Abstract
Red pigmented marine bacteria, Pseudoalteromonas rubra strains PS1 and SB14, were isolated from two sampling locations in different ecosystems on Alor Island, Indonesia, and cultured in the laboratory. We analyzed the 16S rRNA gene sequences and examined the pigment composition and found that both strains produced cycloprodigiosin (3), prodigiosin (4), and 2-methyl-3-hexyl-prodiginine (5) as major compounds. In addition, we detected three minor compounds: prodigiosin derivatives 2-methyl-3-propyl prodiginine (1), 2-methyl-3-butyl prodiginine (2), and 2-methyl-3-heptyl-prodiginine (6). To our knowledge, this is the first report that P. rubra synthesizes not only prodigiosin and cycloprodigiosin but also four prodigiosin derivatives that differ in the length of the alkyl chain. The antimicrobial activity of cycloprodigiosin, prodigiosin, and 2-methyl-3-hexyl-prodiginine was examined by a disk-diffusion test against Escherichia coli, Staphylococcus aureus, Salmonella typhi, and Candida albicans. We found that, at a concentration of 20 μg/mL, cycloprodigiosin showed the greatest inhibition (25.1 ± 0.55 mm) against S. aureus.
Introduction
Antimicrobial agents are drugs generally obtained from microorganisms that are crucial to the prevention and treatment of bacterial infection. However, the use of antibiotics in medicine, which is too frequent and inappropriate, contributes to the rapid spread of antibiotic resistance.1 Antimicrobial resistance (AMR) poses a serious global problem. A previous report from the World Health Organization (WHO) combined available global data on AMR in seven common bacterial pathogens2 and highlighted a lack of systematic data on AMR in Southeast Asia.3 Nevertheless, it was reported that nosocomial infections caused by antimicrobial-resistant bacteria resulted in 38,481 deaths in Thailand.4 New drugs are needed because of decreases in drug efficacy and the need to combat antibiotic resistance. Marine microorganisms represent a significant source of new drugs for development due to their rich biodiversity and genetic capacity to produce unique metabolites.
Prodiginines are a family of microbial red pigments produced as secondary metabolites by many bacterial species.5−7 There are two types of prodiginines:7,8 linear alkyl side chain-containing compounds, such as prodigiosin and undecylprodigiosin, and cyclic compounds, such as cycloprodigiosin, metacycloprodigiosin, prodigiosin R1, and streptorubin B. Prodiginines have received considerable interest because of their numerous biological activities, including antimicrobial,9 anticancer,10,11 antitumor,12 and anti-inflammatory activities.13 Recent studies have shown that the antimicrobial effect of prodigiosin on bacterial physiology was concentration-dependent. In Escherichia coli cells treated with prodigiosin above the minimum inhibitory concentration (MIC), no significant DNA damage or cytoplasmic membrane disintegration was observed.14 However, prodigiosin was observed to be responsible for plasma membrane leakage.15
Although prodiginines were extracted from the terrestrial bacteria Serratia marcescens(16,17) early on, these compounds were later obtained from several bacteria in the genera Pseudomonas, Pseudoalteromonas, Hahella, Vibrio, and Zooshikella, which were isolated from different marine habitats.18−20 The genus Pseudoalteromonas is a group of widespread marine bacteria. There are three species from this genus, Pseudoalteromonas (P.) rubra, P. denitrificans, and P. bacteriolytica, which are known to produce prodigiosin.21−23
Currently, of the 200 Pseudoalteromonas genomes submitted to the National Center for Biotechnology Information (NCBI),61 there are nine genomes that are code for biosynthetic enzymes that responsible for synthesize prodiginines: one belongs to P. denitrificans, and eight belong to P. rubra.24P. rubra strain ATCC 29570 (formerly Alteromonas rubra NCMB 1890) isolated from the Mediterranean Sea is a Gram-negative, red, and motile marine bacterium that has antibiotic activity against Staphylococcus epidermidis.21 This strain has been reported to synthesize prodigiosin; however, there is little information on the prodigiosin itself, except for absorption spectra of the crude pigment extract. Later, fractionation of a prodigiosin extract was conducted using a high-performance liquid chromatography (HPLC) system. Several prodigiosins were separated, but there was no detailed compound identification provided. Gerber and Gauthier25 found a new prodigiosin-like pigment from P. rubra with a molecular mass of (m/z) 321 that is known as cycloprodigiosin. Over more than three decades, other types of prodigiosin derivatives, 2-methyl-3-butyl-prodiginine, 2-methyl-3-hexyl-prodiginine, and 2-methyl-3-heptyl-prodiginine, were identified in the marine bacterium Pseudoalteromonas sp. 1020R, which has 99% homology to P. rubra.26 Strain 1020R produced only prodigiosin and its derivatives with different alkyl chains. Until now, there have been no reports on a P. rubra strain that contains cycloprodigiosin, except for the originally identified P. rubra strain (ATCC 29570). The previous studies indicate that the strains of P. rubra are compositionally heterogeneous in terms of prodigiosins, and information concerning prodigiosins composition in each strain is important to distinguish subspecies among the strains of P. rubra.
Recently, we isolated two new marine bacterial strains that have UV–Vis spectral characteristics of prodigiosins. The strains were designated P. rubra PS1 and P. rubra SB14 according to a 16S rRNA sequence analysis and blast search, which revealed more than 99% similarity to P. rubra ATCC 29570. The prodigiosins were successfully separated and identified by reversed-phase (RP)-HPLC, and the purified compounds were identified by electrospray ionization-mass spectrometry (ESI-MS/MS). Here, we report the bacterial isolation and the determination of the prodigiosin composition and the antimicrobial activity of three types of prodiginines isolated from P. rubra strain PS1 against pathogenic microorganisms such as Escherichia coli, Staphylococcus aureus, Salmonella typhi, and Candida albicans.
Results and Discussion
Bacterial Isolation and Spectrophotometric Analysis
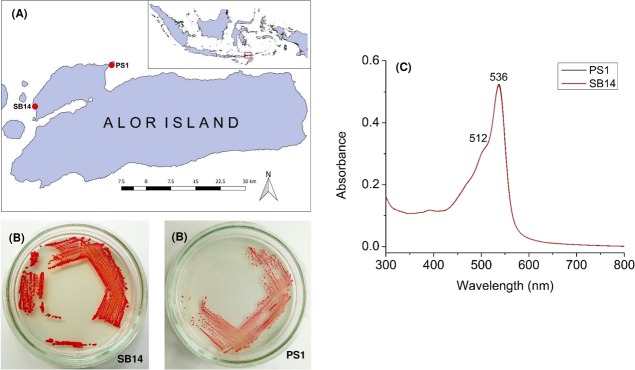
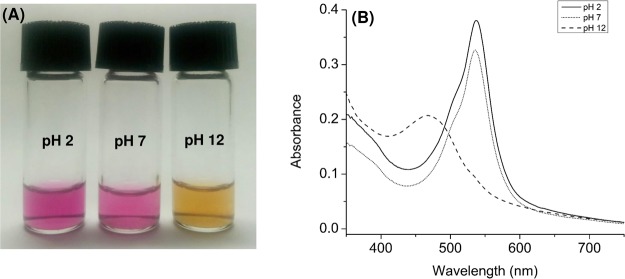
Two strains were successfully isolated from different habitats. Strain PS1 was isolated from seawater in a habitat dominated by seagrasses and mammals such as Dugong dugon in Sika Island. Strain SB14 was isolated from seawater in a habitat of a healthy coral reef ecosystem in Sebanjar Beach (Figure 1A,B). The UV–Vis absorption spectra of the methanol extract of strains PS1 and SB14 were characterized by a broad band in the 400–600 nm wavelength range with an absorption maximum (λmax) at 536 nm and a shoulder at 512 nm (Figure 1C). These spectra were identical to the UV–Vis absorption spectrum of prodiginine pigments.27,28 The UV–Vis spectra of prodiginine were also recorded in 95% MeOH at different pH values. Under acidic conditions (pH 2, adjusted with 0.01 N HCl), its color was red with λmax at 536 nm. Under neutral conditions, the color changed to pink, and the intensity decreased. Under alkali conditions (pH 12, adjusted with 0.01 NaOH), it was orange, and the spectra shifted to the left with λmax at 465 nm (Figure 2), indicating deprotonation of the nitrogen atoms on the three conjugated pyrrole rings by NaOH.29 These spectral changes are in line with the ones reported by Song et al.30
Figure 1.
(A) Sampling locations on Alor Island; inset shows the Indonesian Archipelago. (B) Two isolated strains, PS1 and SB14. (C) UV–Vis absorption spectra of their crude pigment extracts in methanol. (Photos were taken by E. Setiyono.)
Figure 2.
(A) Differences in the color of prodiginine in acidic (pH 2), neutral (pH 7), and alkaline (pH 12) conditions. (B) Associated UV–Vis absorption spectra. (Photos were taken by E. Setiyono.)
Molecular Identification of Bacteria
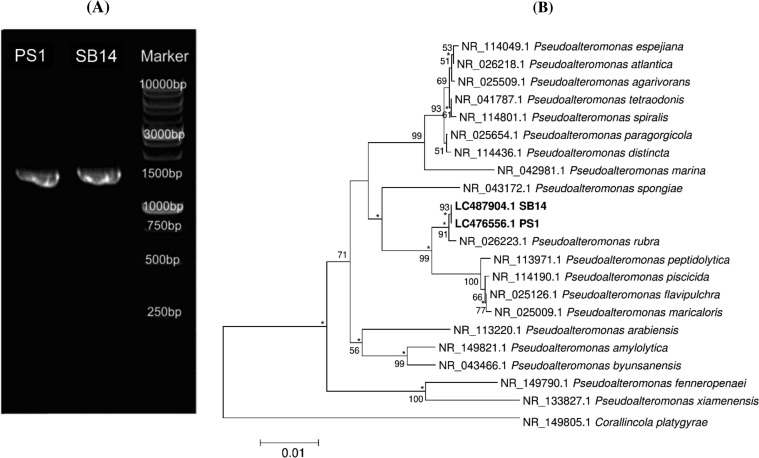
The 16S rRNA gene sequences of strains PS1 and SB14 comprised 1294 and 1343 bp, respectively. The nucleotide data from both strains have been deposited in the DNA Data Bank of Japan (DDBJ; https://ddbj.nig.ac.jp) with accession numbers LC476556 and LC487904 for strains PS1 and SB14, respectively. The similarity of the 16S rRNA genes of these strains was calculated using the nucleotide BLAST program (NCBI) for highly similar sequences (megablast). A comparative study of the 16S rRNA gene sequences showed that strains PS1 and SB14 shared 99.46 and 99.48% sequence similarity, respectively, with P. rubra strain ATCC 29570. In the phylogeny constructed as a neighbor-joining tree, strains PS1 and SB14 were grouped with P. rubra strain ATCC 29570, with a bootstrap resampling value of 91% (Figure 3). The relationship of strains PS1 and SB14 with P. rubra strain ATCC 29570 was also determined in trees constructed with the maximum-likelihood and maximum-parsimony algorithms, and the recovered nodes are marked by asterisks in Figure 3 below.
Figure 3.
(A) 16S rRNA gene PCR amplicons on 0.8% agarose gel of P. rubra strains PS1 and SB14. (B) Phylogeny of strains PS1 and SB14, the type strains of recognized species in the genus Pseudoalteromonas, and representatives of related taxa. Corallincola platygyrae was used as an outgroup. Only bootstrap values >50% (expressed as percentages of 1000 replications) are shown at branch points. Asterisks indicate that the corresponding nodes were also recovered in the trees generated with the maximum-likelihood and maximum-parsimony algorithms. Bar, 0.01 substitutions per nucleotide position.
At the time of writing this report, there were 45 strains of P. rubra, including our two strains, which were isolated from different sources, that have been deposited in the NCBI (www.ncbi.nlm.nih.gov) (Table S1, Supporting Information). P. rubra has been discovered throughout the region spanning the Indian Pacific to Atlantic Oceans. However, most strains have been found in the Indo-Pacific area (Figure S1, Supporting Information). The first P. rubra strain identified with prodiginine production capability was isolated from seawater.21 The 45 strains were isolated from various habitats: 14 from seawater, 7 from coral, 5 from macroalgae, 4 from tunicates, 3 each from sponges and copepods, 2 each from nudibranchs and mussels, and 1 each from sediment, protist, fish, leaf, and larval stone (Figure S2, Supporting Information).
HPLC Analysis of Prodiginine and Its Derivatives
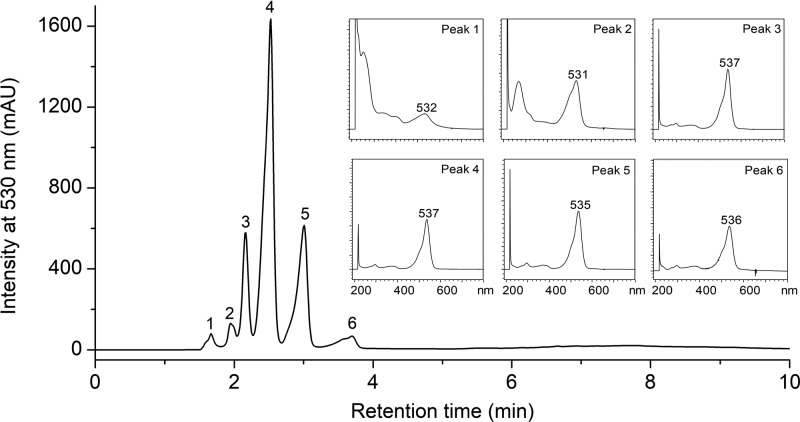
Prodiginine and its derivatives from the pigment extracts of P. rubra strains PS1 and SB14 were separated on a C8 column. Both strains have the same prodiginine composition based on their HPLC chromatograms. The HPLC separation profile contains six well-resolved compounds (Figure 4).
Figure 4.
HPLC chromatogram of the crude pigment extract of P. rubra strain PS1 and the UV–Vis spectra of the eluent peaks.
The first two compounds, peaks #1 and #2, are minor compounds eluted at retention times (tR) of 1.66 and 1.94 min with λmax at 532 and 531 nm, respectively. The third compound (peak #3) eluted at a tR of 2.16 min with λmax at 537 nm. The fourth compound (peak #4) eluted at a slightly more nonpolar tR than peak #3 and was a major compound with a tR of 2.52 min and λmax at 537 nm. The last two compounds (peaks #5 and #6) had a tR of 3.00 and 3.69 min and λmax at 535 and 536 nm, respectively. Next, the six compounds (peaks #1, #2, #3, #4, #5, and #6) were analyzed in highly purified samples (purity of >97%) by ESI-MS/MS and assigned as follows with descriptions below: 2-methyl-3-propyl prodiginine (peak #1), 2-methyl-3-butyl prodiginine (peak #2), cycloprodigiosin (peak #3), prodigiosin (peak #4), 2-methyl-3-hexyl prodiginine (peak #5), and 2-methyl-3-heptyl prodiginine (peak #6). The identification of prodigiosin and four derivatives was confirmed by the linear relationship between the log capacity factor (k′) of the pigments and the number of the carbon atoms in the alkyl side chain of the pigment molecule (Figure S3, Supporting Information), as shown previously for esterifying alcohols in chlorophylls31 and hydroxyl moieties in β-carotene congeners.32 The characteristics of the purified pigments are listed in Table 1.
Table 1. Identification of Prodiginine Pigments in P. rubra Strain PS1a.
| peak # | identification | tR (min) | λmax (nm) | molecular ion (m/z) | product ion (m/z) | CE (V) | compound formula |
|---|---|---|---|---|---|---|---|
| 1 | 2-methyl-3-propyl prodiginine | 1.66 | 532 | 296.1 [M + H]+ | 92.0 [M – 204]+ | –35 | C18H21N3O |
| 252.2 [M – 44]+ | –30 | ||||||
| 281.0 [M – 15]+ | –20 | ||||||
| 2 | 2-methyl-3-butyl prodiginine | 1.94 | 531 | 310.2 [M + H]+ | 92.1 [M – 218]+ | –49 | C19H23N3O |
| 252.2 [M – 58]+ | –31 | ||||||
| 295.3 [M – 15]+ | –22 | ||||||
| 3 | cycloprodigiosin | 2.16 | 537 | 322.4 [M + H]+ | 147.2 [M – 175]+ | –32 | C20H23N3O |
| 292.2 [M – 30]+ | –33 | ||||||
| 307.2 [M – 15]+ | –24 | ||||||
| 4 | prodigiosin | 2.52 | 537 | 324.4 [M + H]+ | 92.2 [M – 232]+ | –53 | C20H25N3O |
| 252.1 [M – 72]+ | –33 | ||||||
| 309.5 [M – 15]+ | –23 | ||||||
| 5 | 2-methyl-3-hexyl prodiginine | 3.00 | 535 | 338.5 [M + H]+ | 92.2 [M – 246]+ | –51 | C21H27N3O |
| 252.1 [M – 86]+ | –33 | ||||||
| 323.2 [M – 15]+ | –23 | ||||||
| 6 | 2-methyl-3-heptyl prodiginine | 3.69 | 536 | 352.5 [M + H]+ | 92.2 [M – 260]+ | –52 | C22H29N3O |
| 252.2 [M – 100]+ | –34 | ||||||
| 337.3 [M – 15]+ | –25 |
Note: Product ions were obtained at the optimized collision energy (CE) determined by multiple reaction monitoring (MRM).
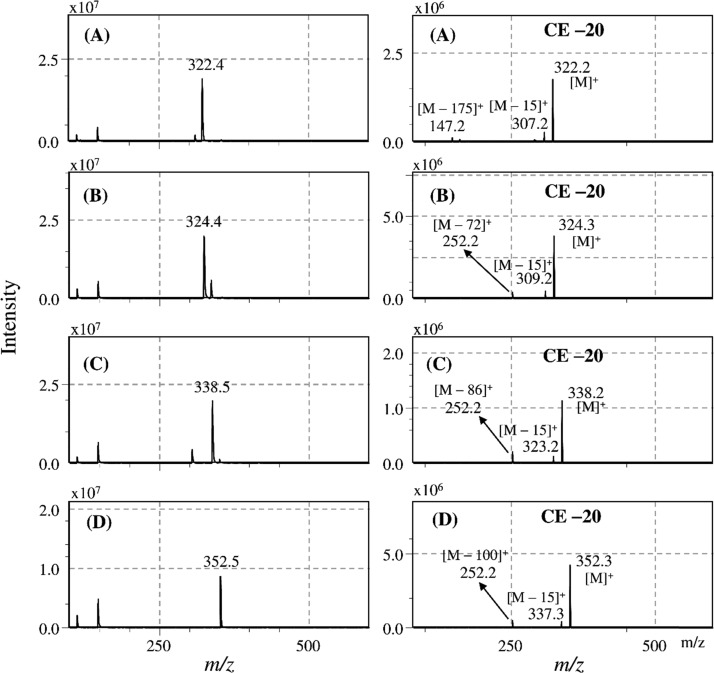
MS/MS Analysis of Prodiginine and Its Derivatives
The six purified compounds were analyzed by ESI-MS/MS to determine the molecular ions and fragment ions. The full Q1 scan mass spectrum of peak #3 showed a molecular ion at a mass-to-charge ratio (m/z) of 322.4 [M + H]+ (Figure 5A, left). Further study of the product ion scan mass spectrum with a CE of −20 V showed a molecular ion at m/z 322.2 [M + H]+ and product ions at m/z 307.2 [M – 15]+ and 147.2 [M – 175]+ (Figure 5A, right), indicating loss of a methyl group [CH3] and C10H9N2O, respectively. The UV–Vis absorption (Figure 4) and mass (Figure 5A) spectra obtained for purified peak #3 were consistent with those of the cycloprodigiosins reported by Gerber and Gauthier25 and Lee et al.,33 which include an immunosuppressant,34 anticancer compound,35 and antimalarial drug.36 Next, a major compound, peak #4, was analyzed by mass spectrometry (Figure 5B). The mass spectra from the Q1 scan showed a molecular ion at m/z 324.4 [M + H]+ and two product ions at m/z 252.2 [M – 72]+ and 309.2 [M – 15]+ (Figure 5B, right). Comparison of the molecular and product ions with those of standard prodigiosin hydrochloride revealed the same mass spectra (Figure S4, Supporting Information). The mass spectra and absorption maxima of the purified sample were consistent with those of prodigiosin (2-methyl-3-pentyl-prodiginine).33 Therefore, the major compound, peak #4 at 2.52 min, was identified as prodigiosin. Based on the molecular ion and product ions, peaks #5 and #6 were identified as 2-methyl-3-hexyl-prodiginine (m/z 338.5 [M + H]+) (Figure 5C) and 2-methyl-3-heptyl-prodiginine (m/z 352.5 [M + H]+) (Figure 5D), respectively, which were also reported in the marine bacterium Hahella chejuensis KCTC 2396.37,38 The minor compounds, peaks #1 and #2, respectively, were identified as 2-methyl-3-propyl prodiginine (m/z 296.1 [M + H]+) and 2-methyl-3-butyl prodiginine (m/z 310.2 [M + H]+) (Figure S5, Supporting Information), respectively. To our knowledge, this is the first report on the presence of four derivatives of prodigiosin with different alkyl side chains in P. rubra in addition to prodigiosin and cycloprodigiosin. These findings largely rely on recently developed MS techniques, such as single ion monitoring (SIM) and MRM, which are based on the selection of a precursor ion and one or more characteristic fragment ions and are powerful tools for determining the structure of target compounds.
Figure 5.
ESI-MS/MS analysis of the purified compounds. Full Q1 scan (left) and product ion scan (right) mass spectra of (A) cycloprodigiosin, (B) prodigiosin, (C) 2-methyl-3-hexyl-prodiginine, and (D) 2-methyl-3-heptyl-prodiginine.
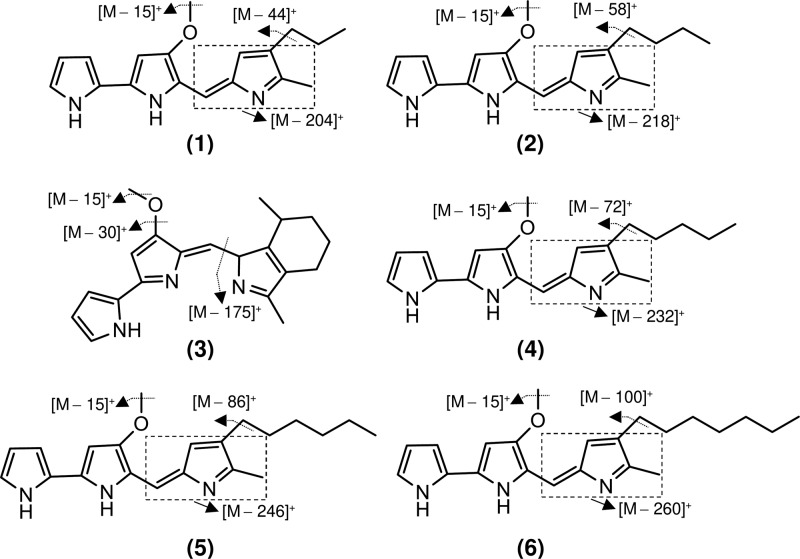
In this study, MS analysis was carried out in positive mode, and product ions were almost all obtained at a CE of −20 V. Interestingly, prodigiosin and its analogs showed similar fragmentations, with losses of a methyl group at m/z [M – 15]+ and alkyl chains at m/z [M – 44]+, [M – 58]+, [M – 72]+, [M – 86]+, and [M – 100]+, respectively, for compounds containing propyl, butyl, pentyl, hexyl, and heptyl side chains (Figure 5 and Figure S5). The complete alkyl chains were lost as shown in agreement to the previously studies by Wang et al. and Lee et al.26,33 The complete alkyl chain might be removed due to strong ESI ionization technique from the prodigiosins compound instead remains as allylic carbocation such as benzylic ions. To obtain candidate product ions, we conducted MRM to determine the optimum CE (Table 1). At a CE of −33 V, an additional product ion was found for cycloprodigiosin at m/z 292.15 [M – 30]+, indicating loss of a methyl group and one oxygen atom [CH3 + O – H]+. Additionally, the product ion at m/z 92 that was found for prodigiosin and its derivatives with different alkyl chain sides due to loss of cyclopentadienecarbonitrile [C6H5N2 + H]+ was obtained at a CE of −51 to −53 V. The optimal CE for the loss of methyl and alkyl groups was −23 to −25 V and −33 to −34 V, respectively. Thus, the CE is a key factor, and the MRM method used in this study to determine the optimum CE is ideal for analyzing candidate product ions (Figure 6).
Figure 6.
Molecular structure and fragmentation of identified molecules at different CEs based on optimized product ions using MRM. For the optimum CE values, see Table 1. 1, 2-methyl-3-propyl prodiginine; 2, 2-methyl-3-butyl prodiginine; 3, cycloprodigiosin; 4, prodigiosin; 5, 2-methyl-3-hexyl prodiginine; 6, 2-methyl-3-heptyl prodiginine.
Antimicrobial Activity
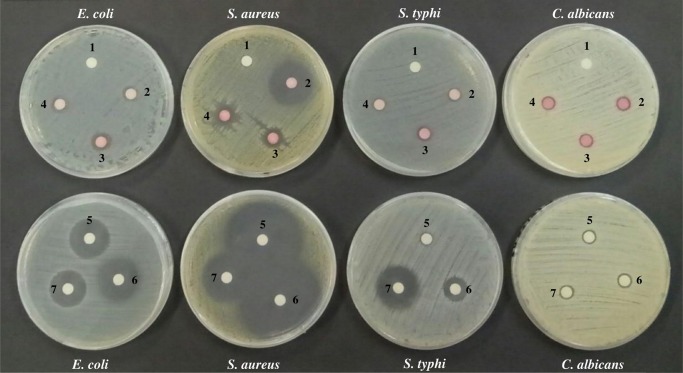
The antimicrobial activities of cycloprodigiosin, prodigiosin, and 2-methyl-3-hexyl prodiginine compared to the standard antibiotic amoxicillin, ampicillin, and chloramphenicol are presented in Table 2 and Figure 7.
Table 2. Antimicrobial Activity (mm) of Cycloprodigiosin, Prodigiosin, and 2-Methyl-3-hexyl Prodiginine Detected in the Disk Diffusion Testa.
| inhibition
zone (mm) |
||||
|---|---|---|---|---|
| compound | E. coli | S. aureus | S. typhi | C. albicans |
| cycloprodigiosin | 9.0 ± 0.49 | 25.1 ± 0.55 | 8.1 ± 0.03 | 7.9 ± 0.07 |
| prodigiosin | 10.5 ± 0.9 | 11.6 ± 0.28 | 8.5 ± 0.13 | 8.2 ± 0.09 |
| 2-methyl-3-hexyl prodiginine | 9.1 ± 0.49 | 10.1 ± 0.44 | 8.1 ± 0.09 | 7.9 ± 0.06 |
| amoxicillin | 23.4 ± 0.26 | 41.5 ± 0.26 | 8.2 ± 0.17 | 8.6 ± 0.12 |
| ampicillin | 24.1 ± 0.20 | 45.7 ± 0.21 | 13.4 ± 0.20 | 8.6 ± 0.07 |
| chloramphenicol | 20.8 ± 0.32 | 27.3 ± 0.18 | 23.5 ± 0.20 | 8.3 ± 0.06 |
The data used are averages of triplicate measurement ± the standard error (±SE).
Figure 7.
Antimicrobial activity of cycloprodigiosin (2), prodigiosin (3), and 2-methyl-3-hexyl prodiginine (4) compared to the standard antibiotic amoxicillin (5), ampicillin (6), and chloramphenicol (7). DMSO as a negative control is marked with number 1. (Photos were taken by E. Setiyono.)
As shown in Table 2, cycloprodigiosin, prodigiosin, and 2-methyl-3-hexyl prodiginine exhibited antimicrobial activity against all tested pathogenic organisms. The inhibition zone varied from 9.0 to 10.5 mm when applied to E. coli. A smaller clear zone of 8.1–8.5 mm was obtained against S. typhi. An interesting result was obtained when the pigments were applied to S. aureus: a significant inhibition zone from 10.1 to 25.1 mm. In this case, cycloprodigiosin showed greater activity than prodigiosin and 2-methyl-3-hexyl prodiginine. The pigments also showed antifungal activity (7.9–8.2 mm) against C. albicans. There was no clear zone in the negative control (DMSO). However, the positive controls amoxicillin, ampicillin, and chloramphenicol demonstrated susceptibility to E. coli, S. typhi, and S. aureus. On the other hand, C. albicans was resistant to all antibiotics with resulting inhibition zone only around 8.3–8.6 mm. The bioactivities of cycloprodigiosin and prodigiosin from the marine bacterium Zooshikella rubidus S1-1 have been studied by Lee et al.33 Disks containing 50 μg of these compounds produced an inhibition zone of approximately 8.0 mm against Salmonella typhimurium, S. aureus, E. coli, Bacillus subtilis, and Candida albicans.33 Another experiment by Lapenda et al.9 showed that 300 μg of prodigiosin from Serratia marcescens produced significant inhibition zones against S. aureus (35 mm), Enterococcus faecalis (22 mm), and Streptococcus pyogenes (14 mm). However, no inhibition zones were found against E. coli, Pseudomonas aeruginosa, or Acenitobacter.9 In our case, cycloprodigiosin, prodigiosin, and 2-methyl-3-hexyl prodiginine at a concentration of 20 μg/mL caused clear zones around the disks, suggesting that these compounds are sensitive to E. coli, S. typhi, S. aureus, and C. albicans. Bioactive prodiginine-producing marine bacteria comprise a wide variety of genera, including Beneckea,38Pseudovibrio,39Vibrio,40−42,59Streptomyces,5,43−45,60Pseudomonas,46Pseudoalteromonas,21,23,26,47Hahella,48,49,58 and Zooshikella.33,50,51 However, the prodiginine types and composition differ among them, as shown in Table S2, Supporting Information. Our isolated bacteria, P. rubra strains PS1 and SB14, are included in one of these genera. Pseudoalteromonas species appear to have a wide habitat, as described above. As shown in this study, strains of P. rubra can be easily isolated from a variety of marine environments and cultured by standard manipulation techniques; therefore, they might be a potential source for the production of bioactive prodigiosin and its derivatives.
Experimental Section
Sampling Location
Sampling was conducted on Alor Island, Eastern Indonesia. It is a tropical island located on the eastern tip of the Nusa Tenggara Islands at 8°15′S and 124°45′E. The island is bordered by the Flores Sea and the Banda Sea in the north, the Ombai Strait in the south (separating it from Timor Island), and the Pantar Strait in the west. Alor Island is one of the two main islands in the Alor Regency, East Nusa Tenggara Province, Indonesia. Two isolated strains were collected from two different locations with different ecosystems: Sika Island coast and Sebanjar Beach (Figure 1). Seawater was sampled in the surface and the bottom layer (depths of 0 and 6 m). Samples were collected in 50 mL sterile tubes and placed in a cold box immediately. Samples were then brought to the Ma Chung Research Center for Photosynthetic Pigments for bacterial isolation. A map of sampling locations was created using QGIS version 2.18.19 and the ESRI continent base map.
Bacterial Isolation and Purification
Up to 35 μL of seawater was spread directly into Petri dishes containing Zobell agar medium (0.5 g of yeast, 2.5 g of peptone, and 13 g of Bacto agar in 1 L of seawater) and incubated for 3 days at 35 °C. After 3 days of incubation, the bacterial colonies on the Petri dishes were observed. The red pigmented bacterium was purified by the streak plate method and cultured in new medium for 3 days at 32 °C. Purification of the pigmented bacterium was based on its color, shape, margin, elevation, and size. The purification was performed repeatedly until the colony was pure. The pure bacterium was then stored at 10 °C until further study.
Bacterial Culture
The pure bacterium was cultured on the same medium as that used in the bacterial isolation. However, the culturing was carried out in a shaking incubator (New Brunswick Scientific Excella E24, Edison, NJ, USA) at 32 °C for 24 h. Red pigment production is very rapid. After 24 h, the pigment begins to fade. The cells of the bacterium were scraped, collected, and placed in 25 mL plastic tubes and then precipitated by centrifugation at 10,000 rpm for 10 min at 4 °C, and the collected cells were stored in a freezer at −30 °C until use.
DNA Extraction
DNA extraction was carried out by the Chelex method.52 The bacterial cells were inoculated into a mixture of 100 μL of ddH2O and 1 mL of 0.5% saponin (w/v) in 1× PBS and incubated overnight. The precipitate was separated and collected by centrifugation at 12,000 rpm for 10 min. Subsequently, 100 μL of ddH2O and 50 μL of 20% Chelex 100 (w/v) were mixed until the precipitate dissolved. The resulting solution was boiled for 10 min and vortexed for 5 min. Then, the mixture containing bacterial DNA was centrifuged at 12,000 rpm for 10 min and stored at −20 °C until use. The bacterial DNA concentrations were quantified and qualified by a NanoDrop 2000 spectrophotometer (Thermo Scientific, Waltham, Massachusetts, USA). The concentration of 1 μL of DNA was analyzed, and the DNA purity was calculated as the 260/280 nm ratio.
PCR Amplification of the 16S rRNA Gene Sequence
The universal primers 27F (5′-AGAGTTTGATCMTGGCTCAG-3′) and 1492R (5′-TACGGTTAACCTTGTTACGACTT-3′) in a PCR mixture were used in this study. For analysis of 16S rRNA gene sequences, GoTaq Green Mix Promega (Promega, Madison, Wisconsin, USA) (25 μL) was used to amplify DNA. The PCR mixture consisted of GoTaq Green Master Mix Promega (25 μL), primer 27F (0.5–5 μL), primer 1492R (0.5–5 μL), DNA extract (1–5 μL), and nuclease-free water (50 μL). PCR was conducted in an MJ Mini Personal Thermal Cycler (Bio-Rad, Hercules, California, USA). The cycling conditions were as follows: initial denaturation at 95 °C for 3 min followed by 30 cycles of denaturation at 95 °C for 1 min, annealing at 55 °C for 1 min, and extension at 72 °C for 1 min. The final extension was carried out at 72 °C for 7 min.53 The PCR products were analyzed using agarose 0.8% gel electrophoresis, and the results were visualized by a UVIDoc HD5 system (UVITEC, Cambridge, UK). The amount of DNA ladder per lane and the sample volume loaded per lane were 0.2 μg and 1 μL, respectively.
DNA Sequencing
DNA sequencing was performed using a QIAquick PCR Purification Kit (QIAGEN, Hilden, Germany), and PCR sequencing was performed using Big Dye Terminator v.3.1. The sequencing analysis was carried out using an ABI 3130XL system (Applied Biosystem, Carlsbad, California, USA). The sequencing data for the amplified 16S rRNA genes from each bacterium were deposited in the GenBank database of the NCBI (http://www.ncbi.nlm.nih.gov). The sequencing data were inserted into the Advanced BLAST search program to identify the sequences of any closely related organisms. The 16S rRNA sequencing data of the bacteria were also deposited in the DDBJ.
Phylogenetic Analysis
The results of DNA sequencing were aligned using ClustalW Multiple Alignment. MEGA 6 software was used for phylogenetic analysis. The phylogenetic trees were determined by the neighbor-joining method with Kimura’s two-parameter model. The tree topology was evaluated by bootstrap analysis of the neighbor-joining method based on 1000 resamplings.54
Pigment Extraction
The collected cells were homogenized in a mixture of methanol and acetone (7:3, v/v, 1 mL of mixture per 0.1 g of cells) by vortexing five times (1 min of vortexing, 1 min on ice) and then disrupted by sonication in pulse mode with 60% amplitude and 10 s on/30 s off for 10 min (QSonica, Newtown, Connecticut, USA). A few grains of CaCO3 and sodium ascorbate were added to the mixture to prevent pigment degradation via oxidation. The pigment extract was separated from the cell debris by centrifugation at 19,230g for 5 min at 4 °C (Kubota 6500 centrifuge, Tokyo, Japan). The resulting extract was collected and dried using a rotary evaporator at 100 rpm and 35 °C in a water bath (Heidolph Laborota 4010 digital, Schwabach, Germany). The dried pigment extract was stored at −30 °C until use.
Spectrophotometric Analysis
The UV–Vis absorption spectra of crude pigment extracts were recorded by a UV–Vis 1700 spectrophotometer (Shimadzu, Kyoto, Japan). Methanol was used for background subtraction in all measurements. The dried pigment extract was diluted in methanol and measured at a wavelength (λ) of 200–1100 nm. Subsequently, the obtained data were analyzed using OriginPro 8.5.1 software (OriginLab, Northampton, Massachusetts, USA).
HPLC Analysis and Purification of Prodiginines and Their Derivatives
The pigments were separated and purified by preparative HPLC (Shimadzu preparative UFLC, Kyoto, Japan) and analyzed by analytical HPLC (Shimadzu analytical-UFLC, Kyoto, Japan) using a Symmetry C8 column (150 × 4.6 mm, 3.5 μm particle size, 100 Å pore size) (Waters, Milford, MA, USA). The HPLC-grade solvents (MERCK, Darmstadt, Germany) were degassed for 5 min with ultrasonication prior to use. The mobile phases used consisted of two solvents. Solvent A was methanol (MeOH), and solvent B was 1% formic acid in water (H2O). The elution gradient was 70% A for min 0–2, 85% A for min 2–4, and 100% A for or min 4–10. The flow rate was 1 mL/min with a column oven temperature of 29 °C. The pigments were detected with a diode array detector (Shimadzu SPD M20A, 190–800 nm) at λ 530 nm. The prodigiosin pigment standard (from Serratia marcescens, >98% purity, CAS number: 56144-17-3) was obtained from Sigma-Aldrich (MERCK).
MS/MS Analysis
The highly purified compounds (>97% purity) were analyzed by ESI-MS with a triple quadrupole mass spectrometer (LCMS-8030, Shimadzu). Determination of the pigments was based on their MS data and spectral properties. The simple MS method was used with isocratic HPLC elution by 0.1% formic acid in a mixture of methanol (90%) and water (10%) for 2 min at a flow rate of 0.3 mL/min without a column. The DL temperature was 200 °C, the nebulizing gas flow rate was 3 L/min, the heat block temperature was 350 °C, the drying gas flow rate was 15 L/min, the column oven temperature was 30 °C, and the cooler temperature was 5 °C. Prodiginine was detected at a wavelength (λ) of 530 nm. The initial identification of precursor ions was conducted by using a Q1Q3 scan in positive and negative modes at m/z 50 to 600 using an event time of 0.1 s and a scan speed of 6000 u/s. The precursor ions were then fragmented in product ion scan mode by using various CEs. Pigment identification was based on the molecular mass of the precursor from the Q1Q3 scan and the product ions and on the product ions optimized by the MRM method. Identification of the pure compounds was performed according to the precursor ion, product ions, and SIM data. The chemical structures of the identified compounds were drawn using ChemDraw software version 12.0.2 (PerkinElmer, Inc., Massachusetts, USA).
Pigment Identification
The pigments were identified by HPLC and MS/MS analyses based on the chromatographic, that is, retention time (tR); spectrophotometric, that is, spectral shape and maximal absorption wavelength (λmax); and mass, that is, precursor and fragment ions, properties compared to those of reference compounds.21,23,25,33,36,50 The prodigiosin in P. rubra PS1 and SB14 was compared to a prodigiosin standard (Sigma-Aldrich, MERCK). The data consisted of a full Q1 scan and product ion scans at the optimized CE of the prodigiosin standard in the LabSolution MS Library (Shimadzu). The prodigiosin from P. rubra PS1 and SB14 was isolated and purified to >97% purity. Then, the isolated prodigiosin was identified by comparison of the recorded chromatographic and spectral data with the data on the prodigiosin standard stored in the library using LabSolution LCMS version 5.4 (Shimadzu). The software compared retention times and aligned MS/MS data to calculate a match factor and produced a degree of similarity between spectra. Cycloprodigiosin, 2-methyl-3-propyl prodiginine, 2-methyl-3-butyl prodiginine, 2-methyl-3-hexyl-prodiginine, and 2-methyl-3-heptyl-prodiginine were analyzed on the basis of MS and fragmentation data.
Antimicrobial Activity
The antimicrobial activity was determined by the disk-diffusion technique according to the Kirby-Bauer disk diffusion susceptibility test protocol.55 Prodigiosin, cycloprodigiosin, and 2-methyl-3-hexyl prodiginine were used as antimicrobial agents against three pathogenic bacteria: Escherichia coli ATCC 8739, Staphylococcus aureus ATCC 6538, Salmonella typhi, from clinical isolation and one pathogenic yeast, Candida albicans ATCC 10231. Briefly, 90 mm dishes were filled with 15 mL of Mueller-Hinton agar (MHA) to a depth of 4 mm for bacteria and Sabouraud dextrose agar (SDA) for yeast. The pathogenic microorganisms were swabbed on the dishes following the McFarland turbidity standard (0.5) using sterile cotton swabs. Blank disks were placed on the surface of the medium, and each antimicrobial agent (20 μg/mL) was injected into the disks. DMSO was used as a negative control. Ten micrograms of amoxicillin, ampicillin, and chloramphenicol were applied as positive controls. Dishes were inverted and incubated at 37 °C for 24 h. The inhibition zone surrounding disks was measured, as was the disk diameter. The prodigiosin concentration was determined by absorption in acidified ethanol (4% v/v of 1 N HCl) with a molar extinction coefficient ε535 of 139,800 ± 5100 M–1 cm–1.56,57
Acknowledgments
The authors would like to thank J. F. Maro from Universitas Tribuana Kalabahi Alor for help during sampling on Alor Island and M. R. Prabowo and Y. S. Kurniawan from Universitas Ma Chung for their assistance in fluorescence study.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.9b04322.
P. rubra strains that have been deposited in the NCBI (Table S1); the distribution map from 45 strains of P. rubra that have been found until now (Figure S1); the number of isolated P. rubra found from different sources (Figure S2); the plot of log k′ value of 2-methyl-3-propyl-prodiginine (k′ = −0.605), 2-methyl-3-butyl-prodiginine (k′ = −0.339), prodigiosin (k′ = −0.048), 2-methyl-3-hexyl-prodiginine (k′ = 0.099), and 2-methyl-3-heptyl-prodiginine (k′ = 0.249) versus the number of the carbon atoms of the alkyl side chains of the pigment molecule showed a linear line with R2 = 0.9755 (Figure S3); the comparison mass spectra of standard prodigiosin hydrochloride and purified pigment peak #4 from P. rubra strain PS1 (Figure S4); ESI-MS/MS analysis of the purified compounds (Figure S5); and prodigiosin-producing marine bacteria (Table S2) (PDF)
This work was supported by the Ministry of Research and Technology of Indonesia/National Research and Innovation Agency through Basic Research Scheme Number 9/E1/KPT/2020.
The authors declare no competing financial interest.
Supplementary Material
References
- Llor C.; Bjerrum L. Antimicrobial resistance: risk associated with antibiotic overuse and initiatives to reduce the problem. Ther. Adv. Drug Saf. 2014, 5, 229–241. 10.1177/2042098614554919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . Antimicrobial Resistance Global Report on Surveillance; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- Zellweger R. M.; Carrique-Mas J.; Limmathurotsakul D.; Day N. P. J.; Thwaites G. E.; Baker S.; Ashley E.; de Balogh K.; Baird K.; Basnyat B.; Benigno C.; Bodhidatta L.; Chantratita N.; Cooper B.; Dance D.; Dhorda M.; van Doorn R.; Dougan G.; Hoa N. T.; Ip M.; Lawley T.; Lim C.; Lin T. K.; Ling C.; Lubell Y.; Mather A.; Marks F.; Mohan V. R.; Newton P.; Paris D.; Thomson N.; Turner P.; Serichantalergs O.; Smithuis F.; Wuthiekanun V.; White N.; Yang H. L. A current perspective on antimicrobial resistance in Southeast Asia. J. Antimicrob. Chemother. 2017, 72, 2963–2972. 10.1093/jac/dkx260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thamlikitkul V.; Rattanaumpawan P.; Boonyasiri A.; Pumsuwan V.; Judaeng T.; Tiengrim S.; Paveenkittiporn W.; Rojanasthien S.; Jaroenpoj S.; Issaracharnvanich S. Thailand antimicrobial resistance containment and prevention program. J. Glob. Antimicrob. Resist 2015, 3, 290–294. 10.1016/j.jgar.2015.09.003. [DOI] [PubMed] [Google Scholar]
- Williamson N. R.; Fineran P. C.; Leeper F. J.; Salmond G. P. C. The biosynthesis and regulation of bacterial prodiginines. Nat. Rev. Microbiol. 2006, 4, 887–899. 10.1038/nrmicro1531. [DOI] [PubMed] [Google Scholar]
- Stankovic N.; Senerovic L.; Ilic-Tomic T.; Vasiljevic B.; Nikodinovic-Runic J. Properties and applications of undecylprodigiosin and other bacterial prodigiosins. Appl. Microbiol. Biotechnol. 2014, 98, 3841–3858. 10.1007/s00253-014-5590-1. [DOI] [PubMed] [Google Scholar]
- Yip C.-H.; Yarkoni O.; Ajioka J.; Wan K.-L.; Nathan S. Recent advancements in high-level synthesis of the promising clinical drug, prodigiosin. Appl. Microbiol. Biotechnol. 2019, 103, 1667–1680. 10.1007/s00253-018-09611-z. [DOI] [PubMed] [Google Scholar]
- Kim S.; Thiessen P. A.; Bolton E. E.; Chen J.; Fu G.; Gindulyte A.; Han L.; He J.; He S.; Shoemaker B. A.; Wang J.; Yu B.; Zhang J.; Bryant S. H. PubChem substance and compound databases. Nucleic Acids Res. 2016, 44, D1202–D1213. 10.1093/nar/gkv951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapenda J. C.; Silva P. A.; Vicalvi M. C.; Sena K. X. F. R.; Nascimento S. C. Antimicrobial activity of prodigiosin isolated from Serratia marcescens UFPEDA 398. World J. Microbiol. Biotechnol. 2015, 31, 399–406. 10.1007/s11274-014-1793-y. [DOI] [PubMed] [Google Scholar]
- Wang Z.; Li B.; Zhou L.; Yu S.; Su Z.; Song J.; Sun Q.; Sha O.; Wang X.; Jiang W.; Willert K.; Wei L.; Carson D. A.; Lu D. Prodigiosin inhibits Wnt/β-catenin signaling and exerts anticancer activity in breast cancer cells. Proc. Nat. Acad. Sci. U. S. A. 2016, 113, 13150–13155. 10.1073/pnas.1616336113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D.; Liu J.; Wang X.; Kong D.; Du W.; Li H.; Hse C. Y.; Shupe T.; Zhou D.; Zhao K. Biological potential and mechanism of prodigiosin from Serratia marcescens subsp. lawsoniana in human choriocarcinoma and prostate cancer cell lines. Int. J. Mol. Sci. 2018, 19, E3465 10.3390/ijms19113465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong B.; Prabhu V. V.; Zhang S.; van den Heuvel A. P. J.; Dicker D. T.; Kopelovich L.; El-Deiry W. S. Prodigiosin rescues deficient p53 signaling and antitumor effects via upregulating p73 and disrupting its interaction with mutant p53. Cancer Res. 2014, 74, 1153–1165. 10.1158/0008-5472.CAN-13-0955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishna P. S.; Vani K.; Prasad M. R.; Samatha B.; Bindu N. S. V. S. S.; Charya M. A. S.; Shetty P. R. In −silico molecular docking analysis of prodigiosin and cycloprodigiosin as COX-2 inhibitors. Springerplus 2013, 2, 172. 10.1186/2193-1801-2-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danevcic T.; Vezjak M. B.; Zorec M.; Stopar D. Prodigiosin-a multifaceted Escherichia coli antimicrobial agent. PLoS One 2016, 11, e0162412 10.1371/journal.pone.0162412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suryawanshi R. K.; Patil C. D.; Koli S. H.; Hallsworth J. E.; Patil S. V. Antimicrobial activity of prodigiosin is attributable to plasma-membrane damage. Nat. Prod. Res. 2017, 31, 572–577. 10.1080/14786419.2016.1195380. [DOI] [PubMed] [Google Scholar]
- Williams R. P.; Green J. A.; Rappo-Port D. A. Studies on pigmentation of Serratia marcescens, I. Spectral and paper chromatographic properties of prodigiosin1. J. Bacteriol. 1955, 71, 115–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreyeva I. N.; Ogorodnikova T. I. Pigmentation of Serratia marcescens and spectral properties of prodigiosin. Microbiology 2015, 84, 28–33. 10.1134/S0026261715010026. [DOI] [PubMed] [Google Scholar]
- Bennett J. W.; Bentley R. Seeing red: the story of prodigiosin. Adv. Appl. Microbiol. 2000, 47, 1–32. 10.1016/S0065-2164(00)47000-0. [DOI] [PubMed] [Google Scholar]
- Ramesh C.; Vinithkumar N. V.; Kirubagaran R. Marine pigmented bacteria: A prospective source of antibacterial compounds. J. Nat. Sci., Biol. Med. 2019, 10, 104–113. 10.4103/jnsbm.JNSBM_201_18. [DOI] [Google Scholar]
- Ramesh C.; Vinithkumar N. V.; Kirubagaran R.; Venil C. K.; Dufossé L. Multifaceted applications of microbial pigments: current knowledge, challenges and future directions for public health implications. Microorganisms 2019, 7, E186 10.3390/microorganisms7070186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier M. J. Alteromonas rubra sp. nov., a new marine antibiotic-producing bacterium. Int. J. Syst. Bacteriol. 1976, 26, 459–466. 10.1099/00207713-26-4-459. [DOI] [Google Scholar]
- Enger O.; Nygaard H.; Solberg M.; Schei G.; Nielsen J.; Dundas I. Characterization of Alteromonas denitrificans sp. nov. Int. J. Syst. Bacteriol. 1987, 37, 416–421. 10.1099/00207713-37-4-416. [DOI] [Google Scholar]
- Sawabe T.; Makino H.; Tatsumi M.; Nakano K.; Tajima K.; Iqbal M. M.; Yumoto I.; Ezura Y.; Christen R. Pseudoalteromonas bacteriolytica sp. nov., a marine bacterium that is the causative agent of red spot disease of Laminaria Japonica. Int. J. Syst. Bacteriol. 1998, 48, 769–774. 10.1099/00207713-48-3-769. [DOI] [PubMed] [Google Scholar]
- Sakai-Kawada F. E.; Ip C. G.; Hagiwara K. A.; Awaya J. D. Biosynthesis and bioactivity of prodiginine analogs in marine bacteria, Pseudoalteromonas: A mini review. Front. Microbiol. 2019, 10, 1715–1715. 10.3389/fmicb.2019.01715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber N. N.; Gauthier M. J. New Prodigiosin-like pigment from Alteromonas rubra. Appl. Environ. Microbiol. 1979, 37, 1176–1179. 10.1128/AEM.37.6.1176-1179.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.; Nakajima A.; Hosokawa K.; Soliev A. B.; Osaka I.; Arakawa R.; Enomoto K. Cytotoxic prodigiosin family pigments from Pseudoalteromonas sp. 1020R isolated from the pacific coast of Japan. Biosci., Biotechnol., Biochem. 2012, 76, 1229–1232. 10.1271/bbb.110984. [DOI] [PubMed] [Google Scholar]
- Rapoport H.; Holden K. G. The synthesis of prodigiosin. J. Am. Chem. Soc. 1962, 84, 635–642. 10.1021/ja00863a026. [DOI] [Google Scholar]
- Fehér D.; Barlow R. S.; Lorenzo P. S.; Hemscheidt T. K. A 2-substituted prodiginine, 2-(p-hydroxybenzyl) prodigiosin, from Pseudoalteromonas rubra. J. Nat. Prod. 2008, 71, 1970–1972. 10.1021/np800493p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo V.; Morelli A.; Pinciroli V.; Sciangula D.; D’Alessio R. Equilibrium and kinetics of rotamer interconversion in immunosuppressant prodigiosin derivatives in solution. J. Pharm. Sci. 1999, 88, 73–78. 10.1021/js980225w. [DOI] [PubMed] [Google Scholar]
- Song M.-J.; Bae J.; Lee D.-S.; Kim C.-H.; Kim J.-S.; Kim S.-W.; Hong S.-I. Purification and characterization of prodigiosin produced by integrated bioreactor from Serratia sp. KH-95. J. Biosci. Bioeng. 2006, 101, 157–161. 10.1263/jbb.101.157. [DOI] [PubMed] [Google Scholar]
- Shioi Y.; Fukae R.; Sasa T. Chlorophyll analysis by high-performance liquid chromatography. Biochim. Biophys. Acta Bioenerg. 1983, 722, 72–79. 10.1016/0005-2728(83)90158-5. [DOI] [Google Scholar]
- Setiyono E.; Heriyanto; Pringgenies D.; Shioi Y.; Kanesaki Y.; Awai K.; Brotosudarmo T. H. P. Sulfur-containing carotenoids from a marine coral symbiont Erythrobacter flavus strain KJ5. Mar. Drugs 2019, 17, 349. 10.3390/md17060349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. S.; Kim Y. S.; Park S.; Kim J.; Kang S. J.; Lee M. H.; Ryu S.; Choi J. M.; Oh T. K.; Yoon J. H. Exceptional production of both prodigiosin and cycloprodigiosin as major metabolic constituents by a novel marine bacterium, Zooshikella rubidus S1-1. Appl. Environ. Microbiol. 2011, 77, 4967–4973. 10.1128/AEM.01986-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azuma T.; Watanabe N.; Yagisawa H.; Hirata H.; Iwamura M.; Kobayashi Y. Induction of apoptosis of activated murine splenic T cells by cycloprodigiosin hydrochloride, a novel immunosuppressant. Immunopharmacology 2000, 46, 29–37. 10.1016/S0162-3109(99)00153-8. [DOI] [PubMed] [Google Scholar]
- Yamamoto C.; Takemoto H.; Kuno K.; Yamamoto D.; Tsubura A.; Kamata K.; Hirata H.; Yamamoto A.; Kano H.; Seki T.; Inoue K. Cycloprodigiosin hydrochloride, a new H+/Cl– symporter, induces apoptosis in human and rat hepatocellular cancer cell lines in vitro and inhibits the growth of hepatocellular carcinoma xenografts in nude mice. Hepatology 1999, 30, 894–902. 10.1002/hep.510300417. [DOI] [PubMed] [Google Scholar]
- Kim H. S.; Hayashi M.; Shibata Y.; Wataya Y.; Mitamura T.; Horii T.; Kawauchi K.; Hirata H.; Tsuboi S.; Moriyama Y. Cycloprodigiosin hydrochloride obtained from Pseudoalteromonas denitrificans is a potent antimalarial agent. Biol. Pharm. Bull. 1999, 22, 532–534. 10.1248/bpb.22.532. [DOI] [PubMed] [Google Scholar]
- Kim D.; Lee J. S.; Park Y. K.; Kim J. F.; Jeong H.; Oh T. K.; Kim B. S.; Lee C. H. Biosynthesis of antibiotic prodiginines in the marine bacterium Hahella chejuensis KCTC 2396. J. Appl. Microbiol. 2006, 0, 937–944. 10.1111/j.1365-2672.2006.03172.x. [DOI] [PubMed] [Google Scholar]
- Harwood C. S. Beneckea gazogenes sp. nov., a red, facultatively anaerobic, marine bacterium. Curr. Microbiol. 1978, 1, 233–238. 10.1007/BF02602849. [DOI] [Google Scholar]
- Sertan-de Guzman A. A.; Predicala R. Z.; Bernardo E. B.; Neilan B. A.; Elardo S. P.; Mangalindan G. C.; Tasdemir D.; Ireland C. M.; Barraquio W. L.; Concepcion G. P. Pseudovibrio denitrificans strain Z143-1, a heptylprodigiosin-producing bacterium isolated from a philippine tunicate. FEMS Microbiol. Lett. 2007, 277, 188–196. 10.1111/j.1574-6968.2007.00950.x. [DOI] [PubMed] [Google Scholar]
- D’Aoust J. Y.; Gerber N. N. Isolation and purification of prodigiosin from Vibrio psychroerythrus. J. Bacteriol. 1974, 118, 756–757. 10.1128/JB.118.2.756-757.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shieh W. Y.; Chen Y. W.; Chaw S. M.; Chiu H. H. Vibrio ruber sp. nov., a red, facultatively anaerobic, marine bacterium isolated from sea water. Int. J. Syst. Evol. Microbiol. 2003, 53, 479–484. 10.1099/ijs.0.02307-0. [DOI] [PubMed] [Google Scholar]
- Borić M.; Danevčič T.; Stopar D. Prodigiosin from Vibrio sp. DSM 14379; A new uv-protective pigment. Microb. Ecol. 2011, 62, 528–536. 10.1007/s00248-011-9857-0. [DOI] [PubMed] [Google Scholar]
- Song Y.; Liu G.; Li J.; Huang H.; Zhang X.; Zhang H.; Ju J. Cytotoxic and antibacterial angucycline- and prodigiosin-analogues from the deep-sea derived Streptomyces sp. SCSIO 11594. Mar. Drugs 2015, 13, 1304–1316. 10.3390/md13031304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki T.; Sakurai F.; Hayakawa Y. A prodigiosin from the roseophilin producer Streptomyces griseoviridis. J. Nat. Prod. 2008, 71, 1265–1267. 10.1021/np7007494. [DOI] [PubMed] [Google Scholar]
- Luti K. J. K.; Mavituna F. Streptomyces coelicolor increases the production of undecylprodigiosin when interacted with Bacillus subtilis. Biotechnol. Lett. 2011, 33, 113–118. 10.1007/s10529-010-0401-y. [DOI] [PubMed] [Google Scholar]
- Gandhi N. M.; Patell J. R.; Gandhi J.; de Souza N. J.; Kohl H. Prodigiosin metabolites of a marine Pseudomonas species. Mar.Biol. 1976, 34, 223–227. 10.1007/BF00388799. [DOI] [Google Scholar]
- Kawauchi K.; Shibutani K.; Yagisawa H.; Kamata H.; Nakatsuji S.; Anzai H.; Yokoyama Y.; Ikegami Y.; Moriyama Y.; Hirata H. A possible immunosuppressant, cycloprodigiosin hydrochloride, obtained from Pseudoalteromonas denitrificans. Biochem. Biophys. Res. Commun. 1997, 237, 543–547. 10.1006/bbrc.1997.7186. [DOI] [PubMed] [Google Scholar]
- Lee H. K.; Chun J.; Moon E. Y.; Ko S. H.; Lee D. S.; Lee H. S.; Bae K. S. Hahella chejuensis gen. nov., sp. nov., an extracellular-polysaccharide-producing marine bacterium. Int. J. Syst. Evol. Microbiol. 2001, 51, 661–666. 10.1099/00207713-51-2-661. [DOI] [PubMed] [Google Scholar]
- Nakashima T.; Kurachi M.; Kato Y.; Yamaguchi K.; Oda T. Characterization of bacterium isolated from the sediment at coastal area of Omura Bay in Japan and several biological activities of pigment produced by this isolate. Microbiol. Immunol. 2005, 49, 407–415. 10.1111/j.1348-0421.2005.tb03744.x. [DOI] [PubMed] [Google Scholar]
- Ramaprasad E. V. V.; Bharti D.; Sasikala C.; Ramana C. V. Zooshikella marina sp. nov. a cycloprodigiosin and prodigiosin-producing marine bacterium isolated from beach sand. Int. J. Syst. Evol. Microbiol. 2015, 65, 4669–4673. 10.1099/ijsem.0.000630. [DOI] [PubMed] [Google Scholar]
- Yi H.; Chang Y. H.; Oh H. W.; Bae K. S.; Chun J. Zooshikella ganghwensis gen. nov., sp. nov., isolated from tidal flat sediments. Int. J. Syst. Evol. Microbiol. 2003, 53, 1013–1018. 10.1099/ijs.0.02521-0. [DOI] [PubMed] [Google Scholar]
- Walsh P. S.; Metzger D. A.; Higuchi R. Chelex 100 as a medium for simple extraction of DNA for PCR-based typing from forensic material. BioTechniques 2013, 54, 134–139. 10.2144/000114018. [DOI] [PubMed] [Google Scholar]
- Lee Y. K.; Jung H. J.; Lee H. K. Marine bacteria associated with the korean brown alga Undaria pinnatifida. J. Microbiol. 2006, 44, 694–698. [PubMed] [Google Scholar]
- Kamei Y.; Isnansetyo A. Lysis of methicillin-resistant Staphylococcus aureus by 2, 4-diacetylphloroglucinol produced by Pseudomonas sp. AMSN isolated from a marine alga. Int. J. Antimicrob. Agents 2003, 21, 71–74. 10.1016/s0924-8579(02)00251-0. [DOI] [PubMed] [Google Scholar]
- Hudzicki J. Kirby-Bauer disk diffusion susceptibility test protocol. Am. Soc. Microbiol. 2012, 1–23. [Google Scholar]; https://www.asmscience.org/content/education/protocol/protocol.3189 (accessed December 16, 2019).
- Domröse A.; Klein A. S.; Hage-Hülsmann J.; Thies S.; Svensson V.; Classen T.; Pietruszka J.; Jaeger K. E.; Drepper T.; Loeschcke A. Efficient recombinant production of prodigiosin in Pseudomonas putida. Front. Microbiol. 2015, 6, 972. 10.3389/fmicb.2015.00972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein A. S.; Domröse A.; Bongen P.; Brass H. U. C.; Classen T.; Loeschcke A.; Drepper T.; Laraia L.; Sievers S.; Jaeger K. E.; Pietruszka J. New prodigiosin derivatives obtained by mutasynthesis in Pseudomonas putida. ACS Synth. Biol. 2017, 6, 1757–1765. 10.1021/acssynbio.7b00099. [DOI] [PubMed] [Google Scholar]
- Kim D.; Kim J. F.; Yim J. H.; Kwon S. K.; Lee C. H.; Lee H. K. Red to red - the marine bacterium Hahella chejuensis and its product prodigiosin for mitigation of harmful algal blooms. J. Microbiol. Biotechnol. 2008, 18, 1621–1629. [PubMed] [Google Scholar]
- D’aoust J. Y.; Kushner D. J. Vibrio psychroerythrus sp. n.: classification of the psychrophilic marine bacterium, NRC 1004. J. Bacteriol. 1972, 111, 340–342. 10.1128/JB.111.2.340-342.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimata S.; Matsuda T.; Suizu Y.; Hayakawa Y. Prodigiosin R2, a new prodigiosin from the roseophilin producer Streptomyces griseoviridis 2464-S5. J. Antibiot. 2018, 71, 393–396. 10.1038/s41429-017-0011-1. [DOI] [PubMed] [Google Scholar]
- Pseudoalteromonas rubra. https://www.ncbi.nlm.nih.gov/nuccore/?term=Pseudoalteromonas+rubra (accessed December 10, 2019).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.