Graphical abstract
Atherosclerotic cardiovascular disease is a major burden on global health and a leading cause of death worldwide. The pathophysiology of this chronic disease is complex, involving inflammation, lipoprotein oxidation and accumulation, plaque formation, and calcification. In 1981, Dr. Jerome Sullivan formulated the ‘Iron Hypothesis’, suggesting that higher levels of stored iron promote cardiovascular diseases, whereas iron deficiency may have an atheroprotective effect. This hypothesis has stimulated research focused on clarifying the role of iron in the development of atherosclerosis. However, preclinical and clinical studies have produced contradictory results and the observation that patients with hemochromatosis do not appear to have an increased risk of atherosclerosis seemed incongruous with Sullivan’s initial hypothesis. The ‘paradox’ of systemic iron overload not being accompanied by an increased risk for atherosclerosis led to a refinement of the iron hypothesis focusing on intracellular macropage iron. More recent in vitro and animal studies have elucidated the complex signaling pathways regulating iron, with a particular focus on hepcidin, the master regulator of body iron homeostasis. Bone morphogenetic protein (BMP) signaling is the major pathway that is required for induction of hepcidin expression in response to increasing levels of iron. Strong links between iron homeostasis, BMP signaling, inflammation and atherosclerosis have been established in both mechanistic and human studies. This review summarizes the current understanding of the role of iron homeostasis and hepcidin in the development of atherosclerosis and discusses the BMP-hepcidin-ferroportin axis as a novel therapeutic target for the treatment of cardiovascular disease.
Introduction
Iron is an essential regulator of many cellular and biological processes including oxygen transport and energy metabolism. Intracellular and circulating iron levels are maintained by the hormone hepcidin, a master regulator of iron homeostasis. The transmembrane protein ferroportin is the only known “iron exporter”, which serves to transport ferrous iron from within cells to the plasma.1 Hepcidin regulates the amount of ferroportin at the surface of enterocytes, hepatocytes, macrophages and enterocytes, and thereby controls the concentration of iron in the circulation.2
Because free iron participates in the Fenton reaction, which leads to the generation of reactive oxygen species (ROS), iron at high levels may contribute to DNA, protein or lipid damage and thereby cause cell death.3 ROS-mediated changes to lipoproteins have a central role in the development of atherosclerosis. Oxidized low-density lipoproteins (oxLDL) trigger endothelial activation and induce inflammatory responses, which promote macrophage recruitment. Macrophages internalize oxLDL and become foam cells within vascular plaques. Vascular plaques can increase in size and thicken the vessel wall causing lumen narrowing. Plaques may also rupture and trigger thrombosis, which leads to acute ischemia and infarction.4–6 Because of the involvement of iron in the formation of ROS, iron may contribute to the development and progression of atherosclerosis. Approximately 40 years ago, the ‘Iron Hypothesis’ was formulated, suggesting that higher levels of stored iron promote cardiovascular diseases, whereas iron deficiency may have an atheroprotective effect.
Atherosclerosis
Atherosclerosis is a major burden on global health and cardiovascular disease (CVD) is the leading cause of death worldwide.7 Modifiable risk factors for the development of atherosclerosis include smoking, hypertension, diabetes, and hyperlipidemia.8 Over the last two decades, there has been increased appreciation of the role of oxidative stress and inflammation in the development of atherosclerosis. The opportunity to inhibit inflammation represents a potentially novel approach to prevent and treat atherosclerotic heart disease.9
The pathophysiology of atherosclerosis is characterized by a chronic inflammatory response to endothelial injury with associated lipid deposition and infiltration by monocyte-derived macrophages, followed by calcification of arterial vessels.5,10 In the early stages of atherogenesis, intimal accumulation of apolipoprotein B (apoB)-containing lipoproteins, particularly low-density lipoprotein cholesterol ( LDL-C), activates the overlying endothelium.11 Activated endothelial cells express leukocyte adhesion molecules that bind circulating monocytes, which then diapedese into the intimal wall where they differentiate into macrophages.12,13 Atherosclerotic plaque progression is characterized by the intracellular accumulation of oxidized lipoproteins (oxLDL) and cholesterol esters within macrophages, leading to foam cell formation, with eventual apoptosis and growth of a necrotic core within the plaque.9,14 Atheromatous plaques consist of lipids, fibrosis, and inflammatory cells.5 Intra-plaque necrosis perpetuates the recruitment of more monocytes, which differentiate into macrophages and release cytokines and growth factors, further fueling the inflammatory process. The density of foam cells within. atherosclerotic plaques and the size of the necrotic core are thought to be key determinants of the vulnerability of plaques to rupture with subsequent vessel occlusion.4,15
In addition to treating hypertension and diabetes, intensive control of plasma lipid concentration is considered part of the first-line medical treatment for primary and secondary prevention of CVD.16–18 Numerous randomized clinical trials support the benefits of decreasing the level of harmful lipids (LDL-C) using-3-hydroxy-3-methyglutaryl-CoA (HMG-CoA) reductase inhibitors (statins), to prevent CVD.19,20 Nevertheless, in clinical practice, some patients do not reach the desired LDL-C target level using statins alone. In addition, some patients still have cardiovascular (CV) events, despite adequate control of LDL-C, diabetes, and hypertension. Efforts have been made to find and test alternative lipid-lowering agents for the primary and secondary prevention of CV-events. Novel lipid-lowering agents, such as cholesterol absorption inhibitors and proprotein convertase subtilisin/kexin type 9 (PCSK-9) inhibitors, have been developed and used in clinical settings.21
Along with the increasing appreciation of the role of inflammation in atherosclerosis, the inflammatory response has become a promising target for novel therapies. However, because inflammation is often required for an adequate defense against infections, there is a narrow therapeutic window for the use of anti-inflammatory medications for the treatment of atherosclerotic disease.22, 23 The use of aggressive immunosuppressive therapy, as an approach to inhibiting inflammation in atherosclerosis, has only gradually been pursued as a therapeutic option.24 Novel approaches are needed to specifically target aspects of inflammatory processes that may be unique to CVD.
Iron modulates Toll-like receptor (TLR) 4-activated inflammatory responses and increases oxidative stress through the generation of reactive oxygen and nitrogen species.25,26 ROS activate the transcription factor NF-kB (nuclear factor kappa-B), which regulates expression of genes important for inflammation.27 Because iron is a regulator of the body’s inflammatory response, one promising approach to the prevention of athrosclerosis is to modify iron homeostasis.28
Regulation of iron homeostasis
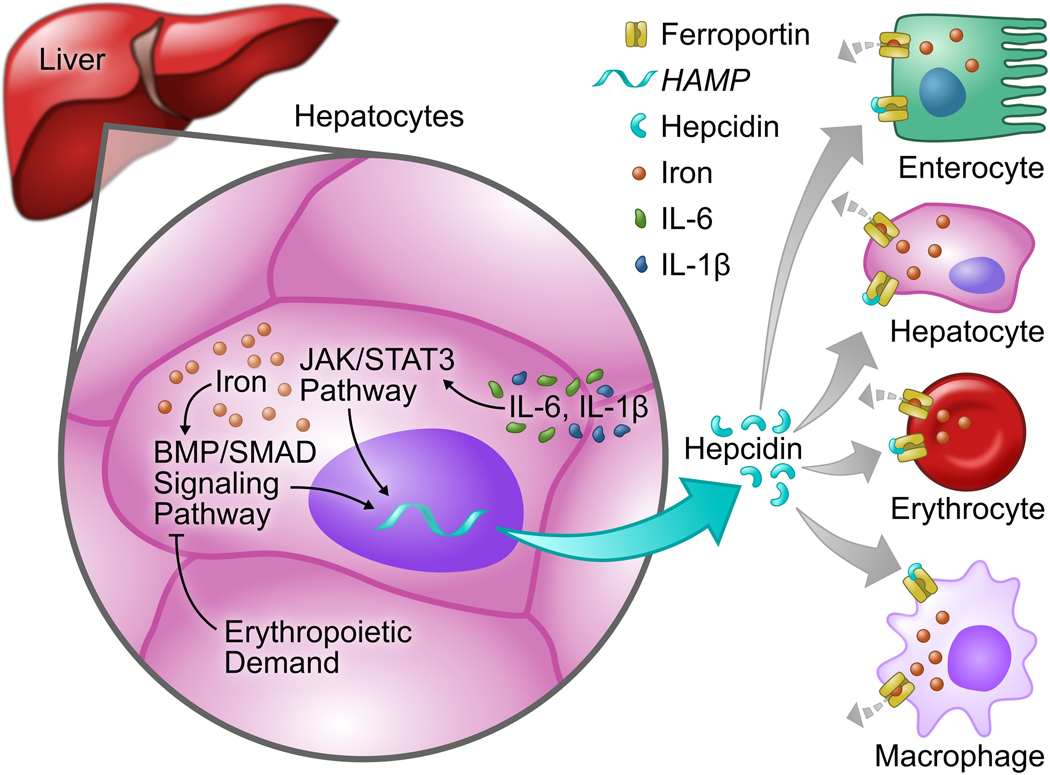
In a healthy individual, plasma iron levels are maintained at a constant level to provide iron for essential biological and cellular processes, while preventing excess iron accumulation, which can lead to the production of ROS. The central regulator of systemic iron homeostasis is the hepatic peptide hormone hepcidin (Figure 1).29 Initially identified as a defensin-like antimicrobial peptide,2, 30,31 hepcidin received its name because of high expression levels in the liver (hep-) and microbicidal activity (-cidin)31. By targeting and down-regulating ferroportin (FPN), hepcidin reduces the export of ion from within duodenal enterocytes, macrophages, and hepatocytes.32,33 Disturbances in the tight regulation of hepcidin expression are associated with various diseases including iron overload disorders, such as hereditary hemochromatosis2,34, as well as anemia of inflammation.35 The expression of the gene encoding hepcidin (HAMP) is regulated at the level of transcription. 29,36 Iron deficiency, hypoxia, and/or erythropoiesis inhibit the transcription of , HAMP, whereas increased iron levels and inflammation stimulate hepcidin transcription 37 Inflammatory mediators, such as IL-6, increase hepcidin expression through the Janus kinase/signal Transducer and Activator of Transcription 3 (JAK/STAT3) pathway, resulting in sequestration of iron in macrophages, reduced duodenal iron absorption, and subsequent hypoferremia, which can lead to the development of anemia.38,39 Increasing the level of hepcidin in states of inflammation (and thereby decreasing serum iron) may have evolved as a defense mechanism against invading pathogens, because iron is an obligate cofactor for the proliferation and growth of some microorganisms.33
Figure 1. Overview of the regulation of iron homeostasis.
The hepatic hormone hepcidin is the central regulator of systemic iron homeostasis. The expression of the gene encoding hepcidin (hepcidin antimicrobial protein, “HAMP”) is regulated at the transcriptional level by iron via the BMP/Smad signaling pathway, by inflammatory cytokines such as IL-6 and by IL-1β via the JAK/STAT3 pathway, and by erythropoietic demand. While iron and inflammation increase the expression of hepcidin, erythropoietic demand and iron deficiency cause suppression of hepcidin. Hepcidin regulate, the level of the sole known iron exporter, ferroportin, at the cell surface of enterocytes, hepatocytes, erythrocytes and macrophages, and thereby controls the amount of iron in the circulation. IL-6, Interleukin-6; BMP, Bone morphogenetic protein; Smad, mothers against decapentaplegic; JAK/STAT3, Janus kinase/Signal Transducer and Activator of Transcription 3.
The bone morphogenetic protein (BMP) signal transduction system is the major pathway that is required for induction of hepcidin expression in response to increasing levels of iron, with BMP2 and BMP6 being the predominant ligands.33–40–43 Upon binding of BMP2 or BMP6 to the BMP receptor complex, type II receptors (ActRIIA, ActRIIB, and BMPR2) phosphorylate type I receptors ALK2 (ACVR1) and ALK3 (BMPR1B). Activated type I BMP receptors phosphorylate SMAD1/5/8 proteins, which translocate to the nucleus together with SMAD4, bind to BMP response elements in the hepcidin promoter and induce the expression of hepcidin.44 In mice, liver-specific disruption of BMP type I receptors ALK2 or ALK3 markedly decreases hepcidin expression, resulting in iron overload.45 This effect is more severe in hepatocyte-specific Alk3 knockout mice, indicating a predominant role for ALK3 in basal hepcidin expression. ALK3 is also required for induction of hepcidin expression by IL-6, showing an essential crosstalk between inflammatory-mediated (JAK/STAT) and iron-mediated (BMP/SMAD) hepcidin regulatory pathways.46 Pharmacological inhibition of BMP type I receptors, with small molecules such as dorsomorphin and its derivative LDN-193189, inhibits hepcidin expression and decreases anemia of chronic disease in rodents treated with inflammatory agents.47,48 Therefore, inhibitors of the BMP pathway may represent new therapeutic options for the treatment of (chronic) inflammatory processes with associated anemia of chronic disease.
The Iron Hypothesis
In 1981, Dr. Jerome Sullivan formulated the ‘Iron Hypothesis’, suggesting that higher levels of stored iron promote cardiovascular diseases, whereas iron deficiency may have an atheroprotective effect.49,50 The hypothesis was primarily based on the observation that women have a lower risk of cardiovascular disease compared to men. The increased incidence of CVD that occurs in women after menopause, and the associated increase in iron stores, further suggested a link between iron and CVD.51 Increased iron, in the form of free non-transferrin bound iron (NTBI), hemoglobin or heme, may catalyze the formation of ROS and promote lipid oxidation, thus enhancing intimal LDL-C retention and macrophage progression to foam cells.52–54
The iron hypothesis was supported by clinical studies that reported an association between atherosclerosis and either increased serum iron55 or increased levels of serum ferritin56–59 (the latter is a marker of tissue iron stores). Studies demonstrated an association between iron depletion, (either by blood donation60–62 or iron chelation63) and a reduced risk of cardiovascular disease. however, several other studies found no evidence for, or demonstrated results that were even contrary to, the iron hypothesis:64–67 Mendelian randomization analysis showed that higher levels of serum iron, as indicated by an increased percentage of transferrin saturation or increased serum ferritin, were associated with a reduced risk of coronary artery disease; 64 In a cohort study, transferrin saturation was inversely associated with cardiovascular mortality in men and post-menopausal women;67 and in a randomized controlled study, reduction of iron stores by phlebotomy in patients with peripheral arterial disease did not decrease all-cause mortality (Table 1).65
Table 1.
Studies on the association or effect of iron measures on atherosclerotic cardiovascular disease
| Study | Year | Sample Size | Design | Iron measure | Association with clinical disease |
|---|---|---|---|---|---|
| Salonen et al.56 | 1992 | 1,931 (men) | Prospective cohort study Endpoint: myocardial infarction | Serum ferritin | Men with high serum ferritin levels had a 2.2-fold (95% CI, 1.2–4.0) higher risk of acute myocardial infarction |
| Kiechl et al.57 | 1997 | 826 | Prospective cohort study Endpoint: progression of carotid atherosclerosis | Serum ferritin | Serum ferritin is a strong risk factor for carotid atherosclerosis progression (odds ratio, 1.50 per SD increase in ferritin; P=0.0002) |
| Salonen et al. 60 | 1998 | 2,862 | Prospective cohort study Endpoint: risk of myocardial infarction | Iron depletion by blood donation | Frequent blood donation was associated with an 88% reduced risk of acute myocardial infarction (95% CI for hazard ratio, 0.02–0.86) |
| Duffy et al. 63 | 2001 | 54 | Acute intervention clinical study with CAD patients and healthy controls. Endpoint: vasomotor function in forearm resistance vessels | Iron depletion by iron chelation | Iron chelation with one dose of deferoxamine improved endothelium-dependent vasodilation in patients with coronary artery disease (p<0.01 by 2-way repeated-measures ANOVA) |
| Meyers et al.61 | 2002 | 2,104 | Retrospective cohort study Endpoint: cardiovascular events | Iron depletion by blood donation | Frequent blood donation was associated with reduced risk of cardiovascular events (odds ratio, 0.60; CI 0.43–0.83) |
| Zacharski et al.65 | 2007 | ,277 | Multicenter, randomized, controlled, single-blinded clinical trial Endpoint: all-cause mortality | Iron depletion by phlebotomy | Phlebotomy in patients with symptomatic peripheral arterial disease did not decrease all-cause mortality (hazard ratio, 0.85; 95% CI, 0.67–1.08) |
| Menke et al.58 | 2009 | 2,662 | Cross-sectional analysis Endpoint: association of ferritin and transferrin saturation with PAD | Serum ferritin and transferrin saturation | Borderline association in men of serum ferritin with peripheral arterial disease (PAD) (men: OR, 1.18; 95% CI, 1.00–1.41; women: OR, 1.04; 95% CI, 0.87–1.25) but not transferrin saturation (men: OR, 1.45; 95% CI, 0.83–2.51; women: OR, 1.55; 95% CI, 0.98–2.45) |
| Sung et al.59 | 2012 | 12,033 | Cross-sectional analysis Endpoint: association of ferritin and coronary artery calcium score (marker of atherosclerosis) | Serum ferritin | Increased ferritin levels were associated with the presence of coronary artery calcium (OR, 1.66; 95% CI, 1.3–1.98) |
| Houschyar et al.62 | 2012 | 64 | Randomized, controlled, single-blind clinical trial Endpoint: clinical markers of metabolic syndrome | Iron depletion by phlebotomy in metabolic syndrome patients | In patients with metabolic syndrome, phlebotomy reduced systolic blood pressure by 16.6 mmHg compared to the control group (95% CI, −20.7 to −12.5 mmHg) |
| Kim et al.67 | 2012 | 5695 | Prospective cohort study Endpoint: all cause-, cancer-, and cardiovascular mortality | Transferrin saturation, serum ferritin | Increasing quintiles of transferrin saturation were associated with reduced all-cause, cancer, and CV mortality, but there was no association with serum ferritin |
| Bagheri et al.55 | 2013 | 337 | Cross sectional analysis Endpoint: association of iron levels with severity of coronary artery disease (CAD) | Serum iron | Increased serum iron levels in patients with severe atherosclerosis (p=0.01) compared to those with mild and moderate amounts of coronary disease |
| Gill et al.64 | 2017 | 294,223 | Mendelian randomization analysis Endpoint: Coronary artery disease risk | Serum iron, transferrin saturation, and ferritin | Protective effect of higher iron status on risk of coronary artery disease: OR 0.94 per SD increase in iron; 95% CI, 0.88–1.00; OR, 0.95 per SD increase in transferrin saturation; 95% CI, 0.910.99; OR, 0.85 per SD increase in log- transformed ferritin; 95% CI, 0.73–0.98 |
Murine models examining the role of iron in the development of atherosclerosis also produced conflicting results. In mice with iron overload disease, administration of iron chelators protected against the development of atherosclerosis.68 Reduced macrophage iron, achieved through either an iron-deficient diet69 or treatment with an iron chelator70, was associated with decreased atherosclerosis suggesting a protective effect of decreased intracellular iron levels. Dietary iron loading worsened atherosclerosis by inducing inflammation in macrophages.71 However, another study found that a high-iron diet decreased atherogenesis.72 Kautz and colleagues reported that iron loading of macrophages had no effect on atherosclerosis. The authors suggested that excess iron was effectively stored in combination with ferritin within macrophages, thereby inhibiting ROS formation.73
Patients with hereditary hemochromatosis (HH), a genetic disorder associated with hepcidin deficiency and progressive iron overload, do not appear to have an increased risk of atherosclerosis and ischemic heart disease.74–76 The low hepcidin levels in hemochromatosis patients and the resulting increase in the amount of cell surface ferroportin leads to iron depletion of monocytes. Iron-depleted monocytes isolated from hemochromatosis patients were found to have a decreased ability to upregulate the cytokines monocyte chemoattractant protein (MCP)-1 and interleukin (IL)-6.77 The ‘paradox’ that chronic systemic iron overload in hemochromatosis was not accompanied by an increased risk for atherosclerosis, and even perhaps conferred protection from atherosclerotic heart disease, was taken as evidence against the original iron hypothesis. The hypothesis has subsequently been refined to focus on the level of intracellular macrophage iron and its impact on the development of atherosclerosis.78
Macrophages in atherosclerosis
Macrophages have a vital role in systemic immunity and tissue remodeling as well as iron homeostasis. Macrophages have pro-and anti-inflammatory effects and these cells both protect from infection and resolve inflammation Multiple macrophage phenotypes have been detected in atherosclerotic lesions, including macrophages with M1, M2, or M(Hb) phenotype (Table 2).79
Table 2. Characteristics of macrophages with a M1, M2, and M(Hb) phenotype.
Multiple macrophage phenotypes have been detected in atherosclerotic lesions, including macrophages with a M1, M2, and M(Hb) phenotype. The different types of macrophages have different effects on plaque progression. IFNγ, Interferon gamma; IL, Interleukin; TNFα, Tumor necrosis factor alpha; iNOS, inducible nitric oxide synthase; PDGF, Platelet-derived growth factor; MMP, Matrix metallopeptidase; VEGF-A, vascular endothelial growth factor; HIF, Hypoxia inducible factor; ABC transporter; ATP-binding cassette transporter; oxLDL, Oxidized low-density lipoprotein; FPN, Ferroportin; HO-1, Heme oxygenase 1.
| Classically activated M1 macrophages | Alternatively activated M2 Macrophages | Hemorrhage-associated M(Hb) macrophages | |
|---|---|---|---|
| Activation by | INFγ, fatty acids, oxidized lipids79 | IL-4, IL-10, IL-1387 | Hemoglobin97 |
| Characteristics | Pro-inflammatory80,85,86
Promote inflammation Promote collagen hydrolysis ↑TNFα, IL-1, IL-6, IL-12, iNOS ↑TGF-β, PDGF production ↑secretion of MMP1, MMP3 and MMP9 |
Anti-inflammatory88,89,93,94
Promote tissue repair and vascular stalility Promote collagen production ↑IL-10, arginase-1, CD206 ↑ornithine, VEGF-A production |
Anti- as well as pro-inflammatory properties97,105,106
Promote tissue repair and vascular stability Promote angiogenesis, vessel permeability, & inflammatory cell recruitment ↑IL-10, CD206 ↑HIF-α and VEGF-A |
| Lipid handling | Increased intracellular lipid levels84
↑oxLDL scavenger receptors CD36 ↑foam ceil formation |
Decreased intracellular lipid levels93,96
↑ABC transporters ↓CD36 ↑efferocytosis |
Decreased intracellular lipid levels97 ↑ABC transporters ↓CD36 |
| Iron phenotype | Iron sequestration phenotype82,83 ↑Ferritin ↓FPN, HO-1, CD163 ↑ intracellular iron |
Iron releasing phenotype83,90
↑Ferritin ↓FPN, HO-1, CD163 ↑intracellular iron |
Iron releasing phenotype103,104 ↓Ferritin ↑FPN, HO-1, CD163 ↓intracellular iron |
| Atherosclerosis | Pro-atherogenic | Atheroprotective | Pro-atherogenic and Atheroprotective |
Classically activated (‘M1’) macrophages promote inflammation and are characterized by high expression of tumor necrosis factor alpha (TNF-α), IL-6, IL-1, and inducible nitric oxide synthase (iNOS).80 M1 macrophages are the predominant phenotype found in advanced, unstable, atherosclerotic plaques, which have a large lipid-rich necrotic core.81 Endogenous TLR ligands, such as free fatty acids and oxidized lipids, as well as cytokines such as interferon Y (IFN-γ), contribute to activation of macrophages to a M1-phenotype.79 M1 polarized macrophages express high levels of the iron storage protein ferritin and low levels of FPN.82,83 M1 macrophages express scavenger receptors on their surface, which internalize oxLDL, and M1 macrophages can transform into foam cells by lipid accumulation, which is a critical step in atherogenesis.84 Factors such as platelet-derived growth factor (PDGF) or TGF-β produced by M1 macrophages prompt proliferation of vascular smooth muscle cells (VSMC) and their migration from the arterial media into the intima.85 Matrix metalloproteases (MMP-1, MMP-3, MMP-9), secreted by M1 macrophages, may destabilize the atherosclerotic plaque by hydrolysis of collagen within the fibrous cap.86 In general, classically activated M1 macrophages have a pro-atherogenic phenotype.
Alternatively activated (M2) macrophages are induced by different cytokines, including IL-4 and IL-13, and produce anti-inflammatory cytokines, such as IL-10.87 M2 macrophages contribute to the resolution of vascular inflammation and to the stabilization of atheromatous plaques by clearing apoptotic cells and debris, by increasing the production of type I collagen within the plaque, and possibly by inducing the proliferation of VSMC within the fibrous cap (before activation of the MMP cascade).80,88,89 Compared to M1 macrophages, M2 macrophages have high constitutive expression of arginase I, mannose receptor (CD206), hemoglobin-haptoglobin complex scavenging receptor (CD163) and IL-10. Relatively high levels of FPN and low levels of ferritin in M2 macrophages favor cellular iron efflux resulting in lower intracellular iron levels.83,90 The activation of heme oxygenase 1 (HO-1), which catalyzes the degradation of he me into ferrous iron and carbon monoxide, further favors iron release.91,92
In contrast to M1 macrophages, M2 macrophages have lower levels of intracellular lipids and do not transform into foam cells. In a process referred to as efferocytosis M2 marophages engulf apoptotic foam cells within atherosclerotic plaques, thereby promoting tissue repair and inhibiting further lesion progression.93,94 However, in advanced atherosclerotic plaques, increased accumulation of cellular debris and lipids derived from apoptotic foam cells may exceed the efferocytosis capacity of M2 macrophages and promote necrotic core formation, contributing to further inflammation, necrosis, and thrombosis.95,96
Hemorrhage-associated macrophages (M(Hb), also referred to as M(hem)), are induced by hemoglobin and are found in areas of neoangiogenesis aM hemorrhage.97 In humans, but not mice, intraplaque hemorrhage (IPH), as a consequence of neovascularization due to local hypoxia from intimal thickening, is observe’ in the late stages of atherosclerosis. IPH is associated with increased vessel permeability, red blood cell lysis, and free hemoglobin (Hb) release.98,99, 100 The CD163 heme-scavenger receptors on the surface of M(Hb) bind and internalize free hemoglobin-haptoglobin complexes. M(Hb) macrophages produce anti-inflammatory factors such as IL-10 101,102 In addition, M(Hb) macrophages are resistant to foam cell formation due to LXRα-induced up-regulation of cholesterol-exporting ATP-binding cassette (ABC) transporters ABCA-1 and ABCG-1.97 M(Hb) macrophages were therefore originally classified as atheroprotective and anti-inflammatory. However, LXRα also upregulates FPN mRNA expression and promotes iron export.103, 104 The resulting iron decrease in M(Hb) macrophages increases argiogenesis, vessel permeability, and inflammatory cell recruitment, leading to plaque progression.105 M(Hb) macrophages are therefore classified as both atheroprotective and pro-atherogenic, as they have anti- as well as pro-inflammatory propertes.106
Differentiated macrophages have a “plastic” cell type in that they maintain the ability to change from one phenotype to another. For example, M1 macrophages can develop features of M2 macrophages, and may then revert to the M1 phenotype, depending on environmental signals.107,108 Macrophage plasticity is reported to occur during atherosclerotic plaque progression, in both humans and mice.109 Iron is one important factor that affects macrophage plasticity.110–112
Effect of intracellular iron on macrophage polarization and function
Intracellular iron levels in macrophages are regulated by the hepcidin-ferroportin axis.37 Iron handling and immune functions of macrophages are linked, and intracellular iron levels directly regulate macrophage polarization.110–112 Although some studies have yielded conflicting results on the directionality of the effect of iron on the inflammatory response,113,114 most studies indicate that increased intracellular iron induces a pro-inflammatory M1 phenotype, 115–119 potentially by increasing ROS production.120 Intracellular iron per se has direct effects on macrophage polarization, as increased iron loading of M2 macrophages induces a rapid switch from an M2 to an M1 phenotype.117 Furthermore, intracellular iron levels contribute alterations in cytokine production. Fpn-deficient macrophages, which are characterized by increased intracellular iron levels due to lack of iron export, have an increased expression of inflammatory cytokines. Moreover, intracellular macrophage iron may catalyze the oxidation of LDL thereby promoting the development of foam cells.121 In contrast, treatment of macrophage with the iron chelator deferoxamine (DFO) has an anti-inflammatory effect and decreases the release of TNFα, IL-6, and the potent monocyte-attracting chemokine MCP-1.122
The pro-inflammatory effects of increased intracellular iron concentration were observed in a mouse model of wound healing. In mice with a macrophage-specific inactivation of FPN, wound closure was delayed, while the inflammatory response was prolonged. In these mice, high intracellular iron content inhibited the switch from pro-inflammatory M1 macrophages to anti-inflammatory M2 phenotype, and prolonged the wound healing phase.123 In humans, an “unrestrained” population of pro-inflammatory M1 macrophages was identified adjacent to chronic venous leg ulcers. Localized iron overloaded induced by release of iron from degenerating, extracellular erythrocytes may contribute to the information of M1 macrophages and impair wound healing.115
Mice that lack the Hfe gene are a well-studied murine model of hemochromatosis. Although the plasma iron concentrations in these mice are high, the intracellular iron levels in macrophages from Hfe−/− mice are low due to systemic hepcidin deficiency. Associated with the low intracellular iron levels ii Hfe−/− macrophages, the cells have an impaired response to activation by TLR4 and impaired expression of inflammatory cytokines, including TNFα and IL-6. The reduced inflammatory response is likely the result of low intracellular iron levels within macrophages, as pro-inflammatory responses of macrophages can be increased by administration of exogenous iron.26,90,124 The results of these studies suggest that macrophage iron content influences cytokine production in macrophages. The effect is, at least in part, attributed to the RNA-binding protein tristetraprolin (TTP). TTP is induced by iron deficiency 125,126 and destabilizes mRNA of pro-inflammatory cytokines including TNFα and IL-6.127
Because high intracellular iron levels induce the pro-inflammatory, pro-atherogenic M1 macrophage phenotype, factors that decrease intracellular iron may promote the switch from the M1 phenotype to the M2 phenotype and thereby protect against the development of atherosclerosis.
The role of hepcidin in atherosclerosis
Hepcidin gene expression is induced by increased levels of iron and by inflammation (Figure 2). High levels of iron increase hepcidin by stimulating the bone morphogenetic protein (BMP) signal transduction pathway. Inhibition of the BMP signaling pathway has been demonstrated to effectively hinder atherogenesis in different mouse models of atherosclerosis.128–131
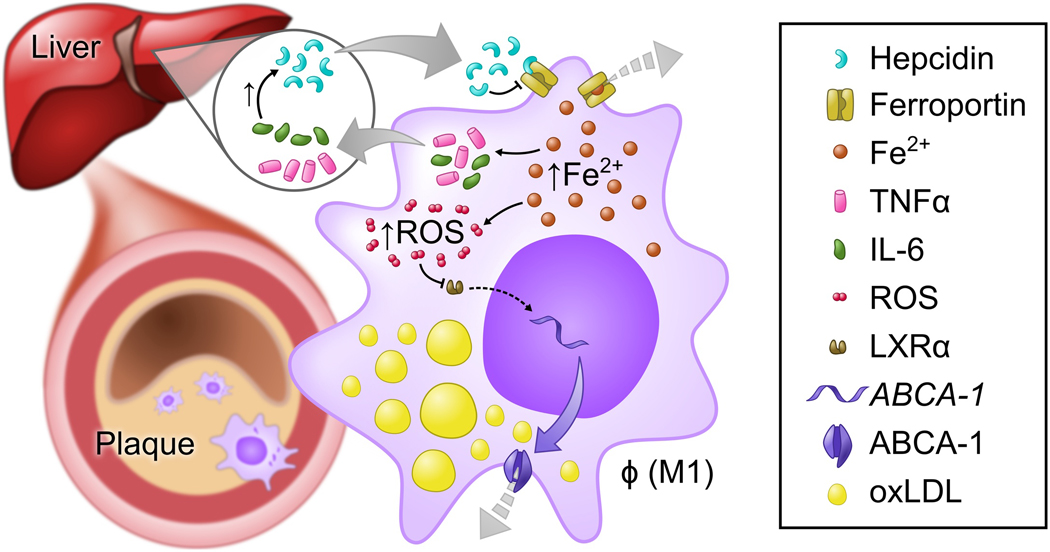
Figure 2. Schematic representation of the role of hepcidin in foam cell formation and the pathogenesis of atherosclerosis.
Hepcidin gene expression in the liver is induced by inflammatory cytokines IL-6 and IL1b. Hepcidin binds to ferroportin, the only-known exporter of iron, and induces the degradation of ferroportin. Iron export from macrophages is therefore reduced, and high intracellular iron levels induce a pro-inflammatory, pro-atherogenic M1 macrophage phenotype. As a result of increased intracellular iron, the production of inflammatory cytokines, including IL-6 and TNFα, is increased. High levels of reactive oxygen species (ROS) are generated, which inhibit LXRα-induced expression of the gene encoding the ABCA-1 cholesterol transporter. As a consequence, cholesterol efflux is decreased, and lipids accumulate in the cell, leading to foam cell formation within the vascular plaque. Vascular plaques increase in size, causing lumen narrowing. The dotted grey arrow indicates reduced export. Fe2+, ferrous iron; TNFα, Tumor necrosis factor α; IL-6, Interleukin-6; ROS, Reactive oxygen species; LXRα, Liver X receptor α; ABCA-1, ATP-binding cassette transporter 1; oxLDL, Oxidized low-density lipoprotein.
LDN-193189 is a small molecule inhibitor of the BMP type I receptor. Treatment of Apolipoprotein E deficient (Apoe−/−) mice (a model for the development of atherosclerosis) with LDN-193189 significantly reduced vascular macrophage accumulation and atherosclerotic lesion formation.130 Inhibition of BMP signaling reduced hepcidin levels and increased the expression of FPN, which resulted in macrophage iron depletion and reduced intracellular ROS production. These changes were associated with an increase in transcription of genes encoding ABC transporters, which raised cholesterol efflux from macrophages and decreased the formation of foam cells and atherosclerosis.130 Finn and colleagues further. supported the interplay between cholesterol and iron metabolism in macrophages. In human monocytes derived from atherosclerotic plaques, hepcidin-induced down-regulation of FPN increased intracellular iron and increased lipid accumulation in macrophages.97 By promoting the production of ROS, high intracellular iron levels inhibited LXRα-mediated transcription of genes encoding ABC transporters and hence hindered cholesterol efflux.
In LDL receptor-deficient (Ldlr−/−) mice, a second model of atherosclerosis, inhibition of BMP signaling, either by treatment with LDN 193189 or with a soluble receptor-antibody fusion protein that sequesters BMP ligands (ALK3-Fc) reduced vascular calcification, vascular inflammation and atheroma formation.129 Besides the direct effect of BMP inhibition on the vasculature, Derwall and colleagues found a potent effect of LDN-193189 on lipoprotein biosynthesis. LDN-193189 treatment reduced serum levels of total cholesterol and LDL, suggesting an additional and previously unknown role for BMP signaling in lipoprotein homeostasis.129
These studies performed in Apoe−/− and Ldlr−/− mice described the essential role of BMP signaling in the development of atherosclerosis with intimal calcification. In addition, we and others have identified he BMP pathway as an important mediator of calcification of the vascular media both in humans and in mouse models with matrix Gla protein (MGP) deficiency or overexpression 128,132,133 Pharmacological inhibition of BMP signaling with LDN-193189 or ALK3-Fc reduced medial vessel calcification in Mgp−/− mice and was associated with a decreased VSMC osteogenic phenotype.128 Furthermore, BMP inhibition by overexpression of MGP, an antagonist o BMP signaling, reduced BMP activity, atherosclerotic lesion size and vascular calcification, without having an effect on cholesterol levels.131 Intimal and medial calcification results from different underlying pathogenic mechanisms: Intimal calcification is associated with inflammation, lipid deposition and macrophage accumulation; medial calcification results from metabolite-induced (e.g., phosphate-induced) upregulation of osteogenic gene programs in the vasculature134. Despite the differing mechanisms of calcification, both processes are linked to BMP signaling.
BMP signaling also has a critical role in maintaining homeostasis in many tissues, including bone and cartilage formation.135 Therefore, chronic inhibition of BMP signal transduction might have a broad range of adverse effects. To specifically determine the role of hepcidin-ferroportin signaling in the development of atherosclerosis, we investigated the effects of hepcidin deficiency on the progression of atherosclerosis in hepcidin- and LDL receptor-deficient (Hamp−/−/Ldlr−/−) mice.119 The absence of hepcidin in these mice, and the resulting increase in ferroportin on the surface of macrophages, was associated with decreased atherosclerosis. A regression analysis showed that the benefits of hepcidin deficiency were not caused by differences in serum iron levels, body weight or serum LDL levels. The results strongly suggest that the protective effect of hepcidin deficiency was caused by macrophage iron depletion. Decreased intracellular macrophage iron was associated with decreased levels of M1 phenotypic markers in aortic macrophages as well as reduced oxidized LD uptake, 119
Although these studies show that mice treated with inhibitors of BMP signaling (thereby decreasing hepcidin levels) or mice with genetic deletion of hepcidin have decreased atherosclerosis, the precise role of hepcidin in the pathogenesis of atherosclerosis remains controversial. In a study published by Vinchi et al., ApoE-deficient/FPN wT/C326S knock-in mice, which carry a mutation in ferroportin that disrupts hepcidin binding, had significantly increased atherosclerosis compared to age-matched ApoE-deficient mice, despite reduced intracellular iron levels in macrophages.68 Resolution of these seemingly contradictory results requires further investigation. One possible explanation for these disparate results68,119 may be the intrinsic difference between the ApoE- and Ldlr-deficient mouse models of atherosclerosis.136 ApoE, unlike LDLR, exerts additional atheroprotective effects (beyond its capacity to lower lipid levels), which include direct anti-inflammatory properties on macrophages that favor M2 polarization.137 A second possible explanation or the differences between the two models of atherosclerosis is the complex interplay between serum and intracellular iron levels and the progression of atherosclerosis. The protective benefits of low macrophage iron, along with reduced hepcidin levels, may be partially offset by the detrimental effects of high serum iron. The degree of intracellular macrophage iron content might affect the anti-inflammatory properties of macrophages and explain the controversial results of the two models: while macrophages of homozygous FPNC326S knock-in mice are iron depleted,138 heterozygous FPNwT/C326S knock-in mic may still have residual intracellular iron in macrophages as indicated by the presence of detectable iron within atherosclerotic plaques in ApoE-deficient/FPNWT/C326S knock-in mice.68 Residual macrophage iron content may counteract the protective effect on atherosclerosis. It is also possible that the effect of hepcidin may be dependent on the stage of atherosclerotic disease. 139 In early- to mid-stage disease, low hepcidin levels might have beneficial effects by constraining the effects of pro-inflammatory macrophages.97 In late-stage lesions however, depletion of macrophage iron might lead to HIF (hypoxia-inducible factor)-α and VEGGF (vascular endothelial growth factor)-A mediated increased angiogenesis, vessel permeability, and inflammatory cell recruitment resulting in plaque progression.105,106
Hepcidin and atherosclerosis in clinical studies
Inflammation induces hepcidin expression, and a variety of chronic inflammatory diseases are associated with increased levels of hepcidin, including rheumatoid arthritis140, heart failure141, chronic kidney disease,142 and non-alcoholic fatty liver disease (Table 3).143,144 In a study of patients with rheumatoid arthritis, increased serum hepcidin levels were associated with higher coronary artery calcium scores.145 In patients with non-alcoholic fatty liver disease, higher serum ferritin and hepcidin levels were associated with increased vascular damage and the presence of carotid plaques.146 In individuals with metabolic syndrome (diabetes mellitus, hypertension, and hyperlipidemia), who have a high risk of atherosclerosis, serum hepcidin levels and intracellular macrophage iron levels correlated with release of the pro-inflammatory cytokine MCP-1 and vascular damage.77,146 Studies in patients with chronic kidney disease undergoing hemodialysis showed a relationship between elevated hepcidin levels and arterial stiffness147 as well as between hepcidin levels and cardiovascular events.148 In a recent population-based cohort study, increased hepcidin and an increased ratio of heicidin to ferritin (an index of the amount of hepcidin relative to iron stores) were significantly associated with the presence of plaques in the carotid artery of post-menopausal women.149 Together, these findings strongly suggest an important role for hepcidin in the pathogenesis of atherosclerosis and CVD events.
Table 3.
Epidemiological studies showing a correlation between circulating hepcidin levels and sub-clinical or clinical atherosclerosis
| Study | Year | Underlying disease | Sample size | Atherosclerosis Marker or Clinical Event |
|---|---|---|---|---|
| Abdel-Khalek et al.145 | 2011 | Rheumatoid Arthritis | 80 | Coronary calcium score (CCS) |
| Valenti et al.146 | 2011 | Nonalcoholic fatty liver disease (NAFLD) | 143 | Carotid plaque |
| Kuragano et al.147 | 2011 | Chronic Kidney Disease patients on maintenance hemodialysis | 168 | Arterial stiffness |
| Van der Weerd et al.148 | 2013 | Chronic Kidney Disease patients on chronic hemodialysis | 405 | Fatal and non-fatal CV events |
| Galesloot et al.149 | 2014 | General population | 766 (346 women) | Presence of plaque |
To date, there have been no clinical studies addressing the specific role of hepcidin in atherosclerosis. However, modulating the inflammatory response has become a promising target to reduce mortality and morbidity in CVD (Table 4 ).22,150,151 Increased expression of hepcidin is induced by inflammatory cytokines, inclining IL-6 and IL-1β.152 Canakinumab is an inhibitor of IL-1β, a cytokine that is highly expressed in atherosclerotic lesions153 and induces the expression of IL-6 and downstream CRP and hepcidin.154 The CANTOS trail was designed to test whether canakinumab prevents recurrent cardiovascular events in men and women with prior myocardial infarction who have elevated levels of inflammation. Treatment with canakinumab resulted in reduction of IL-6 and CRP levels, with no effects on lipid levels. The incidence rate for myocardial infarction stroke, and cardiovascular death was reduced in the canakinumab-treated group compared to the placebo group.22 Reducing the inflammatory mediators IL-1β and IL-6 seems to be one of the key factors that were beneficial in reducing CVD events. The CANTOS trial is the first clinical trial to report that inhibition of inflammatory mediators improved CVD outcome.
Table 4.
Clinical trials on the effect of targeting inflammation in cardiovascular disease
| Study | Year | Sample Size |
Type of study | Drug | Result |
|---|---|---|---|---|---|
| Klevelnad O et al.158 and Holte E et al.159 | 2016, 2017 |
117 | Randomized, double-blind placebo-Controlled phase II trial | Tocilizumab | Tocilizumab attenuated inflammation and Troponin-T release (primarily PCI-related, p<0.001), but did not improve coronary flow reserve (a measure of endothelial function) in NSTEMI patients |
| Ridker P et al.22 CANTOS-trial | 2017 | 10,061 | Randomized, double-blind placebo-controlled phase III trial | Canakinumab | Treatment with canakinumab (at either a 150 mg or 300 mg dose) led to a statistically significant 15% or 14% reduction, respectively, in the composite of nonfatal MI, nonfatal stroke, or CV death |
| Ridker P et al.162 C’RT-trial | 2019 | 4786 | Randomized, double-blind, placebo-controlled trial | Methotrexate | Treatment with low-dose methotrexate did not reduce the occurrence of cardiovascular events including nonfatal myocardial infarction, nonfatal stroke or cardiovascular death (HR, 1.01; 95% CI, 0.82 to 1.25). |
The significance of the inflammatory cytokine IL-6 in CVD became evident with the finding that the ASP358Ala hypofunctional variant of the IL-6 receptor in humans is associated with reduced risk coronary artery disease.155–157 Expression of this variant in hepatocytes, monocytes and macrophages reduces IL-6 signaling and leads to an attenuated expression of IL-6-induced proteins, including CRP. In a double-blind, randomized, placebo-controlled phase II trial, patients with non-ST-elevation MI received the IL-6 receptor antagonist tocilizumab to analyze the effect of IL-6 inhibition on the acute inflammatory response (primary endpoint), and to reduce troponin T release.
Treatment with tocilizumab attenuated inflammation and reduced percutaneous coronary intervention (PCI)-related troponin T release compared to the placebo group, which may indicate that IL-6 receptor antagonism is cardioprotective in ischemia-reperfusion injury. However, tocilizumab did not improve coronary flow reserve (a measure of endothelial function).158,159 The clinical trial was not powered to determine the effect of IL-6 inhibition on clinical outcome.158 A definitive clinical trial to test the effects of specific IL-6 antagonism on CVD outcomes has not yet been performed. However, previous studies160,161 found an association between high IL-6 levels and increased rates of coronary heart disease and CV-related mortality, as well as all-cause mortality. Increased hepcidin levels and iron deficiency were associated with higher IL-6 levels161, suggesting a link between hepcidin, inflammation and CV outcomes. Further investigation is needed to determine if the beneficial clinical effects of decreased IL-6 or IL-1β signaling, as observed in humans carrying the Asp358Ala variant or in patients treated with tocilizumab or canakinumab, are in part mediated by decreased hepcidin expression, increased ferroportin levels, and low intracellular macrophage iron.
Clinical implications/Conclusions
The “Iron Hypothesis” has led to research focused on clarifying the role of iron in the development of atherosclerotic heart disease. Early preclinical and clinical studies produced contradictory results and the observation that patients with hemochromatosis do not appear to have an increased risk of atherosclerosis seemed especially difficult to explain. This ‘paradox’ of systemic iron overload not being accompanied by an increased risk to atherosclerosis led to a refinement of the iron hypothesis focusing particularly on intracellular macrophage iron. More recent in vitro and animal studies have identified intracellular iron as an effector of macrophage polarization, cytokine production as well as lipid metabolism. High intracellular iron promotes a pro-inflammatory M1 macrophage phenotype with an increased expression of cytokines and reduced efflux of cholesterol. The effect on cholesterol transport is a key determinant of foam cell formation. Intracellular iron levels in macrophages are regulated by the hormone hepcidin, which induces the degradation of ferroportin and decreases iron export. BMP signaling is essential for the induction of hepcidin expression, and inhibition of BMP signaling in in vivo models has an atheroprotective effect. The strong links between iron homeostasis, BMP signaling, inflammation and atherosclerosis have been established in both mechanistic and human studies. However, while recent clinical trial results have underscored the benefits of targeting inflammation in cardiovascular disease, the precise clinical impact of modifying iron homeostasis warrants further investigation. The BMP-hepcidin-ferroportin axis may represent a novel therapeutic target for the treatment of cardiovascular disease.
Acknowledgments
Funding Sources
Dr. Traeger was supported by the German Research Foundation (DFG TR 1642/1–1). Dr. Bloch was supported by the Leducq Foundation (Multidisciplinary Program to Elucidate the Role of Bone Morphogenetic Protein Signaling in the Pathogenesis of Pulmonary and Systemic Vascular Diseases) and by RO1DK082971 from the National institute of Diabetes and Digestive and Kidney Diseases and by a donation from Luisa Hunnewell and Larry Newman Dr Malhotra was supported by the National Heart, Lung, and Blood Institute (K08HL111210 and R01HL142809), the American Heart Association (18TPA34230025), the Wild Family Foundation, and the Hassenfeld Scholar Award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nemeth E, Tuttle MS, Powelson J, et al. Hepcidin regulates cellular iron efflux by bindirg to ferroportin and inducing its internalization. Science. 2004;306(5704):2090–2093. [DOI] [PubMed] [Google Scholar]
- 2.Pigeon C, Ilyin G, Courselaud B, et al. A new mouse liver-specific gene, encoding protein homologous to human antimicrobial peptide hepcidin, is overexpressed during iron overload. J Biol Chem. 2001;276(11):7811–7819. [DOI] [PubMed] [Google Scholar]
- 3.Dev S, Babitt JL. Overview of iron metabolism in health and disease. Hemodial Int. 2017;21(1):S6–S20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Falk E. Pathogenesis of atherosclerosis. J Am Coll Cardiol. 2006;47(8 Suppl), C7–12. [DOI] [PubMed] [Google Scholar]
- 5.Libby P, Ridker PM, Hansson GK. Progress and challenges in translating. the biology of atherosclerosis. Nature. 2011;473(7347):317–325. [DOI] [PubMed] [Google Scholar]
- 6.Ross R, Glomset JA. The pathogenesis of atherosclerosis (first of two parts). N Engl J Med. 1976;295(7):369–377. [DOI] [PubMed] [Google Scholar]
- 7.Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2095–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Braunwald E. Shattuck lecture--cardiovascular medicine at the turn of the millennium: triumphs, concerns, and opportunities. N Engl J Med. 1997;337(19):1360–1369. [DOI] [PubMed] [Google Scholar]
- 9.Ross R Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340(2):115–126. [DOI] [PubMed] [Google Scholar]
- 10.Small DM. Cellular mechanisms for lipid deposition in atherosclerosis (first of two parts). N Engl J Med. 1977;297(16):873–877. [DOI] [PubMed] [Google Scholar]
- 11.Williams KJ, Tabas I. The response-to-retention hypothesis of early atherogenesis. Arterioscler Thromb Vasc Biol. 1995;15(5):551–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glass CK, Witztum JL. Atherosclerosis. the road ahead. Cell. 2001;104(4):503–516. [DOI] [PubMed] [Google Scholar]
- 13.Mestas J, Ley K. Monocyte-endothelial cell in tractions in the development of atherosclerosis. Trends Cardiovasc Med. 2008;18(6):228–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352(16):1685–1695. [DOI] [PubMed] [Google Scholar]
- 15.Sakakura K, Nakano M, Otsuka F, Ladich E, Kolodgie FD, Virmani R. Pathophysiology of atherosclerosis plaque progression. Heart Lung Circ. 2013;22(6):399–411. [DOI] [PubMed] [Google Scholar]
- 16.Lawall H, Huppert P, Espinola -Klein C, Zemmrich CS, Ruemenapf G. German guideline on the diagnosis and treatment of peripheral artery disease - a comprehensive update 2016. VASA Zeitschrift fur Gefasskrankheiten. 2017;46(2):79–86. [DOI] [PubMed] [Google Scholar]
- 17.Arnett DK, Blumenthal RS, Albert MA, et al. 2019. ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease. Circulation. 2019:Cir0000000000000678. [Google Scholar]
- 18.Catapan AL, Graham I, De Backer G, et al. 2016 ESC/EAS Guidelines for the Management of Dyslipidaemias: The Task Force for the Management of Dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS) Developed with the special contribution of the European Assocciation for Cardiovascular Prevention & Rehabilitation (EACPR). Atherosclerosis. 2016;253:281–344. [DOI] [PubMed] [Google Scholar]
- 19.Baigent C, Keech A, Kearney PM, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366(9493):1267–1278. [DOI] [PubMed] [Google Scholar]
- 20.Fulcher J, O’Connell R, Voysey M, et al. Efficacy and safety of LDL-lowering therapy among men and women: meta-analysis of individual data from 174,000 participants in 27 randomised trials. Lancet. 2015;385(9976):1397–1405. [DOI] [PubMed] [Google Scholar]
- 21.Fujisue K, Tsujita K. Current status of lipid management in acute coronary syndrome. J Cardiol. 2017;70(2):101–106. [DOI] [PubMed] [Google Scholar]
- 22.Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N Engl J Med. 2017;377(12):1119–1131. [DOI] [PubMed] [Google Scholar]
- 23.Ridker PM, Luscher TF. Anti-inflammatory therapies for cardiovascular disease. European heart journal. 2014;35(27):1782–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jones DP, Patel J. Therapeutic Approaches Targeting Inflammation in Cardiovascular Disorders. Biology (Basel). 2018;7(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weiss G, Werner-Felmayer G, Werner ER, Grunewald K, Wachter H, Hentze MW. Iron regulates nitric oxide synthase activity by controlling nuclear transcription. J Exp Med. 1994;180(3):969–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang L, Harrington L, Trebicka E, et al. Selective modulation of TLR4-activated inflammatory responses by altered iron homeostasis in mice. J Clin Invest. 2009;119(11):3322–3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bubici C, Papa S, Dean K, Franzoso G. Mutual cross-talk between reactive oxygen species and nuclear factor-kappa B: molecular basis and biological significance. Oncogene. 2006;25(51):6731–6748. [DOI] [PubMed] [Google Scholar]
- 28.Cherayil BJ. Iron and immunity: immunological consequences of iron deficiency and overload. Archivum immunologiae et therapiae experimentalis. 2010;58(6):407–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ganz T, Nemeth E. Hepcidin and iron homeostasis. Biochim Biophys Acta. 2012;9(43):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krause A, Neitz S, Magert HJ, et al. LEAP-1, a novel highly disulfide-bonded human peptide, exhibits antimicrobial activity. FEBS Lett. 2000;480(2–3):147–150. [DOI] [PubMed] [Google Scholar]
- 31.Park CH, Valore EV, Waring AJ, Ganz T. Hepcidin, a urinary antimicrobial peptide synthesized in the liver. J Biol Chem. 2001;276(11):7806–7810. [DOI] [PubMed] [Google Scholar]
- 32.Ganz T. Hepcidin, a key regulator of iron. metabolism and mediator of anemia of inflammation. Blood. 2003;102(3):783–788. [DOI] [PubMed] [Google Scholar]
- 33.Ganz T, Nemeth E. Iron homeostasis in host defence and inflammation. Nat Rev Immunol. 2015;15(8):500–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nicolas G, Bennoun M, Devaux l, et al. Lack of hepcidin gene expression and severe tissue iron overload in upstream stimulatory factor 2 (USF2) knockout mice. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(15):8780–8785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fleming RE, Sly WS. Hepcidin: a, putative iron-regulatory hormone relevant to hereditary hemochromatosis and the anemia of chronic disease. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(15):8160–8162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang CY, Babitt JL. Hepcidin regulation in the anemia of inflammation. Curr Opin Hematol. 2016;23(3):189–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muckenthaler MU, Rivella S, Hentze MW, Galy B. A Red Carpet for Iron Metabolism. Cell. 2017;168(3):344–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chung B, Verdier F, Matak P, Deschemin JC, Mayeux P, Vaulont S. Oncostatin M is a potent inducer of hepcidin, the iron regulatory hormone hormone. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2010;24(6):2093–2103. [DOI] [PubMed] [Google Scholar]
- 39.Goodnough LT, Nemeth E, Ganz T. Detection, evaluation, and management of iron-restricted erythropoiesis. Blood. 2010;116(23):4754–4761. [DOI] [PubMed] [Google Scholar]
- 40.Meynard D, Kautz L, Darnaud V, Canonne-Hergaux F, Coppin H, Roth MP. Lack of the bone morphogenetic protein BMP6 induces massive iron overload. Nat Genet. 2009;41(4):478–481. [DOI] [PubMed] [Google Scholar]
- 41.Koch PS, Olsavszky V, Ulbrich F, et al. Angiocrine Bmp2 signaling in murine liver controls normal iron homeostasis. Blood. 2017;129(4):415–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Parrow NL, Fleming RE. Bone morphogenetic proteins as regulators of iron metabolism. Annual review of nutrition. 2014;34:77–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morrell NW, Bloch DB, ten Dijke P, et al. Targeting BMP signalling in cardiovascular disease and anaemia. Nat Rev Cardiol. 2016;13(2):106–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Steinbicker AU, Muckenthaler MU. Out of balance--systemic iron homeostasis in iron-related disorders. Nutrients. 2013;5(8):3034–3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Steinbicker AU, Bartnikas TB, Lohmeyer LK, et al. Perturbation of hepcidin expression by BMP type I receptor deletion induces iron overload in mice. Blood. 2011;118(15):4224–4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mayeur C, Lohmeyer LK, Leyton P, et al. The type I BMP receptor Alk3 is required for the induction of hepatic hepcidin gene expression by interleukin-6. Blood. 2014;123(14):2261–2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yu PB, Hong CC, Sachidanandan C, et al. Dorsomorphin inhibits BMP signals required for embryogenesis and iron metabolism. Nat Chem Biol. 2008;4(1):33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Steinbicker AU, Sachidanandan C, Vonner AJ, et al. Inhibition of bone morprogenetic protein signaling attenuates anemia associated with inflammation. Blood. 2011;117(18):4915–4923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sullivan JL. Iron and the sex difference in heart disease risk. Lancet 1981;1(8233):1293–1294. [DOI] [PubMed] [Google Scholar]
- 50.Sullivan JL. The iron paradigm of ischemic heart, disease. American heart journal. 1989;117(5):1177–1188. [DOI] [PubMed] [Google Scholar]
- 51.Kannel WB, Hjortland MC, McNamara PM, Gondon T. Menopause and risk of cardiovascular disease: the Framingham study. Annals of internal medicine. 1976;85(4):447–452. [DOI] [PubMed] [Google Scholar]
- 52.Kraml PJ, Klein RL, Huang Y, Nareika A, Lopes-Virella MF. Iron loading increases cholesterol accumulation and macrophage scavenger receptor I expression in THP-1 mononuclear phagocytes. Metabolism. 2005;54(4):453–459. [DOI] [PubMed] [Google Scholar]
- 53.Lapenna D, Pierdomenico SD, Ciofani G, et al. Association of body iron stores with low molecular weight iron and oxidant damage of human atherosclerotic plaques. Free radical biology & medicine. 2007;42(4):492–498. [DOI] [PubMed] [Google Scholar]
- 54.Tuomainen TP, Loft S, Nyyssonen K, Punnonen K, Salonen JT, Poulsen HE. Body iron is a contributor to oxidative damage of DNA. Free Radic Res. 2007;41(3):324–32’ [DOI] [PubMed] [Google Scholar]
- 55.Bagheri B, Shokrzadeh M, Mokhberi V, et al. Association between Serum Iron and the Severity of Coronary Artery Disease. International cardiovascular research journal. 2013;7(3):95–98. [PMC free article] [PubMed] [Google Scholar]
- 56.Salonen JT, Nyyssonen K, Korpela H, Tuomilehto J, Seppanen R, Salonen R. High stored iron levels are associated with excess risk of myocardial infarction in eastern Finnish men. Circulation. 1992;86(3):803–811. [DOI] [PubMed] [Google Scholar]
- 57.Kiechl S, Wileit J, Egger G, Poewe W, Oberhollenzer F. Body iron stores and the risk of carotid atherosclerosis: prospective results from the Bruneck study. Circulation. 1997;96(10):3300–3307. [DOI] [PubMed] [Google Scholar]
- 58.Menke A, Fernandez-Real JM, Muntner P, Guallar E. The association of biomarkers of iron status with peripheral arterial disease in US adults. BMC Cardiovasc Disord. 2009;9:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sung KC, Kang SM, Cho EJ, Park JB, Wild SH, Byrne CD. Ferritin is independently associated with the presence of coronary artery calcium in 12,033 men. Arterioscler Thromb Vasc Biol. 2012;32(10):2525–2530. [DOI] [PubMed] [Google Scholar]
- 60.Salonen JT, Tuomainen TP, Salonen R, Lakka TA, Nyyssonen K. Donation of blood is associated with reduced risk of myocardial infarction. The Kuopio Ischaemic Heart Disease Risk Factor Study. Am J Epidemiol. 1998;148(5):445–451. [DOI] [PubMed] [Google Scholar]
- 61.Meyers DG, Jensen KC, Menitove JE. A historical cohort study of the effect of lowering body on through blood donation on incident cardiac events. Transfusion. 2002;42(9):1135–1139. [DOI] [PubMed] [Google Scholar]
- 62.Houschyar KS, Ludtke R, Dobos GJ, et al. Effects of phlebotomy-induced reduction of body iron stores on metabolic syndrome: results from a randomized clinical trial. BMC medicine. 2012;10:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Duffy SJ, Biegelsen ES, Holbrook M, et al. Iron chelation improves endothelial function patients with coronary artery disease. Circulation. 2001;103(23):2799–2804. [DOI] [PubMed] [Google Scholar]
- 64.Gill D, Del Greco MF, Walker AP, Srai SKS, Laffan MA, Minelli C. The Effect of iron Status on Risk of Coronary Artery Disease: A Mendelian Randomization Study-Brief Report. Arterioscler Thromb Vasc Biol. 2017;37(9):1788–1792. [DOI] [PubMed] [Google Scholar]
- 65.Zacharski LR, Chow BK, Howes PS, et al. Reduction of iron stores and cardiovascular outcomes in patients with peripheral arterial disease: a randomized controlled trial. Jama. 2007;297(6):603–610. [DOI] [PubMed] [Google Scholar]
- 66.Munoz-Bravo C, Gutierrez-Bedmar M, Gomez-Aracena J, Garci Rodriguez A, Navajas JF. Iron: protector or risk factor for cardiovascular disease? Still controversial. Nutrients. 2013;5(7):2384–2404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kim KS, Son HG, Hong NS, Lee DH. Associations of serum ferritin and transferrin % saturation with all-cause, cancer, and cardiovascular disease mortality: Third National Health and Nutrition Examination Survey follow-up study. Journal of preventive medicine and public health = Yebang Uihakhoe chi. 2012;45(3):196–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vinchi F, Porto G, Simmelbauer A, et al. Atherosclerosis is aggravated by iron overload and ameliorated by dietary and pharmacological iron restriction. European heart journal 2019. [DOI] [PubMed] [Google Scholar]
- 69.Lee TS, Shiao MS, Pan CC, Chau LY. Iron-deficient diet reduces atherosclerotic lesions in apoE-deficient mice. Circulation. 1999;99(9):1222–1229. [DOI] [PubMed] [Google Scholar]
- 70.Zhang WJ, Wei H, Frei B. The iron chelator, desferrioxamine, reduces inflammation and atherosclerotic lesion development in experimental mice. Exp Biol Mr. (May wood) 2010;235(5):633–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hu X, Cai X, Ma R, Fu W, Zhan C, Du X Iron-load exacerbates the severity of atherosclerosis via inducing inflammation and enhancing the glycolysis in macrophages. j cell Physiol. 2019;29(10):28518. [DOI] [PubMed] [Google Scholar]
- 72.Kirk EA, Heinecke JW, LeBoeuf RC. Iron overload diminishes atherosclerosis in apoE-deficient mice. J Clin Invest. 2001;107(12):1545–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kautz L, Gabayan V, Wang X, et al. Testing the iron hypothesis in a mouse model of atherosclerosis. Cell Rep. 2013;5(5):1436–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ellervik C, Tybjaerg-Hansen A, Grande P, Appleyard M, Nordestgaard BG. Hereditary hemochromatosis and risk of ischemic heart disease: prospective study and a case-control study. Circulation. 2005;112(2):185–193. [DOI] [PubMed] [Google Scholar]
- 75.Sullivan JL. Do hemochromatosis mutations protect against iron-mediated atherogenesis? Circ Cardiovasc Genet. 2009;2(6):652–657. [DOI] [PubMed] [Google Scholar]
- 76.Miller M, Hutchins GM. Hemochromatosis, multiorgan hemosiderosis, and coronary artery disease. Jama. 1994;272(3):231–233. [PubMed] [Google Scholar]
- 77.Valenti L, Dongiovanni P, Motta BM, et al. Serum hepcidin and macrophage iron correlate with MCP-1 release and vascular damage in patients with metabolic syndrome alterations. Arterioscler Thromb Vasc Biol. 2011;31(3):683–690. [DOI] [PubMed] [Google Scholar]
- 78.Sullivan JL, Zacharski LR. Hereditary haemochromatosis and the hypothesis that iron depletion protects against ischemic heart disease. Eur J Clin Invest. 2001;31(5):375–377. [DOI] [PubMed] [Google Scholar]
- 79.Leitinger N, Schulman IG. Phenotypic polarization of macrophages in atherosclerosis. Arterioscler Thromb Vasc Biol. 2013;33(6):1120–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Moore KJ, Sheedy FJ, Fisher EA. Macrophages in atherosclerosis: a dynamic balance. Nat Rev Immunol. 2013;13(10):709–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stoger JL, Gijbels MJ, van der Velden S, et al. Distribution of macrophage polarization marker in human atherosclerosis. Atherosclerosis. 2012;225(2):461–468. [DOI] [PubMed] [Google Scholar]
- 82.Agoro R, Mura C. Inflammation-induced up-regulation of hepcidin and down regulation of ferroportin transcription are dependent on macrophage polarization. Blood cells, molecules & diseases. 2016;61:16–25. [DOI] [PubMed] [Google Scholar]
- 83.Corna G, Campana L, Pignatti E, et al. Polarization dictates iron handling by inflammatory and alternatively activated macrophages. Haematologica. 2010;95(11):1814–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kraml P. The role of iron in the pathogenesis of atherosclerosis. Physio res. 2017;66(Supplementum 1):S55–S67. [DOI] [PubMed] [Google Scholar]
- 85.Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiological reviews. 2004;84(3):767–801. [DOI] [PubMed] [Google Scholar]
- 86.Ley K, Miller YI, Hedrick CC. Monocyte and macrophage, dynamics during atherogenesis. Arterioscler Thromb Vasc Biol. 2011;31(7):1506–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gordon S Alternative activation of macropha.es. Na Rev Immunol. 2003;3(1):23–35. [DOI] [PubMed] [Google Scholar]
- 88.Medbury HJ, James V, Ngo J, et al. Differing association of macrophage subsets with atherosclerotic plaque stability. Int Angiol. 2013;32(1):74–84. [PubMed] [Google Scholar]
- 89.Hanna RN, Shaked I, Hubbeling HG, et al. NR4A1 (Nur77) deletion polarizes macrophages toward an inflammatory phenotype and increases atherosclerosis. Circ Res. 2012;110(3):416–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Recalcati S, Locati M, Marini A, et al. Differential regulation of iron homeostasis during human macrophage polarized activation. Eur J Immunol. 2010;40(3):824–835. [DOI] [PubMed] [Google Scholar]
- 91.Ferris CD, Jaffrey SR, Sawa A, et al. Haem oxygenase-1 prevents cell death by regulating cellular iron. Nat Cell Biol. 1999;1(3):152–157. [DOI] [PubMed] [Google Scholar]
- 92.Cairo G, Recalcati S, Mantovani A, Locati M. Iron trafficking and metabolism in macrophages: contribution to the polarized phenotype. Trends Immunol. 201132(6)241–247. [DOI] [PubMed] [Google Scholar]
- 93.Jetten N, Verbruggen S, Gijbels MJ, Post MJ, De Winther MP, Donners MM. Anti-inflammatory M2, but not pro-inflammatory M1 macrophages promote angiogenesis in vivo. Angiogenesis. 2014;17(1):109–118. [DOI] [PubMed] [Google Scholar]
- 94.Tabas I Macrophage death and defective inflammation resolution in atherosclerosis. Nat Rev Immunol. 2010;10(1):36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zizzo G, Hiliard BA, Monestier M, Cohen PL. Efficient clearance of early apoptotic cells by human macrophages requires M2c polarization and MerTK education. J Immunol. 2012;189(7):3508–3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tabas I. Consequences and therapeutic implications of macrophage apoptosis in atherosclerosis: the importance of lesion stage and phagocytic efficiency. Arterioscler Thromb Vasc Biol. 2005;25(11):2255–2264. [DOI] [PubMed] [Google Scholar]
- 97.Finn AV, Nakano M, Polavarapu R, et al. Hemoglobin directs macrophage differentiation and prevents foam cell formation in human atherosclerotic plaques. J Am Coll Cardiol. 2012;59(2):166–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nielsen MJ, Moller HJ, Moestrup SK. Hemoglobin and heme scavenger receptors. Antioxid Redox Signal. 2010;12(2):261–273. [DOI] [PubMed] [Google Scholar]
- 99.Guo L, Harari E, Virmani R, Finn AV. Linking Hemorrhage, Angiogenesis, Macrophages, and Iron Metabolism in Atherosclerotic Vascular Diseases. Arterioscler Thromb Vasc Biol. 2017;37(4):e33–e39. [DOI] [PubMed] [Google Scholar]
- 100.Parma L, Baganha F, Quax PHA, de Vries MR. Plaque angiogenesis and intraplaque hemorrhage in atherosclerosis. Eur J Pharmacol. 2017;816:107–115. [DOI] [PubMed] [Google Scholar]
- 101.Landis RC, Philippidis P, Domin J, Boyle JJ, Haskard DO. Haptoglobin Genotype-Dependent Anti-Inflammatory Signaling in CD163(+) Macrophages. Int J Inflam. 2013;2013:980327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Philippidis P, Mason JC, Evans BJ, et al. Hemoglobin scavenger receptor CD163 mediates interleukin-10 release and heme oxygenase-1 synthesis: antiinflammatory monocyte-macrophage responses in vitro in resolving skin blisters in vivo, and after cardiopulmonary bypass surgery. Circ Res. 2004;94(1):119–126. [DOI] [PubMed] [Google Scholar]
- 103.Bories G, Colin S, Vanhoutte J, et al. Liver X receptor activation stimulate iron export in human alternative macrophages. Circ Res. 2013;113(11):1196–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Harada N, Kanayama M, Maruyama A, et al. Nrf2 regulates ferroportin 1-mediated iron efflux and counteracts lipopolysaccharide-induced ferroportin 1 mRNA suppression in macrophage. Arch Biochem Biophys. 2011;508(1):101–109. [DOI] [PubMed] [Google Scholar]
- 105.Guo L, Akahori H, Harari E, et al. CD163+ macrophages promote angiogenesis and vascular permeability accompanied by inflammation in atherosclerosis. J Clin Invest. 2018;128(3):1106–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pourcet B, Staels B. Alternative macrophages in therosclerosis: not always protective! J Clin Invest. 2018;128(3):910–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Porcheray F, Viaud S, Rimaniol AC, et al. Macrophage activation switching: an asset for the resolution of inflammation. Clin Exp Immunol. 2005;142(3):481–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lee S, Huen S, Nishio H, et al. Distinct macrophage phenotypes contribute to kidney injury and repair. J Am Soc Nephrol. 2011;22(2):317–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Feig JE, Rong JX, Shamir R, et al. HDL promotes rapid atherosclerosis regression in mice and alters inflammatory properties of plaque monocyte-derived cells. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(17):7166–7171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Habib A, Finn AV. The role of iron metabolism as a mediator of macrophage inflammation and lipid handling in atherosclerosis. Frontiers in pharmacology. 2014;5:195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jung M, Mertens C Brun B. Macrophage iron homeostasis and polarization in the context of cancer. Immunobiology. 2015;220(2): 295–304. [DOI] [PubMed] [Google Scholar]
- 112.Recalcati S, Locati M Gammella E, Invernizzi P, Cairo G. Iron levels in polarized macrophages: regulation of immunity and autoimmunity. Autoimmun Rev. 2012;11(12):883–889. [DOI] [PubMed] [Google Scholar]
- 113.Pagani A, Na A, Corna G, et al. Low hepcidin accounts for the proinflammatory status associated with iron deficiency. Blood. 2011;118(3):736–746. [DOI] [PubMed] [Google Scholar]
- 114.Agoro R, Taleb M, Quesniaux VFJ, Mura C. Cell iron status influences macrophage polarization. PLoS One. 2018;13(5):e0196921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sindrilaru A, Peters T, Wieschalka S, et al. An unrestrained proinflammatory M1 macrophage population induced by iron impairs wound healing in humans and mice. J Clin Invest. 2011;121(3):985–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hoeft K, Bloch DB, Graw JA, Malhotra R, Ichinose F, Bagchi A. Iron Loading Exaggerates the inflammatory Response to the Toll-like Receptor 4 Ligand Lipopolysaccharide by Altering Mitochondrial Homeostasis. Anesthesiology. 2017;127(1):121–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kroner A, Greenhalgh AD, Zarruk JG, Passos Dos Santos R, Gaestel M, David S. TNF and increased intracellular iron alter macrophage polarization to a detrimental M1 phenotype in the injured spinal cord. Neuron. 2014;83(5):1098–1116. [DOI] [PubMed] [Google Scholar]
- 118.Zanganeh S, Hutter G, Spitler R, et al. Iron oxide nanoparticles inhibit tumour growth by inducing pro-inflammatory macrophage polarization in tumour tissues. Nature nanotechnology. 2016;11(11):986–994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Malhotra R, Wunderer F, Barnes HJ, et al. Hepcidin Deficiency Protects Again t Atherosclerosis. Arterioscler Thromb Vasc Biol. 2019;39(2):178–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhou Y, Que KT, Zhang Z, et al. Iron overloaded polarizes macrophage to proinflammation phenotype through ROS/acetyl-p53 pathway. Cancer medicine. 2018;7(8):4012–4022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wen Y, Leake DS. Low density lipoprotein undergoes oxidation within lysosomes in cells. Circ Res. 2007;100(9):1337–1343. [DOI] [PubMed] [Google Scholar]
- 122.Zhang Z, Zhang F, An P, et al. Ferroportin1 deficiency in mouse macophages impairs iron homeostasis and inflammatory responses. Blood. 2011;118(7):1912–1922. [DOI] [PubMed] [Google Scholar]
- 123.Recalcati S, Gammella E, Buratti P, et al. Macrorhage ferrportin is essential for stromal cell proliferation in wound healing. Haematologica. 2019;104(1):47–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wang L, Johnson EE, Shi HN, Walker WA, Wessling-Resnick M, Cherayil BJ. Attenuated inflammatory responses in hemochromatosis reveal a role for iron in the regulation of macrophage cytokine translation. J Immunol. 2008;181(4):2723–2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bayeva M, Khechaduri A, Puig S, et al. mToR regulates cellular iron homeostasis through tristetraprolin. Cell Metab. 2012;16(5):645–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Recalcati S, Gammella E, Cairo G. Ironing out Macrophage Immunometabolism. Pharmaceuticals (Basel). 2019;12(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Brooks SA, Blackshear PJ. Tristetraprolin (TTP): interactions with mRNA and proteins, and current thoughts on mechanisms of action. Biochim Biophys Acta. 2013;1829(6–7):666–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Malhotra R, Burke MF, Martyn T, et al. Inhibition of bone morphogenetic protein signal transduction prevents the medial vascular calcification associated with matrix Gla protein deficiency. PLoS One. 2015;10(1):e0117098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Derwall M, Malhotra P Lai CS, et al. Inhibition of bone morphogenetic protein signaling reduces vascular calcification and atherosclerosis. Arteriosclerosis Thromb Vasc Biol. 2012;32(3):613–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Saeed O, Otsuka F, Polavarapu R, et al. Pharmacological suppression of hepcidin increases macrophage cholesterol efflux and reduces foam cell formation and atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32(2):299–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yao Y, Bennett BJ, Wang X, et al. Inhibition of bone morphogenetic proteins protects against atherosclerosis and vascular calcification. Circ Res. 2010;107(4):485–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Nigwekar SU, Bloch DB, Nazarian RM, et al. Vitamin K-Dependent Carboxylation of Matrix Gla Protein Influences the Risk of Calciphylaxis. J Am Soc Nephrol. 2017;28(6):1717–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Nigwekar SU, Jiramongkolchai P, Wunderer F, et al. Increased Bone Morphogenetic Protein Signaling in the Cutaneous Vasculature of Patients with Calciphylaxis. Am J Nephrol. 2017;46(5):429–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sharma N, Lu Y, Zhou G, et al. Myeloid Kruppel-like factor 4 deficiency augments atherogen is in ApoE−/− mice--brief report. Arterioscler Thromb Vasc Biol. 2012;32(12):2836–2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wan N, Gree J, Wan Z, et al. Bone Morphogenetic Protein (BMP) signaling in development and human diseases. Genes Dis. 2014;1(1):87–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Getz GS, Reardon CA. Do the Apoe−/− and Ldlr−/− Mice Yield the Same Insight on Atherogenesis? Arterioscler Thromb Vasc Biol. 2016;36(9):1734–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Baitsch D, Bock HH, Engel T, et al. Apolipoprotein E induces antiinflammatory phenotype in macrophages. Arterioscler Thromb Vasc Biol. 2011;31(5):1160–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Altamura S, Kessler R, Grone HJ, et al. Resistance of ferroportin to hepcidin binding causes exocrine pancreatic failure and fatal iron overload. Cell Metab. 2014;20(2):359–367. [DOI] [PubMed] [Google Scholar]
- 139.Cornelissen A, Guo L, Sakamoto A, Virmani R, Finn AV. New insights, into the role of iron in inflammation and atherosclerosis. EBioMedicine. 2019;47:598–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Demirag MD, Haznedaroglu S, Sancak B, et al. Circulating hepcidin in the crossroads of anemia and inflammation associated with rheumatoid arthritis. Intern Med. 2009;48(6):421–426 [DOI] [PubMed] [Google Scholar]
- 141.Lewis GD, Malhotra R, Hernandez AF, et al. Effect of Oral Iron, Repletion on Exercise Capacity in Patients With Heart Failure With Reduced Ejection Fraction and Iron Deficiency: The IRONOUT HF Randomized Clinical Trial. Jama. 2017;317(19):1958–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Santos-Silva A, Ribeiro S, Reis F, Belo L. Hepcidin in chronic kidney disease anemia. Vitam Horm. 2019;110:243–264. [DOI] [PubMed] [Google Scholar]
- 143.Zimmermann A, Zimmermann T, Schattenberg J, et al. Alterations in lipid, carbohydrate and iron metabolism in patients with non-alcoholic steatohepatitis (NASH) and metabolic syndrome. Eur J Intern Med. 2011;22(3):305–310. [DOI] [PubMed] [Google Scholar]
- 144.Senates E, Yilmaz Y, Colak Y, et al. Serum levels of hepcidin in patients with biopsy-proven nonalcoholic fatty liver disease. Metab Syndr Relat Disord. 2011;9(4):287–290 [DOI] [PubMed] [Google Scholar]
- 145.Abdel-Khalek MA, El-Barbary AM, Essa SA, Ghobashi AS. Serum hepcidin: a direct link between anemia of inflammation and coronary artery atherosclerosis in patient with rheumatoid arthritis. The Journal of rheumatology. 2011;38(10):2153–2159. [DOI] [PubMed] [Google Scholar]
- 146.Valenti L, Swinkels DW Burdick L, et al. Serum ferritin levels are associated with vascular damage in patients with nonalcoholic fatty liver disease. Nutrition, metabolism, and cardiovascular diseases : NMCD. 2011;21(8):568–575. [DOI] [PubMed] [Google Scholar]
- 147.Kuragano T, Itoh K, shimonaka Y, et al. Hepcidin as well as TNF-alpha are significant predictors of arterial stiffness in patients on maintenance hemodialysis. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2011;26(8):2663–2667. [DOI] [PubMed] [Google Scholar]
- 148.van der Weerd NC, Grooteman MP, Bots ML, et al. Hepcidin-25 is related to cardiovascular events in chronic haemodialysis patients. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2013;28(12):3062–3071. [DOI] [PubMed] [Google Scholar]
- 149.Galesloot TE, Holewijn S, Kiemeney LA, de Graaf J, Vermeulen SH, Swinkels DW. Serum hepcidin is associated with presence of plaque in postmenopausal women of a general population. Arterioscler Thromb Vasc Biol. 2014;34(2):446–456. [DOI] [PubMed] [Google Scholar]
- 150.Aday AW, Ridker PM. Antiinflammatory Therapy in Clinical Care: The CANTOS Trial and Beyond. Front Cardiovasc Med. 2018;5(62). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ridker PM, Danielson E, Fonseca FA, et al. Reduction in C-reactive protein and LDL cholesterol and cardiovascular event rates after initiation of rosuvastatin: a prospective study of the JUPITER trial. Lancet. 2009;373(9670):1175–1182. [DOI] [PubMed] [Google Scholar]
- 152.Sebastian G, Wilkinso N, Pantopoulo K. Pharmacological Targeting of the Hepcidin/Ferroportin Axis. Frontiers in pharmacology. 2016;7(160). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Galea J, Armstrong J, Gadsdon P, Holden H, Francis SE, Holt CM. Interleukin-1 be a in ceronary arteries of patients with ischemic heart disease. Arterioscler Thromb Vasc Biol. 1996;16(8):1000–1006. [DOI] [PubMed] [Google Scholar]
- 154.Reiss AB, Siegart NM, De Leon J. Interleukin-6 in atherosclerosis: atherogenic or atheroprotective? Clinical Lipidology. 2017;12(1):14–23. [Google Scholar]
- 155.Sarwar N, Butterworth AS, Freitag DF, et al. Interleukin-6 receptor pathways in coronary heart disease: a collaborative meta-analysis of 82 studies. Lancet. 2012;379(9822):1205–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Swerdlow DI, Holmes MV, Kuchenbaecker KB, et al. The interleukin-6 receptor as a target for prevention of coronary heart disease: a mendelian randomisation analysis. Lancet. 2012;379(9822):1214–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Yudkin JS, Kumari M, Humphries SE, Mohamed-Ali V. Inflammation obesity, stress and coronary heart disease: is interleukin-6 the link? Atherosclerosis. 2000;148(2):209–214. [DOI] [PubMed] [Google Scholar]
- 158.Kleveland O, Kunszt G, Bratlie M, et al. Effect of a single dose of the interleukin-6 receptor antagonist tocilizumab on inflammation and troponin T release in patients with non-ST-elevation myocardial infarction: a double-blind, randomized, placebo-controlled phase 2 trial. European heart journal. 2016;37(30):2406–2413. [DOI] [PubMed] [Google Scholar]
- 159.Holte E, Kleveland O, Ueland T, et al. Effect of interleukin-6 inhibition on coronary microvascular and endothelial function in myocardial infarction. Heart. 2017;103(19):1521–152, [DOI] [PubMed] [Google Scholar]
- 160.Danesh J, Kaptoge S, Mann AG, et al. Long-term interleukin-6 levels and subsequent risk of coronary heart disease: two new prospective studies and a systematic review. PLoS Med 2008;5(4):0050078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Markousis-Mavrogenis G, Tromp J, Ouwerkerk W, et al. The clinical significance of interleukin-6 in heart failure: results from the BIOSTAT-CHF study. Eur J Heart Fail. 2019;21(8):965–973. [DOI] [PubMed] [Google Scholar]
- 162.Ridker PM, Everett BM, Pradhan A, et al. Low-Dose Methotrexate for the Prevention of Atherosclerotic Events. N Engl J Med. 2019;380(8):752–762. [DOI] [PMC free article] [PubMed] [Google Scholar]