca alloy sample was first incubated in the medium for (a) 3 days and (b) 7 days. Cell proliferation was assessed with the CCK-8 assay. Higher OD450 values indicate a higher number of cells and thus greater cell proliferation. *p < 0.05 and **p < 0.01.
2.3. Cell Adhesion to HT-Coated and Uncoated Mg Alloy Samples
Table 2 shows the BMSC cell adhesion percentage for uncoated and HT-coated Mg–Zn–Ca alloy samples. Briefly, after overnight incubation, the cell adhesion percentage was calculated by counting the remaining cells in the solution after removing the samples. The average cell adhesion percentage for the HT-coated Mg alloy was significantly higher than that of the uncoated Mg alloy (p < 0.01).
Table 2. BMSC Adhesion to the Surface of Different Coated Mg–Zn–Ca Alloy Samplesa.
| alloy sample | remaining cells (×105) | adhesion percentage (%) |
|---|---|---|
| uncoated | 0.744 ± 0.044 | 25.6 ± 0.02% |
| HT coated | 0.633 ± 0.02* | 36.7 ± 0.02%** |
Compared to uncoated samples *p < 0.05 or **p < 0.01.
2.4. Direct Cytoactivity Analysis of Mg Alloy Samples in Vitro
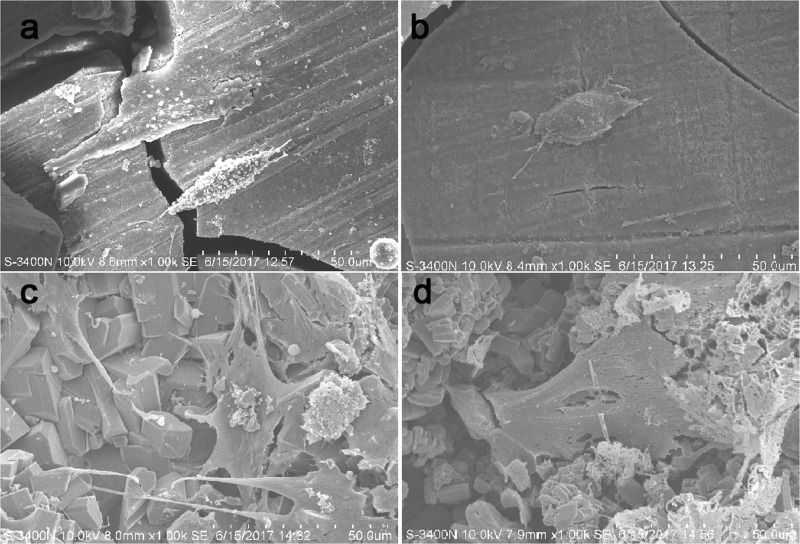
The viability of cells on scaffolds was analyzed via live/dead staining (Figure 4) and scanning electron microscopy (SEM) (Figure 5). When BMSCs were cultured for 3 or 7 days, almost all cells on the uncoated samples were dead (Figure 4a,b). Conversely, there was an abundance of live cells on the coated Mg alloy surface (Figure 4c,d). In SEM analysis conducted after 3 and 7 days in culture, the cells on the uncoated Mg alloy were fusiform at 3 days (Figure 5a) and ellipsosome at 7 days (Figure 5b). In contrast, the cells on the HT-coated Mg alloy had a spread morphology and appeared to be more well adhered by 3 days (Figure 5c), and especially at 7 days (Figure 5d).
Figure 4.
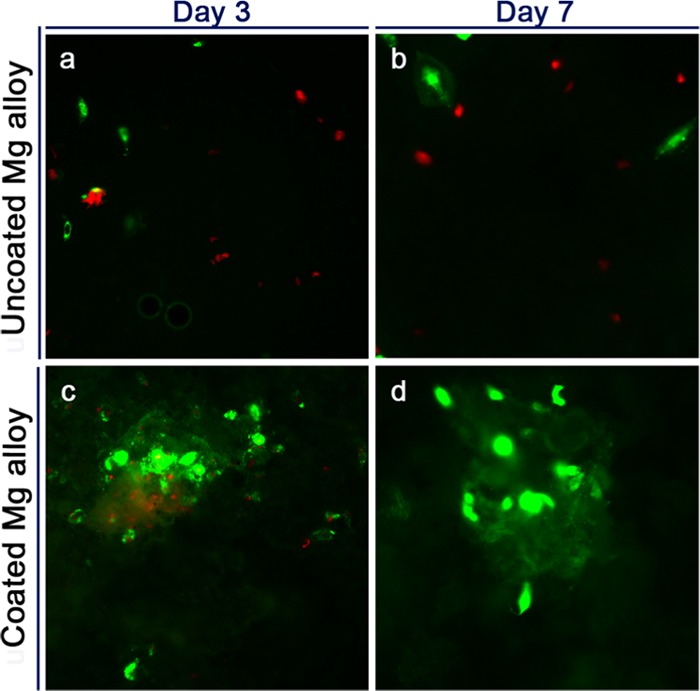
Live/dead staining of BMSCs cultured on uncoated and HT-coated Mg–Zn–Ca alloy samples. (a–d) Cells were cultured on uncoated Mg alloy (a, b) and HT-coated Mg alloy (c, d) for 3 days (a, c) and 7 days (b, d). Green fluorescent cells are alive, whereas red fluorescent cells are dead.
Figure 5.
SEM of the cell-seeded surface of uncoated and HT-coated Mg–Zn–Ca alloy samples. (a–d) Samples of the uncoated Mg alloy (a, b) and HT-coated Mg alloy (c, d) were seeded with BMSCs and cultured for 3 days (a, c) and 7 days (b, d) before analysis by SEM.
2.5. X-ray Images in a Rabbit Model of Bone Defect
X-ray images were then taken at 4, 8, and 12 weeks after surgery (Figure 6). Substantial amounts of radiodense material had accumulated around the Mg alloy by 4, 8, and 12 weeks after surgery. By week 4, both the coated and uncoated Mg–Zn–Ca alloy scaffolds were still mostly intact in the forearms. With the extension to week 8, the uncoated scaffolds were no longer intact, whereas the HT-coated scaffolds were intact. At week 12, the uncoated scaffolds could not be identified within the bone defect, but the HT-coated scaffolds had partially retained their structure. We also noted that less gas had accumulated around the HT-coated scaffolds relative to the uncoated scaffolds at week 8; however, the amount of accumulated hydrogen around the implants was similar in both groups at 12 weeks. Although the bone response was not the main purpose of this study, it is particularly important to note that more mature new bone and calcium deposition had formed in the regions of the defects around the HT-coated implants relative to the uncoated implants by fluorescence and histological sections (Supporting Information Figures S1 and S2).
Figure 6.
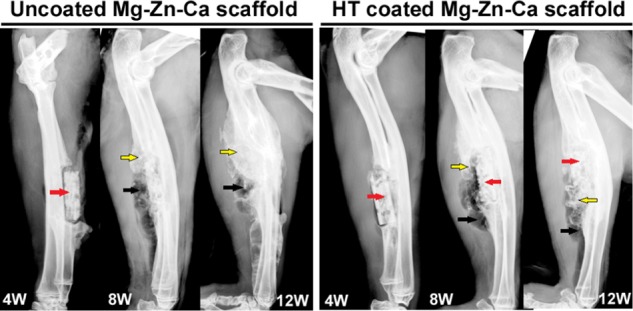
X-ray images of uncoated and HT-coated Mg–Zn–Ca scaffolds in rabbit ulnae 4, 8, and 12 weeks after implantation. The red arrow indicates the position of the scaffold, the black arrow indicates hydrogen produced by degradation, and the yellow arrow indicates bone regeneration.
2.6. Microcomputed Tomography (CT) Analysis of Mg Alloy Scaffolds in a Rabbit Model
Three-dimensional micro-CT images (Figure 7) were then used to calculate the remaining scaffold volume in the operated rabbits. These findings were consistent with the X-ray image results and showed that the uncoated alloy degraded more rapidly. In contrast to the X-ray observations, the accumulation of gas around the HT-coated scaffolds was substantially higher than that around uncoated scaffolds at 12 weeks. We calculated the CR for each implant type based on the CT-measured loss of volume. The CR of the uncoated scaffold was significantly higher than that of the coated scaffold at 4 and 8 weeks after surgery (Table 3). In addition, an intra-group comparison indicated that the CR of the HT-coated scaffold had significantly increased at 8 weeks relative to 4 weeks and was significantly higher at 12 weeks than at 8 weeks (Table 3). These results thus differed from the in vitro findings.
Figure 7.
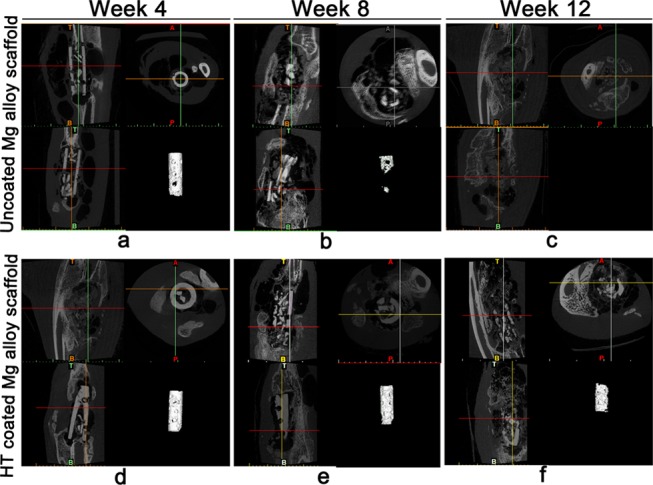
Micro-CT images and volume determination of the uncoated and HT-coated scaffolds in a rabbit model of bone defect. (a–f) Implanted Mg alloy scaffolds that were either uncoated (a–c) or HT coated (d–f) were examined by three-dimensional micro-CT at week 4 (a, d), week 8 (b, e), and week 12 (c, f) after surgery. In each panel, the lower-right image shows the remaining volume of the implant as calculated by micro-CT.
Table 3. CR for the Uncoated and Coated Mg–Zn–Ca Scaffolds in a Rabbit Model of Large Bone Defect at Weeks 4, 8, and 12 after Surgery.
| CR (mm/y) | week 4 | week 8 | week 12 |
|---|---|---|---|
| uncoated Mg–Zn–Ca implants | 2.87 ± 0.56 | 2.63 ± 0.29 | |
| HT-coated Mg–Zn–Ca implants | 0.66 ± 0.32a | 1.13 ± 0.27aΔ | 1.41 ± 0.18aΔ |
As compared between the two groups: p < 0.05. Δ compared within group: p < 0.05.
2.7. Hydrogen Formation in Response to Mg Alloy Scaffolds in a Rabbit Model
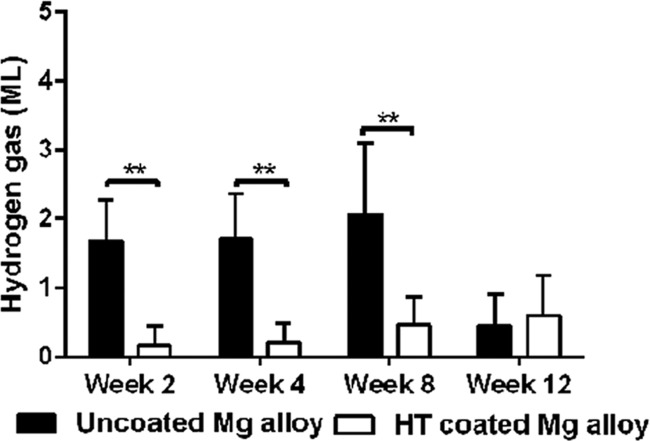
At weeks 2, 4, 8, and 12 after surgery in the rabbit model of large bone defect, hydrogen gas that had accumulated at the implant site was collected by a subcutaneous puncture (Figure 8). The hypodermic bubbles were extracted with a sterile syringe, and no significant body fluid or serum was extracted at the same time. The amount of gas associated with the HT-coated Mg alloy scaffolds was significantly less than that associated with the uncoated scaffolds at 2, 4, and 8 weeks after surgery. Interestingly, this difference between the two groups was absent at 12 weeks after surgery (Figure 8).
Figure 8.
Extracted volumes of hydrogen gas generated by the uncoated and HT-coated Mg alloy scaffolds in a rabbit model of bone defect. Gas was extracted at weeks 2, 4, 8, and 12 after surgery. **p < 0.01.
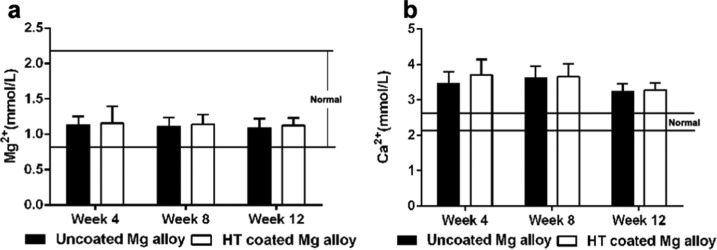
2.8. Serum Mg2+ and Ca2+ Concentrations
Figure 9 shows the serum Mg2+ and Ca2+ concentrations in the two treatment groups at three time points after surgery. The serum Mg2+ concentrations of the two groups were within the normal range (0.82–2.22 mmol/L) over the course of the experiment, and significant differences were not found between the groups. The serum Ca2+ concentrations in the two groups tended to be greater than normal (normal concentrations, 2.1–2.6 mmol/L), but significant differences between groups were also not found.
Figure 9.
Serum Mg2+ and Ca2+ concentrations. (a, b) Serum Mg2+ (a) and Ca2+ (b) levels were determined in rabbits that received Mg alloy scaffold implants that were either uncoated or HT coated. Serum samples were analyzed at weeks 4, 8, and 12 after surgery.
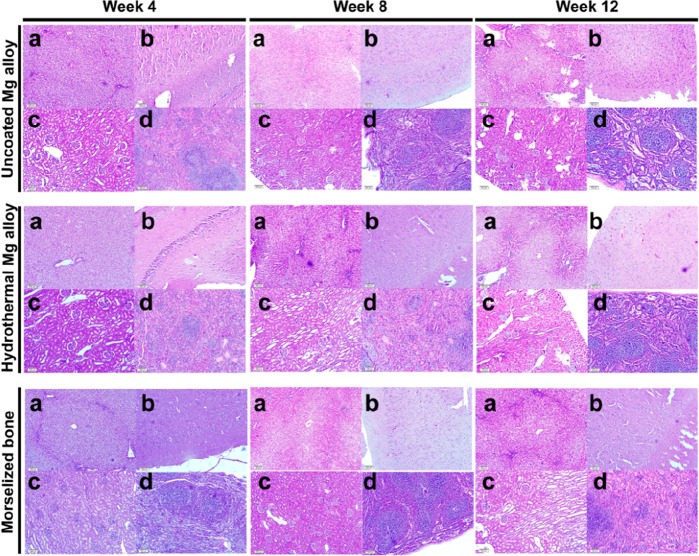
2.9. Organ Pathology
Tissue sections from the liver, brain, kidney, and spleen from the three groups that were stained with hematoxylin and eosin showed that the structures and morphologies of the cells were normal (Figure 10). As compared with the control group (untreated rabbits), the nuclei appeared to be intact and clear, the cytoplasm appeared to be normal, and no abnormal structural features such as those associated with necrosis were observed in the HT-coated and uncoated groups.
Figure 10.
Histochemical analysis of organs in rabbits receiving Mg alloy scaffolds. Images of the liver (a), brain (b), kidney (c), and spleen (d) tissues taken from experimental animals at 4, 8, and 12 weeks after surgery and stained with hematoxylin and eosin.
3. Discussion
3.1. Corrosion Resistance of HT-Coated Mg Alloy in Vitro and in Vivo
In the present study, we adopted heat treatment techniques to prepare a HT composite layer to improve the properties of a medical biodegradable Mg alloy. Although we previously confirmed that the HT coating improves the properties of this Mg alloy,35 its effects on corrosion resistance and biocompatibility had not been analyzed in vivo or in vitro.
In vitro, the CR of uncoated Mg–Zn–Ca samples was higher than that of HT-coated Mg–Zn–Ca samples during the initial 1–7 days of immersion, with the complete corrosion of the sample (and thus its disappearance) by day 14. The CR of the HT-coated Mg–Zn–Ca samples was stable during the 21 days of the experiment. This result is consistent with our previous research.35 The CRs of uncoated and HT-coated Mg samples after implantation in rabbits showed similar trends relative to those obtained in vitro. X-ray and micro-CT analyses also showed that the uncoated implants had disappeared by 12 weeks after surgery, whereas the HT-coated implants were partially intact. Thus, the HT coating effectively improves the corrosion resistance of the Mg–Zn–Ca alloy. We think that this phenomenon can be attributed to the fact that the HT coating may effectively protect the matrix from the active ions in the body fluid.36,37
In these experiments, we found an interesting phenomenon. In vitro, the CRs of both the uncoated and coated Mg alloys were stable during the experiment time course, respectively. However, comparing the CR in HT-coated sample at weeks 4, 8, and 12, we found that it increased over time in vivo. Meanwhile, the CR of the uncoated Mg alloy remained stable through week 8. We believe that initially, the HT coating provides effective protection against corrosion for the Mg alloy matrix. Over time, however, as the coating is degraded, it loses its protective function, which leads to acceleration of the degradation of the Mg matrix. This is supported by the analysis of local hydrogen evolution, as the volume of local hydrogen gas was significantly lower in the HT-coated group than in the uncoated group at 2, 4, and 8 weeks after surgery. Only at 12 weeks was there any difference between the implants with respect to hydrogen gas production. This could also explain why there was more residual gas around the implants in the micro-CT images from HT-coated implants relative to uncoated implants at 12 weeks after surgery. The uncoated implants had likely corroded to the point of no longer being present, whereas the HT coating had lost its protective ability, resulting in acceleration of the degradation of the Mg alloy matrix.
Finally, we observed that the CRs of all samples were substantially lower in vivo than they were in vitro. This is most likely because there are lower concentrations of chloride ions present in blood plasma (103 mM)38 and bone (48.6–56.7 mM)39 than in SBF (147.8 mM). In general, because of electrochemical reactions in metal–fluid systems, the corrosion processes on Mg alloy can generate corrosion products,40 and the corrosion product Mg(OH)2, as the main component of the corrosion layer, is not stable in aqueous solutions, which is especially true in chloride-containing environments.41 The difference noted in the present study was likely increased by our frequent replacement of SBF solution based on changes in its pH. Our results were similar to those from a previous study by Witte et al.,41 who investigated the effects of different environments on the CRs of AZ91D and LAE442. They found that the CRs from in vivo tests were about four orders of magnitude smaller than those from in vitro tests.
3.2. Biocompatibility of HT-Coated Mg Alloy in Vitro and in Vivo
The corrosion of Mg alloy results in the release of hydrogen, which causes local pH changes that have a negative effect on the surrounding cells and can damage the body’s tissues and organs.41 Thus, we further evaluated the effect of the HT coating on the biocompatibility of this Mg–Zn–Ca alloy.
First, the medium that had been preincubated with the uncoated or HT-coated Mg alloy samples did not hinder BMSC proliferation and in fact resulted in higher cell numbers when either the Mg alloy was preincubated in the medium for 7 days, relative to the untreated control medium. However, no significant differences were found between the uncoated and HT-coated groups. Next, through direct contact testing, we found that the cell adhesion frequency associated with the HT-coated Mg alloy was higher than that associated with the uncoated Mg alloy. Meanwhile, the live/dead staining and SEM analysis results showed that more live cells were observed on the HT-coated Mg alloy surface than on the uncoated Mg alloy and that the living cells had better morphology and had attributes consistent with better adhesion on the coated samples.
We also observed changes in Mg2+ levels, because the volume of this novel Mg alloy scaffold is larger and it corrodes more quickly than devices examined in previous studies.2,3,9−12 The Mg2+ concentration maintained within the expected physiological range throughout the experiment. This suggests that the released Mg2+ can be absorbed in experimental animals. In contrast, we observed that the serum Ca2+ concentration was higher than the physiological range. In this rabbit model system, the bone-healing mechanism was presumably activated. In addition, local accumulation of Mg2+ ions promotes bone calcium deposition, and new bone formation may have induced an increase in serum Ca2+.42 Mg ions released from implants contribute to bone formation around the peripheral cortex of cortical bone,43 which may be caused by magnesium ions promoting the expression of calcitonin gene-related peptides in the periosteum.44 In addition, pathological changes were not observed in the liver, brain, kidney, and spleen tissues in animals from any of these treatment groups. These results further demonstrate that this Mg–Zn–Ca alloy matrix is safe for the body regardless of its coating. Zn2+ and Ca2+ are microelements that are present in the human body and are themselves nontoxic. This is also consistent with earlier reports.45,46
In addition, there are some limitations to this study, such as the short time course for the analysis and the quantitative analysis of local gas production is not accurate. In addition, the limited time period during which the anticorrosion property of the HT coating is effective, but the long-term protective effects were required further improved.
4. Conclusions
In the present study, we characterized a Mg–Zn–Ca alloy with a HT coating both in vivo and in vitro. Our results demonstrated that the HT coating can improve the biocompatibility and corrosion resistance of this Mg alloy and thus provides useful data for future studies on Mg alloy biomaterial applications.
5. Materials and Methods
5.1. Hydrothermal Coat Preparation
As in our previous studies,29,47 a Mg–Zn–Ca alloy was used as the initial substrate material, with the major alloying elements (wt %) consisting of approximately 2.5–3.0% Zn, 0.5–1.5% Ca, and 0.5% rare element and pure Mg. After cleaning the surface impurities with an ultrasonic bath, all samples were washed with distilled water after polishing with sandpaper (400-, 800-, and 1200-grit) and dried at room temperature for further use.
The 10-μm-thick MAO bottom bioceramic layers were produced according to the method we previously described.35 In brief, the Mg–Zn–Ca samples and the stainless steel were used as the anode and cathode, which were placed in mixing solution of 10 g/L NaOH, 15 g/L Na2SiO3, and 10 g/L Ca(H2PO4)2, Pulse frequency and duty cycle were fixed at 600 Hz and 8%. The temperature of the electrolyte was kept nearly at 30 °C using a stirring and cooling system. The MAO bottom ceramic layers were produced at a constant voltage of 450 V for 10 min.
To seal any micropores or microcracks on the MAO surface and improve the resistance of the sample to corrosion, a bioactive hydrothermal duplex layer was placed over the MAO layer by the heat treatment method, which was measured to be 60 μm thick in our previous research.47 In brief, the MAO-coated samples were placed in the autoclave (volume 40 mL), which included 20 mL distilled water of Ca(NO3)2·4H2O and KH2PO4·3H2O and the pH was controlled to 3 by dilute nitric acid and heated to 150 °C for 10 h. This composite coating is referred to as the hydrothermal (HT) coating.
5.2. HT-Coated Samples for in Vitro Testing
Samples of the HT-coated or uncoated Mg–Zn–Ca alloy were machined into square plates with a length of 10 mm and thickness of 1 mm and subjected to ethylene-oxide gas sterilization for further research. The control group consisted of samples of the uncoated Mg–Zn–Ca alloy.
5.2.1. Immersion Test
Each sample was weighed before the immersion test. Then, samples were immersed in 50 mL simulated body fluid (SBF) solution (pH = 7.4) in a culture bottle (37 °C) for 1, 3, 7, 14, and 21 days. Every 24 h, the pH of the SBF solution was determined, and the solution was replaced when the pH value was >8.5. The weight of the sample was determined at different times during the immersion. The corrosion rate (CR) of the HT-coated and uncoated Mg–Zn–Ca alloy samples was calculated as follows
| 1 |
where CR is the corrosion rate in units of millimeters per year, m0 is the initial mass and m is the residual mass of a sample at different times, D is the density of Mg alloy, A is the surface area of the sample, and t is the immersion time (in years).
5.2.2. Isolation and Culture of BMSCs
Bone marrow stem cells (BMSCs) are considered to be multipotent, which allows them to differentiate into several cell lineages, including type II chondrocytes, adipocytes, and osteoblasts/osteocytes.48 BMSCs were isolated and cultured, as described in our previous research.49 Briefly, New Zealand white rabbits (25 d after birth) were euthanized via an overdose injection of pentobarbital, and their bilateral femurs were then removed. The bone marrow was flushed from the femurs with Dulbecco’s modified Eagle’s medium (DMEM) (HyClone). After centrifugation, the sediment was resuspended in the culture medium, consisting of DMEM with 10% fetal bovine serum (FBS) (HyClone) and 1% penicillin/streptomycin (Beyotime, Shanghai, China), and then cultured in culture bottles (37 °C, 5% CO2 atmosphere, 95% humidity). Two days later, the nonadherent cells were removed, and the adherent cells were further cultured until 80–90% confluent and then were passaged. After the third passage, the cells were collected for further testing.
5.2.3. Indirect Cytotoxicity
Samples of the Mg–Zn–Ca alloy were machined into round plates with a diameter of 10 mm and thickness of 2 mm. The Mg alloy disc was immersed in 5 mL of culture medium in a culture bottle at 4 °C for 3 and 7 days. We removed the sample and collected the medium to dilute to 50% for subsequent cell culture. BMSCs were cultured at a density of 2 × 103 cells/well in a 96-well plate with a medium that had been preincubated with the uncoated Mg alloy or with the HT-coated Mg alloy or with control medium that had not been incubated with an alloy sample. The cells were analyzed after 1, 3, 5, and 7 days in culture using a CCK-8 assay kit (Biosharp, China). Optical density (OD) values measured the absorbency of cells at 450 nm, with higher absorbency values indicating the presence of more cells. This procedure was repeated three times for each well.
5.2.4. Cell Adhesion
Each Mg alloy disc was immersed in the medium for 2 h for pretreatment. The sample was then mixed with 3 mL of medium with 1 × 105 BMSCs and incubated overnight on a shaking table to assess the dynamic attachment of cells to uncoated and coated Mg alloy disks. The cell-seeded disks were transferred to a 6-well plate and further cultured 3 and 7 days to use for further live/dead test. Then, the nonadherent cells remaining in the medium and the number of adherent cells were counted (n = adherent cells) with the Cell counter (AP-0810401, MARIENFELD, Germany). The cell adhesion percentage for each scaffold was determined as (1 × 105 – n)/(1 × 105) × 100.
5.2.5. Direct Cytoactivity
The viability of cells on disks was analyzed using a live/dead staining kit (Wei Kai, Tianjin, China). Cell-seeded scaffolds were further cultured in the medium, and the medium was refreshed every 2 days. After 3 and 7 days, live/dead staining was performed. Briefly, the kit contains two reagents, calcein AM and EthD-1. When cells are cultured in the medium containing FBS, calcein AM, and EthD-1, calcein AM enters into live cells and serves as the substrate for a specific enzyme. The resulting calcein fluorescent molecules remain within live cells, so live cells appear green. EthD-1 cannot pass the cytomembrane, but it can enter into dead cells, where it binds DNA and becomes highly fluorescent, such that dead cells appear red. Simultaneously, scanning electron microscopy (SEM) (S-4300, Hitachi, Japan) was carried out on parallel samples to observe cells attached to the disks.
5.3. Design of the Mg Alloy Scaffold for in Vivo Testing
The Mg alloy scaffold was manufactured using a die-cast technique as a cylinder with the following dimensions: length, 15 mm; outer diameter, 5 mm; inner diameter, 3 mm; and with 12 holes of 1 mm in diameter drilled into the outside wall (Figure 1a,b). Each scaffold was sterilized under ethylene-oxide gas47 before implantation.
5.3.1. Surgical Procedure
This study was approved by the Harbin Medical University Institutional Animal Care and Use Committee. Thirty-six pathogen-free adult New Zealand white rabbits (2.5–3.0 kg) were randomly placed into one of two groups (n = 18/group), that were provided by Animal Experiment Center of Harbin Medical University. The surgical procedure used was as described in our previous study.47 Briefly, the rabbits were intravenously anesthetized with 3% (w/v) pentobarbital sodium (30 mg/kg). Then, the rabbit’s forearms were shaved and disinfected before an incision was made to expose the ulna. A 15 mm ulna osteotomy was performed to create a critical bone defect. HT-coated or uncoated Mg–Zn–Ca alloy scaffolds (Figure 1a,b) were filled with granular bone and then carefully implanted into the ulna defect (Figure 1c). At 4, 8, and 12 weeks after surgery, the animals (n = 6/group) were sacrificed after being anesthetized. collected forearms (n = 12/group) were stored at −20 °C for further testing.
5.3.2. X-ray Imaging
The X-ray images of the bone defect at 4, 8, and 12 weeks were obtained by an X-ray scanner (Faxitron; 110 kV; anode current, 500 μA). Then. degradation of the scaffold and bone regeneration was evaluated in each rabbit by these images.
5.3.3. Microcomputed Tomography (CT)
The two-dimensional (2-D) image of forearms was scanned using micro-CT (Siemens Medical Solutions, Germany, with 80 kV source voltage and 500 μA source current). Then, reconstructions were performed using Inveon software (Siemens Medical Solutions) and resulted in grayscale images. CT thresholding was performed to enhance the contrast of ossified tissues. The acquired two-dimensional lateral projections were analyzed using Inveon software. The residual scaffold was decisived by three-dimensional morphometric analysis, and the correlated CR was calculated using the Mg volume loss as follows
| 2 |
where CR is the corrosion rate in millimeters per year, V0 is the initial volume of the scaffold and V is the current volume of the scaffold (both in millimeters cubed), A is the surface area of the scaffold (in millimeters squared), and t is time (in years).
5.3.4. Subcutaneous Gas
In an in vivo situation, production of hydrogen gas is accompanied by Mg alloy corrosion with the following formula
| 3 |
Basically, 1 mol of hydrogen gas will be produced with the dissolution of 1 mol of Mg.50 Hydrogen bubbles accumulate in the local host tissue if corrosion rates of Mg or Mg alloy implants are too high. Thus, the animal was anesthetized as described above, and the subcutaneous gas present was punctured to collect in a syringe using a LOGIQ 7 color-Doppler ultrasound diagnostic system (GE), which was used to indirectly evaluate degradation of the scaffold.
5.3.5. Serum Mg2+ and Ca2+ Concentrations
Peripheral blood from each rabbit was collected in a heparinized tube to determine the Mg2+ and Ca2+ concentrations before being sacrificed. Each sample (5–9 mL/animal) was centrifuged (3500 rpm, 10 min) at room temperature, after which the serum was retained. The serum Mg and Ca concentrations (reference ranges: 0.82–2.22 and 2.1–2.6 mM, respectively) were measured using a Hitachi 7600 automatic clinical chemistry analyzer (Hitachi Limited, Japan).
5.3.6. Pathology
Healthy rabbits were used as the control group. The livers, heads, and kidneys of all animals were pathologically examined shortly after they were sacrificed. The samples were fixed in 10% (w/v) formalin (Beijing Yili, China) and embedded in paraffin. Each specimen was sectioned (5 μm thick), and deparaffinized sections were stained with the hematoxylin–eosin (HE) method. Then, the samples were visualized and photographs were taken through an Olympus CKX41 optical microscope (Japan) to evaluate systemic toxicity.
5.4. Statistical Analysis
GraphPad Prism 6.0 software (GraphPad Prism) was used to analyze data. All data are expressed as the mean ± standard error of the mean (SEM). Analysis of variance (ANOVA) and factorial design ANOVA were used, and p < 0.05 was considered significant.
Acknowledgments
This work was supported by the Basic Research Project from the Education Office of Heilongjiang Province (2017-KYYWF-0738) and Special research fund project of Qiqihar medical university (QMSI-201901).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.9b03889.
Calcium ion deposition around the scaffold/bone interfaces is shown in the fluorescence images (Figure S1); histological images showed the morphology and distribution of chondrocytes and osteocytes around the scaffold/bone interfaces (Figure S2) (PDF)
Author Contributions
⊥ Z.X. and Y.W. contributed equally to this work.
The authors declare no competing financial interest.
The version of this paper that was published ASAP February 25, 2020, contained errors in Figure 10. The corrected version was reposted March 10, 2020.
Supplementary Material
References
- Witte F.; Ulrich H.; Rudert M.; Willbold E. Biodegradable magnesium scaffolds: Part I: Appropriate inflammatory response. J. Biomed. Mater. Res., Part A 2007, 81A, 748–756. 10.1002/jbm.a.31170. [DOI] [PubMed] [Google Scholar]
- Qin H.; Zhao Y.; An Z.; Cheng M.; Wang Q.; Cheng T.; Wang Q.; Wang J.; Jiang Y.; Zhang X.; Yuan G. Enhanced antibacterial properties, biocompatibility, and corrosion resistance of degradable Mg-Nd-Zn- Zr alloy. Biomaterials 2015, 53, 211–220. 10.1016/j.biomaterials.2015.02.096. [DOI] [PubMed] [Google Scholar]
- Witte F.; Kaese V.; Haferkamp H.; Switzer E.; Meyer-Lindenberg A.; Wirth C. J.; Windhagen H. In vivo corrosion of four magnesium alloys and the associated bone response. Biomaterials 2005, 26, 3557–3563. 10.1016/j.biomaterials.2004.09.049. [DOI] [PubMed] [Google Scholar]
- Zhao D.; Witte F.; Lu F.; Wang J.; Li J.; Qin L. Current status on clinical applications of magnesium- based orthopedic implants: A review from clinical translational perspective. Biomaterials 2017, 112, 287–302. 10.1016/j.biomaterials.2016.10.017. [DOI] [PubMed] [Google Scholar]
- Vormann J. Magnesium: nutrition and metabolism. Mol. Aspects Med. 2003, 24, 27–37. 10.1016/S0098-2997(02)00089-4. [DOI] [PubMed] [Google Scholar]
- Touyz R. M. Magnesium in clinical medicine. Front. Biosci. 2004, 9, 1278–1293. 10.2741/1316. [DOI] [PubMed] [Google Scholar]
- Witte F.; Hort N.; Vogt C.; Cohen S.; Kainer K. U.; Willumeit R.; Feyerabend F. Degradable biomaterials based on magnesium corrosion. Curr. Opin. Solid State Mater. Sci. 2008, 12, 63–72. 10.1016/j.cossms.2009.04.001. [DOI] [Google Scholar]
- Saris N.-E. L.; Mervaala E.; Karppanen H.; Khawaja J. A.; Lewenstam A. Magnesium: An update on physiological, clinical and analytical aspects. Clin. Chim. Acta 2000, 294, 1–26. 10.1016/S0009-8981(99)00258-2. [DOI] [PubMed] [Google Scholar]
- Chaya A.; Yoshizawa S.; Verdelis K.; Myers N.; Costello B. J.; Chou D. T.; et al. Pal S5, Maiti S, Kumta PN, Sfeir C. In vivo study of magnesium plate and screw degradation and bone fracture healing. Acta Biomater. 2015, 18, 262–269. 10.1016/j.actbio.2015.02.010. [DOI] [PubMed] [Google Scholar]
- Grünewald T. A.; Ogier A.; Akbarzadeh J.; Meischel M.; Peterlik H.; Stanzl-Tschegg S.; Loffler J. F.; Weinberg A. M.; Lichtenegger H. C. Reaction of bone nanostructure to a biodegrading Magnesium WZ21 implanteA scanning small-angle X-ray scattering time study. Acta Biomater. 2016, 31, 448–457. 10.1016/j.actbio.2015.11.049. [DOI] [PubMed] [Google Scholar]
- Lee J. W.; Han H. S.; Han K. J.; Park J.; Jeon H.; Ok M. R.; Seok H. K.; Ahn J. P.; Lee K. E.; Lee D. H.; Yang S. J.; Cho S. Y.; Cha P. R.; Kwon H.; Nam T. H.; Han J. H.; Rho H. J.; Lee K. S.; Kim Y. C.; Mantovani D. Long-term clinical study and multiscale analysis of in vivo biodegradation mechanism of Mg alloy. Proc. Natl. Acad. Sci. U.S.A. 2016, 113, 716–721. 10.1073/pnas.1518238113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshizawa S.; Brown A.; Barchowsky A.; Sfeir Magnesium ion stimulation of bone marrow stromal cells enhances osteogenic activity, simulating the effect of magnesium alloy degradation. Acta Biomater. 2014, 10, 2834–2842. 10.1016/j.actbio.2014.02.002. [DOI] [PubMed] [Google Scholar]
- Guo Y.; Ren L.; Liu C.; Yuan Y.; Lin X.; Tan L.; Chen S.; Yang K.; Mei X. Effect of implantation of biodegradable magnesium alloy on: BMP-2 expression in bone of ovariectomized osteoporosis rats. Mater. Sci. Eng., C 2013, 33, 4470–4474. 10.1016/j.msec.2013.05.042. [DOI] [PubMed] [Google Scholar]
- Yamamoto A.; Hiromoto S. Effect of inorganic salts, amino acids and proteins on the degradation of pure magnesium in vitro. Mater. Sci. Eng., C 2009, 29, 1559–1568. 10.1016/j.msec.2008.12.015. [DOI] [Google Scholar]
- Zhuang J.; Jing Y.; Wang Y.; Zhang J.; Xie H.; Yan J. Degraded and osteogenic properties of coated magnesium alloy AZ31; an experimental study. J. Orthop. Surg. Res. 2016, 11, 30 10.1186/s13018-016-0362-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao D.; Huang S.; Lu F.; Wang B.; Yang L.; Qin L.; Yang K.; Li Y.; Li W.; Wang W.; Tian S.; Zhang X.; Gao W.; Wang Z.; Zhang Y.; Xie X.; Wang J.; Li J. Vascularized bone grafting fixed by biodegradable magnesium screw for treating osteonecrosis of the femoral head. Biomaterials 2016, 81, 84–92. 10.1016/j.biomaterials.2015.11.038. [DOI] [PubMed] [Google Scholar]
- Song G.; Atrens A. Understanding magnesium corrosion-A Framework for Improved Alloy Performance. Adv. Eng. Mater. 2003, 5, 837–858. 10.1002/adem.200310405. [DOI] [Google Scholar]
- Zeng R.; Dietzel W.; Witte F.; Hort N.; Blawert C. Progress and challenge for magnesium alloys as biomaterials. Adv. Eng. Mater. 2008, 10, B3–B14. 10.1002/adem.200800035. [DOI] [Google Scholar]
- Pietak A.; Mahoney P.; Dias G. J.; Staiger M. P. Bone-like matrix formation on magnesium and magnesium alloys. J. Mater. Sci.: Mater. Med. 2008, 19, 407–415. 10.1007/s10856-007-3172-9. [DOI] [PubMed] [Google Scholar]
- Staiger M. P.; Pietak A. M.; Huadmai J.; Dias G. Magnesium and its alloys as orthopedic biomaterials: a review. Biomaterials 2006, 27, 1728–1734. 10.1016/j.biomaterials.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Wong H. M.; Yeung K. W. K.; Lam K. O.; Tam V.; Chu P. K.; Luk K. D.; Cheung K. M. A biodegradable polymer-based coating to control the performance of magnesium alloy orthopaedic implants. Biomaterials 2010, 31, 2084–2096. 10.1016/j.biomaterials.2009.11.111. [DOI] [PubMed] [Google Scholar]
- Friedrich H.; Mordike B.. Magnesium Technology; Springer: Heidelberg, 2006; pp 1–35. [Google Scholar]
- Guo X. H.; Du K. Q.; Ge H.; Guo Q. Z.; Wang Y.; Wang F. H. Good sensitivity and high stability of humidity sensor using micro-arc oxidation alumina film. Electrochem. Commun. 2013, 28, 95–99. 10.1016/j.elecom.2012.11.036. [DOI] [Google Scholar]
- Golshirazi A.; Kharaziha M.; Golozar M. A. Polyethylenimine/kappa carrageenan: Micro-arc oxidation coating for passivation of magnesium alloy. Carbohydr. Polym. 2017, 167, 185–195. 10.1016/j.carbpol.2017.03.025. [DOI] [PubMed] [Google Scholar]
- Razavi M.; Fathi M.; Savabi O.; Vashaee D.; Tayebi L. Improvement of biodegradability, bioactivity, mechanical integrity and cytocompatibility behavior of biodegradable mg based orthopedic implants using nanostructured Bredigite (Ca7MgSi4O16) bioceramic coated via ASD/EPD technique. Ann. Biomed. Eng. 2014, 42, 2537–2550. 10.1007/s10439-014-1084-7. [DOI] [PubMed] [Google Scholar]
- Dou J.; Chen Y.; Chi Y.; Li H.; Gu G.; Chen C. Preparation and characterization of a calcium-phosphate-silicon coating on a Mg–Zn–Ca alloy via two-step micro-arc oxidation. Phys. Chem. Chem. Phys. 2017, 19, 15110–15119. 10.1039/C7CP02672B. [DOI] [PubMed] [Google Scholar]
- Wu Y. F.; Wang Y. M.; Jing Y. B.; Zhuang J. P.; Yan J. L.; Shao Z. K.; Jin M. S.; Wu C. J.; Zhou Y. In vivo study of microarc oxidation coated biodegradable magnesium plate to heal bone fracture defect of 3 mm width. Colloids Surf., B 2017, 158, 147–156. 10.1016/j.colsurfb.2017.06.031. [DOI] [PubMed] [Google Scholar]
- Pan Y.; Chen C.; Feng R.; Cui H.; Gong B.; Zheng T.; Ji Y. Effect of calcium on the microstructure and corrosion behavior of microarc oxidized Mg-xCa alloys. Biointerphases 2018, 13, 011003 10.1116/1.5003320. [DOI] [PubMed] [Google Scholar]
- Wu Y.; Wang Y. M.; Zhao D. W.; Zhang N.; Li H.; Li J.; Wang Y.; Zhao Y.; Yan J.; Zhou Y. In vivo study of microarc oxidation coated Mg alloy as a substitute for bone defect repairing: Degradation behavior, mechanical properties, and bone response. Colloids Surf., B 2019, 181, 349–359. 10.1016/j.colsurfb.2019.05.052. [DOI] [PubMed] [Google Scholar]
- Li B.; Han Y.; Qi K. Formation mechanism, degradation behavior, and cytocompatibility of a nanorod-shaped HA and pore-sealed MgO bilayer coating on magnesium. ACS Appl. Mater. Interfaces 2014, 6, 18258–18274. 10.1021/am505437e. [DOI] [PubMed] [Google Scholar]
- Tian Q.; Liu H. Electrophoretic deposition and characterization of nanocomposites and nanoparticles on magnesium substrates. Nanotechnology 2015, 26, 175102 10.1088/0957-4484/26/17/175102. [DOI] [PubMed] [Google Scholar]
- Razavi M.; Fathi M.; Savabi O.; Vashaee D.; Tayebi L. In vivo assessments of bioabsorbable AZ91 magnesium implants coated with nanostructured fluoridated hydroxyapatite by MAO/EPD technique for biomedical applications. Mater. Sci. Eng., C 2015, 48, 21–27. 10.1016/j.msec.2014.11.020. [DOI] [PubMed] [Google Scholar]
- Sun J.; Zhu Y.; Meng L.; Chen P.; Shi T.; Liu X.; Zheng Y. Electrophoretic deposition of colloidal particles on Mg with cytocompatibility, antibacterial performance, and corrosion resistance. Acta Biomater. 2016, 45, 387–398. 10.1016/j.actbio.2016.09.007. [DOI] [PubMed] [Google Scholar]
- Willbold E.; Gu X.; Albert D.; Kalla K.; Bobe K.; Brauneis M.; Janning C.; Nellesen J.; Czayka W.; Tillmann W.; Zheng Y.; Witte F. Effect of the addition of low rare earth elements (lanthanum, neodymium, cerium) on the biodegradation and biocompatibility of magnesium. Acta Biomater. 2015, 11, 554–562. 10.1016/j.actbio.2014.09.041. [DOI] [PubMed] [Google Scholar]
- Guo J. W.; Sun S. Y.; Wang Y. M.; Zhou Y.; Wei D. Q.; Jia D. C. Hydrothermal biomimetic modification of micro-arc oxidized magnesium alloy for enhanced corrosion resistance and deposition behaviors in SBF. Surf. Coat. Technol. 2015, 269, 183–190. 10.1016/j.surfcoat.2015.02.010. [DOI] [Google Scholar]
- Li L.-Y.; Cui L.-Y.; Zeng R.-C.; Li S.-Q.; Chen X.-B.; Zheng Y.; Kannan M. B. Advances in functionalized polymer coatings on biodegradable magnesium alloys-A review. Acta Biomater. 2018, 79, 23–36. 10.1016/j.actbio.2018.08.030. [DOI] [PubMed] [Google Scholar]
- Chen J.; Tan L.; Yu X.; Etim I. P.; Ibrahim M.; Yang K. Mechanical properties of magnesium alloys for medical application: A review. J. Mech. Behav. Biomed. Mater. 2018, 87, 68–79. 10.1016/j.jmbbm.2018.07.022. [DOI] [PubMed] [Google Scholar]
- Kokubo T.; Takadama H. How useful is SBF in predicting in vivo bone bioactivity?. Biomaterials 2006, 27, 2907–2915. 10.1016/j.biomaterials.2006.01.017. [DOI] [PubMed] [Google Scholar]
- Shaw B. A.Corrosion Resistance of Magnesium Alloys. In ASM Handbook Volume 13A: Corrosion: Fundamentals, Testing and Protection, Stephen D., Ed.; ASM Int.: UK, 2003. [Google Scholar]
- Scharnweber D.Degradation. In Metals as Biomaterials, 4th ed.; Helson J. A.; Breme H. J., Eds.; Wiley: New York, 1998; pp 101–151. [Google Scholar]
- Witte F.; Fischer J.; Nellesen J.; Crostack H.-A.; Kaese V.; Pisch A.; Beckmann F.; Windhagen H. In vitro and in vivo corrosion measurements of magnesium alloys. Biomaterials 2006, 27, 1013–1018. 10.1016/j.biomaterials.2005.07.037. [DOI] [PubMed] [Google Scholar]
- Zhang J.; Tang L.; Qi H.; Zhao Q.; Liu Y.; Zhang Y. Dual Function of Magnesium in Bone Biomineralization. Adv. Healthcare Mater. 2019, 8, 1901030 10.1002/adhm.201901030. [DOI] [PubMed] [Google Scholar]
- Amerstorfer F.; Fischerauer S. F.; Fischer L.; Eichler J.; Draxler J.; Zitek A.; Meischel M.; Martinelli E.; Kraus T.; Hann S.; Stanzl-Tschegg S. E.; Uggowitzer P. J.; Löffler J. F.; Weinberg A. M.; Prohaska T. Long-term in vivo degradation behavior and near-implant distribution of resorbed elements for magnesium alloys WZ21 and ZX50. Acta Biomater. 2016, 42, 440–450. 10.1016/j.actbio.2016.06.025. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; Xu J.; Ruan Y. C.; Yu M. K.; O’Laughlin M.; Wise H.; Chen D.; Tian L.; Shi D.; Wang J.; Chen S.; Feng J. Q.; Chow D. H.; Xie X.; Zheng L.; Huang L.; Huang S.; Leung K.; Lu N.; Zhao L.; Li H.; Zhao D.; Guo X.; Chan K.; Witte F.; Chan H. C.; Zheng Y.; Qin L. Implant-derived magnesium induces local neuronal production of CGRP to improve bone-fracture healing in rats. Nat. Med. 2016, 22, 1160–1169. 10.1038/nm.4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding P.; Liu Y.; He X.; Liu D.; Chen M. In vitro and in vivo biocompatibility of Mg–Zn–Ca alloy operative clip. Bioact. Mater. 2019, 4, 236–244. 10.1016/j.bioactmat.2019.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.-q.; Li Y.; Liu H.; Bai J.; Bao N.-r.; Zhang Y.; He P.; Zhao J.-n.; Tao L.; Xue F.; Zhou G. X.; Fan G. T. Mechanical and Biological Properties of a Biodegradable Mg–Zn–Ca Porous Alloy. Orthop. Surg. 2018, 10, 160–168. 10.1111/os.12378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N.; Zhao D.; Liu N.; Wu Y.; Yang J.; Wang Y.; Xie H.; Ji Y.; Zhou C.; Zhuang J.; Wang Y.; Yan J. Assessment of the degradation rates and effectiveness of different coated Mg–Zn–Ca alloy scaffolds for in vivo repair of critical-size bone defects. J. Mater. Sci.: Mater. Med. 2018, 29, 138 10.1007/s10856-018-6145-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marini J. C.; Forlino A. Replenishing Cartilage from Endogenous Stem Cells. N. Engl. J. Med. 2012, 366, 2522–2524. 10.1056/NEJMcibr1204283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Y.; Cui L.-H.; Xiang S.-Y.; Xu W.-X.; Chen D.-C.; Fu R.; Zhou C.-L.; Liu X.-Q.; Wang Y.-F.; Wang X.-T. Osteoblast-oriented differentiation of BMSCs by co-culturing with composite scaffolds constructed using silicon-substituted calcium phosphate, autogenous fine particulate bone powder and alginate. Oncotarget 2017, 8, 88308–88319. 10.18632/oncotarget.19015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J.; Wang J.; Xie X.; Zhang P.; Lai Y.; Li Y.; Qin L. Surface coating reduces degradation rate of magnesium alloy developed for orthopaedic applications. J. Orthop. Transl. 2013, 1, 41–48. 10.1016/j.jot.2013.06.003. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.