Abstract
Pre-treatment CT imaging is a topic of growing importance in particle therapy. Improvements in the accuracy of stopping-power prediction are demanded to allow for a dose conformality that is not inferior to state-of-the-art image-guided photon therapy. Although range uncertainty has been kept practically constant over the last decades, recent technological and methodological developments, like the clinical application of dual-energy CT, have been introduced or arise at least on the horizon to improve the accuracy and precision of range prediction. This review gives an overview of the current status, summarizes the innovations in dual-energy CT and its potential impact on the field as well as potential alternative technologies for stopping-power prediction.
Introduction
The range and dose distribution of particles are extremely vulnerable to small deviations in the material-specific stopping power relative to the one of water, the so-called stopping-power ratio (SPR). Compared to photon therapy, the CT conversion in the physical relevant quantity (electron density / SPR) is therefore much more challenging and crucial in particle therapy (PT).1 Hence, an accurate SPR prediction from CT is essential for particle therapy planning. Furthermore, the definition of CT scan protocols and image reconstruction settings as well as in particular the conversion from CT number (CTN) to SPR are challenging and error-prone processes demanding special attention and quality assurance. This is especially true for PT centers in the preclinical preparation phase, when it is often difficult to allocate resources for such, at first glance, sideline tasks. Moreover, in the last years, the use of dual-energy CT (DECT) for SPR prediction was intensively investigated from different perspectives and the technology found its first clinical applications. However, it is extremely difficult to overlook the different options of DECT application for non-experts and to judge the clinical value from the massive load of scientific publications on this topic.
In this review article, we give an overview of innovations in CT imaging relevant for PT and the state-of-the-art approach for CTN-to-SPR conversion. Secondly, we summarize the status, benefit and potential of DECT for SPR prediction. Finally, we will update on potential alternative technologies for SPR prediction or its refinement.
Current status
CTN-to-SPR conversion via HLUT
X-ray attenuation, measured in CT scans, is based on photon interactions induced by photoelectric effect as well as incoherent and coherent scattering, whereas particle interactions are described by the Bethe equation. Hence, there is no one-to-one physical relationship between tissues’ CT information and its stopping behavior on particles. Since photon and particle interactions share a linear dependency on electron density for human tissues, a stepwise-linear correlation function between CT number and SPR, the so-called Hounsfield look-up table (HLUT), is the standard approach for SPR prediction. In some treatment planning systems, the HLUT is defined between CT number and mass density, which is then internally translated into a material composition and finally SPR.
However, the CTN-to-SPR correlation carries ambiguities since there is also a dependence on effective atomic number (photon interactions) and on mean excitation energy (particle interactions), which cannot easily be included in such a conversion function that depends on a single parameter, the CT number. Hence, the HLUT-based conversion is an intrinsically heuristic approach.
Two different methods exist for HLUT definition: the empirical and the stoichiometric calibration. For the empirical calibration,2 both the CTN and SPR of tissue surrogates are measured. Tissue surrogates mimic the X-ray attenuation and, ideally, also the particle stopping properties of human tissues. However, the non-perfect tissue equivalency for both photon and particle interactions is actually the biggest disadvantage of this approach. In contrast, for the stoichiometric approach,3,4 measured tissue properties (density, elemental composition) of real human tissue samples,5–7 mostly extracted from corpse, are used to calculate the tissues’ SPR. Only a few datasets of these tissue properties exist - mostly generated by Woodard and White in the 1980s. However, for HLUT definition also the CTN of these tissues is needed, as if they would have been scanned with the institutional CT hardware and the specific scan and reconstruction parameters. Since such a measurement is not possible, the CTN need to be calculated institution-specifically. Hence, tissue surrogates are still required to perform a calibration of such a CTN prediction. To ensure a robust and reliable CTN calculation for tabulated human tissues, a careful selection of calibration materials is still crucial.8,9 In contrast to the empirical approach, the stoichiometric method only uses tissue surrogates to reproduce the X-ray attenuation of human tissues (not their particle stopping behavior) by preserving similar material parameters such as electron density and effective atomic number (EAN).
The limitations of the HLUT approach can be summarized as follows:
Limited material differentiation, e.g. different soft tissues cannot be distinguished in CTN but have a different particle stopping power.
Severe deviations for non-tissue materials (polymers, implant materials), which are usually not tissue-equivalent and are thus not covered by the HLUT.
Not patient-specific, i.e. the potential intra- and inter-patient variability in CTN-to-SPR conversion is not covered. Inter-patient variations arise from differences in tissue composition,7 e.g. different calcium content in bones between adults and children as recently shown10;
From a survey of 12 mostly European particle centers, the variability in HLUT definition became obvious11: two-third of the centers used a stoichiometric calibration whereas the other third applied an empirical calibration. A specific beam hardening correction for bone, leading to a better CT number constancy for varying scan objects (e.g. patient sizes),12 was only applied in one-third of the centers. The number of patient-group specific HLUTs per center varied between 1 (5 of 12 centers) and 6. In addition, the number of used line segments for the stepwise linear conversion varied between 2 and 11. Still, due to the dependence on CT scanner and scan protocol, one cannot directly conclude from differences in HLUT definition to differences in SPR and range prediction.
In a recently presented, yet unpublished, study within the European Particle Therapy Network, this inter-center variation in SPR and subsequent range prediction was investigated with a ground-truth phantom scanned at 17 participating PT centers with their individual scan protocols and institution-specific CTN-to-SPR conversions. Alarmingly, the 2σ inter-center variation in range prediction was 2.6 and 2.9% for typical brain and prostate treatment fields, respectively. On SPR level, the inter-center variation was even higher (6.3% in lung, 8.7% in bone surrogates). For about every fifth center, the range deviation from ground truth was exceeding 2%. These results underline the need for standardization of both, CT scan protocols as well as HLUT definition. This will not only improve the individual treatment planning but will also have a positive impact on multicentric clinical trials that are crucial in particle therapy.
Range uncertainty
The before-mentioned limitations in CT-based SPR prediction are the major contribution to the uncertainty in range prediction for PT planning. We are referring here to the nominal range uncertainty based on the planning CT scan - excluding potentially occurring anatomical changes during the course of treatment. The concept of range uncertainties was introduced in practical treatment planning in the 1980s.13,14 The magnitude of this uncertainty remained practically unchanged since then and can be estimated with a relative term of roughly 3.5% of range plus a 1–2 mm absolute term. Even though, different PT centers use slightly different definitions (e.g. only absolute terms, but different values for different treatment regions), there is a general agreement on the magnitude of range uncertainty.11,15 In view of the mentioned inter-center variations in range prediction, the overall range uncertainty is at least not overestimated with 3–3.5% for a HLUT-based approach.
General CT improvements for treatment planning
Despite the unchanged uncertainty in SPR and range prediction, several innovations in CT imaging, which are also beneficial for CT applications in radiation oncology, have found their way into clinical application.
Techniques for an automatic exposure control, which adjust the tube current according to the patients’ anatomy, became the clinical standard within the last decade and provide a constant diagnostic image quality in body regions with varying size or severe heterogeneities. Hence, streak artifacts in highly attenuating projection angles can be clearly diminished leading to a more consistent dose calculation and differentiation of anatomical structures. Furthermore, this tube current modulation can contribute to a decrease in CT imaging dose by up to 60%.16
With the introduction of iterative image reconstruction,17 it is possible to further decrease the CT imaging dose while maintaining the same image quality (noise), or vice versa, compared to reconstructions using filtered back projection. It was shown that CT dose can be reduced by up to 60% without hampering image quality.12 Especially, in light of the need of frequent repeated imaging during the course of treatment, such a reduction, enabling doses for control CT scans in the range of 5 mGy (CTDI) or even below, cannot be overrated.
The introduction of advanced iterative metal artifact reduction helped to improve the qualitative image impression with a potential positive impact on target and organ-at-risk delineations.18,19 However, since these algorithms can also alter the quantitative image information in regions that were initially not affected by metal artifacts,12,20 their application for dose calculations in particle therapy is not recommended or should at least be carried out with additional inspections and quality control.
Furthermore, algorithms for applications in photon therapy have been developed to estimate the electron density from single-energy CT (SECT) independent from the X-ray tube voltage.21 This so-called DirectDensity method distinguishes between bone and water-like contributions and convolutes the effective thickness of bone and water with the respective electron densities in the projection space. The electron-density dataset derived by DirectDensity shares the same limitations for HLUT definition as a SECT scan and is not expected to improve the accuracy in SPR prediction. However, as the DirectDensity approach is independent from the tube voltage used, the tube voltage can be varied, e.g. depending on the patient diameter, and still one single HLUT could be applied.
Dual-energy CT
Idea and motivation
The basic idea of DECT, acquiring two CT scans with different effective X-ray spectra to gain more information about tissue properties, is anything else but new. It was Godfrey Hounsfield, who proposed the concept when he presented the first clinical CT scanner in 1973.22 Only 4 years later, Michael Goitein saw its potential for improved treatment planning in proton therapy.23 Due to the different relative contributions of photoelectric effect and incoherent/coherent scattering, the two CT scans contain partly complementary attenuation information of tissues. The higher the spectral separation between the two CT scans, the higher is the additional value of the second one. Despite these clear conceptual benefits, it took until 2015 until DECT was first used for clinical treatment planning in PT24 — probably due to the lack of practical hardware and software solutions in the decades before and challenges in data processing, e.g. increased noise level.25–27
Although the main motivation for DECT in radiation oncology is the improvement of SPR prediction for PT28 with broad consensus in the community,11,29 several other potential use cases are discussed and investigated: the calculation of relative electron density (RED) for photon treatment planning including brachytherapy30,31; material classification, e.g. for Monte Carlo applications32,33 ; improved image quality including metal artifact reduction24,34 and variable image contrast24,29 ; target and organ-at-risk delineation35–39 ; the virtual subtraction of contrast agent information to generate a virtual non-contrast and contrast-enhanced dataset from only one single DECT scan40–42; functional imaging43,44 and more.
Technical implementation of DECT acquisition
Within the last decade, various DECT imaging techniques have been technically realized, which can be categorized in single-source and dual-source CT scanners.
Single-source CT scanner
Depending on the type of single-source CT scanner, DECT scans can be acquired
consecutively (dual-spiral mode),22,24
almost simultaneously by fast tube voltage switching,36,45
simultaneously using a dual-layer detector,46,47
simultaneously using a split-beam filter in scan direction (twin-beam mode).48
In some cases, different DECT modes can be realized with the same CT scanner hardware, e.g. dual-spiral and twin-beam.
Dual-spiral DECT benefits from an independent adjustment of tube voltage (commonly 80/140 kVp) as well as current and thus imaging dose. Since motion-induced anatomical changes might occur due to the temporal difference in DECT acquisition (20–90 s), a deformable image registration is performed. For more severe periodic motion, e.g. due to respiration, the dual-spiral technique could in principle be combined with the time-resolved (four-dimensional) CT approach.35 The application of contrast-enhanced DECT scans is limited to a late phase with almost no dynamics starting at least 75 s after injection.
Fast-voltage-switching DECT provides a high spatio-temporal resolution by a fast alternation between low and high tube voltages (commonly 80/140 kV) within 50 µs enabling a projection-based material decomposition. Since the tube current cannot be sufficiently modified for such a rapid voltage change, exposure times for low-energy projections are prolonged (dwell time ratio low:high of 65:35) to allow a higher image dose resulting in comparable image noise. Furthermore, the overall image quality and spectral separation are slightly deteriorated compared to independent acquisitions, because the number of projections per energy and rotation is reduced and a perfectly binary voltage modulation is technically challenging, respectively.
For dual-layer DECT, the energy discrimination is realized solely by the detector arrangement one after the other, leading to different absorption conditions for the same input X-ray spectrum. The rear detector effectively receives a higher photon spectrum. This results in almost perfect temporal correspondence, enabling projection-based reconstruction methods. The spectral separation is considerably smaller compared with other solutions and the measured signal is affected by cross-scatter radiation between detector layers. Thus, no dose modulation is possible between the low- and high-energy dataset.
Twin-beam DECT splits the X-ray spectrum (commonly 120 kVp) into a spatially separated low- and high-energy spectrum by adding an additional filter in a subset of the scanned region (e.g. 0.05 mm gold and 0.6 mm tin filter in a current CT scanner). This technique has an inferior spectral separation and the central part of the X-ray beam cannot be used properly due to the spatial overlap of the two X-ray fields.
Dual-source CT scanner
Dual-source CT scanners consist of two sets of X-ray tube and energy-integrating detector, installed with an angular offset of 9095°.49 While simultaneous acquisition with both X-ray detectors, the tube voltage, current and filter systems can be independently adjusted to increase the spectral separation (up to 70 vs 150 kVp with an additional tin filter) and to individually optimize the CT dose. The contamination of each detector signal with cross-scatter radiation is mitigated by corrections during image post-processing. Due to the temporal shift in projection data (quarter rotation time, at least 66 ms), a projection-based material decomposition is very challenging in the presence of patient motion and thus image-based algorithms are preferably applied. Since the CT gantry is limited in space for the two detectors, DECT information is only available in a field of view (FOV) of up to 35 cm. This is the main limitation of dual-source DECT nowadays. Therefore, if a larger DECT FOV of 50 cm is needed, e.g. for treatment planning in the abdominal or pelvic region and for proper inclusion of immobilization devices, a single-source DECT technique seems currently more convenient.
In general, the choice of the optimal DECT acquisition technique and hardware strongly depends on the purpose of the final application (body site, presence of motion etc.) and the relative impact of a multitude of parameters (spectral separation, impact of scattering, current modulation, etc.), and therefore should be subject to exploratory clinical studies to prove the respective benefit in clinical conditions.
DECT-derived virtual monoenergetic images
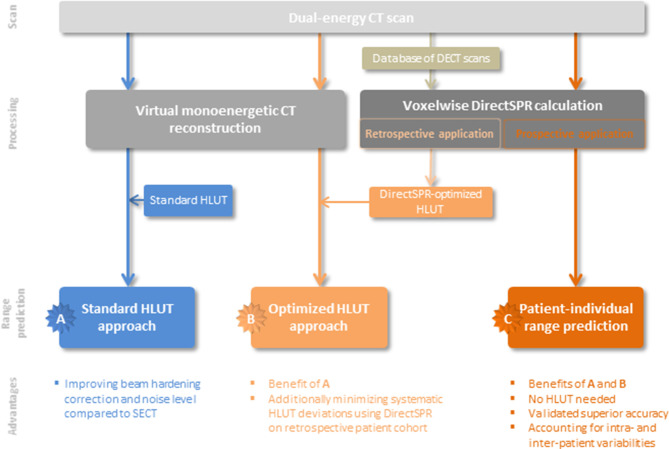
Depending on the purpose, different post-processing options are available for DECT scans, cf. Figure 1 and Figure 2. A very often used and straightforward approach is a weighted superposition of the two CT numbers derived from DECT (CTNlow, CTNhigh), resulting in a so-called virtual monoenergetic CT image (VMI) dataset:
With varying the weighting factor α, that can also become negative, the contribution of incohorent scattering (more dominant in the high-energy CT scan) and photoelectric effect (more dominant in the low-energy CT scan) are changed effectively, resulting in different image contrasts. Hence, from the same DECT scan, a VMI dataset of high image contrast (α > 0) for delineation and another VMI dataset with minimized metal artifacts (α < 0) can be reconstructed. For a specific α, the resulting VMI corresponds to the relative electron density.50 Alternatively, one can superimpose two VMI datasets to obtain RED as shown for dual-layer DECT.51
One can also optimize α to minimize remaining beam hardening effects and thereby increasing CT number constancy. Since this is optimal for treatment planning, this approach was chosen for the first clinical implementation of DECT-based VMI datasets for proton treatment planning.24 When using VMI datasets for PT planning, a conventional HLUT conversion from CTN to SPR is still required and all the limitations of the HLUT approach still apply. Nevertheless, compared to a SECT with the same dose, the CT number constancy is increased while the image noise level is reduced.
Direct stopping-power prediction from DECT
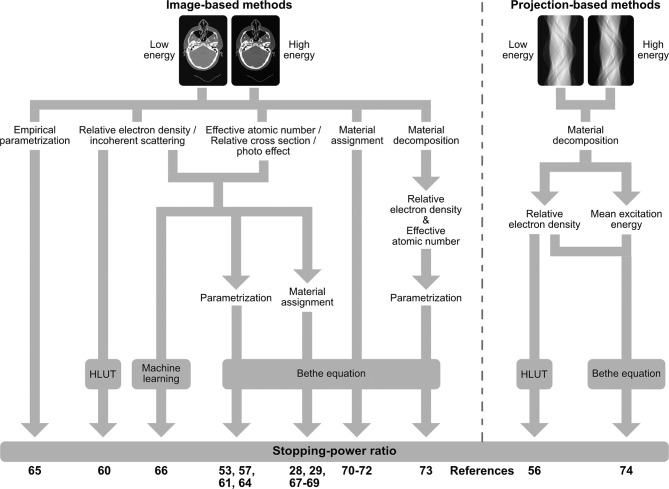
According to the Bethe equation, the material-specific SPR directly follows from two physical quantities, namely electron density and mean excitation energy (I-value). The additional material information gathered by DECT can be used to better distinguish between both parameters and almost fully overcome the intrinsic restrictions of the HLUT approach. For this purpose, a variety of algorithms for direct DECT-based SPR prediction have been developed (Figure 1) and thoroughly validated within the last decade, which have recently started to be implemented in PT for treatment planning (Figure 2). In the following, these algorithms are summarized as DirectSPR approaches. “Direct” refers to the direct determination of material parameters (e.g. RED) that can be inserted in the Bethe equation for SPR determination and thereby substantially reduce the impact of heuristic assumptions.
Figure 1. .
Overview of algorithms for direct stopping-power prediction (DirectSPR) based on dual-energy CT.
Figure 2. .
Different levels of clinical application of dual-energy CT (DECT) scans for particle treatment planning. Option A is the combination of virtual monoenergetic CT image and the classical approach using a Hounsfield look-up table (HLUT), whereas in option B the HLUT is adapted with the retrospective application of DirectSPR for a representative patient cohort. Option C is the prospective application of the DirectSPR approach, possessing the most benefits.
Overview of algorithms
The RED is the dominating parameter in SPR prediction and can be directly derived from DECT in a robust and accurate manner52,53 using either a linear superposition of the two CT scans,54–56 a stoichiometric calibration,57,58 a sophisticated modelling of photon attenuation cross-sections59 or a projection-based material decomposition in water and compact bone.60 Since even with the linear superposition, as the simplest algorithm, a methodological uncertainty within 0.2% can be achieved for a wide range of spectral combinations,53 further algorithmic improvements are not required for PT in regard to other sources of uncertainty.
The EAN is the second relevant quantity for X-ray attenuation and can also be determined by a linear superposition,61,62 an empirical and stoichiometric DECT calibration57,58,63 or a sophisticated cross-section model.59 Many of these algorithms for RED and EAN calculation55,56,61–65 share the same physical principals, differ only in the number of calibration factors and can be mathematically transferred to the equations of Brooks54 by applying simple arithmetic conversions.66
Various approaches for SPR prediction were developed using RED and EAN as input to solve the Bethe equation. Since the I-value cannot be directly derived from DECT in contrast to RED, an heuristic approximation, similar to a SECT-based HLUT, is needed. The impact of this empirical component on SPR accuracy is however substantially reduced compared to the HLUT approach. In general, either a linear conversion from RED to SPR60,64 can be performed or EAN is translated to the (logarithmic) I-value using a stepwise-linear67 or polynomial function57 and then inserted in the Bethe equation.57,61,65 To better account for tissue mixtures within a voxel, Möhler et al68 proposed to determine the relative stopping number (second part in the Bethe equation comprising the I-value) from the photon attenuation cross section (VMI dataset divided by RED) instead of I-value from EAN. Moreover, an empirical SPR parametrization based on the two DECT scans69 or the application of machine learning on DECT-derived image datasets,70 such as VMI or images of incoherent scattering (comparable to RED) and photoelectric effect (comparable to EAN), were proposed.
Since Monte Carlo dose calculation algorithms require the elemental material composition for each CT voxel, DECT can also be used for an improved material assignment. Based on a pre-defined material database, each voxel can be assigned to the material with the shortest distance in the RED/EAN space.33,71–73 However, such a material segmentation does not consider intra- and inter-patient variations in elemental tissue composition. In contrast, a RED and EAN parameterization, resulting in a continuous assignment of elemental weight fractions, considers patient variability.32 With a principal component analysis, directly applied on the two DECT scans, image post-processing for RED and EAN determination would not be required anymore.74–76 Furthermore, an image-77 or projection-based60,78 material decomposition (e.g. water and compact bone) can be performed for material mapping. For all these approaches, the SPR directly follows from the Bethe equation based on the assigned elemental composition.
The comparison between projection- and image-based methods79–81 as well as a broad variety of image-based algorithms26 did not yield relevant differences in SPR accuracy and precision. However, the calibration of each method, i.e. settings of the CT scan protocol, measurement setup and selection of calibration materials, has an immense influence on the overall performance and robustness.8,53 Of note, the same is true for SECT-based HLUT approaches, often used as comparison to assess the performance of DECT-based algorithms.
Experimental validation of algorithms
Several studies assessed the accuracy and precision in RED, EAN and SPR prediction in homogeneous tissue surrogates26,28,32,33,47,53,56–59,61–65,68,70,72,76,78,82–92 or other polymers93,94 within simplified phantoms. The superior accuracy of DECT-based methods compared with the HLUT approach could be confirmed in high-precision experimental studies on different elevated complexity levels: (I) various homogeneous95,96 and heterogeneous91,92 porcine and bovine tissue samples, which closely represent human tissues, as well as (II) an anthropomorphic head phantom of well-known SPR distribution,50 which models usual geometric patient heterogeneities. A DECT-based DirectSPR approach could achieve an SPR accuracy within 0.2% in homogeneous soft tissue samples.95 However, a systematic SPR under- or overestimation in small-volume materials at high-density gradients induced by image smoothing during reconstruction was identified, which similarly impacts every CT-based method50,97 and needs to be considered as CT-related uncertainty.
Due to the high accuracy of DECT-based RED determination and optimized experimental setups for high-precision range measurements, an experimental assessment of I-values in solutions or homogeneous tissues seems feasible.95,98 Hence, current tissue- or element-specific I-values could be verified or adapted to reduce the uncertainty in SPR prediction.99,100
First evaluations also demonstrated the feasibility and benefits of quantitative DECT imaging for dose calculation in preclinical studies with mice.101–103
Clinical relevance for treatment planning
To judge the clinical relevance of the superior SPR accuracy of the DirectSPR method compared with the HLUT approach, dose and range differences were assessed retrospectively on patient datasets.35,89,90,104 The clinical implementation of DECT for routine proton treatment planning on VMI datasets in 2015,24 still using a HLUT for CTN-to-SPR conversion, provided access to a broad variety of standardized patient DECT scans. Large retrospective patient-cohort analyses of brain-, prostate- or non-small cell lung-tumor cases revealed clinically relevant differences in water-equivalent proton range of 1.2, 1.7 and 2.3% on average, respectively.35,90 Considering the high SPR accuracy achievable with DirectSPR, the HLUT could be defined/refined based on retrospectively available DirectSPR information derived from DECT scans of patients, which represent a realistic tissue occurrence and can thus provide guidance for HLUT specification (sampling points, course of HLUT). Thereby, systematic range differences can be substantially reduced (within 0.2% on average) resulting in a clear benefit in clinical practice.12,105 However, to thoroughly account for the intra- and inter-patient tissue variability in SPR,10 the clinical application of a DirectSPR approach is recommended after careful calibration and comprehensive validation.
Clinical implementation and related challenges
DECT-based SPR prediction approaches are often validated in idealized situations (optimized geometry, central position, high CT dose, large homogeneous materials, calibration for specific beam hardening condition) resulting in SPR accuracies within 1%. However, for a robust and reliable clinical implementation, various influences need to be considered for calibration and validation as well as comprehensive assessment of the relative range uncertainty needs to be performed, which results in clinically realistic estimates of approximately 2%.28,106
The calibration of each method is crucial and depends on many CT parameters, such as the tube voltage combination, scanner hardware and software as well as scan protocol and image reconstruction settings. Furthermore, different beam hardening conditions induced by varying patient size have a relevant influence on CT number stability and thus calibration factors, in particular if no beam hardening correction for bone is applied during image reconstruction.12 Since high image noise in DECT datasets can also lead to a systematic SPR deviation,27 iterative image reconstruction and multi-band filtering for noise suppression during image post-processing are highly recommended to obtain an image noise level within 1%, which is sufficient for a precise dose calculation.
Due to the high impact of all these different parameters on SPR accuracy, the calibration should be ideally performed in a standardized and centralized manner or using a commonly agreed scan protocol instead of being center-specific. Hence, CT vendors are encouraged to provide well-calibrated DECT-derived datasets containing material parameters (VMI, RED, EAN, SPR, composition) for their respective DECT technique. These datasets are thus independent from scanner-specific properties and can be universally imported and interpreted by treatment planning systems.
All these influencing variables were included in a recently introduced medical product for patient-specific DECT-based SPR prediction. It comprises a dedicated calibration depending on patient size for a standardized CT scan protocol and a noise-suppression algorithm during image post-processing. The inclusion of these advanced image post-processing algorithms was possible as the SPR calculation was for the first time integrated in CT image reconstruction instead of the treatment planning system. Based on this technology, DirectSPR was clinically implemented for proton treatment planning associated with a reduction of the relative range uncertainty from 3.5% to 1.7–2.0%.106
Alternative technologies for SPR prediction or refinement
Proton computed tomography
The acquisition of CT datasets using protons instead of X-rays is a promising technology to overcome the uncertainty in the conversion from X-ray attenuation to proton stopping power, because the SPR of human tissues can be directly determined in vivo.107–110 Despite ongoing research activities over the last decades, this technique is still in an early stage of development, i.e. first prototype proton CT scanners are constructed and experimentally validated, but a clinical system is currently not available.111 Current applications are mostly limited to head sizes, since today’s proton therapy facilities equipped with cyclotrons are not capable to deliver proton beams with an energy traversing body regions like abdomen and pelvis. As demonstrated in a recent simulation study,112 proton CT scans might be feasible to be acquired with a total dose smaller than 5 mGy, but further improvements in image reconstruction are crucial to reduce the partly severe ring and streak artifacts. Furthermore, it was shown that DECT-based SPR prediction can achieve a comparable SPR accuracy as proton CT.112
Proton radiography and range probing
Proton range assessment in patients with proton radiography or so-called range probing is another possibility to mitigate the inherent restriction of a heuristic SPR prediction based on X-ray attenuation. These methods were suggested for a patient-specific HLUT refinement prior to treatment.113–118 Still, their value for independent validation and quality assurance of the applied range prediction method is barely dispensable. Whereas for proton radiography,109 a two-dimensional image with information on the water-equivalent thickness of the patient is calculated by deconvoluting the measured signal behind the patient, the range probe approach (also called proton-integrating radiography) directly compares the spot-wise measured depth–dose curve with an expectation derived from dose calculation. Hence, range probing appears to be simpler and easier to implement compared to proton radiography, which has similar limitations and requirements as proton CT. First proof-of-principle implementations and phantom-based validations have been performed.116,119 Still, neither of the approaches has yet been clinically applied. As additional dose is deposited in the patient (currently 10–20 mGy as determined experimentally for a head geometry118), an approval by ethical and radiation protection authorities is likely to be required.
Prompt-γ-based range measurements
The measurement of prompt γ-rays can provide in vivo information on the actual proton range in patients during treatment without adding any extra dose to the patient.120–122 Similar to range-probing, a direct in-human validation and potential adaptation of CT-based range prediction for patients seems feasible. For a PGI prototype system, the accuracy and reproducibility was improved, so that the uncertainty in range verification is smaller than the nominal range uncertainty for deep-seated tumors.123 In-human validation of range verification is currently ongoing within a clinical study. Still, the main potential of prompt-γ-based range verification lays in the early and potentially automatic detection of deviations, caused by anatomical changes or patient setup, during fractionated treatments.
MR-only treatment planning
As the application of MRI is substantially increasing in photon therapy, with online-adaptive MR-guided photon therapy being clinical reality nowadays, there is also an increasing need to follow this innovation in PT. This could be by treatment planning solely on MR datasets or, on a longer timescale, even the realization of online adaptive proton therapy guided by MR imaging, so-called MR-guided PT.124 However, MR-only dose calculation and also dosimetry in magnetic fields are much more challenging for PT as it already is for photon therapy. Recently developed approaches for MR-based dose calculations generate synthetic CT datasets with heterogeneous tissue representation by an intensity-based assignment of CT numbers, which differentiates soft tissues and bones obtained by segmentation.125–127 The validation results for small cohorts of prostate- and brain-tumor cases were promising (mean absolute error in CT numbers of 30–80 HU125,126 or 3 mm error in water equivalent path length127). However, further investigations and improvements are required. Depending on the use case, e.g. initial treatment planning or dose re-calculation to assess the adaptation need, different accuracy levels need to be achieved. At the current stage, it remains questionable whether MR-only PT treatment planning can reach a similar level of accuracy and precision as current CT-based approaches, e.g. DirectSPR. For the potential future use of MRI for adaptive treatment purposes, the current research activities are needed to reach and assess clinical applicability.
Photon-counting computed tomography
CT vendors work towards the integration of energy-resolving detectors, allowing for a separation of the X-ray spectrum into multiple energy bins, in future generations of commercial CT scanners. Theoretical and initial experimental studies performed with first photon-counting CT prototypes demonstrated a comparable or slightly better accuracy in SPR and material assignment compared with DECT for the same CT dose.128–131 The assumption that an increase from 2 to 4 energy bins would automatically lead to an improved accuracy could not be verified in current experiments due to a higher noise level per energy bin and a clear overlap of the bin-specific X-ray spectra. Further developments of spectral denoising methods and projection-based corrections for beam hardening and scattering might be promising to improve the overall performance for more advanced future systems.
Conclusion
In the last decade, the field of pre-treatment CT imaging has seen a lot of research efforts focused especially on PT, resulting in several innovations that already found their way into clinical application or this is on the near horizon (Table 1). The most prominent example is the use of DECT for direct SPR prediction: Substantial and broad evidence for its benefits has been demonstrated on a research level and sustained efforts focused on translating them into real-world clinical implementation have been made. Finally, industry picked up the approach for product development enabling its broad consistent use. DirectSPR has the ability to end the several decades lasting era of heuristic, institution-specific HLUT-based SPR prediction with its intrinsic limitations and thus to substantially reduce range uncertainty. Furthermore, if implemented correctly including inter-center consensus on the algorithm and calibration, inter-center variations in range prediction between centers will be decreased compared to the current situation. On the mid- and long-term scale, several further innovations promise further benefits for PT applications - photon-counting CT being just one example. Still, there is a high need for sustained translational research efforts to adapt technologies and assess their specific benefits for the, in radiology-terms small, application field of particle therapy.
Table 1. .
Overview of techniques for range prediction and its validation
| Applicable for pre-treatment SPR prediction | Applicable for SPR re-assessment during treatment | Technical readiness level** | Current estimated range uncertainty | Advantages | Limitations | |
|---|---|---|---|---|---|---|
| SECT-based HLUT | ✔ | Only with inroom-CT or additional CT acquisition outside of treatment room | 9 | 3–3.5% |
|
|
| DECT-based DirectSPR | ✔ | 8 | 2% |
|
|
|
| Photon-counting CT | ✔ | 6 | ≤2% |
|
|
|
| Proton CT | ✔ | (✔) | 4–6 | ? |
|
|
| MRI-only SPR prediction | (✔) probably inferior to CT-based methods | (✔) for MR-PT integration | 6 | >2% |
|
|
| Range probes | ✖ | ✔ | 6 | 1 mma |
|
|
| Prompt-γ-based range assessment | ✖ | ✔ | 7 | 1.5 mma |
|
|
DECT, dual-energy CT; HLUT, Hounsfield look-up table; SECT, single-energy CT; SPR, stopping-power ratio.
The TRL definition is according to the Horizon 2020 programme.
Global verification accuracy of average range shifts within a field only. If subsequently an additional adaptation of the range prediction would be applied, e.g. HLUT refinement, this would bear additional uncertainties - for example an overestimation in one tissue together with an underestimation in another tissue could still exist, even if the integral range shift is minimized on average. Note that both techniques are able to validate an existing range prediction, however cannot replace the (voxelwise) range prediction.
Footnotes
Acknowledgment: The authors would like to thank Dr Aswin Hoffmann for his feedback on MR-guided proton therapy.
The authors Patrick Wohlfahrt and Christian Richter contributed equally to the work.
Conflict of interest statement: The authors received individual funding as lecturer from Siemens Healthineers (2018), which was not related to this study. OncoRay has an institutional research agreement with Siemens Healthineers in the field of dual-energy CT for particle therapy (2016-2020) as well as an institutional agreement as reference center for dual-energy CT in radiotherapy and a software evaluation contract. The authors received no financial support for the present paper.
Author Notes: We will use the abbreviation PT for particle and proton therapy in this review. No differentiation between proton therapy and the use of heavier ions like carbon is needed. Hence, the terms protontherapy and particle therapy are also exchangeable in the article.
Contributor Information
Patrick Wohlfahrt, Email: patrick.wohlfahrt@oncoray.de.
Christian Richter, Email: christian.richter@oncoray.de.
REFERENCES
- 1.Knopf A-C, Lomax A. In vivo proton range verification: a review. Phys Med Biol 2013; 58: R131–60. doi: 10.1088/0031-9155/58/15/R131 [DOI] [PubMed] [Google Scholar]
- 2.Jäkel O, Jacob C, Schardt D, Karger CP, Hartmann GH. Relation between carbon ion ranges and X-ray CT numbers. Med Phys 2001; 28: 701–3. doi: 10.1118/1.1357455 [DOI] [PubMed] [Google Scholar]
- 3.Schneider U, Pedroni E, Lomax A. The calibration of CT Hounsfield units for radiotherapy treatment planning. Phys Med Biol 1996; 41: 111–24. doi: 10.1088/0031-9155/41/1/009 [DOI] [PubMed] [Google Scholar]
- 4.Schaffner B, Pedroni E. The precision of proton range calculations in proton radiotherapy treatment planning: experimental verification of the relation between CT-HU and proton stopping power. Phys Med Biol 1998; 43: 1579–92. doi: 10.1088/0031-9155/43/6/016 [DOI] [PubMed] [Google Scholar]
- 5.Woodard HQ, White DR. The composition of body tissues. Br J Radiol 1986; 59: 1209–18. doi: 10.1259/0007-1285-59-708-1209 [DOI] [PubMed] [Google Scholar]
- 6.White DR, Woodard HQ, Hammond SM. Average soft-tissue and bone models for use in radiation dosimetry. Br J Radiol 1987; 60: 907–13. doi: 10.1259/0007-1285-60-717-907 [DOI] [PubMed] [Google Scholar]
- 7.White DR, Widdowson EM, Woodard HQ, Dickerson JW. The composition of body tissues (II). fetus to young adult. Br J Radiol 1991; 64: 149–59. doi: 10.1259/0007-1285-64-758-149 [DOI] [PubMed] [Google Scholar]
- 8.Wohlfahrt P, Möhler C, Greilich S, Richter C. Comment on „Dosimetric comparison of stopping power calibration with dual-energy CT and single-energy CT in proton therapy treatment planning“. Med Phys 2017; 43: 2845–54. doi: 10.1002/mp.12418 [DOI] [PubMed] [Google Scholar]
- 9.Gomà C, Almeida IP, Verhaegen F. Revisiting the single-energy CT calibration for proton therapy treatment planning: a critical look at the stoichiometric method. Phys Med Biol 2018; 63: 235011. doi: 10.1088/1361-6560/aaede5 [DOI] [PubMed] [Google Scholar]
- 10.Wohlfahrt P, Möhler C, Troost EGC, Greilich S, Richter C. Dual-energy computed tomography to assess intra- and inter-patient tissue variability for proton treatment planning of patients with brain tumor. Int J Radiat Oncol Biol Phys 2019; 105: 504–13. doi: 10.1016/j.ijrobp.2019.06.2529 [DOI] [PubMed] [Google Scholar]
- 11.Taasti VT, Bäumer C, Dahlgren CV, Deisher AJ, Ellerbrock M, Free J, et al. Inter-centre variability of CT-based stopping-power prediction in particle therapy: survey-based evaluation. Physics and Imaging in Radiation Oncology 2018; 6: 25–30. doi: 10.1016/j.phro.2018.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wohlfahrt P. Dual-energy computed tomography for accurate stopping-power prediction in proton treatment planning. Technische Universität Dresden 2018. Available from: https://nbn-resolving.org/urn:nbn:de:bsz:14-qucosa2-317554.
- 13.Goitein M. Calculation of the uncertainty in the dose delivered during radiation therapy. Med Phys 1985; 12: 608–12. doi: 10.1118/1.595762 [DOI] [PubMed] [Google Scholar]
- 14.Urie M, Goitein M, Wagner M. Compensating for heterogeneities in proton radiation therapy. Phys Med Biol 1984; 29: 553–66. doi: 10.1088/0031-9155/29/5/008 [DOI] [PubMed] [Google Scholar]
- 15.Paganetti H. Range uncertainties in proton therapy and the role of Monte Carlo simulations. Phys Med Biol 2012; 57: R99–117. doi: 10.1088/0031-9155/57/11/R99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Söderberg M, Gunnarsson M. Automatic exposure control in computed tomography – an evaluation of systems from different manufacturers. Acta radiol 2010; 51: 625–34. doi: 10.3109/02841851003698206 [DOI] [PubMed] [Google Scholar]
- 17.Hsieh J, Nett B, Yu Z, Sauer K, Thibault J-B, Bouman CA. Recent advances in CT image reconstruction. Curr Radiol Rep 2013; 1: 39–51. doi: 10.1007/s40134-012-0003-7 [DOI] [Google Scholar]
- 18.Gjesteby L, De Man B, Jin Y, Paganetti H, Verburg J, Giantsoudi D, et al. Metal artifact reduction in CT: where are we after four decades? IEEE Access 2016; 4: 5826–49. doi: 10.1109/ACCESS.2016.2608621 [DOI] [Google Scholar]
- 19.Giantsoudi D, De Man B, Verburg J, Trofimov A, Jin Y, Wang G, et al. Metal artifacts in computed tomography for radiation therapy planning: dosimetric effects and impact of metal artifact reduction. Phys Med Biol 2017; 62: R49–80. doi: 10.1088/1361-6560/aa5293 [DOI] [PubMed] [Google Scholar]
- 20.Andersson KM, Dahlgren CV, Reizenstein J, Cao Y, Ahnesjö A, Thunberg P. Evaluation of two commercial CT metal artifact reduction algorithms for use in proton radiotherapy treatment planning in the head and neck area. Med Phys 2018; 45: 4329–44. doi: 10.1002/mp.13115 [DOI] [PubMed] [Google Scholar]
- 21.van der Heyden B, Öllers M, Ritter A, Verhaegen F, van Elmpt W. Clinical evaluation of a novel CT image reconstruction algorithm for direct dose calculations. Physics and Imaging in Radiation Oncology 2017; 2: 11–16. doi: 10.1016/j.phro.2017.03.001 [DOI] [Google Scholar]
- 22.Hounsfield GN. Computerized transverse axial scanning (tomography): Part 1. description of system. Br J Radiol 1973; 46: 1016–22. doi: 10.1259/0007-1285-46-552-1016 [DOI] [PubMed] [Google Scholar]
- 23.Goitein M. The measurement of tissue heterodensity to guide charged particle radiotherapy. Int J Radiat Oncol Biol Phys 1977; 3: 27–33. doi: 10.1016/0360-3016(77)90223-1 [DOI] [PubMed] [Google Scholar]
- 24.Wohlfahrt P, Möhler C, Hietschold V, Menkel S, Greilich S, Krause M, et al. Clinical implementation of dual-energy CT for proton treatment planning on pseudo-monoenergetic CT scans. Int J Radiat Oncol Biol Phys 2017; 97: 427–34. doi: 10.1016/j.ijrobp.2016.10.022 [DOI] [PubMed] [Google Scholar]
- 25.Kelcz F, Joseph PM, Hilal SK. Noise considerations in dual energy CT scanning. Med Phys 1979; 6: 418–25. doi: 10.1118/1.594520 [DOI] [PubMed] [Google Scholar]
- 26.Bär E, Lalonde A, Royle G, Lu H-M, Bouchard H. The potential of dual-energy CT to reduce proton beam range uncertainties. Med Phys 2017; 44: 2332–44. doi: 10.1002/mp.12215 [DOI] [PubMed] [Google Scholar]
- 27.Lee HHC, Li B, Duan X, Zhou L, Jia X, Yang M. Systematic analysis of the impact of imaging noise on dual-energy CT-based proton stopping power ratio estimation. Med Phys 2019; 46: 2251–63. doi: 10.1002/mp.13493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li B, Lee HC, Duan X, Shen C, Zhou L, Jia X, et al. Comprehensive analysis of proton range uncertainties related to stopping-power-ratio estimation using dual-energy CT imaging. Phys. Med. Biol. 2017; 62: 7056–74. doi: 10.1088/1361-6560/aa7dc9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Elmpt W, Landry G, Das M, Verhaegen F. Dual energy CT in radiotherapy: current applications and future outlook. Radiotherapy and Oncology 2016; 119: 137–44. doi: 10.1016/j.radonc.2016.02.026 [DOI] [PubMed] [Google Scholar]
- 30.Landry G, Gaudreault M, van Elmpt W, Wildberger JE, Verhaegen F. Improved dose calculation accuracy for low energy brachytherapy by optimizing dual energy CT imaging protocols for noise reduction using sinogram affirmed iterative reconstruction. Zeitschrift für Medizinische Physik 2016; 26: 75–87. doi: 10.1016/j.zemedi.2015.09.001 [DOI] [PubMed] [Google Scholar]
- 31.Remy C, Lalonde A, Béliveau-Nadeau D, Carrier J-F, Bouchard H. Dosimetric impact of dual-energy CT tissue segmentation for low-energy prostate brachytherapy: a Monte Carlo study. Phys. Med. Biol. 2018; 63: 025013. doi: 10.1088/1361-6560/aaa30c [DOI] [PubMed] [Google Scholar]
- 32.Hünemohr N, Paganetti H, Greilich S, Jäkel O, Seco J. Tissue decomposition from dual energy CT data for MC based dose calculation in particle therapy. Med Phys 2014; 41(6Part1): 061714. doi: 10.1118/1.4875976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Almeida IP, Schyns LEJR, Vaniqui A, van der Heyden B, Dedes G, Resch AF, et al. Monte Carlo proton dose calculations using a radiotherapy specific dual-energy CT scanner for tissue segmentation and range assessment. Phys Med Biol 2018; 63: 115008. doi: 10.1088/1361-6560/aabb60 [DOI] [PubMed] [Google Scholar]
- 34.Kovacs DG, Rechner LA, Appelt AL, Berthelsen AK, Costa JC, Friborg J, et al. Metal artefact reduction for accurate tumour delineation in radiotherapy. Radiotherapy and Oncology 2018; 126: 479–86. doi: 10.1016/j.radonc.2017.09.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wohlfahrt P, Troost EGC, Hofmann C, Richter C, Jakobi A. Clinical feasibility of single-source dual-spiral 4D dual-energy CT for proton treatment planning within the thoracic region. Int J Radiat Oncol Biol Phys 2018; 102: 830–40. doi: 10.1016/j.ijrobp.2018.06.044 [DOI] [PubMed] [Google Scholar]
- 36.Ohira S, Wada K, Hirata T, Kanayama N, Ikawa T, Karino T, et al. Clinical implementation of contrast-enhanced four-dimensional dual-energy computed tomography for target delineation of pancreatic cancer. Radiother Oncol 2018; 129: 105–11. doi: 10.1016/j.radonc.2018.01.012 [DOI] [PubMed] [Google Scholar]
- 37.Wang T, Ghavidel BB, Beitler JJ, Tang X, Lei Y, Curran WJ, et al. Optimal virtual monoenergetic image in “TwinBeam” dual-energy CT for organs-at-risk delineation based on contrast-noise-ratio in head-and-neck radiotherapy. J Appl Clin Med Phys 2019; 20: 121–8. doi: 10.1002/acm2.12539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wohlfahrt P, Agolli L, Krause M, Pilz K, Richter C, Troost EGC. PO-1012: Dual-energy computed tomography for improved delineation in postoperative brain-tumor patients. Radiotherapy and Oncology 2019; 133: S559–60. doi: 10.1016/S0167-8140(19)31432-X [DOI] [Google Scholar]
- 39.van der Heyden B, Wohlfahrt P, Eekers DBP, Richter C, Terhaag K, Troost EGC, et al. Dual-energy CT for automatic organs-at-risk segmentation in brain-tumor patients using a multi-atlas and deep-learning approach. Sci Rep 2019; 9: 4126. doi: 10.1038/s41598-019-40584-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Möhler C, Wohlfahrt P, Nicolay NH, Richter C, Greilich S. EP-2089: Dual-energy CT enables dose calculation on scans with iodinated contrast agent. Radiotherapy and Oncology 2018; 127: S1147–8. doi: 10.1016/S0167-8140(18)32398-3 [DOI] [Google Scholar]
- 41.Lapointe A, Lalonde A, Bahig H, Carrier Jean‐François, Bedwani S, Bouchard H. Robust quantitative contrast‐enhanced dual‐energy CT for radiotherapy applications. Med Phys 2018; 45: 3086–96. doi: 10.1002/mp.12934 [DOI] [PubMed] [Google Scholar]
- 42.Lalonde A, Xie Y, Burgdorf B, O’Reilly S, Ingram WS, Yin L, et al. Influence of intravenous contrast agent on dose calculation in proton therapy using dual energy CT. Phys. Med. Biol. 2019; 64: 125024. doi: 10.1088/1361-6560/ab1e9d [DOI] [PubMed] [Google Scholar]
- 43.Lapointe A, Bahig H, Blais D, Bouchard H, Filion Édith, Carrier J-F, et al. Assessing lung function using contrast-enhanced dual-energy computed tomography for potential applications in radiation therapy. Med Phys 2017; 44: 5260–9. doi: 10.1002/mp.12475 [DOI] [PubMed] [Google Scholar]
- 44.Bahig H, Campeau M-P, Lapointe A, Bedwani S, Roberge D, de Guise J, et al. Phase 1-2 study of dual-energy computed tomography for assessment of pulmonary function in radiation therapy planning. Int J Radiat Oncol Biol Phys 2017; 99: 334–43. doi: 10.1016/j.ijrobp.2017.05.051 [DOI] [PubMed] [Google Scholar]
- 45.Kalender WA, Perman WH, Vetter JR, Klotz E. Evaluation of a prototype dual-energy computed tomographic apparatus. I. phantom studies. Med Phys 1986; 13: 334–9. doi: 10.1118/1.595958 [DOI] [PubMed] [Google Scholar]
- 46.Heismann BJ, Wirth S, Janssen S, Spreiter Q. Technology and image results of a spectral CT system. Medical Imaging 2004: Physics of Medical Imaging, Pts 1 and 2 2004; 5368: 52–9. doi: 10.1117/12.530217 [DOI] [Google Scholar]
- 47.Ohira S, Washio H, Yagi M, Karino T, Nakamura K, Ueda Y, et al. Estimation of electron density, effective atomic number and stopping power ratio using dual-layer computed tomography for radiotherapy treatment planning. Phys Med 2018; 56: 34–40. doi: 10.1016/j.ejmp.2018.11.008 [DOI] [PubMed] [Google Scholar]
- 48.Euler A, Parakh A, Falkowski AL, Manneck S, Dashti D, Krauss B, et al. Initial results of a single-source dual-energy computed tomography technique using a Split-Filter: assessment of image quality, radiation dose, and accuracy of dual-energy applications in an in vitro and in vivo study. Invest Radiol 2016; 51: 491–8. doi: 10.1097/RLI.0000000000000257 [DOI] [PubMed] [Google Scholar]
- 49.Flohr TG, McCollough CH, Bruder H, Petersilka M, Gruber K, Süss C, et al. First performance evaluation of a dual-source CT (DSCT) system. Eur Radiol 2006; 16: 256–68. doi: 10.1007/s00330-005-2919-2 [DOI] [PubMed] [Google Scholar]
- 50.Wohlfahrt P, Möhler C, Richter C, Greilich S. Evaluation of stopping-power prediction by dual- and single-energy computed tomography in an anthropomorphic ground-truth phantom. Int J Radiat Oncol Biol Phys 2018; 100: 244–53. doi: 10.1016/j.ijrobp.2017.09.025 [DOI] [PubMed] [Google Scholar]
- 51.Landry G, Dörringer F, Si-Mohamed S, Douek P, Abascal J, Peyrin F, et al. Technical note: relative proton stopping power estimation from virtual monoenergetic images reconstructed from dual-layer computed tomography. Med Phys 2019. doi: 10.1002/mp.13404 [DOI] [PubMed] [Google Scholar]
- 52.Saito M, Tsukihara M. Technical note: exploring the limit for the conversion of energy-subtracted CT number to electron density for high-atomic-number materials. Med Phys 2014; 41: 071701. doi: 10.1118/1.4881327 [DOI] [PubMed] [Google Scholar]
- 53.Möhler C, Wohlfahrt P, Richter C, Greilich S. Methodological accuracy of image-based electron density assessment using dual-energy computed tomography. Med Phys 2017; 44: 2429–37. doi: 10.1002/mp.12265 [DOI] [PubMed] [Google Scholar]
- 54.Brooks RA. A quantitative theory of the Hounsfield unit and its application to dual energy scanning. J Comput Assist Tomogr 1977; 1: 487–93. doi: 10.1097/00004728-197710000-00016 [DOI] [PubMed] [Google Scholar]
- 55.Heismann BJ, Leppert J, Stierstorfer K. Density and atomic number measurements with spectral X-ray attenuation method. J Appl Phys 2003; 94: 2073–9. doi: 10.1063/1.1586963 [DOI] [Google Scholar]
- 56.Saito M. Potential of dual-energy subtraction for converting CT numbers to electron density based on a single linear relationship. Med Phys 2012; 39: 2021–30. doi: 10.1118/1.3694111 [DOI] [PubMed] [Google Scholar]
- 57.Bourque AE, Carrier J-F, Bouchard H. A stoichiometric calibration method for dual energy computed tomography. Phys Med Biol 2014; 59: 2059–88. doi: 10.1088/0031-9155/59/8/2059 [DOI] [PubMed] [Google Scholar]
- 58.Garcia LIR, Azorin JFP, Almansa JF. A new method to measure electron density and effective atomic number using dual-energy CT images. Phys Med Biol 2016; 61: 265–79. doi: 10.1088/0031-9155/61/1/265 [DOI] [PubMed] [Google Scholar]
- 59.van Abbema JK, van Goethem M-J, Greuter MJW, van der Schaaf A, Brandenburg S, van der Graaf ER. Relative electron density determination using a physics based parameterization of photon interactions in medical DECT. Phys Med Biol 2015; 60: 3825–46. doi: 10.1088/0031-9155/60/9/3825 [DOI] [PubMed] [Google Scholar]
- 60.Vilches-Freixas G, Létang JM, Ducros N, Rit S. Optimization of dual-energy CT acquisitions for proton therapy using projection-based decomposition. Med Phys 2017; 44: 4548–58. doi: 10.1002/mp.12448 [DOI] [PubMed] [Google Scholar]
- 61.Hünemohr N, Krauss B, Tremmel C, Ackermann B, Jäkel O, Greilich S. Experimental verification of ion stopping power prediction from dual energy CT data in tissue surrogates. Phys Med Biol 2014; 59: 83–96. doi: 10.1088/0031-9155/59/1/83 [DOI] [PubMed] [Google Scholar]
- 62.Saito M, Sagara S. A simple formulation for deriving effective atomic numbers via electron density calibration from dual-energy CT data in the human body. Med Phys 2017; 44: 2293–303. doi: 10.1002/mp.12176 [DOI] [PubMed] [Google Scholar]
- 63.Landry G, Seco J, Gaudreault M, Verhaegen F. Deriving effective atomic numbers from DECT based on a parameterization of the ratio of high and low linear attenuation coefficients. Phys Med Biol 2013; 58: 6851–66. doi: 10.1088/0031-9155/58/19/6851 [DOI] [PubMed] [Google Scholar]
- 64.Hünemohr N, Krauss B, Dinkel J, Gillmann C, Ackermann B, Jäkel O, et al. Ion range estimation by using dual energy computed tomography. Zeitschrift für Medizinische Physik 2013; 23: 300–13. doi: 10.1016/j.zemedi.2013.03.001 [DOI] [PubMed] [Google Scholar]
- 65.Saito M, Sagara S. Simplified derivation of stopping power ratio in the human body from dual-energy CT data. Med Phys 2017; 44: 4179–87. doi: 10.1002/mp.12386 [DOI] [PubMed] [Google Scholar]
- 66.Möhler C, Wohlfahrt P, Richter C, Greilich S. On the equivalence of image-based dual-energy CT methods for the determination of electron density and effective atomic number in radiotherapy. Physics and Imaging in Radiation Oncology 2018; 5: 108–10. doi: 10.1016/j.phro.2018.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yang M, Virshup G, Clayton J, Zhu XR, Mohan R, Dong L. Theoretical variance analysis of single- and dual-energy computed tomography methods for calculating proton stopping power ratios of biological tissues. Phys Med Biol 2010; 55: 1343–62. doi: 10.1088/0031-9155/55/5/006 [DOI] [PubMed] [Google Scholar]
- 68.Möhler C, Wohlfahrt P, Richter C, Greilich S. Range prediction for tissue mixtures based on dual-energy CT. Phys Med Biol 2016; 61: N268–75. doi: 10.1088/0031-9155/61/11/N268 [DOI] [PubMed] [Google Scholar]
- 69.Taasti VT, Petersen JBB, Muren LP, Thygesen J, Hansen DC. A robust empirical parametrization of proton stopping power using dual energy CT. Med Phys 2016; 43: 5547–60. doi: 10.1118/1.4962934 [DOI] [PubMed] [Google Scholar]
- 70.Su K-H, Kuo J-W, Jordan DW, Van Hedent S, Klahr P, Wei Z, et al. Machine learning-based dual-energy CT parametric mapping. Phys Med Biol 2018; 63: 125001. doi: 10.1088/1361-6560/aac711 [DOI] [PubMed] [Google Scholar]
- 71.Bazalova M, Carrier J-F, Beaulieu L, Verhaegen F. Tissue segmentation in Monte Carlo treatment planning: a simulation study using dual-energy CT images. Radiother Oncol 2008; 86: 93–8. doi: 10.1016/j.radonc.2007.11.008 [DOI] [PubMed] [Google Scholar]
- 72.Bazalova M, Carrier J-F, Beaulieu L, Verhaegen F. Dual-energy CT-based material extraction for tissue segmentation in Monte Carlo dose calculations. Phys Med Biol 2008; 53: 2439–56. doi: 10.1088/0031-9155/53/9/015 [DOI] [PubMed] [Google Scholar]
- 73.Landry G, Parodi K, Wildberger JE, Verhaegen F. Deriving concentrations of oxygen and carbon in human tissues using single- and dual-energy CT for ion therapy applications. Phys Med Biol 2013; 58: 5029–48. doi: 10.1088/0031-9155/58/15/5029 [DOI] [PubMed] [Google Scholar]
- 74.Lalonde A, Bouchard H. A general method to derive tissue parameters for Monte Carlo dose calculation with multi-energy CT. Phys Med Biol 2016; 61: 8044–69. doi: 10.1088/0031-9155/61/22/8044 [DOI] [PubMed] [Google Scholar]
- 75.Lalonde A, Bär E, Bouchard H. A Bayesian approach to solve proton stopping powers from noisy multi-energy CT data. Med Phys 2017; 44: 5293–302. doi: 10.1002/mp.12489 [DOI] [PubMed] [Google Scholar]
- 76.Shen C, Li B, Chen L, Yang M, Lou Y, Jia X. Material elemental decomposition in dual and multi-energy CT via a sparsity-dictionary approach for proton stopping power ratio calculation. Med Phys 2018; 45: 1491–503. doi: 10.1002/mp.12796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Han D, Siebers JV, Williamson JF, Linear A. A linear, separable two-parameter model for dual energy CT imaging of proton stopping power computation. Med Phys 2016; 43: 600–12. doi: 10.1118/1.4939082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang S, Han D, Williamson JF, Zhao T, Politte DG, Whiting BR, et al. Experimental implementation of a joint statistical image reconstruction method for proton stopping power mapping from dual-energy CT data. Med Phys 2019; 46: 273–85. doi: 10.1002/mp.13287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tremblay JÉ, Bedwani S, Bouchard H. A theoretical comparison of tissue parameter extraction methods for dual energy computed tomography. Med Phys 2014; 41: 081905. doi: 10.1118/1.4886055 [DOI] [PubMed] [Google Scholar]
- 80.Vilches-Freixas G, Taasti VT, Muren LP, Petersen JBB, Létang JM, Hansen DC, et al. Comparison of projection- and image-based methods for proton stopping power estimation using dual energy CT. Physics and Imaging in Radiation Oncology 2017; 3: 28–36. doi: 10.1016/j.phro.2017.08.001 [DOI] [Google Scholar]
- 81.Zhang S, Han D, Politte DG, Williamson JF, O'Sullivan JA. Impact of joint statistical dual-energy CT reconstruction of proton stopping power images: comparison to image- and sinogram-domain material decomposition approaches. Med Phys 2018; 45: 2129–42. doi: 10.1002/mp.12875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Landry G, Reniers B, Granton PV, van Rooijen B, Beaulieu L, Wildberger JE, et al. Extracting atomic numbers and electron densities from a dual source dual energy CT scanner: experiments and a simulation model. Radiother Oncol 2011; 100: 375–9. doi: 10.1016/j.radonc.2011.08.029 [DOI] [PubMed] [Google Scholar]
- 83.Tsukihara M, Noto Y, Hayakawa T, Saito M. Conversion of the energy-subtracted CT number to electron density based on a single linear relationship: an experimental verification using a clinical dual-source CT scanner. Phys Med Biol 2013; 58: N135–44. doi: 10.1088/0031-9155/58/9/N135 [DOI] [PubMed] [Google Scholar]
- 84.Hansen DC, Seco J, Sørensen TS, Petersen JBB, Wildberger JE, Verhaegen F, et al. A simulation study on proton computed tomography (CT) stopping power accuracy using dual energy CT scans as benchmark. Acta Oncol 2015; 54: 1638–42. doi: 10.3109/0284186X.2015.1061212 [DOI] [PubMed] [Google Scholar]
- 85.Almeida IP, Schyns LEJR, Öllers MC, van Elmpt W, Parodi K, Landry G, et al. Dual-energy CT quantitative imaging: a comparison study between twin-beam and dual-source CT scanners. Med Phys 2017; 44: 171–9. doi: 10.1002/mp.12000 [DOI] [PubMed] [Google Scholar]
- 86.Almeida IP, Landry G, Dedes G, Patel R, Pankuch M, Coutrakon G, et al. Evaluating clinical stopping power estimation from a radiotherapy dual energy CT scanner. Acta Phys Pol B 2017; 48: 1619. doi: 10.5506/APhysPolB.48.1619 [DOI] [Google Scholar]
- 87.Michalak G, Taasti V, Krauss B, Deisher A, Halaweish A, McCollough C. A comparison of relative proton stopping power measurements across patient size using dual- and single-energy CT. Acta Oncol 2017; 56: 1465–71. doi: 10.1080/0284186X.2017.1372625 [DOI] [PubMed] [Google Scholar]
- 88.Hua CH, Shapira N, Merchant TE, Klahr P, Yagil Y. Accuracy of electron density, effective atomic number, and iodine concentration determination with a dual-layer dual-energy computed tomography system. Med Phys 2018; 45: 2486–97. doi: 10.1002/mp.12903 [DOI] [PubMed] [Google Scholar]
- 89.Hudobivnik N, Schwarz F, Johnson T, Agolli L, Dedes G, Tessonnier T, et al. Comparison of proton therapy treatment planning for head tumors with a pencil beam algorithm on dual and single energy CT images. Med Phys 2016; 43: 495–504. doi: 10.1118/1.4939106 [DOI] [PubMed] [Google Scholar]
- 90.Wohlfahrt P, Möhler C, Stützer K, Greilich S, Richter C. Dual-energy CT based proton range prediction in head and pelvic tumor patients. Radiother Oncol 2017; 125: 526–33. doi: 10.1016/j.radonc.2017.09.042 [DOI] [PubMed] [Google Scholar]
- 91.Bär E, Lalonde A, Zhang R, Jee K-W, Yang K, Sharp G, et al. Experimental validation of two dual-energy CT methods for proton therapy using heterogeneous tissue samples. Med Phys 2018; 45: 48–59. doi: 10.1002/mp.12666 [DOI] [PubMed] [Google Scholar]
- 92.Xie Y, Ainsley C, Yin L, Zou W, McDonough J, Solberg TD, et al. Ex vivo validation of a stoichiometric dual energy CT proton stopping power ratio calibration. Phys. Med. Biol. 2018; 63: 055016. doi: 10.1088/1361-6560/aaae91 [DOI] [PubMed] [Google Scholar]
- 93.Taasti VT, Høye EM, Hansen DC, Muren LP, Thygesen J, Skyt PS, et al. Technical note: improving proton stopping power ratio determination for a deformable silicone-based 3D dosimeter using dual energy CT. Med Phys 2016; 43: 2780–4. doi: 10.1118/1.4948677 [DOI] [PubMed] [Google Scholar]
- 94.Zhu J, Penfold SN. Dosimetric comparison of stopping power calibration with dual-energy CT and single-energy CT in proton therapy treatment planning. Med Phys 2016; 43: 2845–54. doi: 10.1118/1.4948683 [DOI] [PubMed] [Google Scholar]
- 95.Möhler C, Russ T, Wohlfahrt P, Elter A, Runz A, Richter C, et al. Experimental verification of stopping-power prediction from single- and dual-energy computed tomography in biological tissues. Phys Med Biol 2018; 63: 025001. doi: 10.1088/1361-6560/aaa1c9 [DOI] [PubMed] [Google Scholar]
- 96.Taasti VT, Michalak GJ, Hansen DC, Deisher AJ, Kruse JJ, Krauss B, et al. Validation of proton stopping power ratio estimation based on dual energy CT using fresh tissue samples. Phys Med Biol 2018; 63: 015012. doi: 10.1088/1361-6560/aa952f [DOI] [PubMed] [Google Scholar]
- 97.Polf JC, Mille MM, Mossahebi S, Chen H, Maggi P, Chen‐Mayer H. Determination of proton stopping power ratio with dual‐energy CT in 3D‐printed tissue/air cavity surrogates. Med Phys 2019. doi: 10.1002/mp.13587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Vilches-Freixas G, Quiñones CT, Létang JM, Rit S. Deriving the mean excitation energy map from dual-energy and proton computed tomography. Physics and Imaging in Radiation Oncology 2018; 6: 20–4. doi: 10.1016/j.phro.2018.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bär E, Andreo P, Lalonde A, Royle G, Bouchard H. Optimized I-values for use with the Bragg additivity rule and their impact on proton stopping power and range uncertainty. Phys Med Biol 2018; 63: 165007: aad312. doi: 10.1088/1361-6560/aad312 [DOI] [PubMed] [Google Scholar]
- 100.De Smet V, Labarbe R, Vander Stappen F, Macq B, Sterpin E. Reassessment of stopping power ratio uncertainties caused by mean excitation energies using a water‐based formalism. Med Phys 2018; 45: 3361–70. doi: 10.1002/mp.12949 [DOI] [PubMed] [Google Scholar]
- 101.Vaniqui A, Schyns LEJR, Almeida IP, van der Heyden B, van Hoof SJ, Verhaegen F. The impact of dual energy CT imaging on dose calculations for pre-clinical studies. Radiat Oncol 2017; 12: 181. doi: 10.1186/s13014-017-0922-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Schyns LEJR, Eekers DBP, van der Heyden B, Almeida IP, Vaniqui A, Verhaegen F. Murine vs human tissue compositions: implications of using human tissue compositions for photon energy absorption in mice. Br J Radiol 2019; 92: 20180454. doi: 10.1259/bjr.20180454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Vaniqui A, Schyns LEJR, Almeida IP, van der Heyden B, Podesta M, Verhaegen F. The effect of different image reconstruction techniques on pre-clinical quantitative imaging and dual-energy CT. Br J Radiol 2019; 92: 20180447. doi: 10.1259/bjr.20180447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Taasti VT, Muren LP, Jensen K, Petersen JBB, Thygesen J, Tietze A, et al. Comparison of single and dual energy CT for stopping power determination in proton therapy of head and neck cancer. Physics and Imaging in Radiation Oncology 2018; 6: 14–19. doi: 10.1016/j.phro.2018.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wohlfahrt P, Möhler C, Enghardt W, Krause M, Kunath D, Menkel S, et al. Refinement of the Hounsfield look-up table by retrospective application of patient-specific direct proton stopping-power prediction from dual-energy CT. Med Phys 2019;. [DOI] [PubMed] [Google Scholar]
- 106.Peters N, Wohlfahrt P, Möhler C, Hofmann C, Greilich S, Richter C. Reduction of range uncertainty in particle treatment planning enabled by Patient-individual stopping-power prediction using dual-energy CT. International Journal of Particle Therapy 2019;. [Google Scholar]
- 107.Schulte R, Bashkirov V, Tianfang Li, Zhengrong Liang, Mueller K, Heimann J, et al. Conceptual design of a proton computed tomography system for applications in proton radiation therapy. IEEE Trans Nucl Sci 2004; 51: 866–72. doi: 10.1109/TNS.2004.829392 [DOI] [Google Scholar]
- 108.Penfold SN, Rosenfeld AB, Schulte RW, Schubert KE. A more accurate reconstruction system matrix for quantitative proton computed tomography. Med Phys 2009; 36: 4511–8. doi: 10.1118/1.3218759 [DOI] [PubMed] [Google Scholar]
- 109.Poludniowski G, Allinson NM, Evans PM. Proton radiography and tomography with application to proton therapy. Br J Radiol 2015; 88: 20150134. doi: 10.1259/bjr.20150134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Prall M, Durante M, Berger T, Przybyla B, Graeff C, Lang PM, et al. High-energy proton imaging for biomedical applications. Sci Rep 2016; 6: 27651. doi: 10.1038/srep27651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Johnson RP, Bashkirov V, DeWitt L, Giacometti V, Hurley RF, Piersimoni P, et al. A fast experimental scanner for proton CT: technical performance and first experience with phantom scans. IEEE Trans Nucl Sci 2016; 63: 52–60. doi: 10.1109/TNS.2015.2491918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dedes G, Dickmann J, Niepel K, Wesp P, Johnson RP, Pankuch M, et al. Experimental comparison of proton CT and dual energy X-ray CT for relative stopping power estimation in proton therapy. Phys Med Biol 2019; 64: 165002: 165002. doi: 10.1088/1361-6560/ab2b72 [DOI] [PubMed] [Google Scholar]
- 113.Schneider U, Pedroni E. Proton radiography as a tool for quality control in proton therapy. Med Phys 1995; 22: 353–63. doi: 10.1118/1.597470 [DOI] [PubMed] [Google Scholar]
- 114.Schneider U, Pemler P, Besserer J, Pedroni E, Lomax A, Kaser-Hotz B. Patient specific optimization of the relation between CT-Hounsfield units and proton stopping power with proton radiography. Med Phys 2005; 32: 195–9. doi: 10.1118/1.1833041 [DOI] [PubMed] [Google Scholar]
- 115.Doolan PJ, Testa M, Sharp G, Bentefour EH, Royle G, Lu H-M. Patient-specific stopping power calibration for proton therapy planning based on single-detector proton radiography. Phys Med Biol 2015; 60: 1901–17. doi: 10.1088/0031-9155/60/5/1901 [DOI] [PubMed] [Google Scholar]
- 116.Farace P, Righetto R, Deffet S, Meijers A, Vander Stappen F, et al. Technical note: a direct ray-tracing method to compute integral depth dose in pencil beam proton radiography with a multilayer ionization chamber. Med Phys 2016; 43: 6405–12. doi: 10.1118/1.4966703 [DOI] [PubMed] [Google Scholar]
- 117.Collins-Fekete C-A, Brousmiche S, Hansen DC, Beaulieu L, Seco J. Pre-Treatment patient-specific stopping power by combining list-mode proton radiography and X-ray CT. Phys. Med. Biol. 2017; 62: 6836–52. doi: 10.1088/1361-6560/aa7c42 [DOI] [PubMed] [Google Scholar]
- 118.Krah N, Patera V, Rit S, Schiavi A, Rinaldi I. Regularised patient-specific stopping power calibration for proton therapy planning based on proton radiographic images. Phys Med Biol 2019; 64: 065008. doi: 10.1088/1361-6560/ab03db [DOI] [PubMed] [Google Scholar]
- 119.Farace P, Righetto R, Meijers A. Pencil beam proton radiography using a multilayer ionization chamber. Phys Med Biol 2016; 61: 4078–87. doi: 10.1088/0031-9155/61/11/4078 [DOI] [PubMed] [Google Scholar]
- 120.Richter C, Pausch G, Barczyk S, Priegnitz M, Keitz I, Thiele J, et al. First clinical application of a prompt gamma based in vivo proton range verification system. Radiother Oncol 2016; 118: 232–7. doi: 10.1016/j.radonc.2016.01.004 [DOI] [PubMed] [Google Scholar]
- 121.Xie Y, Bentefour EH, Janssens G, Smeets J, Vander Stappen F, Hotoiu L, et al. Prompt Gamma Imaging for In Vivo Range Verification of Pencil Beam Scanning Proton Therapy. Int J Radiat Oncol Biol Phys 2017; 99: 210–8. doi: 10.1016/j.ijrobp.2017.04.027 [DOI] [PubMed] [Google Scholar]
- 122.Hueso-González F, Rabe M, Ruggieri TA, Bortfeld T, Verburg JM. A full-scale clinical prototype for proton range verification using prompt gamma-ray spectroscopy. Phys Med Biol 2018; 63: 185019. doi: 10.1088/1361-6560/aad513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Berthold J, Khamfongkhruea C, Petzoldt J, Thiele J, Hölscher T, Wohlfahrt P, et al. Improved accuracy of prompt-gamma-based range verification system enabling validation of CT-based stopping-power prediction. International Journal of Particle Therapy 2019. [Google Scholar]
- 124.Oborn BM, Dowdell S, Metcalfe PE, Crozier S, Mohan R, Keall PJ. Future of medical physics: real‐time MRI‐guided proton therapy. Med Phys 2017; 44: e77–90. doi: 10.1002/mp.12371 [DOI] [PubMed] [Google Scholar]
- 125.Koivula L, Wee L, Korhonen J. Feasibility of MRI-only treatment planning for proton therapy in brain and prostate cancers: dose calculation accuracy in substitute CT images. Med Phys 2016; 43: 4634–42. doi: 10.1118/1.4958677 [DOI] [PubMed] [Google Scholar]
- 126.Maspero M, van den Berg CAT, Landry G, Belka C, Parodi K, Seevinck PR, et al. Feasibility of MR-only proton dose calculations for prostate cancer radiotherapy using a commercial pseudo-CT generation method. Phys Med Biol 2017; 62: 9159–76. doi: 10.1088/1361-6560/aa9677 [DOI] [PubMed] [Google Scholar]
- 127.Uh J, Krasin MJ, Hua C-ho. Technical note: feasibility of MRI-based estimation of water-equivalent path length to detect changes in proton range during treatment courses. Med Phys 2018; 45: 1677–83. doi: 10.1002/mp.12822 [DOI] [PubMed] [Google Scholar]
- 128.Taasti VT, Hansen DC, Michalak GJ, Deisher AJ, Kruse JJ, Muren LP, et al. Theoretical and experimental analysis of photon counting detector CT for proton stopping power prediction. Med Phys 2018; 45: 5186–96. doi: 10.1002/mp.13173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lalonde A, Simard M, Remy C, Bär E, Bouchard H. The impact of dual- and multi-energy CT on proton pencil beam range uncertainties: a Monte Carlo study. Phys Med Biol 2018; 63: 195012. doi: 10.1088/1361-6560/aadf2a [DOI] [PubMed] [Google Scholar]
- 130.Saito M. Simulation of photon-counting detectors for conversion of dual-energy-subtracted computed tomography number to electron density. Radiol Phys Technol 2019; 12: 105–17. doi: 10.1007/s12194-018-00497-0 [DOI] [PubMed] [Google Scholar]
- 131.Simard M, Lapointe A, Lalonde A, Bahig H, Bouchard H. The potential of photon-counting CT for quantitative contrast-enhanced imaging in radiotherapy. Phys Med Biol 2019; 64: 115020–17. doi: 10.1088/1361-6560/ab1af1 [DOI] [PubMed] [Google Scholar]