Abstract
Objective:
Large inoperable sacral chordomas show unsatisfactory local control rates even when treated with high dose proton therapy (PT). The aim of this study is assessing feasibility and reporting early results of patients treated with PT and concomitant hyperthermia (HT).
Methods:
Patients had histologically proven unresectable sacral chordomas and received 70 Gy (relative biological effectiveness) in 2.5 Gy fractions with concomitant weekly HT. Toxicity was assessed according to CTCAE_v4. A volumetric tumor response analysis was performed.
Results:
Five patients were treated with the combined approach. Median baseline tumor volume was 735 cc (range, 369–1142). All patients completed PT and received a median of 5 HT sessions (range, 2–6). Median follow-up was 18 months (range, 9–26). The volumetric analysis showed an objective response of all tumors (median shrinkage 46%; range, 9–72). All patients experienced acute Grade 2–3 local pain. One patient presented with a late Grade 3 iliac fracture.
Conclusion:
Combining PT and HT in large inoperable sacral chordomas is feasible and causes acceptable toxicity. Volumetric analysis shows promising early results, warranting confirmation in the framework of a prospective trial.
Advances in knowledge:
This is an encouraging first report of the feasibility and early results of concomitant HT and PT in treating inoperable sacral chordoma.
Introduction
Large inoperable extracranial chordomas represent a therapeutic challenge due to their intrinsic resistance to both systemic and local treatments. High dose proton therapy (PT), which is the first treatment of choice in non-resectable patients, achieves long term local tumor control rates ranging between 50 and 80%,1–3 with worse results in larger tumors.3 As prognosis is poor after tumor recurrence,4 improving the efficiency of the primary treatment is of paramount importance.
Hyperthermia (HT), consisting in raising tissue temperature between 39 and 42°C, has shown improved tumor control rates when used in combination with other treatment modalities such as radiation therapy and chemotherapy in the management of several cancers, including soft tissue sarcomas.5,6 Furthermore, as PT shares a number of the physical dose distribution properties akin to 12C ions and HT has radiobiological features similar to the high linear energy transfer radiation of 12C ions, a combination of PT and HT could possibly mimic a 12C ion therapy,7 which has shown good results in treating unresectable sacral chordoma.8
We therefore hypothesized that HT delivered concomitantly with high dose PT would improve the outcome of patients treated for large inoperable sacral chordomas. In this paper, we aim to report the early results of five such patients treated with this combined modality of PT and HT.
Methods and materials
We performed a retrospective analysis of the early outcome and volumetric response of patients with inoperable sacral chordoma treated with Pencil Beam Scanning PT with concomitant HT. The institutional database was queried and five patients with inoperable, non-metastatic, biopsy-proven chordoma in the sacral region receiving HT in addition to definitive PT were identified. Local staging was performed by MRI. Distant metastasis was excluded with a thorax, abdomen and pelvis CT. The study was approved by the EKNZ Ethics Board (project ID EKNZ 2018-01156).
Pencil Beam Scanning Intensity Modulated Proton Therapy was administered at the Paul Scherrer Institute (PSI), utilizing a 250 MeV cyclotron. Target volume definition was performed on a three-dimensional high resolution planning CT, matched with a planning MRI. Prescribed dose was 70 Gy (relative biological effectiveness, RBE) in 28 fractions of 2.5 Gy (RBE), delivered five times weekly over 5½ weeks. In four patients, the 70 Gy (RBE) was prescribed to the gross tumor volume (GTV) within a simultaneous integrated boost (SIB) concept. The planning target volume (PTV), corresponding to the area of possible microscopic disease (clinical target volume, or CTV) plus a 7 mm security margin, received 56 Gy (RBE). For the patient treated with the non-SIB/sequential boost paradigm, 70 Gy (RBE) were prescribed to the PTV [2.5 Gy (RBE) daily fraction] which was the GTV, expanded to areas of possible microscopic spread for CTV, with an additional 7 mm security margin. Further details about planning protocols are described in our previous publication.9
Patients received local HT at Kantonsspital Aarau, Switzerland. They underwent a HT planning CT scan using the hammock couch as used in the deep HT unit treating with radiofrequency waves at 100 MHz (BSD 2000 unit, M/s Pyrexar Medical Inc. Salt Lake City UT, USA). The GTV was outlined on consecutive slices. HT treatment planning was carried out using the SigmaHyperPlan software (M/s Dr Sennewald Medizintechnik GmbH, Germany) by segmentation and creation of a grid model of the various body tissues according to their dielectric properties (e.g. tumor, muscle, bone, fat) and specific perfusion factors. Using appropriate power and steering parameters, a specific absorption rate (SAR) distribution in the target volume was generated using finite element modelling.
A warm-up heating phase of 30 min was followed by 60 min of steady state (SS) hyperthermia treatment. The resultant SAR distribution and the tumor temperature were evaluated throughout the target volume. A thermal dose–volume histogram (DVH) was generated to evaluate the temperature distribution in the tumor and the other adjacent normal tissues (Figure 1). No invasive thermometry was used; however Bowman temperature sensors were placed on the skin at the bilateral inguinal regions, gluteal fold, rectum and the urinary bladder. Detailed protocol is also described elsewhere.10 We aimed at an interval between the two treatments kept at 90–120 min. However, as patients had to travel from PSI to Kantonsspital Aarau (around 26 kms) for HT, this proved to be challenging at times.
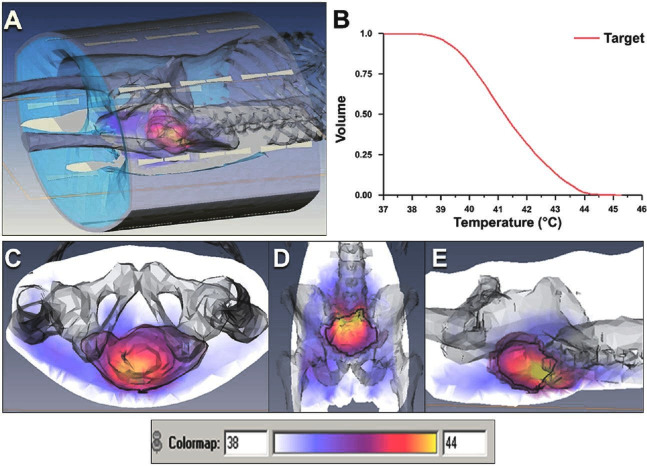
Figure 1.
Hyperthermia treatment plan in Patient 4. (A) Patient outline with the positions of the radiofrequency antennae. (B) Temperature volume histogram. Temperature distributions in the (C) transverse (D) sagittal and (E) coronal planes. Temperature scale representing the color wash is shown in the insert.
Patients were seen at weekly medical consultations during treatment. After treatment, regular clinical and radiological follow-up (FU) were performed by the referring physicians. All data were collected by PSI’s study and Research Office. Reported acute and late adverse events were graded using CTCAE_v4.0.11
A volumetric analysis was performed to assess the tumor response. The pre-PT planning MRI was used as a reference and compared to all available FU MRIs. T1_tse (n = 4) or T2_blade (n = 1) sequences were consistently used for each patient. The choice of sequence type depended on tumor visibility and image availability. GTV was delineated on axial slices with the clinical contouring software Velocity (Varian Medical Systems Inc. Palo Alto, CA), which computed tumor volumes. The contours were reviewed by a senior radiation oncologist. Reference and FU calculated tumor volumes were used to generate volumetric response. An analysis according to the RECIST 1.1 criteria was also performed.12
Results
From May 2016 to October 2017, five male patients referred from centers in Switzerland (n = 2) and the United Kingdom (n = 3) were treated with the combined PT and HT approach with a common treatment protocol. Patient’s characteristics and outcome are detailed in Table 1. Median patient age at diagnosis was 67 years (range, 57–72) and median tumor reference size was 735 cc (range, 369–1142). All patients completed the prescribed PT regimen in a median overall treatment time of 41 days (range, 38–45). They received a median of 5 weekly HT sessions (range, 2–6), either just before (n = 4) or after (n = 1) PT.
Table 1.
Patients’ characteristics and outcome
| Patient number | Sex | Age | Delivered dose [Gy] RBE | Delivered fractions | OTT [days] | HT sessions | Mean maximum rectal SS temp. [°C] | Mean average rectal SS temp [°C] | Mean HT duration [minutes] | Mean HT to PT interval [minutes] | FU [days] | Reference GTV [cc] | GTV on last FU MRI [cc] | Volume reduction [%] | RECIST 1.1 assessment | Initial symptoms (CTCAE_v4) | Acute Toxicity (CTCAE_v4) | Late Toxicity (CTCAE_v4) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Male | 72 | 70.0 | 28 | 41 | 5 | 40.6 | 38.8 | 92 | 161 | 846 | 678 | 171 | 72 | PR | Pain G2 | Pain G3 (sacrum fatigue fracture), Dermatitis G1 | none |
| 2 | Male | 57 | 72.5 | 29 | 45 | 5 | 42.0 | 38.2 | 74 | 139 | 616 | 544 | 293 | 46 | PR | Pain G1 | Dermatitis G2, Pain G2, GI G1 (aerocolia) | Rectal bleeding G1 |
| 3 | Male | 67 | 70.0 | 28 | 42 | 2 | 41.6 | 39.1 | 47 | 142 | 595 | 735 | 400 | 46 | PR | Urinary frequency G2 (G1 after treatment) | Dermatitis G1, Pain G1 | none |
| 4 | Male | 69 | 70.0 | 28 | 38 | 6 | 41.3 | 38.9 | 90 | 96 | 556 | 1142 | 928 | 19 | SD | Pain G1, urinary frequency G1 | Dermatitis G2, Pain G2, Dysuria G2 | Fibrosis G2, Pigmentation G1 |
| 5 | Male | 65 | 70.0 | 28 | 40 | 5 | 41.7 | 39.8 | 91 | 107 | 276 | 369 | 337 | 9 | SD | Pain G2 (G1 after treatment), urinary incontinence G3 | Dermatitis G2, Fatigue G1 | Ala fracture G3 |
CTCAE, common terminology criteria for adverse event; FU, follow up; G1-3, Grade 1–3; GI, gastro intestinal; GTV, gross tumor volume; Gy, grays; HT, hyperthermia; MRI, magnetic resonance imaging; OTT, overall treatment time; PR, partial response; PT, proton therapy; RBE, relative biological effectiveness; RECIST, response evaluation criteria in solid tumors; SD, stable disease; SS, steady state; Temp, temperature.
Mean interval between HT and PT was 123 min (range, 92–172). Median SS maximum and average rectal temperatures were 41.4°C (range, 39.9–42.6) and 39.1°C (range, 37.0–41.7), respectively. Mean and median duration of HT sessions, including the 30 min warmup phase, were 84 and 90 min, respectively (range, 42–94). One patient had to stop HT after two sessions due to pain in the sacral area, thereby making it difficult to maintain the position during the HT treatment sessions. For the same reasons, this patient received shorter HT sessions. Figures 1 and 2 display a PT and a HT treatment plans, respectively.
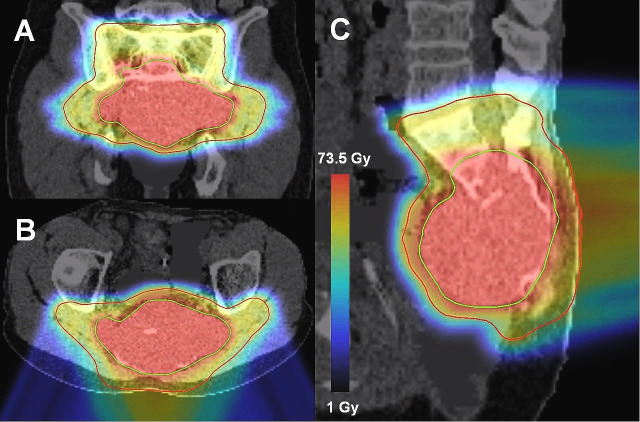
Figure 2.
PBS SIB IMPT plan with two posterior oblique fields in patient 4. (A) coronal, (B) axial and (C) sagittal views. The green and red contours represent the GTV which receives 70 Gy and the PTV which receives 56 Gy, respectively. Radiation dose scale representing the color wash is shown in the insert. IMPT,intensity modulated proton therapy; PBS,Pencil Beam Scanning; SIB, simultaneous integratedboost.
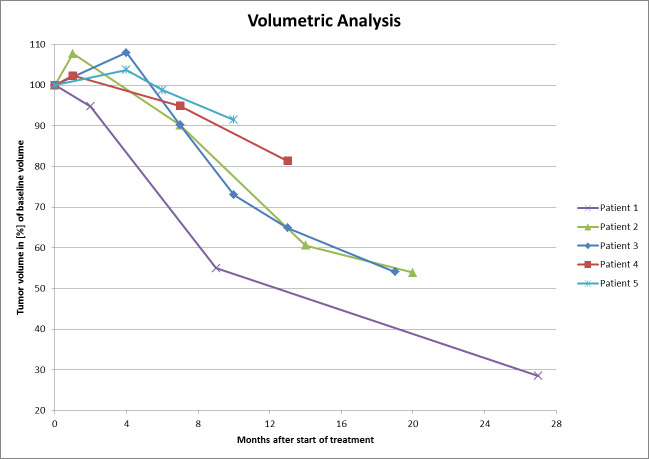
Median FU time after treatment was 18 months (range, 9–26). At last FU, no patient was diagnosed with neither local nor distant recurrence. In the volumetric analysis, we found that all but one patient presented with an initial increase in tumor volume on the first FU MRI, always followed by tumor shrinkage below pre-treatment volume in further FU imaging. Images acquired up to 4 months after the planning MRI showed a 2 to –8% (median, 4%) increase in tumor volume in those four patients. The remaining patient showed an immediate response which continued on subsequent examinations and is illustrated in Figure 3. The median tumor shrinkage was 46% of reference (range, 9–72). According to the revised RECIST criteria, three patients (67%) showed a partial radiological response. The two patients presenting with stable disease had the shortest FU time. Figure 4 illustrates the volumetric evolution of all patients.
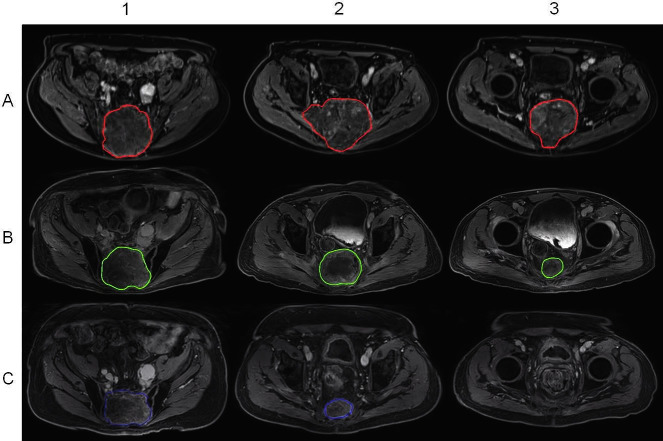
Figure 3.
Tumor response in Patient 1. (A) Baseline, (B) 9 months and (C) 27 months after treatment. Columns represent the (1) cranial, (2) middle and (3) caudal aspects of the tumor. Disease is no longer found in the caudal region 27 months after treatment due to tumor response.
Figure 4.
Volumetric tumor response relative to tumor volume at treatment start.
Local pain was the most common and serious acute adverse effect of treatment and was experienced by all patients, although four (80%) of them were already presenting mild to moderate tumor related pain before treatment. Pain level increased during treatment in all but one patient who reported an improvement from Grade 2 to Grade 1. Nonsteroidal anti-inflammatory drugs were prescribed to all patients, and three additionally received paracetamol, dexamethasone or noramidopyrine and oxycodone, respectively. All patients experienced Grade 1 (n = 1) or 2 (n = 4) radiation dermatitis. One patient presented with Grade 2 dysuria, while another reported improvement in a tumor induced urinary frequency. All acute toxicities resolved completely.
The observed late toxicity is detailed in Table 1. One patient presented with a fracture of the previously involved iliac bone, which required a surgical plasty. This was considered a Grade 3 treatment induced adverse event. Another patient presented with late Grade 2 local fibrosis and Grade 1 skin hyperpigmentation. Grade 1 rectal bleeding was observed in one case.
The patient who interrupted HT after two sessions reported no late toxicity and harbored a tumor response to treatment corresponding to the mean value obtained among the whole cohort.
Discussion
These results provide valuable feasibility data for this combined PT/HT treatment paradigm delivered to patients for which standard treatment provides with suboptimal local tumor control. All patients have been able to complete the prescribed PT, and 80% the prescribed HT. The patient whose HT treatment had to be suspended after two shortened sessions was uncomfortable in lying and maintaining his position in the hyperthermia treatment couch for the entire duration of HT (60 min). Importantly, pain was the major acute side-effect of this combined modality. Proactive pain management was therefore paramount. Of note, acute and late toxicity (Table 1) is in line with other reports.9,13
The 100% local control at 1.5 year found in our study is in line with the previous series of patients treated for spinal chordoma at PSI without HT published by Snider et al.9 Of note, in this previously published analysis of our PSI cohort, we observed that patients with increased PTV size presented with a worse local control, although PTV size does not directly reflect the amount of disease still in place. However, objective radiological response after PT alone was rarely observed. This clinical observation may be in line with the hypothesis of a radiobiological advantage of HT along with PT.7 As failure events started occurring rapidly after the 24 month mark, long-term FU is warranted to fully assess the value of this combined modality approach. Finally, soon to be presented data on our sacral chordoma patients does show worse outcomes in patients with large disease volume present at irradiation.
Kabolizadeh et al performed a volumetric analysis on a cohort of 19 patients with a median tumor volume of 174 cc treated with high dose proton/photon radiation without HT for mobile spine and sacral chordomas.13 They demonstrated a 12 and 24 month target volume reduction of 36 and 55%, respectively. This concurs with the median volumetric tumor response of 46% at 18 months observed in our series, even though our patients had a larger median tumor volume of 735 cc. As tumor shrinkage has been reported to occur in a gradual manner over time, a long-term assessment would be of interest to ascertain the regression patterns of these tumors.
Patients in our series fared at least as well as those treated with 12C ions alone or in combination with photon irradiation in publications by Imai et al8 and Uhl et al14 in terms of short term local control and toxicity. More detailed comparison is difficult as no volumetric analysis was performed in the 12C ion cohorts and due to the short FU time in this study. A randomized Phase II trial comparing 12C and ion and PT is currently underway in Heidelberg and will bring more insight on potential differences between the two treatment modalities.15
As a single institution study, our results can only be viewed as hypothesis generating. There were several limitations of our study. First, the study design was retrospective in nature and thus lacked complete data for certain variables such as quality of life. Second, the small sample size of five patients and short FU limited any conclusion on the success of this combined treatment paradigm to durably control large inoperable sacral chordomas. However, the initial results of this novel approach of combining HT with PT provide with feasibility data and are quite encouraging and merit future consideration.
Conclusion
High dose PT in combination with deep HT in the initial management of large inoperable sacral chordoma is feasible and well tolerated provided proactive pain management. Those early results that show promising tumor response, local and distant disease control rates need to be confirmed by prospective controlled trials.
Footnotes
Acknowledgment: This project was supported by the Forschungsrat Kantonsspital Aarau, The Krebsliga Aargau and the Kelm Stiftung. The contribution to this research study by BM Seddon was supported by the National Institute for Health Research University College London Hospitals Biomedical Research Centre.
Contributor Information
Sebastien Tran, Email: sebastien.tran@me.com.
Emsad Puric, Email: Emsad.Puric@ksa.ch.
Marc Walser, Email: Marc.Walser@psi.ch.
Robert Poel, Email: Robert.Poel@psi.ch.
Niloy Ranjan Datta, Email: NiloyRanjan.Datta@ksa.ch.
Juerg Heuberger, Email: juerg.heuberger@ksa.ch.
Alessia Pica, Email: alessia.pica@psi.ch.
Dietmar Marder, Email: Dietmar.Marder@ksa.ch.
Nicoletta Lomax, Email: nicoletta.lomax@ksa.ch.
Alessandra Bolsi, Email: alessandra.bolsi@psi.ch.
Petra Morach, Email: petra.morach@psi.ch.
Barbara Bachtiary, Email: barbara.bachtiary@psi.ch.
Beatrice M Seddon, Email: beatrice.seddon@nhs.net.
Ralf Schneider, Email: ralf.schneider@helios-kliniken.de.
Stephan Bodis, Email: stephan.bodis@ksa.ch.
Damien C Weber, Email: damien.weber@psi.ch.
REFERENCES
- 1.DeLaney TF, Liebsch NJ, Pedlow FX, Adams J, Weyman EA, Yeap BY, et al. Long-term results of phase II study of high dose photon/proton radiotherapy in the management of spine chordomas, chondrosarcomas, and other sarcomas. J Surg Oncol 2014; 110: 115–22. doi: 10.1002/jso.23617 [DOI] [PubMed] [Google Scholar]
- 2.Park L, Delaney TF, Liebsch NJ, Hornicek FJ, Goldberg S, Mankin H, et al. Sacral chordomas: impact of high-dose proton/photon-beam radiation therapy combined with or without surgery for primary versus recurrent tumor. Int J Radiat Oncol Biol Phys 2006; 65: 1514–21. doi: 10.1016/j.ijrobp.2006.02.059 [DOI] [PubMed] [Google Scholar]
- 3.Chen Y-L, Liebsch N, Kobayashi W, Goldberg S, Kirsch D, Calkins G, et al. Definitive high-dose photon/proton radiotherapy for unresected mobile spine and sacral chordomas. Spine 2013; 38: E930–E936. doi: 10.1097/BRS.0b013e318296e7d7 [DOI] [PubMed] [Google Scholar]
- 4.Pennicooke B, Laufer I, Sahgal A, Varga PP, Gokaslan ZL, Bilsky MH, et al. Safety and local control of radiation therapy for chordoma of the spine and sacrum: a systematic review. Spine 2016; 41 Suppl 20(Suppl 20): S186–S192. doi: 10.1097/BRS.0000000000001831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Datta NR, Ordóñez SG, Gaipl US, Paulides MM, Crezee H, Gellermann J, et al. Local hyperthermia combined with radiotherapy and-/or chemotherapy: recent advances and promises for the future. Cancer Treat Rev 2015; 41: 742–53. doi: 10.1016/j.ctrv.2015.05.009 [DOI] [PubMed] [Google Scholar]
- 6.Aiba H, Yamada S, Mizutani J, Yamamoto N, Okamoto H, Hayashi K, et al. Clinical outcomes of radio-hyperthermo-chemotherapy for soft tissue sarcoma compared to a soft tissue sarcoma registry in Japan: a retrospective matched-pair cohort study. Cancer Med 2018; 7: 1560–71. doi: 10.1002/cam4.1366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Datta NR, Puric E, Schneider R, Weber DC, Rogers S, Bodis S. Could hyperthermia with proton therapy mimic carbon ion therapy? Exploring a thermo-radiobiological rationale. Int J Hyperthermia 2014; 30: 524–30. doi: 10.3109/02656736.2014.963703 [DOI] [PubMed] [Google Scholar]
- 8.Imai R, Kamada T, Araki N. Working Group for B, soft tissue S. carbon ion radiation therapy for unresectable sacral chordoma: an analysis of 188 cases. Int J Radiat Oncol Biol Phys 2016; 95: 322–7. [DOI] [PubMed] [Google Scholar]
- 9.Snider JW, Schneider RA, Poelma-Tap D, Stieb S, Murray FR, Placidi L, et al. Long-term outcomes and prognostic factors after Pencil-Beam scanning proton radiation therapy for spinal chordomas: a large, single-institution cohort. Int J Radiat Oncol Biol Phys 2018; 101: 226–33. doi: 10.1016/j.ijrobp.2018.01.060 [DOI] [PubMed] [Google Scholar]
- 10.Datta NR, Schneider R, Puric E, Ahlhelm FJ, Marder D, Bodis S, et al. Proton irradiation with hyperthermia in unresectable soft tissue sarcoma. International Journal of Particle Therapy 2016; 3: 327–36. doi: 10.14338/IJPT-16-00016.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Common Terminology Criteria for Adverse Events (CTCAE) v4.0 2010.. Available from: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm.. [PubMed]
- 12.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1. Eur J Cancer 2009; 45: 228–47. doi: 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 13.Kabolizadeh P, Chen Y-L, Liebsch N, Hornicek FJ, Schwab JH, Choy E, et al. Updated outcome and analysis of tumor response in mobile spine and sacral chordoma treated with definitive high-dose Photon/Proton radiation therapy. Int J Radiat Oncol Biol Phys 2017; 97: 254–62. doi: 10.1016/j.ijrobp.2016.10.006 [DOI] [PubMed] [Google Scholar]
- 14.Uhl M, Welzel T, Jensen A, Ellerbrock M, Haberer T, Jäkel O, et al. Carbon ion beam treatment in patients with primary and recurrent sacrococcygeal chordoma. Strahlenther Onkol 2015; 191: 597–603. doi: 10.1007/s00066-015-0825-3 [DOI] [PubMed] [Google Scholar]
- 15.Uhl M, Edler L, Jensen AD, Habl G, Oelmann J, Röder F, et al. Randomized phase II trial of hypofractionated proton versus carbon ion radiation therapy in patients with sacrococcygeal chordoma-the ISAC trial protocol. Radiat Oncol 2014; 9: 100. doi: 10.1186/1748-717X-9-100 [DOI] [PMC free article] [PubMed] [Google Scholar]