Abstract
Recent studies on cancer stem cells revealed they are tumorigenic and able to recapitulate the characteristics of the tumour from which they derive, so that it was suggested that elimination of this population is essential to prevent recurrences after any treatment. However, there is evidence that cancer stem cells are inherently resistant to conventional (photon) radiotherapy. Since the use of proton beam therapy in cancer treatment is growing rapidly worldwide, mainly because of their excellent dosimetric properties, the possibility could be considered that they also have biological advantages through preferential elimination of cancer stem cells.
Indeed, a review of preclinical data suggest that protons and photons differ in their biological effects on cancer stem cells, with protons offering potential advantages, although the heterogeneity of cancer stem cells and the different proton irradiation modalities make the comparison of the results not so easy. Further research to understand the mechanisms underlying such effects is important for their possible exploitation in clinics and to perform proton beam therapy optimization.
Introduction
The cancer stem cells issue
Cancer stem cells (CSCs) are considered the most likely cause for cancer recurrence due to their properties of self-renewal, multipotency, plasticity and tumorigenicity.1
CSCs can survive and usually persist in tumours for a substantial length of time as a distinct population. They are supported within a histological niche (CSC microenvironment), composed of connective stroma and vascular tissue, that provides a hypoxic microenvironment that induce the quiescent state in CSCs and gives resistance to oxidative stress. Niches can reside in different locations including hypoxic, perivascular and invasive tumor areas that can dynamically change during tumor development and treatment.2 In addition, epigenetic changes of CSCs may activate the biological programme termed epithelial-to-mesenchymal transition (EMT) with acquisition of an elongated, fibroblast-like morphology as well as an increased capacity for migration and invasion. There is also a strong correlation between an EMT-associated gene-expression signature and treatment resistance.3
There is growing evidence that CSCs play important roles in regulating cancer radiation response. They are inherently resistant to conventional photon radiotherapy, and to other cancer therapies. The CSC radioresistance has been related in particular to their efficient repair capability, hypoxic environment, increased expression of free radical scavenging systems, existence in the quiescent state or slow progression through the cell cycle.2,4,5
An intriguing aspect is that CSCs can paradoxically be induced from non-stem cancer cells by radiotherapy itself, constituting the subpopulation of induced cancer stem cells (iCSCs). This plasticity represents a potential adverse side-effect of radiotherapy, possibly contributing to tumour recurrence and metastasis.6
Furthermore, photon irradiation at clinical doses has been shown to enhance migratory properties of various tumour cell lines, a behaviour attributed to EMT, where epithelial tumour cells may also acquire CSC traits.1,2,7
Rationale for proton beam therapy
During the past decade, the application of proton beam therapy (PBT) has been rapidly increasing worldwide and gradually expanding for the treatment of various malignancies.5,8
Therapeutic proton beams are made of several single Bragg peaks superimposition, giving the so-called spread out Bragg peak (SOBP) where most protons are of relatively high energy and low linear energy transfer (LET), except in the narrow distal region where only stopping protons are mostly relevant. Therefore, they are assumed to have little or no biological advantages, such as decreased radiation resistance of hypoxic tumour cells and reduced cell cycle dependence of radiation response, typical of high-LET radiation. The excellent dosimetric properties achieved with PBT is currently assumed to be their main advantage over conventional photon therapy, as it allows delivery of more conformal treatment, minimizing exposure to healthy tissue, with reduction of unwanted side-effects, and/or allowing for dose escalation with higher probability of cure.
Although limited data exist on long-term outcomes of PBT for cancer care, it was reported that PBT, compared to radiotherapy with photons, might present some advantages such as improving the outcome for breast cancer, increasing the overall survival in non-small cell lung cancer (NSCLC) and neurological patients, and giving a favourable tumour control in high-risk patients with prostate cancer.8
Even if the dosimetric properties of protons are considered the key for improvements on long-term morbidity and mortality, preclinical studies have indicated the possibility that they may have biological advantages through preferential action against CSCs. This might contribute for making PBT more efficient than photon radiotherapy also in terms of reduced recurrence probability.
Evidence from preclinical studies
Preclinical data suggest that proton irradiation, even at the SOBP, may have greater capability of eliminating CSCs than photons at comparable doses.
It has been reported that CSCs isolated from the breast cancer cell line MCF7, besides being more resistant than non-stem cancer cells to either ~2 keV/µm proton or γ-irradiation, are more susceptible to apoptosis as well as to DNA damage after proton irradiation.9
Results obtained irradiating with a therapeutic proton SOBP different subpopulations of NSCLC cells, including CSC-like cells derived from them, have shown that SOBP protons better target CSC-like cells than photons do for many end points, i.e. cell viability, clonogenic survival, apoptosis, cell migration or invasiveness, and tumour sphere formation.10 The suggested reason for such increased sensitivity is a greater reactive oxygen species (ROS) generation by protons. In another study, it was reported that, at equivalent doses, ~13 keV/µm protons are more effective than photons in reducing population of CSC-like cells derived from NSCLC.7 The quantitative and qualitative differences found in the global gene expression profile seem to impart significant biological advantages to protons as compared to photons in terms of G2/M arrest, suppression of EMT and CSCs phenotypes.
Another study, aimed at comparing ~6 keV/µm proton and γ-irradiation in SW620 colon cancer cells, showed no significant difference in clonogenic survival and apoptotic rate, but significantly less accumulation of CSC-like cells in the proton irradiated cells.11
Of special interest are the studies on glioma stem cells (GSCs) from patients affected by glioblastoma multiforme, known to be among the most radioresistant tumours. Comparison of two GSC lines irradiated with protons or C-ions (with LET of ~1.1 keV/µm and 43.2 keV/µm, respectively) indicated that particles are more effective in cell killing than photons, likely because of a different quality of the induced DNA damage.12 This study also indicated some heterogeneity in radiosensitivity of GSCs from different patients. Individual heterogeneity was also observed by other authors13 among four GSC lines resistant to photons as the relative biological effectiveness (RBE) values for clonogenic survival after irradiation with a therapeutic proton SOBP were found to vary in the range 0.7–1.2. Moreover, it was reported that GSCs cytotoxicity (as measured by neurosphere formation) is greater with proton SOBP than with photon radiation. Protons also induce more single- and double-stranded DNA breaks, less H2AX phosphorylation, increased Chk2 phosphorylation, reduced cell cycle recovery from G2 arrest, and stronger cell apoptosis together with a larger quantity of ROS.14
Furthermore, exposure of stem-like cells from human prostate carcinoma and breast carcinoma to a dose of 8 Gy in a therapeutic proton SOBP have little or no significant effect on their viability, while it significantly increases cell-surface expression and translocation of calreticulin, suggesting a modulatory effect in facilitating immune attack of CSCs. These findings offer a rationale for the use of protons in combination with immunotherapy.15
Discussion
It is not easy to compare the preclinical results as they depend on the type of CSC examined, and even cells derived from the same type of cancer, show heterogeneous radiation responses. Moreover, an increasing body of evidence supports the variability of CSC biomarkers during radiotherapy together with consequences for the predictive value of the respective marker.2 Accordingly, the findings based on the enrichment by radiation of the fraction of cells expressing CSC markers have to be taken with cautions. Also, the proton energies considered in the available studies are not always comparable, the mean LET used ranging from ~1 to ~12 keV/μm (Table 1). Nevertheless, overall these results suggest that protons and photons differ in their biological effects, with protons offering potential advantages for CSC eradication and local tumor control.
Table 1.
LET values of the proton beams used in the preclinical studies considered in this paper and corresponding CSC types studied
| Ref. | Facility | Mono-energetic beam | SOBP | CSC type | |
|---|---|---|---|---|---|
| LET (keV/µm) | Position (water eq. depth, cm) | Average LETa (keV/µm) | |||
| Fu et al 20129 | Tandem Peking University (China) | ~2.0 | Breast cancer | ||
| Quan et al 201211 | Tandem Peking University (China) | ~6.0 | Colon cancer | ||
| Narang et al 20157 | Tandem Bhabha Atomic Research Centre, Mumbai (India) | ~13.0 | Lung cancer (NSCLC) | ||
| Pecchia et al 201512 | Istituto Nazionale di Fisica Nucleare, Catania (Italy) | 1.1 | Glioma (GSC) | ||
| Zhang et al 201310 | M.D. Anderson Cancer Center, Houston,TX (USA) | 14 | ~1 | Lung cancer (NSCLC) | |
| [td] | HIT, Heidelberg (Germany) | 12 | ~1 | Glioma (GSC) | |
| Mitteer et al 201514 | Roberts Proton Therapy Center, Philadelphia, PA (USA) | 12–15 | ~1 –~5 | Glioma (GSC) | |
| Gameiro et al 201615 | M.D. Anderson Cancer Center, Houston,TX (USA) | 14 | ~1 | Prostate, breast and lung cancers, chordoma | |
CSC, cancer stem cell; GSC, glioma stem cell; LET, linear energy transfer; NSCLC, non-small cell lung cancer; SOBP, spread out Bragg peak.
Evaluation by the present Authors based on simulations with Monte Carlo approaches as described in Belli et al.16
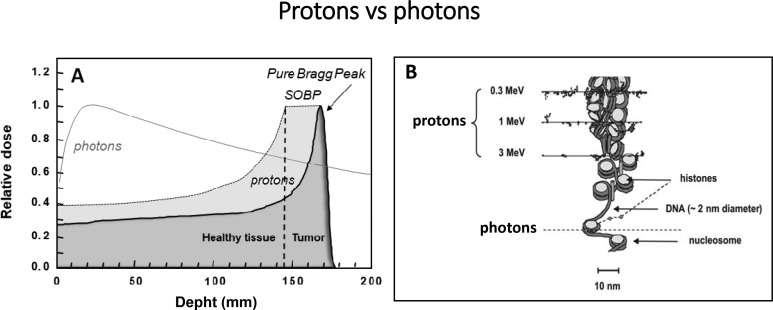
Understanding the mechanisms underlying such effects is important for their exploitation in clinics and PBT optimization. Clearly, the biological differences between photons and protons must stem from their differences in energy deposition at microscopic level (Figure 1). Proton track structure implies events that are spatially correlated to higher degree than those with photons. However, protons in a clinical SOBP have relatively low LET values on average, accounting for the use in clinical practice of an RBE of only 1.1. A possible explanation for the observed proton effectiveness in CSCs is the presence of low-energy, high-LET components, even in the middle of a SOBP. These components are known to induce complex, difficult to repair, DNA damage and could be effective in killing specifically radioresistant CSCs. Moreover, a role could be played by microscopic features of energy deposited by protons that are not described by LET. If proton irradiated CSCs were inactivated with similar effectiveness as non-stem cancer cells, also the effect of plasticity, seen as a critical aspect in photon therapy, would be ruled out.
Figure 1.
Schematic representation of the major features of photons and protons as used in PBT: (A) macroscopic/dosimetric level; (B) microscopic level (track structure) showing the peculiar distribution of energy deposition events of protons at the chromatin scale, the low-energy protons being predominant in the Bragg peak near path end (modified from Belli et al 17). PBT, proton beam therapy.
Future research is expected to consolidate and extend the preclinical data, so as to uncover the relationship between the physical properties of protons (LET and/or track structure features) and the favourable CSC biological responses. The possible underlying mechanisms, such as the increased production of ROS already proposed as a key mechanism of radioresistance for several types of CSCs, the differences in other responses, e.g. de-differentiation, epigenetic changes, and the impact on the tumour microenvironment are essential aspects to be better clarified. Finally, in order to understand if, and how, use of protons can improve cancer therapy by targeting CSCs, it is necessary to substantiate preclinical data with the results coming from CSC focused clinical trials, presently still very few.
Footnotes
Funding: Authors acknowledge funding from Istituto Nazionale di Fisica Nucleare (INFN), Scientific Commission V, ‘RADIOSTEM’ experiment.
Contributor Information
Valentina Dini, Email: valentina.dini@iss.it.
Mauro Belli, Email: mau.belli1@gmail.com.
Maria Antonella Tabocchini, Email: antonella.tabocchini@iss.it.
REFERENCES
- 1.Qiu H, Fang X, Luo Q, Ouyang G. Cancer stem cells: a potential target for cancer therapy. Cell Mol Life Sci 2015; 72: 3411–24. doi: 10.1007/s00018-015-1920-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krause M, Dubrovska A, Linge A, Baumann M. Cancer stem cells: radioresistance, prediction of radiotherapy outcome and specific targets for combined treatments. Adv Drug Deliv Rev 2017; 109: 63–73. doi: 10.1016/j.addr.2016.02.002 [DOI] [PubMed] [Google Scholar]
- 3.Shibue T, Weinberg RA. Emt, CSCs, and drug resistance: the mechanistic link and clinical implications. Nat Rev Clin Oncol 2017; 14: 611–29. doi: 10.1038/nrclinonc.2017.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang L, Graham P, Hao J, Ni J, Deng J, Bucci J, et al. Cancer stem cells and signaling pathways in radioresistance. Oncotarget 2016; 7: 11002–17. doi: 10.18632/oncotarget.6760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Held KD, Kawamura H, Kaminuma T, Paz AES, Yoshida Y, Liu Q, et al. Effects of charged particles on human tumor cells. Front Oncol 2016; 6: 23. doi: 10.3389/fonc.2016.00023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen X, Liao R, Li D, Sun J. Induced cancer stem cells generated by radiochemotherapy and their therapeutic implications. Oncotarget 2017; 8: 17312–17301. doi: 10.18632/oncotarget.14230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Narang H, Kumar A, Bhat N, Pandey BN, Ghosh A. Effect of proton and gamma irradiation on human lung carcinoma cells: gene expression, cell cycle, cell death, epithelial–mesenchymal transition and cancer-stem cell trait as biological end points. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis 2015; 780: 35–46. doi: 10.1016/j.mrfmmm.2015.07.006 [DOI] [PubMed] [Google Scholar]
- 8.Hu M, Jiang L, Cui X, Zhang J, Yu J. Proton beam therapy for cancer in the era of precision medicine. J Hematol Oncol 2018; 11: 136. doi: 10.1186/s13045-018-0683-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fu Q, Quan Y, Wang W, Mei T, Wu J, Li J, et al. Response of cancer stem-like cells and non-stem cancer cells to proton and γ-ray irradiation. Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms 2012; 286: 346–50. doi: 10.1016/j.nimb.2012.01.032 [DOI] [Google Scholar]
- 10.Zhang X, Lin SH, Fang B, Gillin M, Mohan R, Chang JY. Therapy-Resistant cancer stem cells have differing sensitivity to photon versus proton beam radiation. Journal of Thoracic Oncology 2013; 8: 1484–91. doi: 10.1097/JTO.0b013e3182a5fdcb [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Quan Y, Wang W, Fu Q, Mei T, Wu J, Li J, et al. Accumulation efficiency of cancer stem-like cells post γ-ray and proton irradiation. Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms 2012; 286: 341–5. doi: 10.1016/j.nimb.2011.11.019 [DOI] [Google Scholar]
- 12.Pecchia I, Dini V, Ricci-Vitiani L, Biffoni M, Balduzzi M, Fratini E, Ricci-Vitiani L, Pelacchi F, Pallini R, et al. Glioblastoma stem cells: radiobiological response to ionising radiations of different qualities. Radiat Prot Dosimetry 2015; 166(1-4): 374–8. doi: 10.1093/rpd/ncv299 [DOI] [PubMed] [Google Scholar]
- 13.Chiblak S, Tang Z, Campos B, Gal Z, Unterberg A, Debus J, et al. Radiosensitivity of patient-derived glioma stem cell 3-dimensional cultures to photon, proton, and carbon irradiation. Int J Radiat Oncol Biol Phys 2016; 95: 112–9. doi: 10.1016/j.ijrobp.2015.06.015 [DOI] [PubMed] [Google Scholar]
- 14.Alan Mitteer R, Wang Y, Shah J, Gordon S, Fager M, Butter P-P, et al. Proton beam radiation induces DNA damage and cell apoptosis in glioma stem cells through reactive oxygen species. Sci Rep 2015; 5: 13961. doi: 10.1038/srep13961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gameiro SR, Malamas AS, Bernstein MB, Tsang KY, Vassantachart A, Sahoo N, et al. Tumor cells surviving exposure to proton or photon radiation share a common immunogenic modulation signature, rendering them more sensitive to T Cell–Mediated killing. Int J Radiat Oncol Biol Phys 2016; 95: 120–30. doi: 10.1016/j.ijrobp.2016.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Belli M, Campa A, Ermolli I. A semi-empirical approach to the evaluation of the relative biological effectiveness of therapeutic proton beams: the methodological framework. Radiat Res 1997; 148: 592–8. doi: 10.2307/3579735 [DOI] [PubMed] [Google Scholar]
- 17.Belli M, Sapora O, Tabocchini MA. Molecular targets in cellular response to ionizing radiation and implications in space radiation protection. J Radiat Res 2002; 43 Suppl(S): S13–S19. doi: 10.1269/jrr.43.S13 [DOI] [PubMed] [Google Scholar]