Abstract
Colorectal cancer (CRC) is the second leading cause of cancer death worldwide. Growing evidence supports gene fusions as good candidates for molecularly targeted therapy in CRC. Here we describe a case of a 63‐year‐old man who had a radical right hemicolectomy procedure 24 months ago. Pathological diagnosis indicated colorectal adenocarcinoma with stage pT4N2bMx. During re‐examination in December 2016, positron emission tomography/computed tomography scans indicated relapse with multiple lymph nodes metastasis. Then the patient received a nine‐cycle combination treatment of XELOX and bevacizumab and showed progressive disease (PD). Subsequently, the patient was treated with bevacizumab plus FOLFIRI for 2 months before discontinuation because of adverse events. Paraffin sections of postoperative colorectal tissue were subjected to next‐generation sequencing, and epidermal growth factor receptor (EGFR) amplification and rare EGFR–SEPT14 fusion were identified. The patient then received erlotinib, an EGFR tyrosine kinase inhibitor (TKI), and achieved a partial response. However, the patient subsequently showed PD, and a new variant, EGFRvIII, appeared in metastasis, which may be involved in erlotinib resistance. We suggest that there is value in treating patients harboring EGFR fusions with EGFR TKI therapy, and EGFR–SEPT14 fusion may be used as a therapeutic target for CRC.
Key Points
To the authors' knowledge, this is the first report of EGFR–SEPT14 fusion in colorectal cancer.
The patient achieved a partial response after treatment with the epidermal growth factor receptor tyrosine kinase inhibitor erlotinib.
This report expands the list of gene fusions in colorectal cancer and highlights new targets for the therapeutic intervention.
EGFRvIII may be involved in erlotinib resistance, which is rare in colorectal cancer.
Short abstract
Growing evidence supports gene fusions as good candidates for molecularly targeted therapy in colorectal cancer. This article describes a case of a patient who had a radical right hemicolectomy procedure and suggests that there is value in treating patients harboring EGFR fusions with EGFR‐tyrosine kinase inhibitor therapy and that EGFR‐SEPT14 fusion may be used as a therapeutic target for colorectal cancer.
Patient Story
The patient was a 63‐year‐old man who had undergone radical right hemicolectomy in 2014. The postoperative pathological diagnosis indicated a moderately differentiated colorectal adenocarcinoma at stage pT4N2bMx. Immunohistochemical staining showed positive for mutS homolog 2 (MSH2) and mutS homolog 6 (MSH6). Family history revealed that his mother had colorectal cancer at the age of 83. When re‐examined in December 2016, positron emission tomography/computed tomography scans showed a thickened intestinal wall at the anastomosis and multiple pathologically enlarged lymph nodes in his abdominal aorta and root of the mesentery (Fig. 1A), which suggested relapse with multiple lymph nodes metastasis. Meanwhile, genetic testing did not detect the mutations of KRAS, NRAS, BRAF, or PIK3CA in colorectal tissue. The patient went through a nine‐cycle combination treatment of XELOX (oxaliplatin 200 mg intravenously [IV], D1, and capecitabine, 1,500 mg p.o., b.i.d., days 1–14, every 21 days) and bevacizumab (400 mg IV, day 1, every 21 days) from December 2016 to October 2017. In December 2017, a computed tomography (CT) scan showed left adrenal nodules and enlarged lymph nodes in his abdominal aorta and root of the mesentery (Fig. 1B), suggesting progressive disease (PD). The patient was then treated with bevacizumab (300 mg IV, day 1, every 14 days) plus FOLFIRI (irinotecan 300 mg IV, day 1, leucovorin 300 mg IV, day 1, and 5‐fluorouracil 500 mg IV bolus, day 1 plus 4,000 mg over 46 hours, every 14 days) from December 2017 to January 2018. Therapy was discontinued as a result of intestinal perforation around the anastomotic stoma. In December 2017, paraffin sections of postoperative colorectal tissue were subjected to next‐generation sequencing (NGS), and epidermal growth factor receptor (EGFR) amplification and EGFR–SEPT14 fusion were identified. The tumor was microsatellite stable. From January 2018 to May 2018, the patient recuperated and did not receive any further drug treatment.
Figure 1.
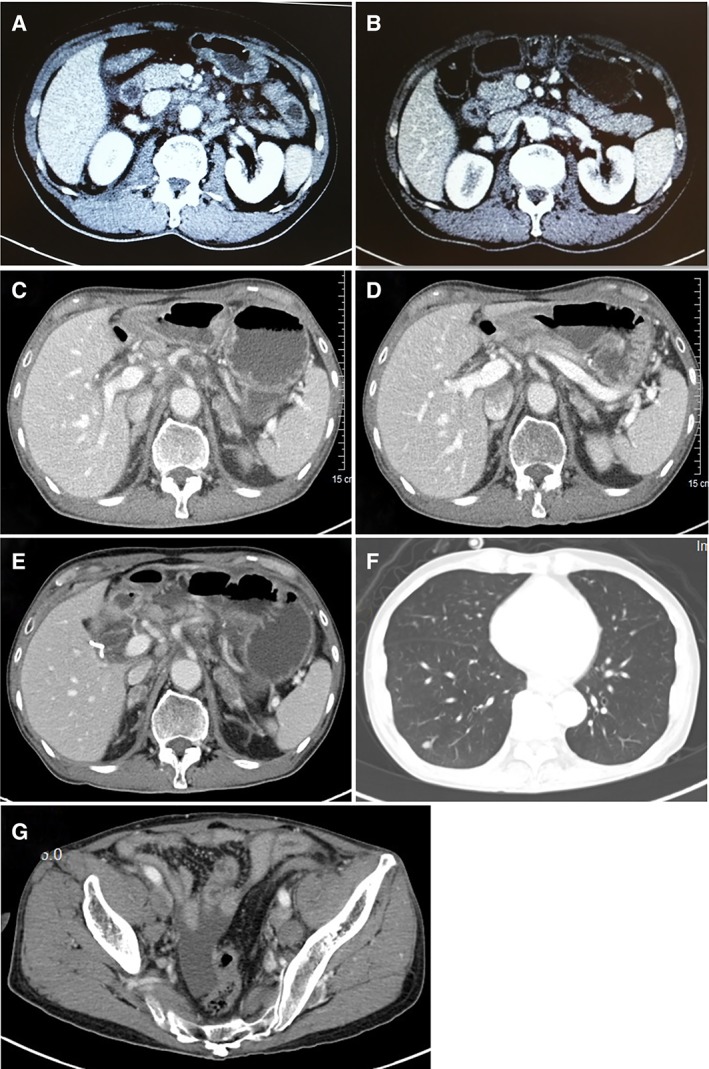
Positron emission tomography/computed tomography scans. (A): Multiple lymph node metastases before drug treatment. (B): Enlarged lymph nodes in abdominal aorta and root of the mesentery after treatment with XELOX regimen. (C): Enlarged lymph nodes in the retroperitoneum and bilateral adrenal metastasis before erlotinib treatment. (D): Reduction of para‐aortic lymph nodes and bilateral adrenal metastasis after treatment with erlotinib for 20 days. Enlarged lymph nodes in the retroperitoneum and bilateral adrenal metastasis (E), enlarged metastatic tumor in right lower lung (F), and new metastatic lymph nodes on both sides of the pelvic cavity (G) after treatment with erlotinib for 82 days.
Molecular Tumor Board
Genotyping Results and Interpretation of the Molecular Results
NGS‐based ultra‐deep panel sequencing was performed on tumor samples and matched blood in a Clinical Laboratory Improvement Amendments–certified and College of American Pathologists–accredited laboratory (OrigiMed) 1. Briefly, genomic DNA from a formalin‐fixed paraffin‐embedded tissue specimen containing more than 20% tumor content was fragmented to ∼250 bp by sonication. A DNA library was constructed using KAPA Hyper Prep Kit (KAPA Biosystems, Wilmington, MA). Hybrid‐capture‐selected libraries were sequenced to a mean coverage of 1,000× on an Illumina NextSeq‐500 Platform (Illumina Incorporated, San Diego, CA). Genomic alterations including single base substitution, copy number variants, short and long insertions/deletions, and gene rearrangement and fusions were assessed. The tumor mutational burden was estimated by analyzing somatic mutations including coding base substitution and indels per megabase of the panel sequences examined. The results showed EGFR amplification and EGFR–SEPT14 fusion.
As members of a highly conserved GTPase family, septins were first described in Saccharomyces cerevisiae 2. Septins have been involved in multiple cellular functions such as cytokinesis, cell cycle control, mitotic spindle formation, and plasma membrane compartmentalization 3. Septin 14 (SEPT14) is a member of septin family molecules and is abundantly expressed in developing cerebral cortex in neuronal development 3. SEPT14 maps to 7p11.2 in humans and includes a conserved GTPase domain and a carboxy‐terminus coil‐coiled domain, which is characteristic of other septins 4.
EGFR (also known as ErbB1) is a 170‐kDa transmembrane tyrosine kinase whose main ligands are epidermal growth factor and transforming growth factor‐α. As one of the most studied receptor tyrosine kinases, EGFR plays an essential role during embryonic development and adult homeostasis and is often aberrantly activated in cancer 5. EGFR contributes to tumor development and progression. In the patient's tumor, EGFR–SEPT14 fusion was detected. The exon 24 on EGFR was fused to the exon 10 on SEPT14, while retaining the receptor tyrosine kinase domain of EGFR (Fig. 2).
Figure 2.
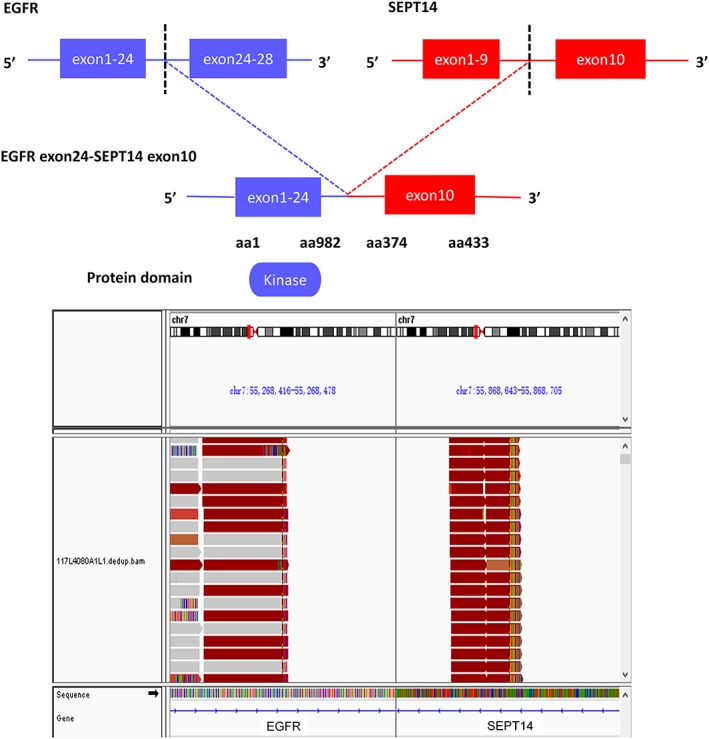
Genomic fusion of EGFR exon 24 with exons 10 of SEPT14.
Abbreviations: EGFR, epidermal growth factor receptor; SEPT14, Septin 14.
Functional and Clinical Significance of the Specific Mutation in the Particular Cancer
EGFR fusions have been previously reported in gliomas 6, and four fusions have been identified in lung cancer, including EGFR–TNS3, EGFR–PURB, EGFR–RAD51, and EGFR–ZCCHC6 7, 8. EGFR–SEPT14 fusion was first reported in glioblastoma, the structure of which involved EGFR at the N terminus, providing a receptor tyrosine kinase domain that was fused to a coiled‐coil domain from SEPT14 9. EGFR–SEPT14 fusion was also identified in a 62‐year‐old never‐smoking female with lung adenocarcinoma 10. This patient responded to icotinib treatment and had no treatment‐related adverse events. One study showed that EGFR–SEPT14 fusion could activate signal transducer and activator of transcription 3 signaling, confer mitogen independence, and impart sensitivity to EGFR kinase inhibition 9. Here, by using a comprehensive NGS assay, we identified a rare EGFR–SEPT14 fusion in advanced colorectal adenocarcinoma. To our knowledge, this is the first report of EGFR–SEPT14 fusion identified in colorectal cancer.
Potential Strategies to Target the Pathway and Implications for Clinical Practice
Cancers with EGFR mutations usually depend on EGFR signaling for growth and survival and are often sensitive to EGFR‐targeted inhibitors 11. Given the function of EGFR in diverse cellular processes, two therapeutic approaches, including tyrosine kinase inhibitors (TKIs) and monoclonal antibodies, are currently being developed and employed for targeting EGFR in various human cancers 12. It has been confirmed that patients with cancer have shown benefit from EGFR‐targeted agents, including non‐small cell lung cancer, colorectal cancer, squamous cell carcinoma of the head and neck, and breast cancer 13, 14, 15, 16, 17.
As a first‐generation EGFR‐mutant‐selective TKI, erlotinib was approved by the Food and Drug Administration for patients with metastatic non‐small cell lung cancer whose tumors have EGFR exon 19 deletions or exon 21 (L858R) substitution mutations 18. Preclinical studies have shown that EGFR–SEPT14 fusion was sensitive to the EGFR inhibitor erlotinib 9. Four cases with lung cancer harboring EGFR–RAD51 fusion showed significant clinical benefit from treatment with erlotinib 7.
Patient Update
On May 4, 2018, a CT scan showed enlarged lymph nodes in the retroperitoneum and mesentery (Fig. 1C), and given that the patient was carrying EGFR–SEPT14 fusion, he started erlotinib (150 mg, once daily) therapy. A CT scan showed a reduction of para‐aortic lymph nodes on May 24, 2018 (Fig. 1D), indicating a partial response. Of note, his rising carcino‐embryonic antigen reduced from 13.02 ng/mL to 7.98 ng/mL during erlotinib treatment. However, during a re‐examination in July 2018, the result of the CT scan suggested PD (Fig. 1E–G). NGS was performed for mediastinal lymph nodes, and a new gene variant, EGFR variant III (EGFRvIII), was detected. As a common EGFR genomic alteration, EGFRvIII results from an in‐frame deletion of exons 2–7 (801 bp) of EGFR 19. It has been shown that EGFRvIII can activate antiapoptotic signals through the PI3K–Akt signaling pathway, which plays a critical role for cell survival, proliferation, and motility 20. EGFRvIII is found in many human cancers, including glioblastomas, lung cancer, and head and neck cancer 21, 22, 23, but is rare in colorectal cancer 24, 25, 26. EGFRvIII is highly oncogenic, and its expression confers resistance to EGFR TKIs. EGFRvIII can regulate resistance to erlotinib in EGFR‐amplified glioblastoma via an increase in PI3Kp110δ 27. A study reported that patients with glioblastomas with EGFRvIII mutant had worse survival after treatment with erlotinib 21, suggesting that EGFRvIII mutation may be involved in erlotinib resistance. Taken together, the new gene variant, EGFRvIII, might be responsible for erlotinib resistance of this patient.
Conclusion
The current report described a Chinese patient with a rare EGFR–SEPT14 fusion and EGFR amplification who showed an antitumor response from treatment with the EGFR TKI erlotinib. EGFRvIII mutation might contribute to erlotinib resistance.
Glossary of Genomic Terms and Nomenclature
- Fusion/Rearrangement
Recombination of two unlinked segments of human DNA, exhibited as sequencing reads uniquely aligned to two different genes or tow apart DNA segments.
Author Contributions
Conception/design: Yong Li, Hai‐Bo Zhang
Provision of study material or patients: Xian Chen, Xiaobing Yang
Collection and/or assembly of data: Yong Li, Hai‐Bo Zhang, Yongsong Ye, Tanios Bekaii‐Saab, Yaojie Zheng, Yihong Zhang
Data analysis and interpretation: Yong Li, Xian Chen, Xiaobing Yang, Yongsong Ye, Tanios Bekaii‐Saab, Yaojie Zheng, Yihong Zhang
Manuscript writing: Yong Li, Hai‐Bo Zhang
Final approval of manuscript: Yong Li, Hai‐Bo Zhang, Xian Chen, Xiaobing Yang, Yongsong Ye, Tanios Bekaii‐Saab, Yaojie Zheng, Yihong Zhang
Disclosures
Yaojie Zheng: OrigiMed (E); Yihong Zhang: OrigiMed (E). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Acknowledgments
Informed consent was obtained from the patient.
Disclosures of potential conflicts of interest may be found at the end of this article.
Footnotes
For Further Reading: Andrea Sartore‐Bianchi, Alessio Amatu, Luca Porcu et al. “HER2 Positivity Predicts Unresponsiveness to EGFR‐Targeted Treatment in Metastatic Colorectal Cancer.” The Oncologist 2019;24:1395–1402.
Implications for Practice: Patients with HER2‐amplified/overexpressed metastatic colorectal cancer (mCRC) harbor a driver actionable molecular alteration that has been shown in preclinical models to hamper efficacy of the anti‐epidermal growth factor receptor (EGFR) targeted therapies. The present study confirmed that this molecular feature was associated with worse objective tumor response and shorter progression‐free survival in response to previous anti‐EGFR therapies. Moreover, it was found that the occurrence of this biomarker is unlikely to be predicted based on main clinicopathological features. Therefore, HER2 status assessment should be included in the molecular diagnostic workup of all mCRC for speedy referral to clinical trials encompassing HER2‐targeted double blockade independently of previous anti‐EGFR treatment.
References
- 1. Tang B, Yan X, Sheng X et al. Safety and clinical activity with an anti‐PD‐1 antibody JS001 in advanced melanoma or urologic cancer patients. J Hematol Oncol 2019;12:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hartwell LH. Genetic control of the cell division cycle in yeast. IV. Genes controlling bud emergence and cytokinesis. Exp Cell Res 1971;69:265–276. [DOI] [PubMed] [Google Scholar]
- 3. Shinoda T, Ito H, Sudo K et al. Septin 14 is involved in cortical neuronal migration via interaction with septin 4. Mol Biol Cell 2010;21:1324–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Peterson EA, Petty EM. Conquering the complex world of human septins: Implications for health and disease. Clin Genet 2010;77:511–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Holbro T, Hynes NE. ERBB receptors: Directing key signaling networks throughout life. Annu Rev Pharmacol Toxicol 2004;44:195–217. [DOI] [PubMed] [Google Scholar]
- 6. Stransky N, Cerami E, Schalm S et al. The landscape of kinase fusions in cancer. Nat Commun 2014;5:4846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Konduri K, Gallant JN, Chae YK et al. EGFR fusions as novel therapeutic targets in lung cancer. Cancer Discov 2016;6:601–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zehir A, Benayed R, Shah RH et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat Med 2017;23:703–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Frattini V, Trifonov V, Chan JM et al. The integrated landscape of driver genomic alterations in glioblastoma. Nat Genet 2013;45:1141–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Xu C, Wx Wang, Chen Y et al. Prevalence of EGFR gene fusions in a large cohort of Chinese patients with non‐small cell lung cancer (NSCLC). J Clin Oncol 2018;36:e13538–e13538. [Google Scholar]
- 11. Tracy S, Mukohara T, Hansen M et al. Gefitinib induces apoptosis in the EGFRl858R non‐small‐cell lung cancer cell line H3255. Cancer Res 2004;64:7241–7244. [DOI] [PubMed] [Google Scholar]
- 12. Yamaoka T, Ohba M, Ohmori T. Molecular‐targeted therapies for epidermal growth factor receptor and its resistance mechanisms. Int J Mol Sci 2017;18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bareschino MA, Schettino C, Troiani T et al. Erlotinib in cancer treatment. Ann Oncol 2007;18(suppl 6):vi35–vi41. [DOI] [PubMed] [Google Scholar]
- 14. Giaccone G, Gonzalez‐Larriba JL, van Oosterom AT et al. Combination therapy with gefitinib, an epidermal growth factor receptor tyrosine kinase inhibitor, gemcitabine and cisplatin in patients with advanced solid tumors. Ann Oncol 2004;15:831–838. [DOI] [PubMed] [Google Scholar]
- 15. Petrelli F, Borgonovo K, Cabiddu M et al. Cetuximab and panitumumab in KRAS wild‐type colorectal cancer: A meta‐analysis. Int J Colorectal Dis 2011;26:823–833. [DOI] [PubMed] [Google Scholar]
- 16. Petrelli F, Barni S. Anti‐EGFR‐targeting agents in recurrent or metastatic head and neck carcinoma: A meta‐analysis. Head Neck 2012;34:1657–1664. [DOI] [PubMed] [Google Scholar]
- 17. Rocha‐Lima CM, Soares HP, Raez LE et al. EGFR targeting of solid tumors. Cancer Control 2007;14:295–304. [DOI] [PubMed] [Google Scholar]
- 18. Khozin S, Blumenthal GM, Jiang X et al. U.S. Food and Drug Administration approval summary: Erlotinib for the first‐line treatment of metastatic non‐small cell lung cancer with epidermal growth factor receptor exon 19 deletions or exon 21 (L858R) substitution mutations. The Oncologist 2014;19:774–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wikstrand CJ, Hale LP, Batra SK et al. Monoclonal antibodies against EGFRvIII are tumor specific and react with breast and lung carcinomas and malignant gliomas. Cancer Res 1995;55:3140–3148. [PubMed] [Google Scholar]
- 20. Li B, Yuan M, Kim IA et al. Mutant epidermal growth factor receptor displays increased signaling through the phosphatidylinositol‐3 kinase/AKT pathway and promotes radioresistance in cells of astrocytic origin. Oncogene 2004;23:4594–4602. [DOI] [PubMed] [Google Scholar]
- 21. van den Bent MJ, Brandes AA, Rampling R et al. Randomized phase II trial of erlotinib versus temozolomide or carmustine in recurrent glioblastoma: EORTC brain tumor group study 26034. J Clin Oncol 2009;27:1268–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ji H, Zhao X, Yuza Y et al. Epidermal growth factor receptor variant III mutations in lung tumorigenesis and sensitivity to tyrosine kinase inhibitors. Proc Natl Acad Sci U S A 2006;103:7817–7822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chau NG, Perez‐Ordonez B, Zhang K et al. The association between EGFR variant III, HPV, p16, c‐MET, EGFR gene copy number and response to EGFR inhibitors in patients with recurrent or metastatic squamous cell carcinoma of the head and neck. Head Neck Oncol 2011;3:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Peciak J, Stec WJ, Treda C et al. Low incidence along with low mRNA levels of EGFRvIII in prostate and colorectal cancers compared to glioblastoma. J Cancer 2017;8:146–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Khelwatty SA, Essapen S, Bagwan I et al. Co‐expression and prognostic significance of putative CSC markers CD44, CD133, wild‐type EGFR and EGFRvIII in metastatic colorectal cancer. Oncotarget 2019;10:1704–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Spindler KL, Olsen DA, Nielsen JN et al. Lack of the type III epidermal growth factor receptor mutation in colorectal cancer. Anticancer Res 2006;26:4889–4893. [PubMed] [Google Scholar]
- 27. Schulte A, Liffers K, Kathagen A et al. Erlotinib resistance in EGFR‐amplified glioblastoma cells is associated with upregulation of EGFRvIII and PI3Kp110delta. Neuro Oncol 2013;15:1289–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]


