Abstract
Background
Treatment of non‐small cell lung cancer (NSCLC) improved substantially in the last decades. Novel targeted and immune‐oncologic drugs were introduced into routine treatment. Despite accelerated development and subsequent drug registrations by the European Medicinal Agency (EMA), novel drugs for NSCLC are poorly accessible in Central and Eastern European (CEE) countries.
Material and Methods
The Central European Cooperative Oncology Group conducted a survey among experts from 10 CEE countries to provide an overview on the availability of novel drugs for NSCLC and time from registration to reimbursement decision in their countries.
Results
Although first‐generation epidermal growth factor receptor tyrosine kinase inhibitors were reimbursed and available in all countries, for other registered therapies—even for ALK inhibitors and checkpoint inhibitors in first‐line—there were apparent gaps in availability and/or reimbursement. There was a trend for better availability of drugs with longer time from EMA marketing authorization. Substantial differences in access to novel drugs among CEE countries were observed. In general, the availability of drugs is not in accordance with the Magnitude of Clinical Benefit Scale (MCBS), as defined by the European Society for Medical Oncology (ESMO). Time spans between drug registrations and national decisions on reimbursement vary greatly, from less than 3 months in one country to more than 1 year in the majority of countries.
Conclusion
The access to novel drugs for NSCLC in CEE countries is suboptimal. To enable access to the most effective compounds within the shortest possible time, reimbursement decisions should be faster and ESMO MCBS should be incorporated into decision making.
Short abstract
Access to novel therapies is a factor contributing to disparities in cancer care. Limited drug availability is a challenge in Central and Eastern European countries, where financial and organizational shortages exist. This article reports a survey that investigated access to novel anti‐cancer drugs for non‐small cell lung cancer.
Introduction
Lung cancer is the most frequent cause of cancer‐related mortality worldwide, with high incidence and mortality rates in Central and Eastern Europe (CEE) 1. Most patients are diagnosed with advanced disease, resulting in poor survival rates 2. However, there is a trend toward better outcomes in developed countries mostly because of improved systemic treatment strategies introduced in the beginning of this century 2, 3, 4.
Nowadays, treatment strategy in advanced non‐small cell lung cancer (NSCLC) mainly depends on molecular markers. The discovery of oncogene drivers such as epidermal growth factor receptor (EGFR) mutations and ALK and ROS1 rearrangements paved the way to effective targeted therapies, whereas immunotherapy with checkpoint inhibitors (CPIs) became the standard treatment for the majority of patients with advanced NSCLC without oncogenic drivers 3, 4.
Access to novel therapies is one of the major factors contributing to disparities in cancer care 5. Limited drug availability remains a prominent aspect of cancer care in CEE countries, still struggling with both financial and organizational shortages 6. The Central European Cooperative Oncology Group (CECOG) created a network of activities to improve quality of cancer care in the region. The most recent CECOG initiative consisted of two surveys on NSCLC. The first survey on molecular testing has recently been published 7. The aim of the present survey was to investigate access to novel anticancer drugs for NSCLC and time from marketing authorization to national reimbursement.
Materials and Methods
A panel of NSCLC experts from 10 CEE countries (Austria, Bulgaria, Croatia, Czech Republic, Hungary, Poland, Romania, Serbia, Slovenia, and Slovakia, each country represented by one expert, respectively) participated in the survey.
Novel drugs with European Medicines Agency (EMA) marketing approval (MA) for particular indication and recommended by European Society for Medical Oncology (ESMO) guidelines 3 were included. In a majority of countries (9 out of 10, i.e., European Union [EU] members), the time from marketing approval was the same, as a result of EMA licensing. Only in Serbia, a national approval procedure was still in place, with 5 out of 17 drugs without national MA at the time of survey.
The obtained answers were further verified on the official websites of National Drug Agencies, National Insurance Houses, and Ministries of Health. The data lock was March 31, 2018.
Each drug was identified by one of three categories: (a) the drug is registered and available for the majority of patients through established governmental or private insurance; (b) the drug is registered and available only to a minority of patients with special insurance or other access programs; or (c) the drug is registered by EMA, but neither reimbursed nor available in the country.
ESMO Magnitude of Clinical Benefit Scale (MCBS) scores available at the moment of survey 3, 8 were included.
Results
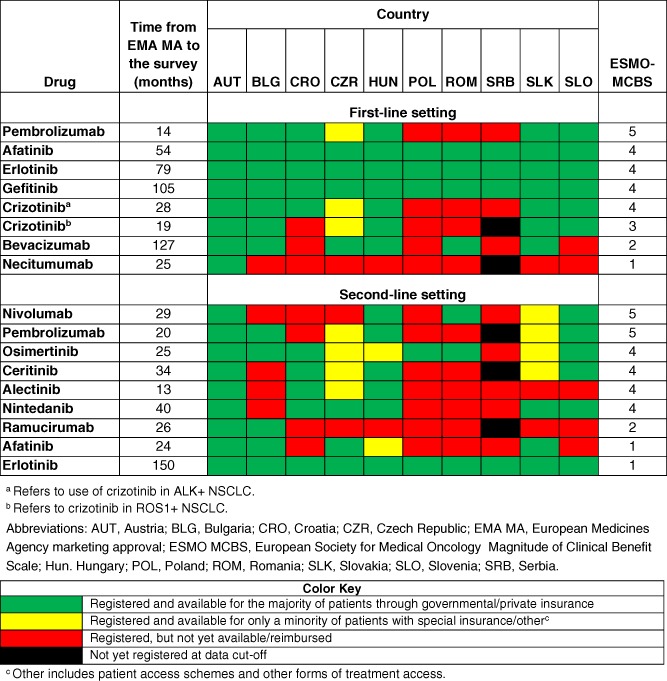
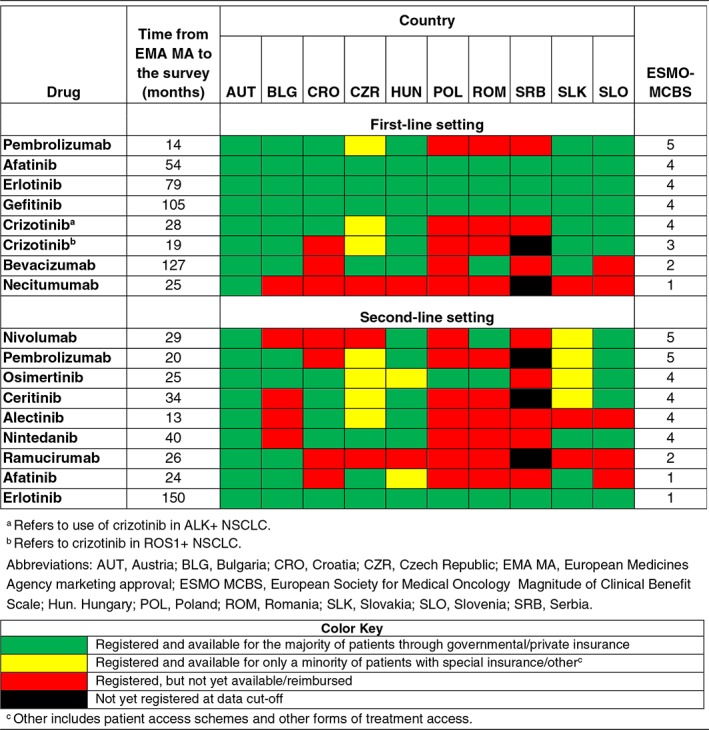
Major gaps and differences in the availability of novel anticancer drugs for NSCLC in the CEE region were recorded (Table 1), with the most profound lack of access observed in countries with lower levels of economic development, such as Serbia and Romania 7. Although first‐generation EGFR tyrosine kinase inhibitors (TKIs) were reimbursed and available in all countries, there were apparent gaps in access to ALK TKIs and CPIs in first‐line. There was a trend for better availability of compounds with longer intervals from EMA MA to the survey. It is quite obvious that availability of drugs was not in accordance with the ESMO MCBS. Drugs with high scores, like crizotinib for ALK‐positive disease or nivolumab (MCBS 4 and 5, respectively) were not available in a number of countries even after a long interval of 2 years from MA.
Table 1.
Availability of novel anticancer drugs for non‐small cell lung cancer in 10 Central and Eastern European countries in relation to time from EMA MA and the ESMO MCBS
|
Time from MA to reimbursement differed between <3 months in a striking minority of countries to >12 months needed for most novel drugs to get reimbursement in a vast majority of countries (Fig. 1). In Croatia and Serbia, the lag time between registration and reimbursement was more than 1 year for all drugs. Almost no reimbursement decision for any novel drug has been made in any country except Austria within a period of <3 months, thus precluding rapid access to effective compounds with high ESMO MCBS.
Figure 1.

Time from marketing authorization to reimbursement of novel drugs for non‐small cell lung cancer in 10 Central and Eastern European countries. Data are expressed as percentage of drugs available in particular timeframe. Abbreviations: AUT, Austria; BLG, Bulgaria; CRO, Croatia; CZR, Czech Republic; HUN, Hungary; POL, Poland; ROM, Romania; SLK, Slovakia; SLO, Slovenia; SRB, Serbia.
Discussion
Based on our survey, the access to novel anticancer drugs for NSCLC in the CEE region is far from satisfactory. Notably, a vast majority of drugs being approved by EMA for 2 years or more and recommended by current ESMO treatment guidelines 3 were not available to CEE patients with NSCLC at the time of our survey. The major reason for poor availability seems to be a long lag interval between EMA or national MA and national reimbursement decisions, which is particularly worrisome for drugs with high ESMO MCBS scores 8. Despite some recent optimistic reports of decreasing time intervals between EMA registrations and national reimbursement decisions of anticancer drugs in Western and Northern European countries 9, our results are not in line with those encouraging data.
The first comprehensive analysis on the availability of anticancer drugs for major cancers in Europe was performed by ESMO in 2014 5. With novel and effective drugs entering the market, the proportion of nonreimbursed and thus unavailable novel drugs for NSCLC has even increased in some CEE countries, based on our observation. This is particularly worrisome for NSCLC, which constitutes a paradigmatic driver of cancer‐related morbidity and mortality in the CEE region.
It has been shown that economic disparities, differences in health care systems, and reimbursement decisions are the main reasons for inequalities in access to novel anticancer drugs across Europe 5, 6, 10. The existing gaps are certainly due to disparities in gross domestic product (GDP), with CEE countries spending about 2.5 times less on anticancer drugs than Western European countries despite using a higher share of their GDP 10. However, more funds do not seem to be the ultimate answer; to retain a sustainability of system and to close the gap in access to novel anticancer drugs, more rational, value‐oriented uptake of novel drugs should be implemented.
Conclusion
With lung cancer representing a major burden in the CEE region, the data of the current survey indicate not only that time intervals between drug registrations on the EU level and reimbursement decisions on the national level should be shortened but also that value scores, like ESMO MCBS, should be taken into account in order to enable patient access to the most effective compounds in the shortest possible time.
Disclosures
Tanja Cufer: AstraZeneca, Boehringer Ingelheim, Roche, Pfizer, Bristol‐Myers Squibb, Takeda, Merck Sharp & Dohme (H); Tudor E. Ciuleanu: A&D Pharma, Amgen, Astellas, AstraZeneca, Boehringer Ingelheim, Bristol‐Myers Squibb, Janssen, Merck, Sanofi (C/A, H); Peter Berzinec: AstraZeneca, Boehringer Ingelheim, Merck Sharp & Dohme, Pfizer, Roche (H), Boehringer Ingelheim, Merck Sharp & Dohme (C/A); Gabriela Galffy: Roche, Bristol‐Myers Squibb, Merck Sharp & Dohme, Boehringer Ingelheim, AstraZeneca, Berlin Chemie, Novartis (C/A), Roche, Bristol‐Myers Squibb, Merck Sharp & Dohme, Boehringer Ingelheim, AstraZeneca, Berlin Chemie, Novartis, Mylen, Ipsen, Pfizer (ET); Marko Jakopovic: AstraZeneca, Roche, Boehringer Ingelheim, Novartis, Novartis Oncology, Merck Sharp & Dohme, Berlin Chemie, Pfizer, Sandoz, Abbot, Eli Lilly (C/A), Roche, Novartis Oncology, Boehringer Ingelheim, AstraZeneca, Eli Lilly (ET); Jacek Jassem: Roche (travel support), G1 Therapeutics (C/A); Dragana Jovanovic: AstraZeneca, Boehringer Ingelheim, Roche, Pfizer (C/A, H); Zhasmina Mihaylova: AstraZeneca, Roche, Eli Lilly, Boehringer Ingelheim, Pfizer (C/A), Bristol‐Myers Squibb, Merck Sharp & Dohme (C/A, ET); Gyula Ostoros: Bristol‐Myers Squibb, Merck Sharp & Dohme, Pfizer, Roche, AstraZeneca (C/A); Milada Zemanova: Novartis, GlaxoSmithKline (C/A, H, ET), Bayer, Novartis (travel grants); Christoph Zielinski: Roche, Novartis, Bristol‐Myers Squibb, Merck Sharp & Dohme, Imugene, Ariad, Pfizer, Merrimack, Merck KGaA, Fibrogen, AstraZeneca, Tesaro, Gilead, Servier, Shire, Eli Lilly, Athenex (H). The other author indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Acknowledgements
We thank MedInteractiv Plus for summarizing survey outcomes and for writing assistance in parts of the manuscript. Support for data collection conducted by CECOG and for writing services were obtained from financial support by AstraZeneca. This research did not receive any specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors.
Disclosures of potential conflicts of interest may be found at the end of this article.
References
- 1. Bray F, Ferlay J, Soerjomataram I et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 2. Allemani C, Matsuda T, Di Carlo V et al. Global surveillance of trends in cancer survival 2000‐14 (CONCORD‐3): Analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population‐based registries in 71 countries. Lancet 2018;391:1023–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Planchard D, Popat S, Kerr K et al. Metastatic non‐small‐cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Ann Oncol 2018;29(suppl 4):iv192–iv237. [DOI] [PubMed] [Google Scholar]
- 4. Ferrera R, Mezquita L, Besse B. Progress in the management of advanced thoracic malignancies in 2017. J Thorac Oncol 2018;13:301–322. [DOI] [PubMed] [Google Scholar]
- 5. Cherny N, Sullivan R, Torode J et al. ESMO European Consortium Study on the availability, out‐of‐pocket costs and accessibility of antineoplastic medicines in Europe. Ann Oncol 2016;27:1423–1443. [DOI] [PubMed] [Google Scholar]
- 6. Vrdoljak E, Bodoky G, Jassem J et al. Cancer control in Central and Eastern Europe: Current situation and recommendations for improvement. The Oncologist 2016;21:1183–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ryska A, Buiga R, Fakirova A et al. Non‐small cell lung cancer in countries of Central and Southeastern Europe: Diagnostic procedures and treatment reimbursement surveyed by the Central European Cooperative Oncology Group. The Oncologist 2018;23:e152–e158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cherny N, Sullivan R, Dafni U et al. A standardized, generic, validated approach to stratify the magnitude of clinical benefit that can be anticipated from anti‐cancer therapies: The European Society for Medical Oncology Magnitude of Clinical Benefit Scale (ESMO‐MCBS). Ann Oncol 2015;26:1547–1573. [DOI] [PubMed] [Google Scholar]
- 9. Hammerman A, Greenberg‐Dotan S, Feldhamer I et al. The ESMO‐Magnitude of Clinical Benefit Scale for novel oncology drugs: Correspondence with three years of reimbursement decisions in Israel. Expert Rev Pharmacoecon Outcomes Res 2018;18:119–122. [DOI] [PubMed] [Google Scholar]
- 10. Vrdoljak E, Bodoky G, Jassem J et al. Expenditures on oncology drugs and cancer mortality‐to‐incidence ratio in Central and Eastern Europe. The Oncologist 2019;24:30–37. [DOI] [PMC free article] [PubMed] [Google Scholar]


