Abstract
Background
Depression warranting intervention is found in ten percent of people over the age of 60. Older depressed people are more likely to die than non‐depressed. Relatively few receive therapeutic interventions, and those that do, tend to receive low dose antidepressant therapy. Depression in older people is thought to differ in terms of aetiology, presentation, treatment and outcome than in younger people. Concomitant physical illness and increasing social, physical and neurophysiological diversity are associated with the ageing process. Consequently drug treatment of older patients is often carried out in institutions and on patients suffering from multiple physical problems.
Objectives
To determine the efficacy of antidepressant medication compared with placebo in the treatment of depression in older patients.
Search methods
The search strategy incorporated: electronic literature searches of databases held by the Cochrane Collaboration Depression, Anxiety and Neurosis Review Group (CCDAN) (see Collaborative Review Group Search Strategy). Reference lists of related reviews and references of located studies. Contact was made with authors working in the field.
Selection criteria
All randomised, placebo controlled trials using antidepressants in the treatment of the presenting episode of depression in patients described as elderly, geriatric senile or older adult.
Data collection and analysis
Two types of data were extracted (if available) from each study. The first type of data was dichotomous data, this consisted of recovered/not recovered. The second, continuous data,included: Hamilton Depression Rating Scale (HAM‐D), Montgomery‐Asberg Rating Scale (MADRS) and other depression rating scale scores. An analysis using Peto Odds ratios for the dichotomous data and weighted mean difference for continuous data was performed using RevMan 3.1. The presence of heterogeneity of treatment effect was assessed.
Main results
Seventeen trials contributed data to the analyses comparing the efficacy of antidepressant treatment and placebo. Analyses of efficacy were based on 245 patients treated with Tricyclic antidepressants (223 with placebo), 365 patients treated with SSRIs (372 with placebo) and 58 patients treated with MAOIs (63 with placebo). The results using a fixed effect model, for the three groups respectively were, TCAs; OR: 0.32 (95% CI: 0.21,0.47), SSRIs; OR; 0.51 (95% CI: 0.36,0.72), MAOIs: OR 0.17 (95% CI: 0.07,0.39).
Authors' conclusions
TCAs, SSRIs and MAOIs are effective in the treatment of older community patients and inpatients likely to have severe physical illness. At least six weeks of antidepressant treatment is recommended to achieve optimal therapeutic effect. There is little evidence concerning the efficacy of low dose TCA treatment. Further trials are required before low dose TCA treatment is routinely recommended.
Keywords: Aged, Female, Humans, Male, Middle Aged, Antidepressive Agents, Antidepressive Agents/therapeutic use, Depression, Depression/drug therapy, Monoamine Oxidase Inhibitors, Monoamine Oxidase Inhibitors/therapeutic use, Odds Ratio, Placebo Effect, Randomized Controlled Trials as Topic, Selective Serotonin Reuptake Inhibitors, Selective Serotonin Reuptake Inhibitors/therapeutic use
Plain language summary
Antidepressants compared with placebos for depressed older people
Seventeen RCTs were identified by the systematic literature search that met our inclusion criteria and provide suitable data for analysis. We analysed these 17 placebo trials examining the efficacy of antidepressant treatment in older people. Just under 2000 patients were entered into the meta analysis. TCAs, SSRIs and MAOIs proved effective in both institutionalised and community patients. Low dose TCA treatment may be effective but further studies are needed.
Background
Approximately ten percent of people over the age of 60 suffer from depression to the degree that warrants intervention (Saunders 1993). Community studies have demonstrated that older depressed men are three times more likely to die and depressed women twice as likely to die compared to non depressed controls (Davidson 1988). It is also acknowledged that relatively few depressed older adults receive therapeutic interventions. Those that do are likely to receive low dose antidepressant treatment (Wilson 1999).
Depression in later life is thought to differ from depression in younger subjects in aspects of aetiology, presentation, treatment and outcome (Schneider 1995). Depression in older people often complicates, or is obscured by co‐morbid physical illness. The depressive syndrome should be viewed in the context of increasing social, physical and neurophysiological diversity associated with the ageing process (Rabbitt 1993). These include frequently experienced changes in immediate social structures, loss of neurological resilience, lowered threshold of cortical arousal, decompensation in homeostatic processes (Gold 1988) and concomitant physical illness. All are identified as potential vulnerability factors for depression. As a consequence drug treatment of older, depressed patients is often carried out in institutions and hospitals on patients suffering from multiple physical illness and handicap.
It is evident that older patients are more prone to side effects and experience greater difficulty in tolerating dosages that are of therapeutic efficacy (Schneider 1995). They may respond to lower dosage as a consequence of changes in metabolic rate and shifts in the body fat ratio associated with the ageing process. Physical illness and handicap are associated with poor outcome and may influence the efficacy and tolerability of antidepressant treatment. Lastly, there is a growing body of literature indicating that older subjects may require longer to respond to antidepressant medication than younger subjects (Reynolds 1996).
It is acknowledged that the definition of an 'older person' presents problems throughout psychogeriatric research (Schneider 1995). We have limited our study to those trials that include older patients described as such, or when not described those trials in which all the patients are over the age of 60, with view to addressing the aforementioned issues.
Objectives
To conduct a review, testing the hypothesis that antidepressants are more effective than placebo in the treatment of depression in older patients.
To identify the duration of treatment required to achieve optimum therapeutic advantage of antidepressant compared to placebo in older patients.
To examine the efficacy of low dose antidepressant treatment in older patients
To test the hypothesis that antidepressants are effective treatment (compared to placebo) in the treatment of older, institutionalised and hospitalised patients likely to suffer from physical illness and handicap.
Methods
Criteria for considering studies for this review
Types of studies
The review will include all randomised, placebo controlled trials using antidepressant drugs in the treatment of depression in subjects described as elderly, geriatric, senile and older adults or in trials where all subjects are over the age of 60. Trials that include subjects under the age of 60 will be excluded unless data concerning subjects over the age of 60, or those described as elderly, geriatric or senile, were randomised and analysed separately.
Types of participants
Diagnoses: Patients are included in the review if diagnosed as suffering from depression (major or minor) by any criteria. Patients suffering from other mental illnesses will be excluded from the review. The review will include patients suffering from concomitant physical illness. Trials including patients with an explicit diagnosis of dementia have been excluded. Gender: Included trials will involve subjects of either sex. Age: Our review will accept studies that include subjects described as elderly, geriatric senile, or older adults or in trials where all subjects are over the aged 55 and over.
Types of interventions
The review will include all randomised, placebo controlled trials using antidepressant drug treatments. Trials that randomise subjects into receiving more than one antidepressant simultaneously were excluded, as were trials that examine the prophylactic efficacy of antidepressant treatment and those that include formal psychotherapeutic treatments.
Types of outcome measures
The primary outcome measure used in this review is the trialists' dichotomous outcome of recovered versus not recovered. This is determined by the number of patients in each group that have shown significant clinical improvement at the end of the trial. This is based on either a change in score of a set amount or achieving a predetermined score, at or below a cut off point on the Hamilton Depression Rating Scale (HAM‐D) (Hamilton 1960), Clinical Global Impression (CGI) scale or other rating scale. The secondary outcome included analyses of continuous data from rating scales.
Only one study (Tollefson 1993) measured quality of life using the Short Form 36 (SF36) (Ware 1992).
Search methods for identification of studies
A two‐stage search strategy was adopted due to the difficulty in locating trials in older patients. In the first stage we validated the electronic search strategy. The abstracts of all trials that were held on the Cochrane Collaboration Depression, Anxiety and Neurosis (CCDAN) register some 7000 abstracts, were read by two reviewers with the intent to identify all trials in older depressed people. When abstracts failed to characterise the trial population sufficiently, the full article was examined. An electronic search strategy was devised that identified all the relevant articles identified through the above procedure.
This produced the following electronic search strategy:
Elder* Geriatri* Senil* Older Old Age Late Life Aged, 80‐And‐Over Combined with (AND) (Depress* OR Dysthymi*) AND #30 = Pharmacotherapy
(1) Electronic bibliographic databases Computer‐assisted searches were undertaken of the following electronic databases: PsycLIT; MEDLINE; EMBASE; CINAHL.
The optimal sensitive search strategy of the Cochrane Collaboration for randomised controlled trials (refer Cochrane Handbook, 1996) was used in conjunction with search terms identified above.
The following databases were searched using the CCDAN Trials Register search strategy (see CCDAN Review Group). PsycLIT (1887‐1999) MEDLINE (1966‐1999) EMBASE (1982‐1999) LILACS (1982‐1999) CINAHL (1982‐1999) SIGLE (19‐‐‐1999) Psyndex (1977‐1999) National Research Register (1999) Dissertation Abstracts International Biological Abstracts
(2) The Cochrane Controlled Trials Register and the Cochrane Collaboration Depression Anxiety and Neurosis Controlled Trials Register were searched using the search terms. (3) Hand‐searching
Additional hand searching of conference abstract and citation lists was undertaken. The International Journal of Geriatric Psychiatry (1989‐1999) The Irish Journal of Psychological Medicine (1996‐1999) and The American Journal of Orthothpsychiatry (1970‐1999) were hand searched.
Six hundred and ninety seven citations concerning older people were identified from the CCDAN database. Of these 366 results concerned depression and the abstracts were assessed for inclusion into the review.
71 articles were excluded as they were not studies on elderly populations.
57 articles were excluded as they were not studying a depressed population or the population included patients with other psychiatric disorders e.g. dementia, alcoholism, bipolar affective disorder.
88 articles were excluded as they reported trials that were not placebo controlled.
16 articles were excluded as they reported on trials that were of continuation or maintenance antidepressant treatment.
14 articles were excluded as they were reporting either dose response trials or trials using a combination of drugs
1 article was excluded as it was a literature review
11 articles were excluded as they were reporting trials on electro convulsive therapy (ECT), prevalence studies, prescribing practice or risk factors for depression.
This left 108 articles that were included at this stage. Full papers were obtained and read to assess for inclusion.
Additional searching of citation lists identified 78 articles of which 5 were included in the review
The search strategy generated 23 placebo‐controlled trials eligible for entry into the review.
However 6 of these failed to provide extractable data: Branconnier (1982) Branconnier (1983) Jansen (1984) Schwiezer (1994) Sunderland (1994) Wallace (1995)
Data collection and analysis
Three reviewers independently assessed the relevance of each trial, blind to decision made by each other (KW, PM, AS). Each trial was assessed against pre‐set criteria and rated on a scoring sheet. In cases of disagreement decisions were reached by consensus through open discussion. Reasons for exclusion and /or inclusion were recorded. Two reviewers were acknowledged experts in the field. Reviewers were blind to authorship of trials, journals and institutions from which citations come. Blindness was tested through reviewer's 'best guessing' authorship, journal and institution. Data were extracted from selected trials and further information requested from authors when insufficient data was available. In trials that examined the efficacy of more than one drug against placebo, drug treatments were considered independent of one another and analysed separately.
Data Collection: Data were extracted from each study, using a pre‐designed form. Data was entered on a Microsoft database and subsequently onto RevMan 3.1 statistical software.
Statistical analyses: In undertaking this meta‐analyses we have 'lumped' studies together which use the same class, as defined by the British National Formulary (BNF), September 1999 (the BNF is a publication authorised by the United Kingdom Joint Formulary Committee) of antidepressants (BMA 1999). Those antidepressants that do not readily fall into specific classes are divided into those that the BNF categorises as antidepressants and those that are not. The main outcome measures included the odds ratios and 95% confidence intervals and pooled estimates using Peto method. Continuous data was pooled by calculating the weighted mean difference (WMD), where studies have used the same instruments. When difference scales have been used the standardised mean differences has been used. Discontinuation rates were identified and reported where possible.
Study quality: Concealment of randomisation was the main quality criteria. The was measured using the Schultz scale (Schulz 1995). Studies were given a quality rating from A = trial that were reported to have taken adequate measures to conceal allocation; B = no adequate details about how the randomisation procedure was carried out; C = inadequately concealed (e.g. via alternation or reference to an open random number table).
Data synthesis: The primary outcome measure was the dichotomous 'recovered vs not recovered' using the trialists' own criteria, an analysis using the Peto odds ratio was conducted (Sackett 1997) using Review Manager 3.1 software. The secondary outcome used weighted mean difference using Review Manager 3.1 software. Heterogeneity of treatment effect was assessed using the Q statistic which approximates the chi square statistic with n ‐ 1 degrees of freedom (DerSimonian 1986). A fixed effect model was used as the primary analyses, if substantial heterogeneity was found, random effects model was used for the analysis.
We anticipated that the outcome of trials might be influenced by variables other than drug vs placebo. We examined outcomes by recruitment source. Trials were categorised by recruitment of patients from hospital inpatient wards and nursing homes and those recruiting from the community, though outpatient services or volunteers. Meta analysis was also conducted on trials using low dose antidepressant treatment (defined by BNF recommendations). The duration of trial was grouped by duration of post randomisation phase. Lastly, we examined the efficacy of antidepressant treatment in those patients diagnosed as suffering from major depressive disorder.
Results
Description of studies
Twenty‐three trials fulfilled the inclusion criteria. Of these, 17 provided sufficient data of acceptable quality to be used in the meta‐analyses. The 17 trials generated 45 publications. Of these 53% were sponsored by industry, 18% non‐commercial sponsors and 29% unknown sponsorship.
Class of Drug Trials were grouped by class of drug as defined by BNF and when not classified by the BNF, drugs were allocated to the pharmacological class to which they were most similar. The principle classes of drugs identified were;
Tricyclic and related antidepressants (TCAs): Nortriptyline, Imipramine, Doxepin, Viloxazine, Lofepramine, and Trazadone. Not included in the BNF: Nomifensine, Diclofensin
Selective serotinergic re‐uptake inhibitors and related antidepressants (SSRIs): Fluoxetine
Monoamine oxidase inhibitors and related antidepressants (MAOIs): Phenelzine and Moclobemide (reversible monoamine oxidase inhibitor).
Atypical Antidepressants: Mirtazepine. (not included in the BNF) Minaprine and Medifoxamine.
Drugs not classed as antidepressants by the BNF; Alprazolam, Bupropion. This was not entered into the meta analysis (data is provided in additional information table).
Twelve trials used TCA versus placebo. Two trials used SSRIs versus placebo. Two trials used MAOI versus placebo. Three trials used other antidepressants versus placebo and two trials used drugs not classed as antidepressants.
Trial design All trials are of parallel group design. Subjects are randomly assigned to treatment/placebo groups. Seven studies include more than one trial drug, tested against placebo: Three trials use imipramine to compare against placebo and a drug of the same class; (Cohn 1984 b; Merideth 1984; Gerner 1980 a). Four studies compare drugs of different pharmacological classes with placebo (Georgotas 1986; Halikas 1995; Nair 1995; Kane 1983). The remaining trials compare a single drug against placebo.
Where two drugs from the same class are compared in the same study, only one appears in the meta‐analyses (appearing twice in analysis of class would over represent the placebo group). This occurs in three trials. CCohn 1984 b and Merideth 1984 compare imipramine with nomifensine (TCAs) against placebo. Data concerning nomifensine were excluded. Trazadone data from the Gerner 1980 a is used at the expense of the imipramine data.
Age Range Trials describing patients as elderly, geriatric, senile or older adult, used different minimum ages for this group, however all patients in the analysis are aged 55 or over. Three studies include patients aged 55 and over (Georgotas 1986; Halikas 1995; Kane 1983). Three studies selected subjects aged 60 and over: (Cohn 1984 b; Jansen 1982; Tollefson 1993). In eight studies the mean age of the study sample is between 60 and 69 years old (inclusive): (Cohn 1984 b; Georgotas 1986; Gerner 1980 a; Halikas 1995; Kane 1983; Merideth 1984; Nair 1995; Tollefson 1993). Five studies have samples with mean ages between 70 and 79 (inclusive): (Beutler 1987; De Leo 1984; Jansen 1982; Lakshmanan 1986; Parnetti 1991). The remaining four studies (Hammond 1993, Katz 1990 a; Meignan‐Debray 1990; Tan 1994) have samples with mean ages of 80 and over. Three studies include subjects aged 90 and over: (Gerner 1980 a; Meignan‐Debray 1990; Nair 1995).
Diagnoses, measurement and severity of depression. Seven trials employ the Diagnostic and Statistical Manual Research Criteria (DSM 111R) for depression: (Beutler 1987; Meignan‐Debray 1990; Nair 1995; Tollefson 1993; De Leo 1984; Halikas 1995; Katz 1990 a). Research Diagnostic Criteria (RDC) are used in five studies; (Georgotas 1986; Gerner 1980 a; Cohn 1984 b; Merideth 1984; Jansen 1982). The remaining studies use a variety of classification systems: Hammond 1993 GMS/AGECAT (Geriatric Mental State/Automated Geriatric Examination for Computer Assisted Taxonomy), Kane 1983 'structured clinical diagnoses'. Parnetti 1991 employed the International Classification of Diseases Issue 9 (ICD 9), 309.1 definition of prolonged depressive disorder. Two studies use rating instrument cut‐off scores as sole depression criteria; Katz 1990 a uses a cut‐off score of 18 on the Hamilton Depression Rating Scale (HAM‐D (21)) and Tan 1994 employs the Geriatric Depression Scale (GDS) cut‐off of 15. Lakshmanan 1986 and Jansen 1982 do not specify inclusion diagnostic criteria.
In addition to diagnostic criteria, eight studies require a severity of 18 on the HAM‐D rating scale. Of the remaining studies; Tollefson 1993 and Georgotas 1986 employ an HAM‐D score of 16 and De Leo 1984 and Cohn 1984 b uses an HAM‐D score of 20. Tan 1994 uses a GDS score of 15 and Meignan‐Debray 1990 uses a Montgomery‐Asberg Depression Rating Scale (MADRS) score of 20. Evans (1997) uses an ELDRS (Evans Liverpool Depression Rating Scale) cut off score of five as a preliminary screening instrument prior to conducting a diagnostic interview using the GMS/AGECAT. Parnetti 1991 entered patients with ECP score (Evaluation Clinique de la Personnalite) between 53 and 104. Jansen 1982 does not employ rating scale entry criteria.
Outcome Measures Few trials used outcome measures other than change in depression scores and recovery/non recovery. Thirteen of the 17 used change in the 17, 21 or 24 version of the HAM‐D as the primary outcome measurement. Only three studies did not include the HAM‐D as an outcome measure (primary or secondary). The remaining four used change in a variety of scales; Tan 1994 Geriatric Depression Scale, Meignan‐Debray 1990 the MADRS, De Leo 1984 used the Zung Self Assessment Rating Scale and Parnetti 1991 used changes in the Evaluation Clinique de la Personnalite (ECP) as a main outcome measure. Fifteen studies used the dichotomised outcome of recovered/non recovered. Of these, nine used a reduction in the HAM‐D and the other six used the CGI score.
Study size There are five multicentre studies included in the review (Tollefson 1993; Cohn 1984 b; Kane 1983; Nair 1995; Parnetti 1991). The remainder are single centre studies. Four studies had 50 (in one case; 48 in one arm) or more subjects randomised to each study arm (Halikas 1995; Meignan‐Debray 1990; Parnetti 1991; Tollefson 1993). Three studies have between 30 and 49 (inclusive) subjects allocated to each experimental arm (Hammond 1993; Nair 1995; Tan 1994). The ten remaining trials included in the review have less than 30 subjects allocated to each experimental arm (Beutler 1987; Cohn 1984 b; De Leo 1984; Georgotas 1986; Gerner 1980 a; Jansen 1982; Kane 1983; Katz 1990 a; Lakshmanan 1986; Merideth 1984). Duration of trials Trials were classified by duration of the post randomisation double blind phase. Beutler 1987 is the longest study (20 weeks/ 140 days). The long duration can be explained by a three‐month follow‐up assessment and the increased time required for psychotherapeutic intervention in two of the experimental arms (excluded from this review). One study (Hammond 1993) had a duration of 8 weeks (56 days). Four studies have duration of seven weeks (49 days) (Georgotas 1986; Katz 1990 a; Nair 1995; Parnetti 1991). Three studies have duration of 6 weeks (42 days) (Halikas 1995; Meignan‐Debray 1990; Tollefson 1993). Merideth 1984 has a duration of five weeks (35 days). Five studies have duration of four weeks (28 days) (Cohn 1984 b; De Leo 1984; Gerner 1980 a; Kane 1983; Tan 1994). Two studies have a duration of 3 weeks (21 days) (Jansen 1982; Lakshmanan 1986). Meta‐analyses were conducted on groups of trials (defined by duration) with 50 or more patients randomised to each experimental arm.
Recruitment source and patient exclusions
Patients with an explicit diagnosis of dementia were excluded from the study. The Hammond 1993 study included patients with a Mini Mental state Score (MMSE) of 10 or more. Katz 1990 a; Lakshmanan 1986; Tan 1994 included some patients with mild to moderate cognitive impairment (MMSE <20). Georgotas 1986 excluded those with moderate to severe dementia, Kane 1983 excluded severe cases of dementia, Parnetti 1991 excluded those with MMSE score of less than 24 and Tollefson 1993 excluded patients with scores <25. the remaining studies excluded dementia sufferers (Halikas 1995; Jansen 1982; Meignan‐Debray 1990; Nair 1995) or did not specify (Beutler 1987; Cohn 1984 b; De Leo 1984; Gerner 1980 a; Merideth 1984). Most excluded patients with 'significant' or 'unstable' medical conditions. However such terms are open to interpretation and there is considerable variability across trials. A minority of trials excluded patients with unstable epilepsy, convulsions and alcohol or drug dependency.
Trials were grouped by source of recruitment: two trials did not provide information concerning recruitment source (De Leo 1984; Nair 1995) and one trial had mixed nursing home and sheltered accommodation patients (Katz 1990 a). Six trials recruited from hospital inpatient unit and rehabilitation units (Hammond 1993; Jansen 1982; Lakshmanan 1986; Meignan‐Debray 1990; Merideth 1984; Tan 1994). Two of these trials (Hammond 1993; Merideth 1984) were conducted on physically ill geriatric patients. Exclusion criteria included hepatic insufficiency, glaucoma, urinary and prostate problems, conductive cardiac conditions and epilepsy. The exclusion criteria reflect the possible complications imposed by the trial drug: Lastly patients requiring Electro Convulsive Therapy (ECT), or severely agitated, suffering from bipolar affective disorder, alcohol dependency or schizophrenia were excluded. Despite a large number of patients being excluded from these studies, most included patients had severe and multiple physical illnesses.
Of the remaining studies included in the review; eight studies were conducted on outpatients or volunteers. It is safe to assume that the majority of these patients are mobile, living relatively independently in the community. In particular, two studies recruited patients through advertisement as well as through outpatient clinics (Beutler 1987; Halikas 1995)). These trials tended to exclude patients with suicidal ideas, physical illness and alcohol abuse. Only three indicate the number of excluded patients that fulfilled the depression inclusion criteria. Twenty of 84 potential patients (24%) were excluded in the Beutler 1987 study, 47 of 137 potential patients (34%) were excluded in the Georgotas 1986 trial, seven of the potential 51 (14%) were excluded in Kane 1983 trial and seven (5%) were excluded from Parnetti 1991 trial.
Low dosage antidepressant trials: Two of studies examined the efficacy of low dose tricyclic treatment: Tan 1994 compared 70 mgs daily of lofepramine and Lakshmanan 1986; 10‐20 mgs daily of doxepin with placebo. Both studies were conducted on small numbers of inpatients. Kane 1983 compared low dose (150 mgs) and high dose bupropion to placebo on an outpatient population. All other trials examined drugs described by the BNF were within the included guidelines.
Discontinuation Meta analysis of discontinuation rates was conducted by class of antidepressant. Discontinuation indicates all patients that were removed from the post randomisation phase. A wide variety of reasons were provided for removing patients. These included death, non‐compliance emergent physical illness, intolerance of side effects and loss to follow‐up.
Risk of bias in included studies
Description of concealment of allocation was rated as B in all studies, no adequate details about how the randomisation procedure was carried out. We are currently contacting the trialists to gather further information and will update the review with this information.
Effects of interventions
Seventeen trials met our inclusion criteria and provided data suitable for use in the analyses.
Efficacy Tricyclic Antidepressants Eleven trials contributed to the efficacy data concerning TCAs. Ten of these contributed dichotomous data: recovered/not recovered. One trial contributed continuous data (Lakshmanan 1986) and one trial contributed both (Katz 1990 a).
The analyses of the dichotomous data gives an estimated pooled fixed effect Odds Ratio of 0.32 (CI 0.21, 0.47); Q statistic 14.7194, df:=9, p=0.989. The numbers needed to treat (NNT) is 3.97 (CI 3.88, 4.05), implying that approximately four patients would need to be treated to produce one that recovers that would not have done so if given placebo. Only one trial provided continuous data using the HAM‐D, however, three trials (active drug N=50, placebo N=52, one of which was low dose treatment) provided continuous data using the CGI; WMD; ‐2.907 (‐5.489, ‐3.24).
Selective Serotonin Reuptake Inhibitors Two trials included in the analyses used fluoxetine. Tollefson 1993 examined its efficacy in a large multi‐centred study of community patients and Hammond 1993 recruited from severely physically ill, medical inpatients. The later study was considerably smaller, possibly effecting the power of the study (type two error). Analyses of the two studies gives an estimated pooled effect and random effect odds ratio of 0.51 (CI 0.36, 0.72): Q statistic 0.1784, df=1, P=0.6727. This gives a numbers needed to treat of 8.45 (CI 8.38, 8.53). This implies that approximately eight patients would need to be treated with an SSRI to produce one recovery from depression that would not have happened if treated with placebo alone.
Monoamine Oxidase Inhibitors The analyses consisted of two trials of two different MAOIs; moclobemide (Nair 1995) and Phenelzine (Georgotas 1986). Fifty‐eight patients were treated with a MAOI and 63 with placebo. The estimated pooled fixed effect odds ratio is 0.17 (CI 0.07, 0.39): Q statistic 1.01, df=1, P=0.3149. This generates a number needed to treat of 3.14 (CI 2.99,3.29), suggesting that approximately three patients would need to be treated to get one better that would not have done so if given placebo, however this is based on small numbers.
Atypical antidepressants Three studies were included, examining different drugs of different pharmacological classes: Minaprine (Parnetti 1991), providing continuous data and Mitrazepine (Halikas 1995) and Medifoxamine (Meignan‐Debray 1990) providing dichotomous data. Analyses of pooled, dichotomous data showed significant effect compared to placebo, generating an Odds ratio of 0.52 (CI 0.29, 0.93) and a numbers needed to treat of 6.63 (CI 6.5, 6.7).
Drugs not classified as antidepressants The two trials examining the efficacy of non‐antidepressants (Beutler 1987, examining alprazolam and Kane 1983, examining Bupropion) were not included in the meta‐analyses. Data from these trials appears in 'Other data'.
The effect of length of trial Trails were classified according to length of the double blind placebo phase. The following groups were generated: 28 days (Cohn 1984 b; De Leo 1984; Gerner 1980 a; Kane 1983) 35 (Merideth 1984), 42 (Halikas 1995; Meignan‐Debray 1990; Tollefson 1993) 56 days (Hammond 1993). They were analysed using dichotomous outcome data (recovery/non recovery). Three groups of trials had more than 50 represented in each arm 28 days, 42 days and 49 days. All three groups of trials demonstrated significant advantage of active drug over placebo. (28 days OR; 0.40, CI; 0.21, 076; 42 days OR 0.22 CI 0.39,0.71; 49 days OR 0.22 CI 0.12,0.42).
The effect of low dose treatment The meta analysis of the two trials (Lakshmanan 1986; Tan 1994) demonstrated efficacy of low dose TCA (lofepramine, doxepin) compared to placebo (WMD ‐2.55 (CI ‐5.70, ‐0.40). However only continuous data was available and the sample size (38 receiving active treatment and 15 receiving placebo) was very small. Patients were highly selected and both studies were carried out on inpatients.
Discontinuation rates All four antidepressant doses had similar discontinuation rates compared to placebo: TCA OR 0.54 CI 0.21,1.39; SSRI OR 1.06 CI 0.74,1.52; MAOI OR 0.84 CI 0.40,1.75: Atypical OR 0.81 CI 0.41, 1.60.
Recruitment source Antidepressants were significantly better than placebo in treating depression in both institutionalised and community recruited patients. The institutionalised patients (153 receiving active drug, 128 receiving placebo) had an OR 0.35 CI 0.21, 0.58;. Community patients (602 received active drug and 468 received placebo) had an OR 0.57 CI 0.35, 0.62.
Depression diagnosis The eight trials employing RDC and DSM criteria for Major Depressive Disorder generated 562 patients receiving active treatment and 538 receiving placebo. Antidepressant treatment was more efficacious that placebo (OR: 0.44 CI 0.34, 0.58). Six studies were included in the meta analysis of other types of depression generating 181 patients receiving active drug and 131 receiving placebo. Again, antidepressant treatment was more effective than placebo (OR 0.29 (CI 0.18,0.48).
Quality of life Tollefson (1993) was the only study to measure quality of life. Using the SF‐36 (Ware 1992) data for 261 Fluoxetine and 271 Placebo subjects were used. The difference in adjusted mean SF‐36 scores for fluoxetine and placebo at end point were analysed. Although some sub‐scales were found significant, differences between the groups overall end score, failed to find a significant difference at this end‐point analysis.
Discussion
1. Methodological considerations. Only seventeen studies generated data that could be included in the meta‐analyses. The majority of these trials excluded large numbers of depressed patients mainly as a consequence of severe depression and physical illness. However those trials that did examine inpatient populations did include patient groups characterised by serious, concomitant physical illness. The 17 trials provided a sample size of less than 2000. The number of different drugs in each class is limited, with only one (fluoxetine) included in the SSRI class. Consequently some caution must be taken in generalising these findings to other drugs of the same class, not included in this review.
Conclusions have to be drawn with some care in view of the small number of patients included in the meta analysis. A number of these studies include patients likely to suffer from dementia. We believe that these represent a minority but are likely to be of greater prevalence in those studies recruiting from inpatient populations. The efficacy of antidepressants in treating depression in dementia sufferers is being reviewed in another Cochrane Review ( Dening 2000). We acknowledge that recruitment source is a poor substitute for physical illness, handicap and dependency. However, in view of the relative under treatment of depressed older people in institutions we believed it necessary to include this sub analysis. 'Discontinuation rates' include a wide variety of causes of drop out it is wrong to make comparative inference concerning side‐effects of different classes of drugs. This issue is being addressed in a subsequent Cochrane review. Attempts to examine how antidepressant dosage and the influence of trial length have been curtailed due to small sample sizes.
2. Quantitative findings. All three major antidepressant classes (tricyclics, selective serotonin re‐uptake inhibitors and monoamine oxidase inhibitors) appear effective in the treatment of depression in older people when compared to placebo. More trials are required before comment can be made concerning the efficacy of individual antidepressants and other drugs falling outside these groups. In all three major antidepressant classes discontinuation rates were no greater than placebo. Despite the small number of trials it is evident that patients recruited from both institutional settings, likely to have serious concomitant physical illness and those recruited from the community respond to antidepressant treatment. Only three trials examined the efficacy of low dose antidepressants. One (Kane 1983) examined the use of bupropion (not classed as an antidepressant) in 18 patients and showed no significant difference from placebo. The other two studies examined doxepin and lofepramine, recruiting a total of 53 patients. Meta‐analyses demonstrated significant efficacy (in observer rated scales) when compared to placebo. However the implications of these findings should be viewed with some caution because of the very small numbers involved. Both studies were conducted on inpatients with large numbers having been excluded through strict entry criteria. Lastly, analyses by duration of trial generated three groups that included more than 50 patients allocated to receiving active drug or placebo. Meta‐analyses of trials by duration demonstrated significant efficacy of antidepressant compared to placebo from four weeks on.
Authors' conclusions
Implications for practice.
The main conclusions from this review are 1. The three major antidepressant classes (TCAs, SSRIs and MAOIs) are effective in the treatment of older people. 2. Despite the relative under‐treatment of older depressed, physically ill and dependant patients in hospitals and nursing homes, antidepressant treatment is effective. 3. The evidence available indicates that antidepressant treatment of four weeks is likely to have a beneficial effect compared to placebo. However further studies are required before the optimum time to recovery can be determined. 4. Low dose tricyclic antidepressants may be superior to placebo in the treatment of physically ill inpatients. However, generalisation of these findings must be treated with caution and the clinician should be encouraged to adopt alternative antidepressant treatment strategies until further trials have been conducted. There are no randomised‐controlled trials demonstrating the antidepressant efficacy of low dose tricyclics in the treatment of older people in community settings. 5. There are relatively few placebo‐controlled studies examining the efficacy of newer antidepressants in older people. Efficacy information concerning newer drugs has to be inferred from trials conducted in younger patients and those suffering from physical illness. They may take longer to work and be effective in lower doses.
Implications for research.
1. Further trials are required before low dose tricyclic antidepressant treatment can be recommended. 2. New antidepressant trials should be subjected to dose response studies in older people.
What's new
| Date | Event | Description |
|---|---|---|
| 1 November 2008 | Amended | Converted to new review format. |
History
Protocol first published: Issue 1, 1998 Review first published: Issue 2, 2001
| Date | Event | Description |
|---|---|---|
| 15 October 2000 | New citation required and conclusions have changed | Substantive amendment |
Notes
The first draft of this review update has been submitted and is currently undergoing peer review.
Acknowledgements
The review is supported by the Wirral and Cheshire Community (NHS) Trust in collaboration with the University of Liverpool.
We would also like to thank Hugh McGuire Trials Coordinator (UK) of the Depression Anxiety and Neurosis Group for his help.
Data and analyses
Comparison 1. TCA and related versus Placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Recovered | 10 | 468 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.32 [0.21, 0.47] |
| 1.2 Diclofensine | 1 | 40 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.30 [0.06, 1.37] |
| 1.4 Imipramine | 3 | 101 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.27 [0.12, 0.60] |
| 1.6 Nortriptyline | 3 | 145 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.19 [0.09, 0.40] |
| 1.7 Lofepramine | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.8 Trazodone | 2 | 136 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.74 [0.35, 1.59] |
| 1.9 Viloxazine | 1 | 46 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.21 [0.07, 0.67] |
| 2 Hamilton Depression Rating Scale | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | ‐8.6 [‐13.78, ‐3.42] |
| 2.2 Diclofensine | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.4 Imipramine | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.5 Nomifensine | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.6 Nortriptyline | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | ‐8.6 [‐13.78, ‐3.42] |
| 2.7 Lofepramine | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.8 Trazodone | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.9 Viloxazine | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Other observer rating scales | 3 | 102 | Mean Difference (IV, Fixed, 95% CI) | ‐2.91 [‐5.49, ‐0.32] |
| 3.2 Diclofensine | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 Doxepin | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐4.8 [‐8.85, ‐0.75] |
| 3.4 Imipramine | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.5 Nomifensine | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.6 Nortriptyline | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | ‐5.90 [‐12.32, 0.52] |
| 3.7 Lofepramine | 1 | 56 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐3.93, 3.93] |
| 3.8 Trazodone | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.9 Viloxazine | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Discontinuation rates | 11 | 543 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.91 [0.61, 1.35] |
| 4.2 Diclofensine | 1 | 40 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.50 [0.05, 5.06] |
| 4.4 Imipramine | 3 | 101 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.54 [0.21, 1.39] |
| 4.5 Nomifensine | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.6 Nortriptyline | 3 | 157 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.88 [0.46, 1.71] |
| 4.7 Lofepramine | 1 | 63 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.12 [0.37, 3.39] |
| 4.8 Trazodone | 2 | 136 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.24 [0.60, 2.57] |
| 4.9 Viloxazine | 1 | 46 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
1.1. Analysis.

Comparison 1 TCA and related versus Placebo, Outcome 1 Recovered.
1.2. Analysis.

Comparison 1 TCA and related versus Placebo, Outcome 2 Hamilton Depression Rating Scale.
1.3. Analysis.

Comparison 1 TCA and related versus Placebo, Outcome 3 Other observer rating scales.
1.4. Analysis.

Comparison 1 TCA and related versus Placebo, Outcome 4 Discontinuation rates.
Comparison 2. SSRI and related versus Placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Recovered | 2 | 737 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.51 [0.36, 0.72] |
| 1.1 Fluoxetine | 2 | 737 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.51 [0.36, 0.72] |
| 2 Hamilton Depression Rating Scale | 1 | 655 | Mean Difference (IV, Fixed, 95% CI) | ‐1.70 [‐2.86, ‐0.54] |
| 2.1 Fluoxetine | 1 | 655 | Mean Difference (IV, Fixed, 95% CI) | ‐1.70 [‐2.86, ‐0.54] |
| 3 Other observer rating scales | 1 | 655 | Mean Difference (IV, Fixed, 95% CI) | ‐0.80 [‐1.98, 0.38] |
| 3.1 Fluoxetine | 1 | 655 | Mean Difference (IV, Fixed, 95% CI) | ‐0.80 [‐1.98, 0.38] |
| 4 Discontinuation rates | 2 | 737 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.06 [0.74, 1.52] |
| 4.1 Fluoxetine | 2 | 737 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.06 [0.74, 1.52] |
2.1. Analysis.

Comparison 2 SSRI and related versus Placebo, Outcome 1 Recovered.
2.2. Analysis.
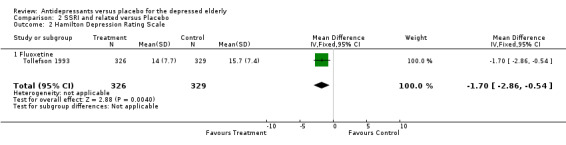
Comparison 2 SSRI and related versus Placebo, Outcome 2 Hamilton Depression Rating Scale.
2.3. Analysis.

Comparison 2 SSRI and related versus Placebo, Outcome 3 Other observer rating scales.
2.4. Analysis.

Comparison 2 SSRI and related versus Placebo, Outcome 4 Discontinuation rates.
Comparison 3. MAOI and related versus Placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Recovered | 2 | 121 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.17 [0.07, 0.39] |
| 1.1 Moclobemine | 1 | 71 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.25 [0.08, 0.81] |
| 1.2 Phenelzine | 1 | 50 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.11 [0.03, 0.37] |
| 2 Hamilton Depression Rating Scale | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.1 Moclobemine | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Phenelzine | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Other observer rating scales | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.1 Moclobemine | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Phenelzine | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Discontinuation rates | 2 | 115 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.84 [0.40, 1.75] |
| 4.1 Moclobemine | 1 | 71 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.84 [0.73, 4.64] |
| 4.2 Phenelzine | 1 | 44 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.22 [0.06, 0.73] |
3.1. Analysis.

Comparison 3 MAOI and related versus Placebo, Outcome 1 Recovered.
3.4. Analysis.
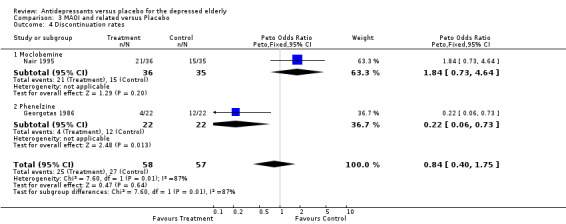
Comparison 3 MAOI and related versus Placebo, Outcome 4 Discontinuation rates.
Comparison 4. Atypical Antidepressants.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Recovered | 2 | 198 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.52 [0.29, 0.93] |
| 1.1 Minaprine | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Mirtazepine | 1 | 97 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.55 [0.24, 1.26] |
| 1.3 Medifoxamine | 1 | 101 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.49 [0.22, 1.11] |
| 2 Hamilton Depression Rating Scale | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.1 Minaprine | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Mirtazepine | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 Medifoxamine | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Other observer rating scales | 1 | 123 | Mean Difference (IV, Fixed, 95% CI) | ‐3.60 [‐7.95, 0.75] |
| 3.1 Minaprine | 1 | 123 | Mean Difference (IV, Fixed, 95% CI) | ‐3.60 [‐7.95, 0.75] |
| 3.2 Mirtazepine | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 Medifoxamine | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Discontinuation rates | 3 | 328 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.81 [0.41, 1.60] |
| 4.1 Minaprine | 1 | 130 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.44 [0.32, 6.57] |
| 4.2 Mirtazepine | 1 | 97 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.77 [0.30, 1.99] |
| 4.3 Medifoxamine | 1 | 101 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.58 [0.16, 2.12] |
4.1. Analysis.

Comparison 4 Atypical Antidepressants, Outcome 1 Recovered.
4.3. Analysis.

Comparison 4 Atypical Antidepressants, Outcome 3 Other observer rating scales.
4.4. Analysis.

Comparison 4 Atypical Antidepressants, Outcome 4 Discontinuation rates.
Comparison 6. Length of Study and recovery.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2 28 days | 4 | 213 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.34 [0.19, 0.63] |
| 4 42 days | 3 | 902 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.52 [0.39, 0.71] |
| 5 49 days | 3 | 201 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.22 [0.12, 0.42] |
6.2. Analysis.

Comparison 6 Length of Study and recovery, Outcome 2 28 days.
6.4. Analysis.

Comparison 6 Length of Study and recovery, Outcome 4 42 days.
6.5. Analysis.
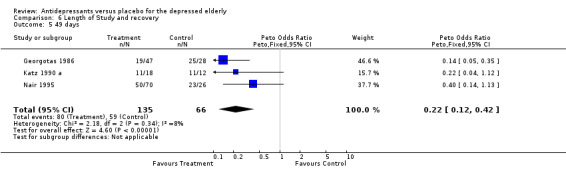
Comparison 6 Length of Study and recovery, Outcome 5 49 days.
Comparison 7. Recruitment source and recovery.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Institutionalised patients | 4 | 281 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.35 [0.21, 0.58] |
| 2 Community dwelling patients | 7 | 1070 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.47 [0.35, 0.62] |
7.1. Analysis.

Comparison 7 Recruitment source and recovery, Outcome 1 Institutionalised patients.
7.2. Analysis.

Comparison 7 Recruitment source and recovery, Outcome 2 Community dwelling patients.
Comparison 8. Types of depression and recovery.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Major depressive disorder | 8 | 1100 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.44 [0.34, 0.58] |
| 2 Other categories of depression | 6 | 312 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.29 [0.18, 0.48] |
8.1. Analysis.

Comparison 8 Types of depression and recovery, Outcome 1 Major depressive disorder.
8.2. Analysis.

Comparison 8 Types of depression and recovery, Outcome 2 Other categories of depression.
Comparison 9. Low Dose antidepressants.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Low dose recovery | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Observer rating scales | 2 | 70 | Mean Difference (IV, Fixed, 95% CI) | ‐2.55 [‐5.50, 0.40] |
| 2.1 Doxepin | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐4.8 [‐8.85, ‐0.75] |
| 2.2 Lofepramine | 1 | 46 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐4.31, 4.31] |
9.2. Analysis.

Comparison 9 Low Dose antidepressants, Outcome 2 Observer rating scales.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Beutler 1987.
| Methods | RCT Concealment of allocation unclear Blindness double Analysis: Endpoint Duration 20 weeks | |
| Participants | Diagnosis Unipolar Major Depression DSMIIIR Community and Advert N=27 Sex unknown Mean age = 71.6 | |
| Interventions | 1. Placebo N=15 2. Alprazolam N=12 0.5mg/d to max 0.8mg/d | |
| Outcomes | HAM‐D BDI Cognitive Error Questionnaire Sleep Efficiency | |
| Notes | Other arms to study Psychotherapy N=64 in total | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Cohn 1984 b.
| Methods | RCT Double Blind Concealment of allocation unclear Analysis: Endpoint Duration: 28 days | |
| Participants | Diagnosis Major Depressive Disorder DSMIII and HAM‐D > 20 Outpatients N= 63 Sex 23M, 40F Mean age = ˜66 | |
| Interventions | 1. Placebo N=21 2. Imipramine N‐21 75mg/d to 200mg/d split dose 3. Nomifensine N=21 75mg/d to 200mg/d split dose | |
| Outcomes | HAM‐D CGI Hopkins symptom Checklist BPRS | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
De Leo 1984.
| Methods | RCT Concealment of allocation unclear Blindness double Duration 28 days | |
| Participants | Diagnosis Primary Depression DSMIII Symptoms present > 5 months Institution for elderly N= 46 Sex 8M, 16F Mean age=74.79+9.51 | |
| Interventions | 1 Placebo N=22 2. Viloxazine N=24 200mg/d to 300mg/d in split dose | |
| Outcomes | Zung SDS HAM‐D CGI TESS | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Georgotas 1986.
| Methods | Concealment of allocation unclear Blindness double Duration 49 days | |
| Participants | Diagnosis Major Depression RDC HAM‐D > 16 Outpatients (Psych) N= 90 Sex Unknown Mean age = 65.7 sd6.8 | |
| Interventions | 1. Placebo N=28 2. Nortriptyline N=25 Plasma levels 50‐180ng/nl 3. Phenelzine N=22 MAO inhibition >70% | |
| Outcomes | HAMD Zung SDS CGI TESS ECG | |
| Notes | Figures given for male/female and age were 75 subjects only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Gerner 1980 a.
| Methods | RCT Concealment of allocation unclear Blindness double Duration 28 days | |
| Participants | Diagnosis Unipolar Depression RDC HAMD >18 Outpatients (Psych) N=60 Sex 23M, 37F Mean age = 68.4 | |
| Interventions | 1. Placebo N=20 2. Trazodone N=19 100 ‐ 400mg/d 3. Imipramine N=21 50 ‐ 200mg/d | |
| Outcomes | HAM‐D HAMA BDI TESS Effects on cognition ECG | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Halikas 1995.
| Methods | RCT Concealment of allocation unclear Blindness double Duration 42 days | |
| Participants | Diagnosis Major Depressive Episode DSMIIIR, HAMD >18 Community & advert recruitment N= 150 Sex 67M, 79F Mean Age = ˜62 | |
| Interventions | 1. Placebo N=50 2. Mirtazapine N=50 5mg/d to 35mg/d 3. Trazodone N=50 40mg/d to 280mg/d | |
| Outcomes | HAMD MADRS Zung SDS CGI | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Hammond 1993.
| Methods | RCT Concealment of allocation unclear Blindness double Duration 56 days | |
| Participants | Diagnosis GMS Case Level Depression with >4 week history MMSE >10 Inpatients (Geriatric) N=82 Sex 20M, 62F Mean age = 80.4+6.6 | |
| Interventions | 1. Placebo N=43 2. Fluoxetine N=39 20 mg/d | |
| Outcomes | HAM‐D MADRS GMS/AGECAT MMSE | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Jansen 1982.
| Methods | RCT Concealment of allocation unclear Blindness double Duration 21 days | |
| Participants | Diagnosis Recurring previously treated depression Inpatients (Psych) N=40 Sex 7M, 33F Mean age = ˜70 | |
| Interventions | 1. Placebo N=20 2.= Diclofensine N=20 50mg/d to 150mg/d | |
| Outcomes | HAMD Befinlichkeitsskala 2 test Mosaik Critical Flicker Frequency 5 point global rating scale for depression | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Kane 1983.
| Methods | RCT Concealment of allocation unclear Blindness double Duration 28 days | |
| Participants | Diagnosis depressed mood and 4 associated symptoms HAM‐D >18 Outpatients (Psych) N=44 Sex 13M, 31F Mean age = 63.9+6.98 | |
| Interventions | 1. Placebo N=7 2. Bupropion (LD)N =11 150mg/d 3. Bupropion (HD)N=13 300mg/d 4. Imipramine N=13 75‐200mg/d | |
| Outcomes | HAM‐D HAMA CGI Zung SDS Zung SAS | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Katz 1990 a.
| Methods | RCT Concealment of allocation unclear Blindness double Duration 49 days | |
| Participants | Diagnosis Major Depressive Disorder DSMIIIR HAM‐D >18 Nursing home or Sheltered housing N=30 Sex 7M, 21F Mean age = 84 | |
| Interventions | 1. Placebo N=7 2. Nortriptyline N=18 start 25mg/d increased to therapeutic plasma levels | |
| Outcomes | HAM‐D GDS CGI | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Lakshmanan 1986.
| Methods | RCT Concealment of allocation unclear Blindness double Duration 21 days | |
| Participants | Diagnosis depressed HAM‐D >18 MMSE >20 Inpatients (Rehab Unit) N=29 Sex unknown Mean age = 75.6 sd4.1 | |
| Interventions | 1. Placebo N= ? 2. Doxepin N= ? 10 ‐ 20mg/d | |
| Outcomes | HAM‐D GDS HVID | |
| Notes | Number completing given but not start number | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Meignan‐Debray 1990.
| Methods | RCT Concealment of allocation unclear Blindness double Duration 42 days | |
| Participants | Diagnosis Major Depressive Disorder DSMIII or MADRS >20 Inpatients (Psych) N= 101 Sex 10M, 91F Mean age = ˜82.5 | |
| Interventions | 1. Placebo N=48 2. Medifoxamine N=53 (100mg/d | |
| Outcomes | MADRS | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Merideth 1984.
| Methods | RCT Concealment of allocation unclear Blindness double Duration 35 days | |
| Participants | Diagnosis Primary Depressive Disorder Feighner Criteria HAM‐D >18 Inpatients N=61 Sex 16M, 45F Mean age = ˜68 | |
| Interventions | 1. Placebo N=19 2. Nomifensine N=22 50 ‐ 200mg/d 3. Imipramine N=20 50 ‐ 200mg/d | |
| Outcomes | HAM‐D CGI | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Nair 1995.
| Methods | RCT Concealment of allocation unclear Blindness double Duration 49 days | |
| Participants | Diagnosis Major Depressive Disorder DSMIIIR HAM‐D >18 CGI >Moderate Unknown recruitment N=109 Sex 32M, 77F Mean age = ˜69 | |
| Interventions | 1. Placebo N=35 2. Moclobemide N=36 100 ‐400mg/d 3. Nortriptyline N=38 25 ‐75mg/d to serum levels 50‐170ng/ml | |
| Outcomes | GDS HAM‐D CGIS MMSE CGIE CGIT | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Parnetti 1991.
| Methods | RCT Concealment of allocation unclear Blindness double Duration 49 days | |
| Participants | Diagnosis Prolonged Reactive Depression ICD9 MMSE >25 Outpatients (Psych) N=130 Sex 47M, 83F Mean age = ˜71 | |
| Interventions | 1. Placebo N=67 2. Minaprine N=63 200mg/d | |
| Outcomes | Evaluation Clinque de la Personalitie Symptom Rating Test CGI | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Tan 1994.
| Methods | RCT Concealment of allocation unclear Blindness double Duration 28 days | |
| Participants | Diagnosis depressed BASDEC >6/21 GDS >15/30 Inpatients (Psych) N=63 Sex 21M, 42F Mean age = 80 | |
| Interventions | 1. Placebo N=31 2. Lofepramine N=32 70mg/d | |
| Outcomes | MADRS GDS | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Tollefson 1993.
| Methods | RCT Concealment of allocation unclear Blindness double Duration 42 days | |
| Participants | Diagnosis Unipolar Major Depression DSMIIIR HAM‐D >16 Outpatients N=671 Sex 305M, 366 Mean age = 67.7 +7.7 | |
| Interventions | 1. Placebo N=336 2. Fluoxetine N=335 20mg/d or every other day | |
| Outcomes | HAM‐D SF‐36 CGI PGI | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Albarede 1983 | Study includes patients with dementia |
| Arcand 1993 | This is a review of literature not a primary source of data |
| Ather 1985 | The trial reported does not have a placebo arm. |
| Balaceanu 1996 | The trial reported does not have a placebo arm. |
| Bella 1990 | Although described as randomised the author describes the randomisation 'Patients were then randomized into two homogenous groups of 30 subjects each with respect to age, sex, education and clinical condition'. |
| Bergener 1968 b | Not all the patients in the study were depressed and other psychiatric diagnosis were included. |
| Bettini 1994 | Patients who were included were described as suffering from 'pathological cerebral involution. |
| Bohm 1990 a | Study included both patients with anxiety or depression |
| Bonavita 1986 | Patients included in the study are not all depressed. |
| Branconnier 1981 | No interpretable data available |
| Branconnier 1983 a | No interpretable data available |
| Cohn 1990 a | Study included both patients with bipolar disorder or depression |
| Cooper 1980 | Patients were included in the study with co‐morbid organicity. |
| Dobie 1992 | Inclusion criteria was tinnitus ‐ not all patients were elderly or depressed. |
| Fabre 1983 | Continuation study |
| Frederiksen 1985 | Patients were included in the study with diagnosis of dementia. |
| Gentili 1984 | Patients with diagnosis of manic depression were included in the study. |
| Hansgen 1993 | Adult population age range 18‐70 years. |
| Hebenstreit 1989 b | Dose titration study. |
| Hubner 1993 | Adult population 20‐64 years |
| Jansen 1984 | No interpretable data available |
| Jarvik 1982 b | Not random allocation 'assignments to one of the trhee groups were made by a non‐blind physician who attampted to keep the goups balanced with regard to age, sex and severity of illness'. |
| Kivela 1987 | Supportive psychotherapy given to all patients by their GP. |
| Koenig 1989 | Study aborted after only three patients randomised. |
| Lapierre 1991 | Study 1. Fixed‐dose double‐blinde controlled study. No ages given so ages checked in paper by Amin et al, 1989 which was cited to have the Canadian arm of the results report ‐ ages given in this 18‐65. Study 2. Forced upward titration double‐blind placebo and amitryptiline controlled study ‐ this study included patients with bipolar affective disorder. Study 3. Dose titration double‐blinde amitriptyline study in elderly depressives ‐ this study did not have a placebo arm. |
| Lavie 1992 | Uses normal controls and demented subjects in study. |
| McNair 1984 | No placebo arm, 12 week double‐crossover double‐blind study of amitriptyline and amoxapine. |
| Mellow 1990 | Non‐randomised crossover trial: 'Six consecutive patients .... every patients received a 2 week placebo period followed by a 2 week active drug period followed by a crossover back to placebo for an additional 2 weeks'. |
| Middleton 1975 | No placebo arm. |
| Monti 1990 | Not a study of elderly patients. |
| Nyth 1992 | Patients were included in the study with a diagnosis of dementia. |
| Petursson 1993 | Study included patients with a diagnosis of dementia. |
| Raffaele 1996 | Not all patients in the study were depressed. |
| Reimherr 1990 | Subjects were adult not aged |
| Rothblum 1982 | All patients received concomitant psychotherapy. |
| Sakalis 1974 a | Study included patients who were demented. |
| Schweizer 1994 | No interpretable data available |
| Siegfried 1986 | No placebo arm. |
| Sunderland 1987 | Study lasted 180 minutes. |
| Sunderland 1994 | No interpretable data available |
| Taeuber 1977 | Reveiw of nomifensine trials ‐ primary sources used. |
| Tammaro 1977 | Patients included in the study were not depressed. |
| Tiller 1990 | Not all patients were elderly or depressed. |
| Truffinet 1990 | Sub‐sample of elderly from much larger study were reanalysed. The elderly had not been separately randomised in the original study. |
| Von Knorring 1980 | Patients with bipolar disorder and dementia were included in the study. |
| Wakelin 1986 | Meta‐analysis paper ‐ primary data in original publications. |
| Wallace 1995 | No interpretable data available |
| Weissman 1992 | All patients received concomitant interpersonal psychotherapy. |
Contributions of authors
K Wilson, Reviewer and main author P Mottram, Reviewer, statistics A Sivanranthan, Reviewer A Nightingale, Search strategy and article retrieval
Declarations of interest
None
Edited (no change to conclusions)
References
References to studies included in this review
Beutler 1987 {published data only}
- Beutler LE, Scogin F, Kirkish P, Schretlen D, Corbishley A, Hamblin D, et al. Group cognitive therapy and alprazolam in the treatment of depression in older adults.. Journal of Consulting and Clinical Psychology 1987;55(4):550‐6. [DOI] [PubMed] [Google Scholar]
Cohn 1984 b {published data only}
- Cohn JB, Varga L, Lyford A. A two‐center double‐blind study of nomifensine, imipramine, and placebo in depressed geriatric outpatients. Journal of Clnical Psychiatry 1984;45(4 Pt 2):68‐72. [PubMed] [Google Scholar]
De Leo 1984 {published data only}
- Leo D, Ceola A, Magni G. Viloxazine against placebo in a double‐blind study in depressed elderly patients. Current Therapeutic Research, Clinical & Experimental 1984;36(2):239‐44. [Google Scholar]
- Leo D, Ceola A, Magni G, Renesto V, Pacchioni A. Viloxazine in the treatment of depression in the aged. Double‐blind placebo study [La viloxazina nel trattamento della depressione dell'anziano. Studio in doppio cieco verso placebo]. Minerva Psichiatrica 1984;25(2):127‐30. [PubMed] [Google Scholar]
Georgotas 1986 {published data only}
- Georgotas A, McCue RE, Cooper T, Chang I, Mir P, Welkowitz J. Clinical predictors of response to antidepressants in elderly patients. Biological Pschiatry 1987;22(6):733‐40. [DOI] [PubMed] [Google Scholar]
- Georgotas A, McCue RE, Cooper TB, Nagachandran N, Friedhoff A. Factors affecting the delay of antidepressant effect in responders to nortriptyline and phenelzine. Psychiatry Research 1989;28(1):1‐9. [DOI] [PubMed] [Google Scholar]
- Georgotas A, McCue RE, Friedman E, Cooper TB. A placebo‐controlled comparison of the effect of nortriptyline and phenelzine on orthostatic hypotension in elderly depressed patients. Journal of Clinical Psychopharmacology 1987;7(6):413‐6. [PubMed] [Google Scholar]
- Georgotas A, McCue RE, Friedman E, Cooper TB. Electrocardiographic effects of nortriptyline, phenelzine, and placebo under optimal treatment conditions. American Journal of Psychiatry 1987;144(6):798‐801. [DOI] [PubMed] [Google Scholar]
- Georgotas A, McCue RE, Friedman E, Cooper TB. Response of depressive symptoms to nortriptyline, phenelzine and placebo. British Journal of Psychiatry 1987;151:102‐6. [DOI] [PubMed] [Google Scholar]
- Georgotas A, McCue RE, Hapworth W, Friedman E, Kim OM, Welkowitz J, et al. Comparative efficacy and safety of MAOIs versus TCAs in treating depression in the elderly. Biological Psychiatry 1986;21(12):1155‐66. [DOI] [PubMed] [Google Scholar]
- Georgotas A, McCue RE, Reisberg B, Ferris SH, Nagachandran N, Chang I, et al. The effects of mood changes and antidepressants on the cognitive capacity of elderly depressed patients. International Psychogeriatrics 1989;1(2):135‐43. [DOI] [PubMed] [Google Scholar]
- Georgotas A, Stokes P, McCue RE, Dubow A, Welkowitz J, Friedman E, et al. The usefulness of DST in predicting response to antidepressants: a placebo‐controlled study. Journal of Affective Disorders 1986;11(1):21‐8. [DOI] [PubMed] [Google Scholar]
Gerner 1980 a {published data only}
- Gerner R, Estabrook W, Steuer J, Jarvik L. Treatment of geriatric depression with trazodone, imipramine, and placebo: a double‐blind study. Journal of Clinical Psychiatry 1980;41(6):216‐20. [PubMed] [Google Scholar]
- Gerner R, Estabrook W, Steuer J, Waltuch L, Kakkar P, Jarvik L. A placebo‐controlled double‐blind study of imipramine and trazodone in geriatric depression. Psychopathology in the Aged 1980;69:167‐82. [PubMed] [Google Scholar]
- Hayes RL, Gerner RH, Fairbanks L, Moran M, Waltuch L. ECG findings in geriatric depressives given trazodone, placebo, or imipramine. Journal of Clinical Psychiatry 1983;44(5):180‐3. [PubMed] [Google Scholar]
Halikas 1995 {published data only}
- Halikas JA. Org 3770 (mirtazapine) versus trazodone: A placebo controlled trial in depressed elderly patients. Human Psychopharmacology 1995;10(Suppl 2):125‐33. [Google Scholar]
Hammond 1993 {published data only}
- Evans M, Hammond M, Wilson K, Lye M, Copeland J. Placebo‐controlled treatment trial of depression in elderly physically ill patients. International Journal of Geriatric Psychiatry 1997;12(8):817‐24. [DOI] [PubMed] [Google Scholar]
- Evans M, Hammond M, Wilson K, Lye M, Copeland J. Treatment of depression in the elderly: Effect of physical illness on response. International Journal of Geriatric Psychiatry 1997;12(12):1189‐94. [PubMed] [Google Scholar]
- Hammond MF, Evans ME, Lye M. Antidepressants and old people. Lancet 1993;342(8865):244‐5. [DOI] [PubMed] [Google Scholar]
Jansen 1982 {published data only}
- Jansen W, Bruckner GW, Henauer S, Omer LM. Clinical double blind comparison of diclofensine and placebo in geriatric patients with depressive syndromes [Klinischer Doppelblindvergleich von Diclofensin und Plazebo bei geriatrischen Patienten mit depressiven Verstimmungszustanden]. Pharmopsychiatria 1982;15(6):205‐9. [DOI] [PubMed] [Google Scholar]
Kane 1983 {published data only}
- Kane JM, Cole K, Sarantakos S, Howard A, Borenstein M. Safety and efficacy of bupropion in elderly patients: preliminary observations. Journal of Clinical Psychiatry 1983;44(5 Pt 2):134‐6. [PubMed] [Google Scholar]
Katz 1990 a {published data only}
- Katz IR, Simpson GM, Curlik SM, Parmelee PA, Muhly C. Pharmacologic treatment of major depression for elderly patients in residential care settings. Journal of Clinical Psychiatry 1990;51(Suppl):41‐7. [PubMed] [Google Scholar]
Lakshmanan 1986 {published data only}
- Lakshmanan M, Mion LC, Frengley JD. Effective low dose tricyclic antidepressant treatment for depressed geriatric rehabilitation patients. A double‐blind study. Journal of the American Geriatric Society 1986;34(6):421‐6. [DOI] [PubMed] [Google Scholar]
Meignan‐Debray 1990 {published data only}
- Meignan‐Debray S, Forette B, Roger M. Double‐blind trial of medifoxamine (Cledial ) versus placebo in elderly patients with depressive disorders [Etude comparative en double aveugle de la medifoxamine (Cledial) versus placebo chez le sujet age deprime]. Psychologie Medicale 1990;22(9):883‐92. [Google Scholar]
Merideth 1984 {published data only}
- Merideth CH, Feighner JP, Hendrickson G. A double‐blind comparative evaluation of the efficacy and safety of nomifensine, imipramine, and placebo in depressed geriatric outpatients. Journal of Clinical Psychiatry 1984;45(4 Pt 2):73‐7. [PubMed] [Google Scholar]
Nair 1995 {published data only}
- Nair NP, Amin M, Holm P, Katona C, Klitgaard N, Ng Ying Kin NM, et al. Moclobemide and nortriptyline in elderly depressed patients. A randomized, multicentre trial against placebo. Journal of Affective Disorders 1995;33(1):1‐9. [DOI] [PubMed] [Google Scholar]
- Ng Ying Kin NM, Klitgaard N, Nair NP, Amin M, Kragh Sorensen P, Schwariz G, et al. Clinical relevance of serum nortriptyline and 10‐hydroxy‐nortriptyline measurements in the depressed elderly: a multicenter pharmacokinetic and pharmacodynamic study. Neuropsychopharmacology 1996;15(1):1‐6. [DOI] [PubMed] [Google Scholar]
- Ng Ying Kin NM, Nair NP, Amin M, Schwartz G, Ahmed SK, Holm P, et al. The dexamethasone suppression test and treatment outcome in elderly depressed patients participating in a placebo‐controlled multicenter trial involving moclobemide and nortriptyline. Biological Psychiatry 1997;42(10):925‐31. [DOI] [PubMed] [Google Scholar]
Parnetti 1991 {published data only}
- Parnetti L, Abate G, Aveni Casucci MA, Balestrieri R, Bartorelli L, Cuzzupoli M, et al. Multicentre, controlled, randomized, double‐blind vs placebo study of minaniprine in elderly patients suffering from prolonged depressive reaction [Studio multicentrico controllato e randomizzato in doppio cieco di minaprina vs placebo in pazienti anziani affetti da disturbo depressivo su base reattiva]. Giornale di Gerontologia 1991;39(3):103‐10. [Google Scholar]
- Parnetti L, Sommacal S, Morselli‐Labate AM, Senin U. Multicentre controlled randomised double‐blind placebo study of minaprine in elderly patients suffering from prolonged depressive reaction. Drug Investigation 1993;6(4):181‐8. [Google Scholar]
Tan 1994 {published data only}
- Tan RS. Lowering antidepressant dosages in the elderly. Clinical Gerontologist 1995;16(1):67‐70. [Google Scholar]
- Tan RS. Prescribing less than recommended doses may be acceptable for geriatric patients. Journal of the American Geriatrics Society 1998;46(4):536. [DOI] [PubMed] [Google Scholar]
- Tan RS, Barlow RJ, Abel C, Reddy S, Palmer AJ, Fletcher AE, et al. The effect of low dose lofepramine in depressed elderly patients in general medical wards. British Journal of Psychopharmacology 1994;37(4):321‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tollefson 1993 {published data only}
- Ackerman DL, Greenland S, Bystritsky A, Small GW. Characteristics of fluoxetine versus placebo responders in a randomized trial of geriatric depression. Psychopharmacology Bulletin 1997;33(4):707‐14. [PubMed] [Google Scholar]
- Beusterien KM, Steinwald B, Ware JE Jr. Usefulness of the SF‐36 Health Survey in measuring health outcomes in the depressed elderly. Journal of Geriatric Psychiatry and Neurology 1996;9(1):13‐21. [DOI] [PubMed] [Google Scholar]
- Goldstein DJ, Hamilton SH, Masica DN, Beasley CM Jr. Fluoxetine in medically stable, depressed geriatric patients: effects on weight. Journal of Clinical Psychopharmacology 1997;17(5):365‐9. [DOI] [PubMed] [Google Scholar]
- Heiligenstein JH, Ware JE Jr, Beusterien KM, Roback PJ, Andrejasich C, Tollefson GD. Acute effects of fluoxetine versus placebo on functional health and well‐being in late‐life depression. International Psychogeriatrics 1995;7(Suppl):125‐37. [DOI] [PubMed] [Google Scholar]
- Koran L, Hertzman M, Meyers B, Holman S, Tollefson G. Predicting response to fluoxetine in geriatric patients with DSM‐III‐R major depression. Psychopharmacology Bulletin 1994;30:76. [DOI] [PubMed] [Google Scholar]
- Koran LM, Hamilton SH, Hertzman M, Meyers BS, Halaris AE, Tollefson GD, et al. Predicting response to fluoxetine in geriatric patients with major depression. Journal of Clinical Psychopharmacology 1995;15(6):421‐7. [DOI] [PubMed] [Google Scholar]
- Schneider LS, Small GW, Hamilton SH, Bystritsky A, Nemeroff CB, Meyers BS. Estrogen replacement and response to fluoxetine in a multicenter geriatric depression trial. American Journal of Geriatric Psychiatry 1997;5(2):97‐106. [PubMed] [Google Scholar]
- Small GW, Birkett M, Meyers BS, Koran LM, Bystritsky A, Nemeroff CB. Impact of physical illness on quality of life and antidepressant response in geriatric major depression. Journal of the American Geriatrics Society 1996;44(10):1220‐5. [DOI] [PubMed] [Google Scholar]
- Small GW, Hamilton SH, Bystritsky A, Meyers BS, Nemeroff CB. Clinical response predictors in a double‐blind, placebo‐controlled trial of fluoxetine for geriatric major depression. International Psychogeriatrics 1995;7(Suppl):41‐53. [DOI] [PubMed] [Google Scholar]
- Small GW, Schneider LS, Hamilton SH, Bystritsky A, Meyers BS, Nemeroff CB. Site variability in a multisite geriatric depression trial. International Journal of Geriatric Psychiatry 1996;11(12):1089‐95. [Google Scholar]
- Tollefson GD, Bosomworth JC, Heiligenstein JH, Potvin JH, Holman S. A double‐blind, placebo‐controlled clinical trial of fluoxetine in geriatric patients with major depression. International Psychogeriatrics 1995;7(1):89‐104. [DOI] [PubMed] [Google Scholar]
- Tollefson GD, Holman SL. Analysis of the Hamilton Depression Rating Scale factors from a double‐blind, placebo‐controlled trial of fluoxetine in geriatric major depression. International Journal of Psychopharmacology 1993;8(4):253‐9. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Albarede 1983 {published data only}
- Albarede JL, Boye JB, Broutin JM, et al. Efficacity of nomifensin (Alival) in geriatrics: Multicentric double blind study against a placebo [Efficacite de la nomifensine (Alival) en geriatrie: Etude multicentrique en double aveugle contre placebo]. Revue de Geriatrie 1983;8(4):195‐201. [Google Scholar]
Arcand 1993 {published data only}
- Arcand M, Hottin P. Treatment of depression in the elderly. The use of psychostimulants [Le traitement de la depression chez les personnes agees. L'utilite des psychostimulants]. Canadian Family Physician 1993;39:2420‐6. [PMC free article] [PubMed] [Google Scholar]
Ather 1985 {published data only}
- Ather SA, Ankier SI, Middleton RS. A double‐blind evaluation of trazodone in the treatment of depression in the elderly. British Journal of Clinical Practice 1985;39(5):192‐9. [PubMed] [Google Scholar]
Balaceanu 1996 {published data only}
- Balaceanu‐Stolnici C, Covic M, Manoiu A, Vrabiescu M. Double blind study concerning the antidepressive effects and the clinical tolerance of Gerovital H‐3 without potassium metabisulphate. Romanian Journal of Gerontology & Geriatrics 1996;17(1‐2):46‐61. [Google Scholar]
Bella 1990 {published data only}
- Bella R, Biondi R, Raffaele R, Pennisi G. Effect of acetyl‐L‐carnitine on geriatric patients suffering from dysthymic disorders. International Journal of Clinical Pharmacology Research 1990;10(6):355‐60. [PubMed] [Google Scholar]
- Fulgente T, Onofrj M, Re ML, Ferracci F, Bazzano S, Ghilardi MF, et al. Laevo‐acetylcarnitine (Nicetile registered ) treatment of senile depression. Clinical Trials Journal 1990;27(3):155‐63. [Google Scholar]
Bergener 1968 b {published data only}
- Bergener M, Undeutsch D. Methods and problems of clinical psychopharmacology. Results of a double blind study with medazepam and placebo in geriatric psychiatry [Methoden und Probleme der klinischen Psychopharmakologie Ergebnisse einer Doppelblindstudie mit Medazepam und Placebo in der Alterspsychiatrie]. Arzneimittelforschung 1968;18(12):1563‐6. [PubMed] [Google Scholar]
Bettini 1994 {published data only}
- Bettini R, Ferrari M, Gobbi G, Volanti F, Piccinelli O. Clinical and instrumental evaluation of the effect of tiapride in senile involutional syndrome [Valutazione clinica e strumentale dell'effetto della tiapride sulla sindrome involutiva senile]. Clinica Terapeutica 1994;144(3):231‐6. [PubMed] [Google Scholar]
Bohm 1990 a {published data only}
- Bohm C, Robinson DS, Gammans RE. Buspirone therapy for elderly patients with anxiety or depressive neurosis. Journal of Clinical Psychiatry 1990;51(7):309. [PubMed] [Google Scholar]
- Bohm C, Robinson DS, Gammans RE, Shrotriya RC, Alms DR, Leroy A, et al. Buspirone therapy in anxious elderly patients: a controlled clinical trial. Journal of Clinical Psychopharmacology 1990;10(3 Suppl):47‐51. [DOI] [PubMed] [Google Scholar]
Bonavita 1986 {published data only}
- Bonavita E. Study of the efficacy and tolerability of L‐acetylcarnitine therapy in the senile brain.. International Journal of Clinical Pharmacology, Therapy and Toxicology 1986;24(9):511‐6. [PubMed] [Google Scholar]
Branconnier 1981 {published data only}
- Branconnier RJ, Cole JO, Ghazvinian S. The therapeutic profile of mianserin in mild elderly depressives. Psychopharmacology Bulletin 1981;17(1):129‐31. [PubMed] [Google Scholar]
- Branconnier RJ, Cole JO, Ghazvinian S, Rosenthal S. Treating the depressed elderly patient: The comparative behavioural pharmacology of mianserin and amitriptyline. In: Costa E, Racagni G editor(s). Typical and atypical antidepressants: Clinical Practice. New York: Raven Press, 1982. [PubMed] [Google Scholar]
Branconnier 1983 a {published data only}
- Branconnier RJ, Cole JO, Ghazvinian S, Spera KF, Oxenkrug GF, Bass JL. Clinical pharmacology of bupropion and imipramine in elderly depressives. Journal of Clinical Psychiatry 1983;44(5):130‐3. [PubMed] [Google Scholar]
Cohn 1990 a {published data only}
- Lapierre YD. Controlling acute episodes of depression. International Clinical Psychopharmacology 1991;6(Suppl 2):23‐35. [DOI] [PubMed] [Google Scholar]
Cooper 1980 {published data only}
- Cooper AJ, Datta SR. A placebo controlled evaluation of L‐tryptophan in depression in the elderly. Canadian Journal of Psychiatry. Revue Canadienne de Psychiatrie 1980;25(5):386‐90. [DOI] [PubMed] [Google Scholar]
Dobie 1992 {published data only}
- Dobie RA, Sakai CS, Sullivan MD, Katon WJ, Russo J. Antidepressant treatment of tinnitus patients: report of a randomized clinical trial and clinical prediction of benefit. American Journal of Otology 1993;14(1):18‐23. [PubMed] [Google Scholar]
Fabre 1983 {published data only}
- Fabre LF, Brodie HK, Garver D, Zung WW. A multicenter evaluation of bupropion versus placebo in hospitalized depressed patients. Journal of Clinical Psychiatry 1983;44(5 Pt 2):88‐94. [PubMed] [Google Scholar]
Frederiksen 1985 {published data only}
- Frederiksen SO, d'Elia G, Bengtsson BO. ACTH 4‐9 analogue (Org 2766) in depressed elderly patients. I. Effect on depressed mood. Acta Psychiatrica Scandinavica 1985;72(4):341‐8. [DOI] [PubMed] [Google Scholar]
- Frederiksen SO, d'Elia G, Bengtsson BO. ACTH 4‐9 analogue (Org 2766) in depressed elderly patients. II. Effect on memory and vigilance. Acta Psychiatrica Scandinavica 1985;72(4):349‐57. [DOI] [PubMed] [Google Scholar]
Gentili 1984 {published data only}
- Gentili P, Maria F, Vanna M, Drago F, Omer LM, Ismail S. Diclofensine in the treatment of depressed geriatric hospitalized patients: a placebo‐controlled double‐blind trial. Current Therapeutic Research, Clinical & Experimental 1984;35(3):386‐95. [Google Scholar]
Hansgen 1993 {published data only}
- Hansgen KD, Vesper J, Ploch M. Multicenter double‐blind study examining the antidepressant effectiveness of the hypericum extract LI 160. Journal of Geriatric Psychiatry and Neurology 1994;7(Suppl 1):S15‐8. [DOI] [PubMed] [Google Scholar]
Hebenstreit 1989 b {published data only}
- Hebenstreit GF, Fellerer K, Zochling R, Zentz A, Dunbar GC. A pharmacokinetic dose titration study in adult and elderly depressed patients. Acta Psychiatrica Scandinavica. Supplementum 1989;350:81‐4. [DOI] [PubMed] [Google Scholar]
Hubner 1993 {published data only}
- Hubner WD, Lande S, Podzuweit H. Hypericum treatment of mild depressions with somatic symptoms. Journal of Geriatric Psychiatry and Neurology 1994;7(Suppl 1):S12‐4. [DOI] [PubMed] [Google Scholar]
Jansen 1984 {published data only}
- Jansen W, Siegfried K. Nomifensine in geriatric inpatients: A placebo‐controlled study. Journal of Clinical Psychiatry 1984;45(4 Pt 2):63‐7. [PubMed] [Google Scholar]
Jarvik 1982 b {published data only}
- Jarvik LF, Mintz J, Steuer J, Gerner R. Treating geriatric depression: A 26‐week interim analysis. Journal of the American Geriatrics Society 1982;30(11):713‐7. [DOI] [PubMed] [Google Scholar]
- Jarvik LF, Read SL, Mintz J, Neshkes RE. Pretreatment orthostatic hypotension in geriatric depression: predictor of response to imipramine and doxepin. Journal of Clinical Psychopharmacology 1983;3(6):368‐72. [PubMed] [Google Scholar]
Kivela 1987 {published data only}
- Kivela SL, Lehtomaki E. Sulpiride and placebo in depressed elderly outpatients: A double‐blind study. International Journal of Geriatric Psychiatry 1987;2(4):255‐60. [Google Scholar]
Koenig 1989 {published data only}
- Koenig HG, Goli V, Shelp F, Kudler HS, Cohen HJ, Meador KG, et al. Antidepressant use in elderly medical inpatients: lessons from an attempted clinical trial. Journal of General Internal Medicine 1989;4(6):498‐505. [DOI] [PubMed] [Google Scholar]
Lapierre 1991 {published data only}
- EXTRA REF Fabre LF, Abuzzahab FS, Amin M, Claghorn JL, Mendels J, Petrie WM, et al. Sertraline safety and efficacy in major depression: a double‐blind fixed‐dose comparison with placebo. Biological Psychiatry 1995;38(9):592‐602. [DOI] [PubMed] [Google Scholar]
- Lapierre YD. Controlling acute episodes of depression. International Clinical Psychopharmacology 1991;6(Suppl 2):23‐35. [DOI] [PubMed] [Google Scholar]
Lavie 1992 {published data only}
- Lavie P, Aharon Peretz J, Klein F, Gruner F, Epstein R, Tzischinsky O, et al. Sleep quality in geriatric depressed patients: Comparison with elderly demented patients and normal controls and the effects of moclobemide. Dementia 1992;3(5‐6):360‐6. [Google Scholar]
McNair 1984 {published data only}
- McNair DM, Kahn RJ, Frankenthaler LM, Faldetta LL. Amoxapine and amitriptyline: II. Specificity of cognitive effects during brief treatment of depression. Psychopharmacology 1984;83(2):134‐9. [DOI] [PubMed] [Google Scholar]
Mellow 1990 {published data only}
- Mellow AM, Lawlor BA, Sunderland T, Mueller EA, Molchan SE, Murphy DL. Effects of daily oral m‐chlorophenylpiperazine in elderly depressed patients. Initial experience with a serotonin agonist. Biological Psychiatry 1990;28(7):588‐94. [DOI] [PubMed] [Google Scholar]
Middleton 1975 {published data only}
- Middleton RS. A comparison between maprotiline (Ludiomil) and imipramine in the treatment of depressive illness in the elderly. Journal of International Medical Research 1975;3(Suppl 2):79‐83. [Google Scholar]
Monti 1990 {published data only}
- Monti JM, Alterwain P, Monti D. The effects of moclobemide on nocturnal sleep of depressed patients. Journal of Affective Disorders 1990;20(3):201‐8. [DOI] [PubMed] [Google Scholar]
Nyth 1992 {published data only}
- Gottfries CG. Depression in the elderly: treatment with selective 5‐HT reuptake blockers. Psychopharmacology 1993;111:B4. [Google Scholar]
- Gottfries CG, Karlsson I, Nyth AL. Treatment of depression in elderly patients with and without dementia disorders. International Clinical Psychopharmacology 1992;6(Suppl 5):55‐64. [DOI] [PubMed] [Google Scholar]
- Nyth AL, Gottfries CG, Lyby K, Smedegaard‐Andersen L, Gylding‐Sabroe J, Kristensen M, et al. A controlled multicenter clinical study of citalopram and placebo in elderly depressed patients with and without concomitant dementia. Acta Psychiatrica Scandinavica 1992;86(2):138‐45. [DOI] [PubMed] [Google Scholar]
Petursson 1993 {published data only}
- Petursson H. A controlled study of moclobemide in elderly depression with cognitive decline. Psychopharmacology 1993;111:B5. [Google Scholar]
- Roth M, Mountjoy CQ, Amrein R. Moclobemide in elderly patients with cognitive decline and depression: an international double‐blind, placebo‐controlled trial. British Journal of Psychiatry 1996;168:149‐57. [DOI] [PubMed] [Google Scholar]
Raffaele 1996 {published data only}
- Raffaele R, Rampello L, Vecchio I, Tornali C, Malaguarnera M. Trazodone therapy of the post‐stroke depression. Archives of Gerontology and Geriatrics 1996;23(Suppl 5):217‐20. [DOI] [PubMed] [Google Scholar]
Reimherr 1990 {published data only}
- Lapierre YD. Controlling acute episodes of depression. International Clinical Psychopharmacology 1991;6(Suppl 2):23‐35. [DOI] [PubMed] [Google Scholar]
Rothblum 1982 {published data only}
- Rothblum ED, Sholomskas AJ, Berry C, Prusoff BA. Issues in clinical trials with the depressed elderly. Journal of the American Geriatrics Society 1982;30(11):694‐9. [DOI] [PubMed] [Google Scholar]
Sakalis 1974 a {published data only}
- Sakalis G, Oh D, Gershon S, Shopsin B. A trial of Gerovital H‐3 in depression during senility. Current Therapeutic Research, Clinical & Experimental 1974;16(1):59‐63. [PubMed] [Google Scholar]
Schweizer 1994 {published data only}
- Schweizer E, Rickels K, Hassman H, Garcia‐Espana F. Buspirone and imipramine for the treatment of major depression in the elderly. Journal of Clinical Psychiatry 1998;59(4):175‐83. [DOI] [PubMed] [Google Scholar]
- Schweizer E, Rickels K, Hassman H. A double‐blind, placebo‐controlled comparison of imipramine and buspirone in the treatment of major depression in the elderly in the community. Pharmocology Bulletin 1994;30(4):639. [Google Scholar]
Siegfried 1986 {published data only}
- Siegfried K, O'Connolly M. Cognitive and psychomotor effects of different antidepressants in the treatment of old age depression. International Clinical Psychopharmacology 1986;1(3):231‐43. [DOI] [PubMed] [Google Scholar]
Sunderland 1987 {published data only}
- Newhouse PA, Sunderland T, Tariot PN, Weingartner H, Thompson K, Mellow AM, et al. The effects of acute scopolamine in geriatric depression. Archives of General Psychiatry 1988;45(10):906‐12. [DOI] [PubMed] [Google Scholar]
Sunderland 1994 {published data only}
- Sunderland T, Cohen RM, Molchan S, Lawlor BA, Mellow AM, Newhouse PA, et al. High‐dose selegiline in treatment‐resistant older depressive patients. Archives of General Psychiatry 1994;51(8):607‐15. [DOI] [PubMed] [Google Scholar]
Taeuber 1977 {published data only}
- Taeuber K. Comparison of nomifensine and placebo. British Journal of Clinical Pharmacology 1977;4(Suppl 2):209‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tammaro 1977 {published data only}
- Tammaro A, Picceo MT, Gemmellaro P, Bonaccorso O. Camazepam versus placebo. A double‐blind clinical study on geriatric patients suffering from psychic complaints. Arzneimittelforschung 1977;27(11):2177‐8. [PubMed] [Google Scholar]
Tiller 1990 {published data only}
- Tiller JW. Antidepressants, alcohol and psychomotor performance.. Acta Psychiatrica Scandinavica, Supplementum 1990;360:13‐7. [DOI] [PubMed] [Google Scholar]
Truffinet 1990 {published data only}
- Truffinet P, Rouillon F, Serrurier D, Phillips R. Relapses of unipolar depression in the elderly and the efficacy of maprotiline [Rechutes de la depression unipolaire chez le sujet age et efficacite de la maprotiline]. Psychologie Medicale 1990;22(8):769‐77. [Google Scholar]
Von Knorring 1980 {published data only}
- Knorring L. A double‐blind trial: vivalan against placebo in depressed elderly patients.. Journal of International Medical Research 1980;8(1):18‐21. [DOI] [PubMed] [Google Scholar]
Wakelin 1986 {published data only}
- Wakelin JS. Fluvoxamine in the treatment of the older depressed patient; double‐blind, placebo‐controlled data.. International Clinical Psychopharmacology 1986;1(3):221‐30. [DOI] [PubMed] [Google Scholar]
Wallace 1995 {published data only}
- Wallace AE, Kofoed LL, West AN. Double‐blind, placebo‐controlled trial of methylphenidate in older, depressed, medically ill patients.. American Journal of Psychiatry 1995;152(6):929‐31. [DOI] [PubMed] [Google Scholar]
Weissman 1992 {published data only}
- Weissman MM, Prusoff B, Sholomskas AJ, Greenwald S. A double‐blind clinical trial of alprazolam, imipramine, or placebo in the depressed elderly. Journal of Clinical Psychopharmacology 1992;12(3):175‐82. [PubMed] [Google Scholar]
Additional references
BMA 1999
- British Medical Association & Royal Pharmaceutical Society of Great Britain. British National Formulary. 38. London: BMA, 1999. [Google Scholar]
Cole 1993
- Cole MG, Primeau FJ. Prognosis of delirium in elderly hospital patients. CMAJ 1993;149(1):41‐6. [PMC free article] [PubMed] [Google Scholar]
Davidson 1988
- Davidson IA, Dewey ME, Copeland JR. The relationship between mortality and mental disorder: Evidence from the Liverpool longitudinal study. International Journal of Geriatric Psychiatry 1988;3(2):95‐8. [Google Scholar]
Dening 2000
- Dening TR, Bains J. Antidepressant drug therapy for depression in people with dementia. Cochrane Library 2000, Issue 2. [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. [DOI] [PubMed] [Google Scholar]
Georgotas 1988
- Georgotas A, McCue RE, Cooper TB, Nagachandran N, Chang I. How effective and safe is continuation therapy in elderly depressed patients?. Archives of General Psychiatry 1988;45(10):929‐32. [DOI] [PubMed] [Google Scholar]
Gold 1988
- Gold PW, Goodwin FK, Chrousos GP. Clinical and biochemical manifestations of depression. Relation to the neurobiology of stress. New England Journal of Medicine 1988;319(7):413‐20. [DOI] [PubMed] [Google Scholar]
Hamilton 1960
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery & Psychiatry 1960;23:56‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mulrow 1996
- Mulrow CD, Oxman AD. Cochrane Collaboration Handbook. Oxford: Update Software, 1996. [Google Scholar]
Murphy 1988
- Murphy E, Smith R, Lindesay J, Slattery J. Increased mortality rates in late‐life depression. British Journal of Psychiatry 1988;152:347‐53. [DOI] [PubMed] [Google Scholar]
Rabbitt 1993
- Rabbitt P. Baseline changes in cognitive performance with age. In: Levy R, Howard R, Burns A editor(s). Treatment and care in old age psychiatry. Petersfield: Wrightson Biomedical, 1993. [Google Scholar]
Reynolds 1996
- Reynolds CF, Frank E, Kupfer DJ, Thase ME, Perel JM, Mazumdar S, et al. Treatment outcome in recurrent major depression: a post hoc comparison of elderly ("young old") and midlife patients. American Journal of Psychiatry 1996;153(10):1288‐92. [DOI] [PubMed] [Google Scholar]
Sackett 1997
- Sackett D. Cochrane Collaboration Handbook. Oxford: Update Software, 1997. [Google Scholar]
Saunders 1993
- Saunders PA, Copeland JR, Dewey ME, Gilmore C, Larkin BA, Phaterpekar H, et al. The prevalence of dementia, depression and neurosis in later life: The Liverpool MRC‐APPHA study. International Journal of Epidemiology 1993;22(5):838‐47. [DOI] [PubMed] [Google Scholar]
Schneider 1995
- Schneider LS, Olin JT. Efficacy of acute treatment for geriatric depression. International Psychogeriatrics 1995;7(Suppl):7‐25. [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias discussions of methological quality associated with estimates of treatment effect in controlled trials. JAMA 1995;273(5):408‐12. [DOI] [PubMed] [Google Scholar]
Ware 1992
- Ware JE Jr, Sherbourne CD. The MOS 36‐item short‐form health survey (SF‐36); 1, Conceptural framework and item selection. Medical Care 1992;30(6):473‐83. [PubMed] [Google Scholar]
Wilson 1999
- Wilson KC, Copeland JR, Taylor S, Donoghue J, McCracken CF. Natural history of pharmacotherapy of older depressed community residents. British Journal of Psychiatry 1999;175:439‐43. [DOI] [PubMed] [Google Scholar]


