Abstract
The National Lung Screening Trial demonstrated improved lung cancer mortality with annual low-dose computed tomography (CT) screening, leading to lung cancer screening endorsement by the United States Preventive Services Task Force and coverage by the Centers for Medicare and Medicaid. Adherence to annual CT screens in that trial was 95%, which may not be representative of real-world, particularly medically underserved populations. This pragmatic trial will determine the effect of patient-focused, telephone-based patient navigation on adherence to CT-based lung cancer screening in an urban safety-net population. 340 adults who meet standard eligibility for lung cancer screening (age 55–77 years, smoking history ≥ 30 pack-years, quit within 15 years if former smoker) are referred through an electronic medical record-based order by physicians in community- and hospital-based primary care settings within the Parkland Health and Hospital System in Dallas County, Texas. Eligible patients are randomized to usual care or patient navigation, which addresses adherence, patient-reported barriers, smoking cessation, and psycho-social concerns related to screening completion. Patients complete surveys and semi-structured interviews at baseline, 6-month, and 18-month follow-ups to assess attitudes toward screening. The primary endpoint of this pragmatic trial is adherence to three sequential, prospectively defined steps in the screening protocol. Secondary endpoints include self-reported tobacco use and other patient-reported outcomes. Results will provide real-world insight into the impact of patient navigation on adherence to CT-based lung cancer screening in a medically underserved population. This study was registered with the NIH ClinicalTrials.gov database ( NCT02758054) on April 26, 2016.
Keywords: Adherence, Lung cancer screening, Navigation, Patient reported outcomes, Pragmatic trial, Smoking cessation
1. Introduction
Lung cancer remains the leading cause of cancer death for both men and women in the United States [1]. While advanced patient age and comorbidities contribute, it is late stage at diagnosis that primarily drives these poor outcomes. Only one-third of patients present with localized, potentially resectable disease [2]. Despite considerable advances in molecular diagnostics, targeted therapy, and immunotherapy, outcomes for patients with advanced disease remain poor, with 5-year survival rates under 5%. Lung cancer also has a disproportionate burden among racial minorities and socioeconomically disadvantaged populations, who experience later-stage diagnoses and higher mortality rates [3–5].
The potential benefit of lung cancer screening has only been realized in recent years. The National Lung Screening Trial (NLST) randomized > 50,000 high-risk individuals to annual CXR or annual low-dose chest computed tomography (CT). The trial met its primary endpoint, demonstrating a 20% reduction in lung cancer mortality, as well as a 6.7% reduction in all-cause mortality, in the low-dose CT arm [6]. These results compare favorably with other established cancer screening platforms. In the NLST, the number needed to screen (NNS) to prevent one lung cancer death was 320, compared to 780 for mammography, 1140 for Pap smears, 1250 for fecal occult blood testing, and 850 for sigmoidoscopy [7–11].
Annual CT-based lung cancer screening has been endorsed by the US Preventive Services Task Force, is now a covered benefit of Medicare, and is well underway in clinical settings across the US [12]. Nevertheless, feasibility and effectiveness of lung cancer screening in the broader population remain critical questions [13]. CT-based lung cancer screening represents a complex clinical undertaking [14]. In the NLST, approximately 40% of patients had “positive” screens that triggered a cascade of complex follow-up steps [6]. Adherence to annual CT screens was 95% over the course of the study, a rate that may not be representative of real-world, particularly medically underserved populations [15]. Compared to the comparable U.S. population, NLST participants were more likely to be white, former (rather than current) smokers, have higher education levels, and have higher socioeconomic status [16]—characteristics associated with participation in and adherence to cancer screening [17–20].
For other malignancies, including breast, colorectal and cervical cancer, patient navigation is associated with improved adherence to screening processes. Its greatest impact occurs among underserved populations. Navigation also improves psychosocial and behavioral outcomes, as well as satisfaction with care, in at-risk populations [21,22]. For lung cancer screening, navigation also provides an opportunity to capitalize on a “teachable moment” for smoking cessation efforts, including harnessing patient motivation, providing resources, and referring patients to empirically supported cessation programs [23–26].
We therefore designed a pragmatic randomized controlled trial to determine the impact of patient navigation on completion of the lung cancer screening process for medically underserved populations. Aim 1 of this study is to compare rates of completion for clinically recommended steps in the lung cancer screening process between patients randomized to the navigation intervention versus patients who receive usual care. Secondary aims include comparing group differences in patient-reported outcomes, and exploring whether differences seen in rates of completion of screening steps, patient-reported outcomes, and tobacco use are moderated by patient attitudes and beliefs.
2. Methods/design
2.1. Study setting and recruitment
Parkland Health and Hospital System (Parkland) is the integrated safety-net health system for Dallas County, Texas, providing care for more than one million under- and uninsured county residents through a central, 982-bed tertiary care hospital, specialty clinics, and 12 community-based primary care clinics [27,28]. These neighborhood-based clinics provide critical outreach for Dallas County, which is the 9th largest and one of the most ethnically diverse counties in the country (39.5% Hispanic, 34.4% white, and 20.8% African American). This diverse but highly vulnerable population has substantial risk factors for lung cancer. Parkland has an enterprise-wide EMR system (EPIC; Verona, WI) that allows electronic tracking of a wide array of patient characteristics and outcomes. Consistent with more substantial smoking histories, more advanced-stage diagnoses, and higher lung cancer-related mortality among racial minorities and lower socioeconomic status individuals, Parkland patients experience a disproportionate lung cancer burden and could receive maximal benefit from an effective lung screening protocol [3,5,29].
Usual care at Parkland is system-wide; however, the process is largely opportunistic, with separate clinic structures for screening and follow-up, and lacks systematic measures to ensure appointment scheduling and receipt of care. Screening results are communicated to the referring physician. Providers have the option of referring patients with radiographic abnormalities suspicious for lung cancer to a central Lung Diagnostics Clinic staffed by Pulmonary Medicine physicians. Patients diagnosed with lung cancer are referred to thoracic surgery, radiation oncology, and/or medical oncology clinics.
2.2. Participant selection & eligibility
Study participants will be comprised of Parkland patients in primary care who have received a physician’s order in their EMR for a low-dose CT for lung cancer screening purposes. Physician completion of the EMR order set for lung cancer screening is possible only if patient age and smoking history (direct EMR extraction, as provided by the ordering clinician) meet eligibility criteria.1 Referred patients are permitted to take part in concomitant care; no interventions will be prohibited during the trial, per SPIRIT guidelines [30].
2.3. Screening and recruitment of participants
Recruitment, intervention delivery, and study data collection will be conducted through a study-specific, interactive research database, populated with weekly EMR data of patients who have received physician referrals for low-dose CT screening. Bilingual study staff will use the database interface to access patient participants’ contact and appointment information as scheduled by Parkland clinics and/or the patient, and to administer research telephone surveys and interviews. Integrating data updates from EMR extraction reports, our system will also flag patients with missed appointments, enabling navigators to conduct timely follow-up to prompt participants to re-schedule missed clinic/procedure visits (see Intervention).
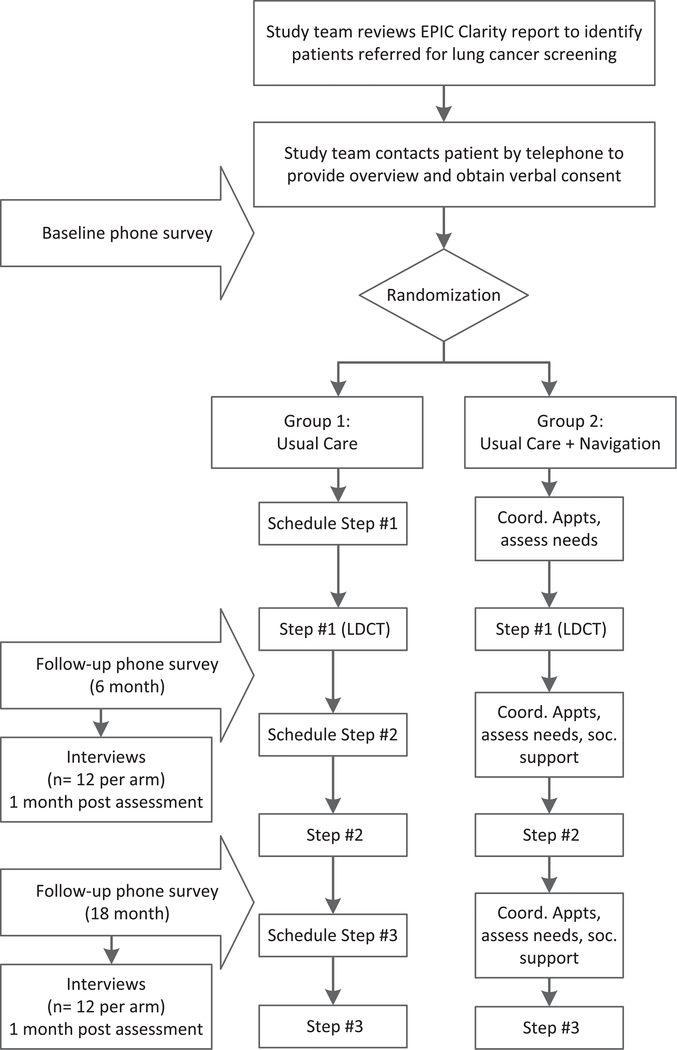
Study staff will call to confirm study eligibility, explain the study and invite patients to participate. Patients who are referred will be excluded from the study only if they do not speak English or Spanish. Verbal informed consent for randomization and data collection, with HIPAA authorization, will be documented, per IRB approval. Verbal instead of written consent will be obtained since all patient interactions take place over the telephone. The Parkland primary care clinics provide access to a patient population that exceeds our sample size several-fold. Patients who consent to participate will be randomized 1:1 to either (1) Usual Care (n = 170) and (2) Usual Care plus telephone-based Navigation Intervention (n = 170; see Fig. 1).
Fig. 1.
Project algorithm. Follow-up surveys will be performed 6 months and 18 months after baseline survey. Depending on clinical findings and events, these may capture the 1st and 2nd CT screen, respectively, or multiple other clinical steps (such as other radiology studies, biopsies, etc.).
Patients are invited to complete the Baseline survey (in either English or Spanish) during this telephone call, and can discontinue participation at any time. Study staff will make up to four call attempts (day, evening, weekend) to reach patients prior to the scheduled CT appointment. Survey administration will be integrated in the study database, using REDCap software [31]. Participants in both arms are asked to complete surveys at baseline, 6 months, and 18 months via telephone with a bilingual research assistant. A subset of patients (n = 48; at 2 time points, 12 per arm) are also recruited to participate in semi-structured qualitative interviews about the navigation experience in the month following each of the post-baseline quantitative assessments. Participants who complete surveys and/or interviews will receive appropriate gift card incentives for their time and effort.
2.4. Baseline survey
Before the initial LDCT scan, study staff will collect baseline demographic, smoking, and health literacy [32] information for each enrolled study participant (see Table 1).
Table 1.
Measures collected at baseline and follow-up.
| Variable | Timing |
|
|---|---|---|
| Baseline (Pre-screen) | Follow-ups (6 months, 18 months) | |
| Participant characteristics | ||
| Age | X | |
| Ethnicity | X | |
| Race | X | |
| Gender | X | |
| Education | X | |
| Health literacy | X | |
| Insurance status/type | X | X |
| Smoking history | X | X |
| Medical comorbidities | X | X |
| Adherence to other cancer screening | X | X |
| Participant attitudes and beliefs (informed by the health belief model) | ||
| Perceived severity of, susceptibility to lung cancer | X | |
| Perceived benefits/barriers to screening | X | |
| Self-efficacy | X | |
| Cancer worry | X | |
| Patient reported outcomes | ||
| Psychosocial distress | X | X |
| Tobacco use (current) | X | X |
| Satisfaction with care | X | |
| Patient involvement in care | X | |
| Decision regret | X | |
2.5. Patient navigation intervention
Our study database will incorporate an algorithm-driven, computer-assisted navigation guide, adapted from our prior Cancer Prevention and Research Institute of Texas (CPRIT)-funded studies of other modalities (breast cancer screening; PP120097) and the NCI-funded Parkland-UTSW PROSPR Center (colorectal and cervical cancer screening; 1U54CA163308).
Patient navigators are bilingual professional research staff members, not from the study population, with experience working with diverse, medically-underserved communities, who will provide a “barrier-focused” intervention [21,33] in a proactive and culturally-appropriate manner. Navigators help participants randomized to the intervention (n = 170) following enrollment to assess, plan, and facilitate each step in the CT screening process through regular telephone contact (Fig. 1). Navigators work individually with study participants by telephone to educate, motivate and empower participants to traverse the county integrated health system, specifically across the lung cancer screening continuum at Parkland. Navigators are trained using an abbreviated curriculum (~20 h) adapted from the George Washington Cancer Institute’s Center for the Advancement of Cancer Survivorship, Navigation & Policy [34]. They receive supplemental training on behavioral aspects of lung cancer screening, especially smoking cessation and modalities, as well as patient resources specific to the Parkland system. All navigator activities are protocolized; a procedure manual was generated to ensure consistency and to provide a reference resource to assist in operationalization.
The navigators do not access the patient EMR directly, but undertake the navigation protocol using the interactive study database. The navigators systematically initiate outreach calls, encouraging participation in the screening process. During these outreach calls, the study database prompts scripted queries appropriate to each stage of the referral and follow-up process. The navigators use motivational interviewing techniques [35–37] to ask about the patient’s status in the care continuum and interest in smoking cessation. They encourage patients to make and keep appointments, and inquire about any challenges to appointment adherence. The navigators track their assigned patients and document their interventions, and patient responses, in the study database.
As appointments are scheduled, the navigators initiate reminder calls 2–3 days in advance and iterate the importance of completing a lung scan, then of adhering to provider-scheduled follow-up appointments, as reflected by clinic orders in the EMR. The navigators address further concerns participants may have about getting a lung scan and/or follow-up procedures by referring patients back to the primary care physician for medical concerns about the lung screen, and to the lung diagnostic specialty clinic for medical concerns about diagnostic follow-up [38–40]. Further, 72 h to 1 week following scheduled appointments, the navigators call patient participants to confirm appointment was or was not kept and related procedures completed.
In addition to appointments prompts and reminders, the study database prompts patient navigators with scripted information about discussion of screening results, hints and reminders to facilitate participant communication with physicians. Navigators systematically address patient-reported barriers to completing the screening and follow-up process. The navigators assess why that was the case and engage participants in problem-solving, making a plan to address these issues going forward. Navigators also address important psychosocial (e.g., coping with indeterminate or abnormal results) and behavioral (e.g., motivation and self-efficacy) aspects of the screening process [15]. Navigators engage participants in discussion of smoking cessation, per individual smoking status, provide evidence-based print materials, the Texas Tobacco Quitline number, and referrals to the Parkland Smoking Cessation clinic, and encourage patients in quit attempts, reiterating the importance of addressing smoking cessation with their primary care team.
2.6. Primary endpoint
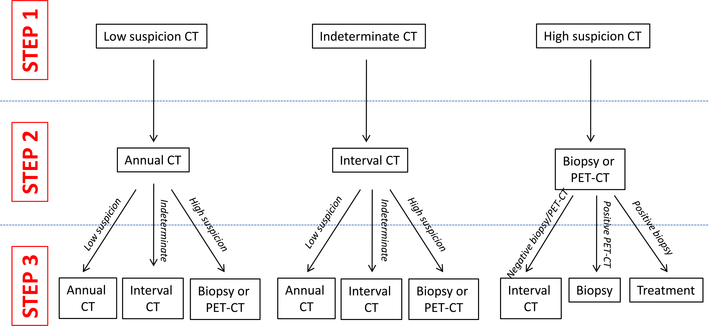
Our primary endpoint for Aim 1 is completion of the first three consecutive steps in the screening algorithm (see Fig. 2). We have selected this endpoint capturing the screening process because (1) a single normal chest CT scan does not rule out development of lung cancer (there was significant incidence of lung cancer in Years 2 and 3 of the NLST) and (2) an abnormal chest CT scan inherently requires subsequent evaluation. For participants with “normal” (“low-suspicion” in Fig. 2) CT scans (≥60%, based on NLST), this endpoint includes the three yearly chest CT scans over a two-year period. For annual CT screens, criteria for adherence is based on those employed in prior population studies of cancer screening modalities [41]. For participants with “positive” (“indeterminate” and “high suspicion” in Fig. 2) scans, the endpoint may include initial CT scan, short-interval repeat CT scan, PET, biopsy, and possibly treatment. At Parkland, low-dose chest CT reports include imaging findings, impression, and specific recommendations following the Lung-RADS system (analogous to BiRADS for mammography) [42,43]. For the primary endpoint, ancillary studies (e.g., pulmonary function tests for assessment of fitness for resection, brain MRI for clinical staging) and clinic visits are not considered algorithm steps, although all of these are captured by EMR abstraction for subanalyses. All information about completion of algorithm steps is accessed from the patient’s Parkland EMR. The study team audits a random sample of patient outcome variables. As part of the consent process, participants provide permission for medical record access for these variables.
Fig. 2.
Screening algorithm and endpoint schema. Steps 1, 2, and 3 may occur over varying time-frames: normal/low suspicion scans will be repeated annually; interval scans to evaluate indeterminate CT scans will be performed at intervals < 12 months; subsequent steps to evaluate high suspicion CT scans are anticipated to occur within weeks of the CT scan. Characterization of suspicion (low, indeterminate, or high) reflects the size, imaging features (e.g., spiculated, calcified), and rate of change of nodules. Follow-up of abnormal CT scans (e.g., biopsy or PET-CT) will be determined by local test availability and practice patterns, such as Fleischner Guidelines or Lung-RADS.
2.7. Secondary endpoints
Aim 2 of this trial elucidates group differences in patient-reported outcomes (satisfaction with care, psychosocial distress, and smoking cessation) of the CT-based lung cancer screening experience using both quantitative and qualitative methodologies. Quantitatively, we will compare groups on pre- (baseline) to post- (6- and 18-month) screen changes in patient-reported outcomes.
Fig. 1 shows the timing of patient-reported outcomes assessments. All study participants, regardless of adherence to CT screens, are asked to complete telephone-based surveys focused on these outcomes at (a) baseline [prior to first CT scan], (b) 6 months post-baseline, and (c) 18 months post-baseline. These timeframes were chosen to capture pre- and post-screening timeframes for most participants while also allowing sufficient time to analyze these data in the 36-month timeframe of the study period. Each survey takes 20–30 min. Table 1 details the content of questionnaires to assess between-group differences in patient-reported outcomes. Brief, well-validated measures used in other studies of cancer control are assessed: (1) psychosocial distress (Patient Health Questionnaire [PHQ]-8, Generalized Anxiety Disorder [GAD]-7 scale) [44–47], (2) satisfaction with care (Patient Satisfaction with Navigation-Logistics [PSN-L] [48]), (3) self-reported tobacco use (NLST smoking-related items [6]), (4) patient involvement in care (PICS) [49], and (5) decision regret (Decision Regret Scale) [50]. Survey items are available in either English or Spanish. Items that did not already have validated Spanish translations were evaluated for conceptual equivalency through the UTSW Language Validation Resource.
Qualitatively, we are conducting semi-structured interviews with a subsample of research participants drawn from each survey wave (6- and 18-month) to complete interviews (12 patients per time-point; 12 patients per arm, n = 48) stratified by arm. Interviews assess experiences of CT-based lung screening and its broader effects on attitudes toward screening, diagnosis, treatment, care quality, quality of life, competing demands/structural barriers to access, and health behaviors. Bilingual staff will randomly invite 24 patients within one month. Study team members with expertise in psychometric and qualitative methods (HAH, SJCL) developed interview guides and provide training and supervision to the research team in conducting patient interviews.
2.8. Exploratory endpoints
Aim 3 is an exploratory aim to investigate whether navigation intervention effects (screening adherence; patient-reported outcomes) are moderated by theory-based patient attitudes and beliefs. These potential moderators are measured by the Lung Cancer Screening Belief Scale, a newly validated measure of attitudes and beliefs specific to lung cancer screening (see Table 1) [51]. This measure incorporates items assessing (a) perceived severity of lung cancer; (b) perceived susceptibility to lung cancer; (c) perceived benefits and barriers to screening; and (d) self-efficacy.
2.9. Randomization to intervention
Following completion of the baseline survey, patients are randomized (1:1 ratio) to the Patient Navigation Intervention or Usual Care (n = 170 per arm). Randomization is facilitated through a computer algorithm and is stratified by gender and smoking status (current vs. former). Within each stratum (combination of gender and smoking status), participants are randomized 1:1 to the two study arms. We developed a randomization list of 0 (Usual Care) and 1 (Patient Navigation) generated from a Bernoulli distribution with probability of 0.5; within each stratum (Female, current smoker; Female, former smoker; Male, current smoker; Male, former smoker), each consecutive patient enrolled receives the next treatment assignment on the list, until the target enrollment of 170 patients in each study arm is achieved. We are not cluster randomizing because the intervention is delivered directly to the patient. Furthermore, the likelihood of contamination is quite low; the 340 patients in our study will be diluted at all levels of care (primary care, radiology department, lung diagnostics clinic, other specialty clinics) by a much larger number of individuals not undergoing lung cancer screening.
2.10. Analysis of primary endpoint and sample size justification
We expect that 25% of the Usual Care group and 43% of the Navigation group will complete three steps of the lung cancer screening process. These adherence rates are consistent with other multi-step screening in similar populations [52–54]. The anticipated intervention effect is based on 32%–245% rates of increase in cancer screening seen in other medically underserved populations [53,55]. Given the wide variability in study outcomes and lack of data specific to lung cancer screening, we conservatively estimate 72% relative and 18% absolute increase in completion of three screening process steps in the Navigation group.
Patients are divided into two strata based on the screening outcome: Stratum 1 for patients with no abnormal screening results, and Stratum 2 for patients with at least one abnormal (“positive”) result. We assume that the ratio comparing the number of patients in Stratum 1 to that in Stratum 2 is 3:2 (based on NLST, in which approximately 40% of patients had ≥1 abnormal screen [6]). We are testing whether there is a significant difference in aggregate completion rates between two intervention groups, across the two strata. Within each stratum, we expect that 25% of the Usual Care group and 43% of the Navigation group will complete three steps of the lung cancer screening process. A sample size of 292 subjects will provide 90% power to detect the difference of 18% in completion rates between the two intervention groups (odds ratio of 2.26), controlling for the screening outcome, based on the Cochran-Mantel-Haenszel test with a 0.05 two-sided significant level. Adding 15% for withdrawal of consent or enrollment of ineligible subjects, our total sample size is 340.
Data will be analyzed using intent-to-treat (ITT) analysis. Missing values will be imputed using appropriate techniques after a careful examination of blinded data sets. For Aim 1, summary statistics for patient characteristics (listed in Table 1) will be reported using means and standard deviations for continuous variables and using counts and percentages for discrete variables. The percentage of screening completion and 95% confidence interval will be reported for each intervention group. The Cochran-Mantel-Haenszel test will be performed to assess whether there is an association between intervention and screening completion, controlling for the screening outcome. Mixed univariable and multivariable logistic regression models will be used to incorporate the nested nature of the data such as participant-, physician-and clinic-level factors to investigate associations between independent variables (i.e., intervention, stratum of screening outcome, etc.) and screening completion. We will run a series of univariable mixed logistic regression models to assess strength and statistical significance of the association between each independent variable and completion of lung cancer screening process. Any variable with a univariable p-value of < 0.25 along with variables of known clinical importance will be entered in a backward selection algorithm to yield the parsimonious multivariable regression model. The screening criterion for variable selection is recommended by Hosmer, Lemeshow and Sturdivant [56]. Multivariable mixed logistic regression analysis will be conducted to identify significant independent predictors for completion of the lung cancer screening process. The PROC GLIMMIX procedure will be used to adjust for the nested nature of the data. Note that the main comparison we considered in this trial is essentially a two-group comparison (usual care vs. patient navigation), controlling for the screening outcome, instead of multiple group comparisons (for example, different combinations between two intervention groups and two strata of screening outcome). Therefore, adjustment for multiple comparisons is not needed in the proposed trial.
2.11. Analysis of secondary and exploratory endpoints
2.11.1. Quantitative analyses
Quantitative analyses of distress, satisfaction, and smoking behavior will be summarized by means and standard deviation for continuous variables and using counts and percentages for discrete variables at each time-point. The relationship between patient-level outcomes and study condition will be assessed by a two-sample t-test or a Chi-square test. Longitudinal analyses will be used to address issues of repeated measures, variable timing and missing data. If patient-reported data are missing at random, we will use mixed linear models to assess the navigation intervention effect on patient-reported outcomes. The mixed model allows for controlling for confounding variables (the screening outcome and other patient characteristics) by specifying fixed effects, and account for heterogeneity among patients by adding random effects that are unique to each patient. A linear mixed-effect model specifying the patient-reported outcome Yij for patient i at the measurement time point j as: Yij = β0 + β1xi + βzZij + b0i + b1itij + eij, where xi denotes the intervention group (1 for navigation; 0 for usual care), Zij denotes a vector of patient characteristics, tij denotes the measurement time j (j = 1,2,3 for baseline, 6 and 18 months, respectively) for patient i. β0, β1 and βz are the fixed intercept and slopes. Particularly, β1 is the fixed effect of the navigation intervention on patient-reported outcomes. Addition of random intercept b0i and random time effect (random slope) b1i will describe individual differences related to the baseline measure as well as trajectory rate; eij’s are sampling or measurement errors. However, because patients with high psychosocial distress tend to have lower chances of completing a questionnaire, missing data might be not missing at random. In that case, we will explore the missing data pattern by comparing average scores among patient subsets defined by their available data and survival status. For example, a sharp distress increase at the last available assessment among surviving patients will suggest a NMAR pattern. The pattern mixture model [57] will be used to adjust for NMAR bias by stratification. Assuming MAR, slope and intercept will be estimated separately for intervention effects within each stratum. Overall, comparison between the two study arms will be obtained by weighting within-stratum estimates with estimated probability of being in each combination of intervention and stratum.
In addition to controlling for potential confounding factors, we will incorporate the main factors that subject is non-adherent to the screening (moderating variables) into the analysis of exploratory endpoints. To explore the effect of moderation for Aim 3, we will test if there are significant interaction effects between intervention groups and moderating variables in mixed logistic regression and mixed linear regression analyses. If the analysis results show significant interaction effects, stratified analyses will be conducted by presence or absence of moderating factors.
2.11.2. Qualitative analyses
We are using the NVivo 9.0 (QSR International) data analysis software program to collate and analyze qualitative data. Research staff will audiotape semi-structured interviews for professional transcription by an approved vendor. The study team (SJCL) works with staff to organize source documents and develop a codebook for deductive analyses following categorical domains laid out in the interview guide. Per method standards, [58,59] we anticipate 48 interviews as stratified will be sufficient to reach thematic and meaning saturation, given prior work [60–62]. To focus inductive analyses, we are developing a matrix of key concepts, populating cells with brief excerpts of raw text to substantiate claims or interpretations. This analytic step facilitates cross-case comparisons (e.g., between patients in the two arms) and in-depth explorations of specific concepts [63]. Additionally, through monthly meetings, we are testing emergent themes and interpretation against the knowledge base of our clinical team (SA, NS, HTC, DEG) [64]. We systematically review coding agreements and resolve discrepancies through consensus [65,66].
3. Ethics
This study was registered with the NIH ClinicalTrials.gov database ( NCT02758054) on April 26, 2016. The protocol was approved by the University of Texas Southwestern Medical Center Institutional Review Board (STU#122015–046), via an expedited review procedure finding no more than minimal risk, and by the Parkland Office of Research; pursuant to that IRB determination, the PIs serve as the Data Monitoring Committee. Study participation is voluntary and can be discontinued at any time; deciding not to take part in this study does not affect a participant’s healthcare. Protocol modifications, adverse events reporting, and annual review are overseen by the IRB. The information provided by participants will only be shared with members of the research team. Every effort is made to keep participant information confidential. All members of the research team are required to undergo extensive training about human subject protections and data security. All personal identifying information is stored securely, per IRB approval; research data is stored on a password protected network and encrypted computers for the duration of the project. All data management procedures and databases are HIPAA compliant. Interviews are transcribed by a professional vendor under a Business Associates Agreement to ensure confidentiality. Transcripts and notes are de-identified and stored on password-protected computers.
4. Discussion
This trial offers unprecedented insight into implementation of routine lung cancer screening for high-risk individuals in underserved settings. Study findings will show whether navigation interventions increase adherence to screening processes and affect patient-reported outcomes among underserved populations. Learning from the experience of established cancer screening programs, our research addresses obstacles to screening process uptake for lung cancer screening at its inception, rather than decades later. Although other efforts to enhance uptake of lung cancer screening are underway [67], this study focuses on patient progress through the entire process, including continued annual scans following normal results and appropriate work-ups for abnormal findings, which are required to achieve the substantial public health benefits of lung cancer screening [68,69]. Pursuant to the pragmatic-explanatory continuum indicator summary (PRECIS) [70], our design reflects key pragmatic dimensions: (1) study eligibility is based on standard of care practice at Parkland, with recruitment from guideline-based referrals to low-dose CT screening by primary care physicians, not researchers; (2) although algorithm-driven, telephone-based patient navigation intervention is similar to usual care available in other Parkland clinical service lines, here tested de novo for lung cancer screening; (3) both patient and provider adherence to screening follow-up is measured unobtrusively by EMR data abstraction; researchers do not intervene on delivery of screening and follow-up processes; and (4) follow an intent-to-treat analysis for primary endpoint, detailed as above. With these features, our trial balances issues of internal and external validity [71,72] with the goal of assessing real-world effectiveness of navigation to improve screening completion within a safety-net health system.
Understanding and intervening on (1) potentially negative psycho-social consequences of lung cancer screening; and (2) continued smoking among those undergoing screening, are of utmost importance. Data from other cancer screening modalities suggest that patient uncertainty associated with indeterminate screens, high-suspicion findings, and follow-up procedures may result in dissatisfaction with medical care and psychosocial distress [73]. Several cancer screening studies suggest that false positive screens (23% in NLST) are associated with lower quality of life, increased psychosocial distress, and dissatisfaction with care [74]. Data also suggest a higher risk of negative psychosocial consequences for screening outside of the coordinated clinical trial system, especially among underserved populations. Furthermore, NLST data show lung cancer screening alone does not facilitate large scale smoking cessation, [23] emphasizing the need for enhanced smoking cessation intervention throughout the screening process, including repeated encouragement and referrals to evidence-based services. Given psychosocial challenges and higher rates of smoking among underserved individuals, understanding ways to address psychosocial concerns and facilitate smoking cessation within the context of screening takes on increased urgency.
As in other cancer screening settings, patient navigation in this study involves instrumental (task-oriented or logistic) support to assist patients with appointment scheduling, reminders, expenses, transportation, and other access to care and adherence issues [75]. Patient navigators also provide emotional support to patients, address patient-provider communication, manage cancer-related distress, and focus on quality of life. Navigation efficacy includes not only strong improvements in cancer screening rates, but also promising results for follow-up of screening abnormalities [21]. This benefit has particular importance for lung cancer screening, where at any time-point an estimated 15–25% of patients have “positive” screens [6]. As conducted for other cancer screening modalities [54], we designed our program for underserved populations to include bilingual navigators familiar with social and cultural issues affecting cancer screening. Targeting navigation for language and cultural concordance has found further beneficial effects among minority patients receiving abnormal test results [76,77].
In collaboration with Parkland partners, it is our intent to disseminate study findings through the Dallas county system, to neighboring healthcare organizations serving similar populations, as well as via Parkland’s leadership in America’s Essential Hospitals[78], formerly the National Association of Public Hospitals and Health Systems (NAPH). Findings will also be presented at scientific proceedings, for example, the Cancer Prevention & Research Institute of Texas (CPRIT), the AACR Science of Cancer Health Disparities annual meeting, the American Society for Preventive Oncology, and the Society for Behavioral Medicine. We anticipate making study data available to qualified researchers through data sharing agreements, as appropriate.
In an era of significant cost constraints, a randomized control trial of telephone-based patient navigation will provide critical evidence to support the provision of these services to strengthen lung cancer screening adherence and patient-reported outcomes. Indeed, if widespread CT-screening for early detection of lung cancer is to be disseminated, sustainability may well hinge on patient capacity to complete all steps of this complex process.
Funding
Research supported by the Cancer Prevention Research Institute of Texas (CPRIT; RP160030 to HAH, SJCL, and DEG); and by a National Cancer Institute Midcareer Investigator Award in Patient-Oriented Research (K24CA201543–01; to DEG). Additional support was received through the Harold C. Simmons Comprehensive Cancer Center (5P30 CA142543) and UT Southwestern Center for Patient-centered Outcomes Research (R24 HS022418).
Footnotes
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This study was approved by the University of Texas Southwestern Institutional Review Board (#122015–046). Informed consent is required for participation. IRB approval includes waiver of documentation of consent because research participant interactions occur by telephone.
Consent to publish
Consent to publish de-identified information is included in the verbal informed consent process. However, no data is being published at this time.
Availability of data and materials
Not applicable.
Per Parkland standard of care, the patient must be deemed “high-risk” according to the USPSTF, whose criteria include between the ages of 55 and 77, ≥30 pack-year smoking history, and if former smoker they must have quit in the last fifteen years.
References
- [1].Cancer Facts & Figures 2013.
- [2].Mulshine JL, Scott F, Molecular markers in early cancer detection. New screening tools, Chest 107 (6 Suppl) (1995) 280S–286S. [DOI] [PubMed] [Google Scholar]
- [3].Hardy D, Xia R, Liu CC, Cormier JN, Nurgalieva Z, Du XL, Racial disparities and survival for nonsmall-cell lung cancer in a large cohort of black and white elderly patients, Cancer 115 (20) (2009) 4807–4818. [DOI] [PubMed] [Google Scholar]
- [4].Bach PB, Cramer LD, Warren JL, Begg CB, Racial differences in the treatment of early-stage lung cancer, N. Engl. J. Med. 341 (16) (1999) 1198–1205. [DOI] [PubMed] [Google Scholar]
- [5].Yorio JT, Yan J, Xie Y, Gerber DE, Socioeconomic disparities in lung cancer treatment and outcomes persist within a single academic medical center, Clinical Lung Cancer 13 (6) (2012) 448–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].National Lung Screening Trial Research Aberle T,DR, Adams AM, Berg CD, Black WC, Clapp JD, Fagerstrom RM, Gareen IF, Gatsonis C, Marcus PM, et al. , Reduced lung-cancer mortality with low-dose computed tomographic screening, N. Engl. J. Med. 365 (5) (2011) 395–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Gerber DE, Should family physicians routinely screen for lung cancer in high-risk populations? Yes: CT-based screening is complex but worthwhile, Am. Fam. Physician 90 (2) (2014) 73B–74B. [PubMed] [Google Scholar]
- [8].Nelson HD, Tyne K, Naik A, Bougatsos C, Chan BK, Humphrey L, Force USPST: screening for breast cancer: an update for the U.S. Preventive Services Task Force, Ann. Intern. Med 151 (10) (2009) 727–737 (W237–742). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Benedet JL, Anderson GH, Matisic JP, A comprehensive program for cervical cancer detection and management, Am. J. Obstet. Gynecol 166 (4) (1992) 1254–1259. [DOI] [PubMed] [Google Scholar]
- [10].Hardcastle JD, Chamberlain JO, Robinson MH, Moss SM, Amar SS, Balfour TW, James PD, Mangham CM, Randomised controlled trial of faecal-occult-blood screening for colorectal cancer, Lancet 348 (9040) (1996) 1472–1477. [DOI] [PubMed] [Google Scholar]
- [11].Elmunzer BJ, Hayward RA, Schoenfeld PS, Saini SD, Deshpande A, Waljee AK, Effect of flexible sigmoidoscopy-based screening on incidence and mortality of colorectal cancer: a systematic review and meta-analysis of randomized controlled trials, PLoS Med 9 (12) (2012) e1001352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Your Medicare coverage: is my test, item, or service covered? Lung cancer screening. https://www.medicare.gov/coverage/lung-cancer-screening.html.
- [13].Kinsinger LS, Atkins D, Provenzale D, Anderson C, Petzel R, Implementation of a new screening recommendation in health care: the veterans health administration’s approach to lung cancer screening, Ann. Intern. Med. 161 (8) (2014) 597–598. [DOI] [PubMed] [Google Scholar]
- [14].Mulshine JL, D’Amico TA, Issues with implementing a high-quality lung cancer screening program, CA Cancer J. Clin 64 (5) (2014) 352–363. [DOI] [PubMed] [Google Scholar]
- [15].Gerber DE, Gillam AO, Hamann HA, Lung cancer screening in the “real world” and the role of nurse navigators, J. Oncol. Navig. Survivorship 4 (2) (2013) 21–23. [Google Scholar]
- [16].Aberle DR, Adams AM, Berg CD, Clapp JD, Clingan KL, Gareen IF, Lynch DA, Marcus PM, Pinsky PF, Baseline characteristics of participants in the randomized national lung screening trial, J. Natl. Cancer Inst. 102 (23) (2010) 1771–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Selvin E, Brett KM, Breast and cervical cancer screening: sociodemographic predictors among White, Black, and Hispanic women, Am. J. Public Health 93 (4) (2003) 618–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Swan J, Breen N, Coates RJ, Rimer BK, Lee NC, Progress in cancer screening practices in the United States: results from the 2000 National Health Interview Survey, Cancer 97 (6) (2003) 1528–1540. [DOI] [PubMed] [Google Scholar]
- [19].Gjelsvik A, Rogers ML, Clark MA, Ombao HC, Rakowski W, Continuum of mammography use among US women: classification tree analysis, Am. J. Health Behav 38 (4) (2014) 492–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ali N, Lifford KJ, Carter B, McRonald F, Yadegarfar G, Baldwin DR, Weller D, Hansell DM, Duffy SW, Field JK, et al. , Barriers to uptake among high-risk individuals declining participation in lung cancer screening: a mixed methods analysis of the UK Lung Cancer Screening (UKLS) trial, BMJ Open (2015) 5(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Paskett ED, Harrop JP, Wells KJ, Patient navigation: an update on the state of the science, CA Cancer J. Clin 61 (4) (2011) 237–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Jean-Pierre P, Fiscella K, Winters PC, Post D, Wells KJ, McKoy JM, Battaglia T, Simon MA, Kilbourn K, Patient navigation research program G: psychometric development and reliability analysis of a patient satisfaction with interpersonal relationship with navigator measure: a multi-site patient navigation research program study, Psycho-Oncology 21 (9) (2012) 986–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Tammemagi MC, Berg CD, Riley TL, Cunningham CR, Taylor KL, Impact of lung cancer screening results on smoking cessation, J. Natl. Cancer Inst. 106 (6) (2014) dju084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Evans WK, Wolfson MC, Computed tomography screening for lung cancer without a smoking cessation program—not a cost-effective idea, J. Thorac. Oncol 6 (11) (2011) 1781–1783. [DOI] [PubMed] [Google Scholar]
- [25].Fiore MC, Jaen CR, A clinical blueprint to accelerate the elimination of tobacco use, JAMA 299 (17) (2008) 2083–2085. [DOI] [PubMed] [Google Scholar]
- [26].Free C, Knight R, Robertson S, Whittaker R, Edwards P, Zhou W, Rodgers A, Cairns J, Kenward MG, Roberts I, Smoking cessation support delivered via mobile phone text messaging (txt2stop): a single-blind, randomised trial, Lancet 378 (9785) (2011) 49–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Pickens S, Boumbulian P, Anderson RJ, Ross S, Phillips S, Community-oriented primary care in action: a Dallas story, Am. J. Public Health 92 (11) (2002) 1728–1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Anderson RJ, Amarasingham R, Pickens S, The quest for quality: perspectives from the safety net, Front. Health Serv. Manag 23 (4) (2007) 15–28. [PubMed] [Google Scholar]
- [29].Hardy D, Liu CC, Xia R, Cormier JN, Chan W, White A, Burau K, Du XL, Racial disparities and treatment trends in a large cohort of elderly black and white patients with nonsmall cell lung cancer, Cancer 115 (10) (2009) 2199–2211. [DOI] [PubMed] [Google Scholar]
- [30].Chan A-W, Tetzlaff JM, Altman DG, Laupacis A, Gøtzsche PC, Krleža-Jerić K, Hróbjartsson A, Mann H, Dickersin K, Berlin JA, et al. , SPIRIT 2013 statement: defining standard protocol items for clinical trials, Ann. Intern. Med. 158 (3) (2013) 200–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Harris PATR, Thielke R, Payne J, Gonzalez N, Conde JG, Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support, J. Biomed. Inform. 42 (2) (2009) 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Bishop WP, Craddock Lee SJ, Skinner CS, Jones TM, McCallister K, Tiro JA, Validity of single-item screening for limited health literacy in English and Spanish speakers, Am. J. Public Health (2016) e1–e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Primeau SW, Freund KM, Ramachandran A, Bak SM, Heeren T, Chen CA, Morton S, Battaglia TA, Social service barriers delay care among women with abnormal cancer screening, J. Gen. Intern. Med. 29 (1) (2014) 169–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Institute GWUC, Guide for patient navigators: a supplement to the oncology patient navigator training: the fundamentals, The GW Cancer Institute’s Center for the Advancement of Cancer Survivorship, Navigation and Policy (caSNP), George Washington University (GW) Cancer Institute, Washington D.C., 2015. [Google Scholar]
- [35].Lindson-Hawley NTT, Begh R, Motivational interviewing for smoking cessation, Cochrane Database Syst. Rev 3 (2015) CD006936. [DOI] [PubMed] [Google Scholar]
- [36].Heckman CJ, Egleston BL, Hofmann MT, Efficacy of motivational interviewing for smoking cessation: a systematic review and meta-analysis, Tob. Control 19 (5) (2010) 410–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Miller WRaR S, Motivational interviewing: Preparing people to change addictive behavior, J. Community Appl. Soc. Psychol 2 (4) (1992) 299–300. [Google Scholar]
- [38].Dohan D, Schrag D, Using navigators to improve care of underserved patients: current practices and approaches, Cancer 104 (4) (2005) 848–855. [DOI] [PubMed] [Google Scholar]
- [39].Ramsey S, Whitley E, Mears VW, McKoy JM, Everhart RM, Caswell RJ, Fiscella K, Hurd TC, Battaglia T, Mandelblatt J, Evaluating the cost-effectiveness of cancer patient navigation programs: conceptual and practical issues, Cancer 115 (23) (2009) 5394–5403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Robinson-White S, Conroy B, Slavish KH, Rosenzweig M, Patient navigation in breast cancer: a systematic review, Cancer Nurs 33 (2) (2010) 127–140. [DOI] [PubMed] [Google Scholar]
- [41].Vernon SW, McQueen A, Tiro JA, del Junco DJ, Interventions to promote repeat breast cancer screening with mammography: a systematic review and meta-analysis, J. Natl. Cancer Inst. 102 (14) (2010) 1023–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Lung CT, Screening reporting and data system (Lung-RADS). www.acr.org/Quality-Safety/Resources/LungRADS.
- [43].Pinsky PFGD, Black W, Munden R, Nath H, Aberle D, Kazerooni E, Performance of Lung-RADS in the National Lung Screening Trial: a retrospective assessment, Ann. Intern. Med. 162 (7) (2015) 485–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Kroenke K, Spitzer RL, Williams JB, The PHQ-9: validity of a brief depression severity measure, J. Gen. Intern. Med. 16 (9) (2001) 606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Thekkumpurath P, Walker J, Butcher I, Hodges L, Kleiboer A, O’Connor M, Wall L, Murray G, Kroenke K, Sharpe M, Screening for major depression in cancer outpatients: the diagnostic accuracy of the 9-item patient health questionnaire, Cancer 117 (1) (2011) 218–227. [DOI] [PubMed] [Google Scholar]
- [46].Randall JM, Voth R, Burnett E, Bazhenova L, Bardwell WA, Clinic-based depression screening in lung cancer patients using the PHQ-2 and PHQ-9 depression questionnaires: a pilot study, Support. Care Cancer 21 (5) (2013) 1503–1507. [DOI] [PubMed] [Google Scholar]
- [47].Spitzer RL, Kroenke K, Williams JB, Lowe B, A brief measure for assessing generalized anxiety disorder: the GAD-7, Arch. Intern. Med. 166 (10) (2006) 1092–1097. [DOI] [PubMed] [Google Scholar]
- [48].Carle AC, Jean-Pierre P, Winters P, Valverde P, Wells K, Simon M, Raich P, Patierno S, Katz M, Freund KM, et al. , Psychometric evaluation of the patient satisfaction with logistical aspects of navigation (PSN-L) scale using item response theory, Med. Care 52 (4) (2014) 354–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Lerman CE, Brody DS, Caputo GC, Smith DG, Lazaro CG, Wolfson HG, Patients’ perceived involvement in care scale: relationship to attitudes about illness and medical care, J. Gen. Intern. Med. 5 (1) (1990) 29–33. [DOI] [PubMed] [Google Scholar]
- [50].Brehaut JC, O’Connor AM, Wood TJ, Hack TF, Siminoff L, Gordon E, Feldman-Stewart D, Validation of a decision regret scale, Med. Decis. Mak 23 (4) (2003) 281–292. [DOI] [PubMed] [Google Scholar]
- [51].Carter-Harris L, Ceppa D, Hanna N, Rawl SM, Screening for lung cancer with low-dose computed tomography: developing measures of individual health beliefs, American Society of Preventive Oncology 39th Annual Meeting Birmingham, AL, 2015. [Google Scholar]
- [52].Han HR, Lee JE, Kim J, Hedlin HK, Song H, Kim MT, A meta-analysis of interventions to promote mammography among ethnic minority women, Nurs. Res 58 (4) (2009) 246–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Lasser KE, Murillo J, Medlin E, Lisboa S, Valley-Shah L, Fletcher RH, Emmons KM, Ayanian JZ, A multilevel intervention to promote colorectal cancer screening among community health center patients: results of a pilot study, BMC Fam. Pract 10 (2009) 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Percac-Lima S, Grant RW, Green AR, Ashburner JM, Gamba G, Oo S, Richter JM, Atlas SJ, A culturally tailored navigator program for colorectal cancer screening in a community health center: a randomized, controlled trial, J. Gen. Intern. Med. 24 (2) (2009) 211–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Han HR, Lee H, Kim MT, Kim KB, Tailored lay health worker intervention improves breast cancer screening outcomes in non-adherent Korean-American women, Health Educ. Res 24 (2) (2009) 318–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Mickey J, Greenland S, A study of the impact of confounder-selection criteria on effect estimation, Am. J. Epidemiol. 129 (1989) 125–137. [DOI] [PubMed] [Google Scholar]
- [57].Hogan JW, Laird NM, Mixture models for the joint distribution of repeated measures and event times, Stat. Med 16 (1–3) (1997) 239–257. [DOI] [PubMed] [Google Scholar]
- [58].Hennink MM, Kaiser BN, Marconi VC, Code saturation versus meaning saturation: how many interviews are enough? Qual. Health Res (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Polit DF, Beck CT, Generalization in quantitative and qualitative research: myths and strategies, Int. J. Nurs. Stud 47 (11) (2010) 1451–1458. [DOI] [PubMed] [Google Scholar]
- [60].Hamann HA, Marks EG, Lee SJC, Gerber DE, Schiller JH, Ostroff JS, A qualitative analysis of continued smoking among patients with lung cancer, Ann. Behav. Med 47 (2014) S188. [Google Scholar]
- [61].Hamann HA, Ostroff JS, Marks EG, Gerber DE, Schiller JH, Lee SJ, Stigma among patients with lung cancer: a patient-reported measurement model, Psychooncology 23 (1) (2014) 81–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Hamann HA, Ostroff JS, McCallister KL, Melhado TV, Martinez-Puente LM, Gerber DE, Schiller JH, Lee SC, A qualitative analysis of perceived stigma in a multiethnic lung cancer patient population, Ann. Behav. Med 43 (2012) S221. [Google Scholar]
- [63].Miles MB, Huberman AM, Qualitative Data Analysis, Sage, Newbury Park, California, 1994. [Google Scholar]
- [64].Morse JM, Constructing qualitatively derived theory: concept construction and concept typologies, Qual. Health Res 14 (10) (2004) 1387–1395. [DOI] [PubMed] [Google Scholar]
- [65].Cohen DJ, Crabtree BF, Evaluative criteria for qualitative research in health care: controversies and recommendations, Ann. Fam. Med 6 (4) (2008) 331–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Bradley EH, Curry LA, Devers KJ, Qualitative data analysis for health services research: developing taxonomy, themes, and theory, Health Serv. Res 42 (4) (2007) 1758–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Quaife SL, Ruparel M, Beeken RJ, McEwen A, Isitt J, Nolan G, Sennett K, Baldwin DR, Duffy SW, Janes SM, et al. , The lung screen uptake trial (LSUT): protocol for a randomised controlled demonstration lung cancer screening pilot testing a targeted invitation strategy for high risk and ‘hard-to-reach’ patients, BMC Cancer 16 (1) (2016) 281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Fischel RJ, Dillman RO, Developing an effective lung cancer program in a community hospital setting, Clin. Lung Cancer 10 (4) (2009) 239–243. [DOI] [PubMed] [Google Scholar]
- [69].Reid AE, Tanoue L, Detterbeck F, Michaud GC, McCorkle R, The role of the advanced practitioner in a comprehensive lung cancer screening and pulmonary nodule program, J. Adv. Pract. Oncol 5 (6) (2014) 440–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Thorpe KE, Zwarenstein M, Oxman AD, Treweek S, Furberg CD, Altman DG, Tunis S, Bergel E, Harvey I, Magid DJ, et al. , A pragmatic-explanatory continuum indicator summary (PRECIS): a tool to help trial designers, Can. Med. Assoc. J 180 (10) (2009) E47–E57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Fransen GA, van Marrewijk CJ, Mujakovic S, Muris JW, Laheij RJ, Numans ME, de Wit NJ, Samsom M, Jansen JB, Knottnerus JA, Pragmatic trials in primary care. Methodological challenges and solutions demonstrated by the DIAMOND-study, BMC Med. Res. Methodol. 7 (2007) 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Godwin M, Ruhland L, Casson I, MacDonald S, Delva D, Birtwhistle R, Lam M, Seguin R, Pragmatic controlled clinical trials in primary care: the struggle between external and internal validity, BMC Med. Res. Methodol. 3 (2003) 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Byrne MM, Weissfeld J, Roberts MS, Anxiety, fear of cancer, and perceived risk of cancer following lung cancer screening, Med. Decis. Mak 28 (6) (2008) 917–925. [DOI] [PubMed] [Google Scholar]
- [74].Bond M, Pavey T, Welch K, Cooper C, Garside R, Dean S, Hyde C, Systematic review of the psychological consequences of false-positive screening mammograms, Health Technol. Assess 17 (13) (2013) 1–170 (v-vi). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Lin CJ, Schwaderer KA, Morgenlander KH, Ricci EM, Hoffman L, Martz E, Cosgrove RH, Heron DE, Factors associated with patient navigators’ time spent on reducing barriers to cancer treatment, J. Natl. Med. Assoc 100 (11) (2008) 1290–1297. [DOI] [PubMed] [Google Scholar]
- [76].Dudley DJ, Drake J, Quinlan J, Holden A, Saegert P, Karnad A, Ramirez A, Beneficial effects of a combined navigator/promotora approach for Hispanic women diagnosed with breast abnormalities. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive, Oncology 21 (10) (2012) 1639–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Percac-Lima S, Ashburner JM, McCarthy AM, Piawah S, Atlas SJ, Patient navigation to improve follow-up of abnormal mammograms among disadvantaged women, J. Women’s Health 24 (2) (2015) 138–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78]. https://essentialhospitals.org/.