Abstract
Lung cancer remains the leading cause of cancer‐related death worldwide. Affected patients frequently experience debilitating disease‐related symptoms, including dyspnea, cough, fatigue, anxiety, depression, insomnia, and pain, despite the progresses achieved in term of treatment efficacy.
Physical activity and exercise are nonpharmacological interventions that have been shown to improve fatigue, quality of life, cardiorespiratory fitness, pulmonary function, muscle mass and strength, and psychological status in patients with lung cancer. Moreover, physical fitness levels, especially cardiorespiratory endurance and muscular strength, are demonstrated to be independent predictors of survival. Nevertheless, patients with lung cancer frequently present insufficient levels of physical activity and exercise, and these may contribute to quality of life impairment, reduction in functional capacity with skeletal muscle atrophy or weakness, and worsening of symptoms, particularly dyspnea.
The molecular bases underlying the potential impact of exercise on the fitness and treatment outcome of patients with lung cancer are still elusive. Counteracting specific cancer cells’ acquired capabilities (hallmarks of cancer), together with preventing treatment‐induced adverse events, represent main candidate mechanisms.
To date, the potential impact of physical activity and exercise in lung cancer remains to be fully appreciated, and no specific exercise guidelines for patients with lung cancer are available. In this article, we perform an in‐depth review of the evidence supporting physical activity and exercise in lung cancer and suggest that integrating this kind of intervention within the framework of a global, multidimensional approach, taking into account also nutritional and psychological aspects, might be the most effective strategy.
Implications for Practice
Although growing evidence supports the safety and efficacy of exercise in lung cancer, both after surgery and during and after medical treatments, most patients are insufficiently active or sedentary. Engaging in exercise programs is particularly arduous for patients with lung cancer, mainly because of a series of physical and psychosocial disease‐related barriers (including the smoking stigma). A continuous collaboration among oncologists and cancer exercise specialists is urgently needed in order to develop tailored programs based on patients’ needs, preferences, and physical and psychological status. In this regard, benefit of exercise appears to be potentially enhanced when administered as a multidimensional, comprehensive approach to patients’ well‐being.
Keywords: Exercise, Physical activity, Lung cancer, Comprehensive approach, Lifestyle intervention
Short abstract
The potential effect of physical activity in lung cancer is not fully understood, and no specific exercise guidelines for lung cancer patients are available. This article reviews the evidence supporting physical activity and exercise in lung cancer and suggests that this type of intervention, along with considerations for the nutritional and psychological aspects of such an intervention, might be the most effective strategy.
Introduction
Historically, patients with cancer were advised to rest, recover, and save energy, avoiding engaging in tiring physical activity. Nevertheless, starting in the late 1980s 1, new data progressively emerged, supporting the notion that physical activity (PA; defined as any bodily movement produced by skeletal muscles that results in energy expenditure) and exercise (EX; including only those planned, structured, and repetitive activities aimed at improving or maintaining one or more components of physical fitness) may provide relevant benefits in oncology. In cancer survivors, an inverse correlation between PA and mortality or recurrence rate was reported 2, 3, 4. Moreover, EX can play a beneficial role during and after oncological treatments, leading to clinically meaningful improvements in physical fitness (aerobic, strength, flexibility, and body composition) 5, 6, 7, quality of life (QoL) 8, treatment‐related side effects 5, 9, and psychological outcomes (such as anxiety, depression, self‐esteem, and energy level and vitality) 5. Nevertheless, the American College of Sport Medicine guidelines for EX in cancer are mainly directed to patients with breast, prostate, colon, gynecologic, and hematological cancer, and no universal recommendations are available for lung cancer.
Lung malignancies are the leading cause of cancer‐related death 10. Non‐small cell lung cancer (NSCLC) and small cell lung cancer (SCLC) are the main histological subtypes of lung cancer, and NSCLC accounts for around 85% of all cases. Despite meaningful recent diagnostic and therapeutic advances, the overall prognosis remains poor for affected patients. In terms of overall survival (OS) according to stage, the eighth edition of TNM classification for lung cancer showed an OS by clinical stage at 24 and 60 months ranging from 97% and 92% for patients at stage I to 10% and 0% for patients at stage IVB 11. Despite crucial progress obtained in terms of availability of innovative treatments (such as targeted therapy and immunotherapy), lung cancer remains associated with physical, psychological, and social difficulties, which exert a negative influence on patients’ QoL. Moreover, various cancer‐ and treatment‐related complications, such as dyspnea, muscle wasting, pain, fatigue, loss of appetite, and deterioration of physical fitness and lung function, may further impair patients’ status 12. All these outcomes have been suggested to be potentially ameliorable with EX, despite the lack of dedicated guidelines for lung cancer. Several biological mechanisms have been proposed in order to explain the link between cancer and EX. The main hypothesis and evidence include the control of chronic low‐grade inflammation and the modulation of metabolic dysregulation substances (e.g., insulin, glucose, and insulin‐like growth factors) and sex hormones. Moreover, it seems that PA and EX could have an impact on oxidative stress and immune‐related function, modifying some crucial mechanisms connected to tumor microenvironment (e.g., angiogenesis, proliferation, and apoptosis) 13.
In this article, we perform an in‐depth revision of available data investigating the role of PA and EX in patients with lung cancer undergoing surgical and/or medical treatments. Moreover, we analyze potential underlying biological mechanisms, peculiar to lung cancer oncogenesis, and suggest a structured, multidimensional way forward to definitively address the potential impact of PA and EX on lung cancer outcomes.
Materials and Methods
A comprehensive Pubmed and http://clinicaltrials.gov search was performed on July 24, 2019, to identify the published and ongoing studies exploring the role of PA and EX in lung cancer. The following keywords were used: exercise, physical activity, lung cancer, non‐small cell lung cancer, small cell lung cancer. In order to acquire a complete and in‐depth perspective on this emerging topic, all original articles (randomized clinical trials, nonrandomized controlled trials, and observational data) investigating PA and EX in lung cancer were considered. Abstracts not published in extenso, case reports, non‐English full texts, and theses were excluded. Participants’ inclusion criteria were adults affected by lung cancer, surgically treated, during or after medical therapies; animal studies were also considered. Regarding intervention, we considered physical activity (including also exercise by definition), defined as supervised or unsupervised interventions including any type of exercise applied to patients with lung cancer and performed for at least 4 weeks. All inclusion criteria were evaluated, in title, abstract, and full text of original papers, by two independent reviewers.
Investigated Outcomes in Lung Cancer
A series of studies have investigated the impact of PA and EX on measurable outcomes such as cardiorespiratory fitness, pulmonary function, strength and muscle mass, fatigue, quality of life, psychological status, and sleep quality (Table 1 and Fig. 1).
Table 1.
Main interventional studies after surgery and/or during medical treatments in lung cancer
| Author | Patients and study type | Duration and type of intervention | Primary outcomes | Secondary outcomes | Main results |
|---|---|---|---|---|---|
| Peddle‐Mclntyre et al. 54 |
14 NSCLC (I–IIIB) Single arm |
12 wks of a distance‐based intervention with printed materials +12 wks follow‐up | Feasibility | Physical activity level, QoL |
↓ eligibility and attrition rate ↑ physical activity level ↑ QoL (some domains) ↑ pain Short‐term benefits |
| Sommer et al. 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64 |
40 NSCLC (I–IIIA) RCT |
2 wks of preoperative +12 wks of postoperative aerobic and strength activity + multidisciplinary intervention | Safety, feasibility, and QoL | Anxiety, depression, distress, perceived social support, smoking, alcohol, and physical activity habits; VO2peak; 6MWT; strength; pulmonary function; patient‐reported outcomes |
↑ QoL (some domains) ↓ anxiety, depression, and distress levels Preoperative interventions not feasible Postoperative interventions safe and feasible ↑ 6MWT ↑ strength |
| Messaggi‐Sartor et al. 42 |
37 NSCLC (I–II) RCT |
8 wks of aerobic exercise and high‐intensity respiratory muscle training vs. UC | VO2peak | Respiratory muscle strength, QoL, IGF‐1 and IGFBP‐3 levels |
↑ QoL (some domains) ↑ VO2peak ↑ respiratory muscle strength ↑ IGFBP‐3 serum level |
| Dhillon et al. 29 |
112 NSCLC (III–IV), SCLC RCT |
2 months of supervised and unsupervised physical activity program +4 months follow‐up +6 months follow‐up vs. education materials only | Fatigue | QoL; ADL; IADL; anxiety, distress, depression; sleep quality; dyspnea; 6MWT; handgrip strength; senior fitness test; physical activity level; sedentary behavior; survival |
↑ physical activity levels in Ex group at 4 and 6 months |
| Cavalheri et al. 43 |
17 NSCLC (I–IIIA) RCT |
8 wks of individual supervised aerobic and strength program vs. control group | Exercise capacity (VO2peak and 6MWT) | Physical activity level; sedentary behavior; strength; QoL; fatigue; anxiety, depression; lung function |
↑ VO2peak ↑ 6MWT |
| Chen et al. 96 |
111 LC (I–IV)
RCT |
12 wks of home‐based walking program and weekly exercise counseling vs. usual care +3 months follow‐up | Sleep quality and rest‐activity rhythms | NA | ↑ sleep quality |
| Solheim et al. 89 |
64 (26 NSCLC, III–IV) RCT |
6 wks of multimodal intervention (anti‐inflammatory drugs, oral supplements, nutritional counseling, and home‐based aerobic and strength exercise) vs. usual care | Feasibility, compliance | Weight; muscle mass; physical activity level, 6MWT, handgrip strength; nutritional status; fatigue; safety; survival |
Feasible 60% compliance for Ex ↑ weight |
| Zhang et al. 24 |
96 NSCLC (I–IV), SCLC RCT |
12 wks of Tai Chi vs. low impact exercise (control group) | Fatigue | NA | ↓ fatigue (some domains) |
| Chen et al. 30 |
116 LC (I–IV) RCT |
12 wks of home‐based walking program and weekly exercise counseling vs. usual care +3 months follow‐up | Anxiety and depression | Cancer‐related symptoms | ↓ anxiety and depression |
| Quist et al. 44 |
114 NSCLC (IIIb–IV), SCLC (ED) Single arm |
6 wks of aerobic and strength program | VO2peak | Strength, 6MWT, FEV1, QoL, cancer‐related symptoms, anxiety, depression |
↑ VO2peak ↑ 6MWT ↑ strength ↑ emotional well‐being ↓ anxiety |
| Sahli et al. 80 |
70 NSCLC (I–IV), SCLC (LD), mesothelioma (I–III) RCT |
12 wks of WBV vs. CRT vs. usual care | 6MWT | Change in exercise capacity, strength, and QoL after radical treatment; maximal exercise capacity; strength; QoL after training |
↑ 6MWT ↑ quadriceps force in CRT after training program |
| Sahli et al. 86 |
45 NSCLC (I–III), SCLC RCT |
12 wks of strength program (whole‐body vibration or conventional resistance training) vs. usual care | Changes in muscle mass and strength | NA |
↓ muscle mass and strength after radical treatment Complete recovery after rehabilitation ↓ muscle mass and strength in control groups over time |
| Edvardsen et al. 52 |
61 NSCLC (I–IV) RCT |
20 wks of high‐intensity aerobic and strength program vs. UC | VO2peak | Pulmonary function; muscle mass; strength; daily physical functioning; QoL |
↑ VO2peak ↑ DLCO ↑ strength ↑ muscle mass ↑ daily physical functioning ↑ QoL |
| Kuehr et al. 31 |
40 NSCLC (IIA–IV) Single arm |
8 wks of aerobic and strength (in patients and home‐based periods) + 8 wks follow‐up | Feasibility | 6MWT; strength; QoL; fatigue; psychological impairment |
Feasible ↑ 6MWT ↑ strength ↓ QoL |
| Chang et al. 70 |
65 LC Quasi‐experimental (2 arms) |
12 wks and of walking vs. usual care +3 months follow‐up | 6MWT; pulmonary function; QoL | NA |
↑ FEV1 at 3 and 6 months ↑ 6MWT at 1, 3, and 6 months |
| Arbane et al. 47 |
53 NSCLC RCT |
5 days of strength and mobility inpatient program +12 wks of aerobic and strength program vs. usual care | QoL | 6MWT; strength; length of stay and postoperative complication | ↓ loss of strength in Ex group |
| Arbane et al. 55 |
131 NSCLC (I–IV) RCT |
4 wks of aerobic and strength (5 days inpatients) and walking program vs. usual care | Physical activity level | Ex tolerance; strength; QoL; length to stay, postoperative complication | ↑ QoL in patients with airflow obstruction |
| Hoffman et al. 25 |
5 NSCLC (IIA–IIIA) Single arm |
16 wks of walking and balance (with Nintendo Wii) | Fatigue | Cancer‐related symptoms; 6MWT; QoL |
↑ 6MWT ↑ QoL ↓ cancer‐related symptoms ↓ fatigue |
| Hoffman et al. 26 |
7 NSCLC (I–IIIA) Single arm |
6 wks of walking and balance (with Nintendo Wii) | Feasibility | Fatigue; self‐efficacy; functional performance (steps/day) |
Feasible ↓ fatigue ↑ walking steps/day ↑ self‐efficacy |
| Stigt et al. 50 |
57 NSCLC RCT |
12 wks of aerobic and strength program +3 months follow‐up +6 months follow‐up vs. usual care | QoL | 6MWT; pain; feasibility in patients undergoing chemotherapy |
↑ 6MWT after 3 months of intervention ↑ pain |
| Henke et al. 53 |
46 NSCLC (IIIA–IV), SCLC RCT |
3 cycles of chemotherapy of aerobic, strength (every other day), and endurance plus breathing techniques (5 days per week) vs. usual care | Activity of daily living (ADL‐Bartel Index) | QoL; 6MWT; strength; dyspnea |
↑ ADL ↑ 6MWT ↑ strength ↓ dyspnea ↑ QoL (some domains) |
| Cheville et al. 32 |
66 (34 LC, IV) RCT |
8 wks of walking and strength home‐based program vs. usual care | Mobility and activity | QoL; fatigue; pain; sleep quality; ability to perform daily activities |
↑ mobility ↑ sleep quality ↓ fatigue |
| Andersen et al. 65 |
59 NSCLC (I–IV), SCLC Pragmatic uncontrolled trial |
9 wks (3wk supervised +3wk unsupervised +3wk supervised) of aerobic interval training and walking | Adherence | FEV1; VO2max; QoL |
44% completed the program 69% continued to be active after rehabilitation |
| Granger et al. 45 |
15 (10 LC I–IV) RCT |
8 wks of aerobic and strength program (inpatient and outpatient home‐based) vs. usual care | Safety and feasibility | 6MWT; functional mobility; QoL |
Safe and feasible ↑ functional mobility ↑ 6MWT |
| Hwang et al. 46 |
24 NSCLC (IIIA–IV) RCT |
8 wks of high‐intensity interval training vs. usual care | VO2peak | Strength; oxygenation during exercise; insulin resistance; inflammatory response; QoL |
↑ VO2peak ↑ circulation ↑ respiratory and muscular function ↑ peak exercise ↓ dyspnea ↓ fatigue |
| Quist et al. 66 |
29 NSCLC (III–IV), SCLC (ED) Single arm |
6 wks of aerobic, strength, relaxation supervised sessions, walking, and relaxation home‐based sessions | Safety and feasibility | VO2peak; strength; 6MWT; FEV1; QoL |
Safe and feasible ↑ VO2peak ↑ 6MWT ↑ strength ↑ emotional well‐being |
| Temel et al. 48 |
25 NSCLC (IIIB–IV) Single arm |
12 wks of aerobic and strength program | Feasibility | QoL; symptom severity; mood; 6MWT; strength; survival |
44% completed the program ↑ elbow extension strength ↓ cancer‐related symptoms |
| Jones et al. 27 |
20 NSCLC (IA–IIIB) Single arm |
14 wks of aerobic training | VO2peak | QoL; fatigue |
↑ peak workload ↑ functional well‐being ↓ fatigue |
| Spruit et al. 67 |
10 NSCLC and SCLC Single arm |
8 wks of aerobic and strength program | 6MWT and peak cycling load | Pulmonary function; dyspnea |
↑ 6MWT ↑ peak cycling load |
| Tarumi et al. 71 |
82 NSCLC (stage IIB–IV) Single arm (retrospective) |
8 wks of relaxation, respiratory training, cough training, lower‐extremity exercise, and training of daily living | Pulmonary function | NA |
↑ FVC ↑ FEV1 |
| Brocki et al. 49 |
78 LC RCT |
10 wks of aerobic and strength exercise vs. usual care +12 months follow‐up | QoL | 6MWT; lung function | ↓ body pain (at 10 weeks) |
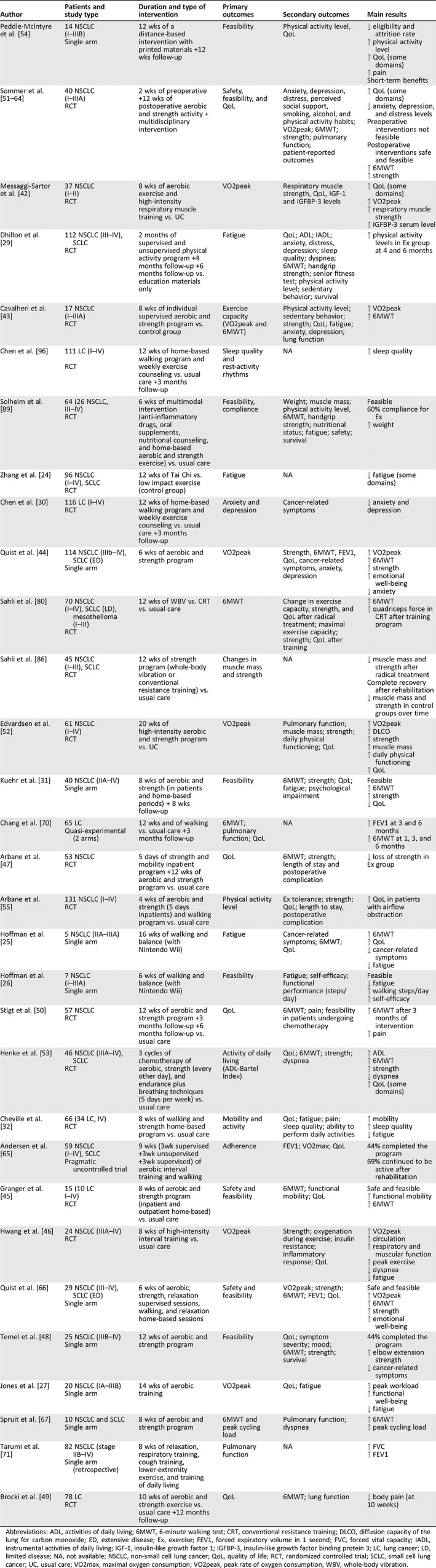
Abbreviations: ADL, activities of daily living; 6MWT, 6‐minute walking test; CRT, conventional resistance training; DLCO, diffusion capacity of the lung for carbon monoxide; ED, extensive disease; Ex, exercise; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; IADL, instrumental activities of daily living; IGF‐1, insulin‐like growth factor 1; IGFBP‐3, insulin‐like growth factor binding protein 3; LC, lung cancer; LD, limited disease; NA, not available; NSCLC, non‐small cell lung cancer; QoL, quality of life; RCT, randomized controlled trial; SCLC, small cell lung cancer; UC, usual care; VO2max, maximal oxygen consumption; VO2peak, peak rate of oxygen consumption; WBV, whole‐body vibration.
Figure 1.
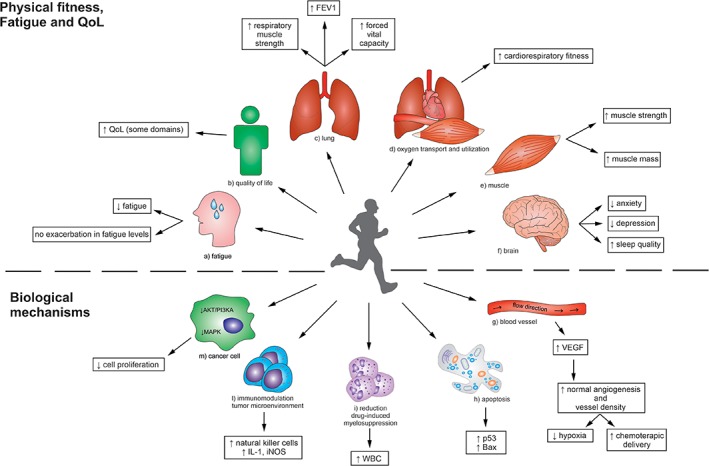
Summary of the effects of exercise on body physiology, psychology, and biology in lung cancer.Abbreviations: FEV1, forced expiratory volume in one second; IGF‐1, insulin‐like growth factor 1; iNOS, inducible nitric oxide synthase; NK, natural killer; PI3KA, phosphoinositide 3‐kinase; QoL, quality of life; VEGF, vascular endothelial growth factor; WBC, white blood cells.
Fatigue
Cancer‐related fatigue is defined as “a distressing, persistent, subjective sense of physical, emotional, and/or cognitive tiredness or exhaustion related to cancer and/or cancer treatment that is not proportional to recent activity and interferes with usual functioning” 14. In lung cancer, about 90% 15 of patients undergoing chemotherapy and 57% 16 of surgically resected patients experience this side effect. Different genetic and behavioral risk factors can predispose patients to cancer‐related fatigue 17, which has numerous manifestations, such as weakness, sleep disturbance, and lower concentration or attention, that cause a negative impact on work, mood, and social relationships, decreasing QoL 17. Several candidate factors have been suggested as underlying mechanisms inducing cancer‐related fatigue. Among them, an increase in proinflammatory cytokines (such as c‐reactive protein, IL‐6, and TNF‐α) and angiogenic modulators (mainly vascular endothelial growth factor [VEGF]), anemia, disturbance in the hypothalamic‐pituitary adrenal axis, altered brain serotonin metabolism, and defect in adenosine triphosphate regeneration seem to play a crucial role 18, 19.
By modulating these biological mechanisms, EX may improve the management of cancer‐related fatigue by reducing symptoms’ severity. Indeed, EX has been demonstrated to assist fatigue management in patients with cancer and cancer survivors affected by different types of malignancies (such as breast, colon, prostate, etc.) 8. Moreover, a recent meta‐analysis has found that EX and psychological intervention are more effective than a pharmacological approach to counteract such distress 20. Observational studies in lung cancer reported an inverse correlation between PA and symptoms of fatigue 21, 22, 23. In particular, D'Silva et al. objectively assessed PA and sedentary time in 127 patients with NSCLC (stage I–IV) and found that moderate‐ to vigorous‐intensity activity was associated with fewer fatigue symptoms, whereas sedentary time was associated with increased fatigue, negatively affecting QoL and physical and functional well‐being 21. Moreover, Janssen et al. described a routine rehabilitation program, offered to patients with lung cancer, composed of aerobic and strength exercises with a frequency of three times per week. Fifty patients (stage I–IIIA) started the program with a completion rate of 86%. After 12 weeks, fatigue, QoL, and cardiorespiratory fitness were significantly improved from the baseline assessment 22. Similarly, other interventional studies showed that EX ameliorates fatigue‐related symptoms 24, 25, 26, 27, especially if initiated early after surgery 28. For instance, a randomized controlled trial including 96 patients with lung cancer (including NSCLC stage I–IV and SCLC) undergoing chemotherapy compared the effect of a tai chi program (performed every other day) versus low impact exercise (as a control group) on cancer‐related fatigue. The tai chi group reported an improvement in total fatigue score compared with the control group at 6 weeks (59.6 ± 11.3 vs. 66.8 ± 11.9, p < .05) and 12 weeks (53.3 ± 11.8 vs. 59.3 ± 12.2, p < .05) 24. Although these findings seem to support the benefit of EX for cancer‐related fatigue in lung cancer, other interventional studies reported no changes in fatigue levels after a targeted exercise program 29, 30, 31, 32. In this regard, a trial randomized 112 patients with lung cancer (NSCLC stage III–IV and SCLC) to 8‐week supervised and unsupervised PA sessions plus a behavioral support program, and general health education materials or control (general health education material only). After the intervention, at 4 and 6 months, no significant changes were detected in terms of fatigue, QoL, symptom severity, physical or functional status, and survival between the two groups 29. Nevertheless, the engagement in an EX program was never associated with an exacerbation in fatigue levels. Further studies are required to consolidate the real contribution of exercise on cancer‐related fatigue in lung cancer.
Quality of Life
QoL is defined by a subjective and multidimensional concept that includes physical, psychological, and social domains and has shown a prognostic impact in lung cancer 33, 34. The World Health Organization describes QoL as “an individual's perception of their position in life, in the context of the culture and value systems in which they live and relation to their goals, expectations, standard and concerns” 35. Patients with lung cancer have a long‐lasting QoL impairment compared with healthy people, especially regarding physical health score, after both surgery and chemotherapy or radiotherapy 36, 37, 38. Nevertheless, the level of QoL reduction might also depend on disease stage, prognosis, and tumor localization 39. A longitudinal study among 107 patients with lung cancer (stage I–IV) showed a direct correlation between QoL level and time dedicated in walking activities over a 6‐month follow‐up period, with a QoL increase by 0.03 points per additional minute of walking time per week 40. A comprehensive systematic review including 16 randomized controlled trials with different cancer types (breast, colorectal, lymphoma, prostate, and lung) concluded that EX significantly improved QoL (mean difference, 5.55; 95% confidence interval [CI], 3.16–7.90; p < .001), during and after medical treatment. Moreover, during treatment, a gain in both psychological and physical variables was observed, whereas after completion of therapies an improvement in only the physical aspects was evident 41.
Focusing on studies including only patients with lung cancer, no clear advantage in terms of QoL after applying a physical exercise program is evident 29, 31, 32, 42, 43, 44, 45, 46, 47, 48, 49. A randomized controlled trial attempted to assess the impact of EX intervention on QoL in 81 patients undergoing thoracotomy. Although the study closed prematurely (after 57 patients randomized) because of the introduction of video‐assisted thoracoscopic surgery, after 12 weeks of intervention and during follow‐up, a very high drop‐out rate was reported (8/23 patients in the active group and 11/25 in the control group performed the functional test), with no changes in QoL and increased pain in the active group 50. On the other hand, there are studies reporting a QoL improvement after training 25, 51, 52, 53, 54, 55. Among them, a study with 40 patients with stage I–IIIA NSCLC showed a significant improvement in global quality of life (p = .0032), emotional well‐being (p < .0001), mental health component (p = .0004), and a reduction in anxiety, depression, and distress after 12 weeks of multidisciplinary intervention including PA (aerobic, strength, and nature activity), dietary guidance, social counseling, and other options (e.g., counseling for smoking cessation) 51. Considering the controversial association between exercise and QoL in lung cancer care, further studies with a solid design and an adequate sample size are required to clarify this issue.
Pulmonary Function
The assessment of respiratory functionality by a global spirometry test is a crucial step to define therapeutic perspectives in lung cancer. In particular, the predicted postoperative of forced expiratory volume in 1 second (FEV1) and the diffusion capacity of carbon monoxide (DLCO) are the most utilized parameters to evaluate the surgical risk 56 and are associated with the prognosis of patients with lung cancer 57, 58. Surgical resection, chemotherapy, radiotherapy, and comorbidities (such as chronic obstructive pulmonary disease [COPD]) may be harmful for the pulmonary system, reducing respiratory functionality 59, 60, 61. Historically, the respiratory system is defined as overbuilt for exercise, and therefore training does not appear to confer a significant adaptation in lung of healthy human subjects 62. Nevertheless, the role of EX is still controversial in pulmonary diseases. EX in patients with COPD is largely studied, and a recent meta‐analysis including 21 randomized controlled trials has analyzed the role of whole‐body exercise on pulmonary function in adult participants with chronic lung diseases (mainly COPD). The results demonstrated a small but significant improvement in spirometry values in the EX group as compared with controls, suggesting that in certain conditions the respiratory system may adapt in response to training 63.
In lung cancer, the effect of EX on pulmonary parameters was investigated with preliminary supportive evidence 43, 64, 65, 66, 67. An individual supervised training (three times per week, 60 minutes per session) was proposed in 17 patients with stage I–IIIA NSCLC. After 8 weeks of training, an increase was observed in exercise capacity (the primary outcome), without any significant improvement in other parameters, such as strength, QoL, fatigue, anxiety, depression, and pulmonary function 43. However, in the first year after surgery, patients usually experience an increase in pulmonary parameters 68 that may be attributed to compensatory mechanisms, such as the expansion of the remaining lobes and vascular tissues 69. In this postoperative context, studies testing different training programs are consistent in detecting an improvement in respiratory muscle strength and/or functionality 42, 52, 70. A retrospective study evaluated the outcome of a comprehensive rehabilitation schedule on pulmonary function in 82 patients with lung cancer (stage IIB–IV). The program included relaxation (at least once per day), respiratory training (at least once day before surgery), cough training (at least once per day before surgery), activities of daily living (after surgery) and lower‐extremity exercise (high‐intensity aerobic exercise, 5 days per week for 45 minutes). At 8–10 weeks, significant increases in forced vital capacity (FVC; +6.4%, p = .0096) and in FEV1 (+ 10.4%, p < .0001) were found, whereas the DLCO decreased. Even in current or former smokers, an improvement in FEV1 was observed, whereas patients with respiratory impairment experienced a greater increase in both FVC (+13.9%, p = .0025) and FEV1 (+ 22.5%, p < .0001) 71. Collectively, these results should be interpreted cautiously, because some studies lack a control group and several aspects need to be further defined, mainly about the potential role of EX on postsurgery compensatory mechanisms.
Cardiorespiratory Fitness
Peak oxygen consumption (VO2peak) and the 6‐minute walking test (6MWT) are the most applied assessments for cardiorespiratory fitness in lung cancer. Cardiorespiratory fitness reflects the capability to introduce, transport, and use oxygen, and it is an important index of functionality, health, and longevity. Similar to pulmonary function, VO2peak can provide clinically relevant diagnostic and prognostic information. It is inversely related to perioperative and postoperative complications, and it is an independent predictor of survival 72. To date, three studies have investigated the relationship between cardiorespiratory fitness and survival in lung cancer 73, 74, 75. In this regard, Jones et al. prospectively found that each 50 meters of improvement in 6MWT was associated with a reduction of 13% in risk of death in patients with metastatic NSCLC. Furthermore, compared with patients in the lowest 6MWT group, the overall reduction of death risk improved together with the increase in functional capacity (from 39% to 52%) 75. Cardiorespiratory fitness was compromised in patients with lung cancer versus healthy participants (mean difference, 0.87 mL × kg−1 × min−1; 95% CI, −12.1 to −5.3; p < .001) 76, and this impairment did not improve after therapies. Fifty patients with NSCLC (stage I–IIIB) were monitored for 6 months, from diagnosis (pretreatment) to the following 10 weeks (during treatment) and 6 months (usually after completion of therapies). 6MWT declined significantly from diagnosis to during treatment (−42.7 m; 95% CI, −71.4 to 14.0; p < .01) and continued to be lower after 6 months (−77.9 m; 95% CI, −144.3 to 11.4; p = .02) 77.
Cardiorespiratory fitness involves several consecutive steps, including respiratory and cardiovascular systems, vasculature, blood, and skeletal muscle. In healthy persons the most important factor that limits exercise capacity is the cardiac muscle 62, but in lung malignancies many cancer‐related factors concur to diminishing the cardiorespiratory fitness 78. First, the presence of a tumor mass, together with related surgical procedures, may affect the respiratory system by reducing diffusion capacity. Second, in case of advanced disease, the oxidative capacity of skeletal muscles is impaired with a reduction in capillarization and mitochondrial density. Moreover, chemotherapeutic agents and radiotherapy may harm cardiac pump, blood cell populations, and vascular function 78.
Although in lung diseases the respiratory system could play a major role in limiting exercise capacity, in long‐term postpneumonectomy patients (mean 5.5 years after surgery), it was suggested that it was mainly limited by the cardiovascular system 79. Nevertheless, physical exercise may mitigate these impairments and improve the cardiorespiratory fitness in lung cancer. A randomized controlled trial investigated the effects of high‐intensity endurance and strength training on cardiorespiratory fitness as primary outcome. Sixty‐one patients with NSCLC (stage I–IV) were enrolled in an exercise program (60 minutes for three times per week). After 20 weeks, with an adherence rate of 88%, the authors found an increase of 4.5 ± 3.4 mL × kg−1 × min−1 in the EX group, whereas the control group reported a decrease of –0.6 ± 2.7 mL × kg−1 × min−1 in cardiorespiratory fitness 52. Similarly, a recent study including patients with surgically resected NSCLC (stage I–II) detected a significant VO2peak increment in the EX group versus control 42. Globally considered, although some studies did not report any significant change in functional capacity following a training period 27, 29, 45, 47, 48, 49, 55, 64, 65, the majority agreed on the potential beneficial effect of exercise on cardiorespiratory fitness 22, 25, 31, 42, 43, 44, 46, 50, 52, 53, 66, 67, 70, 80.
Strength and Muscle Mass
Strength and muscle composition (muscle mass or size) are the most accurate parameters to evaluate muscle function. Patients with lung cancer may suffer from muscle dysfunction for disease‐related metabolic disorders, oncological treatments, physical inactivity, and malnutrition 81. Muscle mass alterations occurring during cancer define pathological conditions, such as cachexia (a multifactorial syndrome characterized by severe muscle wasting, malnutrition, and systemic inflammation) and sarcopenia (decreased muscle mass). The majority of patients affected by advanced lung cancer experience cachexia (69%) or sarcopenia (47%) 82, both related to a poor prognosis 12, 81, 83. Considering that strength is closely linked to muscle mass, patients with lung cancer may also have relevant impairments of this parameter. Indeed, patients with NSCLC (stage I–IIIA) had a significantly lower handgrip strength as compared with healthy controls, with a mean difference of −6 kg (p = .023) 76. Muscular strength is an important parameter, and, in healthy persons, it represents a predictor of all‐cause mortality 84. A study investigating the impact of strength on survival found that handgrip strength is an independent prognostic factor in patients with NSCLC and gastrointestinal cancer with advanced and metastatic disease 85.
EX, especially resistance training, is a potent modulator of skeletal muscle and could counteract muscle dysfunction in patients with lung cancer. The majority of interventional studies that included strength assessment in their secondary outcomes found a positive effect of EX 31, 44, 47, 48, 52, 53, 64, 66, 80, whereas few of them reported no relevant effect 43, 46, 55. However, relatively few studies explored the role of EX on muscle mass in lung cancer. Salhi et al. investigated the impact of a rehabilitation program on muscle mass and strength in 45 patients with lung cancer (stage I–III) who underwent radical oncological treatments (surgery and/or radiotherapy and/or chemotherapy). The rehabilitation consisted of an initial warming‐up (20 minutes), followed by resistance training of upper and lower limb muscles with conventional resistance training or whole‐body vibration training, 3 days per week for 12 weeks after treatment completion. A significant decrease in muscle cross‐sectional area and in quadriceps force with a conservation in fat‐free mass, measured with bioelectrical impedance, was observed after treatments. Following a 12‐week rehabilitation program, full recovery in muscle strength and mass was detected in the intervention arm, whereas the control group experienced a further decline from baseline 86.
As suggested in the context of preclinical studies, aerobic and strength training seem to induce a relevant benefit against cancer cachexia 87, 88. To our knowledge, only one study is available in patients with cachexia. The MENAC trial tests a multimodal intervention to attenuate and/or prevent cancer cachexia, which included anti‐inflammatory drugs, oral nutritional supplements, nutritional counseling, and an exercise program, on patients with lung and pancreatic cancer. To assess intervention safety and feasibility, a phase II cohort randomized 46 patients (26 with advanced NSCLC). The home‐based EX intervention consisted of aerobic training (30 minutes two times per week) and six individualized strength tasks (three times per week). Six weeks later, the intervention was shown to be safe and feasible, with a compliance of 76% for anti‐inflammatory drugs, 60% for exercise, and 48% for nutritional supplements. No significant changes in PA, muscle mass and strength, fatigue, and nutritional status were reported, probably because of the small sample size 89. The phase III cohort of MENAC trial, which will include 240 patients, is currently enrolling patients (NCT02330926) to clarify the efficacy of this multimodal intervention.
Psychological Status and Sleep Quality
Patients with lung cancer may experience several health problems, including psychological distress, because of cancer or undesired effects of its treatment. PA and EX may contribute to limiting these impairments.
The beneficial role of PA and EX in anxiety and depression is well established 90, 91 through the modulation of monoamine and cortisol levels, leading to adaptation in limbic structures 92. The prevalence of anxiety, depression, and sleep disorders among patients with lung cancer is 33%, 34%, and 45%–57%, respectively 93, 94. In the context of lung cancer, few studies have considered the potential role of EX to improve these symptoms, finding positive 44, 51 or neutral effects 31. Chen et al. investigated the impact of EX on anxiety and depression symptoms as a primary outcome in a sample of patients with lung cancer (stage I–IV). Enrolled participants (n = 116) were randomly assigned to a 12‐week moderate‐intensity walking program, three times per week for 40 minutes, or usual care. After the intervention, anxiety (p = .009) and depression (p = .00006) levels were significantly diminished, and the effect was maintained over time (anxiety, p = .006; depression, p = .004) 30.
EX can improve sleep quality in the general population 95, but also in cancer survivors 96. Sleep disturbances are a common problem in oncology care and affect a large portion of patients with lung cancer, especially during the chemotherapy period 94. EX seems to contribute to improving sleep quality in patients with lung cancer 23, 32, 96, although results of different studies are controversial 29, 30. A home‐based walking program proposed by Chen et al. has shown that 12 weeks of moderate‐intensity EX is effective over time in improving both subjective (p = .001) and objective (p = .023) sleep quality in a sample of patients with lung cancer (stage I–IV) compared with the control group 96.
Biological Mechanisms
The molecular mechanisms by which PA and EX could influence lung cancer outcomes remain elusive. Data from literature suggest that PA and EX may counteract some specific cancer cells’ acquired capabilities (hallmarks of cancer) and, at the same time, prevent chemotherapy‐related adverse events (Fig. 1).
The ability to promote an aberrant angiogenesis represents a main hallmark of cancer. In fact, as an adaptive response to hypoxia, cancer cells activate the hypoxia‐inducible factor 1‐alpha (HIF‐1α) pathway to promote angiogenesis through proangiogenetic factors, mainly VEGF‐α 97. Under normal conditions, EX stimulates a physiological angiogenetic process and VEGF release in skeletal muscles in a HIF‐1–independent manner 98. Nevertheless, how EX modulates angiogenesis in an oncological setting is not clear. Treadmill exercise for a period of 4 weeks, five times per week and 60 minutes each session, has demonstrated to significantly increase VEGF serum levels in mice inoculated with Lewis lung cancer (LLC) cells, as compared with baseline (p = .015), but without significant differences in terms of survival rate or tumor growth compared with the control group 99. Alves et al. observed 2.5‐fold higher mRNA levels of VEGF‐α (p < .05) in an LLC mice model undergoing daily high‐intensity interval training after tumor cell injection compared with sedentary mice, with a significant reduction of tumor mass (−52% after 18 days) and benefit in survival 100.
The capability to escape cell death and apoptosis is another hallmark of cancer, and p53 plays a crucial role as a tumor suppressor protein 97. EX may affect oncogenesis through activation of p53‐induced apoptosis. In this regard, daily wheel running for 4 weeks appeared to reduce primary tumor growth (but not distant metastases), as compared with a control group (p < .01), with a significant increase in p53 intratumoral levels (p < .01), in a murine model of lung adenocarcinoma. Similarly, levels of Bax and caspase 3 (two proapoptotic proteins in the p53 pathway) were significantly increased. Interestingly, this cancer model was p53 wild type, suggesting the potential role of EX in stabilizing p53 and avoiding its downregulation 101.
The phosphoinositide 3‐kinase–AKT pathway (through mTOR and S6 kinase) and the RAS‐MAP kinase cascade (through ERK1 and ERK2) are involved in enhancing cell proliferation and survival, as well as in lung cancer cells resistance to chemotherapy and radiation 102, 103. The effect of EX has been studied in lung adenocarcinoma A549 cells incubated with human serum, collected pre‐ or post‐EX, or foetal bovine serum as control. A significant reduction of proliferation and survival for cells treated with post‐EX serum compared with control (p < .05 and p < .001, respectively) was observed. A relevant reduction of cell survival was also evident when comparing cells treated with pre‐ and post‐EX serum (p < .001). To explore the potential underlying reasons, the authors measured activated (phosphorylated) AKT levels through immunoblotting, revealing a significant reduction between cells incubated with pre‐ and post‐EX serum (p < .001). Similar findings were observed for mTOR, S6 kinase, and ERK1 and ERK2 activation 104.
Another possible mechanism underlying the antitumorigenic impact of EX is related to immunomodulation, particularly by increasing proinflammatory cytokine levels and natural killer (NK) cell infiltration in the tumor microenvironment. Pedersen et al. found that EX (wheel running) in LLC mice significantly reduced tumor volume (−58%), with an upregulation of proinflammatory cytokines (IL‐1a and inducible nitric oxide synthase [iNOS]) and markers for NK and T‐cell activity 105. In a prospective randomized study in postsurgical patients with NSCLC, 16 weeks of tai chi chuan training was demonstrated to significantly promote proliferation of peripheral blood mononuclear cells, as compared with both basal levels (p < .001) and a control group (p < .05), with an increase in their cytotoxicity demonstrated by incubation with lung adenocarcinoma A549 cells (p < .001). Moreover, a relevant increase in circulating NK cell percentage, natural killer T, and dendritic CD11c cells between the exercise and control groups was detected 106. In another prospective randomized trial, the control group, including 16 patients with surgically resected NSCLC, experienced a decrease in ratio of IFN‐γ–producing CD3+ T lymphocytes (T1) to IL‐4–producing CD3+ T lymphocytes (T2) and an increase in cortisol levels during recovery time. Conversely, the experimental arm (16 postsurgical patients with NSCLC) who followed a guided 16‐week moderate‐intensity tai chi program (60 minutes per session, three sessions per week) managed to preserve a stable T1‐to‐T2 ratio and cortisol levels 107. Interestingly, preliminary evidence suggests that chemotherapy‐treated patients with lung cancer who joined exercise sessions using resistance bands managed to maintain white blood cell levels during treatment compared with a control group 108.
Considerations About Exercise Prescription in Patients with Lung Cancer
Mounting evidence suggests that EX is safe in patients with lung cancer, both after surgery and during and after medical treatments. Different programs with a variety of activities were explored, such as tai chi, aerobic and strength exercise, walking, balance, and breathing techniques. The most often applied frequency was two or three times per week, and time per session ranged from 5 to 120 minutes. Across the studies, all the levels of training intensity (light, moderate, and vigorous), when reported, appeared to be well tolerated by patients. However, most patients with lung cancer are insufficiently active or sedentary, and a series of studies reported a low adherence and high drop‐out rate from EX programs 43, 48, 65, 109, 110. Among drop‐out reasons, cancer‐related side effects and, mostly, lack of interest and motivation represent key contributors.
There are many barriers limiting the adherence to a PA program. Some of them are also common in healthy people, such as lack of access to services or lack of interest, but others are specifically related to health status, disease course, and therapeutic approach. In addition, environmental and personal exercise preferences, fun, and social implications are important factors that influence the participation and consistency over time to a physical activity program 111. In patients with lung cancer (and their caregivers) there is a higher risk of experiencing exacerbations of psychosocial distress because of the widely shared stigmatization of this disease based on the close link between lung cancer and smoking 112.
Several models, applicable also in cancer populations, are applied to trigger motivation to perform exercise (such as social cognitive theory, theory of planned behavior, and self‐determination theory). Knowledge and integration of these theories in clinical practice may help patients to adopt and maintain EX or PA as part of their lifestyle 113. The American Cancer Society and American College of Sport Medicine recommend avoiding inactivity and suggest that patients with cancer should engage in regular PA. In detail, at least 150 minutes per week of moderate aerobic activity, or 75 minutes of vigorous aerobic activity, with flexibility and strength exercise two or three times per week should be performed 5, 114. This goal could be difficult to achieve, especially for physically deconditioned patients. For this reason, the EX program should be flexible (particularly during treatment periods), start easily, and progress slowly according to patient's rhythm and body response. Moreover, an interpatient heterogeneity in physical, psychological status, and treatment‐related side effects needs to be considered. According to available evidence, an accurate baseline assessment, including clinical, physical, and psychosocial conditions, is fundamental to schedule a tailored EX program. Recognizing the presence of relevant comorbidities to adapt activity and avoid potential EX‐induced risks is fundamental. The presence of extreme fatigue or high physical limitation could be a contraindication to start an EX program, or a low cardiorespiratory fitness may suggest performing EX with low intensity and for short time 5.
Considering all these factors, in clinical practice close collaboration among oncologists and kinesiologists (or cancer exercise specialists or physiotherapists) may allow developing specific EX programs based on patient's needs, preferences, and physical and psychological status. The final aims are to improve patient's physical fitness and quality of life, reduce treatment‐related side effects, and increase the motivation to adopt and maintain an active lifestyle over time (Fig. 2). Several trials are ongoing to enrich the currently available amount of evidence‐based data (Table 2).
Figure 2.
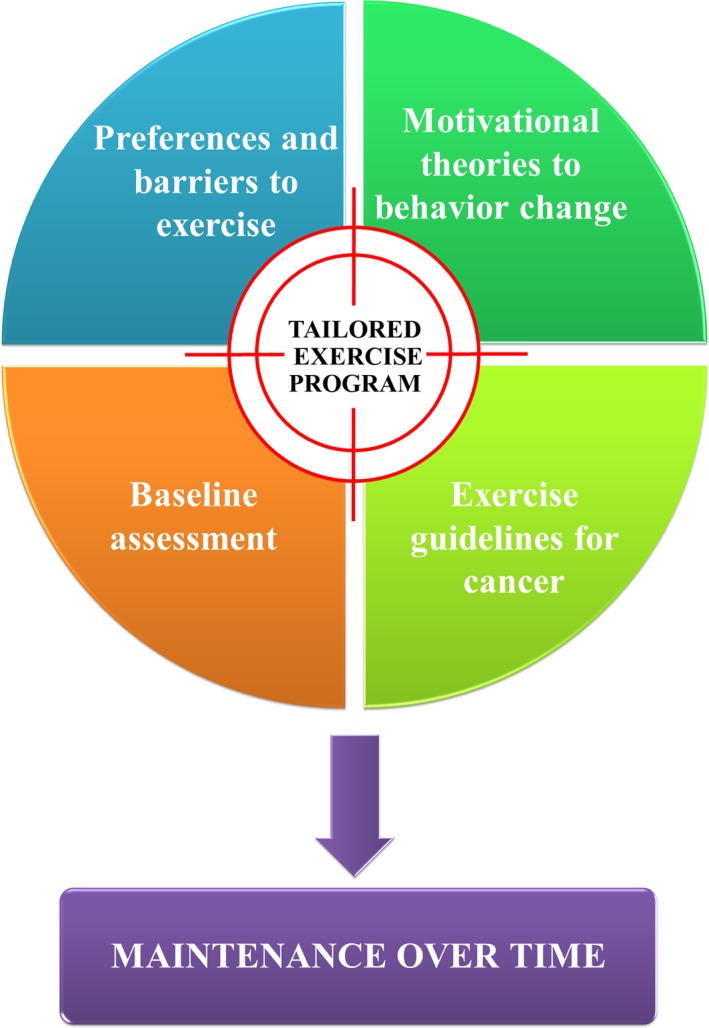
Tailored exercise program: a proposed model.
Table 2.
Randomized controlled trials currently ongoing or recently concluded (without available results) in lung cancer
| PI and Sponsor | Number | Title | Estimated participants | Primary outcome |
|---|---|---|---|---|
|
Chia‐Chin Lin
The University of Hong Kong |
NCT03482323 | Improving Survival in Lung Cancer Patients: A Randomized Controlled Trial of Aerobic Exercise and Tai‐Chi Interventions | 372 NSCLC | 1‐year OS |
|
Tora S. Solheim
Norwegian University of Science and Technology |
NCT02330926 | A Randomized, Open‐Label Trial of a Multimodal Intervention (Exercise, Nutrition and Anti‐Inflammatory Medication) Plus Standard Care Versus Standard Care Alone to Prevent/Attenuate Cachexia in Advanced Cancer Patients Undergoing Chemotherapy [MENAC trial] | 240 LC and PDAC | Body weight |
|
Kathleen Lyons
Dartmouth‐Hitchcock Medical Center |
NCT03500393 | A Remotely Supervised Exercise Program for Lung Cancer Patients Undergoing Chemo‐Radiation (REM) | 50 LC | Recruitment and retention |
|
Lee W. Jones
Memorial Sloan Kettering Cancer Center |
NCT01068210 | Lung Cancer Exercise Training Study: A Randomized Trial of Aerobic Training, Resistance Training, or Both in Lung Cancer Patients | 160 LC | VO2peak |
|
Sandy Jack
University Hospitals Southampton NHS Foundation Trust |
NCT03334071 | Exercise Regimens Before and During Advanced Cancer therapy: A Pilot Study to Investigate Improvements in Physical Fitness with Exercise Training Programme Before and During Chemotherapy in Advanced Lung Cancer Patients | 100 NSCLC | Adherence to exercise training program and adverse events |
|
Morten Quist
Rigshospitalet, Denmark |
NCT03066271 | PRIME ‐ Pre Radiotherapy Daily Exercise Training in Non‐Small Cell Lung Cancer | 40 NSCLC | VO2peak |
|
Paul LaStayo
University of Utah |
NCT03306992 | A Phase III Randomized Study Comparing the Effects of a Personalized Exercise Program (PEP) Against No Intervention in Patients with Stage I–IIIa Primary Non‐Small Cell Lung Cancer or Secondary Lung Cancer Undergoing Surgical Resection | 200 LC (primary or secondary) | 6MWT |
|
Amy Hoffman
University of Nebraska |
NCT03724331 | Understanding the Post‐Surgical Non‐Small Cell Lung Cancer Patient's Symptom Experience | 279 NSCLC | Fatigue |
|
Brett Bade
Yale University |
NCT03352245 | Assessing the Feasibility of a Patient‐centered Activity Regimen in Patients with Advanced Stage Lung Cancer | 40 NSCLC | Steps count and adherence to recommendations |
|
Marta Kramer Mikkelsen
Herlev and Gentofte Hospital |
NCT03411200 | Engaging the Older Cancer Patient; Patient Activation Through Counseling, Exercise and Mobilization ‐ Pancreatic, Biliary Tract, and Lung Cancer (PACE‐Mobil‐PBL) ‐ A Randomized Controlled Trial | 100 NSCLC, PDAC, biliary cancer | Lower body strength |
|
Jesper Holst Pedersen
Rigshospitalet, Denmark |
NCT02439073 | Postoperative Rehabilitation in Operation for LUng CAncer (PROLUCA) ‐ A Randomized Clinical Trial with Blinded Effect Evaluation | 235 LC | VO2peak |
|
Elisabeth Edvardsen
Oslo University Hospital |
NCT01748981 | Cardiorespiratory Fitness and Effect of Training After Lung Cancer Surgery. A Randomized Controlled Trial | 80 NSCLC | VO2peak |
|
Grandes Gonzalo
Basque Health Service |
NCT01786122 | Physical Exercise to Improve the Quality of Life in Cancer Patients During Treatment Process: EFFICANCER Study | 250 NSCLC, GI, and BC | QoL |
|
Liu Jui Fang
Chang Gung Memorial Hospital |
NCT02757092 | The Impacts of Pulmonary Rehabilitation Therapy on Patients After Thoracic Surgery | 120 mixed | Pulmonary complication |
|
Sara Tenconi
Arcispedale Santa Maria Nuova‐IRCCS |
NCT02405273 | Effects of Early Pulmonary Rehabilitation and Long‐Term Exercise on Functioning, Quality of Life and Postoperative Outcome in Lung Cancer Patients | 140 NSCLC | 6MWT |
|
Miklos Pless
Kantonsspital Winterthur KSW |
NCT02585362 | Influence of a Specially Formulated Whey Protein Supplement in Combination with Physical Exercise and Nutrition Program on Physical Performance in Cancer Outpatients | 88 mixed | Physical performance |
|
Ling Xu
Shanghai University of Traditional Chinese Medicine |
NCT03244605 | Clinical Study on the Effect of Comprehensive Rehabilitation Program on Quality of Life and Long‐Term Survival in Postoperative Non‐Small Cell Lung Cancer Patients | 236 NSCLC | QoL |
|
Alice Ryan
University of Maryland |
NCT02991677 | Exercise Effect on Chemotherapy‐Induced Neuropathic Pain, Peripheral Nerve Fibers | 60 mixed | Pain |
|
Young Sik Park
Seoul National University Hospital |
NCT02121379 | Randomized Clinical Trial of 8 Weeks Pulmonary Rehabilitation in Advanced Stage Lung Cancer Patients with COPD During Cytotoxic Chemotherapy | 40 LC | VO2peak |
|
Joseph A. Greer
Massachusetts General Hospital |
NCT03089125 | Brief Behavioral Intervention for Dyspnea in Patients with Advanced Lung Cancer | 200 LC | Dyspnea |
|
Oscar Gerardo Arrieta Rodríguez
Instituto Nacional de Cancerologia de Mexico |
NCT02978521 | Effect of a Pulmonary Rehabilitation Program on Skeletal Muscle Mass, Pulmonary Function, Inflammatory Response and Overall Survival on Patients Diagnosed with Non‐Small‐Cell Advanced Cancer | 94 NSCLC | Pulmonary function |
|
Ling Xu
Shanghai University of Traditional Chinese Medicine |
NCT03372694 | Efficacy Study of Comprehensive Rehabilitation Program Plus Chemotherapy in Postoperative NSCLC Patients | 354 NSCLC | QoL |
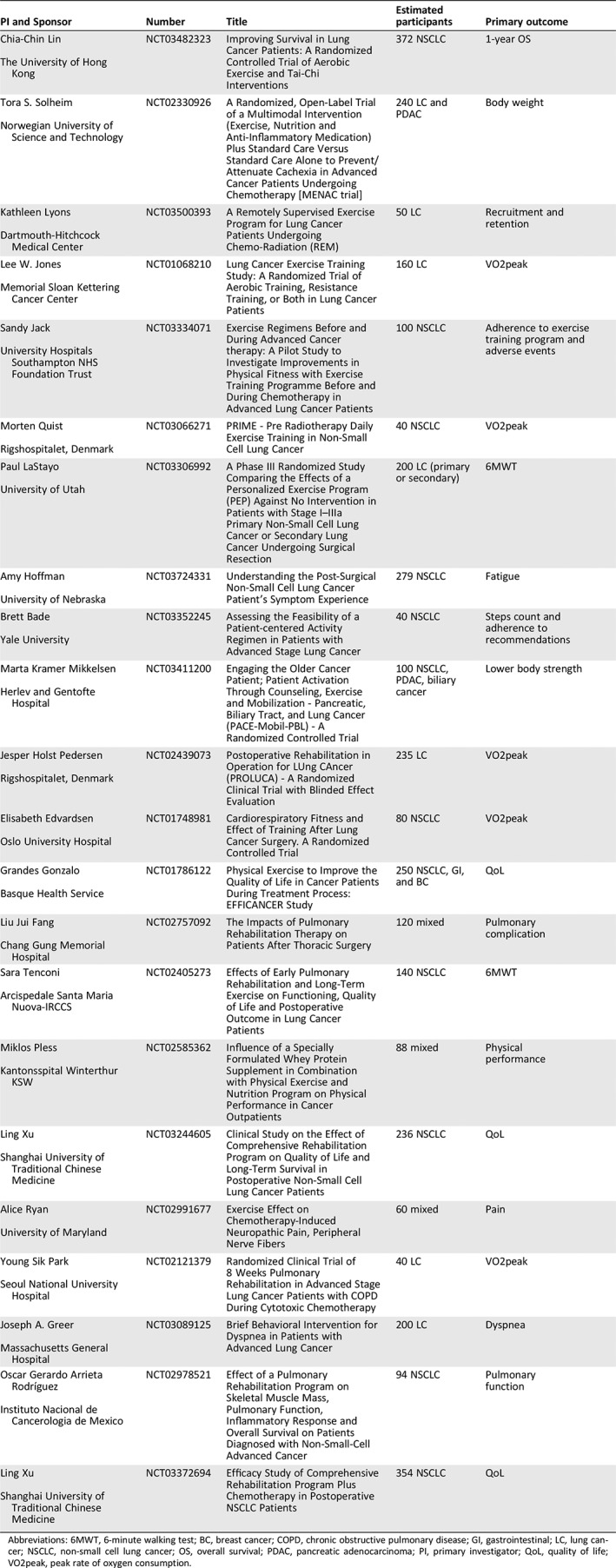
Abbreviations: 6MWT, 6‐minute walking test; BC, breast cancer; COPD, chronic obstructive pulmonary disease; GI, gastrointestinal; LC, lung cancer; NSCLC, non‐small cell lung cancer; OS, overall survival; PDAC, pancreatic adenocarcinoma; PI, primary investigator; QoL, quality of life; VO2peak, peak rate of oxygen consumption.
Conclusion
As highlighted above, many questions are still open regarding optimal exercise prescription and actual impact of EX and PA on survival rate, treatment‐related side effects, and quality of life of patients with lung cancer. Although available evidence provides a strong rationale to continue pursuing and investigating these aspects from both a clinical and translational point of view, current results remain not decisive because of methodological limitations of the performed trials, small numbers of patients included (mainly affected by early‐stage lung cancer), and a general lack of tailored EX programs, taking into account individual patients’ conditions, comorbidities, and preferences.
A common topic emerging from available experiences explores the potential synergistic impact of strongly integrated interdisciplinary approaches, encompassing coordinated EX and PA, nutritional, and psychological and behavioral interventions. From a theoretical standpoint, it is reasonable to speculate that behavioral and psychological intervention or counseling may reinforce motivation and compliance, thus potentially favoring adherence to tailored EX programs. On the other hand, nutritional counseling may help to counteract sarcopenia and muscle wasting, thereby rendering EX more effective in maintaining muscle mass and improving strength. Indeed, a meta‐analysis showed that combined EX and psychological intervention is more effective than a pharmacological approach to counteract fatigue 8. Similarly, an integrated approach encompassing EX, dietary guidance, social counseling, and a smoking cessation program 51 clearly improved QoL, emotional well‐being, and mental health, while reducing anxiety, depression, and distress. Overall, the impact of PA and EX on QoL endpoints appears to be potentially more profound when administered as part of a multidimensional, comprehensive approach to physical, nutritional, and psychological well‐being.
In this regard, a further step to improve the awaited benefit deriving from PA and EX may be achieved by embedding a personalized physical exercise program within a multidimensional teamwork intervention for oncological patients. In this light, we are currently offering a patient‐centered approach provided by an integrated team, including dietitians, kinesiologists, and psychologists coordinated by a medical oncologist (the Focus On Research and CarE team [FORCE]). On one hand, these nonpharmacological interventions may help improve QoL, physical functions, psychological aspects, and treatment‐related adverse events and reduce symptoms and complications occurring during cancer care. On the other, we hypothesize that such a comprehensive approach may influence patients’ immune status, thereby ultimately affecting treatment outcome (in particular for patients undergoing immunotherapy). Based on a rigorous scientific method, we aim to (A) derive tissue‐ and blood‐based immunological signature(s) predicting the outcome of immunological therapy, (B) demonstrate that EX (in context with nutritional counseling and behavioral interventions provided in an integrated fashion by the FORCE team) favorably modifies such predictive signatures, and (C) test (in a formal clinical trial) the hypothesis that specific EX preconditioning schemes (again in the context of a multidisciplinary intervention) improve the outcome(s) of patients with lung cancer undergoing immunotherapy.
Author Contributions
Conception/design: Alice Avancini, Giulia Sartori, Anastasios Gkountakos, Miriam Casali, Ilaria Trestini, Daniela Tregnago, Emilio Bria, Lee W. Jones, Michele Milella, Massimo Lanza, Sara Pilotto
Collection and/or assembly of data: Alice Avancini, Giulia Sartori, Anastasios Gkountakos, Lee W. Jones, Sara Pilotto
Manuscript writing: Alice Avancini, Giulia Sartori, Anastasios Gkountakos, Miriam Casali, Ilaria Trestini, Daniela Tregnago, Emilio Bria, Lee W. Jones, Michele Milella, Massimo Lanza, Sara Pilotto
Final approval of manuscript: Alice Avancini, Giulia Sartori, Anastasios Gkountakos, Miriam Casali, Ilaria Trestini, Daniela Tregnago, Emilio Bria, Lee W. Jones, Michele Milella, Massimo Lanza, Sara Pilotto
Disclosures
Emilio Bria: Roche, Merck Sharp and Dohme, AstraZeneca, Celgene, Pfizer, Helsinn, Eli Lilly & Co., Bristol‐Myers Squibb, Novartis (C/A, SAB), AstraZeneca, Roche (RF); Lee W. Jones: Pacylex (OI); Michele Milella: Eusapharma, AstraZeneca (H), Pfizer (SAB, H); Sara Pilotto: AstraZeneca, Eli Lilly & Co., Bristol‐Myers Squibb, Boehringer Ingelheim, Merck Sharp and Dohme, Roche (C/A, H), Bristol‐Myers Squibb, Boehringer Ingelheim, Merck Sharp and Dohme, Istituto Gentili (SAB), AstraZeneca (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Acknowledgments
E.B. and S.P. are supported by the Italian Association for Cancer Research AIRC‐IG 20583. E.B. and S.P. were supported by the International Association for Lung Cancer (IASLC). L.W.J. is supported by grants from the National Cancer Institute, AKTIV Against Cancer, and the Memorial Sloan Kettering Cancer Center Support Grant/Core (P30 CA008748).
Disclosures of potential conflicts of interest may be found at the end of this article.
References
- 1. Winningham ML, MacVicar MG, Burke CA. Exercise for cancer patients: Guidelines and precautions. Phys Sportsmed 1986;14:125–134. [DOI] [PubMed] [Google Scholar]
- 2. Cormie P, Zopf EM, Zhang X et al. The impact of exercise on cancer mortality, recurrence, and treatment‐related adverse effects. Epidemiol Rev 2017;39:71–92. [DOI] [PubMed] [Google Scholar]
- 3. Holmes MD, Chen WY, Feskanich D et al. Physical activity and survival after breast cancer diagnosis. JAMA 2005;293:2479–2486. [DOI] [PubMed] [Google Scholar]
- 4. Meyerhardt JA, Heseltine D, Niedzwiecki D et al. Impact of physical activity on cancer recurrence and survival in patients with stage III colon cancer: Findings from CALGB 89803. J Clin Oncol 2006;24:3535–3541. [DOI] [PubMed] [Google Scholar]
- 5. Schmitz KH, Courneya KS, Matthews C et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc 2010;42:1409–1426. [DOI] [PubMed] [Google Scholar]
- 6. Scott JM, Zabor EC, Schwitzer E et al. Efficacy of exercise therapy on cardiorespiratory fitness in patients with cancer: A systematic review and meta‐analysis. J Clin Oncol 2018;36:2297–2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stene GB, Helbostad JL, Balstad TR et al. Effect of physical exercise on muscle mass and strength in cancer patients during treatment—A systematic review. Crit Rev Oncol Hematol 2013;88:573–593. [DOI] [PubMed] [Google Scholar]
- 8. Cramp F, Byron‐Daniel J. Exercise for the management of cancer‐related fatigue in adults. Cochrane Database Syst Rev 2012;11:CD006145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hilfiker R, Meichtry A, Eicher M et al. Exercise and other non‐pharmaceutical interventions for cancer‐related fatigue in patients during or after cancer treatment: A systematic review incorporating an indirect‐comparisons meta‐analysis. Br J Sports Med 2018;52:651–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bray F, Ferlay J, Soerjomataram I et al. Global cancer statistics 2018: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 11. Goldstraw P, Chansky K, Crowley J et al. The IASLC lung cancer staging project: Proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J Thorac Oncol 2016;11:39–51. [DOI] [PubMed] [Google Scholar]
- 12. Kimura M, Naito T, Kenmotsu H et al. Prognostic impact of cancer cachexia in patients with advanced non‐small cell lung cancer. Support Care Cancer 2015;23:1699–1708. [DOI] [PubMed] [Google Scholar]
- 13. Ashcraft KA, Peace RM, Betof AS et al. Efficacy and mechanisms of aerobic exercise on cancer initiation, progression, and metastasis: A critical systematic review of in vivo preclinical data. Cancer Res 2016;76:4032–4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bower JE, Bak K, Berger A et al. Screening, assessment, and management of fatigue in adult survivors of cancer: An American Society of Clinical Oncology clinical practice guideline adaptation. J Clin Oncol 2014;32:1840–1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sha F, Zhuang S, Zhou L et al. Biomarkers for cancer‐related fatigue and adverse reactions to chemotherapy in lung cancer patients. Mol Clin Oncol 2015;3:163–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hung R, Krebs P, Coups EJ et al. Fatigue and functional impairment in early‐stage non‐small cell lung cancer survivors. J Pain Symptom Manage 2011;41:426–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bower JE. Cancer‐related fatigue—Mechanisms, risk factors, and treatments. Nat Rev Clin Oncol 2014;11:597–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jager A, Sleijfer S, van der Rijt CC. The pathogenesis of cancer related fatigue: Could increased activity of pro‐inflammatory cytokines be the common denominator? Eur J Cancer 2008;44:175–181. [DOI] [PubMed] [Google Scholar]
- 19. Wang XS. Pathophysiology of cancer‐related fatigue. Clin J Oncol Nurs 2008;12:11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mustian KM, Alfano CM, Heckler C et al. Comparison of pharmaceutical, psychological, and exercise treatments for cancer‐related fatigue: A meta‐analysis. JAMA Oncol 2017;3:961–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. D'Silva A, Gardiner PA, Boyle T et al. Associations of objectively assessed physical activity and sedentary time with health‐related quality of life among lung cancer survivors: A quantile regression approach. Lung Cancer 2018;119:78–84. [DOI] [PubMed] [Google Scholar]
- 22. Janssen SM, Abbink JJ, Lindeboom R et al. Outcomes of pulmonary rehabilitation after treatment for non‐small cell lung cancer stages I to IIIa: An observational study. J Cardiopulm Rehabil Prev 2017;37:65–71. [DOI] [PubMed] [Google Scholar]
- 23. Lin YY, Rau KM, Lin CC. Longitudinal study on the impact of physical activity on the symptoms of lung cancer survivors. Support Care Cancer 2015;23:3545–3553. [DOI] [PubMed] [Google Scholar]
- 24. Zhang LL, Wang SZ, Chen HL et al. Tai chi exercise for cancer‐related fatigue in patients with lung cancer undergoing chemotherapy: A randomized controlled trial. J Pain Symptom Manage 2016;51:504–511. [DOI] [PubMed] [Google Scholar]
- 25. Hoffman AJ, Brintnall RA, von Eye A et al. A rehabilitation program for lung cancer patients during postthoracotomy chemotherapy. Onco Targets Ther 2014;7:415–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hoffman AJ, Brintnall RA, Brown JK et al. Too sick not to exercise: Using a 6‐week, home‐based exercise intervention for cancer‐related fatigue self‐management for postsurgical non‐small cell lung cancer patients. Cancer Nurs 2013;36:175–188. [DOI] [PubMed] [Google Scholar]
- 27. Jones LW, Eves ND, Peterson BL et al. Safety and feasibility of aerobic training on cardiopulmonary function and quality of life in postsurgical nonsmall cell lung cancer patients: A pilot study. Cancer 2008;113:3430–3439. [DOI] [PubMed] [Google Scholar]
- 28. Quist M, Sommer MS, Vibe‐Petersen J et al. Early initiated postoperative rehabilitation reduces fatigue in patients with operable lung cancer: A randomized trial. Lung Cancer 2018;126:125–132. [DOI] [PubMed] [Google Scholar]
- 29. Dhillon HM, Bell ML, van der Ploeg HP et al. Impact of physical activity on fatigue and quality of life in people with advanced lung cancer: A randomized controlled trial. Ann Oncol 2017;28:1889–1897. [DOI] [PubMed] [Google Scholar]
- 30. Chen HM, Tsai CM, Wu YC et al. Randomised controlled trial on the effectiveness of home‐based walking exercise on anxiety, depression and cancer‐related symptoms in patients with lung cancer. Br J Cancer 2015;112:438–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kuehr L, Wiskemann J, Abel U et al. Exercise in patients with non‐small cell lung cancer. Med Sci Sports Exerc 2014;46:656–663. [DOI] [PubMed] [Google Scholar]
- 32. Cheville AL, Kollasch J, Vandenberg J et al. A home‐based exercise program to improve function, fatigue, and sleep quality in patients with stage IV lung and colorectal cancer: A randomized controlled trial. J Pain Symptom Manage 2013;45:811–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lemonnier I, Guillemin F, Arveux P et al. Quality of life after the initial treatments of non‐small cell lung cancer: A persistent predictor for patients’ survival. Health Qual Life Outcomes 2014;12:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pompili C. Quality of life after lung resection for lung cancer. J Thorac Dis 2015;7(suppl 2):S138–S144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. The World Health Organization quality of life assessment (WHOQOL): Position paper from the World Health Organization. Soc Sci Med 1995;41:1403–1409. [DOI] [PubMed] [Google Scholar]
- 36. Rauma V, Sintonen H, Rasanen JV et al. Long‐term lung cancer survivors have permanently decreased quality of life after surgery. Clin Lung Cancer 2015;16:40–45. [DOI] [PubMed] [Google Scholar]
- 37. Akin S, Kas Guner C. Investigation of the relationship among fatigue, self‐efficacy and quality of life during chemotherapy in patients with breast, lung or gastrointestinal cancer. Eur J Cancer Care (Engl) 2018;28:e12898. [DOI] [PubMed] [Google Scholar]
- 38. Ostroff JS, Krebs P, Coups EJ et al. Health‐related quality of life among early‐stage, non‐small cell, lung cancer survivors. Lung Cancer 2011;71:103–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Polanski J, Jankowska‐Polanska B, Rosinczuk J et al. Quality of life of patients with lung cancer. Onco Targets Ther 2016;9:1023–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lin YY, Liu MF, Tzeng JI et al. Effects of walking on quality of life among lung cancer patients: A longitudinal study. Cancer Nurs 2015;38:253–259. [DOI] [PubMed] [Google Scholar]
- 41. Gerritsen JK, Vincent AJ. Exercise improves quality of life in patients with cancer: A systematic review and meta‐analysis of randomised controlled trials. Br J Sports Med 2016;50:796–803. [DOI] [PubMed] [Google Scholar]
- 42. Messaggi‐Sartor M, Marco E, Martínez‐Téllez E et al. Combined aerobic exercise and high‐intensity respiratory muscle training in patients surgically treated for non‐small cell lung cancer: A pilot randomized clinical trial. Eur J Phys Rehabil Med 2019;55:113–122. [DOI] [PubMed] [Google Scholar]
- 43. Cavalheri V, Jenkins S, Cecins N et al. Exercise training for people following curative intent treatment for non‐small cell lung cancer: A randomized controlled trial. Braz J Phys Ther 2017;21:58–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Quist M, Adamsen L, Rorth M et al. The impact of a multidimensional exercise intervention on physical and functional capacity, anxiety, and depression in patients with advanced‐stage lung cancer undergoing chemotherapy. Integr Cancer Therapies 2015;14:341–349. [DOI] [PubMed] [Google Scholar]
- 45. Granger CL, Chao C, McDonald CF et al. Safety and feasibility of an exercise intervention for patients following lung resection: A pilot randomized controlled trial. Integr Cancer Ther 2013;12:213–224. [DOI] [PubMed] [Google Scholar]
- 46. Hwang CL, Yu CJ, Shih JY et al. Effects of exercise training on exercise capacity in patients with non‐small cell lung cancer receiving targeted therapy. Support Care Cancer 2012;20:3169–3177. [DOI] [PubMed] [Google Scholar]
- 47. Arbane G, Tropman D, Jackson D et al. Evaluation of an early exercise intervention after thoracotomy for non‐small cell lung cancer (NSCLC), effects on quality of life, muscle strength and exercise tolerance: Randomised controlled trial. Lung Cancer 2011;71:229–234. [DOI] [PubMed] [Google Scholar]
- 48. Temel JS, Greer JA, Goldberg S et al. A structured exercise program for patients with advanced non‐small cell lung cancer. J Thorac Oncol 2009;4:595–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Brocki BC, Andreasen J, Nielsen LR et al. Short and long‐term effects of supervised versus unsupervised exercise training on health‐related quality of life and functional outcomes following lung cancer surgery ‐ A randomized controlled trial. Lung Cancer 2014;83:102–108. [DOI] [PubMed] [Google Scholar]
- 50. Stigt JA, Uil SM, van Riesen SJ et al. A randomized controlled trial of postthoracotomy pulmonary rehabilitation in patients with resectable lung cancer. J Thorac Oncol 2013;8:214–221. [DOI] [PubMed] [Google Scholar]
- 51. Sommer MS, Trier K, Vibe‐Petersen J et al. Changes in health‐related quality of life during rehabilitation in patients with operable lung cancer: A feasibility study (PROLUCA). Integr Cancer Ther 2018;17:388–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Edvardsen E, Skjonsberg OH, Holme I et al. High‐intensity training following lung cancer surgery: A randomised controlled trial. Thorax 2015;70:244–250. [DOI] [PubMed] [Google Scholar]
- 53. Henke CC, Cabri J, Fricke L et al. Strength and endurance training in the treatment of lung cancer patients in stages IIIa/IIIb/IV. Support Care Cancer 2014;22:95–101. [DOI] [PubMed] [Google Scholar]
- 54. Peddle‐McIntyre CJ, Baker MK, Lee YCG et al. The feasibility of a pragmatic distance‐based intervention to increase physical activity in lung cancer survivors. Eur J Cancer Care (Engl) 2018;27:12722. [DOI] [PubMed] [Google Scholar]
- 55. Arbane G, Douiri A, Hart N et al. Effect of postoperative physical training on activity after curative surgery for non‐small cell lung cancer: A multicentre randomised controlled trial. Physiotherapy 2014;100:100–107. [DOI] [PubMed] [Google Scholar]
- 56. Brunelli A, Kim AW, Berger KI et al. Physiologic evaluation of the patient with lung cancer being considered for resectional surgery: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence‐based clinical practice guidelines. Chest 2013;143(suppl 5):e166S–e190S. [DOI] [PubMed] [Google Scholar]
- 57. Lee JH, Song EM, Sim YS et al. Forced expiratory volume in one second as a prognostic factor in advanced non‐small cell lung cancer. J Thorac Oncol 2011;6:305–309. [DOI] [PubMed] [Google Scholar]
- 58. Berry MF, Yang CJ, Hartwig MG et al. Impact of pulmonary function measurements on long‐term survival after lobectomy for stage I non‐small cell lung cancer. Ann Thorac Surg 2015;100:271–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Linhas A, Campainha S, Conde S et al. P1.04‐027 changes in pulmonary function in lung cancer patients after thoracic surgery. J Thorac Oncol 2017;12:S611–S612. [Google Scholar]
- 60. Lopez Guerra JL, Gomez DR, Zhuang Y et al. Changes in pulmonary function after three‐dimensional conformal radiotherapy, intensity‐modulated radiotherapy, or proton beam therapy for non‐small‐cell lung cancer. Int J Radiat Oncol Biol Phys 2012;83:e537–e543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Rivera MP, Detterbeck FC, Socinski MA et al. Impact of preoperative chemotherapy on pulmonary function tests in resectable early‐stage non‐small cell lung cancer. Chest 2009;135:1588–1595. [DOI] [PubMed] [Google Scholar]
- 62. Dempsey JA. J.B. Wolffe memorial lecture. Is the lung built for exercise? Med Sci Sports Exerc 1986;18:143–155. [PubMed] [Google Scholar]
- 63. Salcedo PA, Lindheimer JB, Klein‐Adams JC et al. Effects of exercise training on pulmonary function in adults with chronic lung disease: A meta‐analysis of randomized controlled trials. Arch Phys Med Rehabil 2018;99:2561–2569.e7. [DOI] [PubMed] [Google Scholar]
- 64. Sommer MS, Trier K, Vibe‐Petersen J et al. Perioperative rehabilitation in operable lung cancer patients (PROLUCA): A feasibility study. Integr Cancer Ther 2016;15:455–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Andersen AH, Vinther A, Poulsen LL et al. A modified exercise protocol may promote continuance of exercise after the intervention in lung cancer patients—A pragmatic uncontrolled trial. Support Care Cancer 2013;21:2247–2253. [DOI] [PubMed] [Google Scholar]
- 66. Quist M, Rorth M, Langer S et al. Safety and feasibility of a combined exercise intervention for inoperable lung cancer patients undergoing chemotherapy: A pilot study. Lung Cancer 2012;75:203–208. [DOI] [PubMed] [Google Scholar]
- 67. Spruit MA, Janssen PP, Willemsen SC et al. Exercise capacity before and after an 8‐week multidisciplinary inpatient rehabilitation program in lung cancer patients: A pilot study. Lung Cancer 2006;52:257–260. [DOI] [PubMed] [Google Scholar]
- 68. Kim HK, Lee YJ, Han KN et al. Pulmonary function changes over 1 year after lobectomy in lung cancer. Respir Care 2016;61:376–382. [DOI] [PubMed] [Google Scholar]
- 69. Kim SJ, Lee YJ, Park JS et al. Changes in pulmonary function in lung cancer patients after video‐assisted thoracic surgery. Ann Thorac Surg 2015;99:210–217. [DOI] [PubMed] [Google Scholar]
- 70. Chang NW, Lin KC, Lee SC et al. Effects of an early postoperative walking exercise programme on health status in lung cancer patients recovering from lung lobectomy. J Clinic Nurs 2014;23:3391–3402. [DOI] [PubMed] [Google Scholar]
- 71. Tarumi S, Yokomise H, Gotoh M et al. Pulmonary rehabilitation during induction chemoradiotherapy for lung cancer improves pulmonary function. J Thorac Cardiovasc Surg 2015;149:569–573. [DOI] [PubMed] [Google Scholar]
- 72. Licker M, Triponez F, Diaper J et al. Preoperative evaluation of lung cancer patients. Curr Anesthesiol Rep 2014;4:124–134. [Google Scholar]
- 73. Jones LW, Watson D, Herndon JE 2nd et al. Peak oxygen consumption and long‐term all‐cause mortality in nonsmall cell lung cancer. Cancer 2010;116:4825–4832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kasymjanova G, Correa JA, Kreisman H et al. Prognostic value of the six‐minute walk in advanced non‐small cell lung cancer. J Thorac Oncol 2009;4:602–607. [DOI] [PubMed] [Google Scholar]
- 75. Jones LW, Hornsby WE, Goetzinger A et al. Prognostic significance of functional capacity and exercise behavior in patients with metastatic non‐small cell lung cancer. Lung Cancer 2012;76:248–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Cavalheri V, Jenkins S, Cecins N et al. Impairments after curative intent treatment for non‐small cell lung cancer: A comparison with age and gender‐matched healthy controls. Respir Med 2015;109:1332–1339. [DOI] [PubMed] [Google Scholar]
- 77. Granger CL, McDonald CF, Irving L et al. Low physical activity levels and functional decline in individuals with lung cancer. Lung Cancer 2014;83:292–299. [DOI] [PubMed] [Google Scholar]
- 78. Lakoski SG, Eves ND, Douglas PS et al. Exercise rehabilitation in patients with cancer. Nat Rev Clin Oncol 2012;9:288–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Vainshelboim B, Fox BD, Saute M et al. Limitations in exercise and functional capacity in long‐term postpneumonectomy patients. J Cardiopulm Rehabil Prev 2015;35:56–64. [DOI] [PubMed] [Google Scholar]
- 80. Salhi B, Haenebalcke C, Perez‐Bogerd S et al. Rehabilitation in patients with radically treated respiratory cancer: A randomised controlled trial comparing two training modalities. Lung Cancer 2015;89:167–174. [DOI] [PubMed] [Google Scholar]
- 81. Nattenmuller J, Wochner R, Muley T et al. Prognostic impact of CT‐quantified muscle and fat distribution before and after first‐line‐chemotherapy in lung cancer patients. PLoS One 2017;12:e0169136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Srdic D, Plestina S, Sverko‐Peternac A et al. Cancer cachexia, sarcopenia and biochemical markers in patients with advanced non‐small cell lung cancer—chemotherapy toxicity and prognostic value. Support Care Cancer 2016;24:4495–4502. [DOI] [PubMed] [Google Scholar]
- 83. Shiroyama T, Nagatomo I, Koyama S et al. Impact of sarcopenia in patients with advanced non‐small cell lung cancer treated with PD‐1 inhibitors: A preliminary retrospective study. Sci Rep 2019;9:2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Garcia‐Hermoso A, Cavero‐Redondo I, Ramirez‐Velez R et al. Muscular strength as a predictor of all‐cause mortality in an apparently healthy population: A systematic review and meta‐analysis of data from approximately 2 million men and women. Arch Phys Med Rehabil 2018;99:2100–2113.e2105. [DOI] [PubMed] [Google Scholar]
- 85. Kilgour RD, Vigano A, Trutschnigg B et al. Handgrip strength predicts survival and is associated with markers of clinical and functional outcomes in advanced cancer patients. Support Care Cancer 2013;21:3261–3270. [DOI] [PubMed] [Google Scholar]
- 86. Salhi B, Huysse W, Van Maele G et al. The effect of radical treatment and rehabilitation on muscle mass and strength: A randomized trial in stages I‐III lung cancer patients. Lung Cancer 2014;84:56–61. [DOI] [PubMed] [Google Scholar]
- 87. Jee H, Chang JE, Yang EJ. Positive prehabilitative effect of intense treadmill exercise for ameliorating cancer cachexia symptoms in a mouse model. J Cancer 2016;7:2378–2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Lira FS, Neto JC, Seelaender M. Exercise training as treatment in cancer cachexia. Appl Physiol Nutr Metab 2014;39:679–686. [DOI] [PubMed] [Google Scholar]
- 89. Solheim TS, Laird BJA, Balstad TR et al. A randomized phase II feasibility trial of a multimodal intervention for the management of cachexia in lung and pancreatic cancer. J Cachexia Sarcopenia Muscle 2017;8:778–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Kvam S, Kleppe CL, Nordhus IH et al. Exercise as a treatment for depression: A meta‐analysis. J Affect Disord 2016;202:67–86. [DOI] [PubMed] [Google Scholar]
- 91. Rebar AL, Stanton R, Geard D et al. A meta‐meta‐analysis of the effect of physical activity on depression and anxiety in non‐clinical adult populations. Health Psychol Rev 2015;9:366–378. [DOI] [PubMed] [Google Scholar]
- 92. Wegner M, Helmich I, Machado S et al. Effects of exercise on anxiety and depression disorders: Review of meta‐analyses and neurobiological mechanisms. CNS Neurol Disord Drug Targets 2014;13:1002–1014. [DOI] [PubMed] [Google Scholar]
- 93. Hopwood P, Stephens RJ. Depression in patients with lung cancer: Prevalence and risk factors derived from quality‐of‐life data. J Clin Oncol 2000;18:893–903. [DOI] [PubMed] [Google Scholar]
- 94. Chen ML, Yu CT, Yang CH. Sleep disturbances and quality of life in lung cancer patients undergoing chemotherapy. Lung Cancer 2008;62:391–400. [DOI] [PubMed] [Google Scholar]
- 95. Banno M, Harada Y, Taniguchi M et al. Exercise can improve sleep quality: A systematic review and meta‐analysis. PeerJ 2018;6:e5172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Chen HM, Tsai CM, Wu YC et al. Effect of walking on circadian rhythms and sleep quality of patients with lung cancer: A randomised controlled trial. Br J Cancer 2016;115:1304–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell 2011;144:646–674. [DOI] [PubMed] [Google Scholar]
- 98. Rowe GC, Jang C, Patten IS et al. PGC‐1β regulates angiogenesis in skeletal muscle. Am J Physiol Endocrinol Metab 2011;301:E155–E163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Tsai MS, Kuo ML, Chang CC et al. The effects of exercise training on levels of vascular endothelial growth factor in tumor‐bearing mice. Cancer Biomark 2013;13:307–313. [DOI] [PubMed] [Google Scholar]
- 100. Alves CRR, das Neves W, Tobias GC et al. High intensity interval training slows down tumor progression in mice bearing Lewis lung carcinoma. J Cachexia Sarcopenia Muscle 2018;1:60. [Google Scholar]
- 101. Higgins KA, Park D, Lee GY et al. Exercise‐induced lung cancer regression: Mechanistic findings from a mouse model. Cancer 2014;120:3302–3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Brognard J, Clark AS, Ni Y et al. Akt/protein kinase b is constitutively active in non‐small cell lung cancer cells and promotes cellular survival and resistance to chemotherapy and radiation. Cancer Res 2001;61:3986–3997. [PubMed] [Google Scholar]
- 103. McCubrey JA, Steelman LS, Kempf CR et al. Therapeutic resistance resulting from mutations in RAF/MEK/ERK and PI3K/PTEN/AKT/mTOR signaling pathways. J Cell Physiol 2011;226:2762–2781. [DOI] [PubMed] [Google Scholar]
- 104. Kurgan N, Tsakiridis E, Kouvelioti R et al. Inhibition of human lung cancer cell proliferation and survival by post‐exercise serum is associated with the inhibition of Akt, mTOR, p70 S6K, and Erk1/2. Cancers (Basel) 2017;9:E46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Pedersen L, Idorn M, Olofsson GH et al. Voluntary running suppresses tumor growth through epinephrine‐ and IL‐6‐dependent NK cell mobilization and redistribution. Cell Metab 2016;23:554–562. [DOI] [PubMed] [Google Scholar]
- 106. Liu J, Chen P, Wang R et al. Effect of tai chi on mononuclear cell functions in patients with non‐small cell lung cancer. BMC Complement Altern Med 2015;15:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Wang R, Liu J, Chen P et al. Regular tai chi exercise decreases the percentage of type 2 cytokine‐producing cells in postsurgical non‐small cell lung cancer survivors. Cancer Nurs 2013;36:E27–E34. [DOI] [PubMed] [Google Scholar]
- 108. Karvinen KH, Esposito D, Raedeke TD et al. Effect of an exercise training intervention with resistance bands on blood cell counts during chemotherapy for lung cancer: A pilot randomized controlled trial. SpringerPlus 2014;3:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Leach HJ, Devonish JA, Bebb DG et al. Exercise preferences, levels and quality of life in lung cancer survivors. Support Care Cancer 2015;23:3239–3247. [DOI] [PubMed] [Google Scholar]
- 110. Missel M, Pedersen JH, Hendriksen C et al. Exercise intervention for patients diagnosed with operable non‐small cell lung cancer: A qualitative longitudinal feasibility study. Support Care Cancer 2015;23:2311–2318. [DOI] [PubMed] [Google Scholar]
- 111. Granger CL, Connolly B, Denehy L et al. Understanding factors influencing physical activity and exercise in lung cancer: A systematic review. Support Care Cancer 2017;25:983–999. [DOI] [PubMed] [Google Scholar]
- 112. Occhipinti S, Dunn J, O'Connell DL et al. Lung cancer stigma across the social network: Patient and caregiver perspectives. J Thorac Oncol 2018;13:1443–1453. [DOI] [PubMed] [Google Scholar]
- 113. Pinto BM, Ciccolo JT. Physical activity motivation and cancer survivorship. Recent Results Cancer Res 2011;186:367–387. [DOI] [PubMed] [Google Scholar]
- 114. Rock CL, Doyle C, Demark‐Wahnefried W et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin 2012;62:243–274. [DOI] [PubMed] [Google Scholar]


