Abstract
Background
Patients with high microsatellite instability (MSI) gastric cancer (GC) show improved survival and no benefit or harm from adjuvant and/or perioperative chemotherapy. The role of immune microenvironment in GC is largely unknown.
Materials and Methods
In the present study, 256 tumor tissue blocks were centrally collected from patients enrolled in ITACA‐S, a randomized adjuvant trial of 5‐FU/LV versus sequential FOLFIRI and cisplatin‐docetaxel. MSI status was assessed by multiplex PCR, inflammatory reaction by H&E morphological assessment, and programmed death‐ligand 1 (PD‐L1) expression by immunohistochemistry.
Results
Overall, 9% patients had MSI‐high tumors, 23% had high inflammatory reaction, 11% had tumor PD‐L1 ≥ 1%, and 11% had stromal PD‐L1 ≥ 1%. A significant association with disease‐free survival (DFS) and overall survival (OS) was found for MSI‐high (hazard ratio [HR], 0.43; p = .02; HR, 0.40; p = .02) and high inflammatory reaction (HR, 0.55; p = .010; HR, 0.53; p = .008) but not for PD‐L1. At multivariable analysis, only MSI showed an independent association with both DFS (p = .02) and OS (p = .01), whereas inflammatory reaction showed an independent association only with OS (p = .04). Patients with tumor PD‐L1 ≥ 1% had a significantly longer DFS in sequential chemotherapy than in than 5‐FU/LV arm (interaction p = .04) and a trend for OS (interaction p = .12).
Conclusion
Our data suggest that MSI status could be a useful prognostic biomarker in patients with radically resected stage II–III GC and should be used as stratification factor in future trials. Tumor PD‐L1 ≥ 1% should be further investigated as a potential predictor of benefit from intensive chemotherapy.
Implications for Practice
In this post hoc analysis of patients with radically resected gastric cancer randomized to an intensive sequential chemotherapy regimen versus 5‐FU/LV monotherapy as adjuvant treatment in the ITACA‐S trial, MSI‐high status was independently associated with better disease‐free survival and overall survival (OS) and inflammatory reaction was independently associated with better OS. Moreover, tumor PD‐L1 expression ≥1% was associated with greater benefit from intensive sequential chemotherapy compared with 5‐fluorouracil plus leucovorin (5‐FU/LV), whereas PD‐L1 expression <1% was not, conditioning a statistically significant interaction between such biomarker and treatment arms. The meta‐analysis of individual patients’ data from available studies could yield data on the role of MSI status that could inform clinical decisions.
Keywords: Gastric cancer, Microsatellite instability, Adjuvant chemotherapy, PD‐L1, Biomarkers
Short abstract
This article reports a post‐hoc analysis of immune‐related biomarkers in patients with radically resected gastric cancer enrolled in the ITACA‐S trial.
Introduction
Gastric cancer (GC) is one of the leading causes of cancer‐related deaths worldwide 1. Surgery is the main treatment for patients with localized GC, although a large number develop recurrence or metastasis even after curative resection. Therefore, various perioperative and adjuvant chemotherapy strategies have been investigated, obtaining a clear survival advantage 2. However, no biomarkers have been validated in patients with radically resected gastric or gastroesophageal junction cancers 3. In fact, to be included in the therapeutic decision algorithms in addition to validated clinicopathological characteristics, such biomarkers should provide a better prognostic stratification and/or should predict the effectiveness of specific anticancer strategies. Robust molecular classifications of GCs based on gene expression profiling have indeed been developed, but their clinical usefulness still awaits to be proven 4, 5, 6.
Microsatellite instability (MSI) is an easily testable biomarker that can serve as a surrogate for a specific GC molecular subgroup identified by both the major molecular classification schemes: TCGA and ACRG 5, 7. Several retrospective studies clearly suggested a positive prognostic value of MSI in patients with radically resected GC and a potentially detrimental or null effect of perioperative and adjuvant chemotherapy in this patients’ subgroup 8, 9, 10. However, the assessment of the potential prognostic role of MSI is complicated by its association with specific clinicopathological features, such as older age, female sex, low grade of differentiation, intestinal histotype, antral localization, and absence of nodal involvement, most of which are good prognostic factors 11, 12. Moreover, the frequency of MSI is below 10% in GC clinical trials 13, 14, which hampers its validation as a prognostic and predictive biomarker with clinical utility and statistical significance. Finally, MSI is associated with other molecular features, including increased inflammation and expression of immune checkpoints, such as programmed death‐ligand 1 (PD‐L1) 10, 15. Specifically, in the absence of large and comprehensive immune‐profiling studies, the prognostic role of PD‐L1 expression in GC is still controversial 16, 17.
Drawing from these considerations, we performed a post hoc analysis of immune‐related biomarkers in patients with radically resected GC enrolled in the ITACA‐S trial.
Materials and Methods
Study Population
In the ITACA‐S trial (http://clinicaltrials.gov identifier: NCT01640782) 18, a total number of 1,106 patients with pT2b‐4 (6th TNM edition) and/or node‐positive, radically resected GC were enrolled, and 1,100 were randomized to adjuvant treatment with either 5‐fluorouracil plus leucovorin (5‐FU/LV) or sequential FOLFIRI regimen for four cycles, followed by cisplatin and docetaxel for three cycles. The trial failed to show any difference in disease‐free survival (DFS) and overall survival (OS) between the two treatment arms. In this analysis, we included all patients whose surgical samples could be centrally collected and were available for immune‐related biomarker testing. Information about relevant clinicopathological characteristics (namely: age, sex, Eastern Cooperative Oncology group performance [ECOG PS], tumor grade, Lauren histotype, tumor site, pT, and lymphnodal status) were retrieved. pT stage was reclassified according to the 7th pTNM edition 19 and Lauren histotype was centrally reassessed by two independent pathologists who were blinded to the clinical and translational data set. All patients who were still alive at the time of this investigation signed a written informed consent, and the institutional review board of Fondazione IRCCS Istituto Nazionale dei Tumori di Milano approved the study. The present study was conducted in accordance with the Declaration of Helsinki for medical research involving human subjects.
Immune‐Related Biomarkers Testing
MSI was assessed by multiplex polymerase chain reaction using five quasimonomorphic mononucleotide repeats, as previously described 20, 21. Histopathologic assessment of the inflammatory reaction at the invasive margin was performed on H&E‐stained sections, as previously described by Klintrup et al. 22. Two senior gastrointestinal pathologists, each of whom was blinded to the other's evaluations and the patient outcomes, revised centrally H&E‐stained sections and tumor representation. In case of disagreement, a consensus among pathologists was reached at the double‐headed microscope. Briefly, the invasive margin was defined as the interface between the host stroma and the invading edge area of a tumor, and inflammatory reaction was classified as high when inflammatory cells formed a band‐ or cup‐like infiltrate at the invasive margin with partial or complete destruction of cancer cell islets and low when these features were absent. Tumor and stromal PD‐L1 expression was assessed by immunohistochemistry (IHC) staining using anti‐PD‐L1 antibodies (clone/SP142, Spring; supplemental online Appendix 1). The expression of PD‐L1 was evaluated by two independent pathologists according to the percentage of stained tumor (tPD‐L1) or immune (sPD‐L1) cells and was defined as positive if ≥1%.
Statistical Analysis
Descriptive statistics were used to summarize patients and disease characteristics. Chi‐square test, Fisher exact test, or Mann‐Whitney test was used, as appropriate, to assess the association between immune‐related biomarkers and other features. DFS was calculated from surgery to relapse or death from any cause. OS was calculated from surgery to death from any cause. The Kaplan‐Meier method and the Cox proportional hazards regression model were used for survival analysis. Specifically, immune‐related biomarkers and relevant clinicopathological characteristics significantly associated with survival outcomes at univariate analysis were used to build the multivariable models. Statistical significance threshold was set to a canonical two‐tailed .05 value. Because of the exploratory nature of our analysis regarding PD‐L1 expression and inflammatory reaction in early GC, we did not consider multiple testing adjustment, so as not to inflate the type II error. R software (version 3.5.0) and RStudio software (version 1.1.453), together with the packages survival, survminer, and epitools, were used for statistical analyses.
Results
Patients and Disease Characteristics
Supplemental online Figure 1 shows the consort diagram of the study. Overall, 1,106 patients were enrolled in ITACA‐S, 6 were excluded before the start of treatment, and a total number of 1,100 patients were included in the final analysis set. We centrally collected 256 archival tumor samples, all of which were analyzed for MSI status (100%), 238 (93%) for inflammatory reaction, and 184 (72%) for PD‐L1 immunohistochemical expression. Table 1 shows patients and disease baseline characteristics in the initial ITACA‐S trial population and in the translational study population, as well as the prevalence of the selected immune‐related biomarkers, overall and per treatment arm. No significant imbalances were observed between the two treatment arms. Specifically, 24 (9%) patients had MSI‐high tumors, 55 (23%) had high inflammatory reaction, 21 (11%) had tumor PD‐L1 expression ≥1%, and 20 (11%) had stromal PD‐L1 expression ≥1%. As expected, MSI was associated with specific clinicopathological features, such as older age, ECOG PS >0 (both p = .030), and intestinal histotype (p = .001). A significant association with the intestinal histotype was also found for all other immune‐related biomarkers, namely high inflammatory reaction (p = .020), tumor PD‐L1 expression ≥1% (p = .003), and stromal PD‐L1 expression ≥1% (p < .001). High inflammatory reaction was associated with lymphnodal negativity (p = .02). Notably, MSI was not associated with either inflammatory reaction or tumor PD‐L1 expression ≥1% but was associated with stromal PD‐L1 expression ≥1% (p < .001). Inflammatory reaction was highly and significantly correlated with both tumor and stromal PD‐L1 expression ≥1% (supplemental online Table 1).
Table 1.
Baseline patients and disease characteristics
| Characteristic | ITACA‐S final data set (n = 1,100), n (%) | Translational study population (n = 256), n (%) | 5‐FU/LV arm (n = 123), n (%) | Sequential arm (n = 133), n (%) | p valuea |
|---|---|---|---|---|---|
| Age, yr | .49 | ||||
| Median | 62 | 63 | 62 | 63 | |
| IQR | 56–67 | 55–68 | 54–68 | 55–68 | |
| Sex | .42 | ||||
| M | 697 (64) | 174 (68) | 87 (71) | 87 (65) | |
| F | 403 (36) | 82 (32) | 36 (29) | 46 (35) | |
| ECOG PS | .99 | ||||
| 0 | 979 (90) | 228 (89) | 110 (89) | 118 (89) | |
| 1 | 115 (10) | 28 (11) | 13 (11) | 15 (11) | |
| NA | 6 | 0 | 0 | 0 | |
| Tumor grade | .89 | ||||
| 1–2 | 103 (30) | 77 (32) | 38 (32) | 39 (31) | |
| 3 | 243 (70) | 166 (68) | 79 (68) | 87 (69) | |
| NA | 754 | 13 | 6 | 7 | |
| Lauren histotype | .19 | ||||
| Diffuse | 340 (53) | 114 (49) | 60 (53) | 54 (44) | |
| Intestinal | 303 (47) | 121 (51) | 53 (47) | 68 (56) | |
| NA | 457 | 21 | 10 | 11 | |
| Tumor site of origin | .38 | ||||
| GC | 914 (86) | 217 (86) | 107 (88) | 110 (85) | |
| GEJ | 155 (14) | 34 (14) | 14 (12) | 20 (15) | |
| NA | 31 | 5 | 2 | 3 | |
| pT | .28 | ||||
| 1–2 | 243 (22) | 59 (23) | 32 (26) | 27 (20) | |
| 3–4 |
848 (78) 9 |
197 (77) | 91 (74) | 106 (80) | |
| Lymphnodal status | .97 | ||||
| Negative | 99 (9) | 21 (8) | 10 (8) | 11 (8) | |
| Positive | 988 (91) | 235 (92) | 113 (92) | 122 (92) | |
| NA | 13 | 0 | 0 | 0 | |
| MSI status | .51 | ||||
| MSS | NA | 232 (91) | 113 (92) | 119 (89) | |
| MSI‐high | NA | 24 (9) | 10 (8) | 14 (11) | |
| Inflammatory reaction | .58 | ||||
| Low | NA | 183 (77) | 91 (78) | 92 (75) | |
| High | NA | 55 (23) | 25 (22) | 30 (25) | |
| NA | ‐ | 18 | 7 | 11 | |
| Tumor PD‐L1 (IHC) | .43 | ||||
| <1% | NA | 163 (89) | 77 (91) | 86 (87) | |
| ≥1% | NA | 21 (11) | 8 (9) | 13 (13) | |
| NA | ‐ | 72 | 38 | 34 | |
| Stromal PD‐L1 (IHC) | .29 | ||||
| <1% | NA | 164 (89) | 78 (92) | 86 (87) | |
| ≥1% | NA | 20 (11) | 7 (8) | 13 (13) | |
| NA | ‐ | 72 | 38 | 34 |
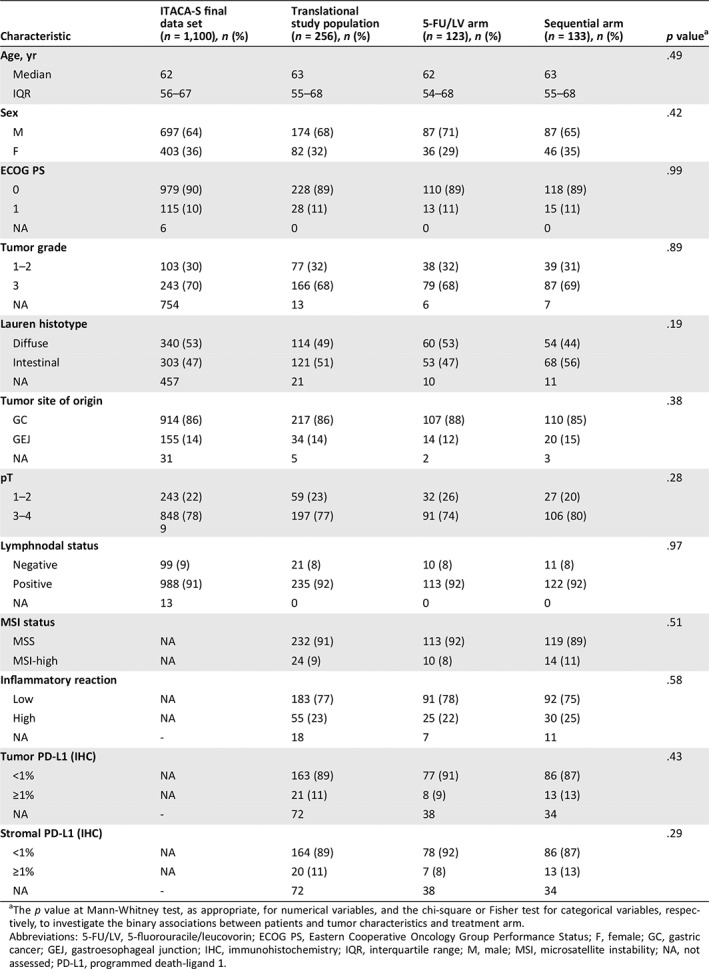
The p value at Mann‐Whitney test, as appropriate, for numerical variables, and the chi‐square or Fisher test for categorical variables, respectively, to investigate the binary associations between patients and tumor characteristics and treatment arm.
Abbreviations: 5‐FU/LV, 5‐fluorouracile/leucovorin; ECOG PS, Eastern Cooperative Oncology Group Performance Status; F, female; GC, gastric cancer; GEJ, gastroesophageal junction; IHC, immunohistochemistry; IQR, interquartile range; M, male; MSI, microsatellite instability; NA, not assessed; PD‐L1, programmed death‐ligand 1.
Prognostic and Predictive Value of MSI Status in Patients with Gastric Cancer
The Kaplan‐Meier curves of DFS and OS, overall and per treatment arm in the translational study population, were similar to those reported in the whole ITACA‐S data set (supplemental online Fig. 2). Table 2 shows the Cox proportional hazard regression models for DFS and OS according to clinicopathological features and each immune‐related biomarker. A significant association with both DFS and OS was found for tumor grade, pT, lymphnodal status, MSI (hazard ratio [HR], 0.43; 95% confidence interval [CI], 0.21–0.88; p = .020 for DFS and HR, 0.40; 95% CI, 0.19–0.87; p = .020 for OS), and high inflammatory reaction (HR, 0.55; 95% CI, 0.35–0.87; p = .010 for DFS and HR, 0.53; 95% CI, 0.33–0.85; p = .008 for OS). In detail, 3‐year DFS was 79.2% (64.5–97.2) in the MSI‐high subgroup versus 49.2% (43.1–56.2) in the microsatellite stable (MSS) one (Fig. 1A), and 5‐year OS was 73.5% (57.2–94.5) versus 44.5% (38.1–52.0), respectively (Fig. 1B); 3‐year DFS was 64.9% (53.3–79.0) in the high inflammatory reaction subgroup versus 44.9% in the low inflammatory reaction one (38.2–52.7; Fig. 1C), and 5‐year OS was 56.1% (42.1–74.6) versus 40.2% (33.4–48.5), respectively (Fig. 1D). No significant differences in DFS and OS were observed according to tumor PD‐L1 expression (Fig. 1E–F) or stromal PD‐L1 expression (supplemental online Fig. 3A–B).
Table 2.
Cox proportional hazard regression models for disease‐free survival and overall survival
| Characteristic | Disease‐free survival | Overall survival | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariable model | Univariate analysis | Multivariable model | |||||
| HR (95% CI) | p value | HR (95% CI) | p value | HR (95% CI) | p value | HR (95% CI) | p value | |
| Age | 1.01 (0.99–1.03) | .35 | — | — | 1.01 (0.99–1.03) | .15 | — | — |
| Sex, M vs. F | 0.96 (0.67–1.36) | .81 | — | — | 0.94 (1.06–1.36) | .75 | — | — |
| ECOG PS, 1 vs. 0 | 1.52 (0.94–2.47) | .09 | — | — | 1.34 (0.79–2.26) | .27 | — | — |
| Tumor grade, 3 vs. 1–2 | 1.17 (1.03–1.32) | .02 | 1.10 (0.97–1.25) | .13 | 1.19 (1.05–1.36) | .009 | 1.12 (0.98–1.28) | .10 |
| Lauren histotype, intestinal vs. other | 0.93 (0.67–1.31) | .71 | — | — | 0.88 (0.62–1.25) | .47 | — | — |
| Tumor site, GEJ vs. GC | 1.01 (0.62–1.66) | .96 | — | — | 1.09 (0.65–1.80) | .76 | — | — |
| pT, 3–4 vs. 1–2 | 2.91 (1.77–4.78) | <.001 | 2.95 (1.76–4.92) | <.001 | 3.34 (1.92–5.82) | <.001 | 3.18 (1.82–5.58) | <.001 |
| Lymphonodal status, pos vs. neg | 2.67 (1.18–6.06) | .02 | 2.98 (1.30–6.81) | .01 | 2.81 (1.15–6.87) | .02 | 3.17 (1.28–7.81) | .01 |
| MSI status, MSI‐high vs. MSS | 0.43 (0.21–0.88) | .02 | 0.39 (0.18–0.83) | .02 | 0.40 (0.19–0.87) | .02 | 0.36 (0.16–0.81) | .01 |
| Inflammatory reaction, High vs. Low | 0.55 (0.35–0.87) | .01 | 0.66 (0.42–1.04) | .07 | 0.53 (0.33–0.85) | .008 | 0.61 (0.38–0.98) | .04 |
| Tumor PD‐L1 (IHC), ≥1% vs. <1% | 0.70 (0.36–1.34) | .28 | — | — | 0.80 (0.42–1.54) | .51 | — | — |
| Stromal PD‐L1 (IHC), ≥1% vs. <1% | 0.81 (0.43–1.51) | .51 | — | — | 0.87 (0.47–1.64) | .68 | — | — |
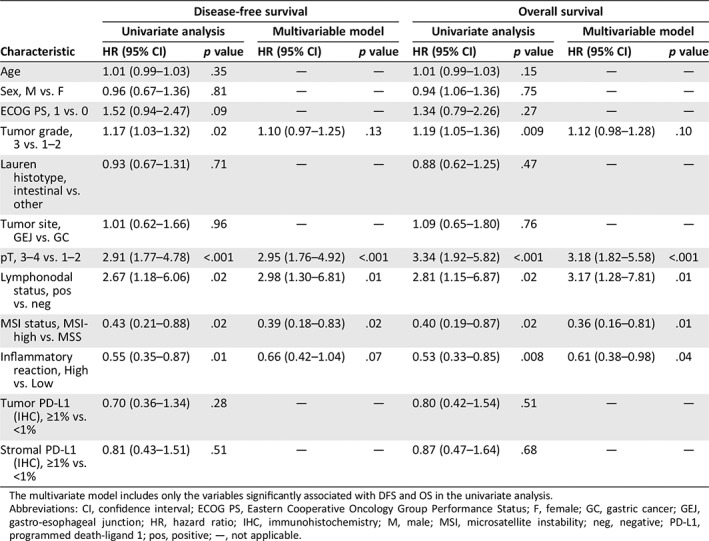
The multivariate model includes only the variables significantly associated with DFS and OS in the univariate analysis.
Abbreviations: CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group Performance Status; F, female; GC, gastric cancer; GEJ, gastro‐esophageal junction; HR, hazard ratio; IHC, immunohistochemistry; M, male; MSI, microsatellite instability; neg, negative; PD‐L1, programmed death‐ligand 1; pos, positive; —, not applicable.
Figure 1.
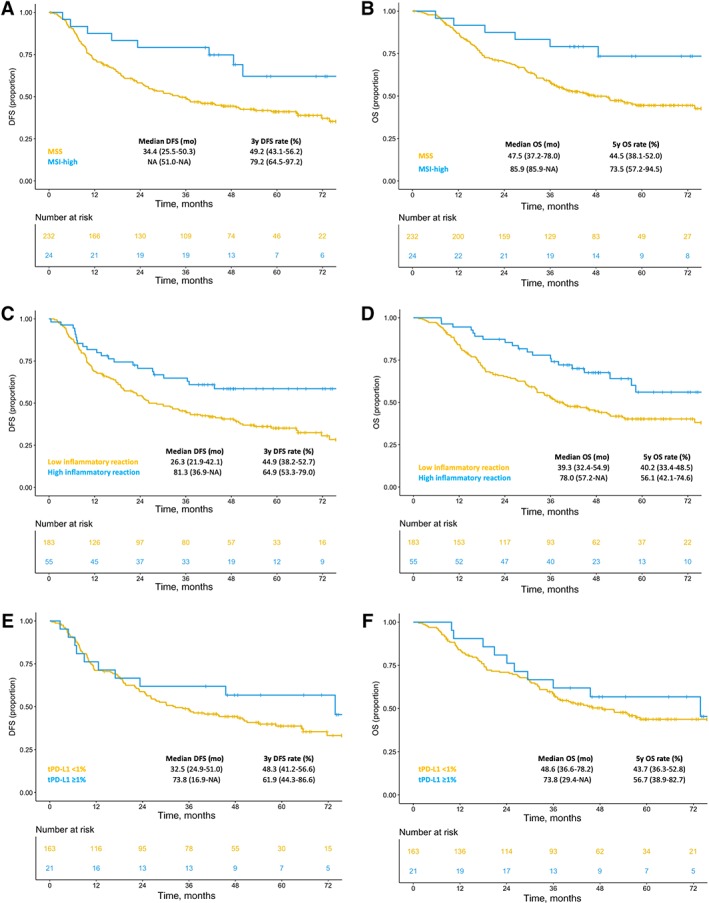
Kaplan‐Meier curves for DFS (A) and OS (B) in MSI‐high versus MSS patients’ subgroups, DFS (C) and OS (D) in patients’ subgroups with high versus low inflammatory reaction, DFS (E) and OS (F) in patients stratified per tumor PD‐L1 expression ≥1% versus <1%.Abbreviations: DFS, disease‐free survival; MSI, microsatellite instability; MSS, microsatellite stable; NA, not applicable; OS, overall survival; tPD‐L1, percentage of stained tumor.
In the multivariable Cox proportional hazard regression models including the variables significantly associated with DFS and OS in the univariate analysis (Table 2), MSI independently confirmed its association with both DFS (HR, 0.39; 95% CI 0.18–0.83; p = .02) and OS (HR, 0.36; 95% CI, 0.16–0.81; p = 0.1), whereas inflammatory reaction was significantly associated only with OS (HR, 0.61; 95% CI, 0.38–0.98, p = .04).
We finally investigated whether each specific immune‐related biomarker was associated with differential efficacy of the two treatment arms in terms of DFS and OS. In both MSS and MSI‐high subgroups, no major differences were observed in the DFS and OS curves according to treatment arm (test of treatment by MSI status interaction: p = .928 for DFS and .824 for OS; Fig. 2A–B). The same was observed in both subgroups with high and low inflammatory reaction (test of treatment by inflammatory reaction interaction: p = .738 for DFS and .955 for OS; Fig. 2C–D). In the subgroup with tumor PD‐L1 expression ≥1%, median DFS was 12.9 months (6.9–not assessable [NA]) in the 5‐FU/LV arm versus not reached (45.5–NR) in the sequential arm, whereas in the subgroup with PD‐L1 expression <1%, median DFS was 36.1 months (26.3–NR) versus 26.2 months (18.2–51.0), respectively, with a significant interaction between tumor PD‐L1 expression and treatment (p = .04; Fig. 2E). A similar trend was observed for OS: in the subgroup with tumor PD‐L1 expression ≥1%, median OS was 26.7 months (20.9–NA) in the 5‐FU/LV arm versus not reached (45.5–NR) in the sequential arm, whereas in in the subgroup with tumor PD‐L1 expression <1%, median OS was 54.9 months (36.1–NR) versus 44.9 months (31.3–NR), respectively, with a nonsignificant interaction test (p = .12; Fig. 2F). No differences were observed in the DFS and OS curves according to treatment arm in the subgroups with stromal PD‐L1 expression <1% vs ≥1% (supplemental online Fig. 3C–D).
Figure 2.
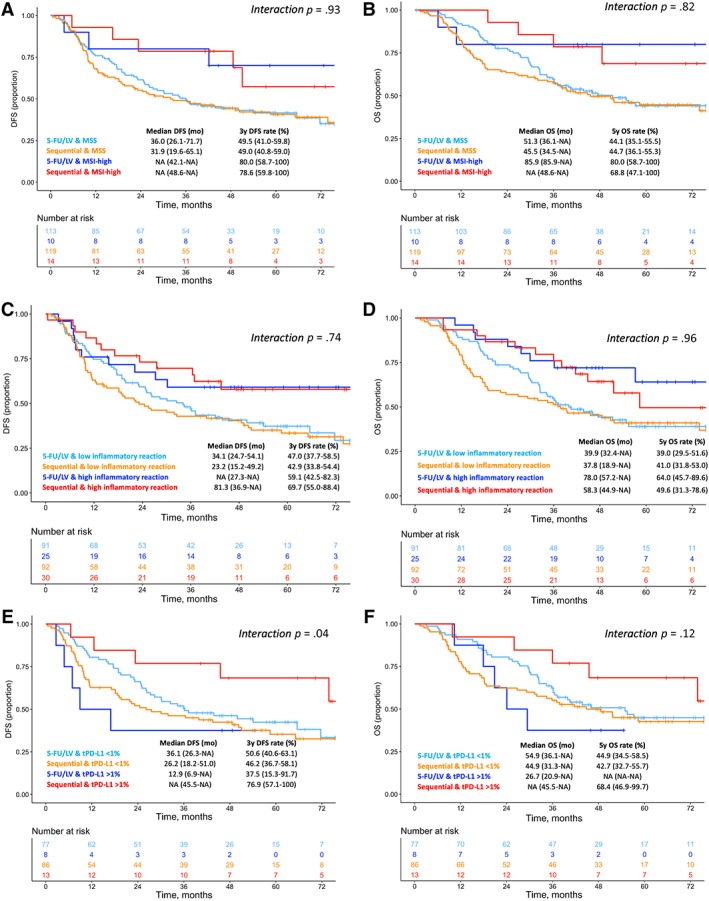
Kaplan‐Meier curves in patients stratified according to treatment arm, for DFS and OS, respectively, in MSI‐high versus MSS patients’ subgroups (A, B), high versus low inflammatory reaction (C, D), tumor PD‐L1 expression ≥1% versus <1% (E, F).Abbreviations: 5‐FU/LV, 5‐fluorouracil plus leucovorin; DFS, disease‐free survival; MSI, microsatellite instability; MSS, microsatellite stable; OS, overall survival; tPD‐L1, percentage of stained tumor.
Discussion
In this analysis of immune‐related biomarkers in patients with GC enrolled in the ITACA‐S trial, tumor and immune microenvironment biomarkers (inflammatory reaction and tumor and stromal PD‐L1) were significantly associated with each other and with intestinal histotype. The hypothesis generating from these results could prompt the identification of a subset of GCs, mainly intestinal‐type ones, in which the induction of T cell exhaustion is promoted by tumor upregulation of immune‐inhibitory checkpoints such as PD‐L1. Notably, the “cold tumor” phenotype of diffuse‐type cancers has been recently confirmed by using circulating immune biomarkers and may be responsible for the relatively worse outcomes compared with intestinal‐type subgroup 23. In agreement with literature data, MSI status was significantly associated with specific clinicopathological features (i.e., older age, ECOG PS, and intestinal histotype) and with stromal PD‐L1 expression but not with inflammatory reaction or tumor PD‐L1 expression. MSI‐high has been associated with increased T cell infiltration and expression of immune checkpoints, such as PD‐L1, in gastrointestinal malignancies, including GCs 15, 24, 25. However, the relatively low prevalence (about 9%) of MSI‐high cancers in our data set makes it difficult to find statistically significant associations with other biomarkers and to establish a causal association. Of note, the relatively low prevalence of MSI‐high status in this and other studies may be mainly due to the different clinicopathological characteristics of patients who had a more locally advanced disease (T3–4 and/or node‐positive status) compared with other data sets such as TCGA.
MSI‐high status was associated with an independent, clinically meaningful survival advantage, with an increase of about 30% in 3‐year DFS and 5‐year OS. MSI is a validated positive prognostic factor in early‐stage colorectal cancers 26, and a significant body of retrospective evidence has pointed out the opportunity of testing its potential value in GC 7, 8, 9, 10. However, post hoc analyses of MAGIC and CLASSIC trials and a recent individual patient data meta‐analysis of four trials have shown that MSI‐status could also be regarded as a potential negative predictive marker in GC patients receiving perioperative and/or adjuvant chemotherapy 9, 10, 27. Also, the post hoc analysis of CRITICS trial showed that patients with MSI‐high GC seem to achieve poorer pathological response to neoadjuvant chemotherapy 28. Our results confirm the positive prognostic value of MSI 8, 11, 29, even in white patients receiving adjuvant chemotherapy. However, the lack of a surgery‐only control arm did not allow us to properly assess the potential negative impact of adjuvant treatment on an MSI‐high population. We could not identify any differential impact on DFS and OS of the two different schedules of adjuvant treatment (sequential combination chemotherapy or 5‐FU/LV) in the MSI‐high versus MSS subgroups.
The development of immunotherapy has led to a deeper knowledge of immune processes in cancer and has raised the so far unmet need to better investigate immune‐related markers, such as inflammatory reaction and PD‐L1 expression, in several cancers. Whereas high inflammatory reaction at the invasive tumor margin was independently and significantly associated with improved OS (and significantly associated with improved DFS in univariate analysis), tumor or stromal PD‐L1 expression was not. Our exploratory observations appear to be consistent with the lack of prognostic value of tumor PD‐L1 expression reported in the CLASSIC trial (positivity rate 2.7%) 10 and in a recent retrospective series reported by Yamashita et al. (positivity rate 20.4%) 30. In particular, the latter work showed that a combined positive score (CPS) evaluating PD‐L1 expression in both tumor and immune cells was associated with poorer outcomes. On the contrary, the lack of independent prognostic impact of both tumor and stromal PD‐L1 expression in our analysis may be due to several reasons, including the specific population with more locally advanced disease and eligible for adjuvant treatment in a western country.
No differential interaction with DFS and OS was observed for the two treatment schedules according to high versus low inflammatory reaction status. Intriguingly, in the subgroup with tumor PD‐L1 ≥ 1%, we observed a DFS benefit in patients randomized to sequential chemotherapy and a negative effect on those receiving 5‐FU/LV, leading to a significant interaction p value between treatment and tumor PD‐L1 status. The shape of the DFS and OS curves was similar, although the interaction was nonsignificant in terms of OS. Based on these results, we can only speculate that tumors with upregulation of PD‐L1 (possibly reflecting an increase of tumor infiltration by cytotoxic T lymphocytes) could benefit from a more intensive cytotoxic treatment (i.e., sequential FOLFIRI followed by cisplatin and docetaxel) that may lead to increased immunogenic cell death and immune activation 31, 32, 33. However, more preclinical and translational evidence should be gathered in this specific setting to formulate stronger biological hypotheses.
Our study has some limitations, including its retrospective nature and the small sample size obtained from a subgroup of the overall trial population. Moreover, we could not properly assess the predictive value of the investigated biomarkers for adjuvant chemotherapy because of the lack of a surgery‐only control arm. A major limitation is the methodology adopted to test for PD‐L1 status, which includes the antibody used (SP142) and the lack of a precise prognostic cutoff value for IHC of PD‐L1, as well as the lack of a validated role for PD‐L1 expression on tumor cells versus immune infiltrate cells in the setting of early GC. However, it should be pointed out that different commercially available antibodies for the immunohistochemical analysis of PD‐L1 have been compared in different tumors, without any significant difference in the measurement of PD‐L1 expression 34, and that the potential predictive role of the CPS has been mainly investigated in the metastatic setting for pembrolizumab efficacy 35. Another major limitation is that Epstein‐Barr virus (EBV), one of the four major TCGA subsets 5, was not evaluated by our study, even if the low prevalence of EBV‐associated GCs makes difficult to show novel information on this subtype. In addition, we assessed and categorized the inflammatory reaction using the Klintrup‐Mäkinen score 22, which was developed for colorectal cancer. The potential advantage of this method is that it includes cells belonging to both innate and adaptive immunity, and not only infiltrating T cells, thus acknowledging the complex role of the immune system in cancer biology. However, a more detailed evaluation of tumor infiltrating lymphocytes and/or immunoscore could be important, particularly to stratify the prognostic outcome of MSI‐high cancers.
Conclusion
Novel immunotherapy‐based strategies should be developed for MSI‐high, resectable GC. Even in need of further confirmations, our observation regarding the prognostic and predictive role of PD‐L1 expression in early GC fascinatingly suggests a possible role for the biomarker as a stratification factor for future trials conducted in the perioperative or adjuvant setting.
Author Contributions
Conception/design: Maria Di Bartolomeo, Filippo Pietrantonio
Provision of study material or patients: ITACA‐S study group
Collection and/or assembly of data: Federica Morano, Alessandra Raimondi, Salvatore Corallo, Elena Tamborini, Federica Perrone, Monica Niger, Alessandro Pellegrinelli, Giovanni Randon, Filippo Pagani, Antonia Martinetti, Giovanni Fucà
Data analysis and interpretation: Maria Di Bartolomeo, Federica Morano, Alessandra Raimondi, Rosalba MiIceli, Elena Tamborini, Giovanni Fucà, Filippo Pietrantonio
Manuscript writing: Maria Di Bartolomeo, Alessandra Raimondi, Giovanni Fucà, Filippo Pietrantonio
Final approval of manuscript: Maria Di Bartolomeo, Federica Morano, Alessandra Raimondi, Rosalba MiIceli, Salvatore Corallo, Elena Tamborini, Federica Perrone, Maria Antista, Monica Niger, Alessandro Pellegrinelli, Giovanni Randon, Filippo Pagani, Antonia Martinetti, Giovanni Fucà, Filippo Pietrantonio, ITACA‐S study group
Disclosures
Federica Morano: Servier (H). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Supporting information
See http://www.TheOncologist.com for supplemental material available online.
Supplemental Appendices
Supplemental Figures
Supplemental Table
Acknowledgments
Partial financial support was provided by no‐profit grant IG 8738 from AIRC (Italian Association for Cancer Research). This report would not have been possible without the valuable contribution of pathologists of the participating Center of the ITACA‐S trial:
1Elisa Giommoni, 2Enrico Aitini, 3Francesca Spada, 4Gerardo Rosati, 5Alberto Marchet, 6Francesca Pucci, 7Alberto Zaniboni, 8Stefano Tamberi, 9Tiziana Pressiani, 10Gianni Sanna, 11Anita Rimanti, 12Stefania Mosconi, 13Paola Bolzoni, 14Carmine Pinto, 15Lorenza Landi, 16Hector Josè Soto‐Parra, 17Luigi Cavanna
1Azienda Ospedaliera‐Università Careggi, Firenze, Italy 2Ospedale di Suzzara, Mantova, Italy; 3Istituto Oncologico Europeo, Milano, Italy; 4Azienda Ospedaliera “San Carlo,” Potenza, Italy; 5Azienda Ospedaliera di Padova, Padova, Italy; 6Azienda Ospedaliera di Parma, Parma, Italy; 7Istituto Ospedaliero Fondazione Poliambulanza, Brescia, Italy; 8AUSL Romagna, Italy; 9Istituto Clinico Humanitas, Rozzano, Milano, Italy; 10Istituto Ospedaliero dell'Università di Sassari, Sassari, Italy; 11Oncologia ASST‐Mantova, Mantova, Italy; 12ASST Papa Giovanni XXIII, Bergamo, Italy; 13Presidio Ospedaliero “Serbelloni” di Gorgonzola, Melegnano, Italy; 14Arcispedale Santa Maria Nuova Azienda Ospedaliera di Reggio Emilia, Reggio Emilia, Italy; 15Presidio Ospedaliero di Livorno, Livorno, Italy; 16Policlinico Vittorio Emanuele, Presidio Gaspare Rodolico, Catania, Italy; 17Ospedale Civile “Guglielmo da Saliceto,” Piacenza, Italy.
Disclosures of potential conflicts of interest may be found at the end of this article.
References
- 1. Bray F, Ferlay J, Soerjomataram I et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 2. Smyth EC, Verheij M, Allum W et al. Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Ann Oncol 2016;27:v38–v49. [DOI] [PubMed] [Google Scholar]
- 3. Corso S, Giordano S. How can gastric cancer molecular profiling guide future therapies? Trends Mol Med 2016;22:534–544. [DOI] [PubMed] [Google Scholar]
- 4. Cristescu R, Lee J, Nebozhyn M et al. Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nat Med 2015;21:449–456. [DOI] [PubMed] [Google Scholar]
- 5. Cancer Genome Atlas Research Network . Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014;513:202–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cheong JH, Yang HK, Kim H et al. Predictive test for chemotherapy response in resectable gastric cancer: A multi‐cohort, retrospective analysis. Lancet Oncol 2018;19:629–638. [DOI] [PubMed] [Google Scholar]
- 7. Sohn BH, Hwang JE, Jang HJ et al. Clinical significance of four molecular subtypes of gastric cancer identified by the Cancer Genome Atlas Project. Clin Cancer Res 2017;23:4441–4449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Marrelli D, Polom K, Pascale V et al. Strong prognostic value of microsatellite instability in intestinal type non‐cardia gastric cancer. Ann Surg Oncol 2016;23:943–950. [DOI] [PubMed] [Google Scholar]
- 9. Smyth EC, Wotherspoon A, Peckitt C et al. Mismatch repair deficiency, microsatellite instability, and survival: An exploratory analysis of the medical research council adjuvant gastric infusional chemotherapy (MAGIC) Trial. JAMA Oncol 2017;3:1197–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Choi YY, Kim H, Shin SJ et al. Microsatellite instability and programmed cell death‐ligand 1 expression in stage II/III gastric cancer: Post hoc analysis of the CLASSIC randomized controlled study. Ann Surg 2019;270:309–316. [DOI] [PubMed] [Google Scholar]
- 11. Polom K, Marano L, Marrelli D et al. Meta‐analysis of microsatellite instability in relation to clinicopathological characteristics and overall survival in gastric cancer. Br J Surg 2018;105:159–167. [DOI] [PubMed] [Google Scholar]
- 12. Leite M, Corso G, Sousa S et al. MSI phenotype and MMR alterations in familial and sporadic gastric cancer. Int J Cancer 2011;128:1606–1613. [DOI] [PubMed] [Google Scholar]
- 13. Lee J, Lim DH, Kim et al. Phase III trial comparing capecitabine plus cisplatin versus capecitabine plus cisplatin with concurrent capecitabine radiotherapy in completely resected gastric cancer with D2 lymph node dissection: The ARTIST trial. J Clin Oncol 2012;30:268–273. [DOI] [PubMed] [Google Scholar]
- 14. Bang YJ, Kim YW, Yang HK et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): A phase 3 open‐label, randomised controlled trial. Lancet 2012;379:315–321. [DOI] [PubMed] [Google Scholar]
- 15. Llosa NJ, Cruise M, Tam A et al. The vigorous immune microenvironment of microsatellite instable colon cancer is balanced by multiple counter‐inhibitory checkpoints. Cancer Discov 2015;5:43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gu L, Chen M, Guo D et al. PD‐L1 and gastric cancer prognosis: A systematic review and meta‐analysis. PLoS One 2017;12:e0182692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhang M, Dong Y, Liu H et al. The clinicopathological and prognostic significance of PD‐L1 expression in gastric cancer: A meta‐analysis of 10 studies with 1,901 patients. Sci Rep 2016;6:37933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bajetta E, Floriani I, Di Bartolomeo M et al. Randomized trial on adjuvant treatment with FOLFIRI followed by docetaxel and cisplatin versus 5‐fluorouracil and folinic acid for radically resected gastric cancer. Ann Oncol 2014;25:1373–1378. [DOI] [PubMed] [Google Scholar]
- 19. Di Bartolomeo M, Pietrantonio F, Pellegrinelli A et al. Osteopontin, E‐cadherin, and beta‐catenin expression as prognostic biomarkers in patients with radically resected gastric cancer. Gastric Cancer 2016;19:412–420. [DOI] [PubMed] [Google Scholar]
- 20. Pietrantonio F, Perrone F, de Braud F et al. Activity of temozolomide in patients with advanced chemorefractory colorectal cancer and MGMT promoter methylation. Ann Oncol 2014;25:404–408. [DOI] [PubMed] [Google Scholar]
- 21. Suraweera N, Duval A, Reperant M et al. Evaluation of tumor microsatellite instability using five quasimonomorphic mononucleotide repeats and pentaplex PCR. Gastroenterology 2002;123:1804–1811. [DOI] [PubMed] [Google Scholar]
- 22. Klintrup K, Mäkinen JM, Kauppila S et al. Inflammation and prognosis in colorectal cancer. Eur J Cancer 2005;41:2645–2654. [DOI] [PubMed] [Google Scholar]
- 23. Pernot S, Terme M, Radosevic‐Robin N et al. Infiltrating and peripheral immune cell analysis in advanced gastric cancer according to the Lauren classification and its prognostic significance. Gastric Cancer 2019. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 24. Grogg KL, Lohse CM, Pankratz VS et al. Lymphocyte‐rich gastric cancer: Associations with Epstein‐Barr virus, microsatellite instability, histology, and survival. Mod Pathol 2003;16:641–651. [DOI] [PubMed] [Google Scholar]
- 25. Kim KJ, Yang HK, Kim WH et al. Combined prognostic effect of PD‐L1 expression and immunoscore in microsatellite‐unstable advanced gastric cancers. Oncotarget 2017;8:58887–58902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sargent DJ, Marsoni S, Monges G et al. Defective mismatch repair as a predictive marker for lack of efficacy of fluorouracil‐based adjuvant therapy in colon cancer. J Clin Oncol 2010;28:3219–3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pietrantonio F, Miceli R, Raimondi A et al. Individual patient data meta‐analysis of the value of microsatellite instability as a biomarker in gastric cancer. J Clin Oncol 2019. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 28. Biesma H, Sikorska K, Hoek D et al. Effect of perioperative treatment on microsatellite instable gastric cancer in the CRITICS trial. Presented at: 13th International Gastric Cancer Congress; May 8–11, 2019: Prague, Czech Republic.
- 29. Miceli R, An J, Di Bartolomeo M, et al. Prognostic impact of microsatellite instability in asian gastric cancer patients enrolled in the ARTIST Trial. Oncology 2019;97:38–43. [DOI] [PubMed] [Google Scholar]
- 30. Yamashita K, Iwatsuki M, Harada K, et al. Prognostic impacts of the combined positive score and the tumor proportion score for programmed death ligand‐1 expression by double immunohistochemical staining in patients with advanced gastric cancer. Gastric Cancer 2019; [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 31. Cerbelli B, Pernazza A, Botticelli A et al. PD‐L1 Expression in TNBC: A predictive biomarker of response to neoadjuvant chemotherapy? Biomed Res Int 2017;2017:1750925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wimberly H, Brown JR, Schalper K et al. PD‐L1 expression correlates with tumor‐infiltrating lymphocytes and response to neoadjuvant chemotherapy in breast cancer. Cancer Immunol Res 2015;3:326–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Voorwerk L, Slagter M, Horlings HM et al. Immune induction strategies in metastatic triple‐negative breast cancer to enhance the sensitivity to PD‐1 blockade: The TONIC trial. Nat Med 2019;25:920–928. [DOI] [PubMed] [Google Scholar]
- 34. O'Malley DP, Yang Y, Boisot S et al. Immunohistochemical detection of PD‐L1 among diverse human neoplasm in a reference laboratory: Observations based upon 62,896 cases. Mod Pathol 2019;32:929–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shitara K, Özgüroğlu M, Bang YJ et al. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro‐oesophageal junction cancer (KEYNOTE‐061): A randomised, open‐label, controlled, phase 3 trial. Lancet 2018;392:123–133. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See http://www.TheOncologist.com for supplemental material available online.
Supplemental Appendices
Supplemental Figures
Supplemental Table


