Abstract
A number of important drugs used to treat cancer—many of which serve as the backbone of modern chemotherapy regimens—have outdated prescribing information in their drug labeling. The Food and Drug Administration is undertaking a pilot project to develop a process and criteria for updating prescribing information for longstanding oncology drugs, based on the breadth of knowledge the cancer community has accumulated with the use of these drugs over time. This article highlights a number of considerations for labeling updates, including selecting priorities for updating; data sources and evidentiary criteria; as well as the risks, challenges, and opportunities for iterative review to ensure prescribing information for oncology drugs remains relevant to current clinical practice.
Keywords: Cancer, Drug prescriptions, Drug legislation, Drug approval, Pharmaceutical research
Short abstract
The U.S. Food and Drug Administration is undertaking a pilot project to develop a process and criteria for updating prescribing information for long‐standing oncology drugs. This article highlights a number of considerations for labeling updates.
Introduction
A number of important drugs used to treat cancer—many of which serve as the backbone of modern chemotherapy regimens—have outdated information in their drug labeling 1. This prescribing information is intended to be an authoritative resource to ensure clinicians are equipped with the information necessary to safely and effectively prescribe drugs to their patients. Outdated drug labeling poses a public health challenge: it undermines the U.S. Food and Drug Administration (FDA) mission to protect public health by ensuring drug safety and efficacy. Thus, the FDA is undertaking a pilot project named Project Renewal to develop a process and criteria for updating prescribing information for longstanding oncology drugs. The process and criteria will leverage the breadth of knowledge the cancer community has accumulated with the use of these drugs over time, often spanning decades of experience.
The FDA requested that the National Academies of Sciences, Engineering, and Medicine convene experts to discuss the challenges and opportunities to update labeling for oncology drugs that are inconsistent with the current evidence base and use in clinical practice. The National Academies’ National Cancer Policy Forum, in collaboration with the Forum on Drug Discovery, Development, and Translation, held a meeting on this topic in March of 2019 2. This article highlights a number of considerations for labeling updates, such as selecting priorities; data sources and evidentiary criteria, including labeling updates for special populations; and the risks, challenges, and opportunities for iterative review, to ensure prescribing information for oncology drugs remains relevant to current clinical practice.
Challenges of Outdated Oncology Drug Labeling
Oncology drug labeling serves several purposes. First, drug labeling is the legal agreement between the federal government and the sponsor of a new drug application (NDA) or a biologics license application (BLA) regarding the drug's prescribing information and represents a license to market the drug for approved uses. Second, drug labeling is intended to provide clinicians with the information necessary for the safe and effective use of a drug 3. Friends of Cancer Research noted that drug labeling, when kept up‐to‐date, is “the most authoritative drug‐related information that is available to prescribers” 4.
However, the prescribing information for a number of longstanding oncology drugs has become outdated and many remain in an older format, making it difficult for prescribers and pharmacists to find important information. The format of drug labeling was revised according to the 2006 Physician Labeling Rule, but drugs that were approved prior to June 2001 were not required to have their drug labeling modernized 5.
A major reason for outdated labeling is the speed and volume at which new information that emerges about drugs in the postmarket setting. Following a drug's approval, research and experience with the drug may indicate revised dosing and administration schedules, or they may provide important information about pharmacology, side effects, safety concerns, and drug performance in special populations (e.g., patients with pediatric cancers or patients with comorbidities or organ dysfunction). Drug indications can also become outdated. Prescribing information for some drugs may contain indications that are no longer supported by the evidence base, and others may not include new indications that would be considered standard of care by the oncology community.
Outdated labeling is particularly problematic in the context of oncology care, because many chemotherapy treatment protocols involve combination therapies. New agents are often used in combination with well‐established drugs, but the labeling of older drugs is generally not updated to reflect those uses. There is also a dearth of information in drug labeling about use in special populations.
Most changes to drug labeling occur at the discretion of a drug manufacturer. However, certain disincentives—such as the cost of preparing supplemental applications or generic competition once a drug goes off patent—may discourage drug manufacturers from pursuing labeling changes 1. Differing regulatory requirements for reference listed drugs (RLDs; i.e., brand‐name drugs) and generic drugs contribute to outdated and inaccurate labeling over time. Generic drugs achieve FDA approval through an abbreviated new drug application (ANDA) and are required to have the same labeling as the RLD at the time of the ANDA approval, with certain exceptions 6, 7, 8. Manufacturers of generic drugs are not able to independently update product labeling without jeopardizing their generic status, because of the “sameness” requirement under the Hatch‐Waxman Amendments to the Federal Food, Drug, and Cosmetic Act. The act mandates that generics have the same active ingredients, strength, dosage, indications, and safety labeling as the RLD. In 2013, the FDA issued a proposed rule to permit generic manufacturers to distribute revised product labeling that differs in certain respects, on a temporary basis, from the labeling of its RLD upon submission to the FDA of a “changes being effected” supplement 9. However, the FDA has not yet issued a final rule.
It is not uncommon for the RLD to be discontinued or withdrawn for reasons other than safety or effectiveness, especially for older products 4. When the RLD has been withdrawn, labeling updates are not initiated by the RLD holder. When an RLD has been discontinued, the RLD holder is required to continue to update the labeling, but is unlikely to be motivated to submit supplemental NDAs for additional indications. Furthermore, the generic drug industry may not have the infrastructure or business model to submit efficacy supplements based on updated evidence.
Project Renewal
A white paper from Friends of Cancer Research noted that outdated prescribing information leads clinicians to rely on sources other than drug labeling to guide their decision making for patient treatment. It also reduces the FDA's ability to convey accurate and reliable information about drugs to patients and clinicians 1, 4. Project Renewal aims to provide clinicians and their patients with the most accurate drug labeling by developing a process and criteria for updating prescribing information for longstanding oncology drugs, based on the breadth of experience the cancer community has accumulated with using these drugs. The FDA has noted that this pilot is not intended to expand indications for drugs on patent or with exclusivity; to review all possible indications for a particular drug; or to affect cost, payment, or coverage decisions.
The FDA is planning to review and update prescribing information for approximately 30–40 longstanding oncology drugs. There are 17 drug labeling sections that may need to be updated for such drugs (Fig. 1). One example of an oncology drug whose prescribing information was recently updated is fluorouracil. The prescribing information was reorganized into the new format, and several sections were updated, including the following changes 10: dosing and administration: indications were reorganized and clarified, and dosing and administration for each indication were updated; safety: contraindications were revised and new warnings were added for several common toxicities associated with warfarin use, such as cardiac and neurologic toxicity; clinical pharmacology: a warning was added regarding increased risk among individuals who have low or absent activity of a specific enzyme; and an outdated black box warning was removed.
Figure 1.

Full prescribing information: Contents, from the FDA Guidance for industry: Labeling for human prescription drug and biological products 26.
For a new indication to be added to drug labeling, the FDA is required to assess whether there is substantial evidence of efficacy, defined as:
Evidence consisting of adequate and well‐controlled investigations, including clinical investigations, by experts qualified by scientific training and experience to evaluate the effectiveness of the drug involved, on the basis of which it could fairly and responsibly be concluded by such experts that the drug will have the effect it purports or is represented to have under the conditions of use prescribed, recommended, or suggested in the labeling or proposed labeling thereof 11.
Five elements of substantial evidence include 10: quantity of evidence (number of clinical trials and supportive evidence); quality of evidence (adequate and well‐controlled clinical trials); magnitude and statistical persuasiveness of the result (e.g., large, statistically significant overall survival improvement); source of evidence (controlled clinical trials, real‐world evidence, published literature); and context (rarity of the disease, unmet need, safety and efficacy data across diseases).
This article describes several factors to consider when assessing the evidence base for labeling updates, including which labeling sections are priorities for updates; what sources of information and study designs could be reviewed; the quality, quantity, and types of evidence that would be considered adequate to update different sections of drug labeling; and challenges and risks to developing a process and criteria for updating drug labeling.
Selecting Priorities for Labeling Updates
Numerous sections of drug labeling could be prioritized for updating. One of the factors that could be considered is whether current information is accessible in an alternate source separate from drug labeling. For example, guidance about off‐label uses of oncology drugs is widely available in various drug compendia. Therefore, updating the indication section may be of lower priority than other labeling sections. Instead, updating important information about safety or use in special populations or ensuring that dosage and administration information is current may be higher priorities. In particular, specifying dose modification strategies that mitigate toxicity while preserving efficacy, or how dosage should be modified based on a patient's comorbidities, may be useful updates to drug labeling. New risks, particularly those severe enough to warrant a warning, or observed drug‐drug interactions would also constitute critical information to update. Another priority is information about use in pediatric, pregnant, or older adult cancer populations, especially given the limited drug development activity focused on these populations. Other areas of emphasis include updated pharmaceutical and admixture data and additional pharmacology information. For example, food effect and organ impairment studies may provide important information for prescribers.
Sources of Information and Study Designs
One of the challenges to updating drug labeling is that much of the data that can be used to update prescribing information for longstanding drugs have not been generated with this purpose in mind. Drug sponsors typically collect data with the intent to submit it to the FDA to review as an NDA, BLA, or supplemental application. However, the data that are available to update longstanding oncology drugs may have been collected by academic investigators, cooperative clinical trial groups, cancer centers, or other stakeholders and may vary in the quality and scientific rigor used to collect them, in quantity, and in completeness. Although these distinctions are important to acknowledge, it is also important to recognize that the oncology community has had years—and sometimes decades—of experience with these drugs, which may help to address some of the scientific gaps in knowledge.
Project Renewal has focused on identifying and codifying existing data and considering how to best evaluate and use this information to update drug labeling. Multiple sources of data exist, and some may be more useful than others for this project. Thus, it will be important to consider all potential data sources that could inform a decision to update labeling. The FDA may place greater reliance on certain sources, but it is important to identify and consider all sources of data, unless it can be concluded that specific data are irrelevant or inaccurate. The following potential data sources and study designs could be considered: meta‐analyses of randomized phase II and phase III clinical trials (study‐level data); randomized controlled clinical trials; prospective, noncomparative clinical trials (e.g., phase II studies); real‐world data (electronic health records, registry data, administrative claims data, adverse events reporting databases); case series based on historical control groups; “case‐control” observational studies; modeling and computer simulation data; case series with literature controls; case series without controls; and case reports.
In addition to reviewing the types of data sources that may be used to update drug labeling, there are other factors that need to be considered to determine whether data is fit‐for‐purpose for a proposed labeling update (Table 1). These considerations can also be useful when compiling information from multiple data sources or multiple studies and are conceptually similar to the checklist for ensuring real‐world data are appropriate for regulatory applications 12.
Table 1.
Examples of factors to consider to determine whether data is fit‐for‐purpose for a labeling update
| Factors | Questions to Consider |
|---|---|
|
Volume of data |
How many data are available to consider a proposed labeling update? Do the data come from one source or multiple sources? Is the volume of data sufficient to draw an appropriate conclusion, acknowledging that this decision may vary depending on the population under consideration (e.g., a lower volume of data may be appropriate for rare cancers or special populations, such as pediatric patients with cancer)? |
|
Volume of experience |
What is the clinical familiarity with the drug? How many years has the drug been in use? Is the proposed labeling update considered standard of care in oncology practice? Is the experience with the drug reflective of modern oncology practice, or is the experience reflective of historical practice? |
|
Longitudinal follow‐up |
Are data available to assess patient outcomes over extended periods of time? Is longitudinal follow‐up reflective of current oncology practice? |
|
Disparate studies |
Are there conflicting studies that call into question a proposed labeling update? How do the quality and quantity of conflicting studies compare to those that support a labeling update? |
|
Quality of the data |
What is the rigor of the study design? Is the proportion of missing data reasonable? What is the follow‐up period? What are the number of events for the primary endpoint? Can data quality be systematically assessed? |
| Relevance of the data |
Are the data available directly related to the proposed labeling update, or are the data being extrapolated from a differing population or geographic region, or clinical question? Are the data able to be directly derived from the drug, as opposed to a drug combination? |
| Risk of being incorrect | If the labeling update is later determined to be wrong, what are the clinical consequences? |

As a general approach, it would be beneficial to review the totality of the evidence when updating prescribing information for longstanding drugs, rather than specifying which data sources may be in scope versus out of scope.
Evidentiary Processes and Criteria
Prospectively specifying criteria that are necessary to update labeling for longstanding oncology drugs and making these criteria transparent to the cancer community would help advance the goal of keeping labels accurate. Because of the rigor required for labeling updates and the consequent impact on prescribing practices, it is understandable that drug labeling will continue to have a conservative bias. The updated prescribing information will not necessarily be inclusive of all possible indications, nor will it reflect all available information on dosing, administration, safety, pharmacology, or other information contained in drug labeling sections.
Numerous evidence assessment methodologies and processes that can inform the these labeling updates, including those used in the development of clinical practice guidelines, drug compendia, and reporting guidelines, such as those compiled by the EQUATOR Network 13. Compendia and clinical practice guidelines represent a synthesis of available information to inform decision making in oncology practice. These resources are compiled in numerous ways according to various criteria, and they often involve incorporation of expert opinion. Because of varying degrees of scientific rigor used within these resources, they may serve as a necessary, but not sufficient, step to identifying potential updates to prescribing information for longstanding oncology drugs. It is also important to recognize that the purpose of clinical practice guidelines and compendia is to inform important clinical questions (e.g., selection of optimal adjuvant chemotherapy and targeted therapy for patients with early stage breast cancer), and not to evaluate one specific oncology drug 14.
Another key component of a high‐quality evidence review process is ensuring appropriate content and process expertise. This requires the engagement of trained systematic reviewers with oncology care knowledge, statisticians, and content experts.
It is likely that updates of certain labeling sections will require more data of higher quality than others. New indications, which need to meet the substantial evidence requirement, will require a high degree of supporting information. The threshold to update safety information in drug labeling may be lower than that for updating a new indication. However, one caveat may be that if a safety signal comes with a recommended course of action (e.g., if a specific adverse event is observed, the dose should be modified), the evidence to support the recommendation needs to be strong to ensure confidence that the strategy is effective in mitigating toxicity while preserving acceptable efficacy. This also ensures that unreliable information about potential harms does not undermine the effectiveness of the treatment.
Figure 2 illustrates the authors’ thinking on which specific sources of information are likely to best inform labeling updates for different types of information, such as indications, dosing, administration, safety, and use in special populations. The data sources are organized conceptually in order of their comparative scientific strength (although the authors recognize that the hierarchy may not be static—e.g., a large, appropriately powered, and well‐designed clinical trial may be stronger evidence than a less‐rigorous meta‐analysis). Because many of these decisions will be subjective and context dependent, Figure 2 classifies the utility as high, medium, and low.
Figure 2.
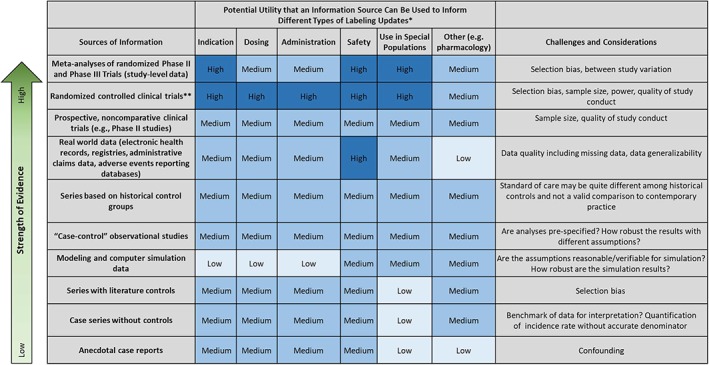
Potential utility of an information source to inform different types of labeling updates*High utility = dark blue; medium utility = blue; low utility = light blue. This table represents a general schematic. Although utility has been visualized as three distinct categories, there can be substantial variability within each category. Further work could refine the utility of different information sources by using a Likert scale. In addition, the quality of the sources of information can vary widely, which could affect assessment of the strength of evidence. **Randomized controlled clinical trials include phase III and some phase II studies. Hierarchy of Strength of Evidence draws on the conceptual framing from Green SB, Byar DP. Using observational data from registries to compare data: The fallacy of omnimetrics. Stat Med 1984;3:361–373 27.
Special Populations
There is a paucity of information about the use of oncology drugs in special populations, including pediatric patients with cancer, older adults, individuals with comorbidities or organ dysfunction, and pregnant women 15, 16, 17. Eligibility criteria in oncology clinical trials often limit participation of special populations in clinical research, particularly very young children or those with very rare diseases 17, 18. However, more recent pediatric oncology clinical trials, particularly for newly diagnosed patients, tend to have broader eligibility criteria. The rarity of certain cancers also contributes to limited information about how drugs perform in these contexts. To help address this challenge, FDA has developed several draft guidances to encourage researchers and drug developers to broaden eligibility criteria and to provide strong scientific rationale for limiting participation on clinical trials 19, 20, 21.
A majority of children with cancer are treated in academic medical centers using drug combination strategies that stem from clinical trial protocols. However, most children are treated off label, owing to the limited number of oncology drugs approved for pediatric indications 22. There are a number of scientific challenges that contribute to limited pediatric cancer drug development, including a lack of understanding drug metabolism in a variety of age cohorts; limited availability of pediatric‐friendly formulations of the drug; a lack of understanding of the acute and long‐term toxicities in the context of normal development; determination of adequate central nervous system penetration in specific disease contexts (e.g., central nervous system tumors, meningeal leukemia); and scarcity of population pharmacokinetic data among children with cancer.
Data to inform pediatric prescribing are an important drug labeling need. The rareness of pediatric cancers limits the availability of randomized controlled clinical trial data that could inform drug labeling.
Oftentimes, research evaluating how oncology drugs perform in older patients and those who have comorbidities or organ dysfunction (which are common conditions among older adults) is conducted following FDA approval and therefore not included in initial drug labeling. However, this information is critical to include in labeling because patients who participate in clinical trials are often not representative of the broader population of patients treated in community oncology practice. Clinical trial participants tend to be younger and healthier, but older adults—including those who have comorbidities or organ dysfunction—often respond differently to treatments than do younger patients. Older patients are also more sensitive to drug toxicities and side effects 16. In addition, chronological age can be an inadequate method for characterizing individuals, and clinicians often perform geriatric assessments to determine whether a patient is fit, vulnerable, or frail. If the assessment suggests a patient is frail, clinicians may modify the dose, schedule, or components of a cancer regimen so that the regimen is better tolerated. For example, a frail, older adult patient may be prescribed a two‐drug combination rather than a three‐drug combination. To better inform prescribing decisions for these populations, pharmacokinetic and adverse event data, as well as effective dose and schedule modification strategies, are particularly important to review for labeling updates.
For patients with rare cancers and those who have comorbidities and organ dysfunction, real‐world data may be a particularly important resource to consider when updating drug labeling. Leveraging real‐world data for labeling updates is consistent with ongoing initiatives to use real‐world evidence to inform regulatory decision making, including a draft FDA guidance on the topic 23 and ongoing research assessing the potential validity of endpoints derived from real‐world data 24.
Challenges and Risks Related to Updating Prescribing Information
A primary objective of developing a process and criteria to update drug labeling is to overcome a common challenge in drug development: after FDA approval, a substantial amount of evidence about oncology drugs is accrued in the postmarket setting, but oftentimes this knowledge is not reflected in drug labeling. Over time, this has resulted in important, well‐established oncology drugs with outdated prescribing information.
Correcting outdated prescribing information presents many risks and challenges. One concern is whether this process will add important new prescribing information or whether the updates will primarily be directed at organization and formatting to meet the requirements of the 2006 Physician Labeling Rule. There is an inherent tension in updating labeling: a reluctance to update prescribing information without a sufficient level of evidence versus the need to use data that were never collected with this purpose in mind. This tension may result in relatively few updates. Differing evidence requirements for distinct labeling sections also adds complexity to this process.
The availability of information to update safety in drug labeling may be an additional challenge. Much of the safety information stems from uncontrolled data sources, such as registries. However, low rates of reporting through MedWatch and other safety registries may restrict the information available to guide labeling updates. Attribution of safety events to a particular oncology drug in a combination regimen may be difficult. For longstanding oncology drugs, some safety data may also be irrelevant to modern oncology practice, because of advances in supportive care and strategies to prevent and manage side effects.
Another challenge is the pace of oncology research. Even if the prescribing information for a specific drug is updated, new research—such as results of pivotal clinical trials presented at scientific conferences—can necessitate additional labeling changes. The speed at which data are acquired in the postmarket setting makes keeping prescribing information up to date difficult. The need for labeling changes, coupled with the time it takes to update labeling, is likely to require significant resources. Thus, it is important to determine whether efforts to update labeling are sustainable and scalable. Is it possible to continue updates to longstanding oncology drugs, given the enormous workload and resources needed to identify outdated prescribing information, review the literature to determine whether and how labeling should be updated, and then prepare and vet the updates? Is this process scalable to update prescribing information for drugs in all therapeutic areas?
Future Directions and Opportunities: Toward Iterative Review and Updating
The FDA is intended to be an impartial, authoritative source of information about oncology drugs. The FDA's process of reviewing data is trusted, rigorous, transparent, and conducted by expert reviewers with no financial ties to industry 14, 25. Data provided in drug labeling are also freely and publicly available to practicing clinicians, whereas information in the published literature may be inaccessible, because of article firewalls and journal subscription fees 25. Thus, establishing a process to iteratively review and update drug labeling serves an important public health objective.
Richard Pazdur, director of the FDA Oncology Center of Excellence, has noted that the drug label “is a living document. Many people have the misconception that the history of the drug ends with the approval of the drug. Really, that is just the beginning….We have to keep that in mind and also have a process of updating these labels.”
Given the speed and volume of new advances in cancer research and drug development, it is unlikely that drug labeling could keep pace. However, FDA could engage with leaders in the oncology community to prioritize drugs that are in need of updated labeling. In addition, important new research studies involving longstanding oncology drugs could serve as triggers to update labeling for specific drugs. Soliciting requests from the public—including oncologists, pharmacists, nurses, patients, and other stakeholders—could also serve as a mechanism to update specific information in drug labeling.
Another potential opportunity is to explicitly list when the prescribing information for a specific drug was last updated. This information could provide important insights to oncology clinicians, especially given the challenge of keeping prescribing information updated.
Novel data sources and analytic methodologies could serve as critical inputs to updating oncology drug labels in the future. Leveraging data collected in real‐world settings could provide important information on the effectiveness and safety of drugs as used in clinical practice, including use in patients with rare cancers, comorbidities, and organ dysfunction.
Glossary of Terms
Compendia: Privately owned pharmaceutical reference guides that are used to inform coverage decisions for oncology drugs that are used in an off label context.a
Discontinued drug: As defined by the FDA, a drug product that has never been marketed, has been discontinued from marketing, is for military use, is for export only, or has had its approval withdrawn for reasons other than safety or efficacy after being discontinued from marketing.b Informally, discontinued drugs are often products that are not marketed, but the relevant new drug application (NDA) is still “approved” by the FDA, such that the applicant could recommence marketing at any time.
Drug labeling: The prescribing information for a prescription drug.
Indication: The disease context in which a drug has approved for use by the FDA (e.g., cancer site, stage, and line of therapy). Oncology clinicians may prescribe drugs “on label” (according to the indications specified in the prescribing information) or “off label” (i.e., for diseases that are not specified in the prescribing information). Many off‐label uses of oncology drugs are considered standard of care and are supported by clinical trials, drug compendia, or clinical practice guidelines. However, some off‐label uses are supported by stronger evidence than others.c
Withdrawn: A drug whose NDA is no longer considered approved by the FDA such that the product cannot be lawfully marketed.d
Notes:
aGreen, AK, Wood WA, Basch EM. Time to reassess the cancer compendia for off‐label drug coverage in oncology. JAMA 2016;316:1541–1542.
bFood and Drug Administration. 2019. Approved drugs with therapeutic equivalence evaluations, 39th ed. Washington, DC. Available at https://www.fda.gov/media/71474/download. Accessed August 14, 2019.
cConti, RM, Bernstein AC, Villaflor VM et al. Prevalence of off‐label use and spending in 2010 among patent‐protected chemotherapies in a population‐based cohort of medical oncologists. J Clin Oncology 2013;31:1134–1139.
dU.S. Food and Drug Administration. 2018. 21 C.F.R. § 314.150(a).
Disclosures
Erin Balogh: Salary support from the sponsors of the National Cancer Policy Forum (RF), which includes Bristol Myers Squibb, Flatiron Health, Helsinn Therapeutics, Novartis Oncology, Pfizer (RF–institution); R. Donald Harvey: AbbVie, Amgen, Arqule, AstraZeneca, Bristol‐Myers Squibb, Boston Biomedical, Calithera, Celgene, Corvus, Eli Lilly & Co., Five Prime Therapeutics, Genmab, Halozyme, Ignyta, Incyte, Meryx, Nektar, Pfizer, Regeneron, Rgenix, Sanofi, Syndax, Takeda, Tesaro, Vertex, Xencor (RF); Takeda, Bristol‐Myers Squibb, GlaxoSmithKline (C/A); Rebecca Miksad: Flatiron Health (E); Sharyl J. Nass: partial salary support from the sponsors of the National Cancer Policy Forum, which includes Bristol‐Myers Squibb, Flatiron Health, Helsinn Therapeutics, Merck, Novartis, Pfizer (RF); Richard L. Schilsky: Astra‐Zeneca, Bayer, Boehringer‐Ingelheim, Bristol Myers Squibb, Eli Lilly & Co., Genentech, Merck, Pfizer (RF–institution). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Acknowledgments
We thank Peter Adamson and Robert Carlson for their review and input on the draft manuscript. We also thank the participants for their contributions to the March 2019 meeting on Updating Labels for Generic Oncology Drugs meeting.
The authors are responsible for the content of this article, which does not necessarily represent the views of the National Academies of Sciences, Engineering, and Medicine.
The Food and Drug Administration provided support to the National Academies of Sciences, Engineering, and Medicine to convene experts on March 26, 2019 to discuss the challenges and opportunities to update labeling.
Disclosures of potential conflicts of interest may be found at the end of this article.
References
- 1. Shea MB, Stewart M, Van Dyke H et al. Outdated prescription drug labeling: How FDA‐approved prescribing information lags behind real‐world clinical practice. Ther Innov Regul Sci 2018;52:771–777. [DOI] [PubMed] [Google Scholar]
- 2. National Academies of Sciences , Engineering, and Medicine. Updating labels for generic oncology drugs. Available at http://nationalacademies.org/hmd/Activities/Disease/NCPF/2019-MARCH-26.aspx. Accessed September 4, 2019.
- 3. Dabrowska, A , Thaul S. How FDA approves drugs and regulates their safety and effectiveness. Washington, DC: Congressional Research Service, 2018. Available at https://fas.org/sgp/crs/misc/R41983.pdf. Accessed July 5, 2019. [Google Scholar]
- 4. Green A, House L, Shea M et al. Enhancing information about older prescription drugs. Washington, DC: Friends of Cancer Research, 2017. Available at https://www.focr.org/sites/default/files/pdf/Panel%203.pdf. Accessed July 17, 2019. [Google Scholar]
- 5. U.S. Food and Drug Administration . PLR requirements for prescribing information. Available at https://www.fda.gov/drugs/laws-acts-and-rules/plr-requirements-prescribing-information. Accessed July 21, 2019.
- 6.Federal Food, Drug, and Cosmetic Act, 21 U.S.C., § 355(j)(2)(A)(v).
- 7.Federal Food, Drug, and Cosmetic Act, 21 U.S.C., § 355(j)(4)(G).
- 8.CFR – Code of Federal Regulations Title 21, § 314.94(a)(8).
- 9. U.S. Food and Drug Administration . Supplemental applications proposing labeling changes for approved drugs and biological products. Federal Register 78(219):67985–67999. Available at https://www.govinfo.gov/content/pkg/FR-2013-11-13/pdf/2013-26799.pdf. Accessed July 5, 2019. [PubMed] [Google Scholar]
- 10. Kluetz P. Oncology Center of Excellence project renewal: Weighing evidence to update oncology drug labels. Presented at: National Academies of Sciences, Engineering, and Medicine meeting on Updating Labels for Generic Oncology Drugs. March 26, 2019; Washington, DC. Available at http://nationalacademies.org/hmd/~/media/Files/Activity%20Files/Disease/NCPF/2019-March%2026/Kluetz.pdf. Accessed July 30, 2019.
- 11.Federal Food, Drug, and Cosmetic Act, 21 U.S.C., § 505(d).
- 12. Miksad RA, Abernethy AP. Harnessing the power of real‐world evidence (RWE): A checklist to ensure regulatory‐grade data quality. Clin Pharmacol Ther 2017;103:202–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. EQUATOR Network. Enhancing the QUAlity and Transparency Of health Research. Available at http://www.equator-network.org/reporting-guidelines. Accessed August 12, 2019. [Google Scholar]
- 14. Basch E. Updating oncology drug labels: Lessons from related efforts. Presented at: National Academies of Sciences, Engineering, and Medicine, meeting on Updating Labels for Generic Oncology Drugs, March 26, 2019; Washington, DC. Available at http://nationalacademies.org/hmd/~/media/Files/Activity%20Files/Disease/NCPF/2019-March%2026/Basch.pdf. Accessed July 30, 2019.
- 15. Hepner A, Negrini D. Hase EA et al. Cancer during pregnancy: The oncologist overview. World J Oncol 2019;10:28–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Levit LA, Balogh EP, Nass SJ et al; Institute of Medicine. Delivering High‐Quality Cancer Care: Charting a Course for a System in Crisis. Washington, DC: The National Academies Press, 2013. [PubMed] [Google Scholar]
- 17. Kim ES, Bruinooge SS, Roberts S et al. Broadening eligibility criteria to make clinical trials more representative: American Society of Clinical Oncology and Friends of Cancer Research joint research statement. J Clin Oncology 2017;35:3737–3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nass SJ, Moses HL, Mendelsohn J, eds; Institute of Medicine. A National Clinical Trials System for the 21st Century: Reinvigorating the NCI Cooperative Group Program. Washington, DC: The National Academies Press, 2010. [PubMed] [Google Scholar]
- 19. U.S. Food and Drug Administration . Cancer clinical trial eligibility criteria: Minimum age for pediatric patients: Draft guidance. Available at https://www.fda.gov/regulatory-information/search-fda-guidance-documents/cancer-clinical-trial-eligibility-criteria-minimum-age-pediatric-patients. Accessed August 1, 2019.
- 20. U.S. Food and Drug Administration . Cancer clinical trial eligibility criteria: Patients with organ dysfunction or prior or concurrent malignancies: Draft guidance. Available at https://www.fda.gov/regulatory-information/search-fda-guidance-documents/cancer-clinical-trial-eligibility-criteria-patients-organ-dysfunction-or-prior-or-concurrent. Accessed August 1, 2019.
- 21. U.S. Food and Drug Administration . Considerations for the inclusion of adolescent patients in adult oncology clinical trials: Draft guidance. Available at https://www.fda.gov/regulatory-information/search-fda-guidance-documents/considerations-inclusion-adolescent-patients-adult-oncology-clinical-trials. Accessed August 1, 2019.
- 22. Gore L. Special considerations for labeling in pediatric oncology. Presented at National Academies of Sciences, Engineering, and Medicine meeting on Updating Labels for Generic Oncology Drugs. March 26, 2019; Washington, DC. Available at http://nationalacademies.org/hmd/~/media/Files/Activity%20Files/Disease/NCPF/2019-March%2026/Gore.pdf. Accessed July 30, 2019.
- 23. U.S. Food and Drug Administration . Submitting documents using real‐world data and real‐world evidence to FDA for drugs and biologics guidance for industry: Draft guidance. Available at https://www.fda.gov/regulatory-information/search-fda-guidance-documents/submitting-documents-using-real-world-data-and-real-world-evidence-fda-drugs-and-biologics-guidance. Accessed August 1, 2019.
- 24. Stewart M, Norden AD, Dreyer N et al. An exploratory analysis of real‐world end points for assessing outcomes among immunotherapy‐treated patients with advanced non‐small‐cell lung cancer. JCO Cancer Clin Informatics 2019;3:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Harvey RD. Generic oncology drug label updates: Implications for patient care. Presented at the National Academies of Sciences, Engineering, and Medicine meeting on Updating Labels for Generic Oncology Drugs. March 26, 2019; Washington, DC. Available at http://nationalacademies.org/hmd/~/media/Files/Activity%20Files/Disease/NCPF/2019-March%2026/Harvey.pdf. Accessed July 30, 2019.
- 26. U.S. Food and Drug Administration . Guidance for industry: Labeling for human prescription drug and biological products—Implementing the PLF content and format requirements. Available at https://www.fda.gov/media/71836/download. Accessed July 30, 2019.
- 27. Green SB, Byar DP. Using observational data from registries to compare data: The fallacy of omnimetrics. Stat Med 1984;3:361–373. [DOI] [PubMed] [Google Scholar]


