Short abstract
Traumatic brain injury (TBI) disrupts the complex arrangement of glia and neuronal cells in the central nervous system. Microglia, the resident immune cells, survey the cellular milieu under homeostatic conditions and play a neuroprotective role via clearance of dead cells and debris such as axons and myelin. Resting (ramified) microglia possess a distinct morphology—small rod-shaped somata with thin processes. After TBI, microglia are activated and transition into an amoeboid morphology. To delineate the spatiotemporal morphological response of microglia after TBI, we used a controlled cortical impact injury model to quantify and characterize microglia at 24 hr and 28 days after TBI in the hippocampus (H) and lateral posterior nucleus of the thalamus (LPNT). Increased numbers of microglia were observed in the H and LPNT at 28 days after controlled cortical impact, but not at 24 hr in comparison to controls. Spatially, controlled cortical impact resulted in an increase of amoeboid microglia bilaterally at 24 hr and 28 days in H and ipsilaterally in LPNT. Temporally, at 28 days, TBI resulted in a significant increase in the number of amoeboid microglia in both H and LPNT. In addition, at 28 days after injury, we observed an increase in translocator protein, a marker for activated microglia, in the ipsilateral thalamus only. TBI results in a spatiotemporal increase in amoeboid microglia in the hippocampus and the LPNT over 28 days. Delineating their spatiotemporal phenotype is critical because it can help identify therapeutic targets with appropriate therapy.
Keywords: cell death, inflammation, microglia, neuro degeneration, neuro glia, neuro immunity, neuro repair, TBI, therapies
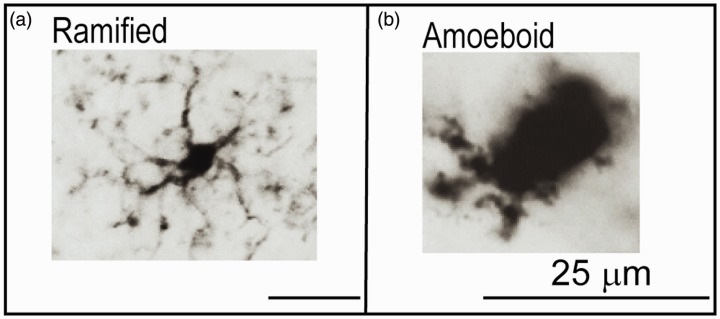
Traumatic brain injury (TBI) affects more than 1.5 million people in the United States each year with a mortality rate near or above 50,000 per year (Coronado et al., 2011; Thurman et al., 1999). TBI causes a prolonged secondary inflammatory response within the central nervous system (CNS) that leads to neurological deficits, both motor and cognitive, beyond that caused by the primary injury (Ramlackhansingh et al., 2011; Sandhir et al., 2008; Smith, 2010). Central to the secondary inflammatory response after TBI are the microglia, resident immune cells within the CNS parenchyma (Nakajima and Kohsaka, 2001). Microglia function to promote learning dependent synapse formation, axonal regeneration, and remove defunct axon terminals (Graeber, 2010; Salter and Beggs, 2014). Under homeostasis, microglia are highly mobile and provide continuous surveillance of their cellular milieu (Davalos et al., 2005; Nimmerjahn et al., 2005). These resting or ramified microglia possess a distinct morphology—small, relatively stable rod-shaped somata with thin ramified withdrawing processes (Figure 1A; Nimmerjahn et al., 2005).
Figure 1.
The Different Morphological Phenotypes of Microglia Using IBA1. Panel A: The ramified, nonactivated microglia (hippocampus, control) has a small cell body, with extensive, fine branching processes. Panel B: The activated, amoeboid microglia (hippocampus, CCI) has very short processes and a large cell body.
After a CNS injury, microglia undergo considerable remodeling. They retract their processes and adopt an amoeboid morphology (Figure 1B; Bedi, Walker, et al., 2013; Bedi et al., 2018; Csuka et al., 2000; Davalos et al., 2005; Smith, 2010). Microglia are responsible for clearance of dead cells and other debris, such as dead axons and myelin and, therefore, have a neuroprotective role (Kalla et al., 2001). However, chronic activation of microglia can negatively affect neuronal function and hippocampal dependent behavior as well as alter migration patterns of developing neurons (Bedi, Hetz, et al., 2013; Belarbi et al., 2012; Hernandez-Ontiveros et al., 2013).
We believe that microglia are key targets for therapies intended to reduce neuroinflammation and improve outcomes after TBI. Cellular therapy has demonstrated promise in attenuating the damage caused by secondary neuroinflammation after TBI; however, the timing of therapy delivery is critical to success (Bedi et al., 2018). We have demonstrated that cellular therapy leads to a decrease in the amoeboid microglia/macrophage response along with a corresponding improvement in spatial learning and memory if given within 24-hr post-injury (Bedi et al., 2018; Bedi, Hetz, et al., 2013). Therefore, we have investigated the microglia response to TBI within the hippocampus, as it is critical in the formation and processing of spatial learning (Morris et al., 1982); furthermore, it is especially vulnerable to neuronal apoptosis after TBI (Sandhir et al., 2008). Hernandez-Ontiveros et al. (2013) demonstrated that chronic TBI reduced neuronal cell survival and led to a significant decrease of neurons in the ipsilateral CA3 region of the hippocampus 8 weeks after the injury. Therefore, the hippocampus is an important locus for monitoring both acute and chronic microglial activation and cellular therapy efficacy. In addition to the hippocampus, another vital locus affected by TBI is the thalamus. Recent preclinical experiments have demonstrated early and prolonged activation of microglia in coronal sections of the ventral posteromedial nucleus of the thalamus after fluid percussion injury 7 and 28 days after injury (Thomas et al., 2018). Evidence from human TBI patients suggests that amoeboid microglia and reactive astrocytes can remain present up to 17 years after injury (Ramlackhansingh et al., 2011). These data are derived from studies using a positron emission tomography (PET) ligand known as [11C](R)PK11195 (PK) to the peripheral benzodiazepine receptor or translocator protein (TSPO), expressed by reactive glia and macrophages (Maeda et al., 2007; Raghavendra Rao et al., 2000). TSPO is localized on the outer mitochondrial membranes of astrocytes, microglia, and macrophages (Papadopoulos, 1998). In patients with TBI, PK binding is significantly elevated in the thalami, putamen, occipital cortices, and posterior limb of the internal capsules even several months to years after the injury (Ramlackhansingh et al., 2011). Recent imaging of TSPO has demonstrated that TSPO is selective for pro-inflammatory polarized astrocytes and microglia (Pannell et al., 2020).
Understanding both acute and chronic microglial activation after TBI is critical for determining effective treatment windows. While the time course of microglial activation after TBI has been extensively studied (Chen et al., 2003; Csuka et al., 2000; Maeda et al., 2007; Sandhir et al., 2008; B. Zhang et al., 2008), here we examined two specific time points critical for cellular therapy intervention: 24 hr and 28 days post-injury. As mentioned earlier, we have seen that cellular therapy is more effective in the acute time point if given at or prior to 24 hr post-injury (Bedi et al., 2018). In addition, we are currently investigating the use of cell therapy to treat TBI patients at subacute and chronic time points after injury (www.clinicaltrials.gov; NCT04063215).
In this study, we interrogated the spatiotemporal response of microglia after TBI in a mouse model via morphologic phenotyping of microglia in the hippocampus and thalamus at 24 hr and 28 days after injury. In addition, we examined the spatiotemporal distribution of TSPO, a marker for activated glial cells.
Methods
Study Design
Adult male (6–7 weeks) C57BL/6 mice (Harlan, Indianapolis, IN) were randomly assigned groups (controlled cortical impact [CCI] or Control). Animals were housed on a 12-hr light/dark cycle with ad libitum access to food and water. We used male mice because of our previous experiments and number of animals used was based on our previous publication (Walker et al., 2012). Two animals died after the injury, one in the 24-hr group and one in the 28-day group. One ipsilateral hippocampus at 28 days was damaged, and we were unable to count microglia in the particular hippocampus. All protocols involving the use of animals were in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee (HSC-AWC-11-023).
Injury
A CCI device (Impact One Stereotaxic Impactor, Leica Microsystems, Buffalo Grove, IL) was used to administer a unilateral brain injury to 6- to 7-week-old normal adult male C57BL/6 mice weighing 17 to 21 g (Walker et al., 2011). Mice were randomly chosen for injury. Mice were anesthetized with 4% isoflurane/O2 and the head mounted in a stereotactic frame. A midline incision was made to expose the cranium and a craniectomy done to the right parietal association cortex (midway between bregma and lambda, 1 mm right to the midline). The bone flap was removed with a pair of forceps. Animals received a single impact to the parietal association cortex of 1.0-mm depth of deformation with an impact velocity of 5.0 m/s and a dwell time of 200 ms (moderate–severe injury). After the injury, the incision was closed with staples. Uninjured mice (Control) were not placed in a stereotaxic frame, nor did they receive an incision, anesthesia, or craniectomy. In previous craniectomy-alone experiments, we did not observe any differences in the hemispheres based on blood–brain barrier permeability, therefore we did not do craniectomy-alone controls in the current experiments (Bedi, Walker, et al., 2013). The animals were group housed.
Tissue Harvest
At time points of 24 hr and 28 days, the animals were euthanized during standard perfusion procedures. Under isoflurane anesthesia, the animals’ chests were opened, and then using a right ventricle puncture technique, the animals were simultaneously exsanguinated and perfused with 20 ml (10 ml/min) of cold phosphate-buffered saline (PBS) followed by 20 ml (10 ml/min) of cold 4% paraformaldehyde (PFA). After fixation, the brains were removed, fixed in 4% PFA for 24 hr, and stored at 4°C.
Immunohistochemistry and Antibody Characterization
After being stored in 4% PFA for 24 hr, the brains were transferred to a 30% sucrose solution, where they were maintained at 4°C for at least 72 hr and allowed to sink. Brains were then put in a 3% agar mold and sectioned into 30-μm-thick slices using a vibrating-blade microtome (Leica Microsystems, Bannockburn, IL, http://www.leica.com). Sections (Bregma −3.30 to −3.60) were stained using a standard free-floating protocol as described previously (Bedi, Hetz, et al., 2013). Sections were washed twice in PBS with 0.01% Triton X-100 (PBST; T-8787; Sigma-Aldrich, St. Louis, MO, http://www.sigmaaldrich.com) for 1 min, incubated for 20 to 30 min in PBS with 0.02% Triton X-100, and blocked for 1 hr at room temperature (RT) in 3% goat serum (no. 005-000-121; Jackson Immunoresearch Laboratories, West Grove, PA, http://www.jacksonimmuno.com) in PBST. IBA1, a rabbit polyclonal primary antibody, was used to identify microglia and stained a pattern of cells identical to previous publications ([1:100]; Wako Chemicals USA, Cat# 019-19741, RRID: AB_2313566; Torres-Platas et al., 2014). Anti-peripheral benzodiazepine receptor, a rabbit monoclonal primary antibody, was used to identify TSPO+ cells, as demonstrated by previous studies (1:200; Abcam Cat# ab109497, RRID: AB_10862345; Karlstetter et al., 2014). Glial fibrillary acidic protein, a rabbit polyclonal antibody, was used to identify astrocytes and stained in an identical fashion to previous reports (1:500; Abcam Cat# ab7260, RRID:AB_305808; Liu et al., 2009).
The antibodies were prepared in PBTB (PBS with 0.01% Triton X-100, 2% bovine serum albumin [A9647; Sigma-Aldrich] and 1% goat serum), and sections were incubated overnight at 4°C. The next day, sections were rinsed briefly and then washed with PBST and incubated with a goat anti-rabbit IgG secondary antibody (1:500; red/568; Molecular Probes (Invitrogen) Cat# A11011, RRID:AB_143157) and a goat anti-mouse IgG secondary antibody (1:500; green/488; Molecular Probes (Invitrogen), Cat# A11029, RRID: AB_A11029) in PBTB for 2 hr at RT. The sections were again rinsed four times with PBST, mounted on slides, and coverslipped with Fluoromount-G (SouthernBiotech, Birmingham, AL, http://www.southernbiotech.com).
Quantification of Immunohistochemistry
Hippocampus
Photomicrographs were taken of the hippocampus at 20× magnification using a Nikon fluorescent microscope (TE2000-U; Nikon, Tokyo, Japan, http://www.nikon.com) and NIS-Elements imaging software (http://www.nikoninstruments.com/Products/Software/NIS-Elements-Basic-Research, RRID: SciRes_000190). Slices for immunohistochemistry were taken approximately mid injury (cavity or bruise left by the impactor tip). Additional photomicrographs (63×) were taken with Carl Zeiss LSM780, Axio Imager 2 microscope and Zen 2.5 lite software (https://www.zeiss.com/microscopy/us/products/confocal-microscopes.html). For control animals, we matched hippocampal structure to the contralateral hippocampus of an injured animal for analysis. A single slice per animal was examined. Two adjacent photomicrographs of each of dentate gyri (DG), CA3, and CA1 at 20× of the ipsilateral and contralateral hippocampus were photomicrographed and identified cells expressing IBA1 were quantified. Total cells in each hemisphere were calculated by adding all IBA1+ cells in each photomicrograph of DG, CA3, and CA1.
We used one or two microglial cells as landmarks while moving to the adjacent photomicrograph to prevent double-counting. If the ipsilateral hippocampus was severely damaged, we did not count the ipsilateral side and did not include that animal in the total count. However, we did examine and count the IBA1+ cells in the intact contralateral side. They were further classified based on morphology of IBA1 labeled cells (ramified or amoeboid) based on previous characterizations (Bedi et al., 2018; Bedi, Hetz, et al., 2013; Torres-Platas et al., 2014; B. Zhang et al., 2008)
Thalamus
We examined the lateral posterior nucleus of the thalamus (LPNT) ipsilateral and contralateral to the injury (CCI and Control) at 24 hr and 28 days. A single image was approximately mid-injury was taken at 40× using a Leica fluorescent microscope Dm4000B LED (https://www.leica-microsystems.com/products/light-microscopes/p/leica-dm4000-b-led) and Leica Application Suite V4.12 (https://www.leica-microsystems.com/products/microscope-software/p/leica-application-suite). A blinded investigator counted all IBA1-labeled cells in the photomicrograph in order for unbiased stereology. They were further classified based on morphology as either ramified or amoeboid. In addition, we also examined TSPO+ cells that were examined in the thalami. All photomicrographs were taken blinded and the analyses were done blinded as well.
Immunohistochemistry and quantification of immunohistochemistry were done concurrently for all groups.
Statistical Analysis
All values are presented as mean ± SD. Between-group comparisons were analyzed using analysis of variance (ANOVA) and if found significant, they were further analyzed using Sidak’s multiple comparison test. Statistical significance is indicated with * for p ≤ .05, ** indicates statistical significance for p ≤ .01, *** indicates statistical significance p ≤ .001, and **** indicates statistical significance p ≤ .0001.
Results
TBI Increased the Total Number of Microglia in the Hippocampus at 28 Days, but Not 24 hr, After Injury
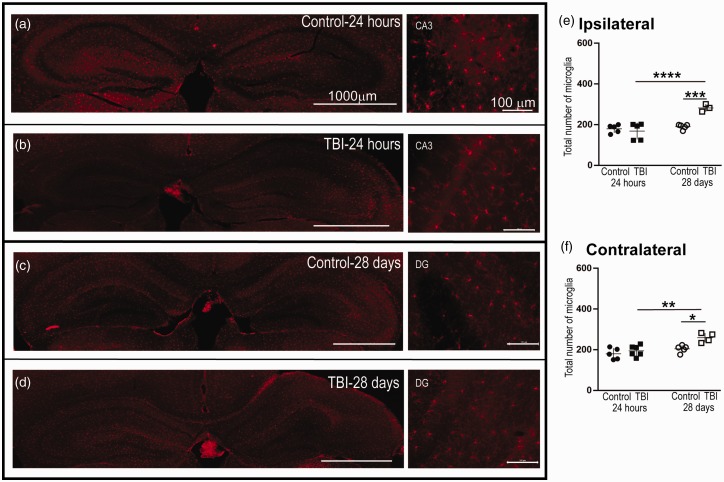
There was an overall significant increase in the microglial population as determined by a one-way ANOVA after TBI—ipsilateral: F(3, 14) = 13.22, p = .0002 and contralateral: F(3, 16) = 8.68, p = .001. At 24 hr after injury, microglia did not significantly change in population as determined by Sidak’s multiple comparisons test in either the ipsilateral (TBI: n = 5, 169 ± 18.5 vs. Control: n = 5, 180 ± 9.0; p = .884, Figure 2E) or contralateral (TBI: n = 6,195 ± 1.8 vs. Control: n = 5, 180 ± 12.5; p = 0.698, Figure 2F) hippocampi. Interestingly, at 28 days after injury, we observed significant increases in IBA1+ cells in ipsilateral (TBI: n = 3, 282 ± 10.7 vs. Control: n = 5, 190 ± 5.6; p = .0009, Figure 2E) and contralateral (TBI: n = 4, 259 ± 11.6 vs. Control: n = 5, 205 ± 8.0; p = .012) hippocampi (Figure 2F). Temporally, there was increase in microglia at 28 days in comparison to 24 hr after injury in the ipsilateral (p = .0001, Figure 2E) and contralateral (p = .002, Figure 2E) hemispheres.
Figure 2.
The Number of Microglia in the Ipsilateral and Contralateral Hippocampi Increased at 28 Days, but Not 24 hr, Post-CCI. Panels A to D: Representative micrographs of IBA1+ cells in the ipsilateral and contralateral hippocampus. Panel E: In the ipsilateral cortex, we observed a significant increase in total IBA1+ cells at 28 days post-injury in comparison to the control and 24-hr post-injury groups. Panel F: In addition, at 28 days, there was a significant increase in IBA1+ cells in the contralateral cortex in comparison to the control and 24-hr post-injury groups. Panels E and F: At 24 hr, we did not observe a significant difference in the number of IBA1+ cells in the ipsilateral or contralateral cortices between the injury group and control. DG = dentate gyrus; 24-hr cohort: TBI ipsilateral n = 5, contralateral n = 6; control ipsilateral n = 5, contralateral n = 5; 28-day cohort: TBI ipsilateral n = 3, contralateral n = 4; control ipsilateral n = 5, contralateral n = 5. Between-group comparisons were analyzed using ANOVA and if found significant, they were further analyzed using Sidak’s multiple comparison test. Statistical significance is indicated with * for p < .05, ** indicates statistical significance for p < .01, *** indicates statistical significance p < .001, and **** indicates statistical significance p < .0001. TBI = traumatic brain injury.
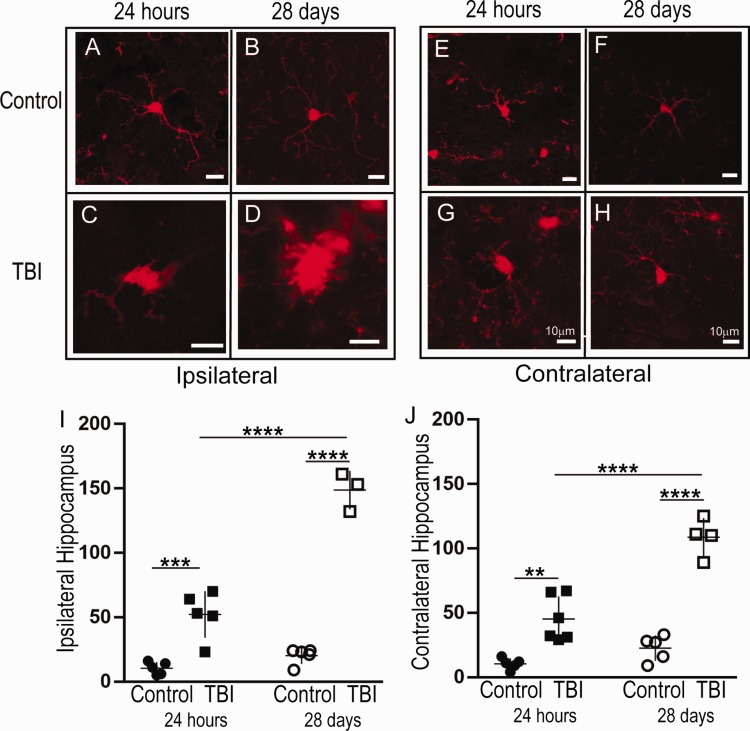
Bilateral Increases in Amoeboid-Shaped Microglia Were Observed in the Hippocampus 24 hr and 28 Days After TBI
There was an overall significant change in the amoeboid microglial population as determined by a one-way ANOVA—ipsilateral: F(3, 14) = 95.9, p < .0001 and contralateral: F(3, 16) = 45.4, p < .001. At 24-hr post-injury, there was no increase in the total number of microglia due to TBI (Figure 2); however, when morphologically characterized, we observed a significant increase in the number of activated ipsilateral microglia (TBI: n = 5, 52.2 ± 18.1 vs. Control: n = 5, 10.4 ± 4.83; p = .0002; Figure 3A, C, and I). Contralateral to the injury, we observed similar significant increases in the number of activated microglia after TBI (n = 6, 45.2 ± 17.6) versus Control (n = 5, 10.4 ± 4.39; p = 0.001, Figure 3E, G, and J).
Figure 3.
The Number of Amoeboid Microglia in the Ipsilateral and Contralateral Hippocampi Increased at 24-hr and 28-Day Post-CCI. Panels A to H: Representative micrographs of IBA1+ cells in the control (A) and injured (B) hippocampus at 24-hr and 28-day post-injury. I-J: There was an acute increase in number of amoeboid IBA1+ cells in the ipsilateral (I) and contralateral (J) hippocampi at 24-hr and 28-day post-injury. In addition, there were significantly more amoeboid IBA1+ cells at 28 days, in comparison to 24 hr, post-injury in both the ipsilateral and contralateral hippocampi; 24-hr cohort: TBI ipsilateral n = 5, contralateral n = 6; Control ipsilateral n = 5, contralateral n = 5. 28 day cohort: TBI ipsilateral n = 3, contralateral n = 4; control ipsilateral n = 5, contralateral n = 5. Between-group comparisons were analyzed using ANOVA and if found significant, they were further analyzed using Sidak’s multiple comparison test. Statistical significance is indicated with * for p < .05, ** indicates statistical significance for p < .01, *** indicates statistical significance p < .001, and **** indicates statistical significance p < .0001. TBI = traumatic brain injury.
There were modest but significant changes ipsilaterally when counting ramified microglia at 24 hr. There was a decrease in the number of ramified microglia in TBI (24 hr: n = 5, 116 ± 50.1) in comparison to Control (24 hr: n = 5, 169 ± 19.7; p = .038, graph not shown). There was no significant difference in the contralateral hemisphere when quantifying ramified microglia (TBI: n = 5, 150 ± 36.8 vs. Control: n = 5, 170 ± 25.8, p > .05, graph not shown).
At 28 days post-injury, TBI-treated animals exhibited increased amoeboid microglia in both the ipsilateral (Figure 3B, D, and I) and contralateral hippocampus compared to controls (Figure 3F, H, and J). On the ipsilateral side there was a significant increase in the number of amoeboid (TBI: n = 3, 149 ± 14.9 vs. Control: n = 5, 20.2 ± 6.38; p < .0001, Figure 3I). There were no significant changes in the number of ramified microglia (TBI: n = 3, 134 ± 13.6 vs. Control: n = 5, 170 ± 6.91; p = .307, graph not shown). Similarly, on the contralateral side, we also observed a significant increase in the number of amoeboid (TBI: n = 4, 109 ± 14.8 vs. Control: n = 5, 22.6 ± 9.8; p < .0001, Figure 3J). There was no significant difference in the number of ramified microglia in the contralateral hemispheres between TBI (n = 4, 151 ± 15.1) versus Control (n = 5, 174 ± 12.4; p = .063, graph not shown).
Temporally, there was a significant increase in the number of amoeboid-shaped microglia when comparing 24 hr (TBI: n = 5, 52.2 ± 18.1) versus 28 days ipsilateral (n = 3, 149 ± 14.9, p < .0001; Figure 3I) and contralateral (24 hr: n = 6, 45.2 ± 17.6 vs. 28 days: n = 4, 109 ± 14.8, p < .0001, Figure 3J). There was no significant difference in the number of ramified microglia ipsilateral (p = .820) or contralateral (p = .999).
TBI Increased the Number of Microglia in the LPNT at 28 Days Ipsilateral to Injury
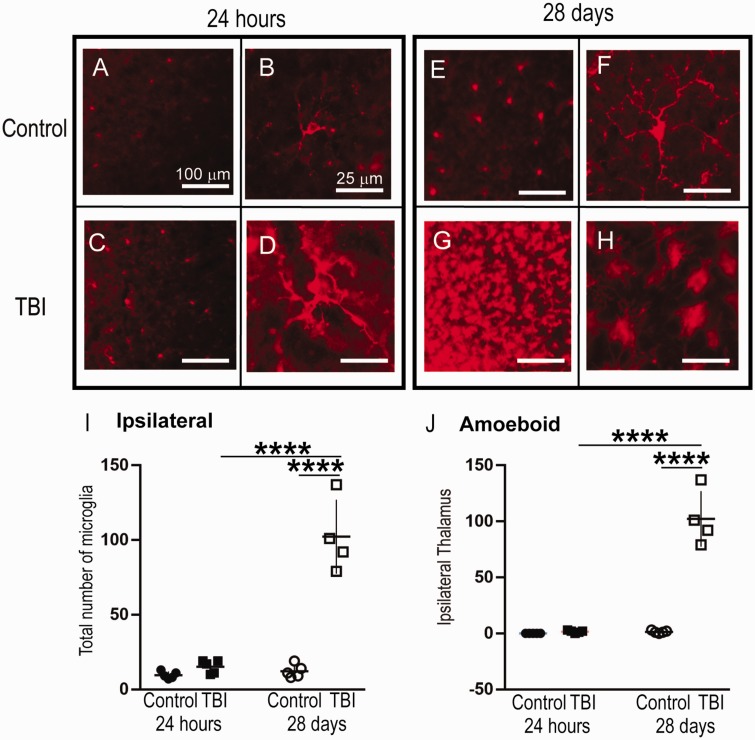
There was an overall significant change in the amoeboid microglial population as determined by a one-way ANOVA in the ipsilateral thalamus only—F(3, 15) = 62.9, p < .0001, Contralateral: F(3, 15) = 1.08, p = .387. At 24 hr after injury, there were no differences in the number of microglia in the ipsilateral LPNT (TBI: n = 5, 15.2 ± 4.38 vs. Control: n = 5, 9.6 ± 2.40; p = .841, Figure 4E) or contralateral LPNT (TBI: n = 5, 11.6 ± 3.78 vs. Control: n = 5, 13.6 ± 3.57; p = .817, graph not shown) when comparing Control versus TBI. At 28 days after injury, we observed significant increases in the number of microglia in the ipsilateral LPNT (TBI: n = 4, 102 ± 24.9 vs. Control: n = 5, 12.2 ± 4.43; p < .0001, Figure 4E) and not in the contralateral LPNT (TBI: n = 4, 16 ± 4.08 vs. Control: n = 5, 12.2 ± 4.26; p = .427; graph not shown). Temporally, there was increase in the number of microglia when comparing 24 hr (TBI) versus 28 days TBI (p < .0001, Figure 4E).
Figure 4.
The Total Number of Microglia and Number of Amoeboid Microglia Increased in the Ipsilateral LPNT at 28 Days, but Not 24 hr, post-CCI. Panels A to H: Representative micrographs of IBA1+ cells in the ipsilateral thalamus. Panel I: There was a significant increase in the total number of IBA1+ cells in the ipsilateral LPNT of the injured brain at 28 days post-injury in comparison to both 24-hr post-injury and control. Panel J: There was a significant increase in the number of amoeboid IBA1+ cells in the ipsilateral LPNT of the injured brain at 28 days post-injury in comparison to both 24-hr post-injury and control; 24-hr cohort: TBI ipsilateral n = 5, contralateral n = 6; control ipsilateral n = 5, contralateral n = 5; 28-day cohort: TBI ipsilateral n = 3, contralateral n = 4; control ipsilateral n = 5, contralateral n = 5. Between-group comparisons were analyzed using ANOVA and if found significant, they were further analyzed using Sidak’s multiple comparison test. Statistical significance is indicated with * for p < .05, ** indicates statistical significance for p < .01, *** indicates statistical significance p < .001, and **** indicates statistical significance p < .0001. TBI = traumatic brain injury.
Ipsilateral Increases in Amoeboid Microglia Were Observed in the LPNT 28 Days After TBI
There was an overall significant change in the amoeboid microglial population as determined by a one-way ANOVA in the ipsilateral LPNT only—F(3, 15) = 86.81, p < .0001, Figure 4F—and not in the contralateral LPNT—F(3, 15) = 3.26, p > .05, graph not shown. At 24 hr after the injury, there was no significant increase in amoeboid microglia in the ipsilateral LPNT (TBI: n = 5, 1.6 ± 1.14 vs. Control: n = 5, 0 ± 0; p = .994, Figure 4F) or in the contralateral LPNT (TBI: n = 5, 0.4 ± 0.54 vs. Control: n = 5, 1.2 ± 1.30; p = .780, graph not shown). At 28 days after injury, there was a significant increase in the number of amoeboid microglia in the ipsilateral LPNT (TBI: n = 4, 102 ± 24.9 vs. Control: n = 5, 1.4 ±1.14; p < .0001 Figure 4F). Temporally, TBI increased the number of activated microglia at 28 days in comparison to 24 hr in the ipsilateral LPNT only (p < .0001, Figure 4). Amoeboid-shaped microglia significantly increased in the ipsilateral LPNT 28 days after injury (TBI: n = 4, 102 ± 24.9) in comparison to 24 hr after injury (TBI: n = 5, 1.6 ± 1.14, p = .016, Figure 4H). There were no significant differences in the number of ramified microglia ipsilateral (p = .909, graph not shown) or contralateral (p = .909, graph not shown).
TBI Results in Chronic Activation of TSPO+ Cells in the Ipsilateral Thalamus
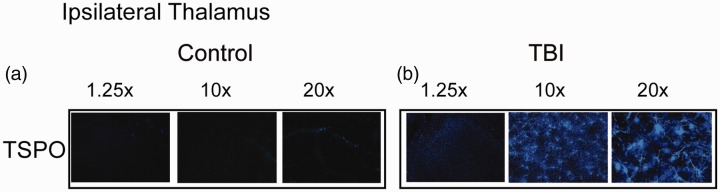
At 28 days post-injury, we only observed TPSO+ cells (a cellular marker for activated microglia found in the outer mitochondrial membrane of astrocytes, microglia, and macrophages) in the ipsilateral thalamus (Figure 5B). In contrast, we did not detect any TSPO+ staining control slices at 28 days (Figure 5A). Furthermore, at 24 hr post-injury, there were no TSPO+ cells in the ipsilateral or contralateral thalami of the TBI or control groups (data not shown). There were no TSPO+ cells in the hippocampus.
Figure 5.
At 28 Days Post-CCI, There Were TSPO+ Cells in the Ipsilateral Thalamus Only. Panels A and B: Representative photomicrographs of TSPO+ cells in the ipsilateral LPNT in the control (A) and 28 days post-injury (B) brains. TBI = traumatic brain injury; TPSO = translocator protein.
Discussion
After CCI, there were significant, but differing, alterations in the number and phenotype of IBA1+ cells in the hippocampus and thalamus of the mouse brain. At 28 days, but not 24 hr, post-injury, there were significant increases in the total IBA1+ cell population in the ipsilateral and contralateral hippocampi. In addition, there was an increase in amoeboid-shaped IBA1+ cells at both 24 hr and 28 days post-injury (Figure 3). In the ipsilateral thalamus, there were increases in both the total number and number of amoeboid IBA1+ cells at both 24 hr and 28 days post-injury; furthermore, these increases were significantly greater at 28 days post-injury in comparison to 24 hr post-injury. Interestingly, there were no significant changes in the IBA1+ cell populations in the contralateral thalamus at either time point. Initially, activated or amoeboid microglia may help at the site of injury by clearing damaged tissue. Yet, persistent microglia at secondary sites such as the hippocampus and thalamus contribute to the secondary pathology of TBI, possibly by phagocytosis of injured—but not necrotic—neurons (Aloisi, 2001; Hernandez-Ontiveros et al., 2013). At 24 hr after injury, there were no changes in the total number of microglia in the injured hippocampi when compared to the control (Figure 2E and F). This is likely due to the lack of a complete microglial activation at this time point. However, there were significant increases in the amoeboid-shaped microglia in comparison to control ipsilateral and contralateral hippocampi (Figure 3I). Since there were no significant differences in the number of microglia between TBI and Control (ipsilateral or contralateral), this indicates that TBI likely causes resident microglia to transition from a ramified to an amoeboid cell. Important to note, perivascular macrophages and other peripheral macrophages may influence counts secondary to an influx of IBA1+ macrophages and myeloid cells from the breach in the blood–brain barrier. A shortcoming of this study is that we did not use a microglia-specific marker to distinguish between infiltrating macrophages and resident microglia. Future studies will include markers such as P2Y12 (purinergic receptor), which are specific to microglia (Haynes et al., 2006). All together, these data indicate that there is a proliferation of microglia after injury, influx of infiltrating macrophages, or both. In recent experiments, we used clodronate liposomes to deplete monocytes/macrophages prior to injury. This resulted in an overall decrease of microglia in the brain as measured by flow cytometry (Aertker et al., 2019). In the absence of infiltrating macrophages, there is less activation of resident microglia (Ma, 2016). Further investigations to delineate between infiltrating macrophages and resident microglia after TBI are warranted.
Interestingly, we observed significant increases in total microglia number and amoeboid microglia in the LPNT at 28 days, but not at 24 hr post-injury (Figure 4I and J). It is possible that there is a delay in the injury effects, and 24 hr is too early to detect any changes in the thalamus, which is further away from the injury site than the hippocampus. This finding is in contrast to other studies that did observe microglia after 24 hr. Specifically, there were changes in immunoreactivity in the cortex, corpus callosum, CA3-Hippocampus, and thalamus after CCI (Chen et al., 2003). Csuka et al. (2000) also observed increases in microglial immunoreactivity within 24 hr after a weight drop injury in the basal subarachnoid space and choroid plexus of the injured animals. In a fluid percussion model of injury, there were also increases of activated microglia 24 hr in the CA 2/3 region (J. Zhang and De Koninck, 2006).
TBI causes chronic microglia activation, and we observed a significant increase in total number and number of amoeboid microglia at 28 days post-injury in both the ipsilateral and contralateral hippocampi (Figures 2E and F and 3I and J). We also observed significant increases in total microglia and amoeboid-shaped microglia in the LPNT 28 days after TBI (Figure 4E to J). The LPNT has multiple connections with other parts of the brain such as the inferior and superior colliculus. However, in contrast to the hippocampus, the increase in number of microglia was only in the ipsilateral hemisphere after injury (Figure 4I). Furthermore, the robust increase cell numbers was due nearly entirely to an increase in amoeboid microglia (Figure 4J). While we did not observe changes in microglia numbers or phenotype at 24 hr post-injury, others have observed (not quantified) amoeboid-shaped microglia as early as 7 days and up to 28 days after injury in the ventral posteromedial nucleus of the thalamus using a different preclinical TBI model (fluid percussion injury; Thomas et al., 2018). Recent studies have also linked TBI and increased activity in the ipsilateral auditory thalamus (medial geniculate nucleus) to a fear conditioning paradigm (amygdala) (Hoffman et al., 2019).
Finally, we observed a dramatic increase in the inflammatory marker TSPO and amoeboid microglia in the ipsilateral thalamus at 28 days post-injury that is absent in control animals. PET studies using PK11195, a TSPO ligand, demonstrated persistent microglia activation for up to 17 years after a single TBI (Ramlackhansingh et al., 2011). Furthermore, recent work has demonstrated that TSPO identifies activated, versus homeostatic, glial cells (Pannell et al., 2020). Reports have demonstrated TSPO expression to overlap significantly with amoeboid microglial cells, via co-staining with IBA1, in mouse and human tissues (Karlstetter et al., 2014). Our studies and those of Ramlackhansingh have shown via PET and immunohistochemistry that this neuroinflammation is primarily due to the presence of amoeboid microglia, with only minor contribution from astrocytes and other cell types (Bedi, Hetz, et al., 2013; Bedi, Walker, et al., 2013; Ramlackhansingh et al., 2011).
Our observations confirmed the results of previous experiments: the thalamus, on the ipsilateral side specifically, is especially susceptible to long-term microglia/macrophage activation (Cao et al., 2012; Ramlackhansingh et al., 2011). As a relay center for motivation, cognition, and motor control, with diffuse connections among many functional areas of the brain, deleterious inflammation in the thalamus may contribute to the chronic, neurodegenerative pathology of TBI (Ramlackhansingh et al., 2011). Other studies have demonstrated that microglia can become primed to activation by previous stimulation and, as a result, do not respond to anti-inflammatory cytokines or neuron-derived anti-inflammatory factors (Eggen et al., 2013). Therefore, the sustained activation of microglia within the thalamus may be a result of priming by the initial injury and resultant microenvironment; furthermore, this provides a potential target for measuring the long-term efficacy of future therapy in vivo. As imaging-based outcomes as increasingly utilized to study the efficacy of therapies in TBI patients, these data presented here may help identify anatomical regions especially susceptible to brain injury at chronic time points and further emphasize the potential advantage of PET-based imaging to identify activated glial cells (Cox et al., 2019).
One significant limitation of this study is the lack of sex as a biological variable. As we and others have previously described, there are likely sex differences in clinical outcomes after TBI (Berry et al., 2009; Caplan et al., 2017; Coimbra et al., 2003; Ley et al., 2013; Phelan et al., 2007). Previous reports of conflicting preclinical data about sex differences in the microglia response to injury only highlight the need for further research in this field (Acaz-Fonseca et al., 2015; Gunther et al., 2015; Loram et al., 2012). Future efforts in our own lab will incorporate both male and female animals.
Conclusion
In summary, our study demonstrates that there is a spatiotemporal increase in amoeboid-shaped IBA1+ cells as early as 24 hr after TBI in the hippocampus but not LPNT. At 28 days post-injury, there is an increase in the number of amoeboid IBA1+ cells over time in both the hippocampus and LPNT. Furthermore, we demonstrated increased TPSO+ cells in the ipsilateral thalamus at 28 days post-injury, indicating sustained glial activation in this region. These data help identify microglia phenotypes at acute and chronic potential treatment windows after TBI and regions of interest to evaluate the efficacy of therapy in preclinical and clinical settings.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported in part by grant 5T35NS064931-04 from the National Institute of Neurological Disorders and Stroke, National Institutes of Health (NIH) 2T32 grant GM0879201-11A1. C. S. C. has received research support from Athersys, CBR Systems, Hope Bio, Biostage, and is on the Scientific Advisory Board of Cellvation, Biostage, CBR, and Hope Bio.
ORCID iDs
Fanni Cardenas https://orcid.org/0000-0002-2026-5351
Supinder S. Bedi https://orcid.org/0000-0003-1938-1790
References
- Acaz-Fonseca E., Duran J. C., Carrero P., Garcia-Segura L. M., Arevalo M. A. (2015). Sex differences in glia reactivity after cortical brain injury. Glia, 63(11), 1966–1981. 10.1002/glia.22867 [DOI] [PubMed] [Google Scholar]
- Aertker B. M., Kumar A., Prabhakara K. S., Smith P., Furman N. E. T., Hasen X., Bedi S. S. (2019). Pre-injury monocyte/macrophage depletion results in increased blood-brain barrier permeability after traumatic brain injury. J Neurosci Res, 97(6), 698–707. 10.1002/jnr.24395 [DOI] [PubMed] [Google Scholar]
- Aloisi F. (2001). Immune function of microglia. Glia, 36(2), 165–179. 10.1002/glia.1106 [DOI] [PubMed] [Google Scholar]
- Bedi S. S., Aertker B. M., Liao G. P., Caplan H. W., Bhattarai D., Mandy F., Mandy F., Fernandez L. G., Zelnick P., Mitchell M. B., Schiffer W., Johnson M., Denson E., Prabhakara K., Xue H., Smith P., Uray K., Olson S. D., Mays R. W., Cox C. S., Jr. (2018). Therapeutic time window of multipotent adult progenitor therapy after traumatic brain injury. J Neuroinflammation, 15(1), 84 10.1186/s12974-018-1122-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedi S. S., Hetz R., Thomas C., Smith P., Olsen A. B., Williams S., Xue H., Aroom K., Uray K., Hamilton J., Mays R. W., Cox C. S., Jr. (2013). Intravenous multipotent adult progenitor cell therapy attenuates activated microglial/macrophage response and improves spatial learning after traumatic brain injury. Stem Cells Transl Med, 2(12), 953–960. 10.5966/sctm.2013-0100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedi S. S., Walker P. A., Shah S. K., Jimenez F., Thomas C. P., Smith P., Hetz R. A., Xue H., Pati S., Dash P. K., Cox C. S., Jr. (2013). Autologous bone marrow mononuclear cells therapy attenuates activated microglial/macrophage response and improves spatial learning after traumatic brain injury. J Trauma Acute Care Surg, 75(3), 410–416. 10.1097/TA.0b013e31829617c6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belarbi K., Arellano C., Ferguson R., Jopson T., Rosi S. (2012). Chronic neuroinflammation impacts the recruitment of adult-born neurons into behaviorally relevant hippocampal networks. Brain Behav Immun, 26(1), 18–23. 10.1016/j.bbi.2011.07.225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry C., Ley E. J., Tillou A., Cryer G., Margulies D. R., Salim A. (2009). The effect of gender on patients with moderate to severe head injuries. J Trauma, 67(5), 950–953. 10.1097/TA.0b013e3181ba3354 [DOI] [PubMed] [Google Scholar]
- Cao T., Thomas T. C., Ziebell J. M., Pauly J. R., Lifshitz J. (2012). Morphological and genetic activation of microglia after diffuse traumatic brain injury in the rat. Neuroscience, 225, 65–75. 10.1016/j.neuroscience.2012.08.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan H. W., Cox C. S., Bedi S. S. (2017). Do microglia play a role in sex differences in TBI? J Neurosci Res, 95(1–2), 509–517. 10.1002/jnr.23854 [DOI] [PubMed] [Google Scholar]
- Chen S., Pickard J. D., Harris N. G. (2003). Time course of cellular pathology after controlled cortical impact injury. Exp Neurol, 182(1), 87–102. [DOI] [PubMed] [Google Scholar]
- Coimbra R., Hoyt D. B., Potenza B. M., Fortlage D., Hollingsworth-Fridlund P. (2003). Does sexual dimorphism influence outcome of traumatic brain injury patients? The answer is no! J Trauma, 54(4), 689–700. 10.1097/01.TA.0000058314.31655.5F [DOI] [PubMed] [Google Scholar]
- Coronado V. G., Xu L., Basavaraju S. V., McGuire L. C., Wald M. M., Faul M. D., Guzman B. R., Hemphill J. D., & Centers for Disease Control and Prevention. (2011). Surveillance for traumatic brain injury-related deaths–United States, 1997-2007. MMWR Surveill Summ, 60(5), 1–32. [PubMed] [Google Scholar]
- Cox C. S., Jr., Juranek J., Bedi S. (2019). Clinical trials in traumatic brain injury: Cellular therapy and outcome measures. Transfusion, 59(S1), 858–868. 10.1111/trf.14834 [DOI] [PubMed] [Google Scholar]
- Csuka E., Hans V. H., Ammann E., Trentz O., Kossmann T., Morganti-Kossmann M. C. (2000). Cell activation and inflammatory response following traumatic axonal injury in the rat. Neuroreport, 11(11), 2587–2590. [DOI] [PubMed] [Google Scholar]
- Davalos D., Grutzendler J., Yang G., Kim J. V., Zuo Y., Jung S., Littman D. R., Dustin M. L., Gan W. B. (2005). ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci, 8(6), 752–758. 10.1038/nn1472 [DOI] [PubMed] [Google Scholar]
- Eggen B. J., Raj D., Hanisch U. K., Boddeke H. W. (2013). Microglial phenotype and adaptation. J Neuroimmune Pharmacol, 8(4), 807–823. 10.1007/s11481-013-9490-4 [DOI] [PubMed] [Google Scholar]
- Graeber M. B. (2010). Changing face of microglia. Science, 330(6005), 783–788. 10.1126/science.1190929 [DOI] [PubMed] [Google Scholar]
- Gunther M., Plantman S., Davidsson J., Angeria M., Mathiesen T., Risling M. (2015). COX-2 regulation and TUNEL-positive cell death differ between genders in the secondary inflammatory response following experimental penetrating focal brain injury in rats. Acta Neurochir (Wien), 157(4), 649–659. 10.1007/s00701-014-2331-2 [DOI] [PubMed] [Google Scholar]
- Haynes S. E., Hollopeter G., Yang G., Kurpius D., Dailey M. E., Gan W. B., Julius D. (2006). The P2Y12 receptor regulates microglial activation by extracellular nucleotides. Nat Neurosci, 9(12), 1512–1519. 10.1038/nn1805 [DOI] [PubMed] [Google Scholar]
- Hernandez-Ontiveros D. G., Tajiri N., Acosta S., Giunta B., Tan J., Borlongan C. V. (2013). Microglia activation as a biomarker for traumatic brain injury. Front Neurol, 4, 30 10.3389/fneur.2013.00030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman A. N., Lam J., Hovda D. A., Giza C. C., Fanselow M. S. (2019). Sensory sensitivity as a link between concussive traumatic brain injury and PTSD. Sci Rep, 9(1), 13841 10.1038/s41598-019-50312-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalla R., Liu Z., Xu S., Koppius A., Imai Y., Kloss C. U., Kohsaka S., Gschwendtner A., Möller J. C., Werner A., Raivich G. (2001). Microglia and the early phase of immune surveillance in the axotomized facial motor nucleus: Impaired microglial activation and lymphocyte recruitment but no effect on neuronal survival or axonal regeneration in macrophage-colony stimulating factor-deficient mice. J Comp Neurol, 436(2), 182–201. [PubMed] [Google Scholar]
- Karlstetter M., Nothdurfter C., Aslanidis A., Moeller K., Horn F., Scholz R., Neumann H., Weber B. H., Rupprecht R., Langmann T. (2014). Translocator protein (18 kDa) (TSPO) is expressed in reactive retinal microglia and modulates microglial inflammation and phagocytosis. J Neuroinflammation, 11, 3 10.1186/1742-2094-11-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley E. J., Short S. S., Liou D. Z., Singer M. B., Mirocha J., Melo N., Bukur M., Salim A. (2013). Gender impacts mortality after traumatic brain injury in teenagers. J Trauma Acute Care Surg, 75(4), 682–686. 10.1097/TA.0b013e31829d024f [DOI] [PubMed] [Google Scholar]
- Liu N., Chen R., Du H., Wang J., Zhang Y., Wen J. (2009). Expression of IL-10 and TNF-alpha in rats with cerebral infarction after transplantation with mesenchymal stem cells. Cell Mol Immunol, 6(3), 207–213. 10.1038/cmi.2009.28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loram L. C., Sholar P. W., Taylor F. R., Wiesler J. L., Babb J. A., Strand K. A., Berkelhammer D., Day H. E., Maier S. F., Watkins L. R. (2012). Sex and estradiol influence glial pro-inflammatory responses to lipopolysaccharide in rats. Psychoneuroendocrinology, 37(10), 1688–1699. 10.1016/j.psyneuen.2012.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y. (2016). Associations of platelet-activating factor acetylhydrolase gene polymorphisms with risk of ischemic stroke. Biomed Rep, 4(2), 246–250. 10.3892/br.2015.560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda J., Higuchi M., Inaji M., Ji B., Haneda E., Okauchi T., Zhang M. R., Suzuki K., Suhara T. (2007). Phase-dependent roles of reactive microglia and astrocytes in nervous system injury as delineated by imaging of peripheral benzodiazepine receptor. Brain Res, 1157, 100–111. 10.1016/j.brainres.2007.04.054 [DOI] [PubMed] [Google Scholar]
- Morris R. G., Garrud P., Rawlins J. N., O’Keefe J. (1982). Place navigation impaired in rats with hippocampal lesions. Nature, 297(5868), 681–683. [DOI] [PubMed] [Google Scholar]
- Nakajima K., Kohsaka S. (2001). Microglia: Activation and their significance in the central nervous system. J Biochem, 130(2), 169–175. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn A., Kirchhoff F., Helmchen F. (2005). Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science, 308(5726), 1314–1318. 10.1126/science.1110647 [DOI] [PubMed] [Google Scholar]
- Pannell M., Economopoulos V., Wilson T. C., Kersemans V., Isenegger P. G., Larkin J. R., Smart S., Gilchrist S., Gouverneur V., Sibson N. R. (2020). Imaging of translocator protein upregulation is selective for pro-inflammatory polarized astrocytes and microglia. Glia, 68(2), 280–297. 10.1002/glia.23716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos V. (1998). Structure and function of the peripheral-type benzodiazepine receptor in steroidogenic cells. Proc Soc Exp Biol Med, 217(2), 130–142. [DOI] [PubMed] [Google Scholar]
- Phelan H. A., Shafi S., Parks J., Maxson R. T., Ahmad N., Murphy J. T., Minei J. P. (2007). Use of a pediatric cohort to examine gender and sex hormone influences on outcome after trauma. J Trauma, 63(5), 1127–1131. 10.1097/TA.0b013e318154c1b8 [DOI] [PubMed] [Google Scholar]
- Raghavendra Rao V. L., Rao A. M., Dogan A., Bowen K. K., Hatcher J., Rothstein J. D., Dempsey R. J. (2000). Glial glutamate transporter GLT-1 down-regulation precedes delayed neuronal death in gerbil hippocampus following transient global cerebral ischemia. Neurochem Int, 36(6), 531–537. 10.1016/S0197-0186(99)00153-9 [DOI] [PubMed] [Google Scholar]
- Ramlackhansingh A. F., Brooks D. J., Greenwood R. J., Bose S. K., Turkheimer F. E., Kinnunen K. M., Gentleman S., Heckemann R. A., Gunanayagam K., Gelosa G., Sharp D. J. (2011). Inflammation after trauma: Microglial activation and traumatic brain injury. Ann Neurol, 70(3), 374–383. 10.1002/ana.22455 [DOI] [PubMed] [Google Scholar]
- Salter M. W., Beggs S. (2014). Sublime microglia: Expanding roles for the guardians of the CNS. Cell, 158(1), 15–24. 10.1016/j.cell.2014.06.008 [DOI] [PubMed] [Google Scholar]
- Sandhir R., Onyszchuk G., Berman N. E. (2008). Exacerbated glial response in the aged mouse hippocampus following controlled cortical impact injury. Exp Neurol, 213(2), 372–380. 10.1016/j.expneurol.2008.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. S. (2010). Activated microglia in nociception. Pain Physician, 13(3), 295–304. [PubMed] [Google Scholar]
- Thomas T. C., Ogle S. B., Rumney B. M., May H. G., Adelson P. D., Lifshitz J. (2018). Does time heal all wounds? Experimental diffuse traumatic brain injury results in persisting histopathology in the thalamus. Behav Brain Res, 340, 137–146. 10.1016/j.bbr.2016.12.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurman D. J., Alverson C., Dunn K. A., Guerrero J., Sniezek J. E. (1999). Traumatic brain injury in the United States: A public health perspective. J Head Trauma Rehabil, 14(6), 602–615. [DOI] [PubMed] [Google Scholar]
- Torres-Platas S. G., Comeau S., Rachalski A., Bo G. D., Cruceanu C., Turecki G., Giros B., Mechawar N. (2014). Morphometric characterization of microglial phenotypes in human cerebral cortex. J Neuroinflammation, 11, 12 10.1186/1742-2094-11-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker P. A., Bedi S. S., Shah S. K., Jimenez F., Xue H., Hamilton J. A., Smith P., Thomas C. P., Mays R. W., Pati S., Cox C. S., Jr. (2012). Intravenous multipotent adult progenitor cell therapy after traumatic brain injury: Modulation of the resident microglia population. J Neuroinflammation, 9, 228 10.1186/1742-2094-9-228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B., West E. J., Van K. C., Gurkoff G. G., Zhou J., Zhang X. M., Kozikowski A. P., Lyeth B. G. (2008). HDAC inhibitor increases histone H3 acetylation and reduces microglia inflammatory response following traumatic brain injury in rats. Brain Res, 1226, 181–191. 10.1016/j.brainres.2008.05.085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., De Koninck Y. (2006). Spatial and temporal relationship between monocyte chemoattractant protein-1 expression and spinal glial activation following peripheral nerve injury. J Neurochem, 97(3), 772–783. 10.1111/j.1471-4159.2006.03746.x [DOI] [PubMed] [Google Scholar]