Abstract
Background
Anti‐programmed cell death 1 antibody is a standard therapy for advanced non‐small cell lung cancer (NSCLC). However, immune‐related adverse events (irAEs), such as skin reactions, are frequently observed. Although skin reactions are associated with clinical efficacy in melanoma, this association in advanced NSCLC and predictors of irAEs remain unclear. Accordingly, this study identified potential correlations of skin reactions with clinical efficacy and clinical predictors of development of skin reactions.
Subjects, Materials, and Methods
We retrospectively surveyed patients with advanced NSCLC who received nivolumab or pembrolizumab monotherapy at Sendai Kousei Hospital (n = 155) during January 2016 to April 2018. Treatment efficacy was evaluated in patients with and without skin reactions, and associated predictive markers were determined. A 6‐week landmark analysis was conducted to assess the clinical benefit of early skin reactions.
Results
Skin reactions were observed in 51 patients with a median time to onset of 6.4 weeks. The overall response rate (ORR) was significantly higher in patients with skin reactions (57% vs. 19%, p < .001). Median progression‐free survival (PFS) durations of 12.9 and 3.5 months and overall survival durations of not reached and 11.4 months were observed in patients with and without skin reactions, respectively. In the 6‐week landmark analysis, the ORR was significantly higher in patients with skin reactions, and skin reactions were significantly associated with increased PFS. A multivariate analysis identified pre‐existing rheumatoid factor (RF) as an independent predictor of skin reactions.
Conclusion
Skin reactions appeared beneficial in patients treated with nivolumab/pembrolizumab for advanced NSCLC and could be predicted by pre‐existing RF. Further large‐scale validations studies are warranted.
Implications for Practice
This single‐institutional medical record review that included 155 patients with advanced non‐small cell lung cancer who were treated with nivolumab or pembrolizumab monotherapy revealed that overall response rate and progression‐free survival were significantly better in patients with skin reactions. Pre‐existing rheumatoid factor was an independent predictor of skin reactions.
Keywords: Programmed cell death 1, Immunotherapy, Immune‐related adverse events, Rheumatoid factor, Lung cancer, Skin reaction
Short abstract
Skin reactions are common immune‐related adverse events associated with PD‐1 therapy. This study investigated the association between the development of skin reactions and clinical benefit of skin reaction, as well as associated predictive markers, in patients with advanced non‐small cell lung cancer who were treated with nivolumab or pembrolizumab monotherapy.
Introduction
Programmed cell death 1 (PD‐1) and programmed cell death ligand 1 (PD‐L1) inhibitors, administered alone or in combination, have demonstrated clear survival benefits relative to standard chemotherapy in both treatment‐naïve and previously treated patients with advanced non‐small cell lung cancer (NSCLC) 1, 2, 3, 4, 5, 6, 7. Accordingly, nivolumab and pembrolizumab have become the new treatments of choice for advanced NSCLC.
However, T‐cell activation may cause immune‐related adverse events (irAEs), including skin reactions, thyroid dysfunction, pneumonitis, and hepatitis 8, that are not triggered by conventional cytotoxic anticancer agents and may require systemic immunosuppression or treatment termination 9. We recently reported that the development of irAEs is also associated with clinical efficacy of nivolumab 10.
Skin reactions are one of the representative irAEs of PD‐1 therapy. In melanoma, some studies have reported that skin reactions are associated with clinical efficacy; however, little is known about this association in NSCLC 11. Additionally, immune‐related pruritus is a frequent adverse event among patients with cancer that is associated with lower quality of life (QOL) 12. Therefore, it is important to identify the predictors of skin reactions.
We investigated the association between the development of skin reactions and clinical benefit, and the predictive markers of skin reaction in patients with advanced NSCLC who were treated with nivolumab or pembrolizumab monotherapy.
Subjects, Materials, and Methods
Patients
The medical records of patients with advanced NSCLC who received nivolumab (3 mg/kg every 2 weeks) or pembrolizumab (200 mg every 3 weeks) monotherapy at Sendai Kousei Hospital between January 2016 and April 2018 were reviewed retrospectively. Treatment was provided until disease progression, unacceptable toxicity, or consent withdrawal. All patients were followed until death, loss of contact, or consent withdrawal.
Assessment
Progression‐free survival (PFS) was defined as the interval from the date of treatment initiation to that of documented disease progression or death from any cause, whereas overall survival (OS) was defined as the interval from the date of treatment initiation to that of death from any cause. Tumor responses to nivolumab or pembrolizumab monotherapy were assessed objectively by two pulmonary physicians (an attending physician and an investigator) via computed tomography scans every 8–9 weeks, according to Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 13. The attending physician and a nurse specialist also performed physical examinations and assessed the irAEs, defined as adverse events with potential immunological etiologies that required potential intervention with immunosuppressive or endocrine therapy, every 2–3 weeks throughout the course of treatment 1, 5, 6, 14, 15. The clinical severity of each irAE was graded according to the Common Terminology Criteria for Adverse Events, version 4.0.
All types of skin problems were considered skin reactions, such as pruritus, rash, erythema, and vitiligo. If the patients developed skin reactions and were judged by their attending physician to require specialized treatment, they also received a physical examination and treatment by the dermatologist.
Blood samples drawn at screening were tested for pre‐existing rheumatoid factor (RF), antinuclear antibody (ANA), antithyroglobulin, and antithyroid peroxidase, using a cutoff of 15 IU/mL for RF and 1:40 for ANA, as previously reported 16, 17. A patient was considered to have pre‐existing antithyroid antibodies if either antithyroglobulin or antithyroid peroxidase was present.
The patients were categorized into two groups comprising those with or without skin reactions. Both groups were evaluated with respect to the objective response rate (ORR), disease control rate (DCR), PFS, and OS. To account for lead‐time bias due to the time‐dependent development of skin reactions, we further performed 6‐week landmark analyses of PFS and OS that included only patients who demonstrated disease control and only those who remained alive at 6 weeks after the initiation of nivolumab or pembrolizumab monotherapy, respectively. Both analyses were based on a landmark assessment of skin reactions that developed within the first 6 weeks. Any skin reaction that occurred after the landmark date was not included in the landmark‐based analyses.
Statistical Analysis
The relationships between the patient variables and responses to nivolumab or pembrolizumab monotherapy were analyzed through univariate and multivariate logistic regression analyses conducted using EZR (Saitama Medical Centre, Jichi Medical University, Saitama, Japan), a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria) 18. Using the same interface, categorical variables were compared via the chi‐square, Student's t, Mann‐Whitney U, or Welch's t test, as appropriate. PFS and OS up to October 19, 2018, were estimated using Kaplan‐Meier curves and compared using a two‐sided log‐rank test. Hazard ratios (HRs) were estimated using the Cox proportional hazards model. All reported p values are two sided, and values <.05 were considered statistically significant.
The present study was approved by the institutional review board of Sendai Kousei Hospital. The requirement to obtain informed consent was waived because the data were anonymized.
Results
Patient Characteristics
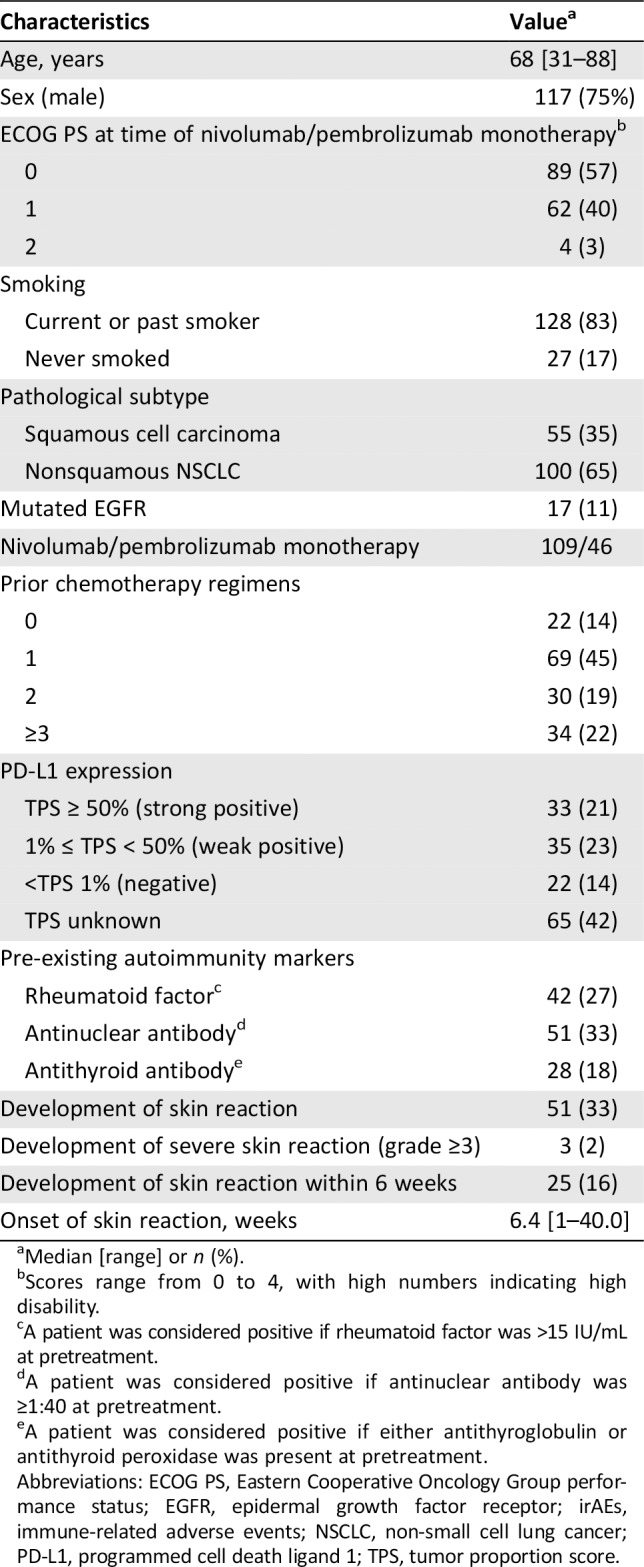
Patients with advanced NSCLC (n = 155; 117 men [75%], 38 women [25%]) who received nivolumab (n = 109) or pembrolizumab (n = 46) monotherapy during the study period were included in our analysis (Table 1). The median patient age was 68 years (range: 31–88 years), and 151 (97%) patients had an Eastern Cooperative Oncology Group Performance Status of 0 or 1. Fifty‐five (35%) and 100 patients (65%) had been diagnosed with squamous cell carcinoma and nonsquamous NSCLC, respectively. Seventeen patients (11%) harbored mutations in the epidermal growth factor receptor (EGFR). Twenty‐two patients (14%) were chemotherapy‐naïve, whereas 69 (45%), 30 (19%), and 34 (22%) had received 1, 2, or ≥3 chemotherapy courses, respectively. PD‐L1 was expressed abundantly (tumor proportion score [TPS] ≥50%) in 33 patients (21%), at low levels (1% to <50%) in 35 (23%), and not at all (<1%) in 22 (14%). The PD‐L1 expression status of the remaining 65 (42%) patients was unknown. Fifty‐one patients (33%) developed skin reactions. Twenty‐five patients (16%) developed skin reactions within 6 weeks. The times to onset of skin reactions varied, with a mean time of 6.4 weeks (range: 1 day to 40 weeks). Grade 1, 2, and 3 skin reactions occurred in 33, 15, and 3 patients, respectively (Table 2).
Table 1.
Patient characteristics at baseline (n = 155)
| Characteristics | Valuea |
|---|---|
| Age, years | 68 [31–88] |
| Sex (male) | 117 (75%) |
| ECOG PS at time of nivolumab/pembrolizumab monotherapyb | |
| 0 | 89 (57) |
| 1 | 62 (40) |
| 2 | 4 (3) |
| Smoking | |
| Current or past smoker | 128 (83) |
| Never smoked | 27 (17) |
| Pathological subtype | |
| Squamous cell carcinoma | 55 (35) |
| Nonsquamous NSCLC | 100 (65) |
| Mutated EGFR | 17 (11) |
| Nivolumab/pembrolizumab monotherapy | 109/46 |
| Prior chemotherapy regimens | |
| 0 | 22 (14) |
| 1 | 69 (45) |
| 2 | 30 (19) |
| ≥3 | 34 (22) |
| PD‐L1 expression | |
| TPS ≥ 50% (strong positive) | 33 (21) |
| 1% ≤ TPS < 50% (weak positive) | 35 (23) |
| <TPS 1% (negative) | 22 (14) |
| TPS unknown | 65 (42) |
| Pre‐existing autoimmunity markers | |
| Rheumatoid factorc | 42 (27) |
| Antinuclear antibodyd | 51 (33) |
| Antithyroid antibodye | 28 (18) |
| Development of skin reaction | 51 (33) |
| Development of severe skin reaction (grade ≥3) | 3 (2) |
| Development of skin reaction within 6 weeks | 25 (16) |
| Onset of skin reaction, weeks | 6.4 [1–40.0] |
Median [range] or n (%).
Scores range from 0 to 4, with high numbers indicating high disability.
A patient was considered positive if rheumatoid factor was >15 IU/mL at pretreatment.
A patient was considered positive if antinuclear antibody was ≥1:40 at pretreatment.
A patient was considered positive if either antithyroglobulin or antithyroid peroxidase was present at pretreatment.
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status; EGFR, epidermal growth factor receptor; irAEs, immune‐related adverse events; NSCLC, non‐small cell lung cancer; PD‐L1, programmed cell death ligand 1; TPS, tumor proportion score.
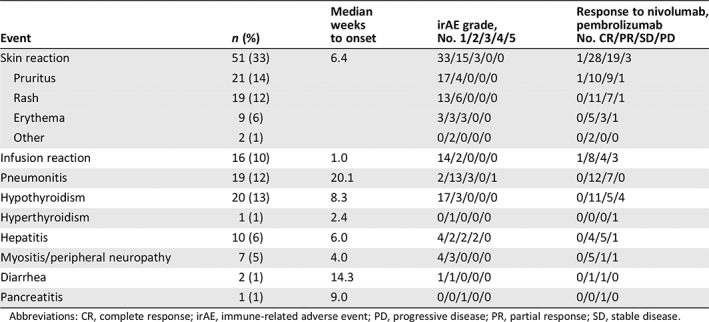
Table 2.
Observed immune‐related adverse events
| Event | n (%) | Median weeks to onset | irAE grade, No. 1/2/3/4/5 | Response to nivolumab, pembrolizumab No. CR/PR/SD/PD |
|---|---|---|---|---|
| Skin reaction | 51 (33) | 6.4 | 33/15/3/0/0 | 1/28/19/3 |
| Pruritus | 21 (14) | 17/4/0/0/0 | 1/10/9/1 | |
| Rash | 19 (12) | 13/6/0/0/0 | 0/11/7/1 | |
| Erythema | 9 (6) | 3/3/3/0/0 | 0/5/3/1 | |
| Other | 2 (1) | 0/2/0/0/0 | 0/2/0/0 | |
| Infusion reaction | 16 (10) | 1.0 | 14/2/0/0/0 | 1/8/4/3 |
| Pneumonitis | 19 (12) | 20.1 | 2/13/3/0/1 | 0/12/7/0 |
| Hypothyroidism | 20 (13) | 8.3 | 17/3/0/0/0 | 0/11/5/4 |
| Hyperthyroidism | 1 (1) | 2.4 | 0/1/0/0/0 | 0/0/0/1 |
| Hepatitis | 10 (6) | 6.0 | 4/2/2/2/0 | 0/4/5/1 |
| Myositis/peripheral neuropathy | 7 (5) | 4.0 | 4/3/0/0/0 | 0/5/1/1 |
| Diarrhea | 2 (1) | 14.3 | 1/1/0/0/0 | 0/1/1/0 |
| Pancreatitis | 1 (1) | 9.0 | 0/0/1/0/0 | 0/0/1/0 |
Abbreviations: CR, complete response; irAE, immune‐related adverse event; PD, progressive disease; PR, partial response; SD, stable disease.
According to RECIST, version 1.1, complete responses were observed in 2 patients (1%), partial responses in 47 (30%), stable disease in 56 (36%), and progressive disease in 50 (32%). Consequently, the ORR was 31% (95% confidence interval [CI]: 24–40) and the DCR was 67% (95% CI: 60–75).
Analysis of Skin Reactions
Table 2 summarizes the development of irAEs. Ninety patients experienced irAEs: 51 (33%) presented with skin reactions, whereas 16 (10%), 19 (12%), 20 (13%), 1 (1%), 10 (6%), 7 (5%), 2 (1%), and 1 (1%) developed infusion reaction, pneumonitis, hypothyroidism, hyperthyroidism, hepatitis, myositis/peripheral neuropathy, diarrhea, and pancreatitis, respectively. Four patients were treated with systemic steroids. No patients with skin reactions withdrew or died after receiving nivolumab or pembrolizumab monotherapy.
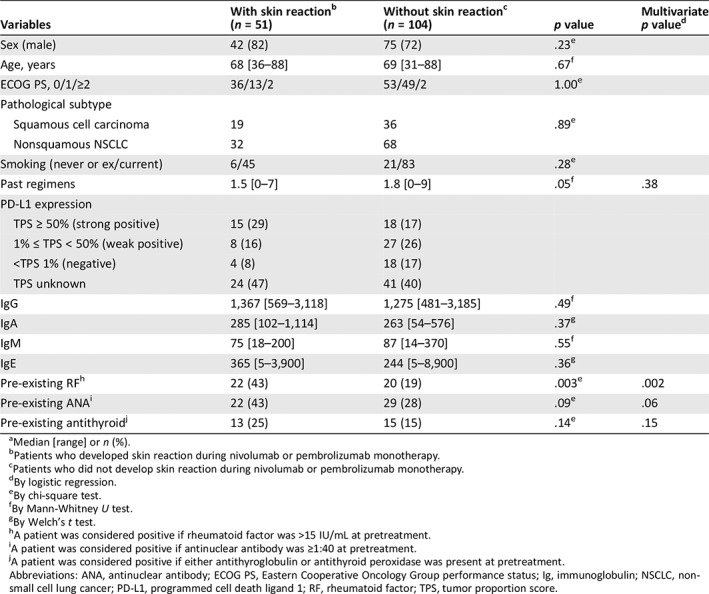
Table 3 compares the patients who did and did not develop skin reactions. No significant intergroup differences were observed regarding sex, age, Eastern Cooperative Oncology Group Performance Status, pathological subtype, smoking history, or number of previous chemotherapy regimens. However, pre‐existing RF was identified significantly more frequently in patients who developed skin reactions.
Table 3.
Characteristics of patients with or without skin reaction during nivolumab or pembrolizumab monotherapy (n = 155)a
| With skin reactionb | Without skin reactionc | Multivariate | ||
|---|---|---|---|---|
| Variables | (n = 51) | (n = 104) | p value | p valued |
| Sex (male) | 42 (82) | 75 (72) | .23e | |
| Age, years | 68 [36–88] | 69 [31–88] | .67f | |
| ECOG PS, 0/1/≥2 | 36/13/2 | 53/49/2 | 1.00e | |
| Pathological subtype | ||||
| Squamous cell carcinoma | 19 | 36 | .89e | |
| Nonsquamous NSCLC | 32 | 68 | ||
| Smoking (never or ex/current) | 6/45 | 21/83 | .28e | |
| Past regimens | 1.5 [0–7] | 1.8 [0–9] | .05f | .38 |
| PD‐L1 expression | ||||
| TPS ≥ 50% (strong positive) | 15 (29) | 18 (17) | ||
| 1% ≤ TPS < 50% (weak positive) | 8 (16) | 27 (26) | ||
| <TPS 1% (negative) | 4 (8) | 18 (17) | ||
| TPS unknown | 24 (47) | 41 (40) | ||
| IgG | 1,367 [569–3,118] | 1,275 [481–3,185] | .49f | |
| IgA | 285 [102–1,114] | 263 [54–576] | .37g | |
| IgM | 75 [18–200] | 87 [14–370] | .55f | |
| IgE | 365 [5–3,900] | 244 [5–8,900] | .36g | |
| Pre‐existing RFh | 22 (43) | 20 (19) | .003e | .002 |
| Pre‐existing ANAi | 22 (43) | 29 (28) | .09e | .06 |
| Pre‐existing antithyroidj | 13 (25) | 15 (15) | .14e | .15 |
Median [range] or n (%).
Patients who developed skin reaction during nivolumab or pembrolizumab monotherapy.
Patients who did not develop skin reaction during nivolumab or pembrolizumab monotherapy.
By logistic regression.
By chi‐square test.
By Mann‐Whitney U test.
By Welch's t test.
A patient was considered positive if rheumatoid factor was >15 IU/mL at pretreatment.
A patient was considered positive if antinuclear antibody was ≥1:40 at pretreatment.
A patient was considered positive if either antithyroglobulin or antithyroid peroxidase was present at pretreatment.
Abbreviations: ANA, antinuclear antibody; ECOG PS, Eastern Cooperative Oncology Group performance status; Ig, immunoglobulin; NSCLC, non‐small cell lung cancer; PD‐L1, programmed cell death ligand 1; RF, rheumatoid factor; TPS, tumor proportion score.
A univariate analysis identified the variables associated with skin reactions, and a multivariate analysis revealed that pre‐existing RF was an independent predictor of these reactions, with an odds ratio of 3.41 (95% CI: 1.58–7.36; p = .002).
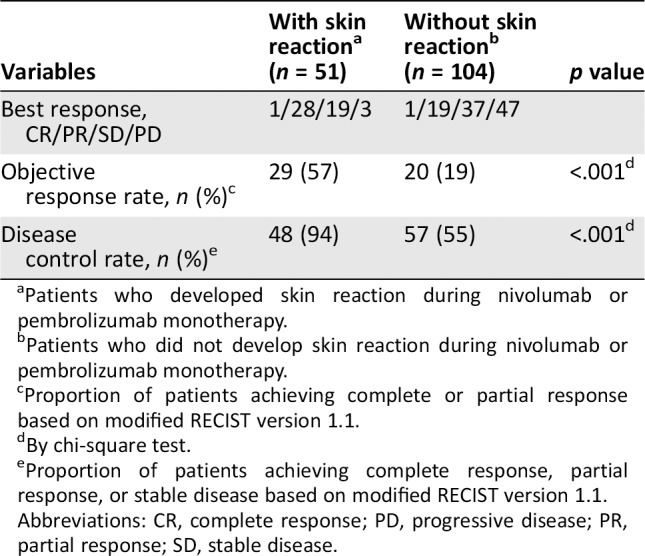
In the skin reaction group, 1 patient (2%) achieved a complete response (CR), 28 (55%) exhibited a partial response (PR), 19 (37%) developed stable disease (SD), and 3 (6%) presented with progressive disease (PD). Among the 104 patients without skin reactions, the corresponding frequencies were 1 (1%), 19 (18%), 37 (36%), and 47 (45%), respectively. The ORR and DCR were significantly higher in patients who developed skin reactions (57% vs. 19%, p < .001 and 94% vs. 55%, p < .001, respectively; Table 4).
Table 4.
Association between the presence of skin reaction and treatment response
| Variables | With skin reactiona(n = 51) | Without skin reactionb (n = 104) | p value |
|---|---|---|---|
| Best response, CR/PR/SD/PD | 1/28/19/3 | 1/19/37/47 | |
| Objective response rate, n (%)c | 29 (57) | 20 (19) | <.001d |
| Disease control rate, n (%)e | 48 (94) | 57 (55) | <.001d |
Patients who developed skin reaction during nivolumab or pembrolizumab monotherapy.
Patients who did not develop skin reaction during nivolumab or pembrolizumab monotherapy.
Proportion of patients achieving complete or partial response based on modified RECIST version 1.1.
By chi‐square test.
Proportion of patients achieving complete response, partial response, or stable disease based on modified RECIST version 1.1.
Abbreviations: CR, complete response; PD, progressive disease; PR, partial response; SD, stable disease.
The median PFS durations were 12.9 (95% CI: 8.3 to not reached [NR]) and 3.5 months (95% CI: 2.5–4.1) for patients who did and did not develop skin reactions, respectively, indicating a significantly longer PFS in those with skin reactions (Fig. 1A). Similarly, the corresponding 1‐year PFS rates were 51% (95% CI: 36–64) or 20% (95% CI: 13–28), respectively. The HR for disease progression or death was 0.38 (95% CI: 0.25–0.58, p < .001). PFS was statistically significantly better in the skin reaction group than in the non–skin reaction group. The median OS durations among patients with and without skin reactions were NR (95% CI: 17.5–NR) and 11.4 months (95% CI: 8.8–15.6), respectively, indicating significantly better survival in the former group (Fig. 1B). Similarly, the corresponding 1‐year OS rates were 76% (95% CI: 60–86) and 49% (95% CI: 38–58), respectively. The HR for death was 0.34 (95% CI: 0.20–0.60, p < .001). OS was statistically significantly better in the skin reaction group than in the non–skin reaction group.
Figure 1.
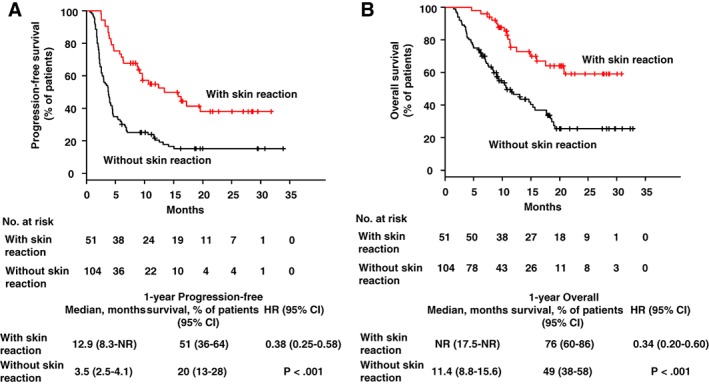
Progression‐free survival and overall survival in patients with or without skin reactions. Kaplan‐Meier curves are shown for progression‐free survival (A) and overall survival (B) in patients with or without skin reaction. The red line indicates patients with skin reaction; the black line represents those without skin reaction. Ticks indicate patients for whom data were censored on October 19, 2018.
Abbreviations: CI, confidence interval; HR, hazard ratio; NR, not reached.
In a 6‐week landmark analysis, we also evaluated PFS and OS only in patients who developed skin reactions within 6 weeks after the start of treatment. Ten patients were excluded from the 6‐week landmark analysis of PFS because of disease progression or death before day 42 of nivolumab treatment, and one patient was excluded from OS analysis because of death.
In this 6‐week group, 1 patient (4%) achieved a CR, 17 (68%) exhibited a PR, 7 (28%) developed SD, and none (0%) presented with PD. The ORR and DCR were significantly higher in patients who developed skin reactions within 6 weeks than in those who did not (72% vs. 24%, p < .001 and 100% vs. 62%, p < .001, respectively; Table 5).
Table 5.
Association between the presence of skin reaction within 6 weeks and treatment response
| With skin reaction within 6 weeksa | Without skin reaction within 6 weeksb | ||
|---|---|---|---|
| Variables | (n = 25) | (n = 129) | p value |
| Best response, CR/PR/SD/PD | 1/17/7/0 | 1/30/49/49 | |
| Objective response rate, n (%)c | 18 (72) | 31 (24) | <.001d |
| Disease control rate, n (%)e | 25 (100) | 80 (62) | <.001d |
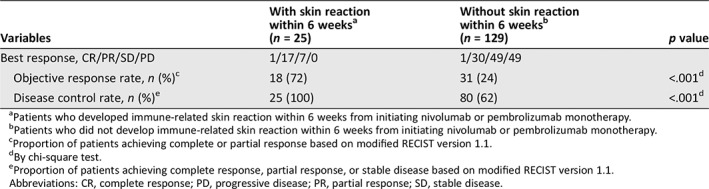
Patients who developed immune‐related skin reaction within 6 weeks from initiating nivolumab or pembrolizumab monotherapy.
Patients who did not develop immune‐related skin reaction within 6 weeks from initiating nivolumab or pembrolizumab monotherapy.
Proportion of patients achieving complete or partial response based on modified RECIST version 1.1.
By chi‐square test.
Proportion of patients achieving complete response, partial response, or stable disease based on modified RECIST version 1.1.
Abbreviations: CR, complete response; PD, progressive disease; PR, partial response; SD, stable disease.
The median PFS durations were 10.3 (95% CI: 5.6–NR) and 4.2 months (95% CI: 3.6–5.9) among patients who did and did not develop skin reactions within 6 weeks, respectively, indicating significantly better survival in the former group (Fig. 2). Similarly, the corresponding 1‐year PFS rates were 44% (95% CI: 24–63) and 30% (95% CI: 22–38), respectively. The HR for disease progression or death within 6 weeks was 0.58 (95% CI: 0.33–0.99, p = .05). PFS was statistically significantly better in the skin reaction group than in the non–skin reaction group. The median OS durations were 20.7 (95% CI: 10.7–NR) and 15.6 months (95% CI 11.4–19.1) among patients who did or did not develop skin reactions within 6 weeks, respectively, indicating better survival in the former group. The HR for death was 0.75 (95% CI: 0.40–1.43, p = .38).
Figure 2.
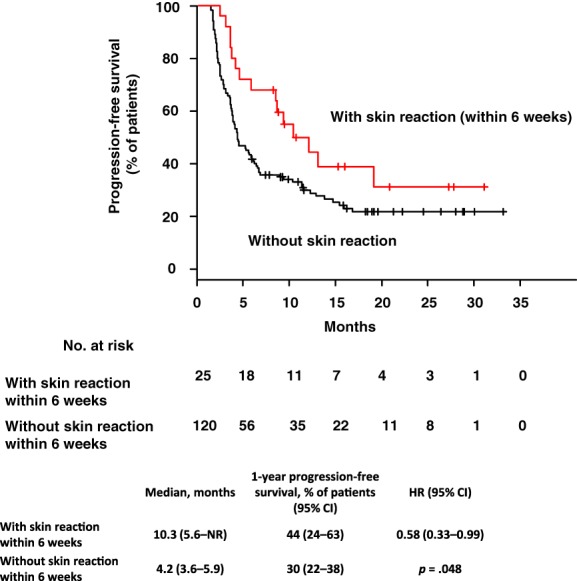
Progression‐free survival in patients with or without skin reactions in 6 weeks. Kaplan‐Meier curves with 6‐week landmark analysis for progression‐free survival in patients with or without skin reactions. The red line indicates patients with skin reaction; the black line represents those without skin reaction. Ticks indicate patients for whom data were censored on October 19, 2018.
Abbreviations: CI, confidence interval; HR, hazard ratio; NR, not reached.
The ORR and DCR were not statistically significantly different between the patients with and without EGFR mutation positive (12% vs. 34%, p = .01 and 47% vs. 70%, p = .01, respectively; supplemental online Table 1). The median PFS durations were 2.3 (95% CI: 1.6–4.1) and 5.1 months (95% CI: 3.8–6.7) among patients with and without EGFR mutation positive, respectively, indicating significantly better survival in the latter group (supplemental online Fig. 1). Similarly, the corresponding 1‐year PFS rates were 18% (95% CI: 4–38) or 32% (95% CI: 24–40), respectively. The HR for disease progression or death was 1.76 (95% CI: 1.00–3.08, p = .05). PFS was statistically significantly better in the patients without EGFR mutation positive than in the patients with EGFR mutation positive.
Among patients with EGFR positive (n = 17), 4 developed skin reactions. The chi‐square test was performed on the correlation between EGFR mutations and skin reactions; there was no statistically significant correlation between the two (supplemental online Table 2).
In addition, among the 17 patients who tested EGFR positive, the Fisher's exact test was performed on the correlation between skin reactions and ORR (supplemental online Table 3). Among EGFR‐positive patients, the ORRs were significantly higher in patients with skin reactions than those without.
We further analyzed the correlation between the presence of skin reactions and treatment response in patients with known TPS and with TPS ≥50%. In patients with known TPS, the ORR and DCR were significantly higher in patients who developed skin reactions (50% vs. 22%, p = .01 and 100% vs. 65%, p = .001, respectively; supplemental online Table 4). Among patients with TPS ≥50%, no statistically significant differences in ORR and DCR between the group with skin reaction and those without (73% vs. 44%, p = .16 and 100% vs. 83%, p = .23, respectively; supplemental online Table 5).
Discussion
The aim of this study was to investigate the association between skin reactions and clinical benefit in patients with advanced NSCLC who received nivolumab or pembrolizumab. Patients who developed skin reactions had better ORR, PFS, and OS than patients who did not. Patients who developed early skin reactions, within 6 weeks, also had a better ORR and PFS than those who did not. The development of skin reactions is considered a useful marker of clinical benefit. We believe that cautious management of skin reactions will permit maximum clinical benefits from PD‐1 inhibitor therapies, regardless of early‐ and late‐onset skin reactions. In addition, RF was an independent predictor of skin reactions. To the best of our knowledge, this is the first report of RF as an independent predictor of skin reactions to PD‐1 inhibitors.
Skin reactions occur in 14%–47% of patients treated with immune checkpoint inhibitors, and these range in severity from mild and localized to debilitating and widespread in 1%–3% of patients 19. In patients with NSCLC who were treated with nivolumab, skin eruptions and pruritus have been reported in approximately 4%–10% of patients or more, and grade 3 or greater skin reactions have been reported in 0.7% of patients 5, 6, 11. Pembrolizumab has been reported to cause skin reactions in approximately 9%–27% of patients, and skin reactions of grade 3 or greater in 1%−4% of patients 1, 14.
In our study, 33% of the total patient population presented skin reactions, and grade 3 or higher reactions occurred in 1.2% of the study population. The development frequency of skin reactions was higher in our study than in past reports; however, the frequency of grade 3 or higher skin reactions that occurred in our study was similar to those of past reports. We suspect that the similarities and differences can be attributed to the irAE management team (Frontline Immunotherapy Team) that we established at our hospital to provide physical examinations carefully and report even mild skin reactions as early as possible.
Wang et al. 20 suggested that a wide range of timelines is associated with skin reactions after PD‐1 inhibitor therapy. In their study, the skin reactions were associated with a median (range) occurrence of 4.2 months (0.5–38 months) in 17 patients who presented with skin reactions that were associated with nivolumab or pembrolizumab. They also found that immune‐related skin reactions may occur after treatment discontinuation. In our study, the mean time of onset of skin reactions was 6.4 weeks. This is earlier than the duration reported in previous studies, which we suspect is due to our astute identification of mild skin reactions. No patients presented skin reactions after treatment discontinuation.
Previous data suggest that the skin irAEs that occur during anti‐PD1 therapy are associated with clinical efficacy and may predict a better therapy response 21, 22. In patients with malignant melanoma, a few prior reports have suggested an association between the appearance of skin reactions and OS 15, 21, 22, 23. Hasan et al. 11 suggested a similar association in patients with NSCLC. However, their study was small sample. In our study, patients who developed skin reactions had better ORR, PFS, and OS than those who did not, and our data support the findings of these previous reports.
In addition, Teraoka et al. 24 found that the expression of early irAEs correlate with the therapeutic effects of immune checkpoint inhibitors. We report that the patients who developed skin reactions within 6 weeks had better ORR and PFS than patients who did not in this landmark analysis study. Therefore, early skin reactions appear to be associated with clinical efficacy in patients treated with anti‐PD‐1 antibody therapy.
We investigated best response and development of skin reactions in patients with and without EGFR mutations. According to previous studies, EGFR mutant lung cancers rarely derive benefit from treatment with anti‐PD‐1 antibody therapy 5, 25, 26, 27. The patients with EGFR mutation positive had worse PFS than those without in this study. This result is consistent with those observed in previous studies. There was no difference in the incidence of skin reactions with and without EGFR mutations; however, the ORRs were significantly higher in patients with skin reactions than those without among EGFR‐positive patients. Even if EGFR mutation positivity is noted in patients, the expression of a skin reaction is considered to indicate clinical efficacy. According to previous reports, immune‐related skin reactions are associated with lower patient QOL 12. In our study, the patients who developed skin reactions had good treatment outcomes, regardless of whether the presentation was early or late onset. Therefore, we believe that it is important to manage the skin reaction symptoms. There is an urgent clinical need to identify those patients more likely to develop skin reactions, as this information would help to personalize patient management and provide early or prophylactic interventions that may mitigate such events. In this study, we found that pre‐existing RF was an independent predictor of skin reactions.
Previous studies have suggested a few predictive biomarkers of irAEs in patients treated with immune‐checkpoint inhibitor. We previously reported that any pre‐existing antibodies are independent predictors of irAEs in patients with advanced NSCLC 28. Osorio et al. 29 found that thyroid dysfunction during pembrolizumab treatment of NSCLC is associated with antithyroid antibodies. Additionally, Suzuki et al. 30 reported 12 patients with myasthenia gravis (0.12%) among 9,869 patients with cancer who had been treated with nivolumab, of whom 10 had pre‐existing antibodies to the acetylcholine receptor. To our best knowledge, we are the first to report that pre‐existing RF is an independent predictor of the development of skin reactions. The mechanism by which pre‐existing RF is associated with the development of a skin reaction remains unclear. PD‐1 is expressed abundantly in activated B cells 31, which are modulated via T‐cell‐independent and ‐dependent mechanisms 32, 33, 34. Earlier analyses of PD‐1 in preclinical models have suggested the antibody‐dependent mitigation of immune‐related toxicity 35, 36. In addition, activated NK cells express PD‐1, while PD‐1 engagement by PD‐L1+ tumor cells potently suppresses NK cell–mediated tumor immunity 37. NK cells, in addition to T cells, mediate the effect of an immune‐checkpoint inhibitor and may, in turn, induce auto‐antibodies in B cells, thereby triggering irAEs. Accordingly, the levels of RF may correlate with irAEs and treatment responses.
This study had several limitations. First, this was a retrospective, nonrandomized, small, single‐center cohort study. Second, PD‐L1 expression was not assayed routinely because diagnostic kits were not commercially available in Japan at the time of this study. Therefore, we were unable to fully consider PD‐L1 in this study. To solve this limitation, we further examined the association between the presence of skin reaction and treatment responses in patients with known TPS and with TPS ≥50%. Among patients with known TPS, the ORR and DCR were significantly higher in patients who developed skin reactions. In patients with TPS ≥50%, although it was not statistically significant, both the ORR and DCR tended to be better with skin reaction group than those without. Regardless of TPS status, the development of skin reaction might be associated with clinical efficacy.
Recently, the combination of chemotherapy and immunotherapy has become mainstream, and therefore, the opportunity to treat patients with anti‐PD‐1 monotherapy has decreased. However, the anti‐PD‐1 monotherapy findings of this study may be useful for predicting clinical efficacy in combination therapies in the near future.
Conclusion
In patients with advanced NSCLC who were treated with nivolumab or pembrolizumab monotherapy, ORR, PFS, and OS were significantly better in the skin reaction group than in the non–skin reaction group. Pre‐existing RF was identified as an independent predictor of skin reactions. In addition to identifying the association between RF and skin reactions, identifying predictors of irAEs can help clinicians determine the risk–benefit ratios for patients and maximize clinical benefits, while minimizing adverse events. Further studies with large patient cohorts are needed to validate these findings.
Author Contributions
Conception/design: Mari Aso, Yukihiro Toi, Shunichi Sugawara
Provision of study material or patients: Mari Aso, Yukihiro Toi, Jun Sugisaka, Tomoiki Aiba, Sachiko Kawana, Ryohei Saito, Takahiro Ogasawara, Kyoji Tsurumi, Kana Ono, Hisashi Shimizu, Yutaka Domeki, Keisuke Terayama, Yosuke Kawashima, Atsushi Nakamura, Shinsuke Yamanda, Yuichiro Kimura, Yoshihiro Honda, Shunichi Sugawara
Collection and/or assembly of data: Mari Aso, Yukihiro Toi, Jun Sugisaka, Tomoiki Aiba, Sachiko Kawana, Ryohei Saito, Takahiro Ogasawara, Kyoji Tsurumi, Kana Ono, Hisashi Shimizu, Yutaka Domeki, Keisuke Terayama, Yosuke Kawashima, Atsushi Nakamura, Shinsuke Yamanda, Yuichiro Kimura, Yoshihiro Honda, Shunichi Sugawara
Data analysis and interpretation: Mari Aso, Yukihiro Toi, Shunichi Sugawara
Manuscript writing: Mari Aso, Yukihiro Toi, Shunichi Sugawara
Final approval of manuscript: Mari Aso, Yukihiro Toi, Jun Sugisaka, Tomoiki Aiba, Sachiko Kawana, Ryohei Saito, Takahiro Ogasawara, Kyoji Tsurumi, Kana Ono, Hisashi Shimizu, Yutaka Domeki, Keisuke Terayama, Yosuke Kawashima, Atsushi Nakamura, Shinsuke Yamanda, Yuichiro Kimura, Yoshihiro Honda, Shunichi Sugawara
Disclosures
Yukihiro Toi: Ono Pharmaceutical, Bristol‐Myers Squibb, Merck Sharp & Dohme (H); Ryohei Saito: Bristol‐Myers Squibb (H); Yutaka Domeki: Ono Pharmaceutical, Bristol‐Myers Squibb (H); Atsushi Nakamura: Merck Sharp & Dohme (H); Shunichi Sugawara: Chugai Pharma, AstraZeneca, Nippon Boehringer Ingelheim, Merck Sharp & Dohme, Taiho Pharmaceutical, Pfizer, Eli Lilly and Company, Novartis, Kyowa Hakko Kirin, Bristol‐Myers Squibb, Ono Pharmaceutical (H). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Supporting information
See http://www.TheOncologist.com for supplemental material available online.
Supplemental Figures
Supplemental Tables
Acknowledgments
We thank Associate Professor Masataka Taguri of Yokohama City University School of Data Science, Chieko Hattori, chief nurse at Sendai Kousei Hospital, and the Frontline Immunotherapy Team at Sendai Kousei Hospital.
Disclosures of potential conflicts of interest may be found at the end of this article.
References
- 1. Reck M, Rodríguez‐Abreu D, Robinson AG et al. Pembrolizumab versus chemotherapy for PD‐L1‐positive non‐small‐cell lung cancer. N Engl J Med 2016;375:1823–1833. [DOI] [PubMed] [Google Scholar]
- 2. Brahmer J, Rodríguez‐Abreu D, Robinson A et al. OA 17.06 updated analysis of KEYNOTE‐024: Pembrolizumab vs platinum‐based chemotherapy for advanced NSCLC with PD‐L1 TPS ≥50%. J Thorac Oncol 2017;12(suppl 2):S1793–S1794. [Google Scholar]
- 3. Gandhi L, Rodríguez‐Abreu D, Gadgeel S et al. KEYNOTE‐189 Investigators. Pembrolizumab plus chemotherapy in metastatic non–small‐cell lung cancer. N Engl J Med 2018;378:2078–2092. [DOI] [PubMed] [Google Scholar]
- 4. Hellmann MD, Ciuleanu TE, Pluzanski A et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med 2018;378:2093–2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Borghaei H, Paz‐Ares L, Horn L et al. Nivolumab versus docetaxel in advanced nonsquamous non–small‐cell lung cancer. N Engl J Med 2015;373:1627–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brahmer J, Reckamp KL, Baas P et al. Nivolumab versus docetaxel in advanced squamous‐cell non–small‐cell lung cancer. N Engl J Med 2015;373:123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rittmeyer A, Barlesi F, Waterkamp D et al. Atezolizumab versus docetaxel in patients with previously treated non–small‐cell lung cancer (OAK): A phase 3, open‐label, multicenter randomised controlled trial. Lancet 2017;389:255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Weber JS, Kähler KC, Hauschild A. Management of immune‐related adverse events and kinetics of response with ipilimumab. J Clin Oncol 2012;30:2691–2697. [DOI] [PubMed] [Google Scholar]
- 9. Linardou H, Gogas H. Toxicity management of immunotherapy for patients with metastatic melanoma. Ann Transl Med 2016;4:272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Toi Y, Sugawara S, Kawashima Y et al. Association of immune‐related adverse events with clinical benefit in patients with advanced non‐small‐cell lung cancer treated with nivolumab. The Oncologist 2018;23:1358–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hasan Ali O, Diem S, Markert E et al. Characterization of nivolumab‐associated skin reactions in patients with metastatic non‐small cell lung cancer. Oncoimmunology 2016;5:e1231292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Phillips GS, Freites‐Martinez A, Wu J et al. Clinical characterization of immunotherapy‐related pruritus among patients seen in 2 oncodermatology clinics. JAMA Dermatol 2018;155:249–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Eisenhauer EA, Therasse P, Bogaerts J et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–247. [DOI] [PubMed] [Google Scholar]
- 14. Herbst RS, Baas P, Kim DW et al. Pembrolizumab versus docetaxel for preciously treated, PD‐L1‐positive, advanced non‐small‐cell lung cancer (KEYNOTE‐010). Lancet 2016;387:1540–1550. [DOI] [PubMed] [Google Scholar]
- 15. Freeman‐Keller M, Kim Y, Cronin H et al. Nivolumab in resected and unresectable metastatic melanoma: Characteristics of immune‐related adverse events and association with outcomes. Clin Cancer Res 2016;22:886–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Aletaha D, Neogi T, Silman AJ et al. 2010 Rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 2010;62:2569–2581. [DOI] [PubMed] [Google Scholar]
- 17. Segni M, Pucarelli I, Truglia S et al. High prevalence of antinuclear antibodies in children with thyroid autoimmunity. J Immunol Res 2014;2014:150239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kanda Y. Investigation of the freely available easy‐to‐use software 'EZR' for medical statistics. Bone Marrow Transplant 2013;48:452–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sibaud V. Dermatologic reactions to immune checkpoint inhibitors: Skin toxicities and immunotherapy. Am J Clin Dermatol 2018;19:345–361. [DOI] [PubMed] [Google Scholar]
- 20. Wang LL, Patel G, Chiesa‐Fuxench ZC et al. Timing of onset of adverse cutaneous reactions associated with programmed cell death protein 1 inhibitor therapy. JAMA Dermatol 2018;154:1057–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lo JA, Fisher DE, Flaherty KT. Prognostic significance of cutaneous adverse events associated with pembrolizumab therapy. JAMA Oncol 2015;1:1340–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sanlorenzo M, Vujic I, Daud A et al. Pembrolizumab cutaneous adverse events and their association with disease progression. JAMA Dermatol 2015;151:1206–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Teulings HE, Limpens J, Jansen SN et al. Vitiligo‐like depigmentation in patients with stage III‐IV melanoma receiving immunotherapy and its association with survival: A systematic review and meta‐analysis. J Clin Oncol 2015;33:773–781. [DOI] [PubMed] [Google Scholar]
- 24. Teraoka S, Fujimoto D, Morimoto T et al. Early Immune‐related adverse events and association with outcome in advanced non‐small cell lung cancer patients treated with nivolumab: A prospective cohort study. J Thorac Oncol 2017;12:1798–1805. [DOI] [PubMed] [Google Scholar]
- 25. Garon EB, Rizvi NA, Hui R et al. Pembrolizumab for the treatment of nonsmall‐cell lung cancer. N Engl J Med 2015;372:2018–2028. [DOI] [PubMed] [Google Scholar]
- 26. Lee CK Man J, Lord S et al. Clinical and molecular characteristics associated with survival among patients treated with checkpoint inhibitors for advanced non‐small cell lung carcinoma: A systematic review and meta‐analysis. JAMA Oncol 2018;4:210–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lisberg A, Cummings A, Goldman JW et al. A phase II study of pembrolizumab in EGFR‐mutant, PD‐L1+, tyrosine kinase inhibitor (TKI) naïve patients with advanced NSCLC. J Thorac Oncol 2018;13:1138–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Toi Y, Sugawara S, Sugisaka J et al. Profiling preexisting antibodies in patients treated with anti‐PD‐1 therapy for advanced non‐small cell lung cancer. JAMA Oncol 2019;5:376–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Osorio JC, Ni A, Chaft JE et al. Antibody‐mediated thyroid dysfunction during T‐cell checkpoint blockade in patients with non‐small‐cell lung cancer. Ann Oncol 2017;28:583–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Suzuki S, Ishikawa N, Konoeda F et al. Nivolumab‐related myasthenia gravis with myositis and myocarditis in Japan. Neurology 2017;89:1127–1134. [DOI] [PubMed] [Google Scholar]
- 31. Velu V, Titanji K, Zhu B et al. Enhancing SIV‐specific immunity in vivo by PD‐1 blockade. Nature 2009;458:206–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Thibult ML, Mamessier E, Gertner‐Dardenne J et al. PD‐1 is a novel regulator of human B‐cell activation. Int Immunol 2013;25:129–137. [DOI] [PubMed] [Google Scholar]
- 33. Kawamoto S, Tran TH, Maruya M et al. The inhibitory receptor PD‐1 regulates IgA selection and bacterial composition in the gut. Science 2012;336:485–489. [DOI] [PubMed] [Google Scholar]
- 34. Sage PT, Francisco LM, Carman CV et al. The receptor PD‐1 controls follicular regulatory T cells in the lymph nodes and blood. Nat Immunol 2013;14:152–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nishimura H, Nose M, Hiai H et al. Development of lupus‐like autoimmune diseases by disruption of the PD‐1 gene encoding an ITIM motif‐carrying immunoreceptor. Immunity 1999;11:141–151. [DOI] [PubMed] [Google Scholar]
- 36. Okazaki T, Tanaka Y, Nishio R et al. Autoantibodies against cardiac troponin I are responsible for dilated cardiomyopathy in PD‐1‐deficient mice. Nat Med 2003;9:1477–1483. [DOI] [PubMed] [Google Scholar]
- 37. Hsu J, Hodgins J, Marathe M et al. Contribution of NK cells to immunotherapy mediated by PD‐1/PD‐L1 blockade. J Clin Invest 2018;128:4654–4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See http://www.TheOncologist.com for supplemental material available online.
Supplemental Figures
Supplemental Tables


