Abstract
Many aspects of cancer can be explained utilizing well-defined ecological principles. Applying these principles to cancer, cancer cells are an invasive species to a healthy organ ecosystem. In their capacity as ecosystem engineers, cancer cells release cytokines that recruit monocytes to the tumor and polarize them to M2-like protumor macrophages. Macrophages, recruited by the cancer cells, act as a secondary invasive species. The ecosystem engineering functions of M2-macrophages in turn support and stimulate cancer cell survival and proliferation. The cooperative ecosystem engineering of both the primary invasive species of the cancer cell and the secondary invasive species of the M2-macrophage thus creates a vicious cycle of tumor promotion. Targeting a specific aspect of this tumor-promoting ecosystem engineering, such as blocking efferocytosis by M2-like macrophages, may improve the response to standard-of-care anticancer therapies. This strategy has the potential to redirect cooperative protumor ecosystem engineering toward an antitumor ecosystem engineering strategy.
Keywords: cancer ecology, ecosystem engineer, invasive species, macrophage, efferocytosis
Introduction
In 2019, it is estimated that about 1,700,000 new cases of cancer will be diagnosed, and there will be approximately 600,000 cancer-related deaths in the United States.1 New paradigms for understanding cancer and cancer lethality are urgently needed. Many aspects of tumor biology are analogous to well-established principles in ecology (Table 1). A tumor itself is not simply an isolated mass of cancer cells but contains many different cell types or “species” that influence each other and the life history of the cancer and therefore the patient. This tumor microenvironment parallels an ecosystem with many interacting cell types and components. As tumors grow, they have substantial impacts on their surrounding local ecosystem and, eventually, the entire human body. Mechanisms including dysregulated cell signaling, cellular proliferation, and recruitment of tumor-promoting host cells lead to ecosystem and ultimately biosphere collapse, resulting in the death of the patient. By applying ecological principles to understand the processes of cancer development and progression, we present a novel strategy to treat cancer: disrupting tumor-promoting ecosystem engineering.
Table 1.
Ecological Definitions in Cancer Biology.
| Term | Definition | Ecology Example | Cancer Biology Example |
|---|---|---|---|
| Ecosystem | Group of interacting species and their physical habitat | Forest, beaver lake | Healthy organ, tumor |
| Habitat | Physical environment of an ecosystem | Soil, water | Blood vessels, extracellular matrix |
| Species | Group of closely related organisms with similar traits | Beaver, kudzu | Cancer cells, macrophages, T cells |
| Invasive species | Non-native species that spreads and cause destruction to an ecosystem | Myrica faya, kudzu | Cancer cells |
| Secondary invasive species | Invasive species that follows and depends on another invasive species | Earthworms | M2-like macrophages |
| Ecosystem engineer | Species that exerts substantial changes to an ecosystem through modifying their habitat | Beaver, kudzu | Cancer cells, M2-like macrophages |
| Biosphere | Global system that is the sum of all ecosystems | Earth | Patient |
Cancer as an Invasive Ecosystem Engineer
An invasive species is a non-native species that enters an ecosystem. Upon entering the new habitat, these invaders eventually become a destructive species of the ecosystem. These invaders can be recruited to the ecosystem by a number of factors including a need for resources, population growth, or desirable characteristics of the ecosystem to be invaded.2-4 Invasive species disrupt homeostasis in the ecosystem, altering the community structure and competing with native species for nutrients and space, ultimately harming the native species. After a lag period, successful invaders have high reproduction and have the ability to survive in a heterogeneous changing environment.5,6 For example, the European green crab is a native species along the coasts of Europe but is invasive to coasts around the world such as those along the United States and South America. These invasive crabs prey on shellfish and oysters, causing destruction to the ecosystem by competing with native species such as the Dungeness crab and American lobster for resources.7-10
Many invasive species are also ecosystem engineers that exert substantial changes to an ecosystem. Ecosystem engineers modify their habitat and by doing so affect other species and the entire adaptive landscape. Allogenic engineers change the ecosystem by mechanically altering the habitat, while autogenic engineers modify their habitat simply by modifying themselves. Beavers are perhaps the most well-known invasive allogenic ecosystem engineer. When beavers build a dam, they exert a substantial and catastrophic influence on the habitat. Both the tree clear-cutting and the resulting redirected water flow have large effects on vegetation and wildlife in the surrounding areas. An autogenic engineer, the kudzu vine is an invasive species in parts of Asia and the Pacific islands as well as the southeastern United States: “the vine that ate the South.” Upon invasion, the kudzu vine spreads and overcomes the native habitat, outcompeting other plants for resources.11 By growing as a vine on native trees and man-made structures, the kudzu vine blocks sunlight and kills the trees it climbs while also introducing new habitats for small animals, effectively destroying the native ecosystem.
During tumorigenesis, cancer cells are invasive ecosystem engineers.12,13 Many of the “Hallmarks of Cancer” such as high proliferative rate, evading predation, and resisting death are parallel to the essential characteristics of an invasive species: rapid growth and reproduction, phenotypic plasticity, and high tolerance for different habitats.14,15 Once the cancer cells are established, long before clinical detection, they engage in ecosystem engineering to modify their environment to favor conditions for their own growth and survival and outcompete native species. As autogenic engineers, the cancer cells proliferate and the tumor grows. For this rapid growth, they consume high amounts of oxygen and nutrients leading to hypoxia and nutrient poverty, generating a “cancer swamp.”12,16,17 In addition, cancer cells are allogenic engineers that secrete matrix metalloproteinases (MMPs) that enzymatically break down the extracellular matrix and secrete cytokines and chemokines that induce angiogenesis and recruit other species to the invaded and engineered ecosystem: the tumor microenvironment. Through their autogenic and allogenic engineering, cancer cells disrupt the native ecosystem of the healthy organ.
Secondary Invasion of the Tumor Ecosystem
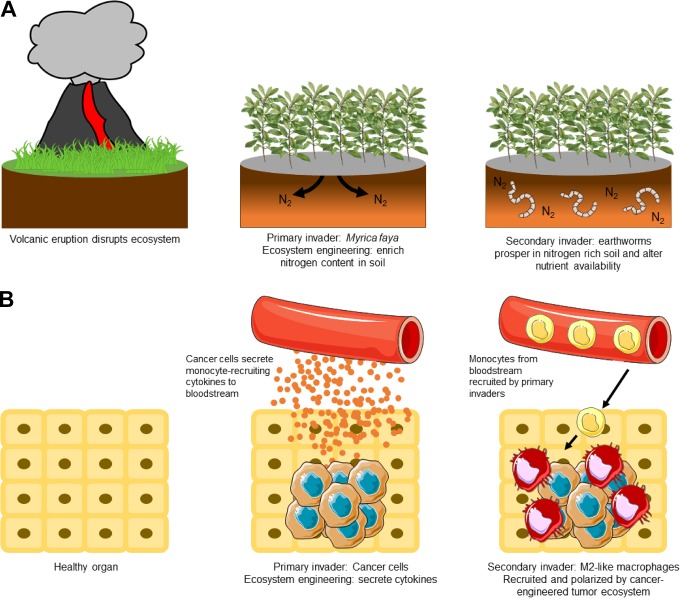
Secondary invasive species follow and depend on invasion of a primary invasive species. These secondary invaders are drawn to the ecosystem due to the changes made by the primary invaders. For example, the Myrica faya tree is a primary invasive species in areas of Hawaii following volcanic activity (Figure 1A). These trees enrich nitrogen content in the soil, allowing certain earthworm species to prosper. In this case, increased nitrogen in the soil due to primary invasion of the Myrica faya trees promotes growth of multiple secondary invasive species, including earthworms.18-20 Together, these invasive species eventually overcome the native ecosystem and generate a new ecosystem in which they all prosper, excluding many of the native species as they struggle to survive in the engineered ecosystem.
Figure 1.
Cancer cells and M2 macrophages as invasive ecosystem engineers. A, Following volcanic eruptions in Hawaii, Myrica faya invade the recovering ecosystem. Myrica faya impact the nitrogen cycle and enriches nitrogen content in the soil through nitrogen fixation, an ecosystem engineering trait. Earthworms, a secondary invasive species, are attracted to the soil with increased nitrogen content. In turn, these earthworms further disrupt the native ecosystem and promote the invasion and success of Myrica faya, such as by serving as an attractive food source for feral pigs. B, This process parallels cancer cell and macrophage invasion. Cancer cells are a primary invasive species that create a protumor environment through ecosystem engineering. Among other engineering functions, cancer cells secrete cytokines that recruit monocytes from the blood stream. Moreover, the cancer cells also secrete M2-polarizing cytokines, promoting the differentiation of monocytes into secondary invasive M2-like macrophages. M2-like macrophages exhibit many protumor ecosystem-engineering functions, thus promoting the success of the cancer cells.
In cancer, one of the most common and destructive secondary invaders is protumor M2-like macrophages, recruited to the tumor microenvironment by the cancer cells and the generation of the cancer swamp (Figure 1B). Macrophages are innate immune cells with roles in antigen presentation, phagocytosis, and modifying different types of immune responses.21,22 In cancer, these cells can play tumor-promoting roles and high macrophage infiltrate correlates with poor prognosis.23-25 After differentiation from their monocyte precursor, macrophages polarize toward an M1-like or M2-like phenotype. These phenotypes are highly plastic and can change when the macrophage encounters different stimuli. While M1 and M2 are simplified subtypes used throughout the field, macrophage phenotypes and functions exist on a continuous spectrum and have both M1-like and M2-like characteristics. M1-like macrophages are part of the Th1 immune response and exert an antitumor effect. In contrast, M2-like macrophages are involved in the Th2 immune response and have protumor roles.
Allogenic engineering by cancer cells, specifically secretion of the cytokines macrophage colony stimulating factor and C-C motif chemokine ligand 2 (CCL2), promotes trafficking of monocytes to the tumor.26-29 After recruitment and intravasation to the tumor, monocytes differentiate into macrophages. Cytokines such as interleukin (IL)-4 and IL-13 released by cancer cells and other immune cells in the ecosystem polarize these recruited macrophages, as well as tissue-resident macrophages, to the M2 tumor-promoting subtype. These M2-like macrophages further disrupt the healthy organ ecosystem and promote cancer growth.
Cooperative Invasive Ecosystem Engineers
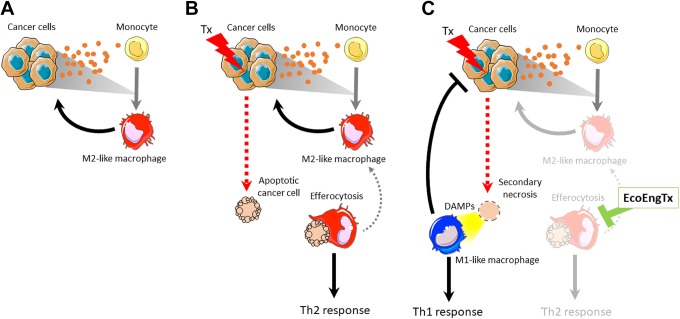
Cancer cells and M2-like macrophages occupy a substantial part of the tumor habitat and have marked influence on the tumor ecosystem as a whole.30-32 Cancer cells and macrophages support each other by secreting factors that stimulate the others’ growth, survival, and, importantly, support tumor-promoting ecosystem engineering (Figure 2A). The ecosystem engineering of the cancer cells, the primary invader population, recruits secondary invasion of M2 macrophages by releasing cytokines that recruit and polarize M2 macrophages. In turn, M2 macrophage ecosystem engineering also supports the cancer cell population. For example, M2 macrophages alter resource availability by secreting growth factors such as epidermal growth factor and hepatocyte growth factor that fuel cancer cell proliferation.33,34 M2 macrophages impact noncancer cell components of the tumor ecosystem by altering the physical habitat and community structure. They also release MMPs that induce extracellular matrix remodeling, physically altering abiotic components of the habitat to permit cancer cell movement.
Figure 2.
Targeting protumor ecosystem engineering by M2-like macrophages. A, Cooperative protumor ecosystem engineering of cancer cells and M2-like macrophages: Cancer cells release cytokines to the bloodstream through ecosystem engineering. Some of these secreted cytokines recruit monocytes to the tumor and differentiate them to M2-like macrophages. These macrophages support the cancer cells through ecosystem engineering. B, One ecosystem engineering function of M2-macrophages is efferocytosis, the phagocytosis of apoptotic cells. This effect is increased following cancer cell apoptosis due to cytotoxic therapies (Tx). By eliminating apoptotic cells from the ecosystem, M2-like macrophages limit the antitumor Th1 response (allogenic engineering) and support the Th2 response through supporting the M2 macrophage phenotype (autogenic engineering). C, Targeting the ecosystem engineering function (EcoEngTx) of efferocytosis will have an antitumor affect by permitting secondary necrosis of the apoptotic cell and the release of DAMPs. Consequently, this redirects the tumor ecosystem engineering toward an antitumor Th1 immune response and limiting the M2-macrophage protumor influences, weakening the cooperative protumor engineering of cancer cells and M2-macrophages. DAMPs indicates danger-associated molecular patterns.
In addition to interacting with cancer cells and noncellular components, M2-like macrophages also interact with many other cell types in the tumor ecosystem. M2 macrophages release Th2 cytokines that suppress that antitumor immune response. For example, M2 macrophages release IL-10 leading to immune suppression via downregulating antigen presentation on dendritic cells and macrophages.35 Tumor-associated macrophages also secrete CCL20 and CCL22 to recruit regulatory T cells to the tumor, and M2-like macrophages secrete vascular endothelial growth factor promote angiogenesis.36-39 M2-like tumor-associated macrophages have interactions with a myriad of other cell types including cancer-associated fibroblasts, natural killer cells, and neutrophils have been described in the literature and were recently reviewed.40,41 Through these mechanisms, among others, M2-like macrophages and cancer cells cooperatively engineer the ecosystem to favor tumor growth.
Phagocytosis of apoptotic cells, or efferocytosis, is one major tumor-promoting ecosystem engineering function of M2-like macrophages. Efferocytosis fundamentally alters the tumor environment by clearing apoptotic cells and preventing secondary necrosis, thus further promoting a protumor Th2 response and restricting the antitumor Th1 immune response (Figure 2B). During secondary necrosis, cells lose membrane integrity resulting in their intracellular contents leaking into the extracellular space. Some of these contents, such as adenosine triphosphate, DNA, and high mobility group box-1, are danger-associated molecular patterns that recruit antitumor M1-like macrophages and other Th1 immune cells (Figure 2C). As allogenic engineers themselves, M1 macrophages present tumor antigens on MHC class II and release Th1 cytokines to stimulate immune cells to help generate an antitumor immune response. M1 macrophages can also directly kill viable cancer cells through phagocytosis. Further, released intercellular contents can serve as antigens to generate an antitumor immune response. Therefore, secondary necrosis overall favors an antitumor Th1 immune response. In addition to preventing secondary necrosis, in vitro evidence suggests that efferocytosing M2 macrophages directly promote increased growth of cancer cells.42 This allogenic engineering function also fuels the M2-macrophage phenotype, thus maintaining the strong autogenic engineering that the tumor ecosystem relies on. After efferocytosis, the macrophage expresses high M2-like characteristics (eg, cytokines IL-4, IL-10, TGF-β), resulting in the maintenance of protumor M2 macrophage effects on the ecosystem.43 Altogether, ecosystem engineering by efferocytosis promotes tumor growth by restricting the antitumor Th1 immune response and supporting the protumor M2 macrophage phenotype.
Novel Therapeutic Strategy to Treat Cancer by Redirecting Ecosystem Engineering
The cooperation of the invasive ecosystem engineers, cancer cells and M2-like macrophages, creates a vicious cycle of cancer progression supporting protumor macrophage engineering. We propose a novel strategy for therapeutic intervention by specifically targeting the ecosystem engineering functions at play in the tumor ecosystem. By combining this strategy with standard-of-care cancer cell-directed therapy, it may be possible to limit cooperative ecosystem engineering and promote antitumor engineering to reduce tumor burden. MerTK is a promising target to interfere with M2 macrophage protumor ecosystem engineering. MerTK is a receptor tyrosine kinase in the Axl and Tyro3 family (TAM receptors) that plays a critical role in binding apoptotic cells and promoting efferocytosis.44-48 Blocking MerTK-mediated efferocytosis may result in altered ecosystem engineering, thus restricting the cancer cell-M2 macrophage cooperative relationship.
Targeting ecosystem engineering to switch the tumor microenvironment from protumor to antitumor by targeting a specific function, rather than a player, of engineering may be a promising treatment strategy. As depicted in Figure 2C, greatest success will likely be found by combination therapy with direct anticancer treatments such as chemotherapy or radiation that engage the targeted engineering function (eg, chemotherapy induces apoptosis that engages M2-macrophages in efferocytosis). Redirecting the ecosystem engineering by targeting MerTK may block efferocytosis and allow apoptotic cells to progress to secondary necrosis. As a result, the dominant immune response will switch from Th2 to Th1. This will fundamentally change the tumor ecosystem and species it contains. In addition to MerTK, other targets on macrophages such as CSF1R, SIRPα, and IL4Rα may similarly target tumor-promoting ecosystem engineering. Overall, this redirection of ecosystem engineering, blocking the vicious cycle of cooperative cancer cell-M2 macrophage engineering, has the potential to improve the antitumor immune response, slow cancer growth, and decrease metastasis.
Acknowledgments
The authors thank Amber de Groot for providing critical feedback to the manuscript. The authors also thank members of the Pienta Lab and the Brady Urological Institute for thoughtful feedback.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Kenneth Pienta is a consultant to Cue Biopharma, Inc.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work is supported by NCI grants U54CA143803, CA163124, CA093900, and CA143055 as well as the Prostate Cancer Foundation, the Patrick C. Walsh Fund and the William and Carolyn Stutt Research Fund.
ORCID iD: Kayla V. Myers  https://orcid.org/0000-0002-0748-1092
https://orcid.org/0000-0002-0748-1092
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. [DOI] [PubMed] [Google Scholar]
- 2. Sakai AK, Allendorf FW, Holt JS, et al. The population biology of invasive species. Annu Rev Ecol Syst. 2001;32:305–332. [Google Scholar]
- 3. Lodge DM. Biological invasions—lessons for ecology. Trends Ecol Evol. 1993;8(4):133–137. [DOI] [PubMed] [Google Scholar]
- 4. Williamson MH, Brown KC. The analysis and modeling of British invasions. Philos T Roy Soc B. 1986;314(1167):505–522. [Google Scholar]
- 5. Kolar CS, Lodge DM. Progress in invasion biology: predicting invaders. Trends Ecol Evol. 2001;16(4):199–204. [DOI] [PubMed] [Google Scholar]
- 6. Crawley MJ. The population biology of invaders. Philos T Roy Soc B. 1986;314(1167):711–731. [Google Scholar]
- 7. Lafferty KD, Kuris AM. Biological control of marine pests. Ecology. 1996;77(7):1989–2000. [Google Scholar]
- 8. Miron G, Audet D, Landry T, Moriyasu M. Predation potential of the invasive green crab (Carcinus maenas) and other common predators on commercial bivalve species found on Prince Edward island. J Shellfish Res. 2005;24(2):579–586. [Google Scholar]
- 9. Williams PJ, MacSween C, Rossong M. Competition between invasive green crab (Carcinus maenas) and American lobster (Homarus americanus). New Zeal J Mar Fresh. 2009;43(1):29–33. [Google Scholar]
- 10. McDonald PS, Jensen GC, Armstrong DA. The competitive and predatory impacts of the nonindigenous crab Carcinus maenas (L.) on early benthic phase Dungeness crab cancer magister dana. J Exp Mar Biol Ecol. 2001;258(1):39–54. [DOI] [PubMed] [Google Scholar]
- 11. Forseth IN, Innis AF. Kudzu (Pueraria Montana): history, physiology, and ecology combine to make a major ecosystem threat. Crit Rev Plant Sci. 2004;23(5):401–413. [Google Scholar]
- 12. Amend SR, Pienta KJ. Ecology meets cancer biology: the cancer swamp promotes the lethal cancer phenotype. Oncotarget. 2015;6(12):9669–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen KW, Pienta KJ. Modeling invasion of metastasizing cancer cells to bone marrow utilizing ecological principles. Theor Biol Med Model. 2011;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. [DOI] [PubMed] [Google Scholar]
- 15. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. [DOI] [PubMed] [Google Scholar]
- 16. Semenza GL. Hypoxia, clonal selection, and the role of HIF-1 in tumor progression. Crit Rev Biochem Mol. 2000;35(2):71–103. [DOI] [PubMed] [Google Scholar]
- 17. Muz B, de la Puente P, Azab F, Azab AK. The role of hypoxia in cancer progression, angiogenesis, metastasis, and resistance to therapy. Hypoxia. 2015;3:83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. O’Loughlin LS, Green PT. Secondary invasion: when invasion success is contingent on other invaders altering the properties of recipient ecosystems. Ecol Evol. 2017;7(19):7628–7637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vitousek PM, Walker LR, Whiteaker LD, Mueller-Dombois D, Matson PA. Biological invasion by myrica faya alters ecosystem development in Hawaii. Science. 1987;238(4828):802–804. [DOI] [PubMed] [Google Scholar]
- 20. Walker LR, Vitousek PM. An invader alters germination and growth of a native dominant tree in Hawaii. Ecology. 1991;72(4):1449–1455. [Google Scholar]
- 21. Gordon S, Martinez-Pomares L. Physiological roles of macrophages. Pflugers Arch. 2017;469(3-4):365–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Elhelu MA. The role of macrophages in immunology. J Natl Med Assoc. 1983;75(3):314–317. [PMC free article] [PubMed] [Google Scholar]
- 23. Nonomura N, Takayama H, Nakayama M, et al. Infiltration of tumour-associated macrophages in prostate biopsy specimens is predictive of disease progression after hormonal therapy for prostate cancer. BJU Int. 2011;107(12):1918–1922. [DOI] [PubMed] [Google Scholar]
- 24. Takayama H, Nishimura K, Tsujimura A, et al. Increased infiltration of tumor associated macrophages is associated with poor prognosis of bladder carcinoma in situ after intravesical bacillus Calmette-Guerin instillation. J Urol. 2009;181(4):1894–1900. [DOI] [PubMed] [Google Scholar]
- 25. Komohara Y, Hasita H, Ohnishi K, et al. Macrophage infiltration and its prognostic relevance in clear cell renal cell carcinoma. Cancer Sci. 2011;102(7):1424–1431. [DOI] [PubMed] [Google Scholar]
- 26. Loberg RD, Ying C, Craig M, Yan L, Snyder LA, Pienta KJ. CCL2 as an important mediator of prostate cancer growth in vivo through the regulation of macrophage infiltration. Neoplasia. 2007;9(7):556–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Van Overmeire E, Stijlemans B, Heymann F, et al. M-CSF and GM-CSF receptor signaling differentially regulate monocyte maturation and macrophage polarization in the tumor microenvironment. Cancer Res. 2016;76(1):35–42. [DOI] [PubMed] [Google Scholar]
- 28. Zou K, Wang Y, Hu Y, Zheng L, Xu W, Li G. Specific tumor-derived CCL2 mediated by pyruvate kinase M2 in colorectal cancer cells contributes to macrophage recruitment in tumor microenvironment. Tumour Biol. 2017;39(3):1010428317695962. [DOI] [PubMed] [Google Scholar]
- 29. Kitamura T, Qian BZ, Soong D, et al. CCL2-induced chemokine cascade promotes breast cancer metastasis by enhancing retention of metastasis-associated macrophages. J Exp Med. 2015;212(7):1043–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zarif JC, Baena-Del Valle JA, Hicks JL, et al. Mannose receptor-positive macrophage infiltration correlates with prostate cancer onset and metastatic castration-resistant disease. Eur Urol Oncol. 2019;2(4):429–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Qi L, Yu HQ, Zhang Y, et al. IL-10 secreted by M2 macrophage promoted tumorigenesis through interaction with JAK2 in glioma. Oncotarget. 2016;7(44):71673–71685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shen L, Li H, Shi Y, et al. M2 tumour-associated macrophages contribute to tumour progression via legumain remodelling the extracellular matrix in diffuse large B cell lymphoma. Sci Rep. 2016;6:30347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dong N, Shi X, Wang S, et al. M2 macrophages mediate sorafenib resistance by secreting HGF in a feed-forward manner in hepatocellular carcinoma. Br J Cancer. 2019;121(1):22–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Goswami S, Sahai E, Wyckoff JB, et al. Macrophages promote the invasion of breast carcinoma cells via a colony-stimulating factor-1/epidermal growth factor paracrine loop. Cancer Res. 2005;65(12):5278–5283. [DOI] [PubMed] [Google Scholar]
- 35. Mittal SK, Roche PA. Suppression of antigen presentation by IL-10. Curr Opin Immunol. 2015;34:22–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Liu J, Zhang N, Li Q, et al. Tumor-associated macrophages recruit CCR6+ regulatory T cells and promote the development of colorectal cancer via enhancing CCL20 production in mice. Plos One. 2011;6(4):e19495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Curiel TJ, Coukos G, Zou L, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10(9):942–949. [DOI] [PubMed] [Google Scholar]
- 38. Kodelja V, Muller C, Tenorio S, Schebesch C, Orfanos CE, Goerdt S. Differences in angiogenic potential of classically vs alternatively activated macrophages. Immunobiology. 1997;197(5):478–493. [DOI] [PubMed] [Google Scholar]
- 39. Jetten N, Verbruggen S, Gijbels MJ, Post MJ, De Winther MPJ, Donners MMPC. Anti-inflammatory M2, but not pro-inflammatory M1 macrophages promote angiogenesis in vivo. Angiogenesis. 2014;17(1):109–118. [DOI] [PubMed] [Google Scholar]
- 40. Najafi M, Goradel NH, Farhood B, et al. Tumor microenvironment: interactions and therapy. J Cell Physiol. 2019;234(5):5700–5721. [DOI] [PubMed] [Google Scholar]
- 41. Ronca R, Van Ginderachter JA, Turtoi A. Paracrine interactions of cancer-associated fibroblasts, macrophages and endothelial cells: tumor allies and foes. Curr Opin Oncol. 2018;30(1):45–53. [DOI] [PubMed] [Google Scholar]
- 42. Jones JD, Sinder BP, Paige D, et al. Trabectedin reduces skeletal prostate cancer tumor size in association with effects on M2 macrophages and efferocytosis. Neoplasia. 2019;21(2):172–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Stanford JC, Young C, Hicks D, et al. Efferocytosis produces a prometastatic landscape during postpartum mammary gland involution. J Clin Invest. 2014;124(11):4737–4752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Myers KV, Amend SR, Pienta KJ. Targeting tyro3, Axl and MerTK (TAM receptors): implications for macrophages in the tumor microenvironment. Mol Cancer. 2019;18(1):94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zagorska A, Traves PG, Lew ED, Dransfield I, Lemke G. Diversification of TAM receptor tyrosine kinase function. Nat Immunol. 2014;15(10):920–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Scott RS, McMahon EJ, Pop SM, et al. Phagocytosis and clearance of apoptotic cells is mediated by MER. Nature. 2001;411(6834):207–211. [DOI] [PubMed] [Google Scholar]
- 47. Seitz HM, Camenisch TD, Lemke G, Earp HS, Matsushima GK. Macrophages and dendritic cells use different Axl/Mertk/Tyro3 receptors in clearance of apoptotic cells. J Immunol. 2007;178(9):5635–5642. [DOI] [PubMed] [Google Scholar]
- 48. A-Gonzalez N, Bensinger SJ, Hong C, et al. Apoptotic cells promote their own clearance and immune tolerance through activation of the nuclear receptor LXR. Immunity. 2009;31(2):245–258. [DOI] [PMC free article] [PubMed] [Google Scholar]