Abstract
BACKGROUND/OBJECTIVE:
Subarachnoid hemorrhage (SAH) is a devastating neurologic event for which markers to assess poor outcome are needed. Elevated cerebrospinal fluid (CSF) protein may result from inflammation and blood-brain barrier (BBB) disruption that occurs during SAH. We sought to determine if CSF protein level is associated with functional outcome after SAH.
METHODS:
We prospectively collected single-center demographic and clinical data for consecutive patients admitted with spontaneous SAH. Inclusion required an external ventricular drain and daily CSF protein and cellular counts starting within 48 hours of symptom onset and extending through 7 days after onset. Seven-day average CSF protein was determined from daily measured values after correcting for contemporaneous CSF red blood cell (RBC) count. Three-month functional outcome was assessed by telephone interview with good outcome defined as modified Rankin score 0–3. Variables univariately associated with outcome at p<0.25 and measures of hemorrhage volume were included for binary logistic regression model development.
RESULTS:
The study included 130 patients (88% aneurysmal SAH, 69% female, 54.8±14.8 years, Glasgow Coma Scale [GCS] 14 [7–15]). Three-month outcome assessment was complete in 112 (86%) patients with good functional outcome in 74 (66%). CSF protein was lower in good outcome (35.3 [20.4–49.7] vs. 80.5 [40.5–115.5] mg/dL; p<0.001). CSF protein was not associated with cerebral vasospasm, but delayed radiographic infarction on three to 12-month neuroimaging was associated with higher CSF protein (46.3 [32.0–75.0] vs. 30.2 [20.4–47.8] mg/dl; p=0.023). Good three-month outcome was independently associated with lower CSF protein (OR 0.39 [0.23–0.70] for 75th versus 25th percentile of protein; p=0.001) and higher admission GCS (OR 1.23 [1.10–1.37] for good outcome per GCS point increase; p<0.001). Parenchymal hematoma predicted worse outcome (OR 6.31 [1.58–25.25]; p=0.009). Results were similar after excluding non-aneurysmal SAH and after including CSF RBC count, CT score, and intraventricular hemorrhage volume in models.
CONCLUSIONS:
Elevated average CSF protein is associated with poor outcome after spontaneous SAH. Further research should investigate if elevated CSF protein identifies patients in whom mechanisms such as BBB disruption contribute to poor outcome.
Keywords: subarachnoid hemorrhage, hemorrhagic stroke, inflammation, outcomes, cerebrospinal fluid, blood-brain barrier
INTRODUCTION:
Spontaneous subarachnoid hemorrhage (SAH) represents a disproportionate loss of quality of life due to high morbidity in a younger patient population than other stroke subtypes.(1) Historically, attention has focused on cerebral vasospasm as the most important mediator of secondary brain injury and poor outcome after SAH. However, interest in additional mechanisms of delayed cerebral ischemia (DCI) and brain injury has grown, especially in light of the CONSCIOUS trial in which clazosentan, an endothelin receptor antagonist, resulted in improved angiographic vasospasm without improved functional outcome.(2) Identifying patients at increased risk of poor outcome from mechanisms besides vasospasm remains a challenge.
Activation of the inflammatory response and blood-brain barrier (BBB) disruption have been considered potential mechanisms of brain injury after SAH.(3) Elevated CSF protein concentration is a biomarker of inflammation and disruption of the blood-cerebrospinal fluid (CSF) barrier in a variety of neurologic diseases.(4) We hypothesized that the average CSF protein during the first seven days after SAH would be associated with functional outcome at three months after SAH, reflecting contributions from the acute inflammatory response and BBB disruption leading to brain injury. As secondary objectives, we investigated if elevated average CSF protein was associated with vasospasm or DCI.
METHODS:
Study Design, Setting, and Patient Population
Consecutive patients with spontaneous SAH admitted to our neurosciences intensive care unit between 2006 and 2017 were enrolled in an institutional review board (IRB) approved observational study. The IRB approved a waiver of consent for patients who died during hospitalization or who were incapacitated and without a legally authorized representative. Otherwise, written informed consent was obtained from each patient or representative. All patients were diagnosed by a board-certified neurologist, with confirmation using computed tomography (CT) or xanthochromia of CSF. Patients with SAH attributable to trauma, arteriovenous malformation, vasculitis, or vasculopathy were excluded. Demographic, medical history, clinical, and outcome data were prospectively recorded in standardized electronic forms. Inclusion in this study required clinically mandated placement of an external ventricular drain (EVD) with collection of daily CSF samples for cellular and protein counts, starting within 48 hours of SAH onset and extending through 7 days after onset. Daily surveillance CSF sampling was customarily performed during the study interval. We excluded patients with a clinical diagnosis of ventriculitis.
Clinical Management
Catheter or CT angiography and aneurysm obliteration by surgical clip or endovascular coiling was performed as soon as possible, usually within 24 hours. Nimodipine was administered unless hypotension developed despite dose-frequency adjustment. Transcranial doppler (TCD) sonography was performed daily by a certified technician. We prospectively recorded the presence of vasospasm as diagnosed by the following: mean TCD flow velocity at two thresholds of greater than 120 or 200 cm/second in MCA flow velocity or arterial stenosis on angiography. Patients with vasospasm were treated with hyperdynamic therapy (fluid administration, blood pressure augmentation, blood transfusion if anemic) and balloon angioplasty and/or intraarterial vasodilators if clinically appropriate.
Data Collection
Clinical variables were prospectively recorded until death or hospital discharge. Laboratory data were retrieved by electronic data extraction. Neurologic status on admission was assessed with the World Federation of Neurosurgical Societies (WFNS) Scale (graded 1 [best] to 5 [worst]), Glasgow Coma Scale (GCS), and National Institutes of Health Stroke Scale (NIHSS). Hemorrhage severity was assessed using the Hunt-Hess scale (symptomatic severity, 1 [mildest] to 5 [severest]) and modified Fisher CT Scale (radiographic severity ranging from 0 to 4). We measured intraventricular hemorrhage (IVH) volume on CT scans obtained immediately following EVD placement using industry standard DICOM images and semi-automated software (Analyze Direct, Overland Park KS), similar to the approach used to measure intraparenchymal hematoma volumes.(5)
Cerebrospinal Fluid Analysis:
Daily CSF white blood cell (WBC), red blood cell (RBC), and protein count were recorded starting with the first CSF sample collected within 48 hours of SAH onset and extending through seven days after onset. A seven-day average CSF protein was determined from daily measured protein values after correcting for contemporaneous CSF RBC count. One mg per dL of protein was subtracted for every 1,000 RBCs per μL and any resulting negative values were truncated to zero.(6) This correction was implemented to account for the quantity of CSF protein due to cellular material present from the patient’s hematoma. Seven-day average CSF WBC and RBC values were also calculated.
Functional Outcome Assessment:
Functional outcomes were obtained using a validated questionnaire to ascertain mRS at 28 days and three months.(7, 8) Data was obtained by a trained study coordinator not involved in the clinical care of the patient using the modified Rankin Scale (mRS), which ranges from 0 (no symptoms) to 6 (death). We categorized mRS scores of 0–3 as favorable functional outcomes.
Presence of Delayed Cerebral Infarction (DCI):
DICOM images of CT and/or MRI scans of the brain performed between 3 and 12 months after SAH were reviewed in those for whom imaging was obtained at our institution. In the case of multiple imaging studies, the first study was used. We used this timeframe because neuroimaging after hospitalization and before this timeframe was uncommon. DCI was considered to be present if there was a new CT hypodensity or T2-weighted MRI intensity present that was not present on admission imaging or imaging performed within 48 hours of aneurysm occlusion, the lesion had not been prospectively adjudicated as being due to surgical or endovascular intervention, and the lesion was not due to EVD placement or intraparenchymal hematoma. All studies were reviewed by a board-certified neuroradiologist and confirmed by a board-certified neurologist that the lesion met the above definition. Imaging review was performed blinded to clinical severity, vasospasm occurrence or treatment, laboratory, and functional outcome data.
Statistical Analysis
Continuous data are presented as mean ± standard deviation for normally distributed variables or median (interquartile range) for non-normally distributed variables. Variables were analyzed using independent samples t-test for normally distributed variables and Mann-Whitney U or Kruskal-Wallis tests for non-normally distributed variables. Categorical data were compared with Pearson Chi-Square or Fisher exact tests. For multivariate analysis of favorable outcome, we used binary logistic regression with prespecified covariates for model inclusion based on biological plausibility as mechanistic contributors to outcome, including: seven-day average CSF protein, age, admission GCS, and modified Fisher CT scale. To account for the possibility of unrecognized contributors to favorable outcome, we included additional covariates with a threshold for inclusion of univariate p<0.25. From this fully adjusted model, we then created a parsimoniously adjusted model by applying a backward stepwise algorithm with a removal criterion of probability of F to remove ≥ 0.1. To address the possibility that CSF total protein could represent a surrogate for hemorrhage volume, we also created a model with the parsimoniously adjusted variables plus hemorrhage burden variables reintroduced (IVH volume, modified Fisher CT score, and seven-day average RBC count). We also created an outcome model excluding cases in which aneurysms were not discovered (i.e. perimesencephalic SAH). After assessing the association between outcome and seven-day average CSF protein, we assessed the associations between outcome and single sample values of CSF protein on each day of collection by substituting the individual daily protein for the seven-day average protein in the parsimonious model. In addition, a last observation carried forward (LOCF) approach was used to assess if missing 3-month outcome data was likely to have influenced the association observed between seven-day average CSF protein and 3-month outcome.(9) For the LOCF approach, a parsimoniously adjusted model of functional outcome was created with the last observed functional outcome assessment (either hospital discharge or 28-day) used in those cases where 3-month outcome assessment data was unavailable. The odds ratios (OR) for continuous variables represent the OR for a one-unit change in the variable unless otherwise listed parenthetically. To facilitate conceptualization of the effect size for CSF protein, we calculated the compounded OR corresponding to the 75th versus the 25th percentile of CSF protein. Our primary study outcome was the adjusted association between seven-day CSF protein and three-month functional outcome, and our secondary study outcome was the association between seven-day CSF protein and occurrence of infarction due to DCI. We also examined associations between CSF protein and vasospasm. A p-value <0.05 was considered statistically significant. Analyses were conducted using SPSS statistical software, v.25 (SPSS, Chicago IL).
RESULTS:
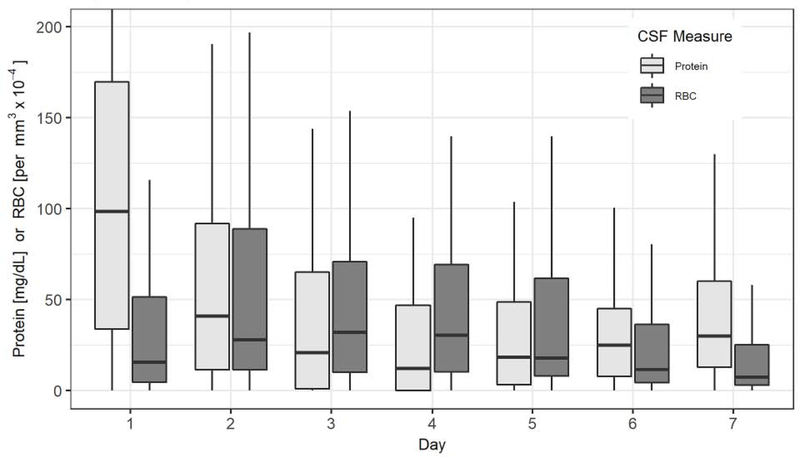
The observational study included 459 consecutive patients with spontaneous SAH. Reasons for exclusion included: patient did not consent (23), no EVD placed (191), ventriculitis (21), absence of CSF studies after initial placement of EVD (19), and absence of daily CSF studies (75). A total of 130 patients (mean 54.8 ± 14.8 years old, 69% female, 115 [88%] aneurysmal) satisfied inclusion criteria, of whom 121 (93%) had 28-day and 112 (86%) had three-month functional outcome data. Table 1 summarizes patient demographic and baseline clinical data. Seven-day average CSF counts were: protein corrected for contemporaneous RBCs 42.2 (24.4–75.9) mg/dL, RBC 37.0 (18.0–66.0) thousand per μL, and WBC 48.1 (16.8–175.7) per μL. Seven-day average CSF protein did not differ between surgical approaches taken for aneurysm obliteration (surgical clip 41.4 (21.1–70.0) versus endovascular coil 43.2 (28.1–84.5) mg/dl, p =0.26) Both daily CSF protein and RBC count differed across the days of collection (p<0.001). The Figure shows the distribution of daily CSF protein corrected for contemporaneous RBCs and the distribution of daily CSF RBC count.
Table 1.
Patient Characteristics
| Variable | Result | |
|---|---|---|
| Age (years) | 54.8 ± 14.8 | |
| Gender (female) | 90 (69.2%) | |
| Race | Unknown | 1 (0.8%) |
| Other | 9 (6.9%) | |
| Black or African-American | 23 (17.7%) | |
| Native Pacific Islander | 1 (0.8%) | |
| White | 96 (73.8%) | |
| Hispanic ethnicity | 15 (11.5%) | |
| Premorbid mRS | 0 [0–0] | |
| History of stroke | 4 (3.1%) | |
| History of hypertension | 66 (50.8%) | |
| Diabetes mellitus | 17 (13.1%) | |
| Smoking history | 49 (37.7%) | |
| Admission GCS score | 14 [7–15] | |
| Admission NIHSS score | 2 [1–18] | |
| WFNS grade | 2 [1–4] | |
| Hunt-Hess scale | 3 [2–4] | |
| Modified Fisher CT score | 3 [3–3] | |
| IVH on presentation | 30 (23.1%) | |
| IVH volume (mL) among those with IVH | 2.6 [1.6–24.2] | |
| Parenchymal hematoma present | 19 (14.6%) | |
| Subarachnoid hemorrhage etiology | ||
| Aneurysmal | 115 (88.5%) | |
| Perimesencephalic | 15 (11.5%) | |
| Aneurysm Location | ||
| ACA/ACOM | 47 (40.9%) | |
| ICA/PCOM | 39 (33.9%) | |
| MCA | 14 (12.2%) | |
| Vertebrobasilar/Posterior | 12 (10.4%) | |
| Other | 3 (2.6%) | |
| Aneurysm maximum diameter (mm) | 6 [4–8] | |
| Procedure performed | ||
| Surgical clip | 63 (48.5%) | |
| Endovascular coil | 51 (39.2%) | |
| None | 16 (12.3%) | |
| Initial CSF protein (RBC corrected, mg/dL) | 98.3 [32.4–164.8] | |
| Initial RBC count (thousand per μL) | 17.0 [5.0–52.0] | |
| Initial WBC count (per μL) | 48.0 [15.0–168.0] | |
| Seven-day average CSF protein (RBC corrected, mg/dL) | 42.2 [24.4–75.9] | |
| Seven-day average RBC count (thousand per μL) | 37.0 [18.0–66.0] | |
| Seven-day average WBC count | 48.1 [16.8–175.7] | |
n (%), Mean ± standard deviation, Median [Interquartile Range]
yr = year, mRS = modified Rankin Scale, GCS = Glasgow Coma Scale, NIHSS = National Institutes of Health Stroke Scale, WFNS = World Federation of Neurosurgical Societies, ICH = intracerebral hemorrhage, CT = computed tomography scan, LMWH = low molecular weight heparin, INR = international normalized ratio, PTT = partial thromboplastin time, HCT = hematocrit, K = 1000, ul = microliter, BP = blood pressure, mmHg = millimeters of mercury, mg = milligrams, dL = deciliter, hr = hour, mL = milliliter
Figure.
Daily Cerebrospinal Fluid Protein (corrected for CSF RBC count) and Red Blood Cell Levels
At three months, 74 (66%) patients had favorable functional outcome (mRS 0–3). Those with favorable functional outcome had lower initial (80.8 [33.4–140.5] vs. 126.9 [54.4–261.3] mg/dL, p=0.046) and seven-day average CSF protein (35.3 [20.4–49.7] vs. 80.5 [40.5–115.5] mg/dL, p<0.001). Other variables univariately associated (p<0.05) with three-month functional outcome were: age, seven-day average WBC count, clinical severity (measured by admission GCS, Hunt Hess, WFNS, and NIHSS scores with GCS having the largest test statistic), modified Fisher CT score, IVH volume, presence of parenchymal hemorrhage, and perimesencephalic SAH. Of note, the rate of good three-month functional outcome did not differ between patients who were treated by surgical clipping versus endovascular coiling (64.4% v 61.0%, p = 0.73). The adjusted OR for favorable outcome comparing the 75th versus 25th percentile of seven-day average CSF protein was 0.39 [95% CI 0.23–0.70], which is equivalent to 2.53 [95% CI 1.43–4.23] times the odds of unfavorable outcome in the 75th compared to the 25th percentile of protein. Favorable outcome was also predicted by higher admission GCS (OR 1.23 [95% CI 1.10–1.37] for good outcome per GCS point increase; p<0.001). Parenchymal hematoma predicted worse outcome (OR 6.31 [95% CI 1.58–25.25]; p=0.009). Table 2 summarizes the three-month functional outcome models. These associations were not meaningfully different if IVH volume, modified Fisher CT score, and seven-day average RBC count were reintroduced or if cases of perimesencephalic SAH were excluded. The effect size of daily CSF protein (75th versus 25th percentile) and three-month functional outcome was similar when single sample values of CSF protein drawn between days two and six were substituted for seven-day average CSF protein, but not for samples from days one or seven as shown in Table 3. The association between favorable 28-day functional outcome and seven-day average CSF protein was similar as for threemonth functional outcome (OR 0.54 [0.24–0.73]; p=0.002).
Table 2.
Binary Logistic Regression Models for Favorable Three-month Functional Outcome (mRS 0-3)
| Variables with univariate p<0.25 | Fully Adjusted Model | Parsimonious Model | Parsimonious Model with Hematoma Burden Variables Reintroduced | ||||||
|---|---|---|---|---|---|---|---|---|---|
| All subarachnoid hemorrhage | |||||||||
| OR | 95% CI | p | OR | 95% CI | p | OR | 95% CI | p | |
| Seven-day average CSF protein (75th versus 25th percentile) | 0.484 | 0.244-0.902 | 0.02 | 0.392 | 0.232-0.696 | 0.001 | 0.392 | 0.220-0.696 | 0.002 |
| Admission GCS score | 1.28 | 1.12-1.47 | <0.001 | 1.23 | 1.10-1.37 | <0.001 | 1.23 | 1.10-1.39 | 0.001 |
| Parenchymal hematoma present† | 0.207 | 0.048-0.902 | 0.04 | 0.159 | 0.040-0.635 | 0.009 | 0.155 | 0.037-0.647 | 0.01 |
| Seven-day average CSF | 1.00 | 1.00-1.00 | 0.46 | -- | -- | -- | 1.00 | 1.00-1.00 | 0.77 |
| RBCs (75th versus 25th percentile) | -- | -- | -- | 1.00 | 1.00-1.00 | 0.73 | |||
| IVH Volume (mL) | 1.00 | 1.00-1.00 | 0.76 | -- | -- | -- | 0.918 | 0.388-2.18 | 0.85 |
| Modified Fisher CT Score | 1.02 | 0.415-2.50 | 0.97 | -- | -- | -- | -- | -- | -- |
| Age (years) | 0.973 | 0.933-1.02 | 0.21 | -- | -- | -- | -- | -- | -- |
| Seven-day average CSF WBCs (75th versus 25th percentile) | 1.10 | 0.81-1.50 | 0.53 | ||||||
| WBCs (75th versus 25th percentile) | -- | -- | -- | -- | -- | -- | |||
| Perimesencephalic SAH | >1000 | 0.00->1000 | 1.00 | -- | -- | -- | -- | -- | -- |
| History of Diabetes Mellitus | 0.538 | 0.099-2.93 | 0.47 | ||||||
| Aneurysm subarachnoid hemorrhage only | |||||||||
| OR | 95% CI | p | OR | 95% CI | p | OR | 95% CI | p | |
| Seven-day average CSF protein (75th versus 25th percentile) | 0.474 | 0.250-0.898 | 0.02 | 0.426 | 0.245-0.740 | 0.002 | 0.392 | 0.220-0.733 | 0.002 |
| Admission GCS score | 1.28 | 1.12-1.47 | <0.001 | 1.24 | 1.01-1.40 | <0.001 | 1.26 | 1.10-1.43 | <0.001 |
| Parenchymal hematoma present | 0.207 | 0.048-0.902 | 0.04 | 0.186 | 0.046-0.749 | 0.02 | 0.195 | 0.047-0.813 | 0.02 |
| Seven-day average CSF | 1.00 | 1.00-1.00 | 0.47 | -- | -- | -- | 1.00 | 1.00-1.00 | 0.53 |
| RBCs (75th versus 25th percentile) | -- | -- | -- | 1.00 | 1.00-1.00 | 0.74 | |||
| IVH Volume (mL) | 1.00 | 1.00-1.00 | 0.82 | -- | -- | -- | 1.03 | 0.425-2.50 | 0.95 |
| Modified Fischer CT Score | 1.02 | 0.415-2.50 | 0.97 | -- | -- | -- | -- | -- | -- |
| Age (years) | 0.973 | 0.933-1.02 | 0.21 | -- | -- | -- | -- | -- | -- |
| Seven-day average CSF | 1.10 | 0.81-1.50 | 0.53 | ||||||
| WBCs (75th versus 25th percentile) | -- | -- | -- | -- | -- | -- | |||
| History of Diabetes Mellitus | 0.538 | 0.099-2.93 | 0.47 | ||||||
GCS was used as the clinical severity variable for modeling since it had the strongest univariate test statistic and is colinear with WFNS score, Hunt Hess scale, and NIHSS score.
The odds ratios for continuous variables represent the odds ratio associated with a one-unit change in the variable unless otherwise listed parenthetically.
Equivalent to a parsimonious model odds ratio of 6.31 (95% CI 1.58–25.25) for parenchymal hematoma presence predicting unfavorable outcome
CI = confidence interval, CSF = cerebrospinal fluid, GCS = Glasgow Coma Scale, IVH =intraventricular hemorrhage OR = Odds Ratio, RBCs = red blood c1ells, SAH = subarachnoid hemorrhage WBCs = white blood cells
Table 3:
Effect Sizes of Daily CSF Protein (75th versus 25th Percentile) in Parsimonious Binary Logistic Regression Models for Three-month Favorable Functional Outcome (mRS 0–3).
| Day After SAH Onset | Odds Ratio | 95% CI | P value |
|---|---|---|---|
| One | 0.874 | 0.667–1.310 | 0.629 |
| Two | 0.616 | 0.378–0.923 | 0.026 |
| Three | 0.406 | 0.241–0.726 | 0.002 |
| Four | 0.328 | 0.181–0.618 | <0.001 |
| Five | 0.287 | 0.135–0.604 | 0.001 |
| Six | 0.546 | 0.344–0.860 | 0.011 |
| Seven | 0.620 | 0.365–1.049 | 0.069 |
Notably, there was no difference between seven-day average CSF protein for those patients with versus without a documented 3-month outcome (43.0 [24.4–78.8] vs. 36.0 [29.0–63.2] mg/dl, p=0.52). Of the 18 (14%) patients with missing 3-month outcome data, 12 patients had 28-day functional outcome data available and 6 patients had hospital discharge functional outcome data available. The LOCF model for functional outcome resulted in a similar effect estimate for the association between CSF protein and functional outcome as for the primary 3-month outcome model (75th versus 25th percentile of seven-day average CSF protein 0.39, 95% CI 0.21– 0.66).
Angiographic vasospasm was confirmed in 42 (32%) of 130 patients. MCA TCD velocities over 120 cm/second occurred in 90 (69%) patients, and MCA TCD velocities over 200 cm/second occurred in 30 (23%) patients. Seven-day average CSF protein did not differ between those with or without angiographic vasospasm or TCD velocity elevation (all p>0.30). Of note, neither angiographic vasospasm nor TCD elevation met criteria for inclusion in multivariate models of functional outcome.
Seventy-one (55%) patients had CT or MRI brain studies performed within three to 12 months after SAH. DCI was identified in 45 (63.4%) of these patients. Those with DCI had higher seven-day average CSF protein (46.3 [32.0–75.0] vs. 30.2 [20.4–47.8] mg/dl, p=0.023) but no significant difference in the rate of vasospasm (18 [40.0%] versus 6 [23.1%], p=0.15).
DISCUSSION:
We found that elevated CSF protein over the seven days after SAH onset was independently associated with unfavorable functional outcomes. We also found evidence that elevated CSF protein was associated with subsequent radiographic delayed cerebral infarction. These data did not suggest an association between CSF protein and surgical method of aneurysm obliteration, angiographic vasospasm nor TCD elevation. While we did not directly study mechanistic pathways, the analyses we performed to adjust for hemorrhage severity by modified Fisher score, IVH volume, and CSF RBC count provide indirect evidence that elevated CSF protein is not simply a marker of total hemorrhage volume, and suggests alternative, independent pathologic mechanisms such as activation of the inflammatory response and BBB disruption.
The results of multiple therapeutic drug trials for patients with aneurysmal subarachnoid hemorrhage have indicated that modifiable risks are not clearly related to vasospasm. Multiple studies have found that oral nimodipine reduces secondary ischemia and increases the rate of good outcomes, but exerts no measurable effect on vasospasm. (10–12) Conversely, the CONSCIOUS-2 and CONSCIOUS-3 trials showed that the endothelin receptor antagonist clazosentan reduced angiographic vasospasm without improving functional outcomes.(2, 13) Others have demonstrated an association between systemic inflammatory markers, global brain atrophy, and worse functional outcome after SAH that was independent of cerebral vasospasm or delayed cerebral infarction.(14) While increased inflammation after aneurysm rupture leading to BBB disruption is a plausible mechanism to explain the association between CSF protein and functional outcome, other explanations are possible. CSF protein may serve as an acute phase reactant and rise in response to early brain injury rather than represent mechanisms causing injury. Efficient clearance of CSF protein may also be a marker of a favorable recovery trajectory, which would be consistent with our data on daily CSF protein trends.
Multiple prior studies have suggested a role for BBB disruption and inflammation in brain injury following SAH. Animal studies have suggested that BBB permeability increases acutely following SAH.(15–17) Consistent with early BBB disruption as a mechanism of brain injury, mouse models of SAH have shown decreased length density of AQP4 on capillaries and astrocyte process retraction and cell body swelling with associated hippocampal volume loss.(18) In patients with SAH, increased BBB permeability has been associated with subsequent DCI and worse functional outcome.(19–21) Blood and CSF proinflammatory cytokines and mediators such as interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α), interleukin-1 receptor antagonist, and high-mobility group box 1 protein have been associated with cerebral vasospasm, DCI, and poor functional outcome in SAH patients.(22–25) Most pre-clinical and clinical studies of SAH implicate BBB disruption for early brain injury and persistent inflammation for more prolonged accumulation of injury.(26–29) Combining a global marker of acute CNS inflammation and BBB disruption, such as average CSF protein, with pathway specific markers, such as IL-6 and TNF-α, may facilitate the study of SAH by elucidating mechanisms of secondary injury and contributions from individual pathways.
There are limitations to our study. First, our data represent a single-center experience and should be replicated. Our study required that patients have an EVD available for daily CSF collection as daily lumbar puncture for the purpose of CSF assessment was impractical. As a result, our study selected patients with greater disease severity. However, it is patients with severe disease phenotypes in whom outcome is most uncertain and biomarkers may be of greatest utility. Caution is warranted in extrapolating our findings to milder disease phenotypes in which an EVD is not therapeutically indicated but CSF is available through alternative means (such as lumbar puncture). We decided a priori to use seven-day average CSF protein as our primary variable of interest. Collecting daily CSF samples is burdensome and becoming less common in clinical practice. However, given the paucity of data on expected CSF protein trends, we felt it was necessary to a priori use CSF protein averaged over several days in order to avoid bias that could be introduced by retrospectively selecting a single day for analysis. While we used seven-day average CSF protein as our primary measure, our analysis of individual daily values suggests that one or two samples from between days 2 and 6 may be sufficient to characterize this association. Additionally, alternative CSF measures of BBB permeability and inflammation, such as IgG/albumin ratio and specific cytokines, were not obtained clinically and, therefore, not available for analysis. While mRS functional outcome was available in the majority of patients at 28-days and three-months, only 54% of patients had clinically indicated neuroimaging available for analysis of DCI at 3–12 months. It is possible that the clinical indications for repeat imaging in these patients represent a source for bias. However, it is unlikely that the 14% of patients without 3-month functional outcome data represent a meaningful source of bias; seven-day CSF protein levels did not differ between those with versus without 3-month outcome, and the LOCF model demonstrated a similar association between CSF protein and functional outcome after accounting for those patients without 3-month outcome data.
CONCLUSIONS:
Elevated average CSF protein within the first week is independently associated with unfavorable functional outcome after SAH. Elevated CSF protein was also associated with DCI but not vasospasm. Further research should investigate if CSF protein levels identify patients in whom mechanisms such as BBB disruption and acute inflammation contribute to poor outcome.
Acknowledgments
Study Funding: Dr. Nadkarni receives support from National Institutes of Neurological Disorders and Stroke Research Education grant R25 NS070695. Dr. Maas receives support from National Institutes of Health grant K23 NS092975. Dr. Naidech receives support from Agency for Healthcare Research and Quality grant K18 HS023437. Dr. Liotta receives support from the National Institutes of Health National Center for Advancing Translational Sciences grant KL2TR001424 and the National Institute of Health grant L30 NS098427.
Research reported in this publication was supported, in part, by the National Institutes of Health’s National Center for Advancing Translational Sciences grant UL1 TR000150. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Agency for Healthcare Research and Quality.
Footnotes
Disclosures:
Dr. Neil A. Nadkarni reports no disclosures.
Dr. Matthew B. Maas reports no disclosures.
Dr. Ayush Batra reports no disclosures.
Dr. Minjee Kim reports no disclosures.
Dr. Edward M. Manno reports no disclosures.
Dr. Farzaneh A. Sorond reports no disclosures.
Dr. Shyam Prabhakaran reports no disclosures.
Dr. Andrew Naidech reports no disclosures.
Dr. Eric M. Liotta reports no disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Johnston SC, Selvin S, Gress DR. The burden, trends, and demographics of mortality from subarachnoid hemorrhage. Neurology 1998;50(5):1413–1418. [DOI] [PubMed] [Google Scholar]
- 2.Macdonald RL, Higashida RT, Keller E, et al. Randomized trial of clazosentan in patients with aneurysmal subarachnoid hemorrhage undergoing endovascular coiling. Stroke 2012;43(6):1463–1469. [DOI] [PubMed] [Google Scholar]
- 3.Chen S, Feng H, Sherchan P, et al. Controversies and evolving new mechanisms in subarachnoid hemorrhage. Prog Neurobiol 2014;115:64–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Killingsworth LM. Clinical applications of protein determinations in biological fluids other than blood. Clin Chem 1982;28(5):1093–1102. [PubMed] [Google Scholar]
- 5.Liotta EM, Prabhakaran S, Sangha RS, et al. Magnesium, hemostasis, and outcomes in patients with intracerebral hemorrhage. Neurology 2017;89(8):813–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seehusen DA, Reeves MM, Fomin DA. Cerebrospinal fluid analysis. Am Fam Physician 2003;68(6):1103–1108. [PubMed] [Google Scholar]
- 7.Banks JL, Marotta CA. Outcomes validity and reliability of the modified Rankin scale: implications for stroke clinical trials: a literature review and synthesis. Stroke 2007;38(3):10911096. [DOI] [PubMed] [Google Scholar]
- 8.Wilson JT, Hareendran A, Grant M, et al. Improving the assessment of outcomes in stroke: use of a structured interview to assign grades on the modified Rankin Scale. Stroke 2002;33(9):2243–2246. [DOI] [PubMed] [Google Scholar]
- 9.Young-Saver DF, Gornbein J, Starkman S, et al. Handling of Missing Outcome Data in Acute Stroke Trials: Advantages of Multiple Imputation Using Baseline and Postbaseline Variables. J Stroke Cerebrovasc Dis 2018;27(12):3662–3669. [DOI] [PubMed] [Google Scholar]
- 10.Barker FG 2nd, Ogilvy CS. Efficacy of prophylactic nimodipine for delayed ischemic deficit after subarachnoid hemorrhage: a metaanalysis. J Neurosurg 1996;84(3):405–414. [DOI] [PubMed] [Google Scholar]
- 11.Feigin VL, Rinkel GJ, Algra A, et al. Calcium antagonists in patients with aneurysmal subarachnoid hemorrhage: a systematic review. Neurology 1998;50(4):876–883. [DOI] [PubMed] [Google Scholar]
- 12.Dorhout Mees SM, Rinkel GJ, Feigin VL, et al. Calcium antagonists for aneurysmal subarachnoid haemorrhage. Cochrane Database Syst Rev 2007(3):CD000277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shen J, Pan JW, Fan ZX, et al. Dissociation of vasospasm-related morbidity and outcomes in patients with aneurysmal subarachnoid hemorrhage treated with clazosentan: a metaanalysis of randomized controlled trials. J Neurosurg 2013;119(1):180–189. [DOI] [PubMed] [Google Scholar]
- 14.Tam AK, Kapadia A, Ilodigwe D, et al. Impact of global cerebral atrophy on clinical outcome after subarachnoid hemorrhage. J Neurosurg 2013;119(1):198–206. [DOI] [PubMed] [Google Scholar]
- 15.Friedrich V, Flores R, Sehba FA. Cell death starts early after subarachnoid hemorrhage. Neurosci Lett 2012;512(1):6–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doczi T, Joo F, Adam G, et al. Blood-brain barrier damage during the acute stage of subarachnoid hemorrhage, as exemplified by a new animal model. Neurosurgery 1986;18(6):733739. [DOI] [PubMed] [Google Scholar]
- 17.Johshita H, Kassell NF, Sasaki T. Blood-brain barrier disturbance following subarachnoid hemorrhage in rabbits. Stroke 1990;21(7):1051–1058. [DOI] [PubMed] [Google Scholar]
- 18.Anzabi M, Ardalan M, Iversen NK, et al. Hippocampal Atrophy Following Subarachnoid Hemorrhage Correlates with Disruption of Astrocyte Morphology and Capillary Coverage by AQP4. Front Cell Neurosci 2018;12:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ivanidze J, Kesavabhotla K, Kallas ON, et al. Evaluating blood-brain barrier permeability in delayed cerebral infarction after aneurysmal subarachnoid hemorrhage. AJNR Am J Neuroradiol 2015;36(5):850–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ivanidze J, Ferraro RA, Giambrone AE, et al. Blood-Brain Barrier Permeability in Aneurysmal Subarachnoid Hemorrhage: Correlation With Clinical Outcomes. AJR Am J Roentgenol 2018;211(4):891–895. [DOI] [PubMed] [Google Scholar]
- 21.Russin JJ, Montagne A, D’Amore F, et al. Permeability imaging as a predictor of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. J Cereb Blood Flow Metab 2018;38(6):973–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mathiesen T, Edner G, Ulfarsson E, et al. Cerebrospinal fluid interleukin-1 receptor antagonist and tumor necrosis factor-alpha following subarachnoid hemorrhage. J Neurosurg 1997;87(2):215–220. [DOI] [PubMed] [Google Scholar]
- 23.Muroi C, Hugelshofer M, Seule M, et al. Correlation among systemic inflammatory parameter, occurrence of delayed neurological deficits, and outcome after aneurysmal subarachnoid hemorrhage. Neurosurgery 2013;72(3):367–375; discussion 375. [DOI] [PubMed] [Google Scholar]
- 24.Nakahara T, Tsuruta R, Kaneko T, et al. High-mobility group box 1 protein in CSF of patients with subarachnoid hemorrhage. Neurocrit Care 2009;11(3):362–368. [DOI] [PubMed] [Google Scholar]
- 25.Lucke-Wold BP, Logsdon AF, Manoranjan B, et al. Aneurysmal Subarachnoid Hemorrhage and Neuroinflammation: A Comprehensive Review. Int J Mol Sci 2016;17(4):497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sokol B, Wasik N, Wieckowska B, et al. Predicting mortality in subarachnoid haemorrhage based on first-week routine blood tests. J Clin Neurosci 2018. [DOI] [PubMed] [Google Scholar]
- 27.Cahill J, Calvert JW, Zhang JH. Mechanisms of early brain injury after subarachnoid hemorrhage. J Cereb Blood Flow Metab 2006;26(11):1341–1353. [DOI] [PubMed] [Google Scholar]
- 28.Kooijman E, Nijboer CH, van Velthoven CT, et al. Long-term functional consequences and ongoing cerebral inflammation after subarachnoid hemorrhage in the rat. PLoS One 2014;9(6):e90584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prunell GF, Svendgaard NA, Alkass K, et al. Inflammation in the brain after experimental subarachnoid hemorrhage. Neurosurgery 2005;56(5):1082–1092; discussion 10821092. [PubMed] [Google Scholar]