 Bevacizumab (BVZ) is a widely investigated angiogenesis inhibitor in cancer patients. Although the anti-tumor effects has been verified, cardiotoxicity may be the most serious side-effect for BVZ.
Bevacizumab (BVZ) is a widely investigated angiogenesis inhibitor in cancer patients. Although the anti-tumor effects has been verified, cardiotoxicity may be the most serious side-effect for BVZ.
Abstract
Bevacizumab (BVZ) is the first recombinant humanized monoclonal antibody against vascular endothelial growth factor (VEGFA) approved by the FDA for the treatment of different kinds of cancers, especially colorectal cancer. Although the anti-tumor effects have been verified, the side effects of BVZ are also noteworthy, among which, cardiotoxicity may be the most serious side effect of BVZ. However, the exact mechanisms of cardiotoxicity induced by BVZ have been little explored. This study was conducted in vitro in a human cardiac myocyte (HCM) model. MTT assay was conducted to determine BVZ-stimulated cell viability. For testing the function and mechanism, the cells were transfected with miR-140-5p mimics, miR-140-5p inhibitor and/or VEGFA small interfering RNA (si-VEGFA). Then, apoptosis of the cells was detected via annexin V/propidium iodide (AV-PI) staining followed by flow cytometry. qRT-PCR and western blot assays were applied to measure gene expression (i.e. mRNA) and protein levels, respectively. The CK, LDH, SOD, CAT and GSH-Px activities and MDA level were determined using commercial kits. ROS levels were determined by DCFH-DA assay. Mitochondrial membrane potential was measured by JC-1 assay. Dual-luciferase reporter assay was used to detect the interaction between miR-140-5p and VEGFA. BVZ could inhibit HCM proliferation and induce apoptosis. miR-140-5p was upregulated in response to BVZ treatment and miR-140-5p restraint could alleviate HCM damage caused by BVZ treatment. In contrast, VEGFA and 14-3-3γ expressions were down-regulated by BVZ, and miR-140-5p could inhibit the expression of 14-3-3γ by directly targeting VEGFA. Moreover, VEGFA suppression enhanced HCM injury stimulated by BVZ and partially reversed the functional role of the miR-140-5p inhibitor in BVZ-treated cells. Taken together, miR-140-5p promoted BVZ-treated cardiomyocyte toxicity by targeting the VEGFA/14-3-3γ signal pathway. Collectively, miR-140-5p mediated the BVZ-induced cytotoxicity to cardiomyocytes by targeting the VEGFA/14-3-3γ signal pathway, indicating that miR-140-5p may be a novel target for treating BVZ-induced cardiotoxicity.
Introduction
Bevacizumab (BVZ) is the first recombinant humanized vascular endothelial growth factor (VEGFA) monoclonal antibody approved by the Food and Drug Administration (FDA) and currently the most investigated angiogenesis inhibitor in cancer patients.1,2 By inhibiting neoplastic vascularization and reducing the formation of new blood vessels, BVZ achieves an anti-tumor effect and initiates the era of anti-angiogenesis therapy in clinical oncology.3,4 BVZ is approved for the treatment of a series of cancers, including metastatic colorectal cancer (CRC), non-small-cell lung cancer (NSCLC), renal cell carcinoma, glioblastoma and cervical cancer.5–9 However, serious side effects of BVZ have recently been reported in clinical therapy, mainly as cardiotoxicity.10–12 BVZ therapy resulted in cardiotoxicity including hypertension, arrhythmias, decline in LVEF (left ventricular ejection fraction) and heart failure in NSCLC patients.12 Nevertheless, the exact mechanisms underlying the cardiotoxicity of BVZ are still unknown.
MicroRNAs (miRNAs) are a class of small molecule (∼22 nucleotides) non-coding RNAs that regulate gene expression through transcriptional or posttranscriptional regulation and participate in a variety of physiological/pathological processes.13 The expression of miRNAs varies in different cardiac disease states, and their expression may lead to the occurrence and development of heart diseases.14 miR-140-5p was reported to aggravate doxorubicin-induced cardiotoxicity by promoting myocardial oxidative stress.15,16 VEGFA is a known effective vascular biological regulator which increases blood flow and improves cardiac function. Tang et al. reported that VEGFA promoted cardiac stem cell engraftment and myocardial repair in the infarcted heart.17 The tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation proteins, 14-3-3, are a class of highly conserved proteins involved in the regulation of apoptosis, adhesion, cell proliferation, differentiation, survival and signal transduction pathways.18 Kleppe et al. demonstrated that 14-3-3 proteins were involved in several cardiovascular disorders.19 Furthermore, the 14-3-3η protein has a protective role against cardiomyocyte apoptosis mediated by mitochondria.20 Whether miR-140-5p mediates bevacizumab-induced cardiotoxicity and the potential molecular mechanisms were still unclear.
In this study, we discovered that miR-140-5p was elevated while VEGFA was downregulated in a BVZ treated human cardiac myocyte (HCM) model. Besides, miR-140-5p repressed the expression of 14-3-3γ by directly targeting VEGFA. Suppression of miR-140-5p could alleviate BVZ-induced cell damage and this was partly inhibited by the knockdown of VEGFA. Therefore, our study indicated that the “miR-140-5p/VEGFA/14-3-3γ” pathway could be a potential target for the treatment of myocardial cytotoxicity caused by BVZ.
Materials and methods
Cell culture and treatment
HCM were purchased from the American Type Culture Collection (Bethesda, MD, USA) and were maintained in Dulbecco's modified Eagle's medium (DMEM) with 10% fetal bovine serum (FBS), penicillin (100 U mL–1) and streptomycin (100 U mL–1) in a 5% CO2 humidified incubator at 37 °C. Cells that grew to 80% confluence were used in the experiments.
BVZ was obtained from Selleck Chemicals and was prepared to make a series of working dilutions. After plating, the cells were treated with BVZ and transfected with the plasmids according to the experimental needs.
MTT assay
HCM were seeded into 96-well plates at 5 × 103 cells per well for 24 h and then treated with 0–400 μg mL–1 BVZ for 0–72 h. For MTT assay, cells were incubated with 120 μL medium containing 20 μL MTT solution per well at 37 °C for 4 h. Then the supernatant was discarded and the cells were washed with PBS. Each well was added with 150 μL DMSO, and the MTT formazan crystals were dissolved. The absorbance was measured at 490 nm using an ELX800 UV universal microplate reader (Bio-Tek Instruments Inc., Vermont, USA).
Cell apoptosis
Apoptosis was assessed by flow cytometry using an Annexin V (AV)/Propidium Iodide (PI) apoptosis detection kit (BD Biosciences, USA) as previously described.21 HCM were harvested, washed, and resuspended in AV binding buffer at a concentration of 5 × 106 cells per mL, and then stained with AV and PI solution. After gentle vortexing and incubation in the dark at 37 °C for 15 min, cell apoptosis was determined by flow cytometry.
RNA extraction and quantitative real-time PCR (qRT-PCR)
The total RNA was extracted using the Trizol reagent (ThermoFisher Scientific, San Jose, CA, USA) and reverse transcribed. qRT-PCR was performed using SYBR GreenMix (Takara) in an ABI 7500 Real-Time PCR system (Applied Biosystems, Foster City, CA, USA). The relative mRNA expression of genes was calculated using the 2–ΔΔCt method and normalized by U6 or GAPDH. The primers used in this study are as follows:
hsa-miR-140-5p forward: 5′-GGGCCAGTGGTTTTACCCTA-3′,
hsa-miR-140-5p reverse: 5′-CGGCCCAGTGTTCAGACTAC-3′;
U6 forward: 5′-CTCGCTTCGGCAGCACA-3′,
U6 reverse: 5′-AACGCTTCACGAATTTGCGT-3′;
VEGFA forward: 5′-CACCAAGGCCAGCACATAGG-3′,
VEGFA reverse: 5′-AGGGAGGCTCCAGGGCATTA-3′;
GAPDH forward: 5′-CCAGGTGGTCTCCTCTGA-3′,
GAPDH reverse: 5′-GCTGTAGCCAAATCGTTGT-3′.
Western blot assay
The total protein samples from the cells were homogenized using a lysis buffer containing protease and phosphatase inhibitors. The total protein concentrations of the samples were determined using a BCA protein assay kit (Bio-Rad, Hercules, CA, USA). 30 μg protein samples were separated by SDS polyacrylamide gel electrophoresis (SDS-PAGE), and then electro-transferred onto a PVDF membrane (Millipore, USA). After blocking with TBST buffer containing 5% non-fat milk for 1 h at room temperature, the membranes were incubated with primary antibodies overnight at 4 °C. Then the membranes were washed and incubated with horseradish peroxidase (HRP)-conjugated secondary antibody for 1 h at room temperature. The blots were detected using an enhanced chemi-luminescence (ECL) detection kit (Jiangsu KeyGEN BioTECH Corp., Ltd, Nanjing, P.R. China). The relative protein levels were normalized to GAPDH. The following primary antibodies were used: anti-VEGFA antibody (#ab46154, Abcam, 1 : 1000), anti-14-3-3γ antibody (#ab155050, Abcam, 1 : 1000), anti-Bax antibody (#2772s, Cell Signaling Technology, 1 : 1000), anti-Bcl-2 antibody (#4223, Cell Signaling Technology, 1 : 1000), anti-cleaved Caspase3 (#9664, Cell Signaling Technology, 1 : 1000), anti-cleaved Caspase9 (#ab2324, Abcam, 1 : 1000), and anti-GAPDH antibody (#5174, Cell Signaling Technology, 1 : 1000).
Cell transfection
miR-140-5p mimics (5′-CAGUGGUUUUACCCUAUGGUAG-3′, 5′-CUACCAUAGGGUAAAACCACUG-3′), negative control oligonucleotides (miR-NC: 5′-UUCUCCGAACGUGUCACGUTT-3′, 5′-ACGUGACACGUUCGGAGAATT-3′), miR-140-5p inhibitor (5′-CAGUGGUUUUACCCUAUGGUAG-3′) and negative control oligonucleotides (inhibitor NC: 5′-CAGUACUUUUGUGUAGUACAA-3′) were purchased from GenePharma Co. Ltd (Shanghai, China). The plasmids containing the wild-type VEGFA (VEGFA-wt) response element and the corresponding mutant (VEGFA-mut) were purchased from RiboBio. Co., Ltd (Guangzhou, China). Upon reaching 70%–80% confluence, the cells were transfected with miR-140-5p mimics, miR-140-5p inhibitor or si-VEGFA using lipofectamine 2000 (Invitrogen, USA) according to the manufacturer's instructions. After 48 h of transfection, the cells were used for subsequent experiments.
Measurement of the malondialdehyde (MDA) level, and lactate dehydrogenase (LDH), creatine kinase (CK), superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GSH-Px) activities
The MDA level, and LDH, CK, SOD, CAT and GSH-Px activities were measured using commercial kits, according to the manufacturer's instructions (Nanjing Jiancheng Bioengineering, China). Briefly, cells (1 × 105 per well) were seeded into 24-well plates and cultured overnight. After treatment, the culture supernatant per well was collected for the measurement of LDH and CK activities. The cells were washed with PBS and then crushed using ultrasonic waves for the determination of the MDA level, and SOD, CAT and GSH-Px activities.
Measurement of intracellular ROS
Measurement of intracellular ROS was performed by DCFH-DA assay as reported.15,22 HCM were plated in 6-well culture plates at a density of 5 × 105 cells per mL and then treated with BVZ for 24 h. After removing the medium, 1.5 mL of DCFH-DA (10.0 μM) was added at 37 °C for 25 min, and the cells were then harvested and washed with PBS. The samples were analyzed by flow cytometry at 488 nm (excitation) and 525 nm (emission).
Assessment of the mitochondrial membrane potential (ΔΨm)
The ΔΨm was determined by JC-1 (5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolyl-arbocyanine iodide) assay as reported.23,24 JC-1 assay kits were purchased from Beyotime Institute of Biotechnology (Shanghai, China). Cells (5 × 105 per well) were seeded into 6-well plates and collected. Then the cells were resuspended in fresh medium containing 10 μg mL–1 JC-1 staining buffer. After incubation at 37 °C for 30 min, the cells were centrifuged at 500g and 4 °C, and the supernatant was discarded. The fluorescence intensity of the cells was determined using a flow cytometer.
Dual-luciferase reporter assay
The procedure for the dual-luciferase reporter assay has been previously reported.15 Plasmid DNA (VEGFA-wt and VEGFA-mut) and miR-140-5p mimics or miR-140-5p negative control (miR-NC) were co-transfected into the cells. Luciferase activity was determined using a Double-Luciferase Reporter Assay Kit (Promega, Madison, WI, USA) according to the manufacturer's instructions, which was normalized to firefly luciferase activity.
Statistical analysis
The statistical analysis was performed using SPSS version 16.0 (IBM, Chicago, IL, USA). The data are presented as mean ± SD of at least 3 separate experiments. The difference between two groups was analyzed by the unpaired two-tailed Student's t-test. One-way analysis of variance (ANOVA) followed by the Tukey post hoc test was used for comparison among multiple groups. Differences were considered statistically significant when p < 0.05.
Results
BVZ induces cytotoxicity to cardiomyocytes
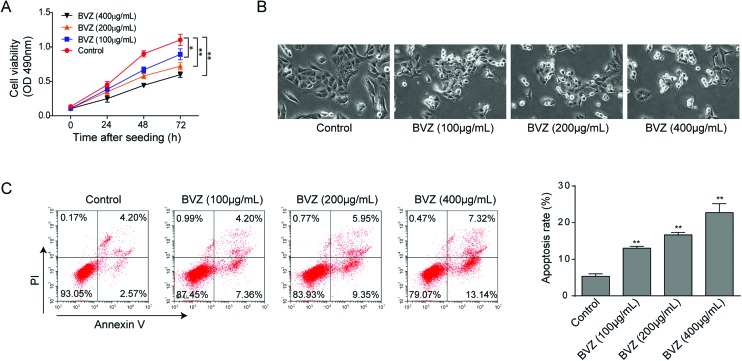
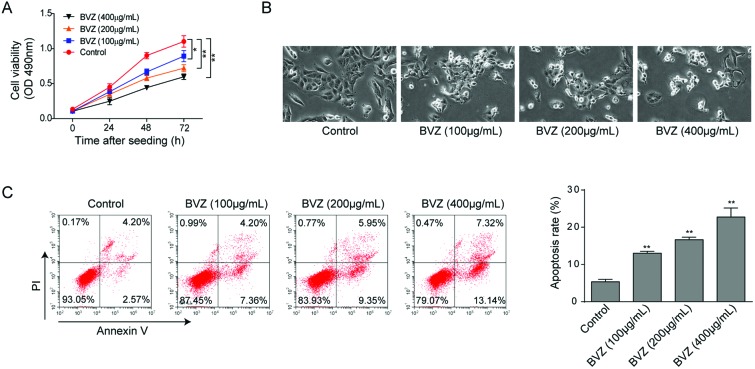
BVZ therapy has been reported to be associated with an increased risk of cardiotoxicity,25 therefore, we first validated the cytotoxicity of BVZ in cardiomyocytes for 24, 48 and 72 hours using MTT assay. As expected, BVZ treatment induced cardiomyocyte death in both concentration-dependent and time-dependent manners (Fig. 1A). We also observed a decrease in the number of cells with BVZ treatment (Fig. 1B). Then we applied AV and PI staining with flow cytometry to detect cell apoptosis. As shown in Fig. 1C, BVZ elicited more apoptotic cells in a concentration-dependent manner, so as to exert cytotoxicity. Taken together, these data suggested that BVZ could induce cytotoxicity to cardiomyocytes.
Fig. 1. BVZ induces cytotoxicity to cardiomyocytes. (A) Cell viability of HCM was determined by MTT assay with BVZ treatment (100, 200 or 400 μg mL–1) for 24, 48 or 72 h. (B) Representative photographs of cardiomyocytes after exposure to different concentrations of BVZ for 24 h. Original magnification, 200×. (C) Cell apoptosis was analyzed by flow cytometry with AV/PI staining. The results are representative of three independent experiments. All data are represented as mean ± SD; p values were determined by ANOVA followed by the Tukey post hoc test. ** p < 0.01, * p < 0.05.
miR-140-5p is upregulated, and VEGFA and 14-3-3γ are downregulated in BVZ-treated cardiomyocytes
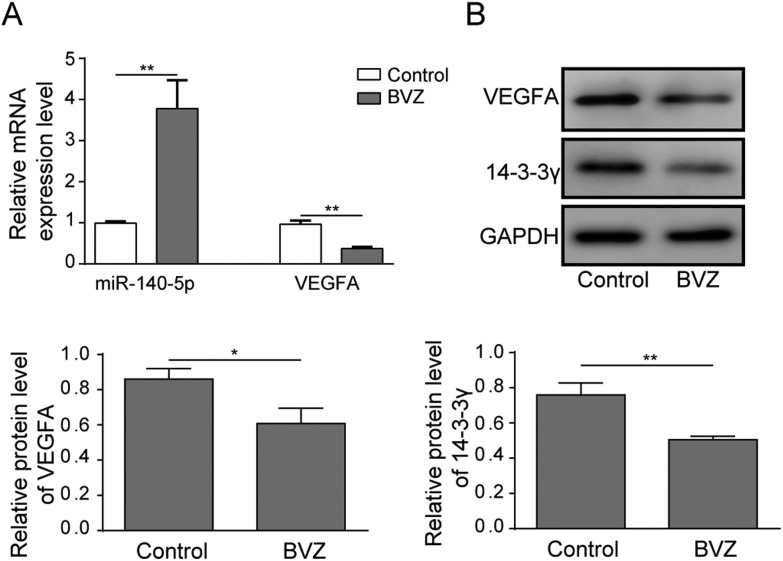
It is reported that miR-140-5p augmented doxorubicin-induced cardiotoxicity by promoting myocardial oxidative stress;15 thus, we doubted whether miR-140-5p played an essential role in BVZ-treated cardiomyocytes. To explore the expression of miR-140-5p in BVZ-treated cardiomyocytes, we applied qRT-PCR assay. As shown in Fig. 2A, miR-140-5p mRNA expression was markedly upregulated in BVZ-treated cardiomyocytes. Furthermore, the mRNA and protein expression of VEGFA and 14-3-3γ protein levels were downregulated with BVZ treatment (Fig. 2A and B).
Fig. 2. miR-140-5p is upregulated, and VEGFA and 14-3-3γ are downregulated in BVZ-treated cardiomyocytes. (A) The mRNA expression levels of miR-140-5p and VEGFA were analyzed by qRT-PCR assay in response to BVZ (200 μg mL–1) treatment. (B) The protein expressions of VEGFA and 14-3-3γ were determined by western blot in HCM with BVZ culture. The results are representative of three independent experiments. All data are represented as mean ± SD; p values were determined by the unpaired two-tailed Student's t-test. ** p < 0.01, * p < 0.05.
miR-140-5p restraint alleviates BVZ treated cardiomyocyte damage
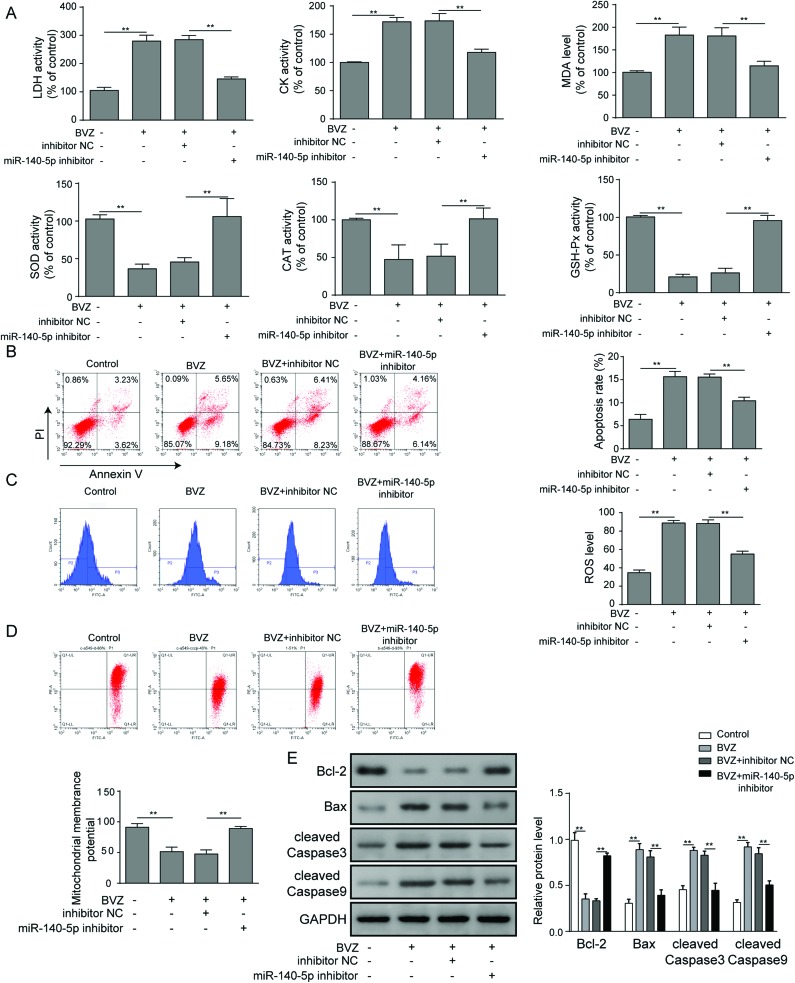
As miR-140-5p was upregulated in BVZ-treated cardiomyocytes, we next asked whether miR-140-5p plays a critical role in BVZ induced cardiomyocyte injury. As shown in Fig. 3A, BVZ could increase the levels of LDH, CK and MDA, and suppress SOD, CAT and GSH-Px levels, which were decreased by the miR-150-5p inhibitor. Then we found that the inhibition of miR-140-5p obviously reduced cell apoptosis (Fig. 3B) and ROS levels (Fig. 3C), while it increased ΔΨm (Fig. 3D) in cells stimulated by BVZ. In addition, BVZ resulted in a remarkable upregulation of Bax, cleaved Caspase3 and cleaved Caspase9, and downregulation of Bcl-2, which were alleviated by the miR-140-5p inhibitor (Fig. 3E). In summary, the above results provided evidence that miR-140-5p repression could abate BVZ-induced cardiomyocyte damage.
Fig. 3. miR-140-5p restraint alleviates BVZ-induced cardiomyocyte damage. HCM were treated with BVZ in the presence of inhibitor NC or the miR-140-5p inhibitor. (A) The LDH, CK, SOD, CAT and GSH-Px activities and MDA level were determined. (B) The apoptosis rate was analyzed by flow cytometry by staining cells with annexin V and PI. (C) Intracellular ROS was determined by DCFH-DA and flow cytometry. (D) ΔΨm was analyzed by flow cytometry via JC-1 staining. (E) The protein expression of Bcl-2, Bax, cleaved Caspase3 and cleaved Caspase9 was determined by western blot. The results are representative of three independent experiments. All data are represented as mean ± SD; p values were determined by ANOVA followed by the Tukey post hoc test. ** p < 0.01, * p < 0.05.
miR-140-5p inhibits the expression of 14-3-3γ by directly targeting VEGFA
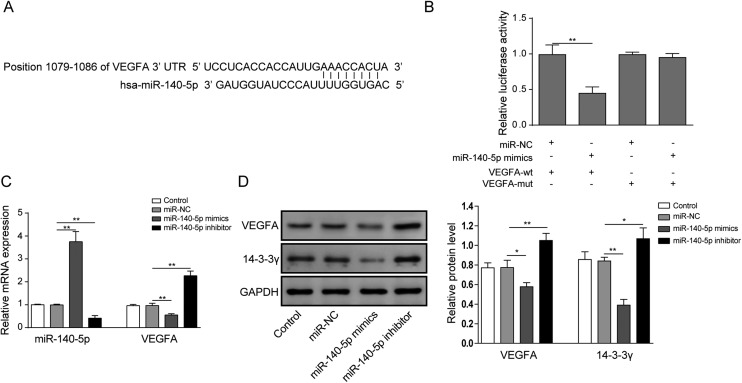
To further explore the relationship between miR-140-5p and VEGFA, we applied bioinformatic methods to predict and obtain the miR-140-5p binding sites in VEGFA 3′-UTR (Fig. 4A). In Fig. 4B, VEGFA 3′-UTR showed lower luciferase activity in the presence of miR-140-5p mimics; by contrast, the binding site mutants of VEGFA 3′-UTR failed to display differences with the control group, implying that miR-140-5p could directly bind to VEGFA 3′-UTR. Moreover, overexpression of miR-140-5p reduces the expression of VEGFA, while the miR-140-5p inhibitor enhanced VEGFA mRNA and protein levels (Fig. 4C and D). Finally, to determine the effects of miR-140-5p on 14-3-3γ expression, we introduced miR-140-5p mimics or the miR-140-5p inhibitor into HCM to determine the expression of the 14-3-3γ protein. As shown in Fig. 4D, 14-3-3γ protein expression was inhibited by miR-140-5p mimics and elevated by miR-140-5p suppression. In conclusion, these data made clear that miR-140-5p inhibits the expression of 14-3-3γ by directly targeting VEGFA.
Fig. 4. miR-140-5p inhibits the expression of 14-3-3γ by directly targeting VEGFA. (A) Alignment of potential miR-140-5p binding sites in the 3′-UTR of VEGFA. (B) Luciferase activity was determined in HCM transfected with constructs containing wild-type VEGFA or mutated VEGFA plasmids in response to the transfection of miR-140-5p mimics and miR-NC. (C) The mRNA levels of miR-140-5p and VEGFA were determined by qRT-PCR in HCM with miR-140-5p mimics or the miR-140-5p inhibitor. (D) The protein levels of VEGFA and 14-3-3γ were determined by western blot assay in response to miR-140-5p mimics or the miR-140-5p inhibitor. The results are representative of three independent experiments. All data are represented as mean ± SD; p values were determined by ANOVA followed by the Tukey post hoc test. ** p < 0.01, * p < 0.05.
miR-140-5p promotes BVZ-induced cytotoxicity to cardiomyocytes by targeting the VEGFA/14-3-3γ signal pathway
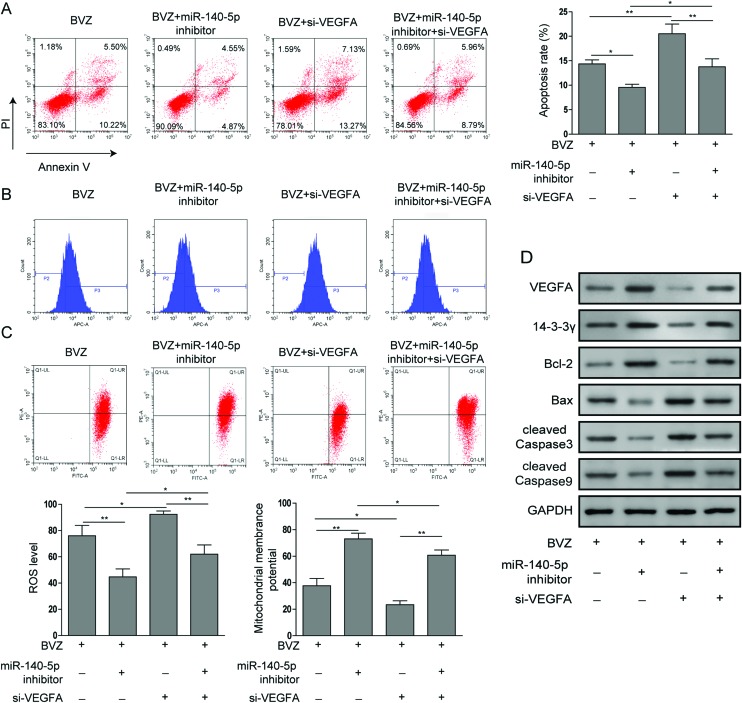
To gain further insight into the mechanisms underlying the BVZ-induced cytotoxicity to cardiomyocytes, we determined the influence of VEGFA on BVZ treated cardiomyocytes. First, we introduced VEGFA siRNA into HCM treated with BVZ and the miR-140-5p inhibitor. As shown in Fig. 5A, flow cytometry with AV/PI staining was used to detect the apoptosis of cells, and the silencing of VEGFA expression not only amplified BVZ-induced apoptosis but also impaired the reduced apoptosis induced by the miR-140-5p inhibitor. In line with the above observation, the ROS levels were decreased and ΔΨm was elevated by the miR-140-5p inhibitor in BVZ-stimulated cells, which were subsequently reversed by the knockdown of VEGFA (Fig. 5B and C). Finally, we further tested the expression of apoptosis-related proteins using western blot. As shown in Fig. 5D, miR-140-5p restraint inhibited the expressions of Bax, cleaved Caspase3 and cleaved Caspase9, and promoted the expressions of Bcl-2 and 14-3-3γ, which were reversed by si-VEGFA. Taken together, these results illustrated that miR-140-5p could promote BVZ-induced cytotoxicity to cardiomyocytes by targeting the VEGFA/14-3-3γ signal pathway.
Fig. 5. miR-140-5p promotes BVZ-induced cytotoxicity to cardiomyocytes by targeting the VEGFA/14-3-3γ signal pathway. In response to BVZ, HCM were treated with or without the miR-140-5p inhibitor and transfected with or without si-VEGFA. (A) The apoptosis rate was analyzed by flow cytometry by staining the cells with annexin V and PI. (B) Intracellular ROS was determined by DCFH-DA and flow cytometry. (C) ΔΨm was measured by JC-1 assay. (D) The protein expression of VEGFA, 14-3-3γ, Bcl-2, Bax, cleaved Caspase3 and cleaved Caspase9 was determined by western blot. The results are representative of three independent experiments. All data are represented as mean ± SD; p values were determined by ANOVA followed by the Tukey post hoc test. ** p < 0.01, * p < 0.05.
Discussion
Anti-tumor drugs generally destroy the physiological homeostasis of the body and cause damage of multiple organs during treatment. As the first recombinant humanized VEGFA monoclonal antibody approved by the FDA, BVZ exerts significant activities against CRC, but causes serious side effects after long-term therapy, including hypertension, third-degree albuminuria, thrombosis and cardiotoxicity.1,10,25 Among them, cardiotoxicity is the most severe one and should be paid more attention.10,25 BVZ and sunitinib (SNT) therapy, recently introduced novel anticancer therapies, could induce Caspase3 cleavage and increase cardiotoxicity risk in mice.25 In this paper, we found that BVZ could inhibit cardiomyocyte proliferation and promote cardiomyocyte apoptosis due to the decrease of Bcl-2 expression and increase of the Bax level, and upregulate the intracellular ROS level, but decrease ΔΨm.
MiRNAs are endogenous small non-coding RNAs that have been recently regarded as essential biomarkers for drug-induced cardiotoxicity.26–30 In recent years, miR-140-5p has also been shown to play an important role in cardiovascular diseases.15,16 Zhao et al. reported that miR-140-5p aggravates doxorubicin-induced cardiotoxicity by promoting myocardial oxidative stress by targeting Nrf2 and Sirt2.15 Furthermore, dioscin exerts a protective effect on doxorubicin-induced cardiotoxicity by regulating miR-140-5p-mediated myocardial oxidative stress.16 However, there is no literature on the effect of miR-140-5p on BVZ-treated cardiomyocytes. In this research, BVZ induced the upregulation of miR-140-5p in cardiomyocytes, and the suppression of miR-140-5p decreased MDA and ROS levels and LDH and CK activities, while it increased SOD, CAT and GSH-Px activities and ΔΨm, suggesting that miR-140-5p inhibition could alleviate oxidative damage in BVZ-treated cardiomyocytes. Therefore, miR140-5p may be considered as one of the potential drug targets for the treatment of BVZ-induced cardiomyocyte toxicity.
VEGFA was classically known to be involved in vascular development including stabilization of neovascularization, vasodilation, and enhancement of vascular permeability, and was considered to play a crucial role in cardiotoxicity.13,31,32 Ju et al. found that the pre-treatment with catalpol might promote the survival and VEGFA secretion of bone mesenchymal stem cells (BMSCs) and improve the efficiency of therapy against myocardial infarction (MI).33 Besides, VEGFA was reported to exert an anti-apoptotic effect in cardiomyocytes.13,34,35 Chen et al. reported that VEGF-C protected the heart from ischemia/reperfusion (I/R) injury by suppressing cardiomyocyte apoptosis.35 Yin et al. reported that miR-320a mediates doxorubicin-induced cardiotoxicity by targeting the VEGFA signal pathway and inducing cell apoptosis.13 Song et al. revealed that VEGFA regulated the expression of miRNA-23a and miRNA-92a, and exhibited anti-apoptotic effects in cardiomyocytes.34 Our study also included the involvement of the VEGFA pathway in BVZ-treated cardiomyocytes. VEGFA was downregulated in BVZ-treated cardiomyocytes and the silencing of VEGFA expression could amplify BVZ-induced oxidative injury and apoptosis, which is shown as changes in the activities of enzymes related to oxidative stress, ROS level, ΔΨm level and expression of apoptotic proteins. Furthermore, the above papers demonstrated that VEGFA was regulated by miR-320a, and VEGFA regulated the expression of miRNA-23a and miRNA-92a, indicating that the interaction between VEGFA and miRNA has not been fully explored. In this study, we found that miR-140-5p directly targeted VEGFA. In addition, VEGFA/VEGFA receptor (VEGFR) signaling is closely related to cell proliferation and apoptosis. VEGFR1 was reported to protect cardiomyocytes from I/R injury by the suppression of oxidative stress and apoptosis.36 And the function and mechanisms of VEGFR in BVZ-induced cardiotoxicity will be included in our future research plan.
The 14-3-3 protein, an intracellular, dimeric, serine-binding molecule, plays a critical role in signal transduction, apoptotic, and checkpoint control pathways.20 Recently, the 14-3-3 protein was reported to be involved in several cardiovascular disorders, and 14-3-3γ was involved in cardiomyocyte oxidative stress and apoptosis.19,37 Furthermore, 14-3-3ζ and VEGFA proteins were co-expressed in tumor sections.38 In our results, the silencing of VEGFA expression could significantly reduce the expression of 14-3-3γ. Moreover, miR-140-5p inhibited the expression of 14-3-3γ by directly targeting VEGFA, leading to the upregulation of Bcl-2 and the downregulation of Bax, cleaved Caspase3 and cleaved Caspase9 in HCM, which was in line with the previous reports. 14-3-3 is a chaperon protein which binds to the phosphorylated serine/tyrosine embedded in certain protein modules. It can bind to many different proteins. Depending on the target protein, the biological function of 14-3-3 can vary a lot. Therefore, we will gain insight into the mechanism and explore which protein 14-3-3 targets to play the biological function.
To sum up, BVZ could induce cytotoxicity to cardiomyocytes, which was impaired by miR-140-5p suppression. Furthermore, miR-140-5p could inhibit the expression of 14-3-3γ by directly targeting VEGFA and mediate BVZ-induced cardiomyocyte toxicity by targeting the VEGFA/14-3-3γ signal pathway. Therefore, our findings provide novel insights into “miR-140-5p/VEGFA/14-3-3γ” in cardiomyocyte toxicity and may help improve the development of a therapy for BVZ-induced cardiotoxicity.
Abbreviations
- BVZ
Bevacizumab
- VEGFA
Vascular endothelial growth factor
- AV-PI
Annexin V/propidium iodide
- CRC
Colorectal cancer
- NSCLC
Non-small-cell lung cancer
- miRNAs
MicroRNAs
- HCM
Human cardiac myocytes
- FBS
Fetal bovine serum
- qRT-PCR
Quantitative real-time PCR
- ROS
Reactive oxygen species
- JC-1
5,5′,6,6′-Tetrachloro-1,1′,3,3′-tetraethylbenzimidazolyl-arbocyanine iodide
- SNT
Sunitinib
- PlGF
Placental growth factor
- BMSCs
Bone mesenchymal stem cells
- MI
Myocardial infarction
- LDH
Lactate dehydrogenase
- CK
Creatine kinase
- MDA
Malondialdehyde
- SOD
Superoxide dismutase
- CAT
Catalase
- GSH-Px
Glutathione peroxidase
- I/R
Ischemia/reperfusion
Conflicts of interest
There is no conflict of interest to report.
Acknowledgments
This work was supported by the Jiangxi Provincial Department of Education Science and Technology Key Project (No. 170022) and the Chinese Medical Association, the Wu Jieping Foundation and the Hengrui Research Foundation (No. LCYX027).
References
- Liu W., Zhang J., Yao X., Jiang C., Ni P., Cheng L., Liu J., Ni S., Chen Q., Li Q., Zhou K., Wang G., Zhou F. Cancer Sci. 2018;109:3294–3304. doi: 10.1111/cas.13779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., Kroetz D. L. Pharmacol. Ther. 2018;182:152–160. doi: 10.1016/j.pharmthera.2017.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Xiao Z., Hong Z., Jiao H., Zhu S., Zhao Y., Bi J., Qiu J., Zhang D., Yan J., Zhang L., Huang C., Li T., Liang L., Liao W., Ye Y., Ding Y. Cancer Lett. 2018;439:78–90. doi: 10.1016/j.canlet.2018.09.026. [DOI] [PubMed] [Google Scholar]
- Jain R. K. Nat. Med. 2001;7:987–989. doi: 10.1038/nm0901-987. [DOI] [PubMed] [Google Scholar]
- Smeets D., Miller I. S., O'Connor D. P., Das S., Moran B., Boeckx B., Gaiser T., Betge J., Barat A., Klinger R., van Grieken N. C. T., Cremolini C., Prenen H., Mazzone M., Depreeuw J., Bacon O., Fender B., Brady J., Hennessy B. T., McNamara D. A., Kay E., Verheul H. M., Maarten N., Gallagher W. M., Murphy V., Prehn J. H. M., Koopman M., Punt C. J. A., Loupakis F., Ebert M. P. A., Ylstra B., Lambrechts D., Byrne A. T. Nat. Commun. 2018;9:4112. doi: 10.1038/s41467-018-06567-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlesi F., Balleyguier C., Besse B., Bonodeau F., Brenac F., Corneloup O., Dansin E., Ferretti G., Gaubert J. Y., Gervais R., Lacombe C., Loundou A., Moro-Sibilot D., Planchard D., Scherpereel A., Menu Y. Ann. Oncol. 2010;21:1682–1686. doi: 10.1093/annonc/mdp590. [DOI] [PubMed] [Google Scholar]
- Voss M. H., Hussain A., Vogelzang N., Lee J. L., Keam B., Rha S. Y., Vaishampayan U., Harris W. B., Richey S., Randall J. M., Shaffer D., Cohn A., Crowell T., Li J., Senderowicz A., Stone E., Figlin R., Motzer R. J., Haas N. B., Hutson T. Ann. Oncol. 2017;28:2754–2760. doi: 10.1093/annonc/mdx493. [DOI] [PubMed] [Google Scholar]
- Pasqualetti F., Gonnelli A., Molinari A., Cantarella M., Montrone S., Cristaudo A., Baldaccini D., Mattioni R., Delishaj D., Mazzotti V., Morganti R., Cocuzza P., Fabrini M. G., Lombardi G., Ruda R., Soffietti R., Paiar F. Anticancer Res. 2018;38:5877–5881. doi: 10.21873/anticanres.12930. [DOI] [PubMed] [Google Scholar]
- Bizzarri N., Ghirardi V., Alessandri F., Venturini P. L., Valenzano Menada M., Rundle S., Leone Roberti Maggiore U., Ferrero S. Expert Opin. Biol. Ther. 2016;16:407–419. doi: 10.1517/14712598.2016.1145208. [DOI] [PubMed] [Google Scholar]
- Chen J., Du F., Hu B., Chi C., Chu H., Jiang L., Li P., Gong Z. Anticancer Res. 2017;37:4557–4561. doi: 10.21873/anticanres.11853. [DOI] [PubMed] [Google Scholar]
- Choueiri T. K., Mayer E. L., Je Y., Rosenberg J. E., Nguyen P. L., Azzi G. R., Bellmunt J., Burstein H. J., Schutz F. A. J. Clin. Oncol. 2011;29:632–638. doi: 10.1200/JCO.2010.31.9129. [DOI] [PubMed] [Google Scholar]
- Zhou F., Lu X., Zhang X. Cardiovasc. Toxicol. 2018;18:284–289. doi: 10.1007/s12012-018-9457-z. [DOI] [PubMed] [Google Scholar]
- Yin Z., Zhao Y., Li H., Yan M., Zhou L., Chen C., Wang D. W. Aging. 2016;8:192–207. doi: 10.18632/aging.100876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt M. N., Werner T., Indenbirken D., Alawi M., Demin P., Kunze A. C., Stenzig J., Starbatty J., Hansen A., Fiedler J., Thum T., Eschenhagen T. J. Mol. Cell Cardiol. 2015;81:1–9. doi: 10.1016/j.yjmcc.2015.01.008. [DOI] [PubMed] [Google Scholar]
- Zhao L., Qi Y., Xu L., Tao X., Han X., Yin L., Peng J. Redox Biol. 2018;15:284–296. doi: 10.1016/j.redox.2017.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L., Tao X., Qi Y., Xu L., Yin L., Peng J. Redox Biol. 2018;16:189–198. doi: 10.1016/j.redox.2018.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J. M., Luo B., Xiao J. H., Lv Y. X., Li X. L., Zhao J. H., Zheng F., Zhang L., Chen L., Yang J. Y., Guo L. Y., Wang L., Yan Y. W., Pan Y. M., Wang J. N., Li D. S., Wan Y., Chen S. Y. Int. J. Cardiol. 2015;183:221–231. doi: 10.1016/j.ijcard.2015.01.050. [DOI] [PubMed] [Google Scholar]
- Neal C. L., Yu D. H. Expert Opin. Ther. Targets. 2010;14:1343–1354. doi: 10.1517/14728222.2010.531011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleppe R., Martinez A., Doskeland S. O., Haavik J. Semin. Cell Dev. Biol. 2011;22:713–719. doi: 10.1016/j.semcdb.2011.08.008. [DOI] [PubMed] [Google Scholar]
- Sreedhar R., Arumugam S., Thandavarayan R. A., Giridharan V. V., Karuppagounder V., Pitchaimani V., Afrin R., Miyashita S., Nomoto M., Harima M., Gurusamy N., Suzuki K., Watanabe K. Cell. Signalling. 2015;27:770–776. doi: 10.1016/j.cellsig.2014.12.021. [DOI] [PubMed] [Google Scholar]
- Huang J., Liu Z., Xu P., Zhang Z., Yin D., Liu J., He H., He M. Eur. J. Pharmacol. 2018;819:43–50. doi: 10.1016/j.ejphar.2017.11.028. [DOI] [PubMed] [Google Scholar]
- Deng J., Liu A. D., Hou G. Q., Zhang X., Ren K., Chen X. Z., Li S. S. C., Wu Y. S., Cao X. J. Exp. Clin. Cancer Res. 2019;38:2. doi: 10.1186/s13046-018-1016-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G. S., Lu J. J., Guo J. J., Huang M. Q., Gan L., Chen X. P., Wang Y. T. Pharmacol. Rep. 2013;65:453–459. doi: 10.1016/s1734-1140(13)71021-1. [DOI] [PubMed] [Google Scholar]
- Jia H., Guo Z., Yao Y. Exp. Ther. Med. 2019;17:199–204. doi: 10.3892/etm.2018.6951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordun K. A., Premecz S., daSilva M., Mandal S., Goyal V., Glavinovic T., Cheung M., Cheung D., White C. W., Chaudhary R., Freed D. H., Villarraga H. R., Herrmann J., Kohli M., Ravandi A., Thliveris J., Pitz M., Singal P. K., Mulvagh S., Jassal D. S. Am. J. Physiol.: Heart Circ. Physiol. 2015;309:H692–H701. doi: 10.1152/ajpheart.00172.2015. [DOI] [PubMed] [Google Scholar]
- Holmgren G., Synnergren J., Andersson C. X., Lindahl A., Sartipy P. Toxicol. in Vitro. 2016;34:26–34. doi: 10.1016/j.tiv.2016.03.009. [DOI] [PubMed] [Google Scholar]
- Nishimura Y., Kondo C., Morikawa Y., Tonomura Y., Torii M., Yamate J., Uehara T. J. Appl. Toxicol. 2015;35:173–180. doi: 10.1002/jat.3044. [DOI] [PubMed] [Google Scholar]
- Rigaud V. O. C., Ferreira L. R. P., Ayub-Ferreira S. M., Avila M. S., Brandao S. M. G., Cruz F. D., Santos M. H. H., Cruz C. B. B. V., Alves M. S. L., Issa V. S., Guimaraes G. V., Cunha-Neto E., Bocchi E. A. Oncotarget. 2017;8:6994–7002. doi: 10.18632/oncotarget.14355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira-Carvalho V., Ferreira L. R. P., Bocchi E. A. J. Appl. Toxicol. 2015;35:1071–1072. doi: 10.1002/jat.3185. [DOI] [PubMed] [Google Scholar]
- Leger K. J., Leonard D., Nielson D., de Lemos J. A., Mammen P. P. A., Winick N. J. J. Am. Heart Assoc. 2017;6:e004653. doi: 10.1161/JAHA.116.004653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J., Kim M., Sun K., An Y. A., Gu X., Scherer P. E. Diabetes. 2017;66:1479–1490. doi: 10.2337/db16-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange C., Storkebaum E., de Almodovar C. R., Dewerchin M., Carmeliet P. Nat. Rev. Neurol. 2016;12:439–454. doi: 10.1038/nrneurol.2016.88. [DOI] [PubMed] [Google Scholar]
- Ju X., Xue D., Wang T., Ge B., Zhang Y., Li Z. Cardiovasc. Toxicol. 2018;18:471–481. doi: 10.1007/s12012-018-9460-4. [DOI] [PubMed] [Google Scholar]
- Song Y. S., Joo H. W., Park I. H., Shen G. Y., Lee Y., Shin J. H., Kim H., Kim K. S. PLoS One. 2017;12:e0179972. doi: 10.1371/journal.pone.0179972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X. G., Lv Y. X., Zhao D., Zhang L., Zheng F., Yang J. Y., Li X. L., Wang L., Guo L. Y., Pan Y. M., Yan Y. W., Chen S. Y., Wang J. N., Tang J. M., Wan Y. Mol. Cell. Biochem. 2016;413:9–23. doi: 10.1007/s11010-015-2622-9. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Cao C., Xin J., Lv P., Chen D., Li S., Yang H., Chen C., Liu B., Li Q. PLoS One. 2018;13:e0202772. doi: 10.1371/journal.pone.0202772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He H., Luo Y., Qiao Y., Zhang Z., Yin D., Yao J., You J., He M. Food Funct. 2018;9:4404–4418. doi: 10.1039/c8fo00466h. [DOI] [PubMed] [Google Scholar]
- Cao W. D., Kawai N., Miyake K., Zhang X., Fei Z., Tamiya T. Brain Tumor Pathol. 2014;31:1–10. doi: 10.1007/s10014-013-0135-3. [DOI] [PubMed] [Google Scholar]