Abstract
Objective
TAS‐102 is effective for treating patients with metastatic colorectal cancer (mCRC). This study determined whether combining bevacizumab (Bmab) with TAS‐102 improves clinical outcomes in refractory mCRC.
Patients and Methods
We retrospectively analyzed data from Japanese patients with refractory mCRC who received TAS‐102 (35 mg/m2, twice a day) with (T‐B group) or without Bmab (TAS‐102 monotherapy; T group) between July 2014 and December 2018. The primary endpoint was median overall survival (OS), and secondary endpoints were median time to treatment failure, overall response rate, and the incidence of adverse events. Clinical outcomes were compared using propensity score matched analysis.
Results
Data from 57 patients were analyzed (T‐B group: 21 patients, T group: 36 patients). Median OS was significantly longer in the T‐B group than the T group (14.4 months vs. 4.5 months, p < .001). Cox proportional hazard analysis showed that combination therapy with Bmab was significantly correlated with OS. Propensity score matched analysis confirmed that the median OS was significantly longer in the T‐B group than the T group (14.4 months vs. 6.1 months, p = .006) and that there was a significant correlation between Bmab and OS. The incidence of hypertension (grade ≥2) as an adverse event was significantly higher in the T‐B group than the T group (23.8% vs. 0.0%, p = .005), whereas other adverse events were comparable between the two groups.
Conclusion
Treatment with Bmab in combination with TAS‐102 is significantly associated with improved clinical outcomes in patients with mCRC refractory to standard therapies.
Implications for Practice
Combining bevacizumab (Bmab) with TAS‐102 significantly improved overall survival and several prognostic indicators in patients with metastatic colorectal cancer (mCRC) refractory to standard therapies, with manageable toxicities. Treatment with Bmab in combination with TAS‐102 is significantly associated with improved clinical outcomes in patients with mCRC.
Keywords: Bevacizumab, Trifluridine, Colorectal neoplasms, Survival, Drug‐related adverse reactions
Short abstract
To determine whether combined treatment with bevacizumab improves clinical outcomes, this retrospective study compared the efficacy and safety of treatment with or without bevacizumab in patients receiving TAS‐102 for metastatic colorectal cancer.
Introduction
TAS‐102 is an oral antitumor drug that contains the thymidine‐based nucleic acid analog trifluridine (FTD) and the thymidine phosphorylase inhibitor tipiracil hydrochloride (TPI) at a molar ratio of 1:0.5. FTD becomes incorporated into DNA and causes DNA dysfunction and damage 1, 2, 3. Although FTD is rapidly degraded to its inactive form in the intestines and liver, TPI assists in maintaining the blood concentration of FTD by inhibiting thymidine phosphorylase 4.
TAS‐102 has been shown to significantly prolong survival in metastatic colorectal cancer (mCRC) with a manageable safety profile. In the phase III RECOURSE trial, Mayer et al. showed that TAS‐102 exhibited clinical superiority over placebo with respect to overall survival (OS) and progression‐free survival (PFS) in patients with mCRC refractory to standard therapies, including fluoropyrimidines, irinotecan, and oxaliplatin (hazard ratio [HR], 0.68 and 0.48 for OS and PFS, respectively; both p < .001) 5. Xu et al. also reported that risk of death was significantly lower in the TAS‐102 arm than in the placebo arm (HR, 0.79; 95% confidence interval [CI], 0.62–0.99; p = .035) in a randomized, double‐blind, placebo‐controlled, phase III trial of TAS‐102 monotherapy in Asian patients with previously treated mCRC 6. Although severe adverse hematological events including neutropenia (38%–50%) have been frequently observed, there have been few TAS‐102–induced nonhematological toxicities 5, 6, 7.
The combination of standard chemotherapy regimens with bevacizumab (Bmab), an antibody that binds to and inhibits vascular endothelial growth factor (VEGF), improves outcomes in Bmab‐naive patients with mCRC 8, 9. Although maintenance of VEGF inhibition with Bmab plus a standard second‐line chemotherapy beyond disease progression has clinical benefits in patients with mCRC 10, the efficacy of continuous administration of Bmab after third‐line chemotherapy has not been clarified.
Tsukihara et al. assessed whether the efficacy of TAS‐102 could be improved by combining with Bmab in a study using colorectal cancer xenografts 11. They found that addition of Bmab enhanced the antitumor effect of TAS‐102 in colorectal cancer xenografts 11. Moreover, an investigator‐initiated, open‐label, single‐arm, multicenter, phase I/II study (C‐TASK FORCE) by Kuboki et al. reported that the median PFS by investigator assessment was 5.6 months (95% CI, 3.4–7.6) and OS was 11.4 months (95% CI, 7.6–13.9) 12. We previously showed that, in salvage‐line therapy for patients with mCRC, Bmab enhances the antitumor effects of TAS‐102 with a median OS (14.1 months) and PFS (6.8 months) superior to those reported in the C‐TASK FORCE study 13. However, neither the C‐TASK FORCE study nor our previous report compared TAS‐102 alone and TAS‐102 plus Bmab, and the effect of Bmab in combination with TAS‐102 was confirmed in a limited number of patients with mCRC. Therefore, the combinatory effect of Bmab in clinical practice is unknown.
Here, to determine whether combined treatment with Bmab improves clinical outcomes, we conducted a retrospective study that compared the efficacy and safety of treatment with or without bevacizumab in patients with mCRC receiving TAS‐102.
Subjects, Materials, and Methods
Patients
The study was conducted under a retrospective observational design. Data were obtained from patients’ electronic medical records in our hospital and analyzed retrospectively. The study subjects were patients with mCRC who were refractory to fluoropyrimidine, irinotecan, oxaliplatin, anti‐VEGF therapy, and anti‐EGFR therapy (for tumors with wild‐type KRAS) who received TAS‐102 in our outpatient chemotherapy clinic between July 2014 and December 2018. The exclusion criteria were reduction of the initial dose of TAS‐102 because of poor performance status (≥2 according to the Eastern Cooperative Oncology Group) or discontinuation without image evaluation. The efficacy and safety were compared between patients who received TAS‐102 plus Bmab (T‐B group) and patients who received TAS‐102 monotherapy (T group). The present study was conducted in accordance with the guideline for human studies adopted by the ethics committee of the Gifu University Graduate School of Medicine and notified by the Japanese government (institutional review board approval no. 2018‐221). Because of the retrospective nature of the study, the need for informed consent from subjects was not mandated.
Chemotherapy
Patients were treated with TAS‐102 (35 mg/m2 of body surface area) orally twice a day on days 1–5 and 8–12 in a 28‐day cycle with or without Bmab (5 mg/kg of bodyweight, administered by intravenous infusion for 30 minutes every 2 weeks). Patients were all administered an initial regular dose of chemotherapy in the first cycle.
Dose reduction was performed in the subsequent chemotherapy cycle for patients who suffered severe adverse events, particularly grade 3–4 neutropenia. In these patients, the dose of TAS‐102 was reduced by 10 mg/day when necessary on a course basis, and no dose escalation was performed, even if the adverse events disappeared. Treatment was delayed when one or more of the following adverse events occurred: grade 3–4 neutropenia or thrombocytopenia, febrile neutropenia, total bilirubin >3.0 mg/dL, aspartate transaminase and alanine transaminase >150 U/L, creatinine >1.5 mg/dL, and grade 3–4 nonhematological toxicity. Treatment was restarted after recovery from the severe adverse events by reducing the dose of TAS‐102 by 10 mg/day.
Efficacy of Chemotherapy
OS was used as the primary endpoint for examining the efficacy of TAS‐102 therapy. The tumor response rate and time to treatment failure (TTF) were used as secondary endpoints. OS was defined as the time from the start of therapy to death. A censored case who was still alive at the end of the follow‐up period was lost to follow‐up.
Tumor response was evaluated using patients’ computed tomography scans as complete response (CR), partial response (PR), stable disease (SD), and progressive disease according to the Response Evaluation Criteria in Solid Tumors guideline version 1.1 14. The overall response rate was defined as CR + PR, and the disease control rate (DCR) as CR+ PR + SD. TTF was defined as the time from the start to the end of therapy.
Assessment of Adverse Events
Adverse events included hematological toxicities, such as neutropenia, anemia, and thrombocytopenia, and nonhematological toxicities, including nausea, vomiting, diarrhea, malaise, proteinuria, and hypertension. The symptoms of adverse events were graded according to the Common Terminology Criteria for Adverse Events version 4.0 15. The incidence rate of adverse events was compared between patients who did and did not receive Bmab.
Statistical Analysis
Data were analyzed using IBM SPSS version 22 (IBM Japan Ltd., Tokyo, Japan) and R software version 3.5.1 (http://www.r-project.org). Values of p < .05 were considered significant. Patient characteristics are summarized as median with 25th and 75th percentiles for continuous variables and frequencies and percentages for categorical variables. For the primary analysis, Cox proportional hazards regression was used to evaluate the association between combination treatment with Bmab and OS with adjustment for covariates. Covariates were restricted to two variables to avoid overfitting and, based on clinical judgment and previous research, included age and modified Glasgow prognostic score (mGPS) owing to their expected strong associations with the outcome and combination treatment with Bmab. The mGPS is a reported prognostic factor in patients with colorectal cancer 16. To adjust for confounding by this factor and age with Bmab on prolongation of survival, we performed multivariable Cox proportional hazard analysis, with mGPS treated as a continuous variable.
To adjust for other baseline factors, sensitivity analysis was conducted using propensity score matching. The propensity score was generated through logistic regression to predict the probability of effectiveness of combination treatment with Bmab as a function of baseline factors (age, sex, height, body mass index [BMI], albumin, aspartate aminotransferase, alanine aminotransferase, serum creatinine, total bilirubin, c‐reactive protein, neutrophils, lymphocytes, white blood cells, hemoglobin, platelets, mGPS, neutrophil‐lymphocyte ratio (NLR), carcinoembryonic antigen (CEA), carbohydrate antigen 19‐9 (CA19‐9), time from start of first‐line chemotherapy, recurrence, primary site, KRAS exon 2 status). Analysis of matched data was performed in addition to the primary analysis to confirm the robustness of the primary results. The reliability of the regression model was internally validated via the bootstrap method by measuring the overfitting quantified using the optimism parameter in a calibration plot. Bootstrap validation was performed by resampling from 150 patients.
Results
Patients
As shown in the Consolidated Standards of Reporting Trials (CONSORT) flow diagram in Figure 1, a total of 62 patients with mCRC who received TAS‐102 were eligible. Among them, five patients were excluded because they were treated with a reduced initial dose of TAS‐102 because of poor performance status (≥2 according to the Eastern Cooperative Oncology Group; n = 2) or discontinued treatment without image evaluation (n = 3). Data from the remaining 57 patients were analyzed.
Figure 1.
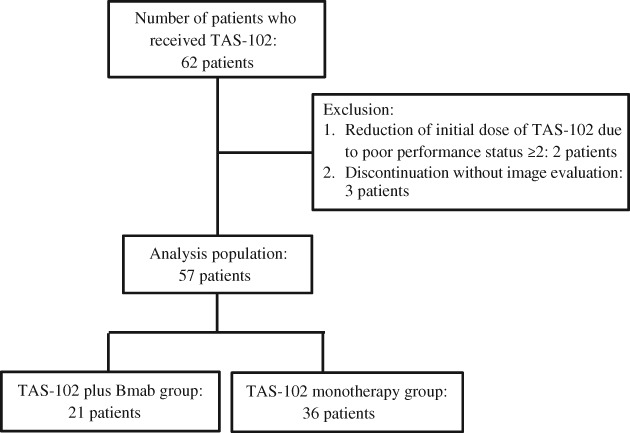
Consolidated Standards of Reporting Trials (CONSORT) flow diagram. Abbreviation: Bmab, bevacizumab.
There were 21 and 36 patients in the T‐B group and T group, respectively (Table 1). All enrolled patients were treated with irinotecan, oxaliplatin, and 5‐fluorouracil (5‐FU). The proportion of patients who received prior VEGF therapy, EGFR therapy, and regorafenib was 100%, 38.1%, and 9.5% in the T‐B group and 75%, 55.6%, and 36.1% in the T group, respectively. In addition, there were no significant differences in the proportion of patients who received post‐treatment between the T‐B group and T group (23.8% vs. 22.2%, p = 1.000). All post‐treatment regimens were regorafenib.
Table 1.
Patient demographics and baseline characteristics among patients in the T‐B group or the T group
| Characteristic | T‐B group (n = 21)a | T group (n = 36)a |
|---|---|---|
| Sex, n | ||
| Male | 13 | 16 |
| Female | 8 | 20 |
| Age, median | 67.0 (50.0–74.0) | 67.5 (59.8–71.2) |
| Height, cm | 164.0 (158.0–168.0) | 159.5 (152.0–164.0) |
| Body weight, kg | 61.8 (55.8–68.4) | 54.9 (47.0–60.5) |
| Body mass index | 23.1 (22.4–23.9) | 21.2 (19.7–23.6) |
| Albumin, mg/dL | 3.9 (3.6–4.1) | 3.7 (3.1–3.9) |
| Aspartate aminotransferase, IU/L | 24.0 (22.0–38.0) | 32.0 (21.0–52.0) |
| Alanine aminotransferase, IU/L | 17.0 (14.0–29.0) | 17.5 (12.8–26.8) |
| Serum creatinine, mg/dL | 0.6 (0.6–0.9) | 0.6 (0.5–0.7) |
| Total bilirubin, mg/dL | 0.6 (0.5–0.8) | 0.7 (0.5–1.0) |
| CRP, mg/dL | 0.4 (0.3–1.8) | 1.7 (0.3–4.0) |
| Neutrophils, /L | 3,670.0 (3,030.0–4,500.0) | 4,515.0 (3,607.8–6,250.0) |
| Lymphocytes, /L | 1,564.0 (1,269.0–1,772.0) | 1,425.0 (980.5–2,102.2) |
| White blood cells, /L | 5,900.0 (5,170.0–7,020.0) | 7,640.0 (5,645.0–9,020.0) |
| Hemoglobin, g/dL | 12.7 (12.1–13.8) | 11.4 (10.3–12.7) |
| Platelets, 104/L | 21.5 (17.5–28.1) | 22.1 (18.1–26.4) |
| mGPS, n | ||
| 0 | 16 | 11 |
| 1 | 4 | 11 |
| 2 | 1 | 9 |
| NLR | 2.5 (1.8–3.4) | 3.2 (2.1–4.6) |
| CEA, U/mL | 37.2 (22.5–98.2) | 101.8 (35.9–260.1) |
| CA19‐9, U/mL | 122.0 (32.0–330.0) | 182.0 (41.2–503.7) |
| Time from start of first‐line chemotherapy, days | 646.0 (534.0–820.0) | 693.0 (410.5–503.7) |
| Primary site, n | ||
| Colon | 17 | 34 |
| Rectal | 4 | 2 |
| Primary site, n | ||
| Right | 14 | 14 |
| Left | 7 | 22 |
| Number of metastatic organs or sites, n | ||
| 1 | 7 | 12 |
| ≥2 | 14 | 24 |
| Metastatic organ, n (%) | ||
| Liver | 15 (71) | 25 (69) |
| Lung | 15 (71) | 28 (78) |
| Lymph nodes | 5 (24) | 11 (31) |
| Peritoneum | 4 (19) | 12 (33) |
| Recurrent/advanced | ||
| Recurrent | 4 | 13 |
| Advanced | 17 | 23 |
| KRAS exon 2 status, n | ||
| Wild type | 10 | 16 |
| Mutant | 11 | 20 |
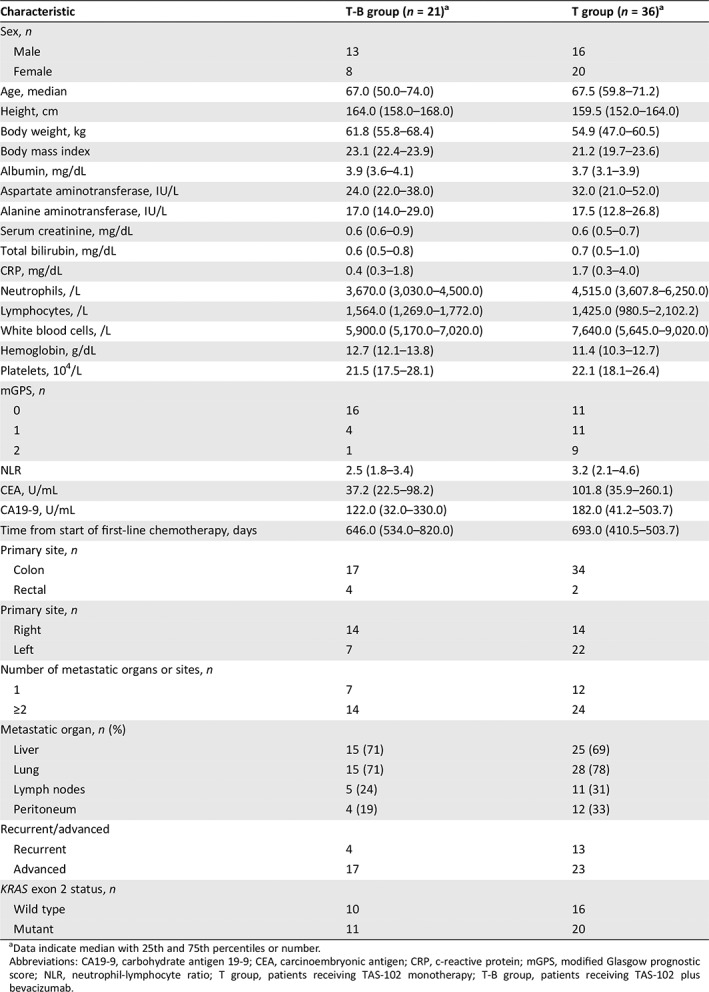
Data indicate median with 25th and 75th percentiles or number.
Abbreviations: CA19‐9, carbohydrate antigen 19‐9; CEA, carcinoembryonic antigen; CRP, c‐reactive protein; mGPS, modified Glasgow prognostic score; NLR, neutrophil‐lymphocyte ratio; T group, patients receiving TAS‐102 monotherapy; T‐B group, patients receiving TAS‐102 plus bevacizumab.
Efficacy of TAS‐102
The relative dose intensity (RDI) of TAS‐102 and Bmab was 0.58 and 0.72 in the T‐B group and 0.57 and 0 in the T group, respectively. Median follow‐up was 14.8 months (interquartile range [IQR], 7.8–23.5). After treatment with TAS‐102, the median OS was significantly longer in patients in the T‐B group than in the T group (14.4 months [95% CI, 7.9–NA] vs. 4.5 months [95% CI, 3.2–6.5]; HR, 0.24 [95% CI, 0.12–0.52]; p < .001; NA indicates that the calculation was impossible; Fig. 2).
Figure 2.
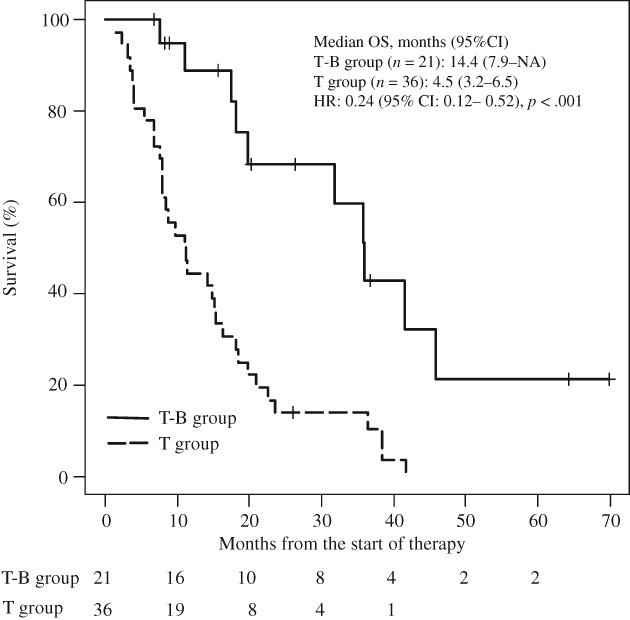
Kaplan‐Meier curves for comparison of overall survival between patients with metastatic colorectal cancer in the T‐B group and the T group. Abbreviations: CI, confidence interval; HR, hazard ratio; NA, not applicable because calculation was impossible; OS, overall survival; T group, patients receiving TAS‐102 monotherapy; T‐B group, patients receiving TAS‐102 plus bevacizumab.
Cox proportional hazards regression showed a significant association between combination treatment with Bmab and OS (HR, 0.30 [95% CI, 0.14–0.66]; p = .003) after adjusting for age and mGPS (Table 2). The regression model was internally validated, and the estimated optimism was 0.17, indicating that there was no evidence of overfitting.
Table 2.
Cox proportional hazard analysis of the risk of overall survival in patients with metastatic colorectal cancer receiving TAS‐102
| Factor | HR (95% CI) | p value |
|---|---|---|
| Combination with bevacizumab | 0.30 (0.14–0.66) | .003 |
| Age (IQR: 56–72) | 1.41 (0.88–2.25) | .151 |
| Modified Glasgow prognostic score | 1.87 (0.86–4.05) | .113 |
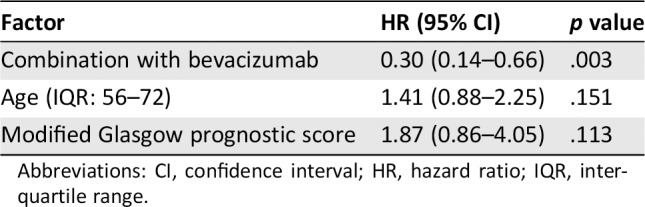
Abbreviations: CI, confidence interval; HR, hazard ratio; IQR, interquartile range.
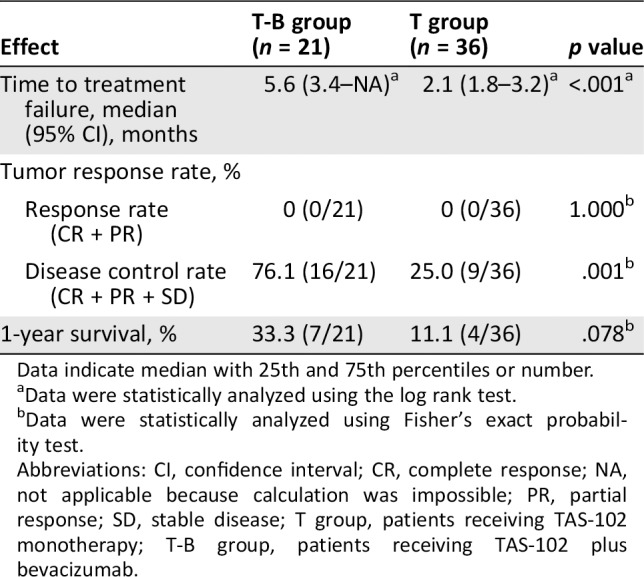
The median TTF was also significantly longer in the T‐B group than the T group (5.6 months [95% CI, 3.4–NA] vs. 2.1 months [95% CI, 1.8–3.2]; HR, 0.30 [95% CI, 0.14–0.66]; p < .001; Table 3). The overall response rate (CR + PR) was zero in both groups, whereas the DCR was significantly higher in the T‐B group than in the T group (76.1% vs. 25.0%; p = .001; Table 3). The 1‐year survival was slightly but not significantly higher in the T‐B group than in the T group (33.3% vs. 11.1%; p = .078).
Table 3.
Comparison of the median time to treatment failure and disease control rate between patients with metastatic colorectal cancer in the T‐B group and the T group
| Effect | T‐B group (n = 21) | T group (n = 36) | p value |
|---|---|---|---|
| Time to treatment failure, median (95% CI), months | 5.6 (3.4–NA)a | 2.1 (1.8–3.2)a | <.001a |
| Tumor response rate, % | |||
| Response rate (CR + PR) | 0 (0/21) | 0 (0/36) | 1.000b |
| Disease control rate (CR + PR + SD) | 76.1 (16/21) | 25.0 (9/36) | .001b |
| 1‐year survival, % | 33.3 (7/21) | 11.1 (4/36) | .078b |
Data indicate median with 25th and 75th percentiles or number.
Data were statistically analyzed using the log rank test.
Data were statistically analyzed using Fisher's exact probability test.
Abbreviations: CI, confidence interval; CR, complete response; NA, not applicable because calculation was impossible; PR, partial response; SD, stable disease; T group, patients receiving TAS‐102 monotherapy; T‐B group, patients receiving TAS‐102 plus bevacizumab.
Adverse Events Due to TAS‐102
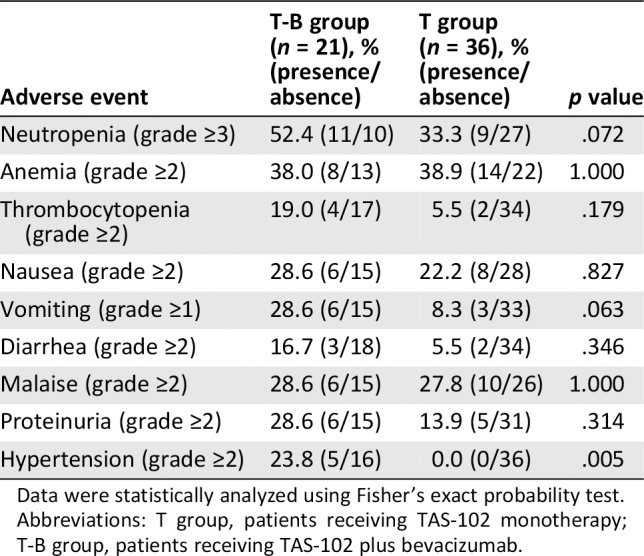
The incidence of hematological adverse events did not significantly differ between the T‐B and T groups. Among nonhematological adverse events, the incidence of hypertension (grade ≥2) was significantly higher in the T‐B group than the T group (23.8% vs. 0.0%, p = .005), and the incidence of vomiting (grade ≥1) was slightly but not significantly higher in the T‐B group than the T group (28.6% vs. 8.3%; p = .063). However, there were no significant differences in the incidence rates of other nonhematological adverse events such as nausea (grade ≥2), diarrhea (grade ≥2), and malaise (grade ≥2) between the two groups (Table 4).
Table 4.
Comparison of the incidence of adverse events between patients with metastatic colorectal cancer in the T‐B group and the T group
| Adverse event | T‐B group (n = 21), % (presence/absence) | T group (n = 36), % (presence/absence) | p value |
|---|---|---|---|
| Neutropenia (grade ≥3) | 52.4 (11/10) | 33.3 (9/27) | .072 |
| Anemia (grade ≥2) | 38.0 (8/13) | 38.9 (14/22) | 1.000 |
| Thrombocytopenia (grade ≥2) | 19.0 (4/17) | 5.5 (2/34) | .179 |
| Nausea (grade ≥2) | 28.6 (6/15) | 22.2 (8/28) | .827 |
| Vomiting (grade ≥1) | 28.6 (6/15) | 8.3 (3/33) | .063 |
| Diarrhea (grade ≥2) | 16.7 (3/18) | 5.5 (2/34) | .346 |
| Malaise (grade ≥2) | 28.6 (6/15) | 27.8 (10/26) | 1.000 |
| Proteinuria (grade ≥2) | 28.6 (6/15) | 13.9 (5/31) | .314 |
| Hypertension (grade ≥2) | 23.8 (5/16) | 0.0 (0/36) | .005 |
Data were statistically analyzed using Fisher's exact probability test.
Abbreviations: T group, patients receiving TAS‐102 monotherapy; T‐B group, patients receiving TAS‐102 plus bevacizumab.
Propensity Score Matched Analysis of OS
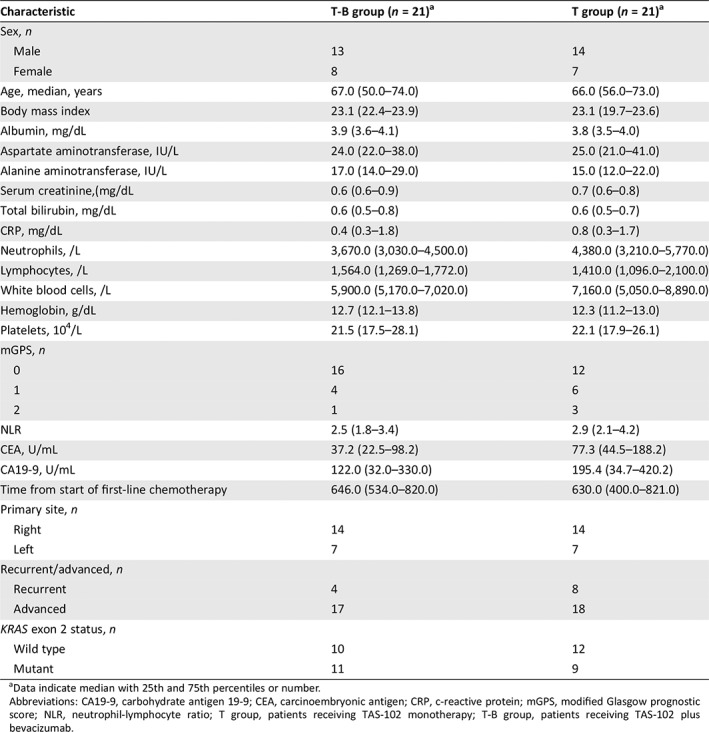
We also performed propensity score matched analysis to minimize indication bias in this retrospective study and subsequently compared patient demographics between the T‐B group and T group. There were no significant differences in factors between the two groups except for BMI (Table 5). In the propensity score matched cohorts, median OS was significantly longer in the T‐B group than in the T group (14.4 months [95% CI, 7.9–NA] vs. 6.1 months [95% CI, 3.3–14.6]; HR, 0.33 [95% CI, 0.15–0.73], p = .006; Fig. 3), and there was a significant association between Bmab and OS (HR, 0.37 [95% CI, 0.16–0.84]; p = .018) after adjusting for age and mGPS (Table 6).
Table 5.
Patient demographics and baseline characteristics among patients in the T‐B group and the T group after propensity score matched analysis
| Characteristic | T‐B group (n = 21)a | T group (n = 21)a |
|---|---|---|
| Sex, n | ||
| Male | 13 | 14 |
| Female | 8 | 7 |
| Age, median, years | 67.0 (50.0–74.0) | 66.0 (56.0–73.0) |
| Body mass index | 23.1 (22.4–23.9) | 23.1 (19.7–23.6) |
| Albumin, mg/dL | 3.9 (3.6–4.1) | 3.8 (3.5–4.0) |
| Aspartate aminotransferase, IU/L | 24.0 (22.0–38.0) | 25.0 (21.0–41.0) |
| Alanine aminotransferase, IU/L | 17.0 (14.0–29.0) | 15.0 (12.0–22.0) |
| Serum creatinine,(mg/dL | 0.6 (0.6–0.9) | 0.7 (0.6–0.8) |
| Total bilirubin, mg/dL | 0.6 (0.5–0.8) | 0.6 (0.5–0.7) |
| CRP, mg/dL | 0.4 (0.3–1.8) | 0.8 (0.3–1.7) |
| Neutrophils, /L | 3,670.0 (3,030.0–4,500.0) | 4,380.0 (3,210.0–5,770.0) |
| Lymphocytes, /L | 1,564.0 (1,269.0–1,772.0) | 1,410.0 (1,096.0–2,100.0) |
| White blood cells, /L | 5,900.0 (5,170.0–7,020.0) | 7,160.0 (5,050.0–8,890.0) |
| Hemoglobin, g/dL | 12.7 (12.1–13.8) | 12.3 (11.2–13.0) |
| Platelets, 104/L | 21.5 (17.5–28.1) | 22.1 (17.9–26.1) |
| mGPS, n | ||
| 0 | 16 | 12 |
| 1 | 4 | 6 |
| 2 | 1 | 3 |
| NLR | 2.5 (1.8–3.4) | 2.9 (2.1–4.2) |
| CEA, U/mL | 37.2 (22.5–98.2) | 77.3 (44.5–188.2) |
| CA19‐9, U/mL | 122.0 (32.0–330.0) | 195.4 (34.7–420.2) |
| Time from start of first‐line chemotherapy | 646.0 (534.0–820.0) | 630.0 (400.0–821.0) |
| Primary site, n | ||
| Right | 14 | 14 |
| Left | 7 | 7 |
| Recurrent/advanced, n | ||
| Recurrent | 4 | 8 |
| Advanced | 17 | 18 |
| KRAS exon 2 status, n | ||
| Wild type | 10 | 12 |
| Mutant | 11 | 9 |
Data indicate median with 25th and 75th percentiles or number.
Abbreviations: CA19‐9, carbohydrate antigen 19‐9; CEA, carcinoembryonic antigen; CRP, c‐reactive protein; mGPS, modified Glasgow prognostic score; NLR, neutrophil‐lymphocyte ratio; T group, patients receiving TAS‐102 monotherapy; T‐B group, patients receiving TAS‐102 plus bevacizumab.
Figure 3.
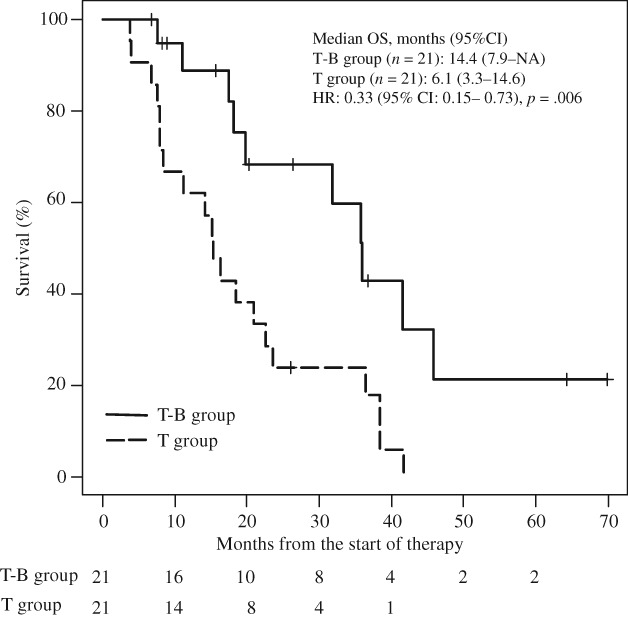
Kaplan‐Meier curves for comparison of overall survival between patients with metastatic colorectal cancer in the T‐B group and the T group after propensity score matched analysis.Abbreviations: CI, confidence interval; HR, hazard ratio; NA, not applicable because calculation was impossible; OS, overall survival; T group, patients receiving TAS‐102 monotherapy; T‐B group, patients receiving TAS‐102 plus bevacizumab.
Table 6.
Cox proportional hazard analysis of the risk of overall survival between patients with metastatic colorectal cancer receiving TAS‐102 after propensity score matched analysis
| Factor | HR (95% CI) | p value |
|---|---|---|
| Combination with bevacizumab | 0.37 (0.16–0.84) | .018 |
| Age (IQR: 53.25–73) | 1.46 (0.77–2.80) | .248 |
| Modified Glasgow prognostic score | 1.30 (0.49–3.44) | .599 |
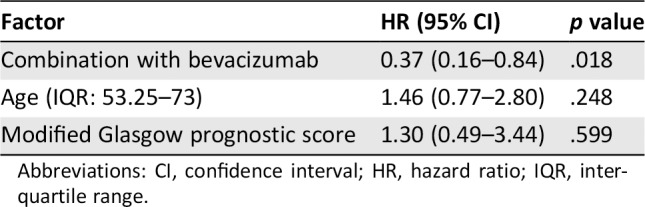
Abbreviations: CI, confidence interval; HR, hazard ratio; IQR, interquartile range.
Discussion
This study compared the clinical outcomes of patients with mCRC who received TAS‐102 in combination with Bmab and TAS‐102 monotherapy. We showed that both the median OS and median TTF were longer in the T‐B group than the T group (OS: 14.4 months vs. 4.5 months; HR, 0.24 [95% CI, 0.12–0.52]; p < .001; TTF: 5.7 months vs. 2.1 months; HR, 0.30 [95% CI, 0.14–0.66]; p < .001). Other clinical responses including the 1‐year OS rate and DCR also tended to be better in the T‐B group than in the T group. Interestingly, the RDI of TAS‐102 was similar between the two groups (0.58 vs. 0.57).
Recent studies have reported the effectiveness of chemotherapy for treating mCRC. The use of molecular target anticancer agents, including Bmab, cetuximab, and panitumumab, in addition of cytotoxic anticancer agents, such as oxaliplatin, irinotecan, and 5‐FU, has led to an OS of over 30 months 17. Furthermore, the CORRECT trial reported that salvage‐line treatment such as regorafenib also prolonged survival time compared with placebo 18. Although TAS‐102 is a later‐line standard chemotherapy for refractory mCRC, the median PFS after TAS‐102 therapy was 2.0 months in the RECOURSE 5, J‐003 7, and TERRA studies 6, which, in comparison with other treatments, may not be sufficient.
The median TTF determined in the T‐B group in the present study was mostly consistent with that reported by Kuboki et al. in the C‐TASK FORCE study (median TTF, 5.6 months) 12. In contrast, the OS in the T‐B group in our study was much longer than that reported previously (median OS, 11.4 months) 12. Tsuchihashi et al. showed that the median OS was 10 months (95% CI, 9.2–11.6 months), 6.5 months (95% CI, 5.3–7.1 months), and 3.9 months (95% CI, 3.3–4.9 months) in patients with mCRC who received regorafenib or TAS‐102 with a mGPS of 0 (n = 285), 1 (n = 71), and 2 (n = 167), respectively, and reported a significant difference according to the mGPS (p < .001) 16. Although the proportion of patients with a mGPS of 0 who received TAS‐102 plus Bmab in the C‐TASK FORCE trial was not disclosed 12, these patients composed as much as 76% of participants in the present study, which may explain the longer OS in the T‐B group compared with that reported previously 12. Cox proportional hazards regression showed a significant correlation between combination treatment with Bmab and OS after adjusting for age and mGPS, suggesting that Bmab improves OS in patients receiving TAS‐102.
In the initial analysis, we found that the mGPS was significantly lower and that NLR and tumor markers (CEA and CA19‐9) tended to be lower in the T‐B group than in the T group. Because mGPS 16, 19, 20, NLR 19, 20, 21, 22, CEA 19, 20, and CA19‐9 20 are prognostic factors for patients with mCRC, our findings suggest that patients in the T group had slightly poorer prognosis in the initial analysis. Using propensity score matched analysis to correct for any biases, we found that the median OS was significantly longer in the T‐B group than in the T group (14.4 months vs. 6.1 months, p < .001) and that there was a significant correlation between Bmab and OS (HR, 0.37; p = .018) after adjusting for age and mGPS, similar to the initial analysis.
Tsukihara et al. reported that levels of phosphorylated FTD, an active form of FTD, were increased when TAS‐102 and Bmab were combined for treatment of human colorectal cancer xenografts 11. Tumor blood vessels are generally poorly organized and hyperpermeable, consequently resulting in diminished blood supply 23. Bmab inhibits angiogenesis by antagonizing VEGF, potentially normalizing tumor vasculature and thereby improving tumor blood supply and increasing FTD accumulation and its subsequent phosphorylation in the tumor. Therefore, therapy comprising Bmab in combination with TAS‐102 is thought to provide high antitumor activity and to prolong survival.
We investigated the adverse events caused by TAS‐102 or Bmab and compared these between the T‐B group and T group. The incidence of hypertension (grade ≥2) was higher in the T‐B group than in the T group (23.8% vs. 0.0%), with the incidence rate of hypertension in the T‐B group being consistent with that reported by Kuboki et al. 12. All cases of hypertension (grade ≥2) were successfully controlled with antihypertensive medication. Although the incidence of severe (grade ≥3) neutropenia tended to be higher in the T‐B group than in the T group (52.4% vs. 33.3%, p = .072), febrile neutropenia only occurred in one patient in the T group. The incidence rate of neutropenia (grade ≥3) caused by TAS‐102 alone was 38% and 33.2% in the RECOUSE study 5 and TERRA study 6, respectively, which is mostly consistent with our findings in the T group. Several clinical trials have reported that the incidence of severe neutropenia tends to be higher in cases in which the therapy comprises combination treatment with an antiangiogenic agent compared with a cytotoxic agent alone 24, 25, 26. Therefore, the incidence of severe neutropenia should be noted in patients receiving combination treatment with Bmab.
Several limitations of our study warrant mention. First, the study was conducted under a retrospective design at a single center. Second, because the sample size was small and the number of factors included in the multivariable analysis was limited to avoid overfitting, consideration of confounding factors may have been insufficient. Confirmation in a large‐scale prospective study is therefore required. Future studies should also investigate the additive antitumor activity effects of Bmab on TAS‐102.
Conclusion
Our retrospective study provides the first evidence of the efficacy and safety of treatment with and without Bmab in patients with refractory mCRC receiving TAS‐102. Combining Bmab with TAS‐102 significantly improved OS and several prognostic indicators in patients with mCRC refractory to standard therapies, with manageable toxicities. Therefore, combination treatment with Bmab may be effective for the treatment of patients with refractory mCRC receiving TAS‐102.
Author Contributions
Conception/design: Hironori Fujii, Nobuhisa Matsuhashi
Provision of study material or patients: Hironori Fujii, Nobuhisa Matsuhashi, Mika Kitahora, Takao Takahashi, Chiemi Hirose, Hirotoshi Iihara, Yunami Yamada, Daichi Watanabe
Collection and/or assembly of data: Hironori Fujii, Nobuhisa Matsuhashi, Mika Kitahora,
Data analysis and interpretation: Takuma Ishihara
Manuscript writing: Hironori Fujii, Nobuhisa Matsuhashi, Akio Suzuki, Kazuhiro Yoshida
Final approval of manuscript: Hironori Fujii, Nobuhisa Matsuhashi, Mika Kitahora, Takao Takahashi, Chiemi Hirose, Hirotoshi Iihara, Yunami Yamada, Daichi Watanabe, Takuma Ishihara, Akio Suzuki, Kazuhiro Yoshida
Disclosures
Takao Takahashi: Takeda Pharmaceutical Co., Ltd., Sanofi (H); Kazuhiro Yoshida: Taiho Pharm, Chugai Pharm, Takeda Pharm, Eli Lilly, Yakult Honsha, Merck Sharp & Dohme, Daiichi Sankyo, Ono Pharm, Merck Serono, Johnson & Johnson, Covidien, Eisai, Otsuka Pharma, Sanofi, Nippon Kayaku, Asahi Kasei, Tsumura, Kyowa Hakko Kirin, Astellas, Toyama Chemical, KCI, Abbott Japan, Toray Medical (H), EA Pharma, Bayer Yakuhin, Olympus, Terumo, Bristol Myers Japan, Denka, Teijin, SBI Pharma, Intuitive Surgical, Novartis Pharma, Pfizer, Kyowa Hakko Kirin, Astellas, Toyama Chemical, KCI, Abbott Japan, Toray Medical (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Disclosures of potential conflicts of interest may be found at the end of this article.
References
- 1. Tanaka N, Sakamoto K, Okabe H et al. Repeated oral dosing of TAS‐102 confers high trifluridine incorporation into DNA and sustained antitumor activity in mouse models. Oncol Rep 2014;32:2319–2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sakamoto K, Yokogawa T, Ueno H et al. Crucial roles of thymidine kinase 1 and deoxyUTPase in incorporating the antineoplastic nucleosides trifluridine and 2'‐deoxy‐5‐fluorouridine into DNA. Int J Oncol 2015;46:2327–2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Emura T, Murakami Y, Nakagawa F et al. A novel antimetabolite, TAS‐102 retains its effect on FU‐related resistant cancer cells. Int J Mol Med 2004;13:545–549. [PubMed] [Google Scholar]
- 4. Fukushima M, Suzuki N, Emura T et al. Structure and activity of specific inhibitors of thymidine phosphorylase to potentiate the function of antitumor 2'‐deoxyribonucleosides. Biochem Pharmacol 2000;59:1227–1236. [DOI] [PubMed] [Google Scholar]
- 5. Mayer RJ, Van Cutsem E, Falcone A et al. Randomized trial of TAS‐102 for refractory metastatic colorectal cancer. N Engl J Med 2015;372:1909–1919. [DOI] [PubMed] [Google Scholar]
- 6. Xu J, Kim TW, Shen L et al. Results of a randomized, double‐blind, placebo‐controlled, phase III trial of trifluridine/tipiracil (TAS‐102) monotherapy in Asian patients with previously treated metastatic colorectal cancer: The TERRA study. J Clin Oncol 2018;36:350–358. [DOI] [PubMed] [Google Scholar]
- 7. Yoshino T, Mizunuma N, Yamazaki K et al. TAS‐102 monotherapy for pretreated metastatic colorectal cancer: A double‐blind, randomised, placebo‐controlled phase 2 trial. Lancet Oncol 2012;13:993–1001. [DOI] [PubMed] [Google Scholar]
- 8. Hurwitz H, Fehrenbacher L, Novotny W et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004;350:2335–2342. [DOI] [PubMed] [Google Scholar]
- 9. Saltz LB, Clarke S, Díaz‐Rubio E et al. Bevacizumab in combination with oxaliplatin‐based chemotherapy as first‐line therapy in metastatic colorectal cancer: A randomized phase III study. J Clin Oncol 2008;26:2013–2019. [DOI] [PubMed] [Google Scholar]
- 10. Bennouna J, Sastre J, Arnold D et al. Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): A randomised phase 3 trial. Lancet Oncol 2013;14:29–37. [DOI] [PubMed] [Google Scholar]
- 11. Tsukihara H, Nakagawa F, Sakamoto K et al. Efficacy of combination chemotherapy using a novel oral chemotherapeutic agent, TAS‐102, together with bevacizumab, cetuximab, or panitumumab on human colorectal cancer xenografts. Oncol Rep 2015;33:2135–2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kuboki Y, Nishina T, Shinozaki E et al. TAS‐102 plus bevacizumab for patients with metastatic colorectal cancer refractory to standard therapies (C‐TASK FORCE): An investigator‐initiated, open‐label, single‐arm, multicentre, phase 1/2 study. Lancet Oncol 2017;18:1172–1181. [DOI] [PubMed] [Google Scholar]
- 13. Matsuhashi N, Takahashi T, Fujii H et al. Combination chemotherapy with TAS‐102 plus bevacizumab in salvage line treatment of metastatic colorectal cancer: A single center, retrospective study examining the prognostic value of the modified Glasgow Prognostic score in salvage‐line therapy of metastatic colorectal cancer. Mol Clin Oncol 2019;11:390–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Eisenhauer EA, Therasse P, Bogaerts J et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–247. [DOI] [PubMed] [Google Scholar]
- 15.U.S. Department of Health and Human Services, National Institutes of Health National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. Available at https://www.eortc.be/services/doc/ctc/. Published 2009. Accessed on September 1, 2018.
- 16. Tsuchihashi K, Ito M, Moriwaki T et al. Role of predictive value of the modified Glasgow prognostic score for later‐line chemotherapy in patients with metastatic colorectal cancer. Clin Colorectal Cancer 2018;17:e687–e697. [DOI] [PubMed] [Google Scholar]
- 17. Elez E, Argilés G, Tabernero J. First‐line treatment of metastatic colorectal cancer: Interpreting FIRE‐3, PEAK, and CALGB/SWOG 80405. Curr Treat Options Oncol 2015;16:52. [DOI] [PubMed] [Google Scholar]
- 18. Grothey A, Van Cutsem E, Sobrero A et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): An international, multicentre, randomised, placebo‐controlled, phase 3 trial. Lancet 2013;381:303–312. [DOI] [PubMed] [Google Scholar]
- 19. Kim IH, Lee JE, Yang JH et al. Clinical significance of changes in systemic inflammatory markers and carcinoembryonic antigen levels in predicting metastatic colorectal cancer prognosis and chemotherapy response. Asia Pac J Clin Oncol 2018;14:239–246. [DOI] [PubMed] [Google Scholar]
- 20. Song A, Eo W, Lee S. Comparison of selected inflammation‐based prognostic markers in relapsed or refractory metastatic colorectal cancer patients. World J Gastroenterol 2015;21:12410–12420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chua W, Charles KA, Baracos VE et al. Neutrophil/lymphocyte ratio predicts chemotherapy outcomes in patients with advanced colorectal cancer. Br J Cancer 2011;104:1288–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dell'Aquila E, Cremolini C, Zeppola T et al. Prognostic and predictive role of neutrophil/lymphocytes ratio in metastatic colorectal cancer: A retrospective analysis of the TRIBE study by GONO. Ann Oncol 1;29:924–930. [DOI] [PubMed] [Google Scholar]
- 23. Jain RK. Normalizing tumor vasculature with anti‐angiogenic therapy: A new paradigm for combination therapy. Nat Med 2001;7:987–989. [DOI] [PubMed] [Google Scholar]
- 24. Wilke H, Muro K, Van Cutsem E et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro‐oesophageal junction adenocarcinoma (RAINBOW): A double‐blind, randomised phase 3 trial. Lancet Oncol 2014;15:1224–1235. [DOI] [PubMed] [Google Scholar]
- 25. Van Cutsem E, Tabernero J, Lakomy R et al. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin‐based regimen. J Clin Oncol 2012;30:3499–3506. [DOI] [PubMed] [Google Scholar]
- 26. Tabernero J, Yoshino T, Cohn AL et al. Ramucirumab versus placebo in combination with second‐line FOLFIRI in patients with metastatic colorectal carcinoma that progressed during or after first‐line therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine (RAISE): A randomised, double‐blind, multicentre, phase 3 study. Lancet Oncol 2015;16:499–508. [DOI] [PubMed] [Google Scholar]


