Abstract
Adverse alterations in the composition of the gut microbiota have been implicated in the development of obesity and a variety of chronic diseases. Re-engineering the gut microbiota to produce beneficial metabolites is a potential strategy for treating these chronic diseases. N-acyl-phosphatidylethanolamines (NAPEs) are a family of bioactive lipids with known anti-obesity properties, Previous studies showed that administration of Escherichia coli Nissle 1917 (EcN) engineered with Arabidopsis thaliana NAPE synthase to produce NAPEs imparted resistance to obesity induced by a high fat diet that persisted after ending their administration. In prior studies, mice were pre-treated with ampicillin prior to administering engineered EcN for 8 weeks in drinking water. If use of antibiotics and long-term administration are required for beneficial effects, implementation of this strategy in humans might be problematic. Studies were therefore undertaken to determine if less onerous protocols could still impart persistent resistance and sustained NAPE biosynthesis. Administration of engineered EcN for only two weeks without pre-treatment with antibiotics sufficed to establish persistent resistance. Sustained NAPE biosynthesis by EcN was required as antibiotic treatment after administration of the engineered EcN markedly attenuated its effects. Finally, heterologous expression of human Phospholipase A/acyl-transferase-2 (PLAAT2) in EcN provided similar resistance to obesity as heterologous expression of A. thaliana NAPE synthase, confirming that NAPEs are the bioactive mediator of this resistance.
Keywords: Gut microbiota, engineered bacteria, N-acyl-phosphatidylethanolamines, N-acyl-ethanolamides, N-acyltransferases, antibiotics
INTRODUCTION
Adverse alterations in the microbial composition of the intestinal tract (gut dysbiosis) has been implicated as contributing to progression of a wide range of chronic diseases including inflammatory bowel disease, obesity, type 1 diabetes, type 2 diabetes, cardiovascular disease, cancer, autism, and depression (Carding et al. 2015; Zhang et al. 2015). For this reason, deliberately modifying the gut microbiota may be an important interventional strategy for the treatment of chronic diseases. One approach is to incorporate within the gut microbiota bacteria engineered to produce therapeutic compounds beneficial in treating disease. We have previously shown proof of concept for this strategy using the human commensal isolate Escherichia coli Nissle 1917 (EcN) engineered to over produce N-acyl-phosphatidylethanolamines (NAPEs) by heterologous expression of the Arabidopsis thaliana NAPE synthase (Chen et al. 2014; May-Zhang et al. 2019).
NAPEs are biosynthesized by a wide range of organisms including bacteria, yeast, plants, and vertebrates (Chapman 2000; Merkel et al. 2005; Mileykovskaya et al. 2009; Wellner et al. 2013). NAPEs are biosynthesized by phosphatidylethanolamine (PE) N-acyltransferases (N-ATs), in mammals these include five members of the phospholipase A/acyl-transferase (PLAAT) family (Mardian et al. 2015) (also known as HRASLS1–5) and a calcium-dependent PE N-AT (PLA2G4e) (Ogura et al. 2016) (Fig. 1). NAPEs are then hydrolyzed to N-acyl-ethanolamides (NAEs) including C18:1NAE (oleoylethanolamide), C16:0NAE (palmitoylethanolamide), C18:0NAE (stearoylethanolamide), and C20:4NAE (anandamide) by NAPE phospholipase D (NAPE-PLD) (Hussain et al. 2017). These bioactive NAEs act on receptors expressed in targets tissues that include PPARα, GPR119, TRPV1, and GPR55 to induce satiety, increase fatty acid oxidation, and enhance resolution of inflammation (Fu et al. 2003; Lauffer et al. 2009; Lo Verme et al. 2005; Rinne et al. 2018; Rodriguez de Fonseca et al. 2001). In lean animals, NAPE and NAE rise rapidly in the small intestine in response to food intake (Fu et al. 2007; Gillum et al. 2008). For reasons that remain to be fully elucidated, extended feeding on high fat diets results in loss of feeding induced intestinal NAPE and NAE biosynthesis (Diep et al. 2011; Gillum et al. 2008; Igarashi et al. 2015). Ablation of biosynthesis of NAE in either the intestine or adipose tissue (via tissue selective deletion of NAPE-PLD) is sufficient to induce obesity in mice (Everard et al. 2019; Geurts et al. 2015), demonstrating the importance of this pathway.
Fig. 1.
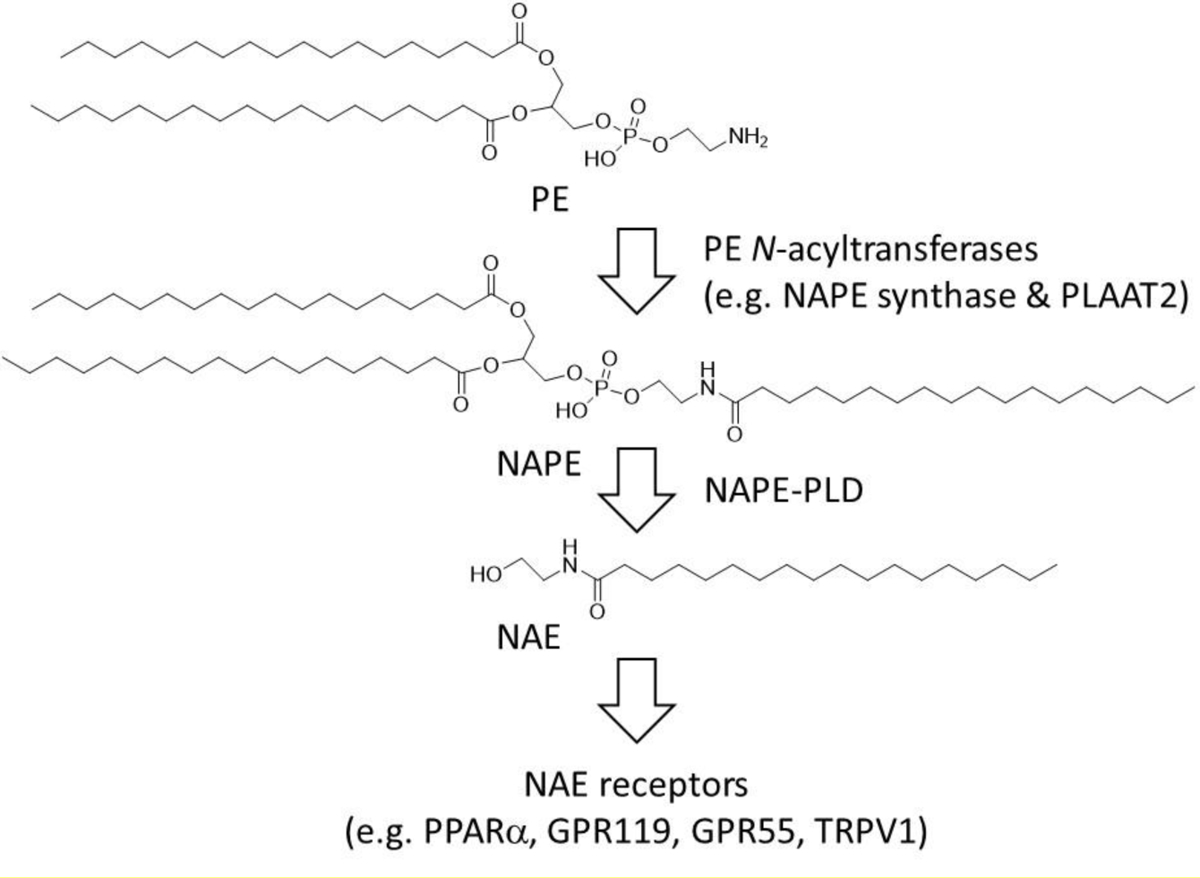
Biosynthetic and signaling pathways for N-acyl-ethanolamides (NAEs). Phosphatidylethanolamine (PE) N-acyltransferase enzymes transfer an acyl chain from a donor phospholipid to PE to form N-acyl-phosphatidylethanolamines (NAPE) which are then converted by NAPE hydrolyzing phospholipase D (NAPE-PLD) to NAEs, which in mammals act on receptors including PPARα, GPR119, GPR55, and TRPV1.
Our previous studies had used EcN engineered to express A. thaliana NAPE synthase (engineered strain pNAPE-EcN) as this was the only PE N-acyltransferase cloned when we initiated our studies. Administration of pNAPE-EcN to mice increased intestinal NAPE levels about two-fold and significantly reduced development of obesity in C57BL6 mice fed a high fat diet (Chen et al. 2014). NAPE delivery via pNAPE-EcN was significantly more effective than oral delivery of purified NAPE (Chen et al. 2017). Administration of pNAPE-EcN also reduced fatty liver disease and atherosclerotic lesion necrosis in Ldlr−/− mice fed a Western diet (May-Zhang et al. 2019).
In our initial studies, pNAPE-EcN was administered in the drinking water of mice continuously for 8 weeks, but significant reductions in food intake, weight gain, and fat gain then persisted for more than 4 weeks post-treatment. Although these studies demonstrated that this strategy could impart persistent resistance to obesity induced by a high fat diet, a number of important questions remain before such strategies could be implemented in humans. For instance, in these previous studies mice were pre-treated with antibiotics prior to giving pNAPE-EcN based on the notion that removal of already extant bacteria was necessary to allow sufficient numbers of the donor engineered bacteria to colonize to have efficacy (Lasaro et al. 2014; Ni et al. 2017; Shen et al. 2015; Willing et al. 2011). Such antibiotic use in humans increases risk for Clostridium difficile infections (Baur et al. 2017) and may further exacerbate gut dybiosis (Cox and Blaser 2015; Cox et al. 2014). Whether beneficial donor bacteria such as pNAPE-EcN can be incorporated into the gut microbiota without the use of antibiotic pre-treatment remains unknown. If antibiotics are indeed required, then would more stringent antibiotic regimes also allow donor bacteria to be administered for a significantly shorter period of time and still achieve persistent resistance?
Our previous studies also raised questions specific to treating obesity with pNAPE-EcN. For instance, do the appetite suppressing effects of NAPEs lead to detrimental weight loss in already lean mice? Since mice undergo significant metabolic changes within a few days of beginning a high-fat diet (de Wilde et al. 2008; Kahle et al. 2013; Lee et al. 2011; Shiwa et al. 2015; Turner et al. 2013), yet previous studies began pNAPE-EcN treatment concurrently with the switch to a high fat diet (Chen et al. 2014), is pNAPE-EcN treatment still effective if started after the onset of these changes? Were the long-term anti-obesity effects of pNAPE-EcN administration the result of ongoing biosynthesis of NAPEs by persisting pNAPE-EcN or simply the result of NAPE biosynthesis during a short window of vulnerability (Cox et al. 2014)? Finally, are EcN expressing a human PE N-AT--which might be favored over A. thaliana NAPE synthase by regulatory agencies for human trials--as effective as pNAPE-EcN?
We conducted a series of studies to answer these questions and found that two weeks of administration of EcN engineered to express NAPEs, regardless of the enzymes used to generate NAPEs, was sufficient to provide persistent resistance to diet induced obesity even in mice that had been pre-fed the high fat diet for 10 days to induce the metabolic and behavioral adaptations of obesity. Pre-treatment with antibiotics did not further enhance these effects, but post-treatment antibiotic administration ablated them. These studies support the utility of E. coli engineered to produce NAPEs as a straightforward method to persistently provide resistance to diet induce obesity and as a general strategy for treatment of chronic diseases.
MATERIALS AND METHODS
Materials
Magnesium sulfate, triethylammonium acetate buffer, vancomycin, neomycin, amphotericin B, and methylamine solution (40 wt. % in water) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Organic solvents including methanol, chloroform, ethanol, 1-butanol and acetonitrile were all HPLC grade purchased from Thermo Fisher Scientific (Waltham, MA, USA). Calcium chloride dihydrate, ammonium chloride, sodium phosphate dibasic anhydrous, potassium phosphate monobasic, acetic acid (glacial), metronidazole, casamino acids (vitamin-free) and sodium chloride were also purchased from Thermo Fisher Scientific. Ampicillin sodium salt and isopropyl-β-D-thiogalactoside (IPTG) were obtained from Research Products International (RPI; Mt. Prospect, IL, USA). Sep-Pak silica cartridges were purchased from the Waters Corporation (Milford, MA, USA). Kinetex 2.6μ C18 100Å column (50×2.1 mm) and Kinetex C8 100Å column (50×2.1 mm) were purchased from Phenomenex (Torrance, CA, USA).
Bacterial strains preparation
E. coli Nissle 1917 (EcN) was obtained as a gift from ArdeyPharm, GmbH (Herdecke, Germany). The generation of pQE-80L1 (the empty expression plasmid), pQE-80L-At (the expression plasmid for A. thaliana NAPE synthase), and pPLAAT2 (the expression plasmid for human PE N-acyltransferase HRASLS2/PLAAT2) and the transformation of EcN with these plasmids to create pEcN (EcN strain with empty expression plasmid), pNAPE-EcN (EcN strain expressing A. thaliana NAPE synthase), and pPLAAT2-EcN (EcN strain expressing PLAAT2), respectively, have been previously described (Chen et al. 2014; Dosoky et al. 2018).
Bacterial culture
Bacteria were grown as described previously (Dosoky et al. 2018). Cultures were allowed to grow overnight at 37°C while shaking at 200 rpm then were diluted (1:50) and grown under the same conditions until they reached an optical density (OD600) of 0.5–0.6. (For calculations of bacterial numbers, 1 OD was defined as 8E8 colony forming units (CFU)/mL). Then isopropyl-β-D-thiogalactoside (IPTG, 1mM) was added to induce NAPE expression and the cultures were allowed to grow overnight at 28°C at 200 rpm. After recording the optical density, cells were harvested by centrifugation at 5000 g and 4°C for 15 min and the pellet was resuspended in 0.125% gelatin to give a final concentration of 5E9 CFU/ml.
Measurement of NAPEs and NAEs by LC/MS/MS
NAPE and NAE concentrations were quantified in feces, plasma, and adipose tissue by LC/MS/MS as previously described (Chen et al. 2017; Dosoky et al. 2018; Guo et al. 2010).
Fecal DNA extraction, pyrosequencing, and bacterial composition analysis
Collected feces were stored at −80C until analysis. In each case, multiple fecal pellets were used for each sample. For analysis of total bacterial number in fecal samples, fecal samples were extracted using Quick-DNA Fecal/Soil Microbe Mini-prep kit (cat# D6010 ZYMO Research, Irvine, CA, USA) and then quantitative real-time PCR performed using the following universal eubacteria primers: Forward (27F): AGAGTTTGATCMTGGCTCAG; Reverse (519R): GWATTACCGCGGCKGCTG with quantitation based on a standard curve with log scale concentrations of EcN.
Determination of the microbial taxa by 16S rRNA gene sequencing was carried out on collected feces by the Vanderbilt Technologies for Advanced Genomics (VANTAGE) Microbiome core (Nashville, TN, USA) using their established protocols for DNA extractions, amplification, library prep and sequencing. The 16S universal Eubacteria primers (PCR primers 515/806) were used to amplify the V4 variable region. Following PCR, all amplicon products from different samples were mixed in equal concentrations and purified using Agencourt Ampure beads (Agencourt Bioscience Corporation, Beverly, MA, USA). Microbial sequencing was analysed by bacterial tag encoded FLX amplicon pyrosequencing (bTEFAP) using a Roche 454 pyrosequencer and titanium reagents (Indianapolis, IN, USA) and 3–5 K nominal sequences per sample of high quality extracted DNA.
Sequences were depleted of barcodes and primers then short sequences <200 bp are removed, sequences with >1 ambiguous base calls removed and sequences with homopolymer runs exceeding 6 bp removed using the statistical software package Quantitative Insights Into Microbial Ecology (QIIME) (http://qiime.org/). A total number of 9,699,765 sequences passed a quality filter with a minimum score of 25 and an average length of 460 bp. Operational taxonomic units were defined after removal of chimeric and singleton sequences, clustering at 3% divergence (97% similarity). Operational taxonomic units were then taxonomically classified using BLASTn against a curated GreenGenes database (http://greengenes.secondgenome.com/?prefix=downloads/greengenes_database/). This pyrosequencing data is publicly archived at https://figshare.com/articles/3469-CRF_Microbiome_zip/7859993.
Animal studies
Four to six week-old male C57BL/6J mice were purchases from Jackson Labs (Bar Harbor, ME, USA) and individually housed in the Vanderbilt University animal facility (Nashville, TN) in a 12-hour light/12-hour dark cycle. During their initial adaptation to the Vanderbilt facility, all mice received a standard low-fat, plant-based diet (LabDiet 5001, 13.5% cal from fat, LabDiet Inc, St. Louis, MO, USA). In the initial study comparing the effect of diet on changes in body weight in response to pNAPE-EcN treatment, three groups of mice (n = 5 mice per group) continued on this chow diet for the duration of the studies. The other three groups of mice in this study (n = 5 mice per group), and all groups of mice in subsequent studies (n = 6 mice per group) were switched to high fat diet (Research Diets D12492, 60% cal from fat, Research Diet Inc., New Brunswick, NJ USA) 10 days prior to the beginning of bacterial treatment and remained on this diet for the duration of the study. Food intake, body weight, and body composition were determined for each mouse. Daily food intake was measured by adding pre-weighed food pellets to each cage and then reweighing the remaining pellets. Body weight was measured using a portable electronic scale. For body composition, mice were scanned by magnetic resonance imaging (MRI) using a Bruker Minispec MQ10 NMR Analyzer (Bruker Inc., Billeria, MA, USA) to determine fat content, lean mass and free fluid. For mice receiving the antibiotics, a cocktail of amphotericin B (0.3mg/mL), metronidazole (10mg/mL), vancomycin (0.5mg/mL), neomycin (10mg/mL), and ampicillin (10mg/mL) were administered by oral gavage (10mL/kg body weight) twice a day for 3 consecutive days either prior to or after the 14 days of bacterial treatment. For comparison of bacterial load after treatment with this cocktail of antibiotics compared to ampicillin alone, ampicillin at 1 mg/mL was administered in drinking water for seven days. For treatment with various bacteria, the appropriate bacterial strains including EcN, pEcN, pNAPE-EcN, and pPLAAT2-EcN were prepared as described above (with 1 OD for A600 defined as 8E8 CFU/mL) and then reconstituted in drinking water with 0.125% gelatin as vehicle at 5×109 CFU/ml. This water containing the various bacteria or vehicle was the only drinking water available to the mice during the treatment period and was changed 3 times per week for a total of 2 weeks (through experimental day 14). After ending bacterial treatment, mice remained on the same diet during the four-week follow-up period (to experimental day 42). Feces were collected before, during and after bacterial treatment. At the end of the study, mice were euthanized in the morning (no fasting prior to euthanasia) and blood and tissues were collected.
Study approval
All animal experiments were performed according to protocols approved by the Institutional Animal Care and Use Committee and the Institutional Biosafety Committee of Vanderbilt University.
Statistics
Statistical analysis was performed using GraphPad Prism 7 for Windows (GraphPad Software, San Diego, CA). A p value of less than 0.05 was considered statistically significant.
RESULTS
We previously used pretreatment with 1 g/L ampicillin in drinking water for 1 week to reduce bacterial load in the gastrointestinal (GI) tract as a strategy for potentially enhancing colonization by donor bacteria. However, cocktails of multiple antibiotics are more effective at clearing the intestinal microbiota than a single antibiotic such as ampicillin (Reikvam et al. 2011). We therefore compared the effect of ampicillin versus a cocktail of five antibiotics (ampicillin, neomycin, vancomycin, metronidazole, amphotericin B) on total bacterial load in feces. Untreated mice had similar bacterial loads on the low-fat chow diet (3.3±1.4E8 CFU/mg feces, mean±SEM) or high fat diet (2.6±0.3E8 CFU/mg feces). While pre-treatment for 1 week with ampicillin in drinking water significantly reduced bacterial load (6.0±1.1E7 CFU/mg feces), 3 days of gavage with the cocktail of five antibiotics reduced bacterial load even further (4.3±1.2E6 CFU/mg feces).
We then performed a study designed to address questions related to the long-term efficacy of pNAPE-EcN treatment after short-term administration. In this study, three groups of male C57BL6 mice were fed a standard low-fat, plant-based chow diet for the entirety of the study, while another three groups of mice were pre-fed a high fat diet (60% cal from fat) for 10 days prior beginning treatment with pNAPE-EcN. This duration of high fat diet pre-feeding has previously been shown to induce many negative metabolic effects including marked increases in fat accumulation in visceral adipose tissue and liver, insulin resistance, dysbiosis of gut microbiota, and reduced responsiveness of neural circuits to various satiety factors secreted by the gut (de Wilde et al. 2008; Kahle et al. 2013; Lee et al. 2011; Shiwa et al. 2015; Turner et al. 2013). For each diet, one group of mice was pretreated with the cocktail of 5 antibiotics for 3 days (experimental day −3 to day 0) (Fig. 2). Then these mice, as well as a second group of mice for each of diets, were administered pNAPE-EcN in drinking water for 14 days (experimental day 0 to 14). The third group in each diet was administered vehicle only (0.125% gelatin) in their drinking water for the 14 days. All six groups were monitored for an additional 4 weeks post-treatment (to experimental day 42).
Fig. 2.
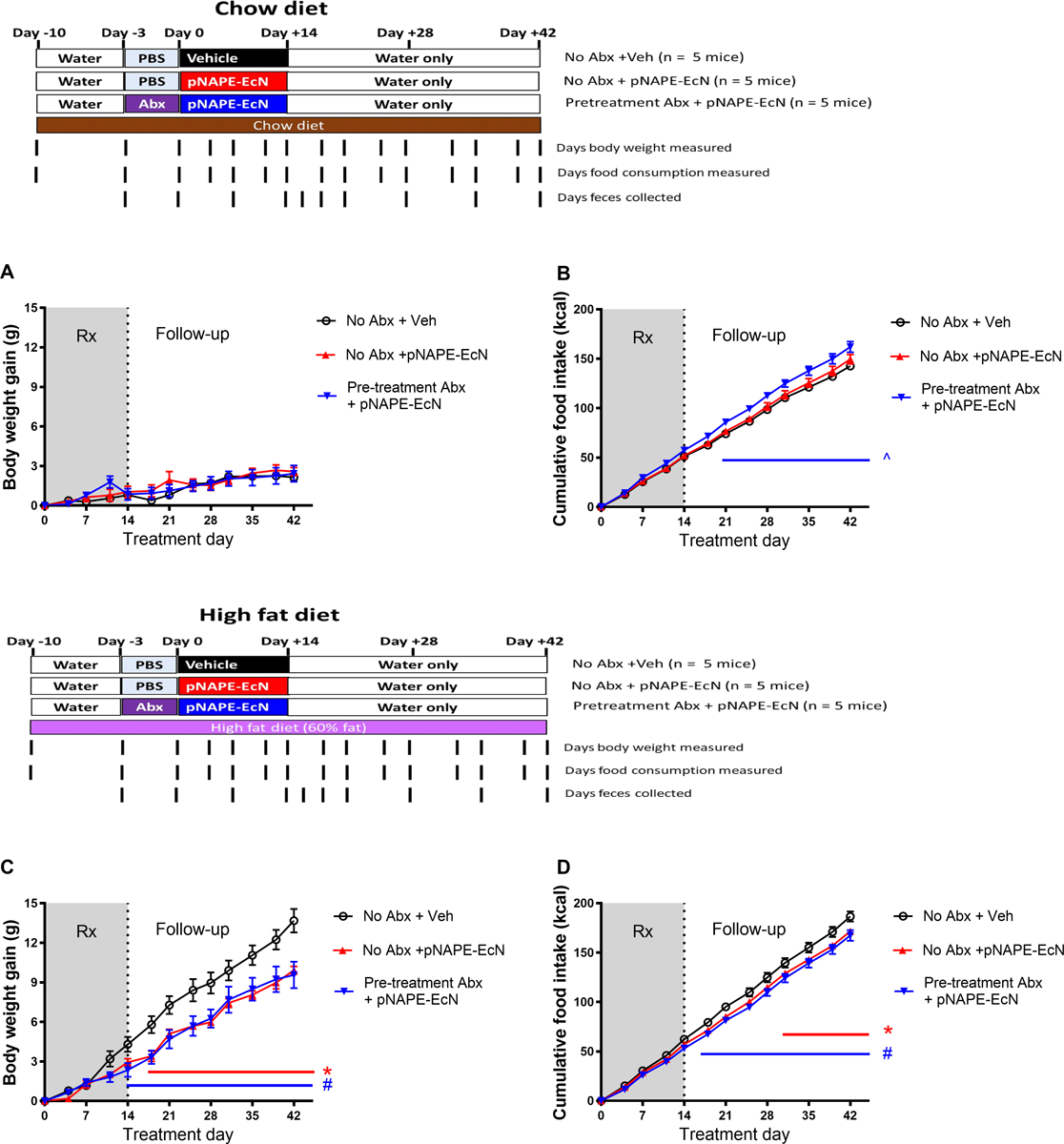
Two-week treatment with pNAPE-EcN induces no effect in mice fed low fat diet, but induces persistent resistance to weight gain in mice fed a high fat diet, and pretreatment with antibiotics (Abx) does not enhance this effect. A. Gain in body weight from start of bacterial treatment for mice fed a low-fat chow diet. (Two-way ANOVA, p<0.0001 for time, p=0.8167 for treatment; B. Cumulative food intake for mice fed a chow diet. (Two-way ANOVA, p<0.0001 for time, p=0.0198 for treatment; Tukey’s multiple comparisons test ^p<0.05 Pretreatment Abx + pNAPE-EcN vs either Veh or pNAPE-EcN). C. Gain in body weight from start of bacterial treatment for mice fed a high fat diet. (Two-way ANOVA, p<0.0001 for time, p=0.0178 for treatment; Tukey’s multiple comparisons test *p<0.05 pNAPE-EcN vs Vehicle, #p<0.05 Abx+pNAPE-EcN vs Vehicle). D. Cumulative food intake for mice fed a high fat diet. (Two-way ANOVA, p<0.0001 for time, p=0.0307 for treatment; Tukey’s multiple comparisons test *p<0.05 pNAPE-EcN vs Vehicle, #p<0.05 Abx+pNAPE-EcN vs Vehicle). All values are shown as mean±SEM (n = 5 mice per group).
As expected, 10 days of pre-feeding a high fat diet was sufficient to significantly increase body weight (27.6±0.6 g, mean±SEM) compared to mice fed the chow diet (24.3±0.3 g). For mice fed a chow diet, pretreatment with antibiotics for the final 3 days prior to the start of pNAPE-EcN treatment did not result in any significant differences in body weight (24.1±1.0 g). In contrast, for mice pre-fed high fat diet for 10 days, pretreatment with the antibiotics on the final 3 days prior to the start of pNAPE-EcN treatment significantly reduced body weight (25.3±1.0 g, p=0.03 Student t-test).
The effect of pNAPE-EcN treatment varied dramatically based on the diet. For mice that remained on the low fat chow diet throughout the study, treatment with pNAPE-EcN (with or without pre-treatment of antibiotics) did not significantly alter weight gain and mice remained lean throughout the study (Fig. 2A). Interestingly, antibiotic pre-treatment did increase food intake (Fig. 2B). In marked contrast to the lack of effect of pNAPE-EcN treatment on chow fed mice, 14 days of pNAPE-EcN treatment of mice fed a high fat diet was sufficient to significantly reduce weight gain (Fig. 2C) and reduce cumulative food intake (Fig. 2D) during the 28 day post-treatment follow-up period.
Unexpectedly, pretreatment with antibiotics did not enhance the efficacy of pNAPE-EcN administration in terms of reduced weight gain or cumulative food intake compared to mice without antibiotic pretreatment. To confirm that the antibiotics reduced intestinal bacterial load prior to pNAPE-EcN as expected, total bacterial load in feces from the mice fed the high fat diet was measured at experimental day −3 (prior to start of antibiotics), experimental day 0 (end of antibiotic treatment), experimental day 7 and 14 (during treatment with pNAPE-EcN), and experimental day 16, 18, 21, 28, 35, and 42 (during the follow-up post-treatment period). Antibiotic pre-treatment reduced bacterial load by approximately 1000-fold (Fig. 3A), confirming that the antibiotic treatment had the intended effect. By experimental day 7, fecal bacterial load had rebounded and did not significantly differ between groups. When the proportion of individual bacterial phyla in feces was analyzed at a few selected time-points using 16S rRNA gene sequencing, bacterial composition differed significantly in the group pre-treated with antibiotics compared to the other groups immediately prior to (day 0) and during pNAPE-EcN treatment (day 7), but not at experimental day 16 (2 days after cessation of pNAPE-EcN treatment) (Fig. 3B). At experimental day 7, the proportion of proteobacteria (e.g. E. coli) in the feces of mice receiving pNAPE-EcN were higher in those that had been pre-treated with antibiotics (33±9%, mean±SEM) compared to those without antibiotics (14±2%), but at experimental day 16 proteobacteria were ≤0.1% of all bacteria in each of the three groups.
Fig. 3.
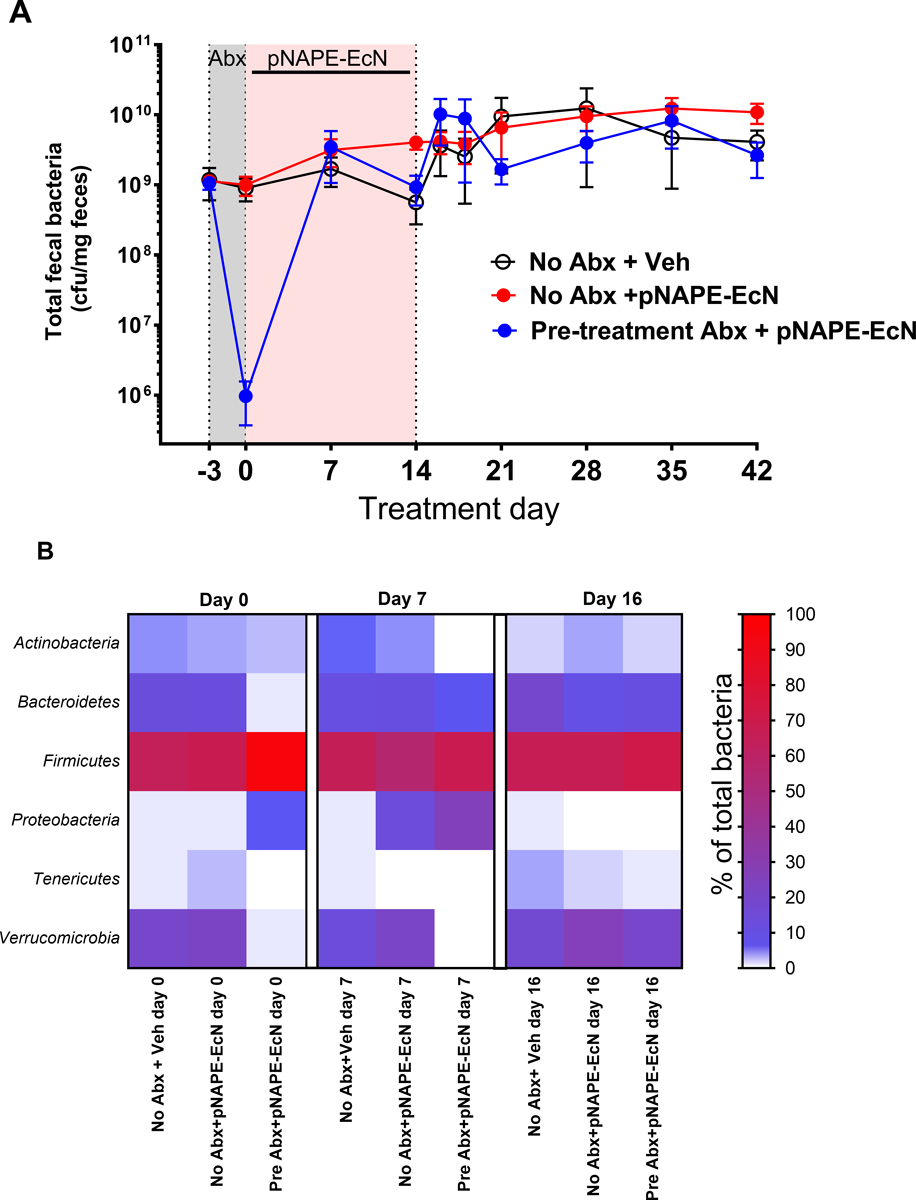
Effect of antibiotic pretreatment and subsequent pNAPE-EcN treatment on fecal bacterial load and 16S microbiota composition of mice fed high fat diet A. Time course of fecal bacterial load. B. Heat map of 16S composition by phylum at experimental day 0, day 7 and day 16.
Although the persistent anti-obesity effect of pNAPE-EcN treatment seemed most likely to be the result of colonized pNAPE-EcN, treatment with pNAPE-EcN for even just 14 days might somehow permanently altered the mouse’s hedonistic and/or metabolic responses to the high fat diet. To distinguish between these two possibilities, mice were treated either with pNAPE-EcN, with an EcN strain expressing only empty expression vector (pEcN) or with vehicle for two weeks and then a subset of mice subsequently treated with antibiotics to eliminate colonized pEcN or pNAPE-EcN. Changes in weight gain and food intake were then measured in the post-treatment period (Fig. 4). As before, the group of mice that had received pNAPE-EcN but no subsequent antibiotics showed reduced weight gain (Fig. 4A) and reduced food intake (Fig. 4D) compared to vehicle or pEcN treated mice. Fat gain (Fig. 4B) was reduced, but not lean body mass gain (Fig. 4C). In contrast, in mice that were subsequently treated with antibiotics, all of these anti-obesity effects were significantly attenuated (Fig. 4A–D).
Fig. 4.
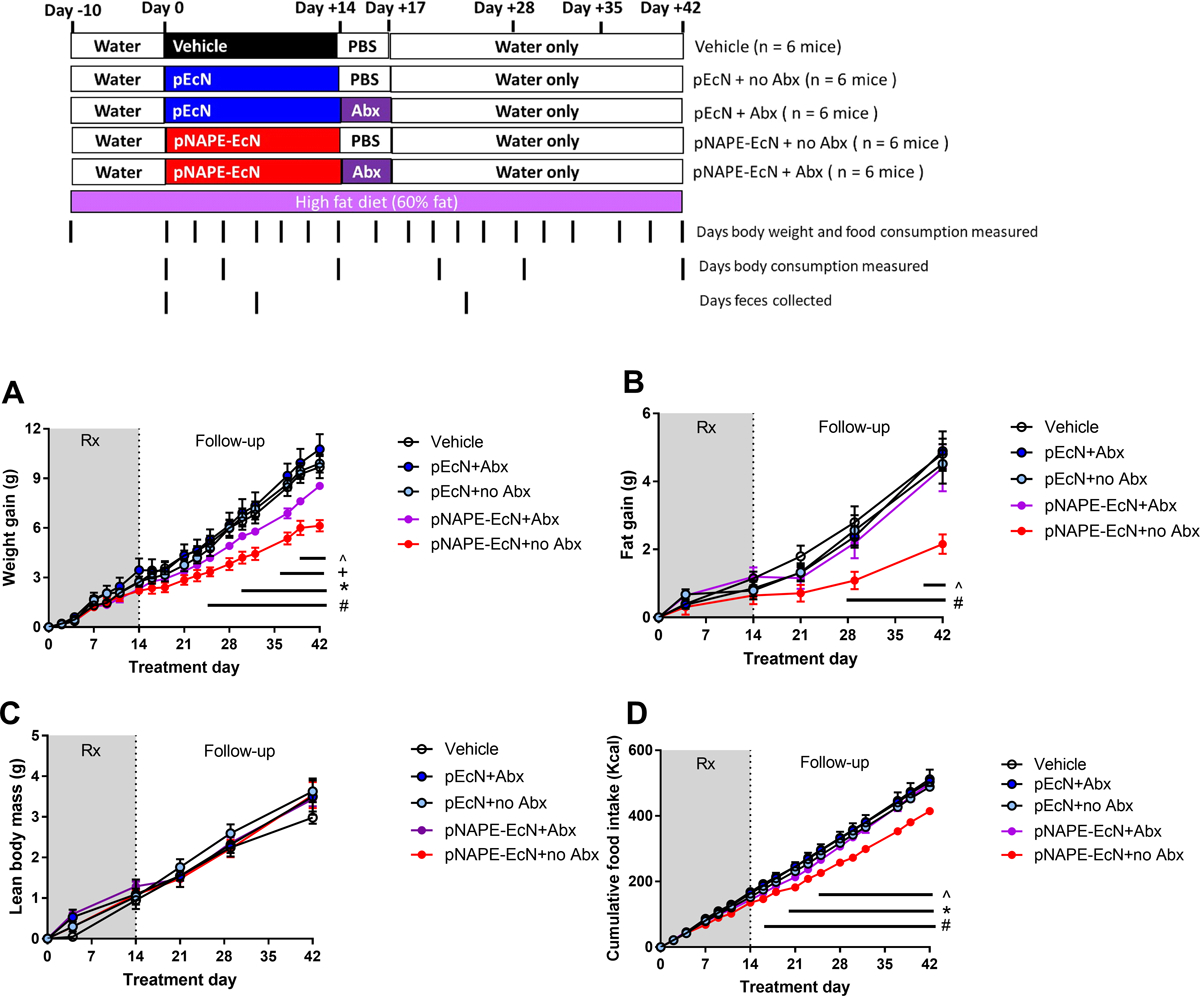
Post-treatment administration of antibiotic cocktail eliminates pNAPE-EcN-induced reductions of weight gain, fat gain, and food intake in mice feeding on high fat diet. All values are mean±SEM (n = 6 mice per group). A. Effect of treatments on change in body weight from start of treatment (2way-ANOVA, treatment p < 0.0001, time p<0.0001, Tukey’s multiple comparison test # p<0.05 pNAPE-EcN+no Abx vs Vehicle or pEcN + Abx; *p <0.05 pNAPE-EcN+no Abx vs pEcN+Abx; +p<0.05 pNAPE-EcN+Abx vs Vehicle or pEcN+Abx or pEcN+no Abx; ^ p<0.05 pNAPE-EcN+no Abx vs pNAPE-EcN+ Abx). B. Effect of treatments on change in body fat from start of treatment (2way-ANOVA, treatment p < 0.0001, time p<0.0001; Tukey’s multiple comparison test # p<0.05 pNAPE-EcN+no Abx vs Vehicle or pEcN+no Abx or pEcN + Abx; ^ p<0.05 pNAPE-EcN+no Abx vs pNAPE-EcN+ Abx). C. Effect of treatments on lean body mass (2way-ANOVA, p=0.9349 treatment, time p<0.0001). D. Effect of treatments on cumulative food intake from start of treatment (2way-ANOVA, treatment p<0.0001, time p <0.0001).
These results suggested that continuous NAPE biosynthesis by colonized pNAPE-EcN led to persistent elevations in tissue levels of NAPEs and their bioactive NAE metabolites. To test that bacterial NAPE biosynthesis continued in the absence of antibiotics, fecal NAPE levels were measured as fecal NAPE levels were previously shown to serve as a useful surrogate for NAPE biosynthesis by pNAPE-EcN in the intestinal lumen (Dosoky et al. 2018). At experimental day 7, fecal NAPE levels were not elevated in pEcN treated mice relative to vehicle treated mice, but NAPE levels were elevated in both groups of mice treated with pNAPE-EcN (Fig. 5A). At experimental day 21 (7 days post-treatment), pNAPE-EcN treated mice that subsequently received antibiotics no longer had elevated NAPE levels but NAPE levels remained elevated in pNAPE-EcN treated mice that did not receive antibiotics (Fig. 5A). At experimental day 42 (4 weeks post-treatment) mice were euthanized and tissue collected. NAPE levels were measured in omental fat as we have previously shown that omental fat is an important target of NAPE produced by engineered bacteria (Chen et al. 2017), and others have shown that adipose-specific deletion of NAPE hydrolyzing phospholipase D (the enzyme that transforms NAPE to its bioactive metabolite) significantly increases obesity and glucose intolerance (Geurts et al. 2015). In mice treated with pNAPE-EcN without subsequent antibiotics, NAPE levels were significantly elevated in omental fat compared to those treated with the antibiotics or the control groups (Fig. 5B). Plasma levels of NAPEs (Fig. 5C) and NAEs (Fig. 5D) showed similar patterns as omental fat.
Fig. 5.
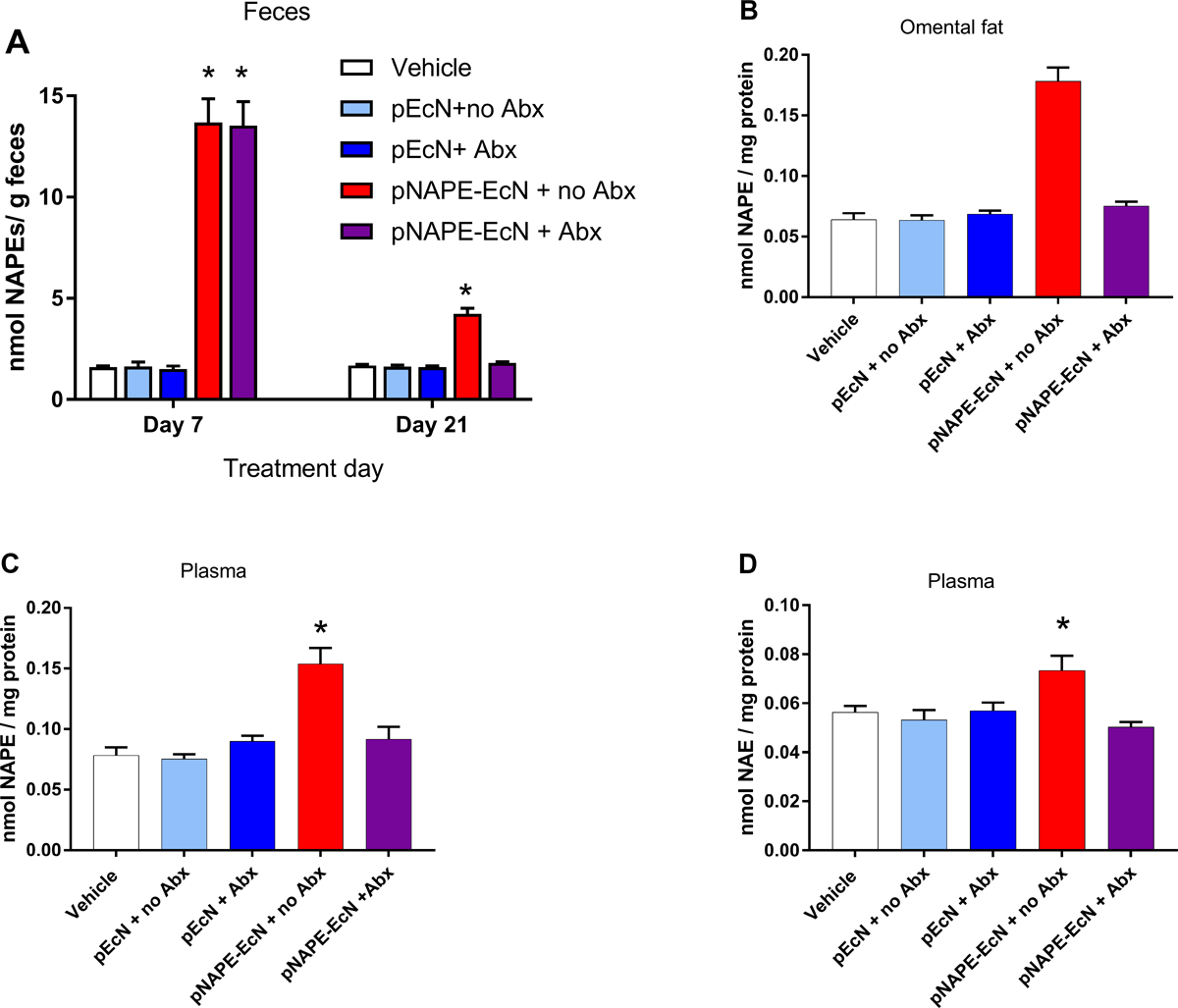
Post-treatment administration of antibiotics (Abx) eliminates elevated NAPE levels. A. Fecal NAPEs on day 7 and day 21. B. Omental fat NAPE levels day 42. C. Plasma NAPE levels day 42. D. Plasma NAE levels day 42.
We then carried out studies to determine if similar persistent resistance to diet induced obesity could be achieved using EcN expressing the human PE N-acyltransferase phospholipase A/acyl transferase-2, (PLAAT2), as therapeutic bacteria expressing human genes by might be viewed more favorably by the general public (and therefore regulatory agencies) than therapeutic bacteria expressing plant genes. Furthermore, pNAPE-EcN also produces small amounts of O-acyl phosphatidylglycerols (Bulat and Garrett 2011; Chen et al. 2014), so we wanted to determine if pPLAAT2-EcN could fully recapitulate the anti-obesity effects of pNAPE-EcN, since PLAAT2 forms NAPE by a different enzymatic mechanisms than NAPE synthase and does not produce O-acyl-phosphatidylglycerols. EcN expressing PLAAT2 (pPLAAT2-EcN) biosynthesize similar levels and species of NAPEs as pNAPE-EcN (Dosoky et al. 2018).
Mice were administered pPLAAT2-EcN in the same manner as for pNAPE-EcN (e.g. no antibiotics and 10-day pre-feeding high fat diet). As with pNAPE-EcN, mice treated with pPLAAT2-EcN gained less weight (Fig. 6A) and had reduced cumulative food intake (Fig. 6D) than mice treated with vehicle or EcN. Mice treated with pPLAAT2-EcN also had less total fat gain (Fig. 6B) but similar lean mass gain as control mice (Fig. 6C). When NAPE levels were measured in feces collected at experimental day 41 (at end of the 4 week post-treatment follow-up period), NAPE levels were elevated two-fold compared to vehicle treated mice (Fig. 7A), similar to the two-fold increase in fecal NAPEs seen at experimental day 21 with pNAPE-EcN treatment. NAPE levels in omental fat (Fig. 7B) and plasma (Fig. 7C) collected from pPLAAT2-EcN treated mice at the end of the 4 week post-treatment follow-up period (day 42) were similar to levels in tissues collected from pNAPE-EcN treated mice at this same time point, and about two-fold higher than those found in vehicle or EcN treated mice. Plasma NAE levels were also increased (Fig. 7D).
Fig. 6.
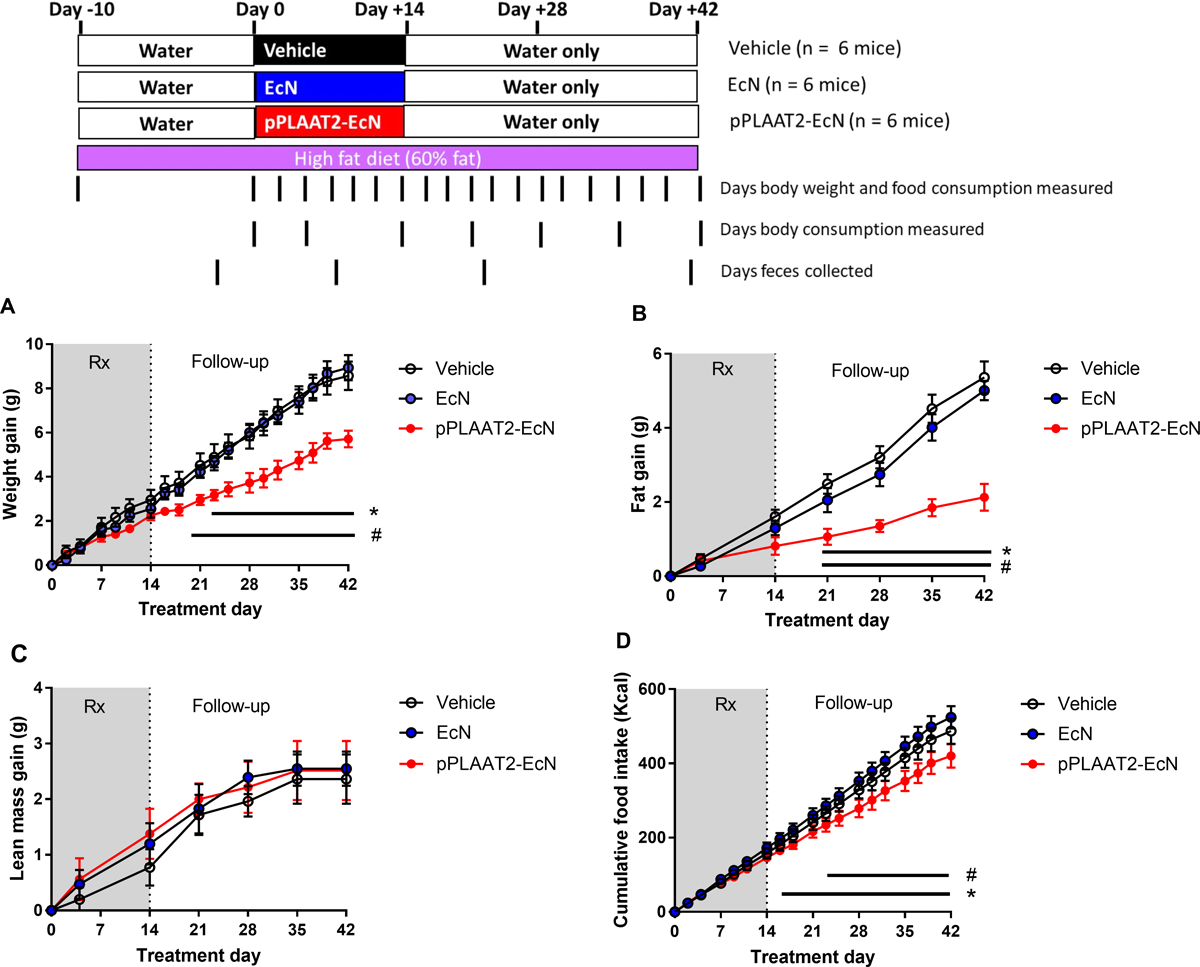
Treatment with pPLAAT2-EcN is effective in reducing obesity in high-fat diet fed mice. All values are mean±SEM (n = 6 mice per group). A . Effect of treatments on gain in body weight from start of treatment (2way-ANOVA p = 0.0129 for treatment, p<0.0001 for time; Tukey’s multiple comparison test #p<0.05 for pPLAAT2-EcN vs Vehicle;*p<0.05 pPLAAT2-EcN vs EcN). B. Effect of treatments on gain of fat mass (2way-ANOVA, p < 0.0001 for treatment, p<0.0001 for time; Tukey’s multiple comparison test #p<0.05 for pPLAAT2-EcN vs Vehicle;*p<0.05 pPLAAT2-EcN vs EcN). C. Effect of treatments on lean body mass (2way-ANOVA, p=0.8205 for treatment, p<0.001 for time). D. Effect of treatments on cumulative food intake (2way-ANOVA, p=0.0001 for treatment, p <0.0001 for time; Tukey’s multiple-comparison #p<0.05 pPLAAT2-EcN vs Vehicle; *p<0.05 pPLAAT2-EcN vs EcN).)
Fig. 7.
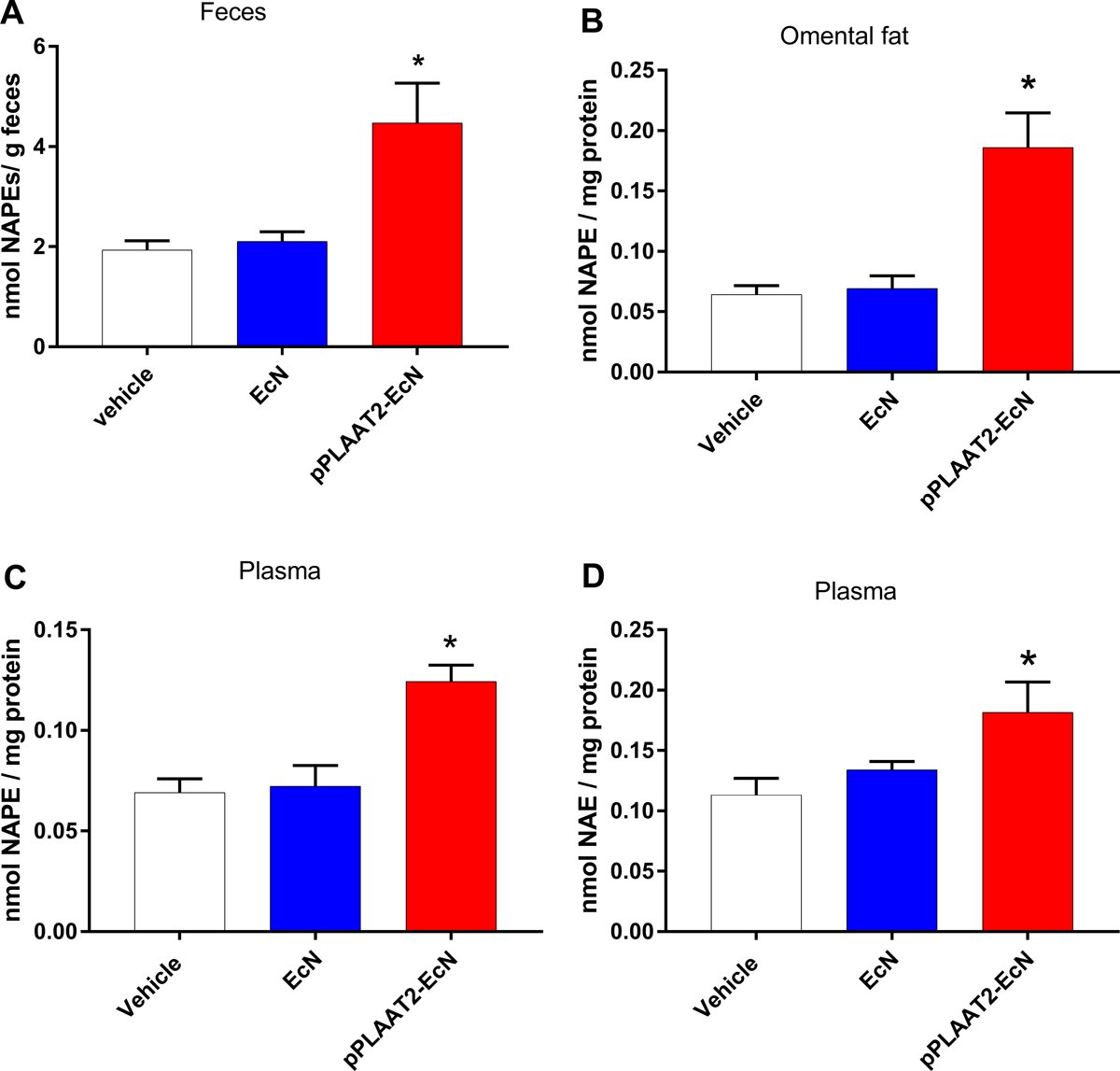
Treatment with pPLAAT2-EcN elevates fecal and tissue NAPE levels at 4 weeks post-treatment. A. Total NAPE levels in feces collected on day 41 from start of treatment. (1way ANOVA p<0.0001; Tukey’s multiple comparison test *p<0.05 pPLAAT2-EcN vs Vehicle or EcN). B. Total NAPE levels in omental fat pads (1way ANOVA p<0.0001; Tukey’s multiple comparison test *p<0.05 pPLAAT2-EcN vs Vehicle or EcN). C. Total NAPE levels in plasma (1way ANOVA p=0.0005; Tukey’s multiple comparison test *p<0.05 pPLAAT2-EcN vs Vehicle or EcN). D. Total NAE levels in plasma (1way ANOVA p<0.0336; Tukey’s multiple comparison test *p<0.05 pPLAAT2-EcN vs Vehicle). All values are mean±SEM, n=6.
DISCUSSION
These studies demonstrate that relatively short-term administration (14 days) of pNAPE-EcN or pPLAAT2-EcN to mice suffices to provide persistent resistance to diet induced obesity even without antibiotic pre-treatment. We found that pre-treatment with a cocktail of five antibiotics by gavage for three days markedly diminished intestinal bacterial load as measured by fecal bacterial levels and that this treatment was more effective at reducing fecal bacterial levels than monotherapy with ampicillin for 7 days. Despite the effectiveness of the antibiotic cocktail in reducing bacterial load, it did not enhance the effectiveness of pNAPE-EcN. For the two groups of mice treated with pNAPE-EcN, the bacterial composition of the feces did show some differences between those pretreated with antibiotics versus without antibiotics at day 7 of pNAPE-EcN treatment. For instance, there were less Actinobacteria and Verrucomicrobia and more Proteobacteria in the group pre-treated with antibiotics. Since pNAPE-EcN is a member of Proteobacteria, this could indicate that more pNAPE-EcN were present in the GI tract as the result of antibiotic pretreatment. Yet at day 16, two days after pNAPE-EcN treatment ended, the fecal bacterial composition of the two groups were fairly similar and mostly resembled the composition of vehicle treated mice. This result suggests that neither antibiotic pre-treatment nor pNAPE-EcN treatment exerted long-term effects on the overall structure of the microbial community, so that the transisent effects of the antibiotics did not substantially enhance pNAPE-EcN colonization beyond the niches that could be accessed simply by continuous administration over a two-week period.
This finding is particularly important for potential translation of this treatment into clinical trials for human obesity because antibiotic treatment in humans significantly increases the risk for C. difficile infection (Baur et al. 2017). Pre-treatment with antibiotics was previously reported to be required for colonization by E. coli strains including EcN, but that study gavaged the bacteria only a single time (Lasaro et al. 2014). Other studies have also suggested that antibiotics were needed for successful incorporation of other donor bacteria (Lasaro et al. 2014; Ni et al. 2017; Shen et al. 2015; Willing et al. 2011). Continuous administration of relatively high levels of pNAPE-EcN or pPLAAT2-EcN over the 14 days may allow these engineered EcN to gradually occupy a small colonization niche. E. coli typically represent only 0.001% to 1.0% of total intestinal bacteria (Tenaillon et al. 2010), and our findings that post-treatment abundance of Proteobacteria were relatively low (≤0.1% of all bacteria) is consistent with our engineered bacteria occupying only a very small niche. Our studies show that this is still sufficient pNAPE-EcN or pPLAAT2-EcN to double the NAPE levels in target extra-intestinal tissues such as omental fat depots and to reduce adiposity and overeating induced by the high fat diet.
That pNAPE-EcN and pPLAAT2-EcN treatment still exert marked anti-obesity effects in mice that were pre-fed a high fat for 10 days is significant. Three to seven days of high fat feeding diet is sufficient to markedly reduce endogenous NAPE biosynthesis and to cause many of the metabolic adaptions associated with obesity and increased morbidity including visceral adiposity, insulin resistance, inflammation, and reduced sensitivity to feeding induced satiety factors (de Wilde et al. 2008; Kahle et al. 2013; Lee et al. 2011; Shiwa et al. 2015; Turner et al. 2013). Therefore, our findings suggest that pNAPE-EcN treatment could potentially be useful even in humans with already established obesity. That pNAPE-EcN did not significantly alter body weight gain in lean mice fed a chow diet suggests that the appetite and weight suppressing effects of NAPE expressing EcN are not potentiated unless mice are in state of significant positive energy balance, so that exposure to NAPE expressing EcN is unlikely to cause adverse weight loss in lean individuals.
Our studies also demonstrate that sustained biosynthesis of NAPE by pNAPE-EcN is essential for the persistent resistance to diet induced obesity induced by pNAPE-EcN. Treatment with antibiotics after pNAPE-EcN treatment almost completely eliminated the increased fecal and tissue levels of NAPE as well as the reductions in weight gain and food intake normally provide by pNAPE-EcN treatment. Furthermore, our studies confirm that it is biosynthesis of NAPE, and not biosynthesis of minor products such as O-acyl phosphatidylglycerol, that are responsible for the persistent satiating effects since pPLAAT2-EcN and pNAPE-EcN show similar efficacy and pPLAAT2-EcN does not produce O-acyl phosphatidylglycerol.
Our finding that 14-day administration of engineered EcN leads to persistent biosynthesis of our therapeutic compound without the use of antibiotics may be of value in considering how to optimize other interventions that utilize donor bacteria. For instance, the fecal microbial transplants used experimentally to treat diseases such as inflammatory bowel disease or metabolic syndrome, may benefit from repeated administrations of donor microbiota rather than the typical single administration typically used currently. It may also be possible to use continuous administration without antibiotics to displace bacterial species associated with disease by administering the same species where disease causing elements (such as the ability to biosynthesize specific metabolites or toxins) have been engineered out. The colonization potential of orally administered bacteria varies widely (Derrien and van Hylckama Vlieg 2015), so studies that determine the persistence of other typically engineered strains such as Bifidobacteria, Lactobacillus, Streptococcus, and Clostridium species after 14 days of continuous administration might be very helpful in establishing the generalizability of our approach.
ACKNOWLEDGEMENTS
We thank Dr. Matthew B. Scholz, the director of the VANTAGE Microbiome Services core for helpful advice in regards to microbial composition studies. EcN was a gift from ArdeyPharm.
Funding: This work was supported in part by National Institutes of Health grants R01 AT007830 (SSD) and U24 DK059637 (Vanderbilt MMPC). VANTAGE is supported by National Institutes of Health grants P30 CA68485, P30 EY08126 and G20 RR030956. The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Footnotes
Conflict of interest: Author 2 (ZC) and Author 6 (SSD) have a patent pending for the use of engineered bacteria expressing NAPE or NAE for treating obesity. Authors 1, 3, 4, and 5 declare that they have no conflict of interest.
Ethical approval: All applicable international, national, and institutional guidelines for care and use of animals were followed. This article does not contain any studies with human participants performed by any of the authors.
REFERENCES
- Baur D, Gladstone BP, Burkert F, Carrara E, Foschi F, Dobele S, Tacconelli E (2017) Effect of antibiotic stewardship on the incidence of infection and colonisation with antibiotic-resistant bacteria and Clostridium difficile infection: a systematic review and meta-analysis. Lancet Infect Dis 17(9):990–1001 doi: 10.1016/S1473-3099(17)30325-0 [DOI] [PubMed] [Google Scholar]
- Bulat E, Garrett TA (2011) Putative N-acylphosphatidylethanolamine synthase from Arabidopsis thaliana is a lysoglycerophospholipid acyltransferase. J Biol Chem 286(39):33819–33831 doi: 10.1074/jbc.M111.269779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carding S, Verbeke K, Vipond DT, Corfe BM, Owen LJ (2015) Dysbiosis of the gut microbiota in disease. Microb Ecol Health Dis 26:10.3402/mehd.v26.26191 doi: 10.3402/mehd.v26.26191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman KD (2000) Emerging physiological roles for N-acylphosphatidylethanolamine metabolism in plants: signal transduction and membrane protection. Chem Phys Lipids 108(1–2):221–229 [DOI] [PubMed] [Google Scholar]
- Chen Z, Guo L, Zhang Y, Walzem RL, Pendergast JS, Printz RL, Morris LC, Matafonova E, Stien X, Kang L, Coulon D, McGuinness OP, Niswender KD, Davies SS (2014) Incorporation of therapeutically modified bacteria into gut microbiota inhibits obesity. J Clin Invest 124(8):3391–33406 doi: 10.1172/JCI72517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Zhang Y, Guo L, Dosoky N, de Ferra L, Peters S, Niswender KD, Davies SS (2017) Leptogenic effects of NAPE require activity of NAPE-hydrolyzing phospholipase D. J Lipid Res 58(8):1624–1635 doi: 10.1194/jlr.M076513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox LM, Blaser MJ (2015) Antibiotics in early life and obesity. Nat Rev Endocrinol 11(3):182–190 doi: 10.1038/nrendo.2014.210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox LM, Yamanishi S, Sohn J, Alekseyenko AV, Leung JM, Cho I, Kim SG, Li H, Gao Z, Mahana D, Zarate Rodriguez JG, Rogers AB, Robine N, Loke P, Blaser MJ (2014) Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell 158(4):705–721 doi: 10.1016/j.cell.2014.05.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wilde J, Mohren R, van den Berg S, Boekschoten M, Dijk KW, de Groot P, Muller M, Mariman E, Smit E (2008) Short-term high fat-feeding results in morphological and metabolic adaptations in the skeletal muscle of C57BL/6J mice. Physiol Genomics 32(3):360–369 doi: 10.1152/physiolgenomics.00219.2007 [DOI] [PubMed] [Google Scholar]
- Derrien M, van Hylckama Vlieg JET (2015) Fate, activity, and impact of ingested bacteria within the human gut microbiota. Trends Microb 23(6):354–366 doi: 10.1016/j.tim.2015.03.002 [DOI] [PubMed] [Google Scholar]
- Diep TA, Madsen AN, Holst B, Kristiansen MM, Wellner N, Hansen SH, Hansen HS (2011) Dietary fat decreases intestinal levels of the anorectic lipids through a fat sensor. FASEB J 25(2):765–774 doi: 10.1096/fj.10-166595 [DOI] [PubMed] [Google Scholar]
- Dosoky NS, Guo L, Chen Z, Feigley AV, Davies SS (2018) Dietary fatty acids control the species of N-acyl-phosphatidylethanolamines synthesized by therapeutically modified bacteria in the intestinal tract. ACS Infect Dis 4(1):3–13 doi: 10.1021/acsinfecdis.7b00127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everard A, Plovier H, Rastelli M, Van Hul M, de Wouters d’Oplinter A, Geurts L, Druart C, Robine S, Delzenne NM, Muccioli GG, de Vos WM, Luquet S, Flamand N, Di Marzo V, Cani PD (2019) Intestinal epithelial N-acylphosphatidylethanolamine phospholipase D links dietary fat to metabolic adaptations in obesity and steatosis. Nat Commun 10(1):457 doi: 10.1038/s41467-018-08051-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J, Astarita G, Gaetani S, Kim J, Cravatt BF, Mackie K, Piomelli D (2007) Food intake regulates oleoylethanolamide formation and degradation in the proximal small intestine. J Biol Chem 282(2):1518–1528 doi: 10.1074/jbc.M607809200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J, Gaetani S, Oveisi F, Lo Verme J, Serrano A, Rodriguez De Fonseca F, Rosengarth A, Luecke H, Di Giacomo B, Tarzia G, Piomelli D (2003) Oleylethanolamide regulates feeding and body weight through activation of the nuclear receptor PPAR-alpha. Nature 425(6953):90–93 doi: 10.1038/nature01921 [DOI] [PubMed] [Google Scholar]
- Geurts L, Everard A, Van Hul M, Essaghir A, Duparc T, Matamoros S, Plovier H, Castel J, Denis RG, Bergiers M, Druart C, Alhouayek M, Delzenne NM, Muccioli GG, Demoulin JB, Luquet S, Cani PD (2015) Adipose tissue NAPE-PLD controls fat mass development by altering the browning process and gut microbiota. Nat Commun 6:6495 doi: 10.1038/ncomms7495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillum MP, Zhang D, Zhang XM, Erion DM, Jamison RA, Choi C, Dong J, Shanabrough M, Duenas HR, Frederick DW, Hsiao JJ, Horvath TL, Lo CM, Tso P, Cline GW, Shulman GI (2008) N-acylphosphatidylethanolamine, a gut- derived circulating factor induced by fat ingestion, inhibits food intake. Cell 135(5):813–824 doi: 10.1016/j.cell.2008.10.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L, Amarnath V, Davies SS (2010) A liquid chromatography-tandem mass spectrometry method for measurement of N-modified phosphatidylethanolamines. Anal Biochem 405(2):236–245 doi: 10.1016/j.ab.2010.06.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain Z, Uyama T, Tsuboi K, Ueda N (2017) Mammalian enzymes responsible for the biosynthesis of N-acylethanolamines. Biochim Biophys Acta 1862(12):1546–1561 doi: 10.1016/j.bbalip.2017.08.006 [DOI] [PubMed] [Google Scholar]
- Igarashi M, DiPatrizio NV, Narayanaswami V, Piomelli D (2015) Feeding-induced oleoylethanolamide mobilization is disrupted in the gut of diet-induced obese rodents. Biochim Biophys Acta 1851(9):1218–1226 doi: 10.1016/j.bbalip.2015.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahle M, Horsch M, Fridrich B, Seelig A, Schultheiß J, Leonhardt J, Irmler M, Beckers J, Rathkolb B, Wolf E, Franke N, Gailus-Durner V, Fuchs H, de Angelis MH, Neschen S (2013) Phenotypic comparison of common mouse strains developing high-fat diet-induced hepatosteatosis. Mol Metabol 2(4):435–446 doi: 10.1016/j.molmet.2013.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasaro M, Liu Z, Bishar R, Kelly K, Chattopadhyay S, Paul S, Sokurenko E, Zhu J, Goulian M (2014) Escherichia coli isolate for studying colonization of the mouse intestine and its application to two-component signaling knockouts. J Bacteriol 196(9):1723–1732 doi: 10.1128/JB.01296-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauffer LM, Iakoubov R, Brubaker PL (2009) GPR119 is essential for oleoylethanolamide-induced glucagon-like peptide-1 secretion from the intestinal enteroendocrine L-cell. Diabetes 58(5):1058–1066 doi: 10.2337/db08-1237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YS, Li P, Huh JY, Hwang IJ, Lu M, Kim JI, Ham M, Talukdar S, Chen A, Lu WJ, Bandyopadhyay GK, Schwendener R, Olefsky J, Kim JB (2011) Inflammation is necessary for long-term but not short-term high-fat diet-induced insulin resistance. Diabetes 60(10):2474–2483 doi: 10.2337/db11-0194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo Verme J, Gaetani S, Fu J, Oveisi F, Burton K, Piomelli D (2005) Regulation of food intake by oleoylethanolamide. Cell Mol Life Sci 62(6):708–716 doi: 10.1007/s00018-004-4494-0 [DOI] [PubMed] [Google Scholar]
- Mardian EB, Bradley RM, Duncan RE (2015) The HRASLS (PLA/AT) subfamily of enzymes. J Biomed Sci 22:99 doi: 10.1186/s12929-015-0210-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- May-Zhang LS, Chen Z, Dosoky NS, Yancey PG, Boyd KL, Hasty AH, Linton MF, Davies SS (2019) Administration of N-acyl-phosphatidylethanolamine expressing bacteria to low density lipoprotein receptor(−/−) mice improves indices of cardiometabolic disease. Sci Rep 9(1):420 doi: 10.1038/s41598-018-37373-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkel O, Schmid PC, Paltauf F, Schmid HH (2005) Presence and potential signaling function of N-acylethanolamines and their phospholipid precursors in the yeast Saccharomyces cerevisiae. Biochim Biophys Acta 1734(3):215–219 doi: 10.1016/j.bbalip.2005.03.004 [DOI] [PubMed] [Google Scholar]
- Mileykovskaya E, Ryan AC, Mo X, Lin CC, Khalaf KI, Dowhan W, Garrett TA (2009) Phosphatidic acid and N-acylphosphatidylethanolamine form membrane domains in Escherichia coli mutant lacking cardiolipin and phosphatidylglycerol. J Biol Chem 284(5):2990–3000 doi: 10.1074/jbc.M805189200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni J, Shen T-CD, Chen EZ, Bittinger K, Bailey A, Roggiani M, Sirota-Madi A, Friedman ES, Chau L, Lin A, Nissim I, Scott J, Lauder A, Hoffmann C, Rivas G, Albenberg L, Baldassano RN, Braun J, Xavier RJ, Clish CB, Yudkoff M, Li H, Goulian M, Bushman FD, Lewis JD, Wu GD (2017) A role for bacterial urease in gut dysbiosis and Crohn’s disease. Sci Transl Med 9(416) doi: 10.1126/scitranslmed.aah6888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogura Y, Parsons WH, Kamat SS, Cravatt BF (2016) A calcium-dependent acyltransferase that produces N-acyl phosphatidylethanolamines. Nat Chem Biol 12(9):669–671 doi: 10.1038/nchembio.2127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reikvam DH, Erofeev A, Sandvik A, Grcic V, Jahnsen FL, Gaustad P, McCoy KD, Macpherson AJ, Meza-Zepeda LA, Johansen F-E (2011) Depletion of murine intestinal microbiota: effects on gut mucosa and epithelial gene expression. PLOS ONE 6(3):e17996 doi: 10.1371/journal.pone.0017996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinne P, Guillamat-Prats R, Rami M, Bindila L, Ring L, Lyytikainen LP, Raitoharju E, Oksala N, Lehtimaki T, Weber C, van der Vorst EPC, Steffens S (2018) Palmitoylethanolamide promotes a proresolving macrophage phenotype and attenuates atherosclerotic plaque formation. Arterioscler Thromb Vasc Biol 38(11):2562–2575 doi: 10.1161/ATVBAHA.118.311185 [DOI] [PubMed] [Google Scholar]
- Rodriguez de Fonseca F, Navarro M, Gomez R, Escuredo L, Nava F, Fu J, Murillo-Rodriguez E, Giuffrida A, LoVerme J, Gaetani S, Kathuria S, Gall C, Piomelli D (2001) An anorexic lipid mediator regulated by feeding. Nature 414(6860):209–212 doi: 10.1038/35102582 [DOI] [PubMed] [Google Scholar]
- Shen T-CD, Albenberg L, Bittinger K, Chehoud C, Chen Y-Y, Judge CA, Chau L, Ni J, Sheng M, Lin A, Wilkins BJ, Buza EL, Lewis JD, Daikhin Y, Nissim I, Yudkoff M, Bushman FD, Wu GD (2015) Engineering the gut microbiota to treat hyperammonemia. J Clin Invest 125(7):2841–2850 doi: 10.1172/JCI79214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiwa M, Yoneda M, Okubo H, Ohno H, Kobuke K, Monzen Y, Kishimoto R, Nakatsu Y, Asano T, Kohno N (2015) Distinct time course of the decrease in hepatic AMP-activated protein kinase and Akt phosphorylation in mice fed a high fat diet. PLoS One 10(8):e0135554 doi: 10.1371/journal.pone.0135554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenaillon O, Skurnik D, Picard B, Denamur E (2010) The population genetics of commensal Escherichia coli. Nat Rev Microbiol 8(3):207–217 doi: 10.1038/nrmicro2298 [DOI] [PubMed] [Google Scholar]
- Turner N, Kowalski GM, Leslie SJ, Risis S, Yang C, Lee-Young RS, Babb JR, Meikle PJ, Lancaster GI, Henstridge DC, White PJ, Kraegen EW, Marette A, Cooney GJ, Febbraio MA, Bruce CR (2013) Distinct patterns of tissue-specific lipid accumulation during the induction of insulin resistance in mice by high-fat feeding. Diabetologia 56(7):1638–1648 doi: 10.1007/s00125-013-2913-1 [DOI] [PubMed] [Google Scholar]
- Wellner N, Diep TA, Janfelt C, Hansen HS (2013) N-acylation of phosphatidylethanolamine and its biological functions in mammals. Biochim Biophys Acta 1831(3):652–662 doi: 10.1016/j.bbalip.2012.08.019 [DOI] [PubMed] [Google Scholar]
- Willing BP, Vacharaksa A, Croxen M, Thanachayanont T, Finlay BB (2011) Altering host resistance to infections through microbial transplantation. PLOS ONE 6(10):e26988 doi: 10.1371/journal.pone.0026988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y-J, Li S, Gan R-Y, Zhou T, Xu D-P, Li H-B (2015) Impacts of gut bacteria on human health and diseases. Int J Mol Sci 16(4):7493–7519 doi: 10.3390/ijms16047493 [DOI] [PMC free article] [PubMed] [Google Scholar]


