Abstract
Background
NEPA, a combination antiemetic of a neurokinin‐1 (NK1) receptor antagonist (RA) (netupitant [oral]/fosnetupitant [intravenous; IV]) and 5‐HT3RA, palonosetron] offers 5‐day CINV prevention with a single dose. Fosnetupitant solution contains no allergenic excipients, surfactant, emulsifier, or solubility enhancer. A phase III study of patients receiving cisplatin found no infusion‐site or anaphylactic reactions related to IV NEPA. However, hypersensitivity reactions and anaphylaxis have been reported with other IV NK1RAs, particularly fosaprepitant in patients receiving anthracycline‐cyclophosphamide (AC)‐based chemotherapy. This study evaluated the safety and efficacy of IV NEPA in the AC setting.
Materials and Methods
This phase IIIb, multinational, randomized, double‐blind study enrolled females with breast cancer naive to highly or moderately emetogenic chemotherapy. Patients were randomized to receive a single 30‐minute infusion of IV NEPA or single oral NEPA capsule on day 1 prior to AC, in repeated (up to 4) cycles. Oral dexamethasone was given to all patients on day 1 only.
Results
A total of 402 patients were included. The adverse event (AE) profiles were similar for IV and oral NEPA and consistent with those expected. Most AEs were mild or moderate with a similarly low incidence of treatment‐related AEs in both groups. There were no treatment‐related injection‐site AEs and no reports of hypersensitivity or anaphylaxis. The efficacy of IV and oral NEPA were similar, with high complete response (no emesis/no rescue) rates observed in cycle 1 (overall [0–120 hours] 73.0% IV NEPA, 77.3% oral NEPA) and maintained over subsequent cycles.
Conclusion
IV NEPA was highly effective and safe with no associated hypersensitivity and injection‐site reactions in patients receiving AC.
Implications for Practice
As a combination of a neurokinin‐1 (NK1) receptor antagonist (RA) and 5‐HT3RA, NEPA offers 5‐day chemotherapy‐induced nausea and vomiting prevention with a single dose and an opportunity to improve adherence to antiemetic guidelines. In this randomized multinational phase IIIb study, intravenous (IV) NEPA (fosnetupitant/palonosetron) was safe and highly effective in patients receiving multiple cycles of anthracycline‐cyclophosphamide (AC)‐based chemotherapy. Unlike other IV NK1RAs, the IV NEPA combination solution does not require any surfactant, emulsifier, or solubility enhancer and contains no allergenic excipients. Hypersensitivity reactions and anaphylaxis have been reported with other IV NK1RAs, most commonly with fosaprepitant in the AC setting. Importantly, there were no injection‐site or hypersensitivity reactions associated with IV NEPA.
Keywords: Antiemetic, CINV, NEPA, Netupitant, Palonosetron
Short abstract
Netupitant and palonosetron (NEPA) is a combination antiemetic that offers five‐day prevention of chemotherapy‐induced nausea and vomiting with a single dose. This article evaluates the safety and efficacy of intravenous NEPA in the setting of anthracycline/cyclophosphamide chemotherapy.
Introduction
The evolving landscape for prevention of chemotherapy‐induced nausea and vomiting (CINV) now includes an armamentarium of antiemetic agents, with the standard of care for highly (HEC) and moderately emetogenic chemotherapy (MEC) settings requiring multiagent prophylactic combinations that target different receptors involved in CINV 1, 2, 3. Although use of these combinations of agents has dramatically improved prevention of CINV for more than 25 years, it also comes with the complexity of administering antiemetics with different doses, schedules, and formulations for each patient. This complexity may contribute to the poor adherence seen in studies examining adherence with antiemetic guideline recommendations 4, 5, 6, 7. Two recent published surveys of oncology nurses in the U.S. 8 and Europe 9 also revealed inconsistencies between antiemetic practice patterns and antiemetic guideline recommendations, with older and less effective agents often being administered. The consequences of CINV can impact both patient outcomes and cost; a recent study found CINV to be a leading factor in the 25% prevalence of avoidable acute care among patients receiving HEC 10.
NEPA, the only fixed antiemetic combination product, is comprised of netupitant, a highly selective long‐lasting neurokinin‐1 (NK1) receptor antagonist (RA), and palonosetron, a second‐generation, pharmacologically and clinically distinct 5‐HT3RA 11, 12. As a combination of an NK1RA and 5‐HT3RA, NEPA uniquely offers 5‐day CINV prevention with a single dose and an opportunity to improve adherence to guidelines. Approval of oral NEPA (netupitant 300 mg and palonosetron 0.50 mg) in the U.S. and Europe was based on studies in which a single oral NEPA capsule plus dexamethasone (DEX), given prior to cisplatin‐ and anthracycline‐cyclophosphamide (AC)‐based chemotherapy, demonstrated superior efficacy in preventing CINV over palonosetron plus DEX for 5 days postchemotherapy 13, 14. The overall and cardiac safety of oral NEPA was also well established in almost 1,200 NEPA‐treated patients 15.
To offer additional ease and efficiency for patients and clinicians, a fixed intravenous (IV) combination formulation of NEPA was developed using fosnetupitant, a water‐soluble phosphorylated pro‐drug of netupitant. Unlike other NK1RAs, fosnetupitant does not require any surfactant (e.g., polysorbate 80), emulsifier, or solubility enhancer and contains no allergenic excipients (e.g., egg, soy), thereby minimizing infusion‐related toxicities 16. Following demonstrated pharmacokinetic bioequivalence of 235 mg of IV fosnetupitant and 300 mg of oral netupitant, a phase III registration study leading to the approval of IV NEPA in the U.S. was conducted to evaluate the safety and tolerability of IV NEPA (relative to oral NEPA) in patients with various solid tumors receiving mainly cisplatin‐based HEC 17. In this study, IV NEPA was well tolerated, with a similar safety and efficacy profile as oral NEPA. Importantly, there were no injection‐site reactions related to IV NEPA over repeated cycles and no instance of anaphylaxis with either formulation of NEPA. This is particularly noteworthy as hypersensitivity reactions and anaphylaxis have been reported with IV fosaprepitant 18, 19, 20, 21, 22, 23, HTX‐019 (IV aprepitant) 24, 25, and IV rolapitant 26, 27. Studies have shown a differential impact of fosaprepitant on infusion‐site adverse events (AEs), with patients receiving AC chemotherapy at higher risk 19, 20, 21, 22, 23 compared with those receiving cisplatin‐based chemotherapy, possibly because of the potential for vascular endothelial damage with both fosaprepitant and anthracycline 23. As a result, it was deemed important to evaluate the safety, particularly relating to infusion‐site AEs, for IV NEPA in the AC setting.
Therefore, this phase IIIb study was designed to primarily evaluate the overall safety and tolerability of a single dose of IV NEPA administered with DEX over initial and repeated cycles of AC‐based chemotherapy. A secondary objective was to explore the efficacy of IV NEPA in this setting, and an exploratory objective was to assess the impact on CINV‐related resource use and costs.
Materials and Methods
Study Design
This was a phase IIIb, multinational, multicenter, randomized, double‐blind, double‐dummy, parallel‐group, multiple cycle study (NCT03403712) conducted at 51 enrolling centers in the U.S, Russia, Ukraine, and Georgia between March 2018 and September 2018. The protocol was approved by ethical review committees at each institution and all patients gave written informed consent. The study was performed in accordance with the principles outlined in the Declaration of Helsinki as amended by the World Medical Association in Fortaleza in 2013, the International Conference on Harmonization (ICH) Good Clinical Practice guidelines, and all local laws and regulations of the countries in which the study was conducted.
Patient Selection
Eligible study participants were women ≥18 years with histologically or cytologically confirmed breast cancer, including recurrent or metastatic. Patients were naive to MEC or HEC at study entry and scheduled to receive at least four consecutive cycles of an AC‐based chemotherapy regimen. Concomitant non‐, low‐, or minimally emetogenic chemotherapy was permitted at any time after AC on day 1; concomitant MEC or HEC was permitted after AC on day 1 only, provided the administration was completed within 6 hours of the initiation of AC. At study entry, patients were required to have an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1.
Patients were not eligible to enter the study or continue in repeated cycles if they were scheduled to receive (a) MEC or HEC any time beyond 6 hours after AC initiation on day 1 up to day 1 of the subsequent cycle, (b) radiotherapy to the abdomen or pelvis within 1 week prior to day 1 or between days 1 and 5, or (c) a bone marrow or stem‐cell transplant. Patients were not allowed to receive any drug with potential antiemetic efficacy within 24 hours or any systemic corticosteroid within 72 hours before the start of AC chemotherapy administration on day 1. Patients could not receive strong or moderate CYP3A4 inhibitors within 1 week or any CYP3A4 inducers within 4 weeks prior to day 1 or during days 1–5. Patients were excluded if they experienced any vomiting, retching, or at least mild nausea within 24 hours before AC administration on day 1. Patients were ineligible if they had a symptomatic primary or metastatic central nervous system malignancy, an active peptic ulcer, gastrointestinal obstruction, increased intracranial pressure, hypercalcemia, an active infection, or any medical condition that could represent another potential etiology for nausea and vomiting. Patients were also excluded if they had a history or predisposition to cardiac conduction abnormalities, except for incomplete right bundle branch block and were ineligible if they had a history of risk factors for Torsade de Pointes, or any severe or uncontrolled cardiovascular diseases within 3 months prior to day 1 of the first cycle.
Treatment
Eligible patients, stratified by region (U.S.; non‐U.S.) and age class (<55 years; ≥55 years) were randomized 1:1 to receive a single 30‐minute 50 mL infusion of IV NEPA or a single capsule of oral NEPA on day 1 prior to initiation of AC (plus the corresponding matching placebo). IV NEPA was initiated 30 minutes prior to chemotherapy and completed before starting AC administration, whereas oral NEPA was taken 60 minutes prior to chemotherapy. Open‐label oral DEX 12 mg was given to all patients 30 minutes before AC chemotherapy on day 1 only. The DEX doses were those recommended in the oral NEPA package insert. For subsequent cycles, patients received the same study treatment they were randomized to in cycle 1.
Rescue medication was permitted on an as‐needed basis at the investigator's discretion and choice only to alleviate breakthrough nausea or vomiting after AC on day 1–5 of each cycle. NK1RAs, commercial NEPA (Akynzeo®; Helsinn Birex Pharmaceuticals Ltd., Dublin, Ireland), and 5‐HT3RAs were not permitted as rescue treatment.
Assessments
The safety assessment was primarily based on the evaluation of treatment‐emergent AEs (i.e., those occurring after the first dose of study treatment); physical examination, vital signs, and locally performed clinical laboratory tests were also assessed. Investigators classified treatment‐emergent AEs based on severity (as per Common Terminology Criteria for Adverse Events [CTCAE] version 4.0) and causal relationship to the study treatment. Treatment‐related AEs were those deemed by the investigator to be possibly, probably or definitely related to study drug or those for which causality was not evaluable or missing.
Infusion‐site AEs were of special interest. Conservatively, all MedDRA preferred terms containing one of the following words were selected as reflecting potential infusion‐site events: pain, erythema, swelling, urticaria, extravasation, thrombosis, phlebitis, discoloration, inflammation, induration, scar, pruritus, warmth, burning, and catheter site related reaction. All these MedDRA preferred terms were then reviewed in a blinded fashion.
The assessment of efficacy was based on the patient's diary, which captured emetic episodes and use of rescue medication from the start of chemotherapy on day 1 up to day 5 (0–120 hours) of each cycle. Severity of nausea (evaluated using a 100‐mm visual analog scale [VAS]) was assessed daily on the same diary.
The Functional Living Index‐Emesis (FLIE), a reliable and valid patient‐reported instrument, was used to assess the impact of CINV on important aspects of daily living 28, 29 during the 5 days (0–120 hours) following AC of cycles 1 and 2. The FLIE has 9 questions specific to each domain (i.e., nausea and vomiting); each response is based on a 7‐point 100‐mm VAS anchored by “a great deal” and “none/not at all”. “No impact on daily life” was operationally defined as an average item score of >6 (nausea and vomiting domain scores ≥54, and total FLIE score ≥108).
Resource use assessments included capturing information pertaining to (a) “acute care visits” (i.e., emergency department visits or inpatient hospitalization associated with CINV) and (b) unplanned outpatient treatment for hydration.
Statistical Analysis
The primary aim of this study was to characterize the safety and tolerability of a single dose of IV NEPA over a duration of time consistent with its intended use in a population of patients receiving AC‐based chemotherapy. Although no formal comparisons between treatment groups were planned, the inclusion of the oral NEPA control group was intended to help interpret any unexpected safety finding in the IV NEPA group, taking into consideration the cancer population receiving AC.
Four hundred patients were to be randomized 1:1 to either IV NEPA or oral NEPA (i.e., 200 patients per group). Study drug assignment for cycles 2–4 was to close 7 days after the last (400th) patient was randomized such that patients already screened for a subsequent cycle would receive study drug. Patients still participating in the study at that time completed their current cycle but were not allowed to enter a subsequent cycle.
The safety population consisted of all patients who received active study drug (either IV NEPA, including partial infusion, or oral NEPA). Demographics and baseline characteristics were summarized by treatment group using descriptive statistics.
Treatment‐emergent AEs were summarized by frequency tables for cycle 1 and throughout the study. Treatment‐emergent AEs of special interest (i.e., infusion‐site AEs) were summarized for each cycle and throughout the study.
The full analysis set (FAS) population consisted of all patients who were randomized, received active study drug and AC. For efficacy endpoints during each cycle, numbers and percentages (including 95% confidence interval [CI] using the Wilson score method) of patients with complete response (defined as no emetic episodes and no use of rescue medication), with no emetic episodes, with no use of rescue medication, and with no significant nausea and no nausea (maximum VAS score <25 mm and <5 mm, respectively) were descriptively summarized by treatment for the acute (0–24 hours), delayed (25–120 hours), and overall (0–120 hours) phases. Differences between groups in response rate and 95% CI for the difference were calculated using the stratum‐adjusted Cochran‐Mantel‐Haenszel (CMH) method for the risk difference. Age and region were used as strata. The number and percentage (including 95% CI) of patients with no impact on daily living (overall, by domain and by individual item) based on FLIE, were also summarized by treatment.
For resource use assessments, CINV involvement with events was defined as a patient with no complete response during the overall phase and occurrence of the respective resource use within the same 5‐day period. This approach may overstate the incidence of CINV resource use due to simultaneous hospitalization or hydration not specifically for nausea or vomiting (e.g., a patient coming to the emergency department for a urinary infection would be “counted” as an acute care visit for CINV if the above criteria for CINV involvement was met, regardless of the reason for the visit).
Results
A total of 404 patients were randomized. Two patients were not treated; therefore, the safety population was comprised of 200 patients in the IV NEPA group and 202 patients in the oral NEPA group. Almost all patients (97%) completed cycle 1, whereas approximately 50% of patients completed all 4 cycles of treatment (Fig. 1). Of note, study closure at completion of accrual, as per protocol, was responsible for 91% of those patients who exited the study prior to the end of cycle 4.
Figure 1.
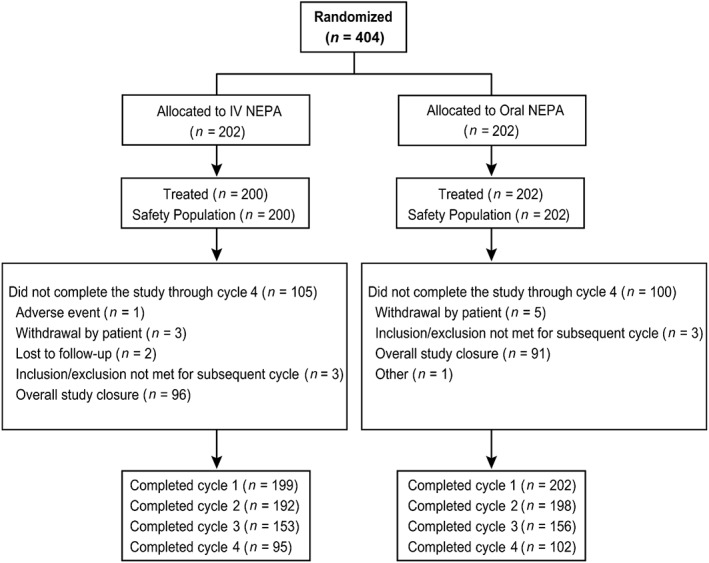
Consort diagram. Abbreviation: IV, intravenous.
The majority of patients (79% at cycle 1 and similar percentages in subsequent cycles) received IV NEPA/placebo via a peripheral line. A total of 641 infusions of IV NEPA and 660 capsules of oral NEPA were administered during the entire study.
Baseline characteristics were similar in the two treatment groups (Table 1). The mean age was 55.4 years; most patients were white (93.3%), with an ECOG performance status of 0 (75.3%), and most did not smoke (95%) or drink alcohol (79.9%).
Table 1.
Patient baseline and disease characteristics (safety population)
| Characteristic | IV NEPA (n = 200), n (%) | Oral NEPA (n = 202), n (%) |
|---|---|---|
| Female | 200 (100) | 202 (100) |
| Fertility status | ||
| Child‐bearing potential | 59 (29.5) | 52 (25.7) |
| Postmenopausal | 120 (60.0) | 128 (63.4) |
| Surgically sterile | 21 (10.5) | 22 (10.9) |
| Mean age (SD), y | 55.6 (9.94) | 55.2 (9.73) |
| Race | ||
| White | 189 (94.5) | 186 (92.1) |
| Black | 4 (2.0) | 9 (4.5) |
| Other | 7 (3.5) | 7 (3.5) |
| ECOG performance statusa | ||
| 0 | 150 (76.5) | 148 (74.0) |
| 1 | 46 (23.5) | 52 (26.0) |
| Extent of cancer | ||
| Localized | 80 (40.0) | 74 (36.6) |
| Locally advanced | 94 (47.0) | 97 (48.0) |
| Metastatic | 26 (13.0) | 31 (15.3) |
| AC regimens | ||
| Epirubicin + cyclophosphamide | 114 (57.0) | 128 (63.4) |
| Doxorubicin + cyclophosphamide | 86 (43.0) | 74 (36.6) |
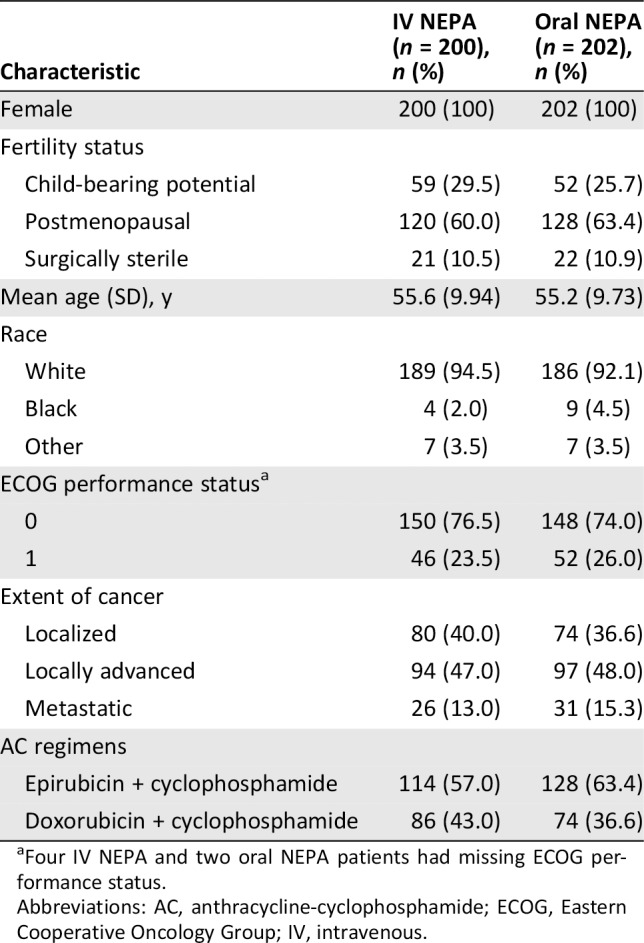
Four IV NEPA and two oral NEPA patients had missing ECOG performance status.
Abbreviations: AC, anthracycline‐cyclophosphamide; ECOG, Eastern Cooperative Oncology Group; IV, intravenous.
Safety
The overall incidence and intensity of treatment‐emergent AEs were similar between the two treatment groups in cycle 1 and throughout the entire study (Table 2). The majority of patients experienced treatment‐emergent AEs of mild/moderate severity, with 18.5% (IV NEPA) and 14.3% (oral NEPA) of patients experiencing at least one treatment‐emergent severe (grade ≥3) AE during the entire study.
Table 2.
Summary of treatment‐emergent adverse events in cycle 1 and throughout the study (safety population)
| Patients with: | Cycle 1 | Entire study | ||
|---|---|---|---|---|
| IV NEPA (n = 200), n (%) | Oral NEPA (n = 202), n (%) | IV NEPA (n = 200), n (%) | Oral NEPA (n = 203),a n (%) | |
| At least one treatment‐emergent adverse event (TEAE) | 121 (60.5) | 122 (60.4) | 184 (92.0) | 187 (92.1) |
| Severe (grade ≥3) TEAEs | 11 (5.5) | 10 (5.0) | 37 (18.5) | 29 (14.3) |
| Serious TEAE | 2 (1.0) | 1 (0.5) | 5 (2.5) | 4 (2.0) |
| Any treatment‐related adverse event (TRAE) | 13 (6.5) | 12 (5.9) | 16 (8.0) | 22 (10.8) |
| Severe (grade ≥3) TRAE | 0 | 0 | 1 (0.5) | 2 (1.0) |
| Serious TRAE | 0 | 0 | 0 | 1 (0.5)b |
| Any TEAE leading to discontinuation | 0 | 0 | 1 (0.5) | 0 |
| Any TRAE leading to discontinuation | 0 | 0 | 0 | 0 |
| Any TEAE resulting in death | 0 | 0 | 0 | 0 |
| Any infusion‐site TEAE | 2 (1.0) | 0 | 3 (1.5) | 9 (4.4) |
| Any treatment‐related infusion site TEAE | 0 | 0 | 0 | 0 |
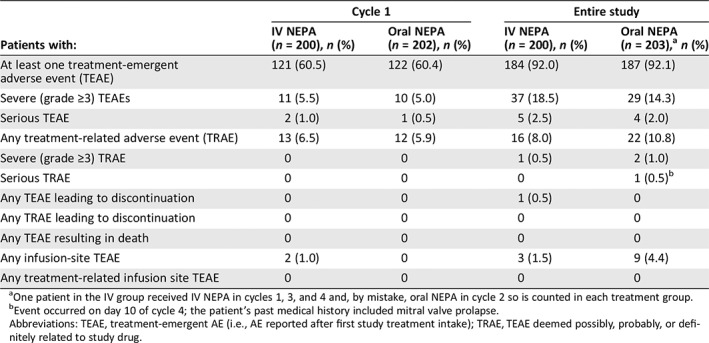
One patient in the IV group received IV NEPA in cycles 1, 3, and 4 and, by mistake, oral NEPA in cycle 2 so is counted in each treatment group.
Event occurred on day 10 of cycle 4; the patient's past medical history included mitral valve prolapse.
Abbreviations: TEAE, treatment‐emergent AE (i.e., AE reported after first study treatment intake); TRAE, TEAE deemed possibly, probably, or definitely related to study drug.
The most frequently reported treatment‐emergent AEs (i.e., those reported by ≥5% of patients in either treatment group) were consistent with those expected for cancer patients undergoing AC‐based chemotherapy (e.g., alopecia, leukopenia); similar incidence rates were seen for IV NEPA and oral NEPA (Table 3). The most frequently reported severe treatment‐emergent AEs with an incidence of ≥2% of patients in either treatment group are also summarized in Table 3, with leukopenia and neutropenia being most common. Overall, grade 3 treatment‐emergent AEs were experienced by 15.0% of patients in the IV NEPA group and 12.3% of patients in the oral NEPA group, whereas grade 4 treatment‐emergent AEs were experienced by 3.5% and 2.0% of patients in the IV NEPA and oral NEPA groups, respectively.
Table 3.
Treatment‐emergent all‐gradea adverse events with an incidence ≥5% and severe (grade ≥3)a adverse events with an incidence ≥2% in either treatment group throughout the study (safety population)
| Patients with: | All‐grade TEAEs | Grade ≥3 TEAEs | ||
|---|---|---|---|---|
| IV NEPA (n = 200), n (%) | Oral NEPA (n = 203),b n (%) | IV NEPA (n = 200), n (%) | Oral NEPA (n = 203),b n (%) | |
| Alopecia | 141 (70.5) | 133 (65.5) | ‐ | ‐ |
| Leukopenia | 40 (20.0) | 33 (16.3) | 13 (6.5) | 7 (3.4) |
| Fatigue | 30 (15.0) | 34 (16.7) | ‐ | ‐ |
| Anemia | 31 (15.5) | 24 (11.8) | 4 (2.0) | 2 (1.0) |
| Neutropenia | 25 (12.5) | 24 (11.8) | 11 (5.5) | 9 (4.4) |
| Asthenia | 27 (13.5) | 17 (8.4) | ‐ | ‐ |
| Nausea | 14 (7.0) | 9 (4.4) | ‐ | ‐ |
| Headache | 10 (5.0) | 12 (5.9) | ‐ | ‐ |
| Diarrhea | 10 (5.0) | 9 (4.4) | ||
| Gamma‐glutamyltransferase increased | 11 (5.5) | 7 (3.4) | ‐ | ‐ |
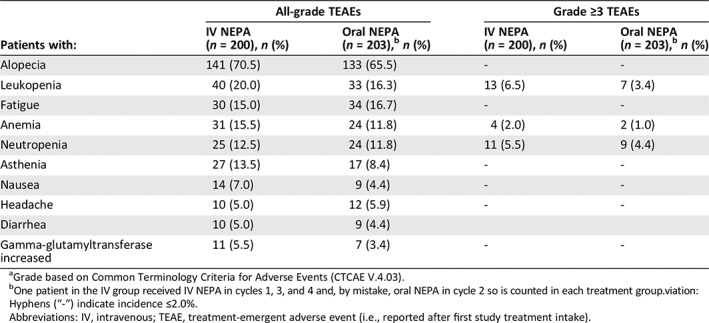
Grade based on Common Terminology Criteria for Adverse Events (CTCAE V.4.03).
One patient in the IV group received IV NEPA in cycles 1, 3, and 4 and, by mistake, oral NEPA in cycle 2 so is counted in each treatment group.viation:
Hyphens (“‐”) indicate incidence ≤2.0%.
Abbreviations: IV, intravenous; TEAE, treatment‐emergent adverse event (i.e., reported after first study treatment intake).
The most common treatment‐related AEs during the entire study were headache (2.5% IV NEPA, 3.4% oral NEPA), dizziness (2.5% in both groups), fatigue (2.0% in both groups), and constipation (2.5% IV NEPA, 1.0% oral NEPA); all other treatment‐related AEs occurred in <2% of patients.
There were no serious treatment‐related AEs associated with IV NEPA; one serious treatment‐related event was reported in the oral NEPA group (a patient with a history of valve prolapse receiving chronic atenolol 50 mg daily experienced atrial fibrillation on day 10 of cycle 4). The patient was recovering when she was discharged from the hospital. No patients died on study and no treatment‐related AEs led to study discontinuation in either treatment group (Table 2).
Infusion‐Site Adverse Events and Hypersensitivity Reactions
The review of infusion‐site specific MedDRA preferred terms for the entire study revealed only 3 (1.5%) treatment‐emergent AEs for IV NEPA patients (peripheral swelling and injection‐site phlebitis, occurring 7 and 3 days, respectively, after IV NEPA administration and catheter site‐related reaction occurring on day 1); none of these treatment‐emergent AEs were deemed by the investigator to be related to IV NEPA infusion. By comparison, there were nine (4.4%) infusion‐site AEs in the oral NEPA (IV placebo infusion) treatment group. There were no infusion‐site, hypersensitivity, or anaphylaxis‐like treatment‐emergent AEs reported as treatment‐related in either treatment group (Table 4).
Table 4.
Summary of infusion‐site adverse events in cycle 1 and throughout the study (safety population)
| Patients with: | Cycle 1 | Entire study | ||
|---|---|---|---|---|
| IV NEPA (n = 200), n (%) | Oral NEPA (n = 202), n (%) | IV NEPA, (n = 200), n (%) | Oral NEPA (n = 203),a n (%) | |
| At least one treatment‐related infusion‐site TEAE | 0 | 0 | 0 | 0 |
| At least one infusion‐site TEAE | 2 (1.0) | 0 | 3 (1.5) | 9 (4.4) |
| Pruritus | 0 | 0 | 0 | 3 (1.5) |
| Deep vein thrombosis | 0 | 0 | 0 | 2 (1.0) |
| Axillary pain | 0 | 0 | 0 | 1 (0.5) |
| Catheter site‐related reaction | 1 (0.5) | 0 | 1 (0.5) | 0 |
| Extravasation | 0 | 0 | 0 | 1 (0.5) |
| Injection site phlebitis | 0 | 0 | 1 (0.5) | 0 |
| Musculoskeletal pain | 0 | 0 | 0 | 1 (0.5) |
| Peripheral swelling | 1 (0.5) | 0 | 1 (0.5) | 0 |
| Phlebitis | 0 | 0 | 0 | 1 (0.5) |
| Vascular site access pain | 0 | 0 | 0 | 1 (0.5) |

One patient in the IV group received IV NEPA in cycles 1, 3, and 4 and, by mistake, oral NEPA in cycle 2 so is counted in each treatment group.
Abbreviations: IV, intravenous; TEAE, treatment‐emergent adverse event.
Efficacy
Complete response rates in cycle 1 were 86.5% and 88.6% during the acute phase, 75.5% and 78.7% during the delayed phase, and 73.0% and 77.3% during the overall phase, for IV and oral NEPA, respectively. Complete response, no emesis, no significant nausea, no nausea and no rescue medication use rates were similar between treatment groups and were maintained over cycles 1–4 (Table 5). As measured by the FLIE, similar proportions of IV and oral NEPA patients reported no impact on daily life due to nausea (nausea domain), vomiting (vomiting domain), or both (overall FLIE) during both cycles 1 and 2 (Fig. 2).
Table 5.
Efficacy response rates during the overall (0‐120 hours) phase over repeated cycles (FAS population)
| Cycle, n a | Complete response, % | No emesis, % | No rescue medication, % | No significant nausea, % | No nausea, % | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| IV NEPA | Oral NEPA | IV NEPA | Oral NEPA | IV NEPA | Oral NEPA | IV NEPA | Oral NEPA | IV NEPA | Oral NEPA | |
| Cycle 1 (n = 200/202) | 73.0 | 77.2 | 82.5 | 86.1 | 81.5 | 86.6 | 70.0 | 74.8 | 42.0 | 48.0 |
| Cycle 2 (n = 193/198) | 80.3 | 81.3 | 90.2 | 90.4 | 85.5 | 86.4 | 76.7 | 73.2 | 49.7 | 51.5 |
| Cycle 3 (n = 153/157) | 80.4 | 83.4 | 90.2 | 89.2 | 84.3 | 89.8 | 77.8 | 80.9 | 52.9 | 59.9 |
| Cycle 4 (n = 96/102) | 85.4 | 87.3 | 94.8 | 94.1 | 88.5 | 88.2 | 83.3 | 82.4 | 57.3 | 61.8 |
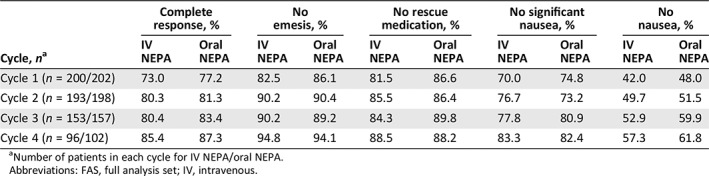
Number of patients in each cycle for IV NEPA/oral NEPA.
Abbreviations: FAS, full analysis set; IV, intravenous.
Figure 2.
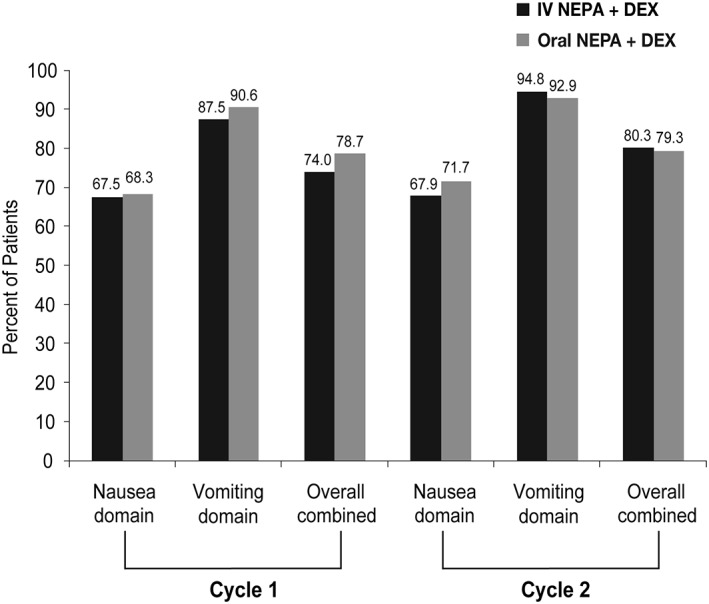
Proportion of patients with no impact on daily living based on Functional Living‐Index Emesis.Abbreviations: DEX, dexamethasone; IV, intravenous.
Resource Use
No patients had inpatient admissions and only one (0.2%) patient (oral NEPA) had an emergency department visit involving CINV. Three (0.7%) patients (2 IV NEPA, 1 oral NEPA) received an unplanned hydration associated with CINV.
Discussion
Different routes of administration of effective and safe drugs increase flexibility and efficiency, especially in highly symptomatic patients with cancer. Some patients with cancer cannot tolerate oral treatments and/or may have difficulty in swallowing due to mucositis or disease 30.
All currently available NK1RAs have been developed initially as oral and subsequently as IV formulations. There are pertinent differences in the IV formulations of these agents. The fosaprepitant formulation contains polysorbate 80, a synthetic surfactant that has been used in other oncology products such as docetaxel and erythropoietin‐stimulating agents 31. Rolapitant IV contains the synthetic surfactant polyoxyl 15 hydroxystearate 26, 31, and HTX‐019 (IV aprepitant) contains potentially allergenic excipients including egg lecithin, soybean oil, and ethanol but does not contain either polysorbate 80 or synthetic surfactants 24. Unlike these IV NK1RAs, the IV NEPA combination solution does not require any surfactant, emulsifier, or solubility enhancer and contains no allergenic excipients 17, 32.
In general, the NK1RAs as a class are considered safe and well tolerated 32. However, hypersensitivity reactions and infusion‐site AEs have been associated with fosaprepitant, most likely due to the polysorbate 80 surfactant 31. In the large registration clinical trial in cancer patients receiving cisplatin, the incidence rate of infusion‐site AEs was 2.2% 18. However, in fosaprepitant postmarketing retrospective trials incidence rates ranged from 28% to 96% 31, with higher rates associated with increased speed of delivery 22, use of peripheral venous access 19, 20, 21, 22, 23, and AC‐based chemotherapy 19, 20, 21, 22, 23. Rolapitant IV and HTX‐019 were both approved in the U.S. based only on pharmacokinetic bioequivalence studies (to the oral formulations) in healthy volunteers 25, 33, 34. Following approval of rolapitant, the U.S. Food & Drug Administration issued a safety alert to clinicians warning that serious hypersensitivity reactions, including anaphylaxis and anaphylactic shock, had been reported in the postmarketing setting 27; shortly thereafter, the manufacturer suspended distribution of IV rolapitant. At the approval of HTX‐019, the product label mimicked that of fosaprepitant; however, it was updated recently with language indicating that serious hypersensitivity reactions, including anaphylaxis, have occurred during or soon after administration of HTX‐019 22. The product labels for all these NK1RAs include contraindications/warnings/precautions regarding the potential for hypersensitivity reactions and anaphylaxis 18, 24. The IV NEPA label includes no such warnings/precautions related to fosnetupitant; only a warning or precaution is included for the palonosetron component.
The current study (NCT03403712) was conducted in follow‐up to the registration study for IV NEPA to further evaluate its safety in patients receiving AC‐based chemotherapy, previously shown with fosaprepitant to be at the greatest risk of experiencing hypersensitivity reactions and infusion‐site AEs. Consistent with the IV NEPA registration study in patients receiving cisplatin 17 and also with the phase III multiple cycle safety study of oral NEPA 35, descriptive statistical methods were applied for evaluation of the comparative adverse event profiles. This study showed that the IV formulation of NEPA is safe and well tolerated in patients with breast cancer receiving AC‐based chemotherapy and that IV NEPA has a similar safety profile to that observed with oral NEPA.
The adverse event profile in the current study was consistent with that expected for breast cancer patients receiving AC and the treatment‐related AEs seen were consistent with those expected for the NK1RA and 5‐HT3RA classes 36. The majority of treatment‐emergent AEs were of mild/moderate intensity with ≤1% of patients experiencing severe treatment‐related AEs. The incidence of all treatment‐related AEs was similar and low in both groups, both during cycle 1 and during the entire study. Moreover, no patients died on study and no treatment‐related AEs led to study discontinuation in either treatment group.
Importantly, only three patients (1.5%) receiving IV NEPA experienced any infusion‐site AE during any cycle in the study and none of these events were deemed to be related to IV NEPA. Similarly, there were no reports of hypersensitivity or anaphylaxis. Given the reports of hypersensitivity reactions and infusion‐site AEs related to the other NK1RAs, it is reassuring that in this study and the prior study 17 neither reaction was observed after more than 1,300 IV NEPA infusions. This is likely due to the unique chemical characteristics of fosnetupitant and simplified formulation of IV NEPA which requires no surfactant, emulsifier, solubility enhancer, or allergenic excipient.
The efficacy of IV NEPA was similar to that for oral NEPA, with high response rates in cycle 1 that were maintained over subsequent cycles. This pattern of sustained efficacy over multiple cycles is similar to that seen in the oral NEPA multiple cycle registration trials 35, 37, despite that just 50% of patients completed four cycles in this study (due to study closure when enrollment was met). It is particularly noteworthy that these response rates were consistent with those seen for oral NEPA (n = 724) in the large phase III registration study in patients with breast cancer receiving AC 14, 37. The overall CR rates in cycle 1 were 73.0% for IV NEPA and 77.2% for oral NEPA in the current study compared with 74.3% for oral NEPA in the prior AC study. These results were consistent across all efficacy endpoints, including the patient‐reported FLIE capturing their evaluation of the impact of nausea and vomiting on quality of life. The CINV control seen in this study was also associated with almost no acute care visits or unplanned clinic visits for hydration because of dehydration secondary to CINV, representing a particularly important factor for quality and cost. Decreased avoidable acute care use will become increasingly more important, especially given the U.S. Centers for Medicare and Medicaid's institution of the OP‐35 oncology outcomes measure tracking avoidable acute care use involving CINV and 8 other chemotherapy‐related toxicities within 30 days of chemotherapy 38.
Although there are inherent challenges in comparing results across trials, it is encouraging that the complete response rates in this (and the prior oral NEPA) study are higher than those seen for other NK1 triplet regimens in the AC setting (e.g., overall complete response rates in phase III trials: oral aprepitant 51% 39, fosaprepitant (51%–56%) 40, and oral rolapitant 63% 41). It is noteworthy that these NK1 RAs were combined with first‐generation 5‐HT3 RAs ondansetron (aprepitant/fosaprepitant trials) and granisetron (rolapitant trial). In addition, a real‐world observational study revealed a very low overall complete response rate (34%) in the subset of patients with breast cancer receiving AC 42, suggesting that a simplified combination agent may minimize patient error or nonadherence by nature of its formulation, and could be beneficial in improving CINV control in clinical practice.
As with most studies, there are limitations. In this case, the population was predominantly white. The study was not designed nor powered for a formal statistical efficacy comparison of IV NEPA and oral NEPA, as the focus was a safety evaluation of IV NEPA. In addition, as the cardiac safety of NEPA has been carefully evaluated previously, ECG assessments were not included in the current trial. Prior cardiac safety evaluations for NEPA have included a thorough QTc study 43, the oral NEPA phase III study in the AC setting 44, and the prior IV NEPA study in the cisplatin setting 13. Previously, there were no cardiac safety concerns based on ECGs observed with either IV or oral NEPA formulations during initial or repeated cycles in >1,500 patients participating in phase III studies. The cardiac AE profile in the current study was consistent with that observed in the previous oral NEPA study in the AC setting 14, 37.
Conclusion
As a fixed single‐dose prophylactic antiemetic combination given with DEX prior to AC chemotherapy, NEPA delivers guideline‐concordant treatment, thereby minimizing the complexity of multiagent dosing and decision‐making. In this study, IV NEPA was shown to be safe and highly effective in patients receiving multiple cycles of AC, with an associated minimal level of CINV‐related resource utilization. Unlike results seen with other NK1RA regimens, IV NEPA was not associated with injection‐site or hypersensitivity reactions.
Author Contributions
Conception/design: Lee Schwartzberg, Daniel Voisin, Giada Rizzi
Provision of study material or patients: Ekaterine Arkania, Irena Radyukova, Kamal Patel
Collection and/or assembly of data: Ekaterine Arkania, Irena Radyukova, Kamal Patel, Daniel Voisin, Giada Rizzi
Data analysis and interpretation: Lee Schwartzberg, Rudolph Navari, Rebecca Clark‐Snow, Daniel Voisin, Giada Rizzi, Rita Wickham, Richard J. Gralla, Matti Aapro, Eric Roeland
Manuscript writing: Lee Schwartzberg, Rudolph Navari, Rebecca Clark‐Snow, Ekaterine Arkania, Irena Radyukova, Kamal Patel, Daniel Voisin, Giada Rizzi, Rita Wickham, Richard J. Gralla, Matti Aapro, Eric Roeland
Final approval of manuscript: Lee Schwartzberg, Rudolph Navari, Rebecca Clark‐Snow, Ekaterine Arkania, Irena Radyukova, Kamal Patel, Daniel Voisin, Giada Rizzi, Rita Wickham, Richard J. Gralla, Matti Aapro, Eric Roeland
Disclosures
Lee Schwartzberg: Helsinn Healthcare, Tesaro, Merck, Heron (C/A), Helsinn Healthcare, Tesaro (RF); Irina Radyukova: Helsinn Healthcare Switzerland (RF); Kamal Patel: Helsinn (RF); Daniel Voisin: Helsinn Healthcare SA, Lugano, Switzerland (E); Giada Rizzi: Helsinn Healthcare SA (E); Rita Wickham: Insys Therapeutics (H), Helsinn Healthcare SA (SAB); Richard J Gralla: Helsinn (C/A), Merck (SAB, RF); Matti Aapro: Helsinn (RF), G1, Helsinn, Mundipharma, Tesaro, Merck (C/A); Eric Roeland: Helsinn Therapeutics, Heron Pharmaceuticals (SAB), BASF, Napo (C/A). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Acknowledgments
The authors acknowledge and thank the patients, investigators, and study teams at each of the participating centers. Editorial, medical writing, and production assistance for this manuscript was provided by Jennifer Vanden Burgt, Minneapolis, MN, and funded by Helsinn Healthcare, Lugano, Switzerland. This trial was sponsored and supported by Helsinn Healthcare, SA, Lugano, Switzerland who provided the study drugs and the funding for this study.
Disclosures of potential conflicts of interest may be found at the end of this article.
References
- 1. Hesketh PJ, Kris MG, Basch E et al. Antiemetics: American Society of Clinical Oncology Clinical practice guideline update. J Clin Oncol 2017;35:3240–3261. [DOI] [PubMed] [Google Scholar]
- 2. Roila F, Molassiotis A, Herrstedt J et al. 2016 MASCC and ESMO guideline update for the prevention of chemotherapy‐ and radiotherapy‐induced nausea and vomiting and of nausea and vomiting in advanced cancer patients. Ann Oncol. 2016;27(suppl 5):v119–v133. [DOI] [PubMed] [Google Scholar]
- 3. Berger MJ, Ettinger DS, Aston J et al. NCCN guidelines insights: Antiemesis, Version 2.2017. J Natl Compr Canc Netw 2017;15:883–893. [DOI] [PubMed] [Google Scholar]
- 4. Aapro M, Molassiotis A, Dicato M et al. The effect of guideline‐consistent antiemetic therapy on chemotherapy‐induced nausea and vomiting (CINV): The Pan European Emesis Registry (PEER). Ann Oncol 2012;23:1986–1992. [DOI] [PubMed] [Google Scholar]
- 5. Gilmore JW, Peacock NW, Gu A et al. Antiemetic guideline consistency and incidence of chemotherapy‐induced nausea and vomiting in US community oncology practice: INSPIRE study. J Oncol Pract 2014;10:68–74. [DOI] [PubMed] [Google Scholar]
- 6. O'Sullivan C, Van Houten HK, Sangaralingham LR et al. Ten year trends in antiemetic prescribing in cancer patients receiving highly emetogenic chemotherapy (HEC). J Natl Compr Canc Netw 2018;16:294–299. [DOI] [PubMed] [Google Scholar]
- 7. Roeland E, Ruddy KJ, LeBlanc TW et al. Gaps in compliance with current antiemetic guidelines for highly emetogenic chemotherapy. J Natl Compr Canc Netw 2018;16(suppl):AB2018‐36a. [Google Scholar]
- 8. Clark‐Snow R, Affronti ML, Rittenberg CN. Chemotherapy‐induced nausea and vomiting (CINV) and adherence to antiemetic guidelines: Results of a survey of oncology nurses. Support Care Cancer 2019;26:557–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dielesenger P, Börjeson S, Vidall C et al. Evaluation of antiemetic practices for prevention of chemotherapy‐induced nausea and vomiting: Results of a European Oncology nurse survey. Support Care Cancer 2019;27:4099–4106. [DOI] [PubMed] [Google Scholar]
- 10. Roeland E, LeBlanc T, Ruddy K et al. Avoidable acute care use associated with nausea and vomiting among patients receiving highly emetogenic chemotherapy or oxaliplatin. J Natl Compr Canc Netw 2019;17:BP19‐019a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rojas C, Raje M, Tsukamoto T et al. Molecular mechanisms of 5‐HT(3) and NK(1) receptor antagonists in prevention of emesis. Eur J Pharmacol 2014;722:26–37. [DOI] [PubMed] [Google Scholar]
- 12. Stathis M, Pietra C, Rojas C et al. Inhibition of substance P‐mediated responses in NG108‐15 cells by netupitant and palonosetron exhibit synergistic effects. Eur J Pharmacol 2012;689:25–30. [DOI] [PubMed] [Google Scholar]
- 13. Hesketh PJ, Rossi G, Rizzi G et al. Efficacy and safety of NEPA, an oral combination of netupitant and palonosetron, for prevention of chemotherapy‐induced nausea and vomiting following highly emetogenic chemotherapy: A randomized dose‐ranging pivotal study. Ann Oncol 2014;25:1340–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Aapro M, Rugo H, Rossi G et al. A randomized phase III study evaluating the efficacy and safety of NEPA, a fixed‐dose combination of netupitant and palonosetron, for prevention of chemotherapy‐induced nausea and vomiting following moderately emetogenic chemotherapy. Ann Oncol 2014;25:1328–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Aapro M, Hesketh PJ, Jordan K et al. Safety of an oral fixed combination of netupitant and palonosetron (NEPA): Pooled data from the phase II/III clinical program. The Oncologist 2016;21:494–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Akynzeo (netupitant and palonosetron) capsules; Akynzeo (fosnetupitant and palonosetron) for injection . U.S. Prescribing information. Dublin, Ireland: Helsinn Birex Pharmaceuticals Ltd; 2018. [Google Scholar]
- 17. Schwartzberg L, Roeland E, Andric Z et al. Phase III safety study of intravenous NEPA: A novel fixed antiemetic combination of fosnetupitant and palonosetron in patients receiving highly emetogenic chemotherapy. Ann Oncol 2018;29:1535–1540. [DOI] [PubMed] [Google Scholar]
- 18. Emend (fosaprepitant) for injection, for intravenous use . US Prescribing Information. Whitehouse Station, NJ: Merck & Co, Inc.; 2017. [Google Scholar]
- 19. Hegerova LT, Leal AD, Grendahl DC et al. An analysis of fosaprepitant‐induced venous toxicity in patients receiving highly emetogenic chemotherapy. Support Care Cancer 2015;23:55–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tsuda T, Kyomori C, Mizukami T et al. Infusion site adverse events in breast cancer patients receiving highly emetic chemotherapy with prophylactic anti‐emetic treatment with aprepitant and fosaprepitant: A retrospective comparison. Mol Clin Oncol 2016;4:603–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Leal AD, Kadakia KC, Looker S et al. Fosaprepitant induced phlebitis: A focus on patients receiving doxorubicin/cyclophosphamide therapy. Support Care Cancer 2014;22:1313–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sato Y, Kondo M, Inagaki A et al. Highly frequent and enhanced injection site reaction induced by peripheral venous injection of fosaprepitant in anthracycline‐treated patients. J Cancer 2014;5:390–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fujii T, Nishimura N, Urayama KY et al. Differential impact of fosaprepitant on infusion site adverse events between cisplatin‐ and anthracycline‐based chemotherapy regimens. Anticancer Res 2015;35:379–383. [PubMed] [Google Scholar]
- 24. Cinvanti (aprepitant) injectable emulsion . US prescribing information. San Diego, CA: HERON Therapeutics; 2018. [Google Scholar]
- 25. Ottoboni T, Lauw M, Keller MR et al. HTX‐019 via 2‐min injection or 30‐min infusion in healthy subjects. Future Oncol 2019;15:865–874. [DOI] [PubMed] [Google Scholar]
- 26. Varubi (rolapitant) injectable emulsion, for intravenous use . US Prescribing Information. Waltham, MA: TESARO, Inc.; 2018. [Google Scholar]
- 27. U.S. Food & Drug Administration . Safety alerts for human medicinal products. Healthcare provider letter. Available at https://www.fda.gov/media/110258/download. Accessed November 2019.
- 28. Lindley CM, Hirsch JD, O'Neill CV et al. Quality of life consequences of chemotherapy‐induced emesis. Qual Life Res 1992;1:331–340. [DOI] [PubMed] [Google Scholar]
- 29. Martin AR, Pearson JD, Cai B et al. Assessing the impact of chemotherapy induced nausea and vomiting on patients'daily lives: A modified version of the Functional Living Index‐Emesis (FLIE) with 5‐day recall. Support Care Cancer 2003;11:522–527. [DOI] [PubMed] [Google Scholar]
- 30. Kraut L, Fauser AA. Anti‐emetics for cancer chemotherapy‐induced emesis: Potential of alternative delivery systems. Drugs 2001;61:1553–1562. [DOI] [PubMed] [Google Scholar]
- 31. Schwartzberg LS, Navari RM. Safety of polysorbate 80 in the oncology setting. Adv Ther 2018;35:754–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Navari R, Schwartzberg L. Evolving role of neurokinin 1‐receptor antagonists for chemotherapy‐induced nausea and vomiting. Onco Targets Ther 2018;11:6459–6478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang X, Zhang ZY, Powers D et al. Bioequivalence of intravenous and oral rolapitant: results from a randomized, open‐label pivotal study. J Clin Pharmacol 2017;57:1600–1606. [DOI] [PubMed] [Google Scholar]
- 34. Ottoboni T, Keller MR, Cravets M et al. Bioequivalence of HTX‐019 (aprepitant IV) and fosaprepitant in healthy subjects: A phase I, open‐label, randomized, two‐way crossover evaluation. Drug Des Devel Ther 2018;12:429–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gralla R, Bosjnak SM, Hontsa A et al. A phase III study evaluating the safety and efficacy of NEPA, a fixed‐dose combination of netupitant and palonosetron, for prevention of chemotherapy‐induced nausea and vomiting over repeated cycles of chemotherapy. Ann Oncol 2014; 25:1333–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Navari RM. The safety of antiemetic medications for the prevention of chemotherapy‐induced nausea and vomiting. Expert Opin Drug Saf 2016;15:343–356. [DOI] [PubMed] [Google Scholar]
- 37. Aapro M, Karthaus M, Schwartzberg L et al. NEPA, a fixed oral combination of netupitant and palonosetron, improves control of chemotherapy‐induced nausea and vomiting (CINV) over multiple cycles of chemotherapy: Results of a randomized, double‐blind, phase 3 trial versus oral palonosetron. Support Care Cancer 2017;25:1127–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. The Centers for Medicare & Medicaid Services . CMS proposes hospital outpatient prospective payment changes for 2017. Available at https://www.cms.gov/Newsroom/MediaReleaseDatabase/Fact-sheets/2016-Fact-sheets-items/2016-07-06.html. Accessed May 11, 2018.
- 39. Warr D, Hesketh PJ, Gralla R et al. Efficacy and tolerability of aprepitant for the prevention of chemotherapy‐induced nausea and vomiting in patients with breast cancer after moderately emetogenic chemotherapy. J Clin Oncol 2005;23:2822–2830. [DOI] [PubMed] [Google Scholar]
- 40. Schnadig ID, Agajanian R, Dakhil C et al. APF530 versus ondansetron, each in a guideline‐recommended three‐drug regimen, for the prevention of chemotherapy‐induced nausea and vomiting due to anthracycline plus cyclophosphamide‐based highly emetogenic chemotherapy regimens: A post hoc subgroup analysis of the phase III randomized MAGIC trial. Cancer Manag Res 2017;19:179–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Schwartzberg LS, Modiano MR, Rapoport BL et al. Safety and efficacy of rolapitant for prevention of chemotherapy‐induced nausea and vomiting after administration of moderately emetogenic chemotherapy or anthracycline and cyclophosphamide regimens in patients with cancer: A randomised, active‐controlled, double‐blind, phase 3 trial. Lancet Oncol 2015;16:1071–1078. [DOI] [PubMed] [Google Scholar]
- 42. Schwartzberg LS, McLaughlin T, Geller RB et al. Real‐world efficacy: Intravenous palonosetron three‐drug regimen for chemotherapy‐induced nausea and vomiting with highly emetogenic chemotherapy. J Comp Eff Res 2018;7:1161–1170. [DOI] [PubMed] [Google Scholar]
- 43. Spinelli T, Moresino C, Baumann S et al. Effects of combined netupitant and palonosetron (NEPA), a cancer supportive care antiemetic, on the ECG of healthy subjects: An ICH E14 thorough QT trial. Springerplus. 2014;3:389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Karthaus M, Aapro M, Rizzi G et al. Cardiac safety of NEPA, a fixed‐dose antiemetic combination, administered prior to anthracycline‐based chemotherapy. Blood 2014;124:4821a. [Google Scholar]


