Abstract
There are no specific therapies for autosomal dominant polycystic kidney disease (ADPKD), and clinical data evaluating the effects of non-specific therapies on ADPKD patients are scarce. We therefore evaluated those effects using data from a longitudinal health insurance database collected from 2000-2010. We individually selected patients with and without ADPKD from inpatient data files as well as from the catastrophic illness registry in Taiwan based on 1:5 frequency matching for sex, age, and index year. The hazard ratios (HR) of all-cause mortality, ischemic stroke, hemorrhagic stroke and end-stage renal disease (ESRD) in ADPKD inpatients were elevated as compared to the controls. Similarly, ADPKD patients from the catastrophic illness registry had an increased risk of hemorrhagic stroke and ESRD. Allopurinol users also had an increased risk of all-cause mortality. The HR for developing ESRD after medication exposure was 0.47-fold for statin and 1.93-fold for pentoxifylline. These results reveal that patients with ADPKD (either inpatient or from the catastrophic illness registry) are at elevated risk for hemorrhagic stroke and ESRD, and suggest that allopurinol and pentoxifylline should not be prescribed to ADPKD patients due to possible adverse effects.
Keywords: autosomal dominant polycystic kidney disease, hemorrhagic stroke, end-stage renal disease, all-cause mortality, time-dependent Cox proportional hazard regression
INTRODUCTION
Autosomal dominant polycystic kidney disease (ADPKD) is the most common hereditary chronic kidney disease (CKD) and can cause end-stage renal disease (ESRD), which is associated with cardiovascular morbidity and mortality [1]. ADPKD patients have a 1.6 to 3.2-fold higher risk of all-cause mortality than the general population, with cardiac-related deaths being the most common [2, 3]. There are no specific therapies for ADPKD; thus, clinical interventions focus on non-specific approaches to slow CKD progression [4–6].
ADPKD increases cardiovascular morbidity and mortality and its clinical management is more restricted than that for other forms of CKD. Inhibition of the renin-angiotensin-aldosterone system with an angiotensin converting enzyme inhibitor (ACEI) or an angiotensin II receptor blocker (ARB) delays CKD progression in patients with or without diabetes and stage 1–3 CKD, and also in patients without diabetes with stage 4 CKD [7–12]. In a small number of patients with ADPKD, statins improve renal blood flow and renal function [13]. Monotherapy with pentoxifylline decreases proteinuria excretion in patients with proteinuric diabetic and non-diabetic kidney disease [14, 15]. Previous studies reported associations between high serum uric acid levels and early-onset hypertension, large kidney volume, and increased risk of ESRD [16] or progression of renal dysfunction in ADPKD patients [17].
In our retrospective cohort study here, we used a nationwide inpatient database to investigate the clinical burden of ADPKD patients, including all-cause mortality, ischemic stroke, hemorrhagic stroke, and ESRD. We also conducted a sensitivity analysis to compare our results with the catastrophic illness registry. Finally, our study is the first to evaluate the effects of non-specific medications (ACEI, ARB, statins, pentoxifylline and allopurinol) on clinical burden risk for ADPKD patients.
RESULTS
Inpatient database
We analyzed data from 4342 ADPKD patients and performed 21710 comparisons. The mean age of the study population was 58.1 years (standard deviation=17.2). There were a few more men in the ADPKD group than women (55.2% vs. 44.8%) (Table 1). Compared to controls, more patients with ADPKD lived in provinces and urban villages, more lived in southern and eastern Taiwan, and more had comorbidities (all p values < 0.05). During the study period of 2000-2010, the ADPKD group had higher incidence of all-cause mortality (26.32 vs. 10.28 person-years), ischemic stroke (15.39 vs. 8.96 per 1000 person-years), hemorrhagic stroke (4.95 vs. 1.70 per 1000 person-years), and ESRD (66.21 vs. 1.18 per 1000 person-years) compared to controls (Table 2). After adjusting for age, sex, geographical area of residence and all comorbidities, the hazard ratios (HRs) of all-cause mortality, ischemic stroke, hemorrhagic stroke and ESRD in the ADPKD group were 2.47, 1.56, 3.19, and 33.1, respectively, compared to controls [corresponding to 95% confidence intervals (CIs) of 2.19–2.78, 1.35–1.81, 2.41–4.22, and 27.6–39.8, respectively].
Table 1. Demographic and clinical characteristics of patients with autosomal dominant polycystic kidney disease (ADPKD) and controls.
| Characteristic | Inpatient patients | P-value | Catastrophic illness patients | P-value | |||
| No. (%) of individuals | No. (%) of individuals | ||||||
| With ADPKD (N=4342) | Control (N=21710) | With ADPKD (N=651) | Control (N=3255) | ||||
| Gender | 0.99 | 0.99 | |||||
| Female | 1946 (44.8) | 9730 (44.8) | 336 (51.6) | 1680 (51.6) | |||
| Male | 2396 (55.2) | 11980 (55.2) | 315 (48.4) | 1575 (48.4) | |||
| Age Group | 0.99 | 0.99 | |||||
| 20-39 | 696 (16.0) | 3480 (16.0) | 190 (29.2) | 950 (29.2) | |||
| 40-59 | 1673 (38.5) | 8365 (38.5) | 350 (53.8) | 1750 (53.8) | |||
| ≥60+ | 1973 (45.5) | 9865 (45.5) | 111 (17.1) | 555 (17.1) | |||
| Mean (SD) | 58.1 (17.2) | 58.0 (17.2) | 0.64 | 47.3 (12.8) | 47.2 (12.9) | 0.86 | |
| Urbanization | 0.0002 | 0.02 | |||||
| Provinces | 1175 (27.1) | 6105 (28.1) | 218 (33.5) | 1042 (32.0) | |||
| Counties | 1399 (32.2) | 6334 (29.2) | 234 (35.9) | 1020 (31.3) | |||
| Districts | 716 (16.5) | 3519 (16.2) | 86 (13.2) | 551 (16.9) | |||
| Urban villages | 1052 (24.2) | 5752 (26.5) | 113 (17.4) | 642 (19.7) | |||
| Geography | 0.003 | <0.0001 | |||||
| North | 1936 (44.6) | 9545 (44.0) | 312 (47.9) | 1553 (47.7) | |||
| Central | 818 (18.8) | 4371 (20.1) | 89 (13.7) | 629 (19.3) | |||
| South | 1298 (29.9) | 6621 (30.5) | 181 (27.8) | 916 (28.1) | |||
| East | 290 (6.68) | 1173 (5.40) | 69 (10.6) | 157 (4.82) | |||
| Income (NTD) | 0.76 | 0.19 | |||||
| <18000 | 1912 (44.0) | 9637 (44.4) | 243 (34.3) | 1339 (41.1) | |||
| 18000-34999 | 1933 (44.5) | 9539 (43.9) | 311 (47.8) | 1468 (45.1) | |||
| ≥35000 | 497 (11.5) | 2534 (11.7) | 97 (14.9) | 448 (13.8) | |||
| Comorbidity | |||||||
| Diabetes | 489 (11.3) | 1114 (5.13) | <0.0001 | 63 (9.69) | 292 (8.97) | 0.57 | |
| Hypertension | 1969 (45.4) | 1999 (9.21) | <0.0001 | 461 (70.8) | 635 (19.5) | <0.0001 | |
| Hyperlipidemia | 233 (5.37) | 393 (1.81) | <0.0001 | 180 (27.7) | 464 (14.3) | <0.0001 | |
| CAD | 548 (12.6) | 1041 (4.80) | <0.0001 | 87 (13.4) | 258 (7.93) | <0.0001 | |
| Atrial fibrillation | 119 (2.74) | 184 (0.85) | <0.0001 | 6 (0.92) | 15 (0.46) | 0.14 | |
| Congestive heart failure | 260 (5.99) | 330 (1.52) | <0.0001 | 22 (3.38) | 36 (1.11) | <0.0001 | |
| Obesity | 5 (0.12) | 4 (0.02) | 0.009 | 0 (0.00) | 4 (0.12) | 0.99 | |
| Gouty arthritis | 247 (5.69) | 235 (1.08) | <0.0001 | 74 (11.4) | 107 (3.29) | <0.0001 | |
| Medications | |||||||
| ACE-Is | 207 (31.8) | 331 (10.2) | <0.0001 | ||||
| ARBs | 392 (60.2) | 405 (12.4) | <0.0001 | ||||
| Statins | 159 (24.4) | 393 (12.1) | <0.0001 | ||||
| Allopurinol | 71 (10.9) | 48 (1.47) | <0.0001 | ||||
| Pentoxifylline | 113 (17.4) | 92 (2.83) | <0.0001 | ||||
Chi-square test and t-test; CAD, coronary artery disease; SD, standard deviation; Medicine users were defined during the study period
Table 2. Risk of various outcomes in ADPKD patients compared to controls.
| Variable | Inpatient database1 | Catastrophic illness patients2 | ||
| With ADPKD | Control | With ADPKD | Control | |
| N | 4310 | 21550 | 651 | 3255 |
| Person-years | 16373 | 121626 | 2540 | 14774 |
| All-cause mortality | ||||
| Event no | 431 | 1250 | 15 | 53 |
| Incidence density | 26.32 | 10.28 | 5.90 | 3.59 |
| Crude HR (95% CI) | 2.54 (2.27-2.83)*** | Ref. | 1.78 (0.99-3.17) | Ref. |
| Adjusted HR (95% CI) | 2.47 (2.19-2.78)*** | Ref. | 1.71 (0.84-3.48) | Ref. |
| Ischemic stroke | ||||
| Event no | 252 | 1090 | 7 | 49 |
| Incidence density | 15.39 | 8.96 | 2.76 | 3.32 |
| Crude HR (95% CI) | 1.69 (1.47-1.94)*** | Ref. | 0.85 (0.38-1.87) | Ref. |
| Adjusted HR (95% CI) | 1.56 (1.35-1.81)*** | Ref. | 0.49 (0.20-1.22) | Ref. |
| Hemorrhagic stroke | ||||
| Event no | 81 | 207 | 14 | 8 |
| Incidence density | 4.95 | 1.70 | 5.51 | 0.54 |
| Crude HR (95% CI) | 2.87 (2.22-3.72)*** | Ref. | 9.83 (4.12-23.5)*** | Ref. |
| Adjusted HR (95% CI) | 3.19 (2.41-4.22)*** | Ref. | 4.41 (1.41-13.8)* | Ref. |
| ESRD | ||||
| Event no | 1084 | 144 | 116 | 5 |
| Incidence density | 66.21 | 1.18 | 45.66 | 0.34 |
| Crude HR (95% CI) | 49.1 (41.3-58.5)*** | Ref. | 132 (54.0-324)*** | Ref. |
| Adjusted HR (95% CI) | 33.1 (27.6-39.8)*** | Ref. | 56.4 (21.4-146)*** | Ref. |
1Adjusted for age, gender, geography, and all comorbidity using extended Cox proportional hazard regression.
2Adjusted for age, gender, geography, all comorbidity, and medications using Cox proportional hazard regression.
* p<0.05, *** p<0.001.
Longitudinal health insurance database for catastrophic illness patients (LHID-CIP)
In total, data from 651 patients with ADPKD and 3255 age- and sex-matched controls were analyzed. Among the ADPKD group, there were female patients more than males (51.6% vs. 48.4%, respectively), and the mean age was 47.3 years (standard deviation=12.8) (Table 1).
Compared to controls, more than 75% of patients with ADPKD lived in northern and southern Taiwan, had more comorbidities, including hypertension, hyperlipidemia, coronary artery disease (CAD), congestive heart failure, and gouty arthritis. In addition, ADPKD patients were prescribed various medications such as ACEIs, ARBs, statins, allopurinol, and pentoxifylline.
During the study period, the ADPKD group had a 1.78-fold higher incidence of all-cause mortality (5.90 vs. 3.59 person-years), 0.85-fold higher incidence of ischemic stroke (2.76 vs. 3.32 per 1000 person-years), 9.83-fold higher incidence of hemorrhagic stroke (5.51 vs. 0.54 per 1000 person-years), and 132-fold higher incidence of ESRD (45.66 vs. 0.34 per 1000 person-years) compared to the controls (Table 2). After adjusting for age, sex, geographical area of residence, all comorbidities, and all medications, the ADPKD group had increased risk of hemorrhagic stroke and ESRD (HR=4.41 and 56.4, 95% CI, 1.41−13.8 and 21.4−146, respectively).
Table 3 shows the effects of medications on each outcome in the ADPKD group. ADPKD patients receiving allopurinol treatment had a 6.44-fold higher risk of all-cause mortality than no-treatment controls (95% CI, 1.72−24.2). In addition, ADPKD patients receiving pentoxifylline treatment had an increased risk of ESRD (HR = 1.93, 95% CI, 1.20−3.09), whereas those who received statin treatment had a lower risk of ESRD (HR = 0.47, 95% CI, 0.27−0.85). There were no medication effects on ischemic or hemorrhagic stroke.
Table 3. Association between clinical medications and ADPKD patient outcomes using time-dependent Cox proportional hazard regression.
| Medication (yes vs. no) | All-cause mortality | Ischemic stroke | Hemorrhagic stroke | ESRD | ||||
| Crude | Adjusted | Crude | Adjusted | Crude | Adjusted | Crude | Adjusted | |
| ACE-Is | 3.44 (1.19-9.96)* | 2.35 (0.69-8.07) | 14.5 (1.65-126)* | 9.50 (0.92-97.9) | 2.26 (0.76-6.73) | 1.88 (0.57-6.18) | 1.49 (1.01-2.20)* | 1.29 (0.86-1.94) |
| ARBs | 0.78 (0.28-2.18) | 0.70 (0.20-2.50) | 0.66 (0.14-3.04) | 0.48 (0.08-2.74) | 1.23 (0.42-3.63) | 1.05 (0.32-3.52) | 1.72 (1.18-2.53)** | 1.26 (0.84-1.90) |
| Statins | 1.08 (0.30-3.87) | 0.76 (0.18-3.32) | 1.64 (0.30-8.91) | 1.93 (0.26-14.2) | 1.72 (0.52-5.66) | 1.04 (0.26-4.25) | 0.73 (0.43-1.24) | 0.47 (0.27-0.85)* |
| Allopurinol | 10.2 (3.55-29.3)*** | 6.44 (1.72-24.2)** | NA | NA | 2.42 (0.53-11.0) | 1.45 (0.26-8.09) | 1.72 (0.92-3.21) | 1.12 (0.56-2.24) |
| Pentoxifylline | 3.41 (1.15-10.1)* | 2.97 (0.75-11.8) | 1.03 (0.12-8.92) | 2.00 (0.17-23.6) | 3.86 (1.25-11.9)* | 3.27 (0.94-11.4) | 2.33 (1.51-3.61)*** | 1.93 (1.20-3.09)** |
Adjusted for age, gender, geography, all comorbidity, and medications.
* p<0.05, ** p <0.01, *** p<0.001
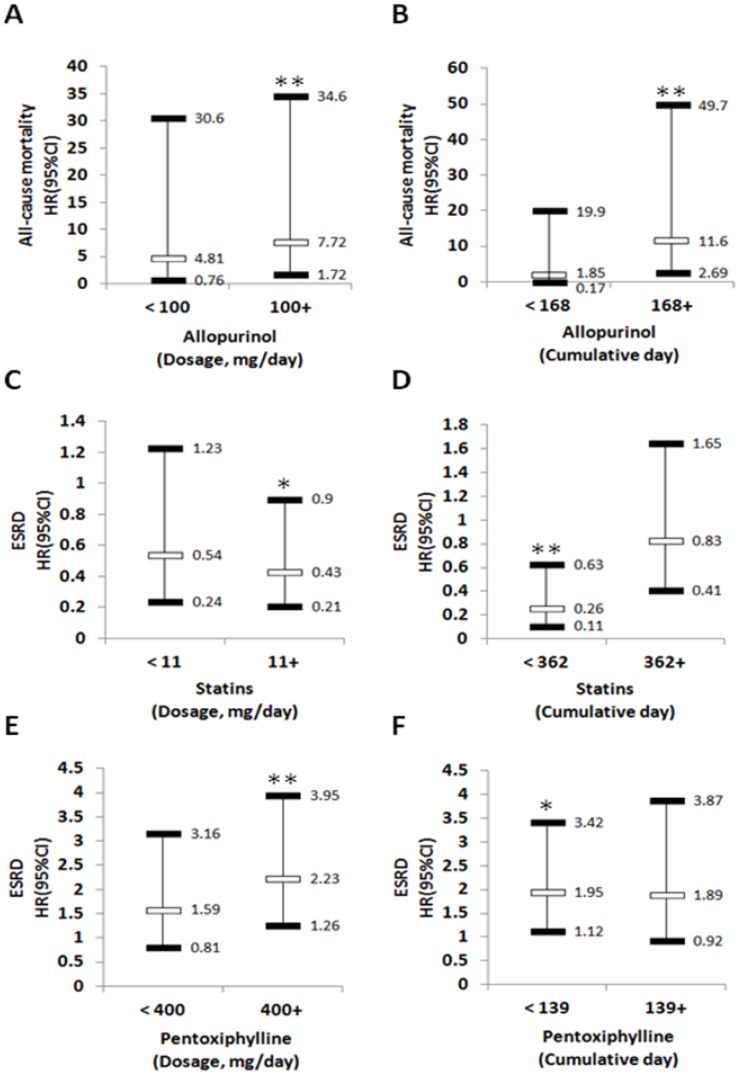
Multivariable-adjusted time-dependent Cox proportional hazard regression showed that allopurinol doses ≥100 mg/day were associated with 7.72 (95% CI, 1.72−34.6) hazards of all-cause mortality compared with allopurinol non-users (Figure 1A). Longer allopurinol use duration was associated with higher hazard of all-cause mortality (HR = 11.6, 95% CI, 2.69–49.7; P<0.01) compared with allopurinol non-users (Figure 1B). Compared with statin non-users, higher statin doses of ≥ 11 mg/day (HR = 0.43, 95% CI, 0.21–0.9) or shorter statin use duration (HR = 0.26, 95% CI, 0.11–0.63) were associated with lower hazard of incident ESRD (Figure 1C and 1D). In this analysis, we also found that, compared with pentoxifylline non-users, higher doses of pentoxifylline ≥400 mg/day (HR = 2.23, 95% CI, 1.26–3.95) or shorter use duration of pentoxifylline (HR = 1.95, 95% CI, 1.12–3.42) were associated with decreased risk of ESRD (Figure 1E and 1F).
Figure 1.
The association of medication dose and duration with various outcomes. Risk of all-cause mortality according to dosage (A) or cumulative (B) of Allopurinol use. Risk of ESRD according to dosage (C) or cumulative (D) of Statins use. Risk of ESRD according to dosage (E) or cumulative (F) of Pentoxiphylline use. Adjusted for age, gender, geography, all comorbidity and medications. ESRD: end-stage renal disease; HR: hazard ratio; CI: Confidence interval. * p<0.05, ** p <0.01.
DISCUSSION
To the best of our knowledge, the present study is the first nationwide population-based cohort study to compare the incidence rates of all-cause mortality, ischemic stroke, hemorrhagic stroke, and ESRD in patients with ADPKD with matched controls from the general population. More specifically, we used multivariate regression analysis to retrospectively compare the development of the above outcomes between patients with ADPKD and controls without ADPKD. Our results indicated that both inpatients and patients with ADPKD had increased risks of hemorrhagic stroke and ESRD. In addition, the use of statins correlated with a reduced risk of ESRD. However, the use of allopurinol and pentoxifylline were associated with an increased risk of all-cause mortality and ESRD, respectively.
In our analysis, the incidence of hemorrhagic stroke was higher in the patients with ADPKD compared to controls, with an adjusted HR of 3.19 for the inpatients and 4.41 for those with a catastrophic illness. An underlying defect in the connective tissue matrix has been associated with intracranial aneurysms, hepatic cysts, diverticulosis, spontaneous coronary artery dissection, and other cardiovascular abnormalities in patients with ADPKD [18–20]. The prevalence of intracranial aneurysm in patients with ADPKD is also higher than that among the general population (9–12% vs. 2–3%), and aneurysm rupture causing subarachnoid or intracerebral hemorrhage is among the most serious complications of ADPKD [18–20]. However, little is known about the incidence of hemorrhagic stroke in patients with ADPKD compared to those without ADPKD. Thus, further studies on therapies to prevent aneurysm rupture and reduce the risk of hemorrhagic stroke may improve outcomes during the transition from CKD to ESRD in ADPKD patients.
ADPKD patients had an increased incidence of ESRD in our study. ADPKD differs from most other causes of ESRD because it can be detected early in life, and approximately 50% of affected individuals experience advanced kidney failure by 60 years of age [21]. Thus, a multidrug approach might benefit ADPKD patients, not only to slow CKD progression, but also to decrease cardiovascular complications, the major cause of morbidity and mortality in these patients [22]. Aggressive blood pressure control with an ACEI or ARB forms the basis of this therapy. Since there is currently no clinically approved specific therapy for ADPKD [23], current consensus guidelines [24] recommend managing hypertension, decline in renal function and renal complications to prevent the progression of ADPKD to ESRD. Our analysis concentrated on cardiorenal protection as a whole and found that the use of statins might moderately reduce the incidence of ESRD in patients with ADPKD without increasing the risk of death. Improvements in endothelial dysfunction in the setting of simvastatin treatment for ADPKD were demonstrated in a prospective cohort study of 16 patients [25], although no direct clinical endpoint was apparent. A pediatric study of 110 participants treated with lisinopril and randomized to receive a placebo or pravastatin for three years demonstrated benefits in height-corrected total kidney volume expansion associated with randomization to pravastatin [26]. In contrast, a small randomized open-label study of 49 patients with ADPKD who received pravastatin therapy for two years showed no effect on renal dysfunction [27]. Further research is needed to investigate the mechanisms underlying the effects of statins on ADPKD.
A 6.44-fold increased risk of mortality was associated with the use of allopurinol in our ADPKD cohort with gouty arthritis. In patients with ADPKD and CKD, serum uric acid may be an independent factor for renal progression [28], and it has been associated with the earlier onset of larger kidney volume, hypertension, and increased risk of ESRD [29–31]. Recent epidemiologic studies have shown a relationship between uric acid level and progression of kidney disease [16, 32]; however, to the best of our knowledge, no data have been published characterizing the effect of allopurinol on the long-term progression of CKD in patients with ADPKD. While several previous studies evaluated the influence of allopurinol treatment on all-cause mortality, they reported conflicting results [33–35]. Yang et al. found that CKD was strongly associated with allopurinol hypersensitivity (OR = 1.49, 95% CI, 1.38−1.61; P < 0.001) and all-cause mortality (OR = 2.20, 95% CI, 1.69−2.87; P < 0.001) in Taiwan [36]. Additionally, older age, CKD and cardiovascular disease are non-genetic risk factors for allopurinol hypersensitivity and hypersensitivity-related mortality [37, 38]. Our findings here showed a consistent effect for allopurinol use on all-cause mortality in ADPKD patients. However, studies with a longer observation period are needed to confirm our findings.
Pentoxifylline is used to treat peripheral vascular disease and microcirculatory disorders. In addition to its hemorrheologic activity, pentoxifylline provides antiproliferative and anti-inflammatory effects that are organoprotective, reduces proteinuria in patients with diabetic and non-diabetic proteinuric kidney disease [39, 40], and improves renal function [41, 42]. However, our analysis here showed that pentoxifylline increased the risk of ESRD for ADPKD patients.
There are several limitations to the present study. First, ADPKD patients applying for catastrophic illness certificates were selected on the basis of their first claim date; therefore, there may be a time delay. However, as the NHIRD is a deidentified secondary database, it is difficult to determine whether or not underestimation occurred. Second, data on personal habits such as lifestyle, smoking, alcohol use, body weight, and disease severity are not available in the NHIRD. These factors are also important and could influence both propensity for active gout and other outcomes. Third, we did not have imaging data for ADPKD patients, which might have been potential confounders. Fourth, a prospective randomized control clinical trial would be the gold standard to compare outcomes. Thus, this study did not demonstrate the causal link (but only concomitance) between allopurinol use and mortality. The same applies to pentoxifylline use and the risk of ESRD. Fifth, the causal link between allopurinol and mortality as well between pentoxifylline and ESRD cannot be demonstrated in this study. Finally, diagnosis bias and loss to follow-up are inherent in cohort studies [43]. In this study, the cohorts were based on the Registry for Catastrophic Illness Patient Database and in-hospital patients, with well-established diagnoses for ADPKD and ESRD [44], which helps avoid diagnostic bias.
In conclusion, to the best of our knowledge, this is the first report of an association between ADPKD and the risk of hemorrhagic stroke and ESRD with matched controls from the general population. CKD increases mortality, and CKD is accompanied by hyperuricaemia which is treated by allopurinol; similarly, it is proteinuria that increases the risk of ESRD, but not the treatment of proteinuria with pentoxifylline. The risk of developing ESRD after medication exposure was reduced for statins and increased for pentoxifylline. In addition, the use of allopurinol was associated with an increased risk of all-cause mortality in ADPKD patients. Health care professionals should be aware of this risk when treating ADPKD patients.
MATERIALS AND METHODS
Data source
We used the inpatient database and longitudinal health insurance database for catastrophic illness patients from the National Health Insurance Research Database (NHIRD) in Taiwan. The NHIRD was established by the Taiwan National Health Insurance Administration Ministry of Health and Welfare based on the National Health Insurance program in Taiwan, and it is maintained by the Taiwan National Health Research Institutes. The NHI program covers over 99% of the population in Taiwan. The inpatient database included all hospital claims from 1996 to 2011, and the LHID-CIP included all inpatient claims, outpatient claims, and medical treatment of patients with catastrophic illnesses from 1997 to 2011. Twenty-nine diseases are classified as being catastrophic illnesses in Taiwan, including ESRD, ADPKD, and organ transplant. Patients are diagnosed as having a catastrophic illness according to guidelines from the Bureau of NHI. These patients can then apply for a catastrophic illness card, which has to be formally reviewed by specialist physicians, and are then exempt from copayments. In the NHIRD, all diseases are defined according to the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). Medications are defined based on the Anatomical Therapeutic Chemical (ATC) classification system, which was established by the World Health Organization Collaborating Centre for Drug Statistics Methodology (http://www.whocc.no). All patient data are deidentified and encrypted before being released for research purposes. This retrospective cohort study was approved by the Research Ethics Committee of China Medical University Hospital (IRB CMUH104-REC2-115(CR-4)).
Study subjects
Inpatient database
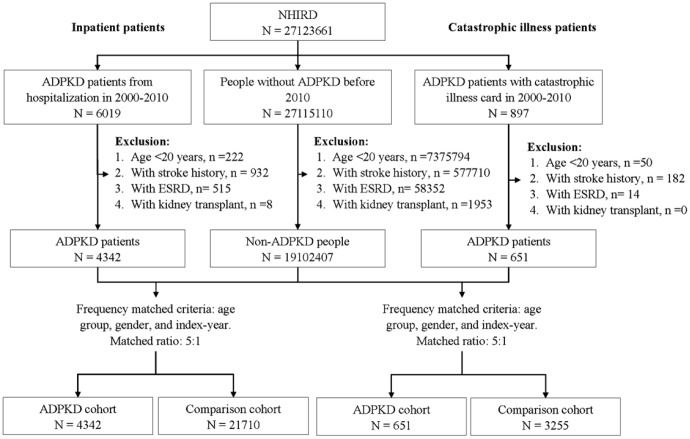
Data for patients with admission for ADPKD (ICD-9-CM 753.12, and 753.13) from 2000-2010 were collected as the ADPKD cohort (n = 6019). The date of this admission was defined as the index date. The exclusion criteria were: 1) age < 20 years (n = 222); 2) a history of stroke (n = 932); 3) ESRD (n = 515); and 4) kidney transplant (n = 8). Dara from patients without ADPKD or any of the exclusion criteria were used as controls, and were randomly assigned with an index date as per the ADPKD cohort. Five controls were randomly selected for each patient and frequency matched by age group (20-39, 40-59 and ≥60 years), sex, and index year.
LHID-CIP
Data for 897 patients with catastrophic illness cards for ADPKD from 2000-2010 were used as the ADPKD cohort. The date of application for a catastrophic illness card was defined as the index date. The exclusion criteria were: 1) age < 20 years (n = 50); 2) a history of stroke (n = 182); 3) ESRD (n = 14); and 4) kidney transplant (n = 0). Data from patients without a catastrophic illness card for ADPKD or any of the exclusion criteria were used as controls. They were randomly assigned with an index date as the ADPKD cohort. Five controls were randomly selected for each patient and frequency matched by age group (20-39, 40-59 and ≥60 years), sex, and index-year (Figure 2).
Figure 2.
Flow chart of study recruitment.
Outcome and covariate
All of the subjects were followed from the index date until a study outcome of death, stroke [including ischemic stroke (ICD-9-CM 433-438), and hemorrhagic stroke (ICD-9-CM 430-432)], or the development of ESRD (ICD-9-CM 585), whichever occurred first. To improve accuracy, outcome diagnoses were defined from catastrophic illness files. The detailed recruitment procedure is shown in Figure 2. Covariates included demographics, baseline comorbidities, and medications, and demographic data included sex, age, urbanization, geographical area of residence, and income. Comorbidities (ICD-9-CM) included diabetes (250), hypertension (401-405), hyperlipidemia (272), coronary artery disease (CAD: 410-414), atrial fibrillation (427.31), congestive heart failure (428), obesity (278.0), and gouty arthritis (274). Medications (ATC) included ACEIs (C09AA, C09BA, and C09BB), ARBs (C09CA, C09DA, C09DB, and C09DX), statins (C10AA), allopurinol (M04AA01), and pentoxifylline (C04AD03). Patients who were prescribed medications during the study period were defined as users.
Statistical analysis
SAS software (version 9.4 for Windows; SAS Institute Inc., Cary, NC, USA) was used for all analyses. A two-tailed P value of < 0.05 was considered to be statistically significant. Differences in categorical variables between patients with and without ADPKD were tested using the chi-square test, and continuous variables were compared using the t-test. All-cause mortality, and the incidence rates of ischemic stroke, hemorrhagic stroke, and ESRD were calculated in the ADPKD and comparison cohorts. HR and 95% CIs of different outcomes were evaluated using a Cox proportional hazard model. The multivariate model was adjusted for other potential covariates. The assumption of the Cox proportional hazard model was certified through testing the scaled Schoenfeld residuals. The results of testing in the LHID-CIP did not violate the assumption of each outcome. However, in the inpatient database, there was an association between Schoenfeld residuals for ADPKD and follow-up time for each outcome. Therefore, we used an extended Cox regression model to estimate the risk of different outcomes between the patients with ADPKD in the inpatient database. The effect of medications was assessed only in patients with ADPKD using time-dependent Cox proportional hazard regression analysis.
ACKNOWLEDGMENTS
This study was based in part on data from the National Health Insurance Research Database provided by the Bureau of National Health Insurance (Department of Health, Taiwan), which is managed by the National Health Research Institutes. The interpretations and conclusions contained herein do not represent those of the Bureau of National Health Insurance, Department of Health, or the National Health Research Institutes. The authors would also like to acknowledge the support of Dr. Tsung-Hsien Chen in the preparation of this manuscript.
Abbreviations
- ACEI
angiotensin converting enzyme inhibitor
- ADPKD
autosomal dominant polycystic kidney disease
- ARB
angiotensin II receptor blocker
- CIs
confidence intervals
- CKD
chronic kidney disease
- ESRD
end-stage renal disease
- HR
hazard ratios
- LHID-CIP
longitudinal health insurance database for catastrophic illness patients
- NHI
National Health Insurance
- NHIRD
National Health Insurance Research Database
Footnotes
AUTHOR CONTRIBUTIONS: PHH, CHL, MCC, CHC and KYH designed the research; CHM and CJC analyzed the data; PHH, CHM, CJC and CHL wrote the first draft of the paper. All authors participated in the revision of the paper.
CONFLICTS OF INTEREST: The authors declare that they have no conflicts of interests.
FUNDING: This study is supported in part by Taiwan Ministry of Health and Welfare Clinical Trial Center (MOHW108-TDU-B-212-133004), China Medical University Hospital, Academia Sinica Stroke Biosignature Project (BM10701010021), MOST Clinical Trial Consortium for Stroke (MOST 108-2321-B-039-003-), Tseng-Lien Lin Foundation, Taichung, Taiwan, and Katsuzo and Kiyo Aoshima Memorial Funds, Japan.
REFERENCES
- 1.Torres VE, Harris PC, Pirson Y. Autosomal dominant polycystic kidney disease. Lancet. 2007; 369:1287–301. 10.1016/S0140-6736(07)60601-1 [DOI] [PubMed] [Google Scholar]
- 2.Fick GM, Johnson AM, Hammond WS, Gabow PA. Causes of death in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 1995; 5:2048–56. [DOI] [PubMed] [Google Scholar]
- 3.Florijn KW, Noteboom WM, van Saase JL, Chang PC, Breuning MH, Vandenbroucke JP. A century of mortality in five large families with polycystic kidney disease. Am J Kidney Dis. 1995; 25:370–74. 10.1016/0272-6386(95)90096-9 [DOI] [PubMed] [Google Scholar]
- 4.Schrier R, McFann K, Johnson A, Chapman A, Edelstein C, Brosnahan G, Ecder T, Tison L. Cardiac and renal effects of standard versus rigorous blood pressure control in autosomal-dominant polycystic kidney disease: results of a seven-year prospective randomized study. J Am Soc Nephrol. 2002; 13:1733–39. 10.1097/01.ASN.0000018407.60002.B9 [DOI] [PubMed] [Google Scholar]
- 5.van Dijk MA, Breuning MH, Duiser R, van Es LA, Westendorp RG. No effect of enalapril on progression in autosomal dominant polycystic kidney disease. Nephrol Dial Transplant. 2003; 18:2314–20. 10.1093/ndt/gfg417 [DOI] [PubMed] [Google Scholar]
- 6.Meijer E, de Jong PE, Peters DJ, Gansevoort RT. Better understanding of ADPKD results in potential new treatment options: ready for the cure? J Nephrol. 2008; 21:133–38. [PubMed] [Google Scholar]
- 7.Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, Remuzzi G, Snapinn SM, Zhang Z, Shahinfar S, and RENAAL Study Investigators. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001; 345:861–69. 10.1056/NEJMoa011161 [DOI] [PubMed] [Google Scholar]
- 8.Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, Lewis JB, Ritz E, Atkins RC, Rohde R, Raz I, and Collaborative Study Group. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001; 345:851–60. 10.1056/NEJMoa011303 [DOI] [PubMed] [Google Scholar]
- 9.Ruggenenti P, Perna A, Gherardi G, Garini G, Zoccali C, Salvadori M, Scolari F, Schena FP, Remuzzi G. Renoprotective properties of ACE-inhibition in non-diabetic nephropathies with non-nephrotic proteinuria. Lancet. 1999; 354:359–64. 10.1016/S0140-6736(98)10363-X [DOI] [PubMed] [Google Scholar]
- 10.The GISEN Group (Gruppo Italiano di Studi Epidemiologici in Nefrologia). Randomised placebo-controlled trial of effect of ramipril on decline in glomerular filtration rate and risk of terminal renal failure in proteinuric, non-diabetic nephropathy. Lancet. 1997; 349:1857–63. 10.1016/S0140-6736(96)11445-8 [DOI] [PubMed] [Google Scholar]
- 11.Hou FF, Zhang X, Zhang GH, Xie D, Chen PY, Zhang WR, Jiang JP, Liang M, Wang GB, Liu ZR, Geng RW. Efficacy and safety of benazepril for advanced chronic renal insufficiency. N Engl J Med. 2006; 354:131–40. 10.1056/NEJMoa053107 [DOI] [PubMed] [Google Scholar]
- 12.Hou FF, Xie D, Zhang X, Chen PY, Zhang WR, Liang M, Guo ZJ, Jiang JP. Renoprotection of Optimal Antiproteinuric Doses (ROAD) Study: a randomized controlled study of benazepril and losartan in chronic renal insufficiency. J Am Soc Nephrol. 2007; 18:1889–98. 10.1681/ASN.2006121372 [DOI] [PubMed] [Google Scholar]
- 13.van Dijk MA, Kamper AM, van Veen S, Souverijn JH, Blauw GJ. Effect of simvastatin on renal function in autosomal dominant polycystic kidney disease. Nephrol Dial Transplant. 2001; 16:2152–57. 10.1093/ndt/16.11.2152 [DOI] [PubMed] [Google Scholar]
- 14.Navarro JF, Mora C, Rivero A, Gallego E, Chahin J, Macía M, Méndez ML, García J. Urinary protein excretion and serum tumor necrosis factor in diabetic patients with advanced renal failure: effects of pentoxifylline administration. Am J Kidney Dis. 1999; 33:458–63. 10.1016/S0272-6386(99)70182-4 [DOI] [PubMed] [Google Scholar]
- 15.Chen YM, Lin SL, Chiang WC, Wu KD, Tsai TJ. Pentoxifylline ameliorates proteinuria through suppression of renal monocyte chemoattractant protein-1 in patients with proteinuric primary glomerular diseases. Kidney Int. 2006; 69:1410–15. 10.1038/sj.ki.5000302 [DOI] [PubMed] [Google Scholar]
- 16.Helal I, McFann K, Reed B, Yan XD, Schrier RW, Fick-Brosnahan GM. Serum uric acid, kidney volume and progression in autosomal-dominant polycystic kidney disease. Nephrol Dial Transplant. 2013; 28:380–85. 10.1093/ndt/gfs417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Panizo N, Goicoechea M, García de Vinuesa S, Arroyo D, Yuste C, Rincón A, Verdalles U, Ruiz-Caro C, Quiroga B, Luño J. Chronic kidney disease progression in patients with autosomal dominant polycystic kidney disease. Nefrologia. 2012; 32:197–205. 10.3265/Nefrologia.pre2011.Dec.11177 [DOI] [PubMed] [Google Scholar]
- 18.Irazabal MV, Huston J 3rd, Kubly V, Rossetti S, Sundsbak JL, Hogan MC, Harris PC, Brown RD Jr, Torres VE. Extended follow-up of unruptured intracranial aneurysms detected by presymptomatic screening in patients with autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol. 2011; 6:1274–85. 10.2215/CJN.09731110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Belz MM, Fick-Brosnahan GM, Hughes RL, Rubinstein D, Chapman AB, Johnson AM, McFann KK, Kaehny WD, Gabow PA. Recurrence of intracranial aneurysms in autosomal-dominant polycystic kidney disease. Kidney Int. 2003; 63:1824–30. 10.1046/j.1523-1755.2003.00918.x [DOI] [PubMed] [Google Scholar]
- 20.Perrone RD, Malek AM, Watnick T. Vascular complications in autosomal dominant polycystic kidney disease. Nat Rev Nephrol. 2015; 11:589–98. 10.1038/nrneph.2015.128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Torres VE. Treatment strategies and clinical trial design in ADPKD. Adv Chronic Kidney Dis. 2010; 17:190–204. 10.1053/j.ackd.2010.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ecder T. Cardiovascular complications in autosomal dominant polycystic kidney disease. Curr Hypertens Rev. 2013; 9:2–11. 10.2174/1573402111309010002 [DOI] [PubMed] [Google Scholar]
- 23.Mochizuki T, Tsuchiya K, Nitta K. Autosomal dominant polycystic kidney disease: recent advances in pathogenesis and potential therapies. Clin Exp Nephrol. 2013; 17:317–26. 10.1007/s10157-012-0741-0 [DOI] [PubMed] [Google Scholar]
- 24.Chapman AB, Devuyst O, Eckardt KU, Gansevoort RT, Harris T, Horie S, Kasiske BL, Odland D, Pei Y, Perrone RD, Pirson Y, Schrier RW, Torra R, et al. , and Conference Participants. Autosomal-dominant polycystic kidney disease (ADPKD): executive summary from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2015; 88:17–27. 10.1038/ki.2015.59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Namli S, Oflaz H, Turgut F, Alisir S, Tufan F, Ucar A, Mercanoglu F, Ecder T. Improvement of endothelial dysfunction with simvastatin in patients with autosomal dominant polycystic kidney disease. Ren Fail. 2007; 29:55–59. 10.1080/08860220601038892 [DOI] [PubMed] [Google Scholar]
- 26.Cadnapaphornchai MA, George DM, McFann K, Wang W, Gitomer B, Strain JD, Schrier RW. Effect of pravastatin on total kidney volume, left ventricular mass index, and microalbuminuria in pediatric autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol. 2014; 9:889–96. 10.2215/CJN.08350813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fassett RG, Coombes JS, Packham D, Fairley KF, Kincaid-Smith P. Effect of pravastatin on kidney function and urinary protein excretion in autosomal dominant polycystic kidney disease. Scand J Urol Nephrol. 2010; 44:56–61. 10.3109/00365590903359908 [DOI] [PubMed] [Google Scholar]
- 28.Han M, Park HC, Kim H, Jo HA, Huh H, Jang JY, Kang AY, Kim SH, Cheong HI, Kang DH, Yang J, Oh KH, Hwang YH, Ahn C. Hyperuricemia and deterioration of renal function in autosomal dominant polycystic kidney disease. BMC Nephrol. 2014; 15:63. 10.1186/1471-2369-15-63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tomita M, Mizuno S, Yamanaka H, Hosoda Y, Sakuma K, Matuoka Y, Odaka M, Yamaguchi M, Yosida H, Morisawa H, Murayama T. Does hyperuricemia affect mortality? A prospective cohort study of Japanese male workers. J Epidemiol. 2000; 10:403–09. 10.2188/jea.10.403 [DOI] [PubMed] [Google Scholar]
- 30.Weiner DE, Tighiouart H, Elsayed EF, Griffith JL, Salem DN, Levey AS. Uric acid and incident kidney disease in the community. J Am Soc Nephrol. 2008; 19:1204–11. 10.1681/ASN.2007101075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bellomo G, Venanzi S, Verdura C, Saronio P, Esposito A, Timio M. Association of uric acid with change in kidney function in healthy normotensive individuals. Am J Kidney Dis. 2010; 56:264–72. 10.1053/j.ajkd.2010.01.019 [DOI] [PubMed] [Google Scholar]
- 32.Kocyigit I, Yilmaz MI, Orscelik O, Sipahioglu MH, Unal A, Eroglu E, Kalay N, Tokgoz B, Axelsson J, Oymak O. Serum uric acid levels and endothelial dysfunction in patients with autosomal dominant polycystic kidney disease. Nephron Clin Pract. 2013; 123:157–64. 10.1159/000353730 [DOI] [PubMed] [Google Scholar]
- 33.Málek F, Ošťádal P, Pařenica J, Jarkovský J, Vítovec J, Widimský P, Linhart A, Fedorco M, Coufal Z, Miklík R, Krűger A, Vondraková D, Špinar J. Uric acid, allopurinol therapy, and mortality in patients with acute heart failure—results of the Acute HEart FAilure Database registry. J Crit Care. 2012; 27:737.e11–24. 10.1016/j.jcrc.2012.03.011 [DOI] [PubMed] [Google Scholar]
- 34.Luk AJ, Levin GP, Moore EE, Zhou XH, Kestenbaum BR, Choi HK. Allopurinol and mortality in hyperuricaemic patients. Rheumatology (Oxford). 2009; 48:804–06. 10.1093/rheumatology/kep069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dubreuil M, Zhu Y, Zhang Y, Seeger JD, Lu N, Rho YH, Choi HK. Allopurinol initiation and all-cause mortality in the general population. Ann Rheum Dis. 2015; 74:1368–72. 10.1136/annrheumdis-2014-205269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang CY, Chen CH, Deng ST, Huang CS, Lin YJ, Chen YJ, Wu CY, Hung SI, Chung WH. Allopurinol Use and Risk of Fatal Hypersensitivity Reactions: A Nationwide Population-Based Study in Taiwan. JAMA Intern Med. 2015; 175:1550–57. 10.1001/jamainternmed.2015.3536 [DOI] [PubMed] [Google Scholar]
- 37.Ng CY, Yeh YT, Wang CW, Hung SI, Yang CH, Chang YC, Chang WC, Lin YJ, Chang CJ, Su SC, Fan WL, Chen DY, Wu YJ, et al. , and Taiwan Severe Cutaneous Adverse Reaction Consortium. Impact of the HLA-B(*)58:01 Allele and Renal Impairment on Allopurinol-Induced Cutaneous Adverse Reactions. J Invest Dermatol. 2016; 136:1373–81. 10.1016/j.jid.2016.02.808 [DOI] [PubMed] [Google Scholar]
- 38.Chung WH, Chang WC, Stocker SL, Juo CG, Graham GG, Lee MH, Williams KM, Tian YC, Juan KC, Jan Wu YJ, Yang CH, Chang CJ, Lin YJ, et al. Insights into the poor prognosis of allopurinol-induced severe cutaneous adverse reactions: the impact of renal insufficiency, high plasma levels of oxypurinol and granulysin. Ann Rheum Dis. 2015; 74:2157–64. 10.1136/annrheumdis-2014-205577 [DOI] [PubMed] [Google Scholar]
- 39.Lin SL, Chen YM, Chien CT, Chiang WC, Tsai CC, Tsai TJ. Pentoxifylline attenuated the renal disease progression in rats with remnant kidney. J Am Soc Nephrol. 2002; 13:2916–29. 10.1097/01.ASN.0000034909.10994.8A [DOI] [PubMed] [Google Scholar]
- 40.Navarro JF, Mora C, Muros M, García J. Additive antiproteinuric effect of pentoxifylline in patients with type 2 diabetes under angiotensin II receptor blockade: a short-term, randomized, controlled trial. J Am Soc Nephrol. 2005; 16:2119–26. 10.1681/ASN.2005010001 [DOI] [PubMed] [Google Scholar]
- 41.Perkins RM, Aboudara MC, Uy AL, Olson SW, Cushner HM, Yuan CM. Effect of pentoxifylline on GFR decline in CKD: a pilot, double-blind, randomized, placebo-controlled trial. Am J Kidney Dis. 2009; 53:606–16. 10.1053/j.ajkd.2008.11.026 [DOI] [PubMed] [Google Scholar]
- 42.Navarro-González JF, Mora-Fernández C, Muros de Fuentes M, Chahin J, Méndez ML, Gallego E, Macía M, del Castillo N, Rivero A, Getino MA, García P, Jarque A, García J. Effect of pentoxifylline on renal function and urinary albumin excretion in patients with diabetic kidney disease: the PREDIAN trial. J Am Soc Nephrol. 2015; 26:220–29. 10.1681/ASN.2014010012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lash TL, Fox MP, MacLehose RF, Maldonado G, McCandless LC, Greenland S. Good practices for quantitative bias analysis. Int J Epidemiol. 2014; 43:1969–85. 10.1093/ije/dyu149 [DOI] [PubMed] [Google Scholar]
- 44.Booth CM, Rapoport B. Uptake of novel medical therapies in the general population. Curr Oncol. 2011; 18:105–08. 10.3747/co.v18i3.858 [DOI] [PMC free article] [PubMed] [Google Scholar]