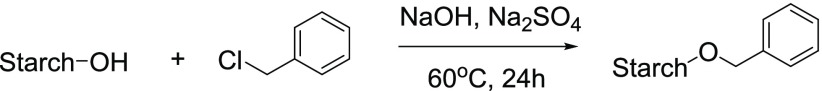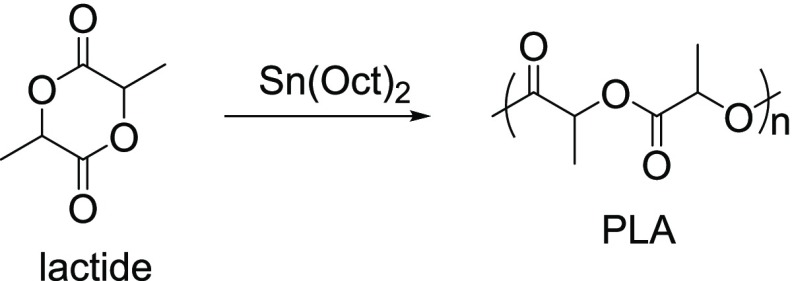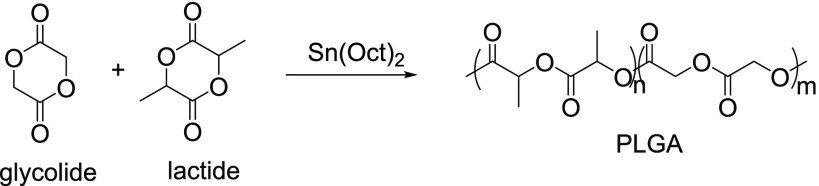Abstract
Polymeric materials obtained from petroleum resources are nonbiodegradable. Defying degradation, they damage the environment as a result of their ending up in the landfills. Synthesized biodegradable polymeric materials (BPMs) have received increasing interest owing to the difficulty in procuring reproducibility when using natural polymeric materials. Through the modification of natural polymeric materials or materials via chemical, microbiological, enzyme-mediated, and chemo-enzymatic synthesis, a comprehensive range of variegated BPMs can be reaped. Amended natural polymeric materials such as starch, cellulose, and chitin have enhanced properties, while synthetic BPMs such as PLA, PGA, PCL, PDS, and PLGA are explicitly designed to pursue coveted applications in multifarious domains such as whole diagnostics and therapeutics. Synthesized BPMs can be embedded with tailored characteristics to justify the neoteric entails of mankind.
1. Introduction
Upsurge prevailing in developing countries regarding the ecological detriment due to pollution has stretched to perilous heights as a result of the use of polymeric materials. Similar durability characteristics of this type of polymeric material for varied execution in packaging supplies, structure materials, and basic commodities can remedy disposal snags for traditional petroleum-derived polymers, which are not readily biodegradable. Being resistant to biodegradation, they accumulate in the environment. This is the primary reason for the interest in biodegradable polymeric materials.1
Biodegradable polymeric materials (BPMs) represent a growing field. Owing to their wide-ranging properties, both synthetic and natural polymeric materials perform a vital and ubiquitous role in everyday life. Having a period of effectiveness, BPMs divulge the phenomenon of biodegradation. It is valuable to distinguish between BPMs on the basis of their origin: native or synthetic. Native BPMs represent the synthesis developed during a long course of evolution in nature, while synthetic BPMs are the result of a mere century of research and development, both resulting in materials possessing tailored characteristics for diverse applications. They include proteins (collagen, gelatin, and albumin), polysaccharides (cellulose, chitin, and alginate), nucleic acids, and lipids, which illustrate totally diverse characteristics reliant on the conditions under which they are used. Synthesized BPMs have received increasing interest owing to the difficulty in obtaining reproducibility when using natural polymeric materials. Although first introduced in the 1980s, synthetic BPMs have been attracting attenention in the last two decades, primarily due to ecological fouling and the realization that our natural resources are finite.1
As for all synthetic polymeric materials, BPMs can be produced with reproducible quality and indigenous purity with no immunogenicity concerns and can be fabricated into a variety of forms in the required bulk with desired morphology. The aptness to tailor the degradation kinetics and mechanical properties while synthesizing BPMs contributes to their multifarious applications by inducing biocompatibility, making them more predictable with respect to polymers isolated from natural sources.2
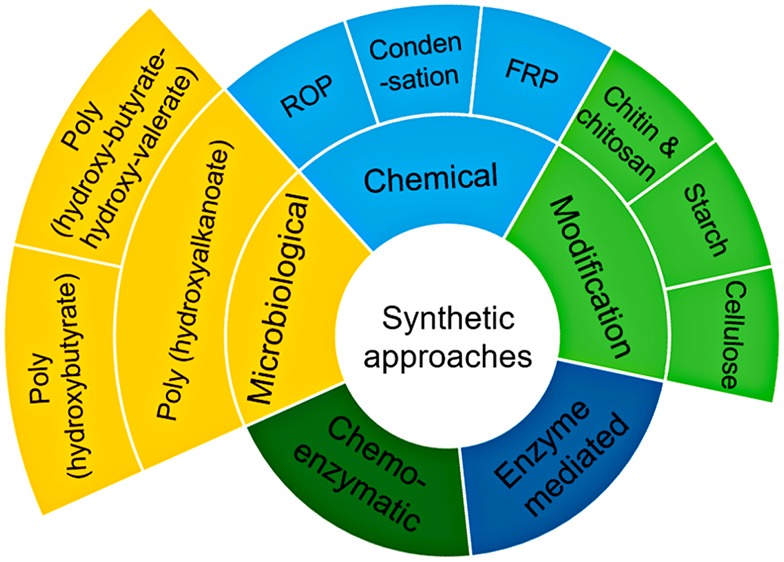
BPMs can be devised broadly via either modification of natural polymeric materials or through diversified routes used to synthesize BPMs comprising chemical synthesis, microbiological synthesis, enzyme-mediated synthesis, and chemo-enzymatic synthesis.2
Synthetic BPMs such as poly(lactic acid) (PLA), poly(glycolic acid) (PGA), poly(lactic-co-glycolic acid) (PLGA), poly(butylene succinate) (PBA), polycaprolactone (PCL), poly(ethylene adipate) (PEA), poly(p-dioxanone) (PDS), and their copolymers play an imperative role in clinical applications such as nonviral gene delivery vectors, drug-delivery systems,2 resorbable sutures, biosensors, tissue engineering scaffolds, regenerative medicine including implants, and orthopedic fixation devices such as pins, rods, and screws. With respect to the varied applications, research on synthetic BPMs has been focused on the simulation of different biopolymeric materials or polymeric materials with degradable backbones, for instance, polyanhydrides, polycarbonates, and polylactones.
This review focuses on the synthesis methods of BPMs. It also briefly summarizes the applications to heighten future research advances in the field of BPMs.
2. Synthesis Methods for the Preparation of BPMs
2.1. Modification of Natural Polymeric Materials
Being readily biodegradable in nature, polysaccharide polymeric materials such as chitosan, chitin, starch, and cellulose can be modified into new BPMs by coblending.
Natural polymers can be modified via various chemical modifications, in particular, nitration, hydroxylation, sulfonation, acylation, alkylation, phosphorylation, thiolation, xanthation, quaternization, and graft copolymerization, of which graft copolymerization is the most promising approach leading to a wide variety of molecular designs.3
2.1.1. Modification of Chitin and Chitosan
Among several methods, graft copolymerization is an influential method used to enhance the antibacterial, chelating, and complexation properties of chitosan. The grafting of chitin and chitosan by covalently binding a molecule onto the backbone enables the synthesis of functional derivatives.
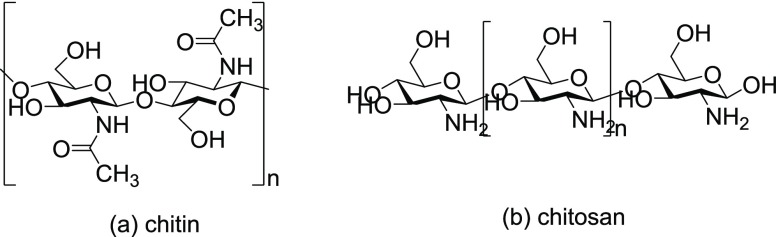
Chitosan contains two reactive groups that can act as grafting sites: the free amino groups (on deacetylated units of chitin) and the hydroxyl groups (on the C3 and C6 carbons on acetylated and deacetylated units of chitin, respectively) (Figure 1).4
Figure 1.
Structures of (a) chitin and (b) chitosan.
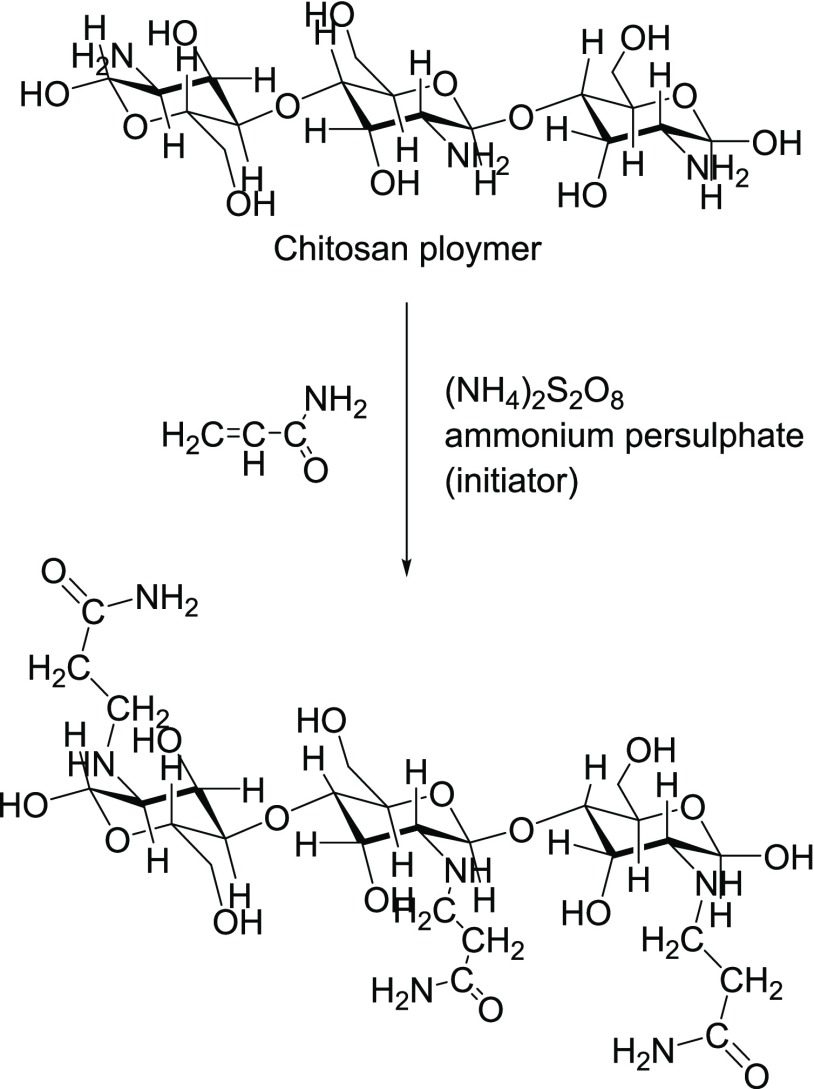
Molatlhegi et al.4 showed that chitosan-grafted polyacrylamide (CGP) was a better flocculant than pure chitosan (Figure 2). Furthermore, the existence of an extremely high adsorption affinity of CGP toward kaolin surfaces was also demonstrated.
Figure 2.
Synthesis of acrylamide-grafted chitosan.
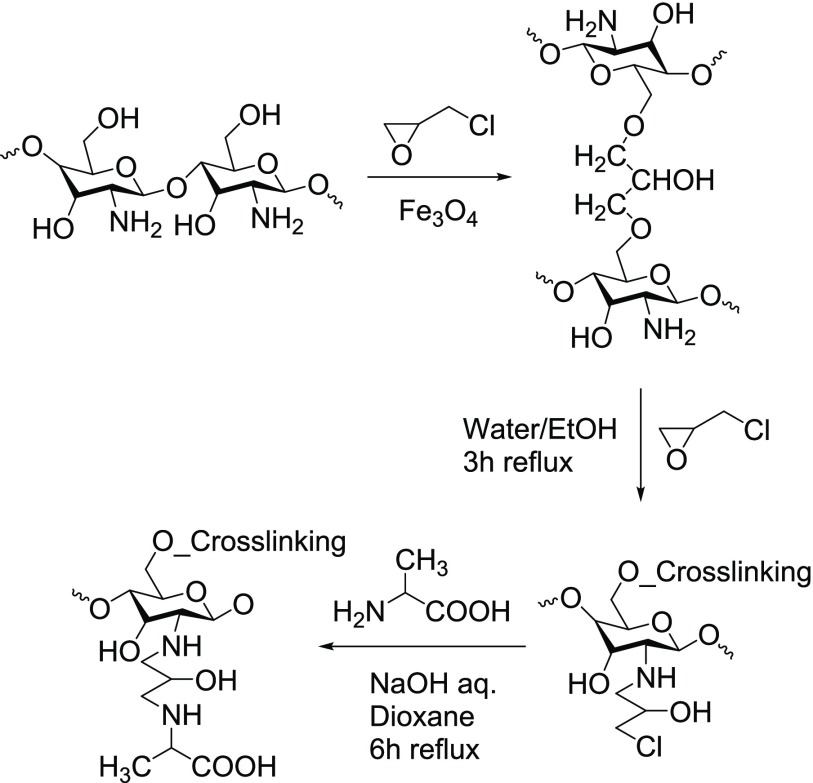
Dong et al.5 formed and stabilized nanometric-sized epichlorohydrin cross-linked chitosan magnetic particles by adding sodium hydroxide to the mixture of chitosan/Fe(II)/Fe(III) dropwise with constant stirring (Figure 3). The hydroxyl groups are preferentially reacted with a cross-linking agent in the presence of iron salts (Fe(II) and Fe(III) ions) in the chitosan solution, suggesting the possible interaction of these ions with the protected amino groups. The protection of amino groups due to weak interaction with iron, resulted in the cross-linking of epichlorohydrin-grafted chitosan with the following:
-
(a)
–NH2, in the case of alanine, where the −CH3 group is poorly reactive,
-
(b)
–NH2 and −CH2OH groups in the case of serine, and
-
(c)
–NH2 and −CH2SH groups in the case of cysteine
Figure 3.
Synthesis of alanine-functionalized epichlorohydrin-grafted magnetic chitosan.
2.1.2. Modification of Starch
The large number of hydroxyl groups on starch molecules provide the active sites for chemical modification. The chemical modification involves introducing various functional groups, such as carboxyl, acetyl, and hydroxypropyl, into starch structure (Figure 4). The properties of modified starch are greatly impacted by the source of starch, reaction conditions, and methods used for its modification. Table 1(6) shows some properties of starch prepared via different modifications.
Figure 4.
Structure of starch.
Table 1. Modifications of Starch and Their Propertiesa.
| modification | properties |
|---|---|
| cross-linking | better freeze–thaw |
| granule stability | |
| higher heat | |
| shear stabilities | |
| lower swelling power | |
| decreased solubility | |
| reduced enthalpy of gelatinization | |
| grafting | better biodegradability |
| enhanced stability | |
| stronger water absorbency | |
| larger hydrodynamic radius and volume | |
| film making | |
| esterification | more moisture-resistant and thermoplasticity |
| better compatibility | |
| lower biodegradability and thermal stability | |
| etherification | better thermal stability |
| enhanced solubility | |
| better flowability | |
| improved permeability | |
| good strength | |
| oxidization | higher solubility |
| better viscosity | |
| enhanced gelatinization temperature | |
| better water absorbency | |
| lower enthalpy value | |
| acidification | better recovery |
| enhanced solubility | |
| lower pasting viscosity | |
| reduced gelatinization enthalpy | |
| dual | combination of properties |
Reproduced from ref (6) with permission from the Royal Society of Chemistry.
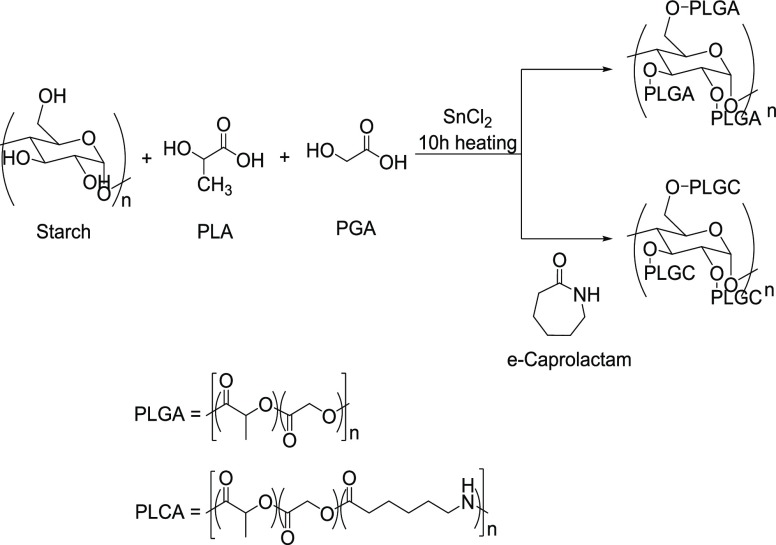
Starch-graft-poly(lactic acid) (SGPLA), starch-graft poly(lactic acid-co-glycolic acid) (SGPLGA), and starch-graft poly(lactic acid-co-glycolic acid-co-e-caprolactam) (SGPLGC) copolymers were yielded through the direct polycondensation of starch with lactic acid, glycolic acid, and e-caprolactam sequentially in aqueous media using stannous chloride (SnCl2) as the catalyst at 120–170 °C (Figure 5).7a
Figure 5.
Graft polymerization of starch with lactic acid, glycolic acid, and e-caprolactum.
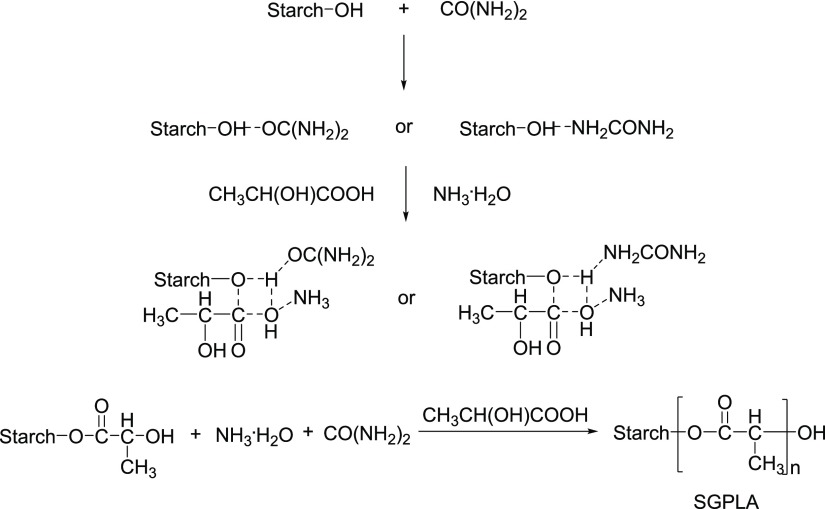
The synthesis of SGPLA was performed in the presence of NH3·H2O with urea as the solvent (Figure 6). The effect of urea and NH3·H2O dosage concentration on the graft copolymerization of starch with lactic was obvious. The reason was proposed that the dissolution of starch was boosted because of its hydrogen bond with urea to attain homogeneous conditions and favor the esterification of starch with lactic acid. Being easily available and reaction-friendly, ammonia–water was selected as the catalyst to promote the above reaction. It is found that the grafting degree of starch increases with the increase in the amount of ammonia–water in 12 initial stages, but because it is a reversible reaction, excess ammonia–water could promote the hydrolysis of ester, which would decrease the grafting degree of starch.7b
Figure 6.
Synthesis of SGPLA.
Misman et al.7c etherified sago starch by reacting it with benzyl chloride in sodium hydroxide and found that the product resulting from solvent-based etherification had a higher degree of substitution, good thermal stability, and a better ability to flow (Figure 7).
Figure 7.
Etherification of starch.
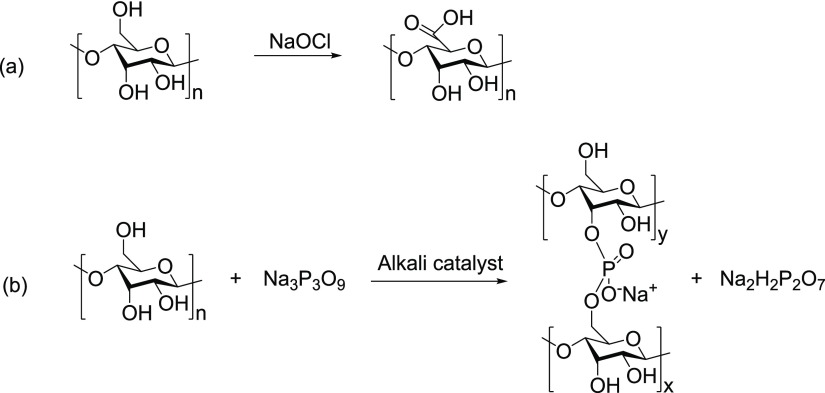
Sometimes when single modification does not impart the desired properties, further modification is used to meet the requirements for the better application of such a modified starch. Sukhija et al.7d performed the dual modification of elephant foot yam starch in two different ways to yield modified starches, i.e., oxidized cross-linked starch (OCS) and cross-linked oxidized starch (COS), to check the change in the physiochemical properties because of chemical modification. The oxidation of starch in the presence of sodium hypochlorite (NaOCl) followed by its cross-linking with sodium trimetaphosphate (STMP) (Na3P3O9) produced OCS, whereas the cross-linking of starch with STMP followed by its oxidation through NaOCl yielded COS. OCS possessed improved gel clarity, solubility, and thermal characteristics with respect to COS. The oxidation and cross-linking reactions of starch are shown in Figure 8.
Figure 8.
(a) Oxidation of starch and (b) cross-linking of starch.
2.1.3. Modification of Cellulose
Naturally obtained cellulose can be chemically modified by treating it via esterification, etherification, or deacylation.
Baiardo et al.8 modified the surface fibers of cellulose without appreciably altering their native structure and morphology. By the simple esterification and etherification reactions, the high-polarity and high-hydrophilicity properties of natural cellulose fibers were suppressed such that the surface property qualities of the most common thermoplastics are fulfilled. Esterification reactions were carried out using acyl chloride and pyridine, while for performing the etherification of cellulose hydroxyls with ethyl groups, alkylation was carried out in two different alkaline media. Mild reaction conditions were employed in order to maintain the reinforcement properties and preserve the biodegradability of the cellulose fibers.
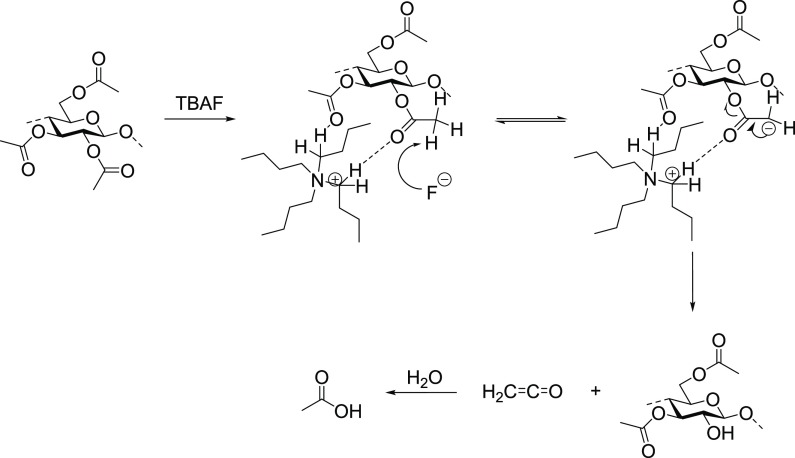
The acetate groups suffered cleavage during the deprotection of silylated cellulose acetate with TBAF·3H2O/THF or TBAF·3H2O/DMSO. Further investigations revealed regioselective deacetylation (Figure 9) at C2 and C3 carbon positions of the aminoguanidine (AGU). Here, TBA+ acts as a general-acid catalyst, whereas F– induces deacetylation via a ketene intermediate. The formation of the latter was inferred from a kinetic isotope which affects the experiment.
Figure 9.
Deacetylation of cellulose.
2.2. Chemical Synthesis of BPMs
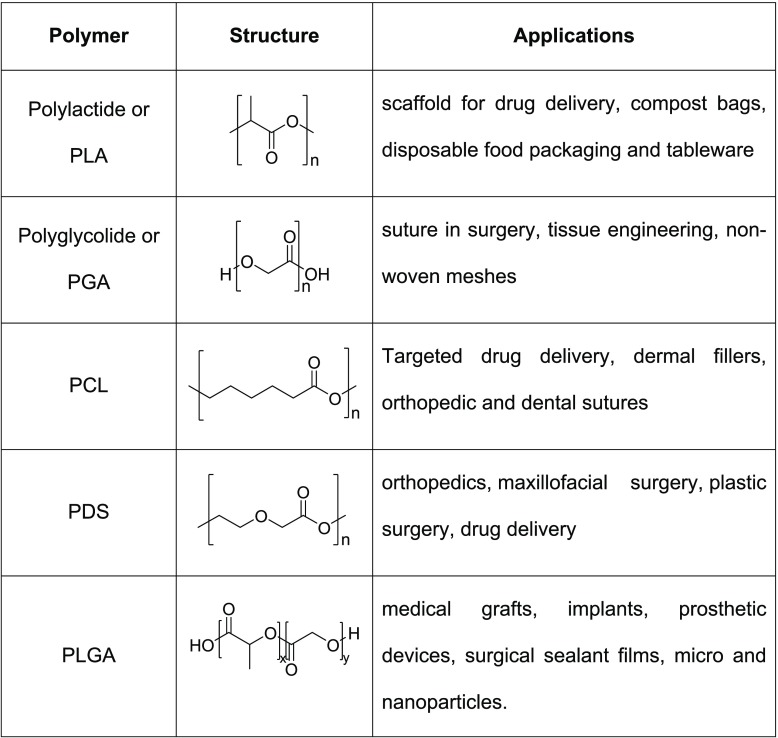
Polymeric materials are chemically synthesized with their structures resembling natural polymeric materials. The polymeric materials containing chains of ester, amide, and peptide bonds are readily biodegradable. The process where many small molecules, called monomers or repeating units, combine to form a covalently bonded chain or network is coined polymerization. The loss of some chemical groups from each monomer may occur during the process. A few synthetic BPMs accompanied by their structures and applications are shown in Table 2.9
Table 2. Some Synthetic Biodegradable Polymers.
Synthetic polymerization reactions can be carried out with or without the help of a catalyst. BPMs synthesized in the laboratory are an area of intensive research.
2.2.1. Ring-Opening Polymerization (ROP)
Polymerization where an acyclic monomeric unit is produced from a cyclic monomer is ROP. It is a form of chain-growth polymerization where the terminal end of a polymer chain acts as a reactive center to form a longer polymer chain by opening the ring system of cyclic monomers. There can be three propagating centers: radical, anionic, or cationic. ROP continues to be the most versatile method for the synthesis of major groups of biopolymers to procure product in large quantities.10a
PLA is synthesized either by the polycondensation of lactic acid or by the chain growth ROP of lactide (Figure 10). Several catalysts and initiators can be used to carry out the ROP of lactide. A commonly used robust catalyst/initiator is Sn(Oct)2.10a
Figure 10.
ROP of lactide.
High-molecular-weight PLGA is produced by the ROP of lactide and glycolide, the cyclic diesters of lactic acid and glycolic acid, respectively, under catalyst Sn(Oct)2 (Figure 11).10a
Figure 11.
ROP of glycolide and lactide.
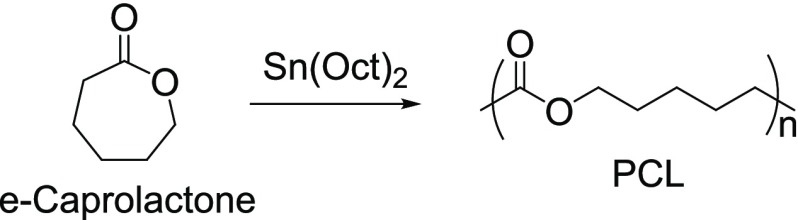
PCL is synthesized via ROP of e-caprolactone with Sn(Oct)2 as a catalyst (Figure 12).10a
Figure 12.
ROP of e-caprolactone.
ROP of p-dioxanone produces PDS (Figure 13), a relatively weak, rapidly biodegrading polymer which was introduced to the market in the 1980s as a new degradable material to be used for suturing. It generates degradation products that are less acidic than PGA and PLA.10b
Figure 13.
ROP of p-dioxanone.
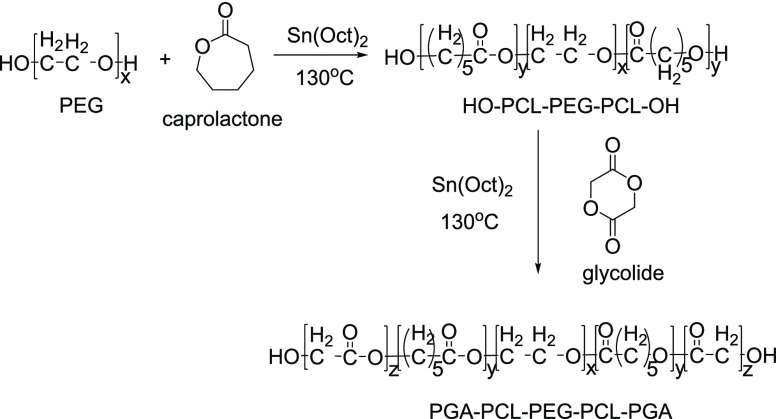
Gou et al.10c synthesized three novel pentablock (PB) copolymers by the bulk ROP method:
-
a.
poly(glycolic acid)-poly(caprolactone)-poly(ethylene glycol)-poly(caprolactone)-poly(glycolic acid) (PGA-PCL-PEG-PCL-PGA, i.e., PB-A) (Figure 14),
-
b.
poly(lactic acid)-poly(caprolactone)-poly(ethylene glycol)-poly(caprolactone)-poly(lactic acid) (PLA-PCL-PEG-PCL-PLA, i.e., PB-B),
-
c.
poly(ethylene glycol)-poly(caprolactone)-poly(lactic acid)-poly(caprolactone)-poly(ethylene glycol) (PEG-PCL-PLA-PCL-PEG, i.e., PB-C)
Figure 14.
Synthesis scheme for the PB-A (PGA-PCL-PEG-PCL-PGA) copolymer.
2.2.2. Free-Radical Polymerization (FRP)
The consecutive addition of building blocks as free radicals, formed by several mechanisms involving separate initiator molecules, to produce polymers is termed FRP. The newly generated initiating free radicals add monomer units to the polymer chain, resulting in its growth. Owing to the nonspecific nature of free-radical chemical interactions, FRP is the most versatile forms of polymerization which allows facile reactions of polymeric free-radical chain ends with other chemicals or substrates.11
2.2.3. Condensation
A condensation reaction is a class of organic addition reaction that typically proceeds in a stepwise fashion, occurring under acidic or basic conditions or in the presence of a catalyst and yielding the addition product in equilibrium. As the name suggests, condensation produces a water molecule. The reaction may also involve the functional groups of the molecule to form a small molecule such as ammonia, ethanol, or acetic acid instead of water. These reactions are a vital part of life as they play an essential role in the generation of peptide bonds between amino acids and the biosynthesis of fatty acids.12
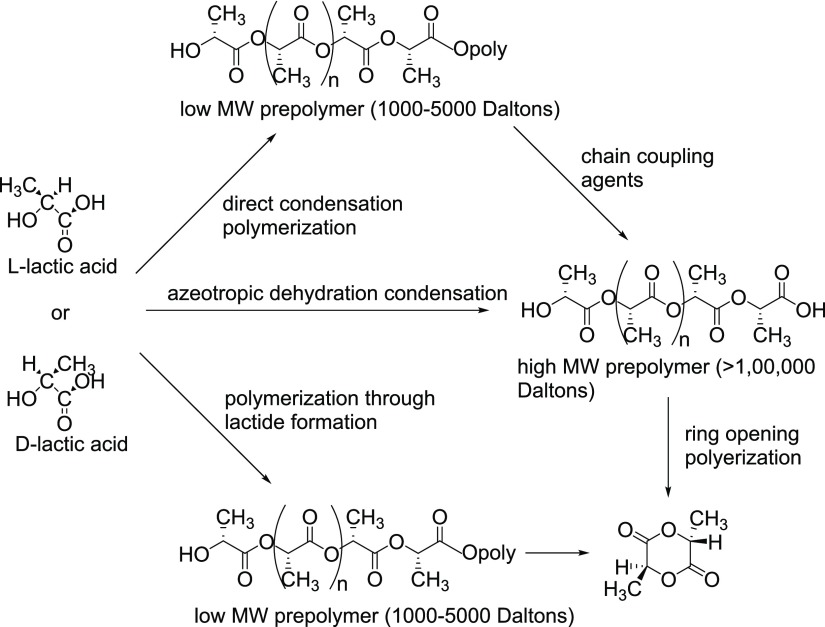
Figure 15 shows different techniques such as direct condensation polymerization, azeotropic dehydrative condensation, and polymerization through lactide formation to produce high-molecular-weight PLA. However, currently most of them are produced by lactide ROP.12
Figure 15.
Synthesis of PLA from l- and d-lactic acids.
However, the required synthesis conditions were harsh, which resulted in many byproducts, making this method less cost-efficient for the synthesis of BPMs.
2.3. Microbiological Synthesis of BPMs
Microorganisms use organic matter such as glucose and starch as food sources to synthesize a series of complex polymeric materials. These polymeric materials cover a varied sort of silk, polysaccharides, polyesters including poly(hydroxyalkanoates) (PHAs) such as poly(hydroxybutyrate) (PHB) and poly(hydroxy-butyrate-hydroxy-valerate) (PHBV), and PCL. The similarity in their chemical properties makes the separation of these products difficult. Table 3(13) shows the list of the few bacterial polyester PHAs mentioned with the respective radicals as shown in Figure 16.
Table 3. Types of Bacterial Polyester PHAs with Their Specified Radicalsa.
| polymer | –R group | applications |
|---|---|---|
| poly(hydroxylbutyrate) PHB | –CH3 | seam threads for the healing of wounds and blood vessels, creating bioplastics |
| poly(hydroxylvalerate) PHV | –CH2–CH3 | controlled release of drugs, clinical repairs, orthopedic devices |
| poly(hydroxyhexanoate) PHHex | –(CH2)2–CH3 | medical implants, adhesion barriers, stents, vein valves |
| poly(hydroxyloctanoate) PHO | –(CH2)4–CH3 | biomedical grafting, bone marrow scaffolds |
| poly(hydroxyldecanoate) PHD | –(CH2)6–CH3 | drug delivery |
| poly(hydroxy phenylvalerate) PHPV | –CH2–C6H5 | surgical implants, biofuels |
Reproduced with permission from ref (13).
Figure 16.
General structure of PHA.
2.3.1. Poly(hydroxyalkanoates)
PHAs are a class of intracellular biopolymers that are produced by the bacterial fermentation of sugar or lipids to store carbon and energy. Bacterially synthesized PHAs are highly biocompatible and have renewable sources. PHAs are formed mainly from saturated and unsaturated hydroxyalkanoic acids (HAA). The monomer of PHA can be branched or unbranched 3-HAA, and those with substituted side chains are 4- and 5-HAA. PHA can be a homo-, co-, or terpolymer depending on the kind of monomer.14
2.3.1.1. Poly(hydroxybutyrate)
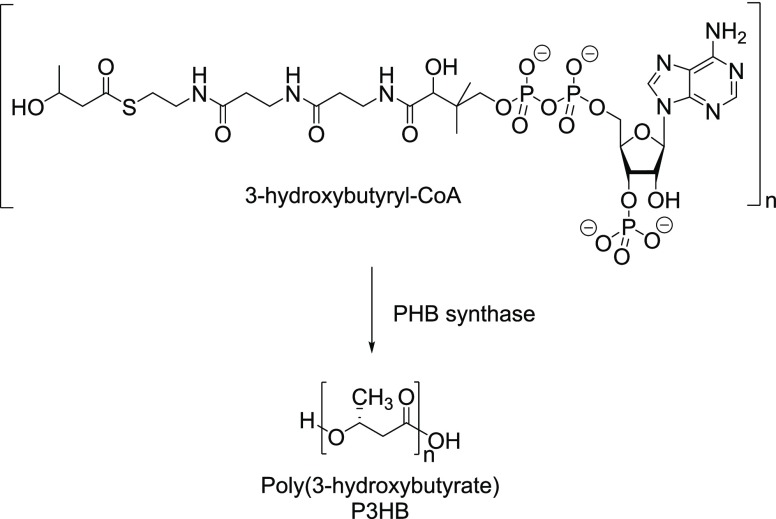
PHB is a member of the bioderived and biodegradable polyesters that were synthesized from bacteria Bacillus megaterium by Lemoigne in 1925.15 The generalized chemical structure of PHB is
Takehara16 biosynthesized poly(3-hydroxybutyrate) (P3HB) through the formation of 3-hydroxybutyryl-CoA and then polymerized it in the presence of enzyme PHB synthase (Figure 17). Microbially produced PHAs have molecular weights of 500 000 to >1 000 000, much higher than that achieved by conventional polycondensation involving hydroxy acid monomers. PHAs typically possess a natural ability to make high-molecular-weight condensation polymers such as proteins, starch, cellulose, and nucleic acids.
Figure 17.
Microbial synthesis of PHB.
Degli et al.17 studied bone tissue regeneration scaffolds made from PHB, which was obtained by a thermally induced phase separation (TIPS) technique, and the osteoinductivity and osteoconductivity were enhanced through the incorporation of HA to form a block copolymer.
2.3.1.2. Poly(hydroxy-butyrate-hydroxy-valerate)
Ma et al.18 synthesized high levels of PHBV with a 3-hydroxyvalerate fraction through l-isoleucine producing Corynebacterium glutamicum recombinant strain WM001.
2.4. Enzyme-Mediated Synthesis of BPMs
Enzyme-mediated polymerization is an emerging approach which can compete against conventional chemical synthesis methods and physical modification techniques. Some enzymes show different properties by catalyzing specific polymerizations. The products can be separated easily as these reactions do not produce any byproducts due to the high specificity of enzymes.19
Via enzyme-mediated synthesis, BPMs such as polyamide, polysaccharide, and polyester can be produced. Furthermore, enzymes can be recycled. Because the catalytic reaction conditions while using enzymes are mild (generally room temperature and atmospheric pressure), the processing cost can be reduced significantly.20
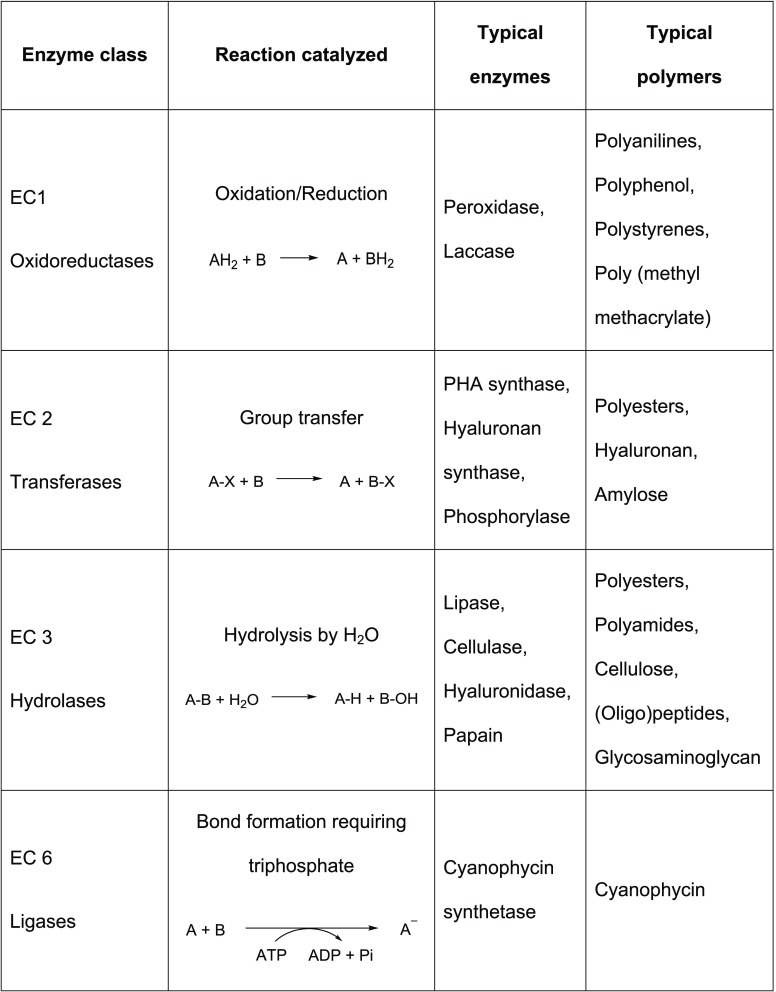
Table 4. Enzymes and Typical Examples of Their Use in Polymer Synthesis and Typical Polymers Synthesized via Enzymatic Polymerizationa.
Reproduced with permission from ref (19).
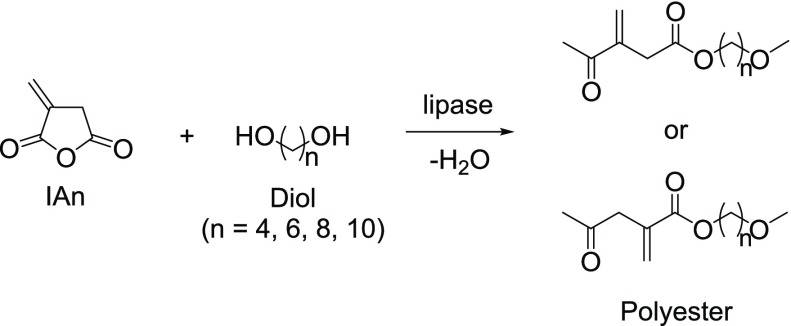
Lipase-catalyzed polymerization (LCP) has become an important method for the synthesis of aliphatic polyesters. Itaconic anhydride (IAn) derived from renewable starting materials was used in a ring-opening addition condensation polymerization (ROACP) monomer to undergo LCP and produce good-to-high yields of reactive polyesters possessing vinylidene double bonds (Figure 18).21
Figure 18.
ROACP using IAn and diol.
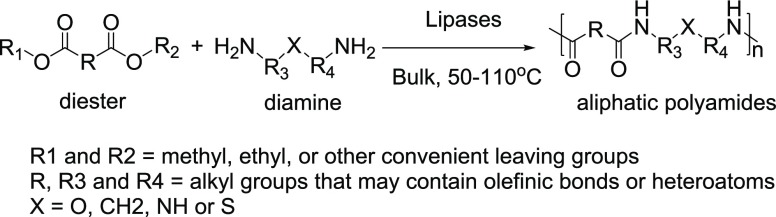
Cheng et al.22 investigated the bulk LCP of diamines and diesters (Figure 19), which resulted in aliphatic polyamides having around a 3000–15 000 g/mol molecular weight. This is the first report showing that high-molecular-weight polyamides can be produced via LCP.
Figure 19.
LCP for aliphatic polyamides.
2.5. Chemoenzymatic Synthesis of BPMs
Enzymatic synthesis adjusts the polymer molecular weight and endows the polymer with both high specificity and stereoselectivity. The chemoenzymatic synthesis method combines conventional polymerization with a highly efficient enzymatic approach. Hence, it can be coined as an attractive strategy for the preparation of high-molecular-weight BPMs.
Researchers have fabricated some optically active polymeric prodrugs as nonsteroidal anti-inflammatory substrates having high molecular weights by using an efficient chemoenzymatic route.
Gutman et al.23 investigated the lipase-catalyzed ROP of ε-caprolactone (ε-CL) in n-hexane to successfully produce PCL having up to a 4400 g/mol molecular weight (Figure 20).
Figure 20.
Lipase-catalyzed ROP of caprolactone.
3. Conclusions and Future Perspectives
This review summarizes the synthetic preparations for biodegradable polymeric materials. Time was needed for the endangered possibility of the survival of mankind to ensue through nonbiodegradable polymers, and vanishing off-the-shelf natural BPMs have led to increased interest in the synthesis of BPMs. Synthetic BPMs have progressed and touched new heights of synergistic research. Scientists, engineers, biologists, and physicians have tailored their efforts to the properties of synthesized materials, which certainly possess potential. They not only have a dynamically regulated structure and morphology but also show their discrete functions to offer a flexible and robust platform for the design and synthesis of smart BPMs with tailored specifications.
The future prospect of synthetic BPMs seems promising. Current drifts have transposed the focus toward biology in order to understand and then mimic the physiological interactions and signaling. Functional BPMs are a concept which has become crucial in biomedical utilization. The well-defined structure, biodegradability/biostability, mechanical properties, multiple functionalities, stimuli-responsiveness, biocompatibility, and minimum cytotoxicity of these BPMs make them suitable candidates for multifarious applications comprising the development of potential drug/gene delivery systems along with combination therapies, biotemplates, wound healing materials, orthopedic and dental sutures, fixatures and implants, and antimicrobial surfaces; in tissue engineering, cryopreservation, food packaging; and as polymeric biocides and herbicides, biofuels, and superabsorbent polymers.
Owing to the advancement of nanotechnology, particularly when consolidating it with the utilization of BPMs, one can envision this cutting-edge technology as one of the most powerful tools in modern society. BPM-based nanovehicles provide a stable platform for bioimaging and diagnosis as they can potentially survive diverse external stimuli in vitro and in vivo. Due to their tailored self-assembly behavior, BPMs can disperse in aqueous media to retain their nanosized distribution for bioapplications. As the old saying goes, “opportunity always sides with challenges”. Although BPMs provide perfect platforms for biomedical applications, this area still faces several crucial challenges. Many BPMs have been used for in vitro studies, thus one can contemplate a protracted course of action to be explored prior to their application in clinical theragnostic for attaining rehabilitation in the future to avail a revolutionary influence on every aspect of human life.
Acknowledgments
The authors acknowledge the Department of Chemistry, School of Sciences, Gujarat University, Ahmedabad, Gujarat, India 380009, for basic infrastructural facilities. The authors also appreciate all lab mates for their support.
Glossary
Abbreviations
- BPMs
biodegradable polymeric materials
- PGA
poly(glycolic acid)
- PLA
poly(lactic acid)
- PLGA
poly(lactic-co-glycolic acid)
- PEG
poly(ethylene glycol)
- PBA
poly(butylene succinate)
- PCL
polycaprolactone
- PEA
poly(ethylene adipate)
- PDS
poly(p-dioxanone)
- PVL
poly(valerolactone)
- CGP
chitosan-grafted-polyacrylamide
- (NH4)2S2O8
ammonium persulfate
- Fe3O4
iron oxide
- EtOH
ethanol
- SGPLA
starch-graft-poly(lactic acid)
- SGPLGA
starch-graft-poly(lactic acid-co-glycolic acid)
- SGPLGC
starch-graft-poly(lactic acid-co-glycolic acid-co-e-caprolactam)
- OCS
oxidized cross-linked starch
- COS
cross-linked oxidized starch
- STMP
sodium trimetaphosphate
- TBAF
tetra-n-butylammonium fluoride
- DMSO
dimethyl sulfoxide
- AGU
aminoguanidine
- ROP
ring-opening polymerization
- PB
pentablock
- FRP
free-radical polymerization
- PHB
poly(hydroxybutyrate)
- PHBV
poly(hydroxy-butyrate-hydroxy-valerate)
- PHHex
poly(hydroxyhexanoate)
- PHO
poly(hydroxyloctanoate)
- PHD
poly(hydroxyldecanoate)
- PHPV
poly(hydroxy phenylvalerate)
- HAA
hydroxyalkanoic acids
- TIPS
thermally induced phase separation
- IAn
itaconic anhydride
- ROACP
ring-opening addition condensation polymerization
Biographies

Siddhi S. Panchal received her bachelor’s degree in chemistry (2013–2016) from Gujarat University and her master’s degree in organic chemistry (2016–2018) from the Department of Chemistry, School of Sciences, Gujarat University. Now she is pursuing her doctoral degree in biodegradable polymeric nanomaterials under the guidance of Dr. Dilip V. Vasava in the Department of Chemistry at Gujarat University.

Dilip V. Vasava, B.Ed., Ph.D., assistant professor, Department of Chemistry, School of Sciences, Gujarat University, received his doctoral degree in polymer chemistry from the Veer Narmad South Gujarat University, Surat, Gujarat, India. He has been working on high-performance fluorescent polymers, biodegradable polymers, nanoparticles and nanocomposites, green chemistry, and drug design. He served at St. Xaviers’ College, Ahmedabad, Gujarat, India, for 8 years (2007–2015) and has been at his current affiliation since 2015.
The authors declare no competing financial interest.
References
- Vasava D. V.; Shah T. V. A glimpse of biodegradable polymers and their biomedical applications. e-Polym. 2019, 19 (1), 385–410. 10.1515/epoly-2019-0041. [DOI] [Google Scholar]
- Rodrigues P. R.; Vieira R. P. Advances in atom-transfer radical polymerization for drug delivery applications. Eur. Polym. J. 2019, 115, 45–58. 10.1016/j.eurpolymj.2019.03.023. [DOI] [Google Scholar]
- El-magied M. O. A.; Galhoum A. A.; Maize M. S.; Vincent T.; Guibal E.; Atia A. A.; Tolba A. A. Cellulose and chitosan derivatives for enhanced sorption of erbium (III). Colloids Surf., A 2017, 529, 580–593. 10.1016/j.colsurfa.2017.05.031. [DOI] [Google Scholar]
- Molatlhegi O.; Alagha L. Adsorption characteristics of chitosan grafted copolymer on kaolin. Appl. Clay Sci. 2017, 150, 342–353. 10.1016/j.clay.2017.09.032. [DOI] [Google Scholar]
- Dong C.; Chen W.; Liu C.; Liu Y.; Liu H. Synthesis of magnetic chitosan nanoparticle and its adsorption property for humic acid from aqueous solution. Colloids Surf., A 2014, 446, 179–189. 10.1016/j.colsurfa.2014.01.069. [DOI] [Google Scholar]
- Chen Q.; Yu H.; Wang L.; ul Abdin Z.; Chen Y.; Wang J.; Zhou W.; Yang X.; Khan R. U.; Zhang H.; Chen X. Recent progress in chemical modification of starch and its applications. RSC Adv. 2015, 5, 67459–67474. 10.1039/C5RA10849G. [DOI] [Google Scholar]
- a Lu D.; Duan P.; Guo R.; Yang L.; Zang H. Synthesis and characterization of starch graft biodegradable polyester and polyesteramide by direct polycondensation. Polym.-Plast. Technol. Eng. 2013, 52, 200–205. 10.1080/03602559.2012.735313. [DOI] [Google Scholar]; b Hu Y.; Tang M. Synthesis of starch-g-lactic acid copolymer with high grafting degree catalyzed by ammonia water. Carbohydr. Polym. 2015, 118, 79–82. 10.1016/j.carbpol.2014.11.011. [DOI] [PubMed] [Google Scholar]; c Misman M. A.; Azura A. R.; Hamid Z. A. The Physical and Degradation Properties of Starch-graft-acrylonitrile/Carboxylated Nitrile Butadiene Rubber Latex Films. Ind. Crops Prod. 2015, 65, 397–405. 10.1016/j.indcrop.2014.11.009. [DOI] [PubMed] [Google Scholar]; d Sukhija S.; Singh S.; Riar C. S. Effect of oxidation, cross-linking and dual modification on physicochemical, crystallinity, morphological, pasting and thermal characteristics of elephant foot yam (Amorphophallus paeoniifolius) starch. Food Hydrocolloids 2016, 55, 56–64. 10.1016/j.foodhyd.2015.11.003. [DOI] [Google Scholar]
- Baiardo M.; Frisoni G.; Scandola M.; Licciardello A. Surface chemical modification of natural cellulose fibers. J. Appl. Polym. Sci. 2002, 83, 38–45. 10.1002/app.2229. [DOI] [Google Scholar]
- Kim J. A.; Van A. D. Neocollagenesis in human tissue injected with a polycaprolactone-based dermal filler. Journal of Cosmetic and Laser Therapy 2015, 17, 99–101. 10.3109/14764172.2014.968586. [DOI] [PubMed] [Google Scholar]
- a Viness P.; Ahmed S.; Choonara Y. E., du Toit L. C.; Valence P. K.; Ndesendo M. K.. AAPS PharmSciTech 2013, 14, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Modjarrad K., Ed. Handbook of Polymer Applications in Medicine and Medical Devices; Ebnesajjad S., Eds; Elsevier; 2013; p 318319. [Google Scholar]; c Gou M.; Gong C.; Zhang J.; Wang X.; Gu Y.; Guo G.; Chen L.; Luo F.; Zhao X.; Wei Y. Polymeric matrix for drug delivery: Honokiol-loaded PCL-PEG-PCL nanoparticles in PEG-PCL-PEG thermosensitive hydrogel. J. Biomed. Mater. Res., Part A 2010, 93, 219–226. 10.1002/jbm.a.32546. [DOI] [PubMed] [Google Scholar]
- Patel S. P.; Vaishya R.; Mishra G. P.; Tamboli V.; Pal D.; Mitra A. K. Tailor-made pentablock copolymer-based formulation for sustained ocular delivery of protein therapeutics. J. Drug Delivery 2014, 2014, 1. 10.1155/2014/401747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng S. H.; Duan P. P.; Shen M. X.; Xue Y. J.; Wang Z. Y.. Preparation and degradation mechanisms of biodegradable polymer: a review. IOP Conf. Ser. Mater. Sci. Eng. 2016, 137, 12003. [Google Scholar]
- Yao Y. C.; Zhan X. Y.; Zhang J.; Zou X. H.; Wang Z. H.; Xiong Y. C.; Chen J.; Chen G. Q. A specific drug targeting system based on polyhydroxyalkanoate granule binding protein PhaP fused with targeted cell ligands. Biomaterials 2008, 29, 4823–4830. 10.1016/j.biomaterials.2008.09.008. [DOI] [PubMed] [Google Scholar]
- Arrieta M. P.; Lopez J.; Hernandez A.; Rayon E. Ternary PLA–PHB–Limonene blends intended for biodegradable food packaging applications. Eur. Polym. J. 2014, 50, 255–270. 10.1016/j.eurpolymj.2013.11.009. [DOI] [Google Scholar]
- Nair A. B.; Sivasubramanian P.; Balakrishnan P.; Kumar K. A.; Sreekala M. S.. Environmental Effects, Biodegradation, and Life Cycle Analysis of Fully Biodegradable ‘‘Green’’Composites. In Polymer Composites; Sabu T., Kuruvilla J., Malhotra S. K., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA, 2014. [Google Scholar]
- Takehara T.Regulation of molecular weight of biopolyesters produced by bacteria. NIST; 2005. Young Investigator Research Grant http://www.nisr.or.jp/englishHP/report2005/NISR05tsuge.pdf. [Google Scholar]
- Esposti M. D.; Chiellini F.; Bondioli F.; Morselli D.; Fabbri P. Highly porous PHB-based bioactive scaffolds for bone tissue engineering by in situ synthesis of hydroxyapatite. Mater. Sci. Eng., C 2019, 100, 286–296. 10.1016/j.msec.2019.03.014. [DOI] [PubMed] [Google Scholar]
- Ma W.; Wang J.; Li Y.; Yin L.; Wang X. Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) co-produced with l-isoleucine in Corynebacterium glutamicum WM001. Microb. Cell Fact. 2018, 17, 93. 10.1186/s12934-018-0942-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y.; Loos K. Enzymatic Synthesis of Biobased Polyesters and Polyamides. Polymers 2016, 8, 243. 10.3390/polym8070243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellis A.; Comerford J. W.; Weinberger S.; Guebitz G. M.; Clark J. H.; Farmer T. J. Enzymatic synthesis of lignin derivable pyridine-based polyesters for the substitution of petroleum derived plastics. Nat. Commun. 2019, 10, 1762. 10.1038/s41467-019-09817-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H. N.; Gu Q. M.; Maslanka W. W.; inventors; Hercules Inc, assignee. Enzyme-catalyzed polyamides and compositions and processes of preparing and using the same. United States patent US 6,677,427. Jan 13, 2004.
- Qu-Ming G.; Maslanka W. W.; Cheng H. N.. Enzyme-catalyzed polyamides and their derivatives. In Polymer Biocatalysis and Biomaterials II; American Chemical Society: Washington, D.C., 2008; Vol 999 pp 309–319. [Google Scholar]
- Gutman A. L.; Knani D.; Kohn D. H. Enzymatic polyesterification in organic media. Enzyme-catalyzed synthesis of linear polyesters. I. Condensation polymerization of linear hydroxyesters. II. Ring-opening polymerization of ϵ-caprolactone. J. Polym. Sci., Part A: Polym. Chem. 1993, 31, 1221–1232. 10.1002/pola.1993.080310518. [DOI] [Google Scholar]