Abstract
As the use of immune checkpoint inhibitors for several different malignancies becomes more mainstream, their side‐effect profile raises new challenges. In 2011, the Food and Drug Administration approved the first checkpoint inhibitor for the treatment of advanced melanoma, and since then, checkpoint inhibitors have demonstrated efficacy in many other tumor types. Given the frequent use of immune checkpoint inhibitors in a wide range of cancers today, the diagnosis and management of their immune‐mediated toxicities need special attention. One of the most common is immune‐mediated colitis. Workup and management of immune‐mediated colitis can be challenging and is the purpose of this review.
Key Points
Rate of immune mediated colitis differ from different kind of immune checkpoint inhibitor treatment.
To work up immune‐mediated colitis, tests to rule out infectious etiologies of diarrhea, colonoscopy and abdominal image will help to differentiate immune mediated colitis from colitis from other etiology.
Patients with mild colitis can be managed with supportive therapies alone, but more severe cases may require immunomodulators such as steroid. Refractory cases may require tumor necrosis factor (TNF) inhibitors, such as infliximab in addition to steroid treatment.
Short abstract
A common immune‐related adverse event is colitis. This review focuses on the mechanisms and management of immunotherapy‐induced colitis.
Case Report
Herein we present a case of a 51‐year‐old female with stage IIIC melanoma of unknown primary origin who underwent tumor resection of the medial left breast and adjuvant radiation before receiving adjuvant pembrolizumab. Shortly thereafter, her cancer progressed, involving a recurrence of malignancy in her medial left breast, which also involved the left chest wall. This recurrence was resected before the patient was enrolled onto the ECOG1609 trial to receive adjuvant ipilimumab.
She received her first dose of intravenous (IV) ipilimumab (10 mg/kg) on July 6, 2017, and then developed grade 1 diarrhea, which lasted for 1.5 weeks. The nonbloody diarrhea was associated with intermittent nausea and lack of appetite. She did not experience any fevers, chills, vomiting, abdominal pain, cramps, or bloating. She was initially started on treatment with loperamide at a dose of 2 mg by mouth as needed but did not notice any improvement in her diarrhea. As a result, the patient's ipilimumab was put on hold, and treatment with oral budesonide (9 mg daily) and lomotil (diphenoxylate hydrochloride and atropine sulfate; 5 mg as needed) was initiated. Her diarrhea subsequently improved enough to be characterized as one loose stool daily. Her lab test results were within normal limits, and as a result, her ipilimumab treatment was restarted on August 4, 2017, but at 3 mg/kg. After the third dose of ipilimumab on August 23, 2017, she again developed diarrhea, but this time it was a grade 2. The patient was again treated with loperamide and also underwent a computed tomography (CT) scan, which showed several right lower quadrant bowel loops featuring submucosal fat proliferation, vasa recta engorgement, and several subcentimeter lymph nodes (Fig. 1A). Despite loperamide treatment, her diarrhea increased to five stools daily and she was started on oral prednisone at a dose of 80 mg on September 4, 2017. On September 5, 2017, her diarrhea worsened and she reported having eight loose stools daily. The patient presented to the emergency room and was started on IV methylprednisolone (solumederol; 80 mg b.i.d.). Slowly, her diarrhea began to improve, and her steroid treatment was switched to oral prednisone at a dose of 80 mg twice daily for 7 days, followed by a tapering dose. Unfortunately, when the prednisone was decreased to 60 mg twice daily, she developed persistent large‐volume, watery diarrhea, which occurred 1–2 times per day for 6 days. The decision was then made to treat the patient with infliximab. She received the first dose of IV infliximab (5 mg/kg) on September 28, 2017. Her diarrhea resolved after the first dose, and her treatment team was able to taper her off steroids. However, on November 15, 2017, the patient again developed grade 1 diarrhea and nausea for 1 week. She was restarted on prednisone but did not experience any improvement in her symptoms. She was then given a second dose of infliximab. Her diarrhea resolved after the second dose of infliximab and she was tapered off prednisone entirely.
Figure 1.
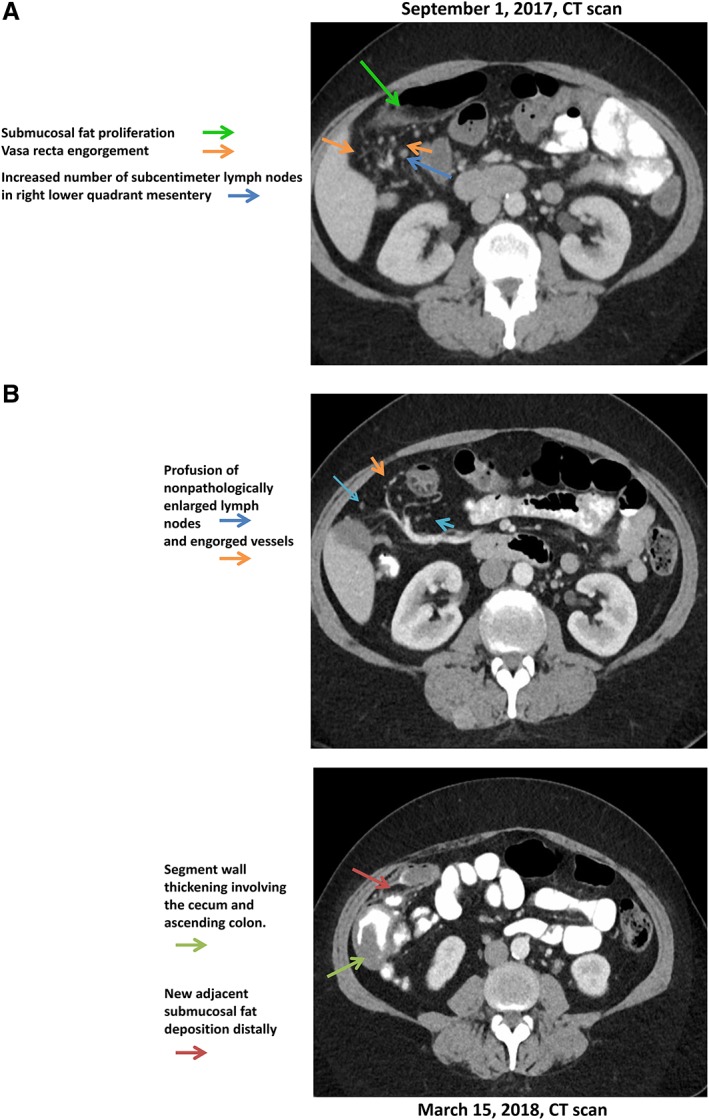
Image findings in a patient with immune mediated colitis at initial presentation (A); image findings in this patient with a recurrence of immune mediated colitis (B).
Abbreviation: CT, computed tomography.
The patient underwent restaging scans on December 14, 2017, including CT C/A/P, which did not show any active disease. However, magnetic resonance imaging showed two new brain lesions; she underwent Cyberknife to both lesions. She was monitored off‐treatment from December 2017 through March 2018. She developed recurrent colitis in March 2018, and on March 15, 2018, she had a CT scan, which showed wall thickening of the cecum and ascending colon with associated vascular engorgement and profusion of nonpathologically enlarged lymph nodes (Fig. 1B). These imaging results confirmed the patient's diagnosis of immunotherapy‐associated colitis. She was restarted on 60 mg of prednisone daily, and the number of bowel movements reduced to 1–2 times daily.
Introduction
Immunotherapy aims to unleash the immune response against cancer cells. In the last several years, inhibition of immune checkpoint proteins including programmed death receptor‐1 (PD‐1), programmed death receptor ligand‐1 (PD‐L1), and cytotoxic T‐lymphocyte‐associated protein 4 (CTLA‐4) have changed the landscape for immunotherapy. PD‐1 is expressed on activated T cells. When bound by PD‐L1, PD‐1 causes T‐cell exhaustion and a favorable environment for tumor growth 1. One mechanism of resistance to immune surveillance in tumors is the expression and activation of the PD‐1/PD‐L1 pathway. On the other hand, CTLA‐4 is homologous to the T‐cell costimulatory protein, CD28, and both molecules bind to CD80 (B7‐1) and CD86 (B7‐2) on antigen‐presenting cells. CTLA‐4 binds CD80 and CD86 with greater affinity and avidity than CD28, thus enabling CTLA‐4 to outcompete CD28 for its ligands. CTLA‐4 transmits an inhibitory signal, whereas CD28 transmits a stimulatory signal to T cells 2, 3, 4.
Ipilimumab, a monoclonal antibody targeting CTLA‐4, was the first agent approved by the Food and Drug Administration in 2011 for the treatment of unresectable melanoma 5. Since that time, there has been an influx of several agents targeting both pathways, including the approved PD‐1/PD‐L1 inhibitors nivolumab, pembrolizumab, atezolizumab, durvalumab, and avelumab, in addition to several others still in development. As immunotherapy becomes more integrated as the standard of care for several different malignancies, it is important to focus on the toxicities associated with this treatment. Immune checkpoint proteins play an important role in healthy tissue, such as peripheral tolerance and prevention of autoimmunity, and for this reason it is not surprising that immune‐related adverse events (irAEs) are common side effects 6, 7, 8. These adverse events are often due to overactivation of the immune system, causing activated T cells to infiltrate healthy tissue 9. This can occur at any point during a patient's treatment course, and the most commonly observed irAEs are rash, colitis, pneumonitis, hepatitis, nephritis, and endocrinopathies.
The incidence of irAEs reported in clinical trials is variable. Much of this variability may be due to the lack of consensus on the definition of an irAE, although more recently there have been attempts to standardize the classification of and grading criteria for irAEs. In clinical trials, a 15% to 90% incidence of irAEs was reported (any grade), and toxicities severe enough to require drug discontinuation and the initiation of immunosuppressive medications occurred between 0.5% and 13% of the time 10, 11.
For the purposes of this review, we will focus on immunotherapy‐induced colitis, the rate of which varies between studies. When ipilimumab was used at a dose of 3 mg/kg, the rate of grade 3 or 4 diarrhea was 2.8%–6.1% 12, 13, 14. At higher doses of 10 mg/kg, grade 3 or 4 diarrhea occurred in 15%–16% of patients 13, 15. During PD‐1 inhibitor therapy, the rate of immune‐mediated diarrhea was approximately 19% 14, and grade 3 or 4 diarrhea was seen to occur at a rate of up to 2.2% 14. The rate of diarrhea increased when anti‐PD‐1 and anti‐CTLA‐4 therapies were combined, and increased further with increasing doses of either or both of these combined agents 14, 16, 17. For example, when patients were dosed with IV nivolumab and ipilimumab every 3 weeks for four doses, the reported rate of grade 3 or 4 diarrhea was 4.3% for nivolumab at 3 mg/kg plus ipilimumab at 1 mg/kg (N3I1); up to 14.9% for nivolumab at 1 mg/kg plus ipilimumab at 3 mg/kg (N1I3); and 16.7% for nivolumab at 3 mg/kg plus ipilimumab at 3 mg/kg (N3I3). Each dosing regimen was followed by nivolumab monotherapy at 3 mg/kg every 2 weeks until progression or toxicity, respectively 17. Colitis often presents 6 to 8 weeks after starting immunotherapy and may lead to serious complications if left untreated 18. Often, patients with mild colitis can be managed with supportive therapies alone, but more severe cases may require immunomodulators such as steroids. Refractory cases like the one presented here may require tumor necrosis factor (TNF) inhibitors such as infliximab in addition to steroid treatment 19. Among 1,994 patients treated with nivolumab, 58 patients (0.7%) were diagnosed with immune‐mediated colitis, and 91% of these patients received corticosteroids with a median duration of steroid treatment of 23 days (1 day to 9.3 months). Among the 58 patients, 4 (7%) received infliximab in addition to high‐dose steroids, 43 (74%) had complete resolution of their diarrhea, and 17 (29%) had recurrent diarrhea after reinitiation of nivolumab treatment (nivolumab package insert). The incidence of immune‐mediated colitis due to immune checkpoint inhibitor therapy is unknown because it was not reported whether patients had a history of underlying inflammatory bowel disease (checkpoint inhibitor treatment is currently contraindicated in patents with underlying inflammatory or autoimmune disease). It is noteworthy that immune‐mediated colitis seems not to be linked to other autoimmune toxicities, such as pneumonitis or hepatitis.
Immunotherapy‐Induced Colitis
Common presenting symptoms of immunotherapy‐induced colitis include abdominal pain, diarrhea, blood or mucous in stool, or ileus 11. Given these nonspecific symptoms, it is essential to rule out infectious etiologies of diarrhea including Clostridium difficile, as well as other common bacterial and parasitic pathogens. Of note, diarrhea may also be a symptom of more severe bowel inflammation such as that seen in enterocolitis. Usually, enterocolitis presents within 2 months of beginning checkpoint inhibitor therapy and is characterized by abdominal pain, hematochezia, or ileus.
Imaging may include an upright abdominal film to rule out perforation, but abdominal CT may be helpful to determine the diagnosis of colitis. In cases of more severe colitis requiring hospitalization, an endoscopic evaluation of the enteric tract should be considered in addition to consultation with a gastroenterologist. During endoscopy, biopsies should be taken to determine if there are inflammatory mucosal changes present in order to definitively diagnose colitis. In one study, investigators biopsied the colon and small bowel of patients with CTLA‐4 inhibitor–induced enterocolitis in addition to newly diagnosed patients with ulcerative colitis, and endoscopic results of the immunotherapy group showed inflammatory mucosal changes similar to those seen with severe inflammatory bowel disease 20.
Endoscopic characteristics of immune therapy‐related colitis are very diverse, and there are no validated scoring systems. Common histological findings include an increase in lamina propria cellularity, patchy neutrophilic infiltrate, and in some cases, crypt abscesses. One research group reported an association between endoscopic features and the need for immune suppression beyond high‐dose corticosteroids 21. These endoscopic findings included the presence of edematous and erythematous mucosa in addition to mucosal ulceration. These changes are often not as severe as those observed in patients with ulcerative colitis and are often more uniformly spread throughout the colon. Interestingly, the study investigators found that the presence of ulcers and pancolitis in greater than three affected colon segments predicted steroid‐refractory colitis, perhaps warranting immediate start of infliximab on colonoscopy. No set of histopathological features was specific to any particular immune checkpoint inhibitor.
Mechanism of Immune Therapy–Induced Colitis
The process of immune therapy‐induced colitis is not fully understood. Different mechanisms have been proposed. For example, immune‐related T‐cell activation induced by immune checkpoint inhibitors leads to the secretion of high levels of CD4 T helper cells, cytokines, and cytolytic CD8 T‐cell infiltration into tissues, possibly causing colitis 22. Alternatively, for anti‐CTLA‐4 antibody treatment, the colitis mechanism may involve immunosuppressive CD25+ CD4+ regulatory T cells (Tregs). These immunosuppressive Tregs constitutively express high levels of CTLA‐4, and increased incidence of autoimmune disease was found in mice lacking Tregs 23. The lamina propria and epithelium of the colon in anti‐PD‐1‐induced colitis have been predominantly characterized by CD8+ T cells and Tregs, whereas the lamina propria of the colon in anti‐CTLA‐4‐induced colitis has been predominantly characterized by CD4+ T cells and high mucosal TNF‐α concentrations 24. Colitis related to anti‐CTLA‐4 treatment has features similar to graft‐versus‐host disease 25. It has been proposed that intestinal microflora antigens contribute to colitis and that therapeutics targeting the intestinal microflora may prevent immune therapy–induced colitis 26.
Management of Colitis
The Common Terminology Criteria for Adverse Events (CTCAE version 4.03) are often used to define grades of diarrhea in patients treated in clinical trials (Table 1). Diarrhea is generally characterized by frequent and watery bowel movements, and grade 1 diarrhea is defined as an increase of up to four stools over baseline daily; grade 2 as between four and six stools over baseline; grade 3 as greater than or equal to seven; grade 4 as life‐threatening consequences; and grade 5 as death. Treatment of colitis varies based on the severity of diarrhea as defined by the CTCAE. Recommended treatment guidelines for immune‐mediated colitis can be found in Table 2. For grade 1 and 2 immune‐mediated colitis or diarrhea, use of antimotility agents such as loperamide, dephenoxylate/atropine, or tincture of opium is recommended. Additionally, dietary modification following the American Dietetic Association guidelines is often recommended. There is an emphasis on eating smaller meals more frequently in addition to foods low in fiber. If symptoms are stable but not improving after 3 days of these interventions, enteric budesonide (9 mg daily) may be tried 27. For patients with grade 3 or 4 symptoms, or less severe symptoms persisting for at least 7 days, treatment with systemic steroids should be initiated. Depending on the severity of diarrhea and the presence of electrolyte abnormalities, either daily prednisone (1 to 2 mg/kg orally) or twice‐daily methylprednisolone (2 mg/kg IV) can be used.
Table 1.
Adverse event grading for colitis according to the National Cancer Institute's Common Terminology Criteria for Adverse Events, version 4
| Grade | Description | Intervention |
|---|---|---|
| 1 | Asymptomatic; clinical or diagnostic observations only | Intervention not indicated |
| 2 | Abdominal pain; mucus or blood in stool | |
| 3 | Severe abdominal pain; peritoneal signs | |
| 4 | Life‐threatening consequences | Urgent intervention indicated |
| 5 | Death |
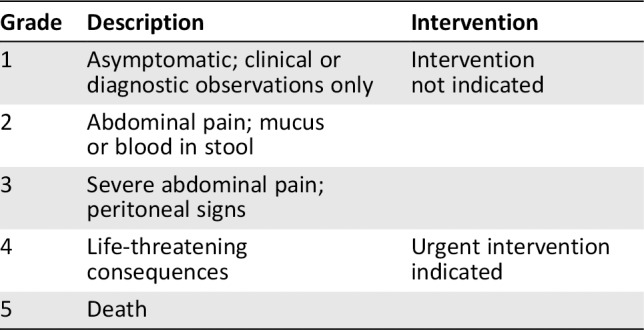
Table 2.
Algorithm for treatment of colitis by grade
| Grade | Management |
|---|---|
| 1 |
Fluid and electrolyte replacement Loperamide, diphenoxylate hydrochloride, or atropine sulfate Colitis diet per the American Dietetic Association |
| 2 |
Same measures as grade 1 If diarrhea persists >3 days, start oral corticosteroids (prednisone) 0.5–1 mg/kg/day tapered over 4–8 weeks Consider addition of oral budesonide, 9 mg daily, if no improvement after 3 days |
| 3–4 |
Start oral corticosteroids (prednisone), 1–2 mg/kg/day If severe symptoms: IV methylprednisone, 2 mg/kg twice a day for 1–2 days before transitioning to oral corticosteroids Consider addition of oral budesonide, 9 mg daily Initiate slow tapering of oral prednisone over 4–8 weeks If steroid refractory, defined by no improvement after 3–7 days on IV corticosteroids, consider infliximab treatment, 5 mg/kg as a single dose. If there is no improvement after 2 weeks, consider one more dose. |
| Refractory disease/perforation | Consider partial/total colectomy |
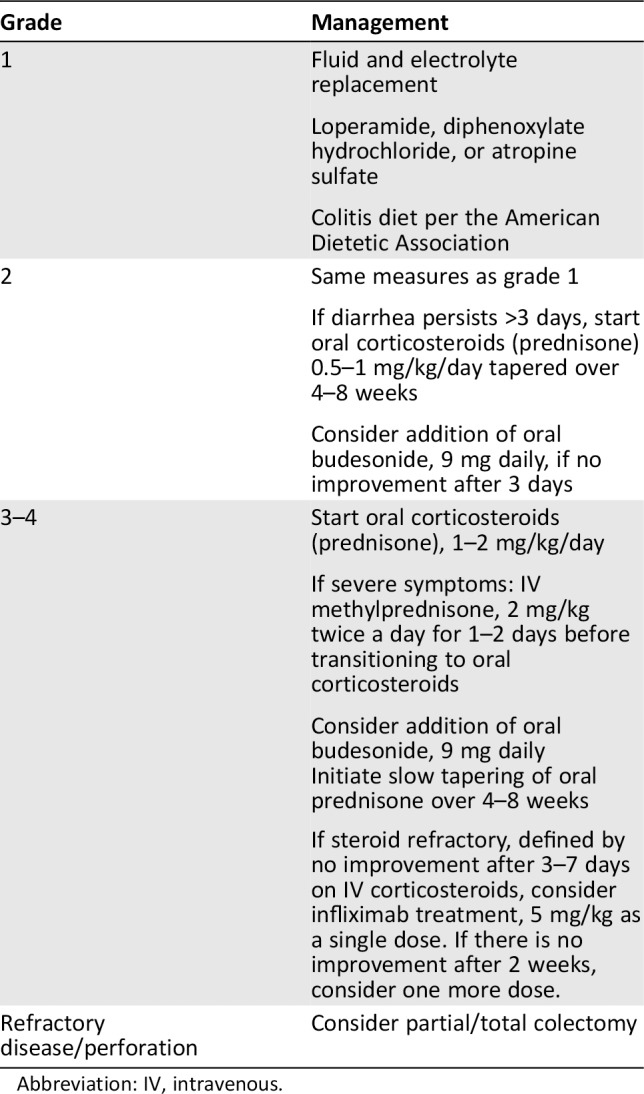
Abbreviation: IV, intravenous.
For steroid‐refractory colitis, intensified treatment with other immunosuppressive medication is necessary. TNF‐α has been unequivocally validated as a therapeutic target in a variety of immune‐mediated inflammatory disorders. Inhibition of the TNF‐α pathway can be achieved by several methods, and the currently available approaches include monoclonal antibodies (infliximab, adalimumab, and golimumab), either chimeric or humanized, a PEGylated anti‐TNF‐α biologic named certolizumab pegol, and an IgG1‐TNFR2 fusion protein named etanercept 28. Patients are usually considered steroid refractory if they do not improve after 3–7 days of IV corticosteroid treatment 19, 29. After 3 days of high‐dose corticosteroids, it is reasonable to initiate anti‐TNF therapy with infliximab. Infliximab works by blocking the ability of TNF to recruit neutrophils to the site of inflammation in the colon. Additionally, the mechanism of action does not impact the efficacy of immunotherapy, which is a major limitation of corticosteroids 19. Infliximab has been used in steroid‐refractory colitis as a rescue therapy to avoid colectomy 30. Usually, infliximab at an IV dose of 5 mg/kg every 2 weeks is used, and most patients respond to a single dose of this medication 20. On average, responses are seen within 1 to 3 days of administration of infliximab. If no response is seen following the first dose, it is reasonable to administer an additional dose of infliximab 2 weeks after the first dose. Infliximab is contraindicated in the setting of intestinal perforation. Also, there is an increased risk of reactivation of tuberculosis, where relevant, with recurrent use of infliximab.
Given the interest in the role of interleukin 6 (IL‐6) in the pathology of Crohn's disease and as a target for treatment of inflammatory bowel disease 31, 32, the humanized anti‐IL‐6‐receptor antibody tocilizumab has been evaluated in nivolumab‐induced colitis with some clinical activity 33. However, randomized trials are needed to better elucidate the relative efficacy and safety of any new agent in comparison with infliximab.
There are also some emerging data supporting the use of the anti‐integrin α4β7 antibody vedolizumab, which acts by preventing the adhesion and migration of memory T lymphocytes into the gut endothelium; this treatment has been effective in some patients with enterocolitis refractory to infliximab 34. Fortunately, with the exception of endocrinopathies, the majority of irAEs can be reversed. It is important to note that up to 12% of patients with colitis may develop severe enterocolitis unresponsive to immunosuppressive therapy 25. If this occurs, these individuals may require a subtotal colectomy for bleeding, perforation, or intractable diarrhea.
Immune toxicities do not appear to be easily preventable. A randomized trial found that prophylactic budesonide during immunotherapy did not prevent the development of grade 2 diarrhea. In a phase II trial, 115 patients with advanced melanoma were randomized to daily prophylactic budesonide or placebo while being treated with ipilimumab 35. No difference in rate of grade ≥2 diarrhea was noted between the two arms (32.7% vs. 35.0%). Therefore, prophylactic budesonide is not recommended for routine use with ipilimumab or PD‐1/PD‐L1 inhibitors. However, high‐dose budesonide (9–12 mg daily) did seem to be useful as a rescue therapy in patients with steroid‐refractory diarrhea 36. High‐dose budesonide is an attractive steroid‐sparing agent, but further studies of its efficacy in this setting are needed. At this point, it should be noted that nonsteroidal anti‐inflammatory drugs (NSAIDs) are not recommended in this setting because of an observed correlation between NSAID use and the development of colitis 37.
Conclusion
Immune checkpoint inhibitors represent an emerging class of therapy for a growing number of cancers. The use of these drugs is associated with potent effects on several different malignancies in addition to the potential for substantial toxicity. Immune‐mediated colitis remains a major side effect of therapy, and much of our understanding is based on limited data and extrapolation from other inflammatory disorders. Patients who develop diarrhea while receiving immune checkpoint inhibitors need to be assessed for immune‐mediated colitis. Patients with possible immune‐mediated colitis need to be monitored closely and treated with steroids, or infliximab if they have colitis that is refractory to steroid treatment. Immune colitis requires very early recognition and education of patients on how to recognize the early signs; it requires prompt and aggressive medical treatment to avoid grade 3 or 4 colitis, which can be life‐threatening or lead to a colectomy. Patients should be encouraged to carry a card that details their current treatment, management algorithm, and physician contact information. Given the growing use of immunotherapy, priority should be given to the further study of the pathogenesis of immune‐mediated colitis and its associated risk factors, as well as effective treatment options.
Author Contributions
Conception/design: Bhavana Pendurthi Singh, John L. Marshall, Aiwu Ruth He
Provision of study material or patients: Bhavana Pendurthi Singh, John L. Marshall, Aiwu Ruth He
Manuscript writing: Bhavana Pendurthi Singh, John L. Marshall, Aiwu Ruth He
Final approval of manuscript: Bhavana Pendurthi Singh, John L. Marshall, Aiwu Ruth He
Disclosures
John L. Marshall: Merck (C/A, H). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Acknowledgments
We thank Marion L. Hartley, Ph.D., for her edits and suggestions during the composition of this manuscript.
Disclosures of potential conflicts of interest may be found at the end of this article.
References
- 1. Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: A common denominator approach to cancer therapy. Cancer Cell 2015;27:450–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Krummel MF, Allison JP. CD28 and CTLA‐4 have opposing effects on the response of T cells to stimulation. J Exp Med 1995;182:459–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Walunas TL, Bakker CY, Bluestone JA. CTLA‐4 ligation blocks CD28‐dependent T cell activation [published erratum appears in J Exp Med 1996;184:301]. J Exp Med 1996;183:2541–2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Walunas TL, Lenschow DJ, Bakker CY et al. CTLA‐4 can function as a negative regulator of T cell activation. Immunity 1994;1:405–413. [PubMed] [Google Scholar]
- 5. Lacroix M. Targeted Therapies in Cancer. Hauppauge, NY: Nova Sciences Publishers, 2014. [Google Scholar]
- 6. Takahashi T, Tagami T, Yamazaki S et al. Immunologic self‐tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte‐associated antigen 4. J Exp Med 2000;192:303–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Francisco LM, Sage PT, Sharpe AH. The PD‐1 pathway in tolerance and autoimmunity. Immunol Rev 2010;236:219–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Maughan BL, Bailey E, Gill DM et al. Incidence of immune‐related adverse events with program death receptor‐1 and program death receptor‐1 ligand‐directed therapies in genitourinary cancers. Front Oncol 2017;7:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Marin‐Acevedo JA, Harris DM, Burton MC. Immunotherapy‐induced colitis: An emerging problem for the hospitalist. J Hosp Med 2018;13:413–418. [DOI] [PubMed] [Google Scholar]
- 10. Kumar V, Chaudhary N, Garg M et al. Current diagnosis and management of immune related adverse events (irAEs) induced by immune checkpoint inhibitor therapy. Front Pharmacol 2017;8:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Friedman CF, Proverbs‐Singh TA, Postow MA. Treatment of the immune‐related adverse effects of immune checkpoint inhibitors: A review. JAMA Oncol 2016;2:1346–1353. [DOI] [PubMed] [Google Scholar]
- 12. Hodi F, O'Day S, McDermott D et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wolchok JD, Neyns B, Linette G et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: A randomised, double‐blind, multicentre, phase 2, dose‐ranging study. Lancet Oncol 2010;11:155–164. [DOI] [PubMed] [Google Scholar]
- 14. Eggermont AM, Chiarion‐Sileni V, Grob JJ et al. Adjuvant ipilimumab versus placebo after complete resection of high‐risk stage III melanoma (EORTC 18071): A randomised, double‐blind, phase 3 trial. Lancet Oncol 2015;16:522–530. [DOI] [PubMed] [Google Scholar]
- 15. Larkin J, Chiarion‐Sileni V, Gonzalez R et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 2015;373:23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sznol M, Ferrucci PF, Hogg D et al. Pooled analysis safety profile of nivolumab and ipilimumab combination therapy in patients with advanced melanoma. J Clin Oncol 2017; 35:3815–3822. [DOI] [PubMed] [Google Scholar]
- 17. Hammers HJ, Plimack ER, Infante JR et al. Safety and efficacy of nivolumab in combination with ipilimumab in metastatic renal cell carcinoma: The CheckMate 016 Study. J Clin Oncol 2017;35:3851–3858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tirumani SH, Ramaiya NH, Keraliya A et al. Radiographic profiling of immune‐related adverse events in advanced melanoma patients treated with ipilimumab. Cancer Immunol Res 2015;3:1185–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Minor DR, Chin K, Kashani‐Sabet M. Infliximab in the treatment of anti‐CTLA4 antibody (ipilimumab) induced immune‐related colitis. Cancer Biother Radiopharm 2009;24:321–325. [DOI] [PubMed] [Google Scholar]
- 20. Yanai S, Nakamura S, Matsumoto T. Nivolumab‐induced colitis treated by infliximab. Clin Gastroenterol Hepatol 2017;15:e80–e81. [DOI] [PubMed] [Google Scholar]
- 21. Geukes Foppen MH, Rozeman EA, van Wilpe S et al. Immune checkpoint inhibition‐related colitis: symptoms, endoscopic features, histology and response to management. ESMO Open 2018;3:e000278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tarhini A. Immune‐mediated adverse events associated with ipilimumab ctla‐4 blockade therapy: The underlying mechanisms and clinical management. Scientifica (Cairo) 2013;2013:857519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Read S, Malmström V, Powrie F. Cytotoxic T lymphocyte‐associated antigen 4 plays an essential role in the function of CD25(+)CD4(+) regulatory cells that control intestinal inflammation. J Exp Med 2000;192:295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Coutzac C, Adam J, Soularue E et al. Colon immune‐related adverse events: Anti‐CTLA‐4 and anti‐PD‐1 blockade induce distinct immunopathological entities. J Crohns Colitis 2017;11:1238–1246. [DOI] [PubMed] [Google Scholar]
- 25. Beck KE, Blansfield JA, Tran KQ et al. Enterocolitis in patients with cancer after antibody blockade of cytotoxic T‐lymphocyte‐associated antigen 4. J Clin Oncol 2006;24:2283–2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Guthery SL, Heubi JE, Filipovich A. Enteral metronidazole for the prevention of graft versus host disease in pediatric marrow transplant recipients: Results of a pilot study. Bone Marrow Transplant 2004;33:1235–1239. [DOI] [PubMed] [Google Scholar]
- 27. Postow MA. Managing immune checkpoint‐blocking antibody side effects. Am Soc Clin Oncol Educ Book 2015:76–83. [DOI] [PubMed] [Google Scholar]
- 28. Taylor PC. Pharmacology of TNF blockade in rheumatoid arthritis and other chronic inflammatory diseases. Curr Opin Pharmacol 2010;10:308–315. [DOI] [PubMed] [Google Scholar]
- 29. Merrill SP, Reynolds P, Kalra A et al. Early administration of infliximab for severe ipilimumab‐related diarrhea in a critically ill patient. Ann Pharmacother 2014;48:806–810. [DOI] [PubMed] [Google Scholar]
- 30. Lawlor G, Moss AC. Cause for controversy? Infliximab in the treatment of ulcerative colitis: An update. Clin Exp Gastroenterol 2009;2:149–161. [PMC free article] [PubMed] [Google Scholar]
- 31. Ito H. IL‐6 and Crohn's disease. Curr Drug Targets Inflamm Allergy 2003;2:125–130. [DOI] [PubMed] [Google Scholar]
- 32. Ito H. Novel therapy for Crohn's disease targeting IL‐6 signalling. Expert Opin Ther Targets. 2004;8:287–294. [DOI] [PubMed] [Google Scholar]
- 33. Stroud CR, Hegde A, Cherry C et al. Tocilizumab for the management of immune mediated adverse events secondary to PD‐1 blockade. J Oncol Pharm Pract 2019;25:551–557. [DOI] [PubMed] [Google Scholar]
- 34. Bergqvist V, Hertervig E, Gedeon P et al. Vedolizumab treatment for immune checkpoint inhibitor‐induced enterocolitis. Cancer Immunol Immunother 2017;66:581–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Weber J, Thompson JA, Hamid O et al. A randomized, double‐blind, placebo‐controlled, phase II study comparing the tolerability and efficacy of ipilimumab administered with or without prophylactic budesonide in patients with unresectable stage III or IV melanoma. Clin Cancer Res 2009;15:5591–5598. [DOI] [PubMed] [Google Scholar]
- 36. De Felice KM, Gupta A, Rakshit S et al. Ipilimumab‐induced colitis in patients with metastatic melanoma. Melanoma Res 2015;25:321–327. [DOI] [PubMed] [Google Scholar]
- 37. Marthey L, Mateus C, Mussini C et al. Cancer immunotherapy with anti‐CTLA‐4 monoclonal antibodies induces an inflammatory bowel disease. J Crohns Colitis 2016;10:395–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Prieux‐Klotz C, Dior M, Damotte D et al. Immune checkpoint inhibitor‐induced colitis: Diagnosis and management. Target Oncol 2017;12:301–308. [DOI] [PubMed] [Google Scholar]


