Short abstract
In 2018, a multi‐disciplinary workshop was held at the Massachusetts General Hospital to discuss challenges in defining, diagnosing, and treating immune‐related adverse events (irAE), including those that occur in patients administered PD‐1/L1 inhibitor combination therapy. In this commentary, the workshop participants present a clinical case that illustrates the complexity of irAE diagnosis and management in a patient receiving PD‐1/L1 combination therapy, summarize the current state of PD‐1/L1 combination therapy, and discuss challenges and opportunities for the evaluation of irAEs as these combinations become more widely used to treat patients with cancer.
Keywords: Immune checkpoint inhibitor, Immune‐related adverse events, Immunotherapy, PD‐1/L1 inhibitor, Immunotherapy toxicity
Introduction
As of November 1, 2018, the U.S. Food and Drug Administration (FDA) had approved six immune checkpoint inhibitors (ICIs) targeting the programmed death‐1 (PD‐1) pathway, including three PD‐1 inhibitors (pembrolizumab, nivolumab, and cemiplimab) and three PD‐1 ligand (PD‐L1) inhibitors (atezolizumab, avelumab, and durvalumab). With indications spanning multiple tumor types 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, PD‐1 and PD‐L1 inhibitors have become the standard of care for many cancers. Because of their mechanism of action, PD‐1 and PD‐L1 inhibitors can cause a distinct set of inflammatory side effects, known as immune‐related adverse events (irAEs). Whereas mild irAEs can generally be treated supportively, severe toxicity requires urgent intervention and, in some cases, may be fatal 48, 49. The recognition that irAEs may arise in patients receiving PD‐1/L1 inhibitors has prompted the coordination of multidisciplinary groups to scrutinize these toxicities 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61 and the development of consensus guidelines by professional organizations to diagnose and manage them 62, 63, 64, 65.
Despite the clinical success of PD‐1 and PD‐L1 inhibitors for a considerable proportion of patients with cancer, it has become apparent that they do not elicit responses in all tumors or individuals. Thus, efforts are underway to potentiate the activity of these agents by administering them with one or more additional types of therapies (e.g., another immunotherapy, cytotoxic chemotherapy, or targeted therapy), herein referred to as “PD‐1/L1 inhibitor combination therapy”, and expand the population of patients who may experience clinical benefit. Combining PD‐1/L1 inhibitors with a cytotoxic T‐lymphocyte–associated antigen 4 (CTLA‐4) inhibitor has shown promise, as evidenced by the approval of nivolumab in combination with ipilimumab for the treatment of metastatic melanoma, advanced renal cell carcinoma (RCC), and microsatellite instability–high or mismatch repair‐deficient colorectal cancer 22, 28, 47. The combination of pembrolizumab with cytotoxic chemotherapy demonstrated clinical benefit 4, 5 and was FDA approved for first‐line treatment of metastatic non‐small cell lung cancer (NSCLC) irrespective of PD‐L1 expression; because PD‐L1 expression is required for pembrolizumab monotherapy in this setting, the combination regimen broadens the eligible patient population. More recently, there were FDA approvals for pembrolizumab or avelumab in combination with axitinib, a vascular endothelial growth factor receptor tyrosine kinase inhibitor, as first‐line treatment for advanced RCC, based on two phase III trials 19, 43, and for atezolizumab in combination with chemotherapy for extensive‐stage small cell lung cancer 40 and for certain women with advanced triple‐negative breast cancer 39.
Possible synergies between PD‐1/L1 inhibitors and agents with different mechanisms of action 66 are increasingly being investigated in clinical trials 67, 68, 69, with one analysis reporting 1,105 studies testing PD‐1/L1 inhibitor combinations in 2017 67, and a recent update identifying 1,716 such trials 69. Optimistically, the outcomes of these trials may render PD‐1/L1 inhibitor combination therapy the dominant treatment strategy for many tumor types. Parenthetically, the sheer number of open combination trials assumes that adding a second agent to PD‐1/L1 inhibitors has the potential to offer more than additive benefit and reverse the inactivity of PD‐1/L1 inhibitors in some tumor types, a hypothesis that remains largely unproven. Nonetheless, in anticipation of a paradigm shift toward combination therapy, it is crucial to consider how administration of PD‐1/L1 inhibitors with other agents may confound the clinical presentation, diagnosis, and management of irAEs, as well as to equip the medical community with sound strategies to identify and treat these side effects. A systematic review of 35 clinical trials showed a different pattern of toxicity regarding frequency and specific organ involvement depending on the type of therapeutic scheme that was administered (i.e., PD‐1/L1 inhibitor monotherapy, CTLA‐4 monotherapy, immunotherapy combination therapies, or concomitant administration of immunotherapy and chemotherapy; Fig. 1) 70. The current guidelines for management of irAEs, based largely on toxicities with CTLA‐4 and PD‐1/L1 monotherapies, will likely require revision as experience with combination therapies continues to accrue. Guidance and management for irAEs related to administration of different ICIs together will be essential, as well as combination of immunotherapies with chemotherapy and/or targeted therapies, where the resulting toxicity can be challenging to diagnose and treat.
Figure 1.
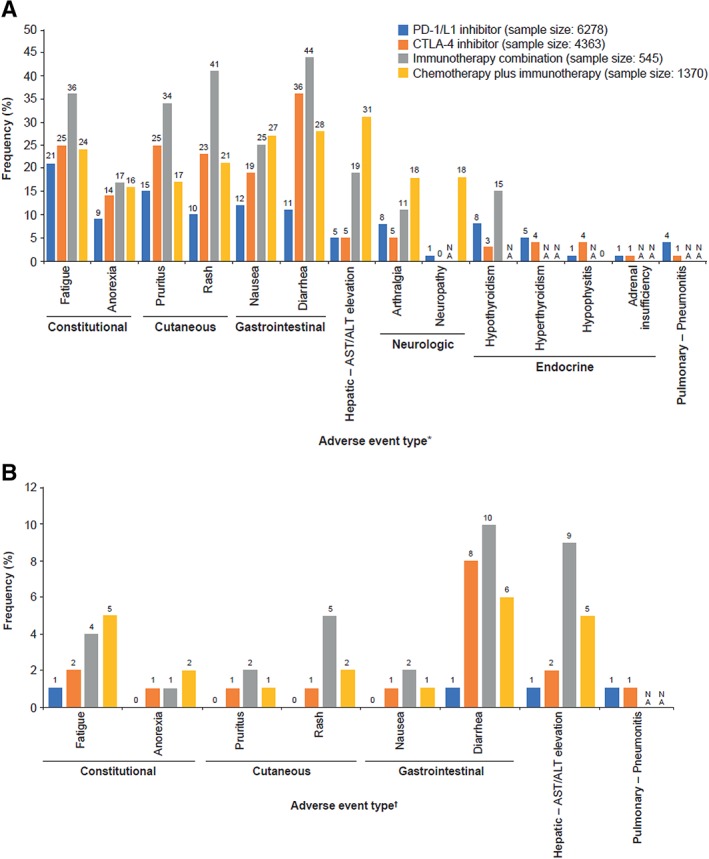
Frequency of (A) any grade and (B) grade 3/4 adverse events in clinical trials of PD‐1/L1 inhibitors, CTLA‐4 inhibitors, immunotherapy combinations, and chemotherapy plus immunotherapy combinations 70. *, Myositis and mucositis not included because these adverse events were NA for all groups except for PD‐1/L1 inhibitor. †, Adverse events from panel A with grade 3/4 frequency of 0 or NA in ≥3 groups were excluded. Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; CTLA‐4, cytotoxic T‐lymphocyte–associated antigen 4; NA, not available; PD‐1, programmed death‐1; PD‐L1, PD‐1 ligand.
A multidisciplinary workshop was held at the Massachusetts General Hospital to discuss challenges in defining, diagnosing, and treating irAEs, including those that occur in patients administered PD‐1/L1 inhibitor combination therapy. Here, the workshop participants present a clinical case that illustrates the complexity of irAE diagnosis and management in a patient receiving PD‐1/L1 combination therapy, summarize the current state of PD‐1/L1 combination therapy based on an analysis of abstracts from the 2018 American Society of Clinical Oncology (ASCO) Annual Meeting, and discuss challenges and opportunities for the evaluation of irAEs as these combinations become more widely used to treat patients with cancer.
Case in Point: An Immune‐Related Adverse Event with PD‐1/L1 Inhibitor Combination Therapy
Strategies for the diagnosis and management of immune‐related colitis in patients receiving PD‐1/L1 inhibitor monotherapy or the CTLA‐4 inhibitor ipilimumab have been described in detail 62, 63, 64, 65, 71, 72. Briefly, immune‐related colitis can generally be diagnosed based on patient symptoms and medical history and following exclusion of infectious colitis, although endoscopy and biopsy may be warranted. Low‐grade immune‐related colitis may be treated symptomatically, but persistent or higher‐grade events typically require administration of systemic corticosteroids. Patients who do not respond to corticosteroids or who have recurring immune‐related colitis following a corticosteroid taper may require treatment with the antitumor necrosis factor‐α antibody infliximab. As noted above, published guidelines describing irAEs 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65 are focused on toxicity secondary to CTLA‐4 and PD‐1/L1 monotherapies or to the concurrent administration of both types of ICIs. Since co‐administration of another type of therapy (e.g., chemotherapy with a PD‐1/L1 inhibitor) may confound the diagnosis of immune‐related colitis and lead to the delay of a suitable remedy, current immunotherapy management guidelines should be applied judiciously.
Here, we describe a 74‐year‐old man with NSCLC treated with first‐line pembrolizumab in combination with pemetrexed and carboplatin. After two cycles of therapy, he developed grade 1 diarrhea, which slowly escalated. One month after symptom onset (following cycle 4 of therapy), he presented with grade 2 diarrhea and rectal bleeding. Outpatient flexible sigmoidoscopy showed significant mucosal inflammation (Fig. 2A), and biopsies revealed neutrophilic cryptitis with dilated, “withered” crypts and without prominent apoptosis, pathology that was not pathognomonic of pembrolizumab‐associated inflammation (Fig. 2B and 2C); thus, a chemotherapy‐related toxicity was presumed. He was initially treated conservatively using stool‐bulking measures (cholestyramine) and antidiarrheal medications (loperamide and diphenoxylate‐atropine). After 1 week of supportive management, his diarrhea persisted, and high‐dose corticosteroids (oral prednisone 60 mg per day) were initiated. Despite high‐dose corticosteroids, diarrhea worsened to grade 3–4 after another week, requiring hospitalization. Repeat flexible sigmoidoscopy showed persistent inflammation (Fig. 2D), and biopsies showed similar characteristics to the prior results but with progressive expansion of the lamina propria and a mixed inflammatory infiltrate more consistent with PD‐1 inhibitor‐induced colitis 71, 72, in spite of rare apoptotic bodies (Fig. 2E and 2F). Clostridium difficile toxin testing of stool cultures and cytomegalovirus stains on the biopsies were negative. Intravenous methylprednisolone was followed by a second‐line immunosuppressive agent, infliximab, to treat the refractory diarrhea. Diarrhea symptoms improved to grade 1 within 3 days of the start of infliximab, and the patient was transitioned to oral prednisone (60 mg per day) and discharged. The timely identification and treatment of irAEs is imperative, and in this example, the addition of chemotherapy to a PD‐1 inhibitor made the diagnosis even more challenging and led to a delay in appropriate management. This new era of combination therapy requires individuals to be aware of anchor bias and continue to reassess a patient's condition, at times with repeat testing, to avoid diagnostic inaccuracies.
Figure 2.
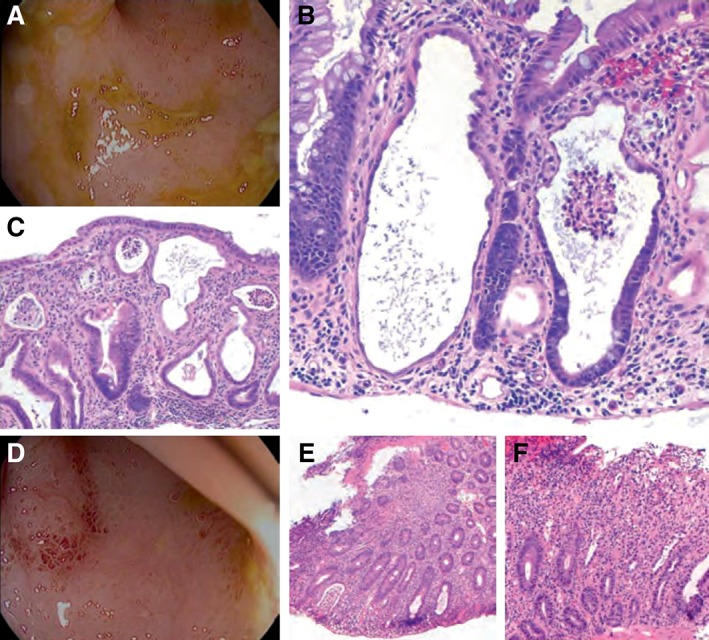
Colitis following treatment with a programmed death‐1 inhibitor (pembrolizumab) plus chemotherapy (pemetrexed and carboplatin). Images are following (A–C) onset of symptoms and (D–F) treatment with corticosteroids; symptoms subsided following treatment with intravenous methylprednisolone and infliximab. Initial biopsies (B–C) show prominent neutrophilic cryptitis with crypt epithelial injury, loss of goblet cells, and rare apoptotic bodies and lymphocytes. Subsequent biopsies (E–F) show similar features but also with expansion of the lamina propria by a mixed inflammatory infiltrate. Original magnification ×100 (C and E) and ×200 (B and F).
Reports of PD‐1/L1 Inhibitor Combination Therapy Trials at ASCO 2018
Current trends in PD‐1/L1 inhibitor combination therapy were evaluated using the abstract database from the 2018 ASCO Annual Meeting. We identified 359 abstracts that presented information on immuno‐oncology agents, of which ICIs are a subset. We excluded abstracts that included unspecified agents, monotherapy, preclinical or health economic and outcomes research studies, or meta‐analyses, finally identifying 183 abstracts reporting on clinical trials of PD‐1/L1 inhibitors in combination with other agents, representing 51% of the full set of ASCO 2018 abstracts on immuno‐oncology agents.
The majority (n = 134; 72%) of combination regimens presented at ASCO 2018 used pembrolizumab or nivolumab as the backbone (Fig. 3A), as expected, given the timing of FDA approvals. PD‐1/L1 inhibitors are being combined with a multitude of other therapies (Fig. 3B), most frequently within the categories of immunotherapy (n = 60; 31%), targeted therapy (n = 53; 27%), or cytotoxic chemotherapy (n = 37; 19%). Although most combinations (n = 173; 88%) consisted of a PD‐1/L1 inhibitor plus one other category of therapy, there were also studies of a PD‐1/L1 inhibitor administered with two additional types of therapies (e.g., chemotherapy plus targeted therapy).
Figure 3.
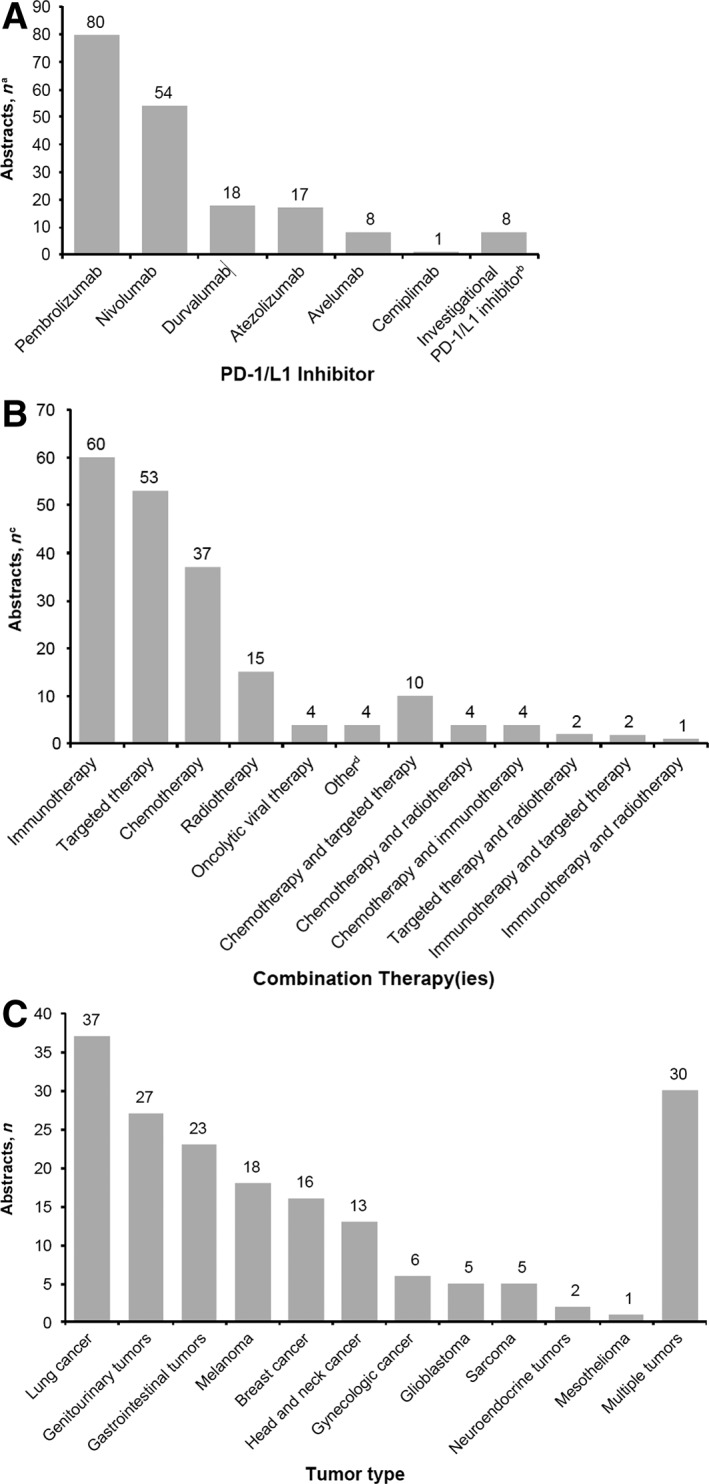
Analyses of programmed death‐1 (PD‐1) and PD‐1 ligand (PD‐L1) inhibitor combination trials at American Society of Clinical Oncology 2018. Analysis according to (A) PD‐1/L1 inhibitor tested, (B) type of combination therapy(ies) tested, and (C) cancer type. aSome trials had >1 PD‐1/L1 inhibitor arm (n = 186). bInvestigational PD‐1/L1 inhibitors were spartalizumab (n = 2) and BGBA333, CX‐072, JS001, M7824, MGA012, and SHR‐1210 (n = 1 each). cSome trials had >1 combination agent arm (n = 196). dOther agents were paricalcitol, ADI‐PEG 20, metformin, and LTX‐315 (n = 1 each).
Consistent with the current indications for PD‐1/L1 inhibitor monotherapy 1, 2, 3, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 20, 21, 23, 24, 25, 26, 27, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 41, 42, 44, 45, 46, combination regimens were most commonly being studied for the treatment of lung cancer, genitourinary cancer, gastrointestinal cancer, and melanoma (Fig. 3C). Tumor types thought to be less susceptible to ICIs were also under study, as well as trials with multiple tumor types. Combination regimens were most frequently being tested as second‐line or later treatment (n = 71; 39% overall). Nevertheless, it is notable that 22 (12%) abstracts described first‐line or subsequent therapy, 40 (22%) focused on first‐line treatment only, and 23 (13%) analyzed neoadjuvant and/or adjuvant therapy. The proportion of trials testing first‐line or neoadjuvant and/or adjuvant PD‐1/L1 inhibitor combination therapy is noteworthy, given that the majority of monotherapy approvals to date are for second‐line or later treatment, and approved indications of PD‐1/L1 inhibitors in an adjuvant setting are scarce (e.g., for nivolumab in patients with melanoma 23 and durvalumab in patients with unresectable stage III NSCLC following chemoradiotherapy 45).
Furthermore, our analysis indicated that PD‐1/L1 inhibitor combination regimens are predominantly in early stages of clinical development, with 41 (22%) abstracts reporting on phase I trials, 41 (22%) on phase I–II trials, 61 (33%) on phase II trials, and only 29 (16%) on phase III trials; the trial phase was not identified in 11 (6%) of the abstracts. Nearly half (n = 81; 44%) of the abstracts reported trials in progress, an indication that an abundance of PD‐1/L1 inhibitor combination data will be forthcoming. The number of trials in early development, in progress, or both, signals that we are still at the beginning of the era of PD‐1/L1 inhibitor combination therapy.
Immune‐Related Adverse Events with PD‐1/L1 Inhibitor Combination Therapy: Challenges and Opportunities
Toxicities associated with PD‐1/L1 inhibitor combination therapy come with an additional layer of complexity, as ICI use may magnify or alter the presentation of adverse events typically seen with traditional cancer therapies and may produce challenges in identifying the etiology. As illustrated in the case above, presentations of PD‐1/L1 inhibitor–associated colitis in the setting of combination treatment may be atypical. Both the endoscopic appearance and the pathology of the colitis seen in this case did not fall into established patterns, which typically demonstrate colonic edema, erythema, and, in severe cases, superficial ulcerations with pathology showing an acute colitis with prominent epithelial apoptosis. Because presentations may differ from established patterns, maintaining a high level of vigilance for potentially atypical presentations and failure of initial supportive therapy, as well as the ability to provide additional diagnostics to guide second‐line therapeutics, are essential for prompt diagnosis and treatment of irAEs in the setting of PD‐1/L1 inhibitor combination therapy. Moreover, this case illustrates the wide morphologic spectrum that may be seen on biopsy in bona fide cases of irAEs, which is increasingly recognized to be broader than that reported in initial case series. Delay or deferral of appropriate diagnostics may lead to inaccurate diagnoses, and empiric therapy may obscure distinctions between true irAEs and non‐irAEs. Downstream consequences could include unnecessary use of corticosteroids, which carries the possibility of immunosuppression that may increase the risk of infection and alter wound healing, and/or the delay or even discontinuation of additional courses of potentially life‐saving ICI treatment.
A retrospective analysis of patients with melanoma who were treated with the CTLA‐4 inhibitor ipilimumab at Massachusetts General Hospital and experienced hypophysitis found that use of higher doses of glucocorticoids to treat this irAE was associated with reduced survival and earlier time to treatment failure 73, and another retrospective analysis of patients with NSCLC treated with PD‐1/L1 inhibitors found that corticosteroid use at baseline was associated with inferior outcomes 74. These contrast with an earlier retrospective study that clearly demonstrated that patients receiving immunotherapy who are treated with corticosteroids can have durable antitumor responses 75. Although further study is needed, recent reports highlight the potential detrimental effect on anticancer response of high‐dose steroids at the onset or during PD‐1/L1 inhibitor treatment 73, 74 and thus the need to continue to refine treatment algorithms for irAEs based on available evidence. Similar to the approach used for PD‐1/L1 inhibitor monotherapy 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, the diagnosis and management of irAEs in patients receiving combination therapy will require an integrated team of oncologists and specialists.
The benefit of combining PD‐1/L1 inhibitors with other types of therapies has already been demonstrated with the approvals of nivolumab plus ipilimumab for melanoma, RCC, and colorectal cancer 22, 28, 47; the approval of pembrolizumab plus chemotherapy for NSCLC 4, 5; and the approvals of pembrolizumab 19 or avelumab 43 plus axitinib for advanced RCC. Our review of the 2018 ASCO abstracts, as well as analyses of clinical trials 67, 69, point to a likely rapid transition to combination therapy for additional cancers. Current management approaches for irAEs rely heavily on empiric treatment, with diagnostic testing typically playing a role only in the most severe clinical scenarios. It is critical that action is taken now to gather and evaluate information on irAEs in the context of PD‐1/L1 inhibitor combination therapy and to update guidelines for diagnosis and management accordingly in order to circumvent incorrect diagnoses, which may limit the use of these promising treatments, and empirical administration of steroids, which may diminish anticancer efficacy 73, 74.
The large quantity of PD‐1/L1 inhibitor combination data on the horizon will provide an opportunity to gain valuable insights into the management of irAEs. A collaborative approach involving academia, industry, and regulatory agencies will enable the formulation of appropriate irAE definitions and reporting, biomarker development, and monitoring and management algorithms. Moreover, this advanced strategy may aid in the selection of the most appropriate immunotherapy treatment for individual patients, in terms of possible toxicity and predicted outcomes.
Author Contributions
Conception/design: Leyre Zubiri, Ian M. Allen, Amanda C. Guidon, Steven T. Chen, Sara R. Schoenfeld, Tomas G. Neilan, Meghan E. Sise, Meghan J. Mooradian, Krista M. Rubin, Rebecca Karp Leaf, Aparna R. Parikh, Alexander Faje, Justin F. Gainor, Justine V. Cohen, Florian J. Fintelmann, Minna J. Kohler, Michael Dougan, Kerry L. Reynolds
Collection and/or assembly of data: Leyre Zubiri, Ian M. Allen, Martin S. Taylor, Michael Dougan, and Kerry L. Reynolds
Data analysis and interpretation: Leyre Zubiri, Ian M. Allen, Martin S. Taylor, Amanda C. Guidon, Steven T. Chen, Sara R. Schoenfeld, Tomas G. Neilan, Meghan E. Sise, Meghan J. Mooradian, Krista M. Rubin, Rebecca Karp Leaf, Aparna R. Parikh, Alexander Faje, Justin F. Gainor, Justine V. Cohen, Florian J. Fintelmann, Minna J. Kohler, Michael Dougan, Kerry L. Reynolds
Manuscript writing: Leyre Zubiri, Ian M. Allen, Martin S. Taylor, Amanda C. Guidon, Steven T. Chen, Sara R. Schoenfeld, Tomas G. Neilan, Meghan E. Sise, Meghan J. Mooradian, Krista M. Rubin, Rebecca Karp Leaf, Aparna R. Parikh, Alexander Faje, Justin F. Gainor, Justine V. Cohen, Florian J. Fintelmann, Minna J. Kohler, Michael Dougan, Kerry L. Reynolds
Final approval of manuscript: Leyre Zubiri, Ian M. Allen, Martin S. Taylor, Amanda C. Guidon, Steven T. Chen, Sara R. Schoenfeld, Tomas G. Neilan, Meghan E. Sise, Meghan J. Mooradian, Krista M. Rubin, Rebecca Karp Leaf, Aparna R. Parikh, Alexander Faje, Justin F. Gainor, Justine V. Cohen, Florian J. Fintelmann, Minna J. Kohler, Michael Dougan, Kerry L. Reynolds
Disclosures
Amanda C. Guidon: Momenta (C/A), Alexion, RaPharma, PCORI (RF), Oakstone Publishing (H), Alexion (SAB); Tomas G. Neilan: Bristol‐Myers Squibb, Intrinsic Imaging, Parexel, Aprea Therapeutics (C/A); Krista M. Rubin: Bristol‐Myers Squibb, Merck, Novartis (C/A); Aparna R. Parikh: Driver, Eisai, Puretech (C/A); Justin F. Gainor: Bristol‐Myers Squibb, Novartis, Pfizer, Merck, Genentech/Roche, Loxo, Incyte, Array, Agios, Regeneron, Amgen, Oncorus, Jounce, ARIAD/Takeda (C/A, H); Bristol‐Myers Squibb, Novartis, Merck, Genentech/Roche, Blueprint, Array, Jounce, Adaptimmune, Alexo, Tesaro, Genentech, ARIAD/Takeda, Novartis (RF); Justine V. Cohen: Sanofi‐Genzyme (C/A); Florian J. Fintelmann: McKesson Health Solutions, Jounce Therapeutics (C/A), William M. Wood Foundation, Society of Interventional Oncology, BTG PLC (RF); Michael Dougan: Genentech (C/A), Novartis (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Acknowledgments
The content for this project was developed at a multidisciplinary irAE workshop held at the Massachusetts General Hospital on April 30, 2018. Organizational support was provided by Project Data Sphere, LLC. Medical writing support was provided by Joanna Bloom, Ph.D., of Engage Scientific Solutions, and was funded by Pfizer. We thank Mazen Nasrallah, Riley Fadden, Ryan Sullivan, Molly Thomas, Jocelyn Farmer, and Alexandra‐Chloe Villani of Massachusetts General Hospital for their contributions during the “Future State: Reporting of Adverse Events” session.
Disclosures of potential conflicts of interest may be found at the end of this article.
References
- 1. Robert C, Schachter J, Long GV et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med 2015;372:2521–2532. [DOI] [PubMed] [Google Scholar]
- 2. Ribas A, Puzanov I, Dummer R et al. Pembrolizumab versus investigator‐choice chemotherapy for ipilimumab‐refractory melanoma (KEYNOTE‐002): A randomised, controlled, phase 2 trial. Lancet Oncol 2015;16:908–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Eggermont AMM, Blank CU, Mandala M et al. Adjuvant pembrolizumab versus placebo in resected stage III melanoma. N Engl J Med 2018;378:1789–1801. [DOI] [PubMed] [Google Scholar]
- 4. Gandhi L, Rodriguez‐Abreu D, Gadgeel S et al. Pembrolizumab plus chemotherapy in metastatic non‐small‐cell lung cancer. N Engl J Med 2018;378:2078–2092. [DOI] [PubMed] [Google Scholar]
- 5. Paz‐Ares L, Luft A, Vicente D et al. Pembrolizumab plus chemotherapy for squamous non‐small‐cell lung cancer. N Engl J Med 2018;379:2040–2051. [DOI] [PubMed] [Google Scholar]
- 6. Mok TSK, Wu YL, Kudaba I et al. Pembrolizumab versus chemotherapy for previously untreated, PD‐L1‐expressing, locally advanced or metastatic non‐small‐cell lung cancer (KEYNOTE‐042): A randomised, open‐label, controlled, phase 3 trial. Lancet 2019;393:1819–1830. [DOI] [PubMed] [Google Scholar]
- 7. Reck M, Rodriguez‐Abreu D, Robinson AG et al. Pembrolizumab versus chemotherapy for PD‐L1‐positive non‐small‐cell lung cancer. N Engl J Med 2016;375:1823–1833. [DOI] [PubMed] [Google Scholar]
- 8. Herbst RS, Baas P, Kim DW et al. Pembrolizumab versus docetaxel for previously treated, PD‐L1‐positive, advanced non‐small‐cell lung cancer (KEYNOTE‐010): A randomised controlled trial. Lancet 2016;387:1540–1550. [DOI] [PubMed] [Google Scholar]
- 9. Chung HC, Piha‐Paul SA, Lopez‐Martin J et al. Abstract CT073: Pembrolizumab after two or more lines of prior therapy in patients with advanced small‐cell lung cancer (SCLC): Results from the KEYNOTE‐028 and KEYNOTE‐158 studies. Cancer Res 2019;79:CT073–CT073. [DOI] [PubMed]
- 10. Rischin D, Harrington KJ, Greil R et al. Protocol‐specified final analysis of the phase 3 KEYNOTE‐048 trial of pembrolizumab (pembro) as first‐line therapy for recurrent/metastatic head and neck squamous cell carcinoma (R/M HNSCC). J Clin Oncol 2019;37(suppl 15):6000. [Google Scholar]
- 11. Chen R, Zinzani PL, Fanale MA et al. Phase II study of the efficacy and safety of pembrolizumab for relapsed/refractory classic Hodgkin lymphoma. J Clin Oncol 2017;35:2125–2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zinzani PL, Thieblemont C, Melnichenko V et al. Efficacy and safety of pembrolizumab in relapsed/refractory primary mediastinal large B‐cell lymphoma (rrPMBCL): Updated analysis of the Keynote‐170 phase 2 trial. Blood 2017;130:2833. [Google Scholar]
- 13. Balar AV, Castellano D, O'Donnell PH et al. First‐line pembrolizumab in cisplatin‐ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE‐052): A multicentre, single‐arm, phase 2 study. Lancet Oncol 2017;18:1483–1492. [DOI] [PubMed] [Google Scholar]
- 14. Bellmunt J, de Wit R, Vaughn DJ et al. Pembrolizumab as second‐line therapy for advanced urothelial carcinoma. N Engl J Med 2017;376:1015–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fuchs CS, Doi T, Jang RW et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: Phase 2 clinical KEYNOTE‐059 trial. JAMA Oncol 2018;4:e180013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chung HC, Ros W, Delord JP et al. Efficacy and safety of pembrolizumab in previously treated advanced cervical cancer: Results from the phase II KEYNOTE‐158 study. J Clin Oncol 2019;37:1470–1478. [DOI] [PubMed] [Google Scholar]
- 17. Zhu AX, Finn RS, Edeline J et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE‐224): A non‐randomised, open‐label phase 2 trial. Lancet Oncol 2018;19:940–952. [DOI] [PubMed] [Google Scholar]
- 18. Nghiem PT, Bhatia S, Lipson EJ et al. PD‐1 blockade with pembrolizumab in advanced Merkel‐cell carcinoma. N Engl J Med 2016;374:2542–2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rini BI, Plimack ER, Stus V et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal‐cell carcinoma. N Engl J Med 2019;380:1116–1127. [DOI] [PubMed] [Google Scholar]
- 20. Weber JS, D'Angelo SP, Minor D et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti‐CTLA‐4 treatment (CheckMate 037): A randomised, controlled, open‐label, phase 3 trial. Lancet Oncol 2015;16:375–384. [DOI] [PubMed] [Google Scholar]
- 21. Robert C, Long GV, Brady B et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 2015;372:320–330. [DOI] [PubMed] [Google Scholar]
- 22. Larkin J, Chiarion‐Sileni V, Gonzalez R et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 2015;373:23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Weber J, Mandala M, Del Vecchio M et al. Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N Engl J Med 2017;377:1824–1835. [DOI] [PubMed] [Google Scholar]
- 24. Borghaei H, Paz‐Ares L, Horn L et al. Nivolumab versus docetaxel in advanced nonsquamous non‐small‐cell lung cancer. N Engl J Med 2015;373:1627–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brahmer J, Reckamp KL, Baas P et al. Nivolumab versus docetaxel in advanced squamous‐cell non‐small‐cell lung cancer. N Engl J Med 2015;373:123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Antonia SJ, Lopez‐Martin JA, Bendell J et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small‐cell lung cancer (CheckMate 032): A multicentre, open‐label, phase 1/2 trial. Lancet Oncol 2016;17:883–895. [DOI] [PubMed] [Google Scholar]
- 27. Motzer RJ, Escudier B, McDermott DF et al. Nivolumab versus everolimus in advanced renal‐cell carcinoma. N Engl J Med 2015;373:1803–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Motzer RJ, Tannir NM, McDermott DF et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal‐cell carcinoma. N Engl J Med 2018;378:1277–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Younes A, Santoro A, Shipp M et al. Nivolumab for classical Hodgkin's lymphoma after failure of both autologous stem‐cell transplantation and brentuximab vedotin: A multicentre, multicohort, single‐arm phase 2 trial. Lancet Oncol 2016;17:1283–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ansell SM, Lesokhin AM, Borrello I et al. PD‐1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. N Engl J Med 2015;372:311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ferris RL, Blumenschein G, Jr., Fayette J et al. Nivolumab for recurrent squamous‐cell carcinoma of the head and neck. N Engl J Med 2016;375:1856–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sharma P, Retz M, Siefker‐Radtke A et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): A multicentre, single‐arm, phase 2 trial. Lancet Oncol 2017;18:312–322. [DOI] [PubMed] [Google Scholar]
- 33. Overman MJ, McDermott R, Leach JL et al. Nivolumab in patients with metastatic DNA mismatch repair‐deficient or microsatellite instability‐high colorectal cancer (CheckMate 142): An open‐label, multicentre, phase 2 study. Lancet Oncol 2017;18:1182–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. El‐Khoueiry AB, Sangro B, Yau T et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): An open‐label, non‐comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017;389:2492–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rosenberg JE, Hoffman‐Censits J, Powles T et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum‐based chemotherapy: A single‐arm, multicentre, phase 2 trial. Lancet 2016;387:1909–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Balar AV, Galsky MD, Rosenberg JE et al. Atezolizumab as first‐line treatment in cisplatin‐ineligible patients with locally advanced and metastatic urothelial carcinoma: A single‐arm, multicentre, phase 2 trial. Lancet 2017;389:67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rittmeyer A, Barlesi F, Waterkamp D et al. Atezolizumab versus docetaxel in patients with previously treated non‐small‐cell lung cancer (OAK): A phase 3, open‐label, multicentre randomised controlled trial. Lancet 2017;389:255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Socinski MA, Jotte RM, Cappuzzo F et al. Atezolizumab for first‐line treatment of metastatic nonsquamous NSCLC. N Engl J Med 2018;378:2288–2301. [DOI] [PubMed] [Google Scholar]
- 39. Schmid P, Adams S, Rugo HS et al. Atezolizumab and nab‐paclitaxel in advanced triple‐negative breast cancer. N Engl J Med 2018;379:2108–2121. [DOI] [PubMed] [Google Scholar]
- 40. Horn L, Mansfield AS, Szczesna A et al. First‐Line atezolizumab plus chemotherapy in extensive‐stage small‐cell lung cancer. N Engl J Med 2018;379:2220–2229. [DOI] [PubMed] [Google Scholar]
- 41. Kaufman HL, Russell J, Hamid O et al. Avelumab in patients with chemotherapy‐refractory metastatic Merkel cell carcinoma: A multicentre, single‐group, open‐label, phase 2 trial. Lancet Oncol 2016;17:1374–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Patel MR, Ellerton J, Infante JR et al. Avelumab in metastatic urothelial carcinoma after platinum failure (JAVELIN Solid Tumor): Pooled results from two expansion cohorts of an open‐label, phase 1 trial. Lancet Oncol 2018;19:51–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Motzer RJ, Penkov K, Haanen J et al. Avelumab plus axitinib versus sunitinib for advanced renal‐cell carcinoma. N Engl J Med 2019;380:1103–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Powles T, O'Donnell PH, Massard C et al. Efficacy and safety of durvalumab in locally advanced or metastatic urothelial carcinoma: Updated results from a phase 1/2 open‐label study. JAMA Oncol 2017;3:e172411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Antonia SJ, Villegas A, Daniel D et al. Durvalumab after chemoradiotherapy in stage III non‐small‐cell lung cancer. N Engl J Med 2017;377:1919–1929. [DOI] [PubMed] [Google Scholar]
- 46. Migden MR, Rischin D, Schmults CD et al. PD‐1 blockade with cemiplimab in advanced cutaneous squamous‐cell carcinoma. N Engl J Med 2018;379:341–351. [DOI] [PubMed] [Google Scholar]
- 47. Overman MJ, Lonardi S, Wong KYM et al. Durable clinical benefit with nivolumab plus ipilimumab in DNA mismatch repair‐deficient/microsatellite instability‐high metastatic colorectal cancer. J Clin Oncol 2018;36:773–779. [DOI] [PubMed] [Google Scholar]
- 48. Postow MA, Sidlow R, Hellmann MD. Immune‐related adverse events associated with immune checkpoint blockade. N Engl J Med 2018;378:158–168. [DOI] [PubMed] [Google Scholar]
- 49. Wang DY, Salem JE, Cohen JV et al. Fatal toxic effects associated with immune checkpoint inhibitors: A systematic review and meta‐analysis. JAMA Oncol 2018;4:1721–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Fecher LA, Agarwala SS, Hodi FS et al. Ipilimumab and its toxicities: A multidisciplinary approach. The Oncologist 2013;18:733–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Weber JS, Postow M, Lao CD et al. Management of adverse events following treatment with anti‐programmed death‐1 agents. The Oncologist 2016;21:1230–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gonzalez‐Rodriguez E, Rodriguez‐Abreu D, Spanish Group for Cancer Immuno‐Biotherapy (GETICA). Immune checkpoint inhibitors: Review and management of endocrine adverse events. The Oncologist 2016;21:804–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Champiat S, Lambotte O, Barreau E et al. Management of immune checkpoint blockade dysimmune toxicities: A collaborative position paper. Ann Oncol 2016;27:559–574. [DOI] [PubMed] [Google Scholar]
- 54. Neilan TG, Rothenberg ML, Amiri‐Kordestani L et al. Myocarditis associated with immune checkpoint inhibitors: An expert consensus on data gaps and a call to action. The Oncologist 2018;23:874–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Reynolds K, Thomas M, Dougan M. Diagnosis and management of hepatitis in patients on checkpoint blockade. The Oncologist 2018;23:991–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Leonardi GC, Gainor JF, Altan M et al. Safety of programmed death‐1 pathway inhibitors among patients with non‐small‐cell lung cancer and preexisting autoimmune disorders. J Clin Oncol 2018;36:1905–1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Reynolds KL, Guidon AC. Diagnosis and management of immune checkpoint inhibitor‐associated neurologic toxicity: Illustrative case and review of the literature. The Oncologist 2019;24:435–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sise ME, Seethapathy H, Reynolds KL. Diagnosis and management of immune checkpoint inhibitor‐associated renal toxicity: Illustrative case and review. The Oncologist 2019;24:735–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Reed VA, Rizvi N. Managing pulmonary toxicities associated with immunotherapy: A case discussion. The Oncologist 2019;24:730–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Patil PD, Fernandez AP, Velcheti V et al. Cases from the irAE tumor board: A multidisciplinary approach to a patient treated with immune checkpoint blockade who presented with a new rash. The Oncologist 2019;24:4–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Warner BM, Baer AN, Lipson EJ et al. Sicca syndrome associated with immune checkpoint inhibitor therapy. The Oncologist 2019;24:1259–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Haanen J, Carbonnel F, Robert C et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Ann Oncol 2017;28:iv119–iv142. [DOI] [PubMed] [Google Scholar]
- 63. Puzanov I, Diab A, Abdallah K et al. Managing toxicities associated with immune checkpoint inhibitors: Consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer 2017;5:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Rapoport BL, van Eeden R, Sibaud V et al. Supportive care for patients undergoing immunotherapy. Support Care Cancer 2017;25:3017–3030. [DOI] [PubMed] [Google Scholar]
- 65. Brahmer JR, Lacchetti C, Schneider BJ et al. Management of immune‐related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2018;36:1714–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zou W, Wolchok JD, Chen L. PD‐L1 (B7‐H1) and PD‐1 pathway blockade for cancer therapy: Mechanisms, response biomarkers, and combinations. Sci Transl Med 2016;8:328rv4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Tang J, Shalabi A, Hubbard‐Lucey VM. Comprehensive analysis of the clinical immuno‐oncology landscape. Ann Oncol 2018;29:84–91. [DOI] [PubMed] [Google Scholar]
- 68. Gong J, Chehrazi‐Raffle A, Reddi S et al. Development of PD‐1 and PD‐L1 inhibitors as a form of cancer immunotherapy: A comprehensive review of registration trials and future considerations. J Immunother Cancer 2018;6:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Tang J, Yu JX, Hubbard‐Lucey VM et al. Trial watch: The clinical trial landscape for PD1/PDL1 immune checkpoint inhibitors. Nat Rev Drug Discov 2018;17:854–855. [DOI] [PubMed] [Google Scholar]
- 70. Arnaud‐Coffin P, Maillet D, Gan HK et al. A systematic review of adverse events in randomized trials assessing immune checkpoint inhibitors. Int J Cancer 2019;145:639–648. [DOI] [PubMed] [Google Scholar]
- 71. Dougan M. Checkpoint blockade toxicity and immune homeostasis in the gastrointestinal tract. Front Immunol 2017;8:1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Chen JH, Pezhouh MK, Lauwers GY et al. Histopathologic features of colitis due to immunotherapy with anti‐PD‐1 antibodies. Am J Surg Pathol 2017;41:643–654. [DOI] [PubMed] [Google Scholar]
- 73. Faje AT, Lawrence D, Flaherty K et al. High‐dose glucocorticoids for the treatment of ipilimumab‐induced hypophysitis is associated with reduced survival in patients with melanoma. Cancer 2018;124:3706–3714. [DOI] [PubMed] [Google Scholar]
- 74. Arbour KC, Mezquita L, Long N et al. Impact of baseline steroids on efficacy of programmed cell death‐1 and programmed death‐ligand 1 blockade in patients with non‐small‐cell lung cancer. J Clin Oncol 2018;36:2872–2878. [DOI] [PubMed] [Google Scholar]
- 75. Horvat TZ, Adel NG, Dang TO et al. Immune‐related adverse events, need for systemic immunosuppression, and effects on survival and time to treatment failure in patients with melanoma treated with ipilimumab at Memorial Sloan Kettering Cancer Center. J Clin Oncol 2015;33:3193–3198. [DOI] [PMC free article] [PubMed] [Google Scholar]


