Abstract
Proton therapy (PT) has been administered for many years to a number of cancers, including brain tumours. Due to their remarkable physical properties, delivering their radiation to a very precise brain volume with no exit dose, protons are particularly appropriate for these tumours. The decrease of the brain integral dose may translate with a diminution of neuro-cognitive toxicity and increase of quality of life, particularly so in children. The brain tumour patient’s access to PT will be substantially increased in the future, with many new facilities being planned or currently constructed in Europe, Asia and the United States. Although approximately 150’000 patients have been treated with PT, no level I evidence has been demonstrated for this treatment. As such, it is this necessary to generate high-quality data and some new prospective trials will include protons or will be activated to compare photons to protons in a randomized design. PT comes however with an additional cost factor that may contribute to the ever-growing health’s expenditure allocated to cancer management. These additional costs and financial toxicity will have to be analysed in the light of a more conformal radiation delivery, non-target brain irradiation and lack of potential for dose escalation when compared to photons. The latter is due to the radiosensitivity of organs at risk in vicinity of the brain tumour, that photons cannot spare optimally. Consequentially, radiation-induced toxicities and tumour recurrences, which are cost-intensive, may decrease with PT resulting in an optimized photon/proton financial ratio in the end.
Advances in knowledge:
This review details the indication of brain tumors for proton therapy and give a list of the open prospective trials for these challenging tumors.
Introduction
Radiotherapy (RT) is an important treatment modality in the management of Central Nervous System (CNS) tumours for the optimization of tumour local control. Unlike other non-CNS malignancies, brain tumours have rarely a propensity for distant non-CNS metastases and consequentially local control is key for the cure of these challenging malignancies. Recent advances in radiation techniques include the use of intensity- (IMRT) or volumetric- modulated radiotherapy/arc therapy (VMAT), stereotactic radiosurgery/fractionated RT (SRS/SFRT) and particle therapy (mostly protons and carbon ions). As a result of optimal dose conformation provided by the latter modality, particles can be used in a dose-escalation paradigm and/or for dose sparing of critical structures/organs at risks. The former could be applied to radio-resistant CNS tumours,1 such as skull base chordoma and chondrosarcoma, or non-benign meningiomas2 and the latter in patients with a favorable prognosis, such as those with benign/low-grade brain tumours. For children, RT has been associated with a number of acute and late adverse events detailed later in this paper. Protons may decrease the rate of acute-3 and, more importantly, late toxicity4 usually seen with photon therapy and would thus increase substantially the therapeutic ratio of RT. The present paper details the most recent data for proton therapy delivered to patients with CNS tumours. Noteworthy, no data regarding carbon ions for CNS malignancies will be summarized in this manuscript and we have included skull base tumors in this brain tumor review, as it is a major indication for protons. First, this paper will detail the rational of using protons for treating brain tumors. Second, an overview of the existing trials will be described and an attempt to discuss the limitations of such studies will be made. A summary of existing data for CNS tumours in adults and children alike will be provided and each section will finish with statements pertaining to the informed analysis. Finally, the additional costs and potential financial toxicity for patients of protons will be discussed at the end of this paper.
Proton beam therapy for brain tumours
This section elaborates on the physical rationale and dosimetric evidence for PT. Dose distribution in PT is characterized by a well-defined maximum range, which is a function of the initial energy, and a sharply defined Bragg Peak, where the bulk of the dose is deposited.5 Beyond the Bragg Peak, the dose drops to zero within a few millimetres. Compared to photons, this dose deposition results in a superior dose conformality and lower total integral dose delivered to surrounding tissue. This remarkable dose-profile is even more pronounced for Intensity Modulated PT (IMPT) available for Pencil Beam Scanning (PBS) systems, which can achieve particularly steep dose gradients.6 CNS tumours are inevitable in vicinity to many critical organs at risk (OARs),7 making them an especially relevant indication for PT. This rationale is supported by clear evidence for a dose-response relationship for many radiation-induced toxicities seen after RT for brain tumours. As an example, dose to the hypothalamus and pituitary correlates with the degree of endocrine dysfunction,8 and dose to the hippocampus correlates with memory outcomes.9
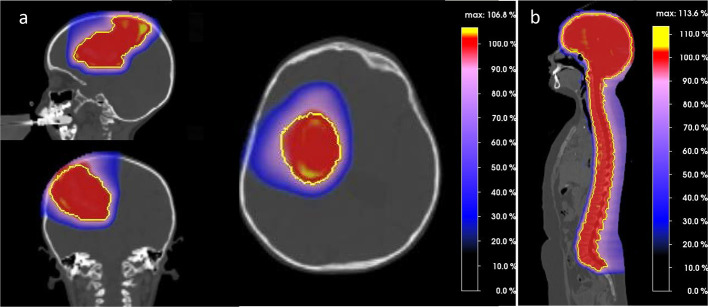
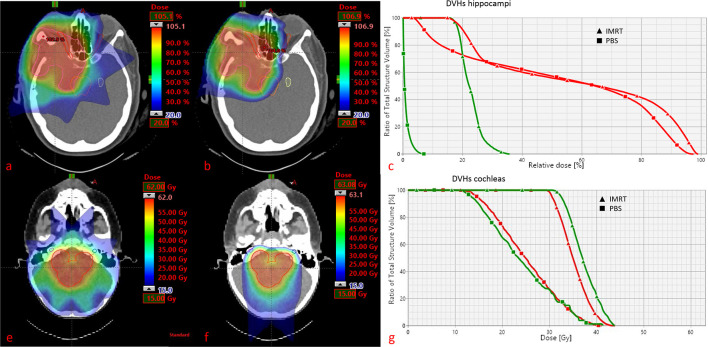
The dosimetric advantage of PT compared to photons is undisputed, but the magnitude of clinical benefit is unknown. This benefit may substantially differ from individual cases to cases and is to a large degree dependent on tumor location. Adeberg et al10 evaluated the relative benefit of protons for five typical brain tumor locations and suggested that in general parietal tumours seem to benefit the most in terms of brain sparing. An exemplary PT dose distribution for a parieto-frontal located brain tumor is shown in Figure 1a. Even for very complex target volumes (Figure 2) involving large parts of the brain, such in whole-ventricular RT for intracranial germ cell tumours, a dosimetric comparison study showed an approximately one-third reduction in integral dose to the brain, and also a better sparing of the circle of Willis with PT.11 This may be clinically significant, as radiation dose delivered to the circle of Willis was recently proposed as the best predictor of stroke in childhood brain cancer survivors.12 Moreover the dosimetric advantage is particularly striking for large target volumes, such in the case of craniospinal irradiation (CSI), where PT is able to completely spare OARs anterior to the vertebrae, as it is demonstrated in Figure 1b. Compared with modern photon techniques, PT obtained the lowest mean doses for OARs in CSI, with dose reductions of >10.0 Gy for parotid glands, thyroid and pancreas.13 Figure 2 details the decrease of dose delivered with PT as opposed to IMRT to the hippocampus and cochleas’ for a supra- and infratentorial tumor, respectively. Tamura et al14 estimated by in silico modelling that the use of PT instead of photons may result in a decrease in the lifetime attributable risk of radiation-induced secondary cancer after CSI. Although these results of in silico modelling have been confirmed by a previous study,15 caution should be taken not to over interpret these data stemming from a modelling computational paradigm, which do not represent ‘real-life’ data. Preliminary evidence suggests that this PT dosimetric gain also translates into a clinical benefit such as, for example, reduced neuro-cognitive disability16 and improved quality of life.17
Figure 1.
Sagittal, axial and coronal views of proton dose distribution for a parieto-frontal tumor. PTV is shown in yellow. Figure 1b. Sagittal view of proton dose distribution for craniospinal irradiation. PTV is shown in yellow.Noteworthy, the color wash dose level display all dose levels. As such, absence of colors equals to absence of dose.
Figure 2.
Supratentorial meningioma case. Doses as % of the prescribed dose and minimum dose level set at 20%. (a) IMRT dose distribution; (b) PBS dose distribution; (c) DVHs for homolateral (red) and controlateral (green) hippocampus. VOIs represented are PTV, brainstem, optic nerves and contralateral hippocampus. Infratentorial ependymoma case. Doses as absolute (GyRBE), minimum dose level set at 15GyRBE. (e) IMRT dose distribution; (f) PBS dose distribution; (g) DVHs for both cochleas (red and green). VOIs represented are PTV and both cochleas.
The superior dose conformality of PT (Figure 2) has also however its dose-delivery hazards, in terms of increased sensitivity to range and setup uncertainties, particularly so for IMPT. Robust planning18 and robust optimization19 can help to mitigate these dosimetric uncertainties. Another concern is the clinically use of a constant value of 1.1 for the relative biological effectiveness (RBE) for PT planning, whereas it is well known that RBE increases with increasing linear energy transfer (LET), thus presenting the highest value in the distal fall-off.20 Other factors, not limited but including total dose, fractional dose, biological endpoint, oxygenation and tissue or cell type (characterized normally by α/β) have an influence on RBE.20,21 LET/RBE evaluation and LET optimization of PT plans can avoid high LET areas, and therefore unintended increase in biological dose, in critical structures such as the brainstem22 or periventricular area23 where the brain stem-cells are located. There is a concern within the community that high-LET values at the distal range of the beam may cause toxicities be it radiological24 or clinical.25 For example, adjusting treatment field angles for posterior fossa tumours can substantially reduce LET values in this OAR.26 Future developments in these and other areas are likely to only enhance the benefits of PT even further.
Establishing the role of proton therapy for the management of brain tumours: clinical trials
In the era of evidence-base medicine (EBM), high quality of data27 is needed to justify the additional cost factor associated with proton therapy for brain and non-CNS tumours alike. Although some authors have challenged the hierarchal evidence paradigm in EBM,28 it remains that randomised controlled trials (RCTs), representing the so-called level I evidence, are of paramount importance in the assessment of the ‘value’ of any treatment modality in cancer care. The ethical issues of such RCTs for proton therapy have been long debated and are not the focus of this section.29 Clinical validation of proton therapy can also be achieved with a non-RCT paradigm: specifically, a model-based driven validation approach, with an enrichment of the experimental arm.30 It is foreseeable that a combination of these trial strategies will best generate data that will create scientifically sound evidence on how best to select patients for protons and increase the therapeutic balance of a number of malignancies, including CNS tumours for value-based cancer management. Caution however should be stressed that, depending on the selected study endpoint, such as late toxicity including but not limited to radiation-induced tumours, the event can be observed after a long interval after PT. As such, the follow-up time of studies should be consequentially sufficient, which creates a significant challenges for prospective trials, one of which is the trial funding that should be appropriate to fund an extended period of follow-up.
A number of RCTs and prospective Phase II trials have been proposed and are currently accruing patients worldwide. Exclusion criteria of the majority of trials are previous radiation to the head and neck or brain and very extensive lesions, which would have been previously defined as gliomatosis cerebri. Several databases were queried (clinicaltrials.gov, CTSU/NRG, EORTC, PTCOG) and 43 prospective brain tumour trials activated between 1996 and 2019 were identified. Trials that assessed the value of targeted agent/immune checkpoint inhibitors or hypoxic target agents with RT including protons were excluded. Median accrual target of these trials was 80 patients, ranging from 12 to 625. Only a minority (n = 3; 7%) of trials had no age limit. Most trials were for adults (n = 23; 53%) or pediatric (n = 12; 30%) patients. Three (7%) studies were for children and adolescent and young adults. Interestingly, a substantial number of studies (n = 9; 21%) were for all brain tumours. The most common brain tumours for these trials were chordoma or chondrosarcoma (n = 7; 16%), meningioma (n = 6; 14%) and low-grade glioma (n = 6; 14%). Most of the studies were however not accruing (n = 17; 39%) or were in the process of activation (n = 2; 5%). Five (12%) studies were closed and 3 (7%) had an unknown status. The 16 (37%) remaining studies accruing patients in Europe and in the United States are detailed in Table 1. One of the low-grade glioma trials has been recently closed, achieving target-accrual. Noteworthy, WHO grade II glioma patients could be included in this trial from Boston, providing that progressive/recurrent disease was observed, neurological symptoms were uncontrolled and/or patient was aged >40 years or presented MIB-1 of ≥3%. Mean age of this cohort was 37.5 years (range, 22–56) and the gender male/female ratio was 1.9. 40% of the cohort have been followed at 5 year. The results will be published soon.
Table 1.
Prospective trials and tumor registries currently accruing patients in Europe and in the United States for brain tumours
| Tumor type | NCT number | Allocation | Activation (closed) [year] |
# of patients | Age limit | Hypothesis | Primary endpoint | Total dose (dose per fx) [GyRBE] |
status |
|---|---|---|---|---|---|---|---|---|---|
| Europe (lead) | |||||||||
| All brain tumorsa (Dresden, D) |
02824731 | Non-randomized Phase II |
1997 | 418 | no | rate of chronic 1 year toxicity: 15% lower with protons | Chronic toxicity @ 1 year and QoL | 54-60(27-30) | accruing |
| WHO grade II/WHO grade III and IDH mutated (Essen, D) |
DRKS 00015160 NOA-25 |
prospective, randomized (Photons vs Protons) |
2019 | 80 | ≥18 years | Less impairment of neurocognition after proton therapy when compared to photon radiotherapy | Neurocognition after 3 years | WHO II: 54 Gy (30 × 1,8 Gy) WHO III: 60 Gy (30 × 2 Gy) Or 59,4 Gy (33 × 1,8 Gy) |
accruing |
| United States (lead) | |||||||||
| All brain tumors Washington Uni. School of Medicine |
02559752 | Non-randomized Phase II |
2015 | 80 | 4–21 years | Testing as measured by an acceptance rate of 60% of eligible patients administered PT | Feasibility of obtaining serial computer-based neurocognitive testing for patients administered PT | NR | accruing |
| Craniopharyngioma St Judes Children Hospital |
02792582 | Non-randomized Phase II |
1996 | 140 | ≤21 years | Increase of PFS @ 3 years compared to photon data | PFS @ 3 years | 54 (1.8) | accruing |
| Meningioma (Recurrent) Washington Uni. School of Medicine | 03267836 | Phase Ib | 2018 | 12 | ≥18 years | Proof of concept to demonstrate on-target effect of the PT-ICI | Immunogenicity as measured by changes of CD8+/CD4 + TILs | 20(5) with concomitant Avelumab | accruing |
| Meningioma (non-benign) Mass. General Hospital |
02693990 | Non-randomized Phase I/II |
2016 | 60 | ≥18 years | Dose escalation | Assess Safety and Utility of Increased Dose IMPT (DLT) | Dose escalation 3 × 3 design | accruing |
| Low-grade brain tumours Mass. General Hospital |
03286335 | Observational study | 2018 | 100 | ≥18 years | None (observational) | Tumor control @ 2 years | NR | accruing |
| Vestibular Schwannoma Mass. General Hospital |
01199978 | Observational study | 2010 | 30 | ≥18 years | None (observational) | Incidence of late toxicity @ 2 year | 54(27) | Accruing |
| All brain tumours requiring CSA Mass. General Hospital |
03281889 | Feasibility | 2018 | 20 | 3–18 years | To assess if IMPT is feasible for CSA vertebral body sparing | Rate of G3/4 haematological toxicity < 5% within 3 months | NR | Accruing |
| Recurrent Ependymoma St Judes Children Hospital |
02125786 | Non-Randomised Phase II | 2014 | 99 | 1–21 years | To assess if surgery and fractionated re-irradiation with either proton or photon is effective and safe | PFS and OS @ 3 years | NR | Accruing |
| Glioblastoma NRG Oncology |
02179086 | Randomised Phase II | 2014 | 606 | ≥18 years | Dose escalation with IMRT or PT is better than standard dose photon radiation therapy | OS dose escalation vs standard dose | NR | Accruing |
| IDH mutant Glioma (GII/III) NRG Oncology |
03180502 | Randomised Phase II | 2017 | 120 | ≥18 years | PT will preserve cognition compared with IMRT | Change in cognition (CTB COMP scoreb) up to 10 years | NR | Accruing |
| Medulloblastoma St Judes Children Hospital |
01878617 | Phase II | 2013 | 625 | 3–39 years | Assess clinical and molecular risk directed therapy | PFS @ 2 years, neurocognition @ baseline and 12 weeks | NR | Accruing |
| Brain tumours Mayo |
03055364 | Observational study | 2017 | 160 | ≥4 years | None (observational) | Cognitive performance change (CogState) within 12 months of radiotherapy | NR | Accruing |
| Meningioma (G II) NRG Oncology |
03180268 | Randomised Phase III | 2017 | 148 | ≥18 years | Observation vs adjuvant RT in the completely resected setting | PFS up to 10 years | 59.4 (1.8) | Accruing |
| Leptomeningeal metastases MSKCC |
03520504 | Phase I | 2018 | 26 | ≥10 years | Identification of safe and effective dose for PT in leptomeningeal metastases | Number of patients with DLT | 30 (3) or 25 (2.5) CSA | Accruing |
| Patient Registries | |||||||||
| All tumours Washington U |
02040467 | Observational (patient registry) | 2013 | 3200 | no | None (observational) | All treatment data | NR | Accruing |
| All Paediatric tumours Paediatric Consortium Registry (PCCR) |
01696721 | Observational (national patient registry) | 2012 | 5000 | ≤21 years | None (observational) | Establish registry | NR | Accruing |
| All tumours Proton Collaborative Group |
01255748 | Observational (patient registry) | 2010 | 20,000 | All | None (observational) | Establish registry and track outcomes | NR | Accruing |
CSA, Craniospinal axis; DLT, dose-limiting toxicity; IMPT, intensity-modulated proton therapy; IMRT, intensity-modulated photon radiotherapy; NCT: NCI/Clinical Trials.NR, not reported; OS, Overall Survival; PFS, progression-free survival; QoL, quality of life; RT, radiotherapy; TIL, tumour infiltrating lymphocytes; fx, proton therapy dose per fraction.
Upfront radiotherapy or re-irradition 30 Gy/RBE in 5 Gy RBE per fraction or 36 Gy/RBE in 2 Gy RBE per fraction.
Clinical Trial Battery Composite score (calculated from the Hopkins Verbal Learning Test Revised [HVLT-R]) Total Recall, HVLT-R Delayed Recall, HVLT-R Delayed Recognition, Controlled Oral Word Association (COWA) test, Trail Making Test (TMT) part A and part B.
Table 1 displays also a number of tumour registries that are active in the United States, one of which is dedicated to children only. The target of total number of patient’s registration is over 28’000. In Europe, it is also foreseen to have a prospective data collection of patients treated with protons in the framework of PARTICLECare. This project is a joint collaboration of the European Organisation for the Research and Treatment of Cancer (protocol: E2RADIatE, EORTC 1811) and the European Society for Radiotherapy and Oncology. It is foreseen that the first patient will be included into this prospective database by the end of 2019.
Adult brain tumours
Patient with benign brain tumours and low-grade CNS tumours might show some clinical benefit from PT. A substantial number of Low Grade Glioma (LGG) patients are long-term survivors.31 The negative impact of photon radiation therapy on cognition has been demonstrated.32 Plan comparative studies have shown the potential of proton therapy to decrease radiation dose delivered to OARs.33 Currently, there are no published data of randomized trials comparing protons with photons for these tumours (Table 1). Reported results however regarding long-term toxicities from clinical proton studies show encouraging results,34–36 although the patient numbers are small. More elucidation on the real benefit regarding the use of protons in treating LGGs can be expected from the results from the ongoing randomized trial.
Due to the poor prognosis of high-grade gliomas, a dose escalation paradigm has been advocated for these challenging tumours. A study applying an escalated boost with protons to a total dose up to 90 Gy(RBE) lead to improvement in tumor control rate as well as median survival time.37 Nevertheless, the study enclosed only a small number of patients with no molecular analysis and a substantial number of patients had to undergo surgery due to radiation brain necrosis.
Some of the meningiomas can also be considered low-grade benign tumours. Total surgical resection is the treatment of choice for symptomatic/progressive meningioma. However, not all meningioma are suitable for surgery and therefore radiation therapy is often indicated. In particular, patients with residual non-benign, recurrent or high-grade tumours are candidates for radiation therapy. Large and complex shaped meningioma located close to brainstem (Figure 2), optical nerve, pituitary gland and cochlea may present however a therapeutic challenge and proton may provide dose escalation possibilities for non-benign meningiomas.2 Eight retrospective studies delivering PT were conducted across a number of countries including Switzerland, Germany, Sweden and USA.38–45 The sample size within the studies ranged from 39 to 170 participants (Table 2). Four studies included meningioma Grad 1–3,40,42,44,45 while two included Grade 1–239,41 and one Grade one meningioma only.43 In two studies a combination of photons and carbon ions was given to patients with Grade 2–3 meningioma whereas patients with Grad one tumours received proton therapy only.42,45 Five-year local control rates for low-risk meningioma were better (94–100%) when compared with high-risk meningioma (49–88%) and tumor grading was found to be of prognostic significance in univariate analysis in four studies.39,40,44,45 Proton therapy induced toxicity was moderate with a rate of 3.6–12.8% Grade ≥ 3 late effects. The results stemming from these small PT series are in line with modern photon series and cannot prove that proton are superior to conventional radiotherapy.
Table 2.
Recent proton therapy studies for meningioma in adult patients
| Author [ref] | year | Tumor type | # patients | Median Dose (GyRBE) (Range) |
Median FU (months) (range) |
Proton Therapy only |
PBS only | Outcome | Positive prognostic factors [Univariate analysis] |
Toxicity |
|---|---|---|---|---|---|---|---|---|---|---|
| Halasz et al.39 | 2011 | Menigioma WHO Grade 1–2 |
n = 50 Grade 1: n = 12 (24%) Grade 2: n = 6 (12%) Grade not known: n = 32 (64%) |
13 (10–15.5) in one fraction | 32 (6–133) | yes | Scattering (stereotactic) |
3y-LC: 94% |
WHO Grade one vs atypical histology (p = 0.03) Recurrent vs non-recurrent meningioma (p = 0.006) |
Acute Transient facial pain 4% Late Seizures associated with cerebral edema 12% Panhypopituitarism 2% |
| Weber et al.40 | 2012 | Menigioma WHO Grade 1–3 |
n = 39 (three re-irradiation) |
Grade 1–2: 52.2–56 Grade 3: 60.8 (±5.3) |
54.8 (6.2–146.8) | yes | yes |
5y-LC: Grad1: 100% Grade 2–3: 49.1% |
WHO Grade 1 vs 2/3 (p = 0.001) GTV < 21.5 vs.>=21.5 ml (p = 0.03) |
CTCA Acute: Grade 2: 12.5% Grade 3: 0% Late: Grade ≥ 3: 12.8% |
| Slater et al.41 | 2012 | Meningioma Grade 1–2 | Entire cohort n = 72 Grade 1: n = 47 (65%) Grade 2: n = 4 (6%) Grade not known: 21 (29%) |
Grade 1: 50.4–66.6 Grade 2: 54–70.2 |
74 (3–183) | yes | Scattering |
5y-LC Overall: 96% Grade 1: 99% Grade 2: 50% 5y-OS 99% (disease-specific) |
No significant differences for tumor size (GTV), dose, number of surgeries and WHO Grad | optic neurologic symptoms: 4.2% brain edema: 2.8% Transient diplopia 1.4% Panhypopituitarism 4.2% |
| Combs et al.42 | 2013 | Meningioma WHO Grade 1–3 | Entire cohort n = 107 WHO Grade 1: 71 (66%) WHO Grad 2/3: n = 36 (34%) |
Grade 1: 57.6 Grade 2/3: N.R. |
12 (2–39) | Grade 1: PT only Grade2/3: photons/carbon ion boost |
Grade 1. Yes Grade 2/3: combination of photon/carbon |
2y-LC: Grade 1: 100% Grade 2/3: 33% |
N.R. | N.R. |
| McDonald et al.38 | 2015 | Meningioma WHO Grade 2 |
n = 22 | 63 (54–68.4) | 39 (7–104) | yes | N.R. | 5y-LC: 71.1% | Dose > 60 Gy vs =<60 Gy (RBE) (p = 0.038) |
Acute ≥Grade III: 0% Late ≥Grade III: one pt. |
| Vlachogiannis et al.43 | 2017 | Meningioma WHO Grade 1 | n = 170 | 21.9 (14–46) 2–6 Gy/fx |
84 (range N.R) | yes | Scattering (stereotactic) |
5y-PFS: 93% 10y-PFS: 85% |
Multivariate analysis: Age: p = 0.009 Localization: middle fossa p = 0.04 |
Pituitary insuffiency: 3.5% Radiation necrosis: 2.9% Visual impairment: 2.9% Expansive tumour cyst: 0.5% |
| Murray et al.44 | 2018 | Menigioma WHO Grad 1–3 |
Entire cohort: n = 96 Grade 1: n = 61 (63%) Grade 2: n = 33 (34.1%) Grade 3: 2 (2.1%) |
Grade I: 54 (50.4–64) Grade II and II: 62 (54-68) |
56.9 (range, 12–207) |
Yes | Yes |
5y-LC: Entire cohort: 86,4% Grade 1: 95.7% Grade 2/3: 68% 5y-OS: entire cohort: 88.2% Grade 1: 92.1% Grad 2/3: 80.7% |
5y-LC WHO Grade 1 vs 2/3 (p < .001) Timing of PT: Initial vs recurrent (p = .006) Tumor site: Skull base vs non-skull base (p = 0.014) Gender: Female vs Male (p = 0.32) 5y-OS |
CTCA Acute ≥Grade III: 0.96% Late ≥Grade III overall: 10% optic toxicity: 6.7% brain edema: 0.96% brain necrosis: 1.9% |
| El Shafie et al.45 | 2018 | Meningioma WHO Grade 1–3 | Entire cohort: n = 110 Grade 1: 60 Grade 2: 7 Grade 3: 1 not known:42 |
Protons: 54 (50-60); 1.8–2.0/fx Carbon ion: 18; 3.0/fx |
46.8 (95%CI: 39,9–53.7) | Proton: n = 104 Photons/Carbon ion: n = 6 |
Yes Grade 2/3: combination of photon/carbon |
5y-PFS: Entire cohort: 96.6% Low risk: 96.6% High risk: 75%, 5y-OS: 96.2 % |
Histology: low vs high risk : p = 0.02 | CTCAE Acute: Grade III: 1.8% (mucositis, nausea) Late: Grade III:3.6% (hypopituitarism, radionecrosis) |
FU, follow-up; LC, local control; NR, not reported; OS, overall survival; PBT, proton beam therapy; PFS, progression free survival; fx, fraction.
In summary, it is unlikely that proton therapy delivered for high-grade brain tumors might translate into a substantial clinical benefit for CNS-tumor patients. Protons could however be administered to low-grade (i.e. glioma) or benign (i.e. meningioma) brain tumors, as these patients experience substantial long survival times, in order to possibly decrease the probability of long term toxicity. Alternatively, proton therapy could be administered to patients with non-benign meningioma with a dose-escalation paradigm.
Skull-base tumours
Skull-base chondrosarcoma (sbChS) and chordoma (sbC) are very rare tumours with an incidence of <1 per million.46 They are usually in direct vicinity of OARs, including but not limited to the optic apparatus, brainstem, pituitary gland and cochleae and are considered radio-resistant.47,48 Local tumour control is associated with overall survival and is thus of paramount importance.49,50 SbChS and sbC are usually managed with cytoreductive surgery and postoperative radiotherapy. The importance of optimal surgery (i.e. optimizing the tumor geometry/debulking) with potentially sequential surgical procedures, before radiotherapy has been advocated by many groups.50–54 The outcome of patients with sbChS/sbC treated with adjuvant or salvaged photon radiation therapy is not optimal. When gauging the benefit of protons for these skull-base tumours, it is important to acknowledge that, due to the rarity of this condition, only observational studies stemming usually from one institution, with few exceptions,50 have been published. Consequentially, no level I or II evidence have been proven on the superiority of protons over photon radiotherapy, although the outcome data published by non-particle radiotherapy is somewhat poor. Two photon series reporting on 17 and 48 sbC and extra cranial chordomas patients have shown that delivering a median dose of 50 Gy with conventional radiotherapy resulted in a 5 year PFS and 5 year LC of 17 and 23%, respectively.55,56 As such, conventional radiotherapy may provide valuable palliation for these challenging patients but chordomas are rarely cured with this therapeutic modality. It has been claimed that historical photon series, such as those reported above, do not reflect the accurate efficiency of modern photon radiotherapy series. A recent study from the UCLA group reported on 57 sbC patients treated with a median dose of 17.8 and 63.4 Gy delivered with SRS and SFRT, respectively.57 The observed 5 year PFS for the entire cohort was only 35.2%. Of note, SRS and SFRT produced comparable rates of tumour control. Numerous modern SRS and SFRT series have shown suboptimal outcomes for sbC patients treated with these radiation modalities.57–59 These suboptimal results may be best explained by the stereotactic margins defined during the planning process and radiation dose delivered to these patients. Regarding the former, Snider et al have shown undisputedly the importance of margins for extracranial chordomas.60 The seminal paper by Pearlman et al have shown a dose-response for chordomas.47 More recently, a South Korean study reported on 35 sbC patients treated with a median 75.5 EQD2 delivered by proton therapy. The observed 5 year local tumour control was 92.8 and 63.0% for patients treated with ≥69.6 and<69.6 Gy, respectively.61 Likewise, an analysis of 863 chordoma patients captured in the National Cancer Data base has shown undisputedly that dose for chordoma was associated with a significant increase in OS on univariate analysis.1 Other proton series have shown such a dose-response with sbC.62 This dose-response relationship has also been observed with photons55,63 : in the above-referred US series, higher dose of SFRT was associated with a significant higher rate (p = 0.013) of tumour local control.57 As such, high-dose radiation therapy, with non-stereotactic margins, have to be delivered to chordoma patients postoperatively. Table 3 details the outcome and prognostic factors of sbChS and sbC adult patients treated with proton therapy, mostly delivered with a passive scattering paradigm. Noteworthy, the prognostic impact of gender is unproven, as all but two series with contradictory results,64,70 have shown that chordoma is gender-neutral. Tumour volume before proton therapy, with various cut-offs ranging from 20 to 70 cc, is a major prognosticator. Interestingly, the outcome of sbC and scChSa patients have improved substantially in recent years (Table 3). Finally, delivering high dose proton radiation to the skull base tumours may induce toxicity,52,65–69,71–74 including but not limited to the brainstem. At the Paul Scherrer Institute, we have seen no brainstem radiation toxicity in adult skull-base tumour patients treated with protons. Debus et al reported on 367 skull base tumours patients treated in Boston with combined proton/photon radiotherapy.49 Brainstem toxicity was observed in 4.6% of cases and the estimated toxicity-free survival was 88%.
Table 3.
Recent proton therapy studies for skull-base tumors (chordoma and chondrosarcoma) in adult patients
| Author [ref] | year | Tumor type | apatients | Mean Dose (GyRBE) (Range) |
Mean FU (months) |
Proton Therapy only |
PBS only | Outcome | Positive prognostic factors LC (p < 0.05) [Univariate analysis] |
|---|---|---|---|---|---|---|---|---|---|
| Youn et al.61 | 2018 | chordoma | 34 | 69.6 | 42.8 | yes | yes | 5yLC: 87.3%b 5yOS: 92.9%b |
Skull-base vs cervical |
| Fung et al.53c | 2018 | chordoma | 106 | (68.4–73.8) | 61.0 | no | no | 5yLC: 88.6% | Tumor volume < 25 cc |
| Weber et al.51c | 2018 | ChSa | 251 | 70.2 | 88.0 | no | no | 5yLC: 93.1% 5yOS: 93.6% |
Tumor volume < 25 cc Non-OA compression |
| Takagi et al.54 | 2018 | chordoma | 11 | 65.0/20 fx | 71.5 | yes | 5yLC: 85.0%b 5yOS: 86%b |
Surgery before PTb | |
| Demizu et al.64 | 2017 | Chordoma & ChSa | 68 | 70 | 52.6 | yes | yes | 5yLC: 71.1% 5yOS: 75.3% |
Female gender |
| Weber et al.51 | 2016 | Chordoma & ChSa | 222 | 72.5 | 50.0 | yes | yes | 7yLC: 70.9% 7yLC: 93.6%d 7yOS: 81.7%b |
Non-compression BS GTV ChSa vs chordoma |
| Hayashi et al.62 | 2016 | chordoma | 19 | 77.4–78.4a | 61.7 | yes | yes | 5yLC: 75.0% 5yOS: 83.2% |
NR |
| Feuvret et al.65c | 2016 | ChSa | 159 | 70.2 | 77.0 | no | no | 5yLC: 96.4% 5yOS: 94.9% |
Age < 40 yearse primary disease statuse |
| Deraniyagala et al.66 | 2014 | chordoma | 33 | (77.4–79.4) | 21.0 | yes | no | 2yLC: 86.0% 2yOS: 92.0% |
NR |
| Yasuda et al.67c | 2012 | chordoma | 17 | 68.9 | 46.1 | no | no | 5yLC: 70.0%b 5yOS: 83.4%b |
≥47 years Skull-base vs CCJ |
| Fuji et al.68 | 2011 | Chordoma & ChSa | 16 | 63.0 | 42.0 | yes | yes | 3yLC: 86.0% (chordoma) 3yOS: 100.0% (chordoma) 3yLC: 100.0% (ChSa) 3yOS: 100.0% (ChSa) |
NR |
| Noel et al.69 | 2001 | Chordoma & ChSa | 45 | 67.0 | 30.5 | no | no | 3yLC: 83.1% (chordoma) 3yOS: 91.0% (chordoma) 3yLC: 90.0% (ChSa) 3yOS: 90.0% (ChSa) |
<55 years Tumor volume < 29 cc |
| Hug et al.52 | 1999 | Chordoma & ChSa | 58 | 70.7 | 33.0 | yes | no | 5yLC: 54.0%(chordoma) 5yOS: 88.0%(ChSa) |
Tumor volume < 25 cc Non-compression BS |
| Terahara et al.70c | 1999 | Chordoma | 115 | 68.9 | NR | no | no | 5yLC: 79.0% (chordoma) 5yOS: 59.0% (chordoma) 5yLC: 94.0% (ChSa) 5yOS: NR (ChSa) |
Male gender Minimum dose Target dose EUD |
| Munzenrider et al.71 | 1999 | Chordoma & ChSa | 621 | (66.0–83.0) | 41d | no | no | 10LC: 54.0% (chordoma) 10OS: 54.0% (chordoma) 10yLC: 88.0% (ChSa) 10yOS: 90.0% (ChSa) |
Male gender Tumor volume < 70 cc |
| Austin-Seymour et al.72 | 1989 | ChSa | 68 | 69.0 | 41 | no | no | 5yLC: 82% 5yDFS: 76% |
NR |
| Berson et al.73 | 1988 | Chordoma & ChSa | 45 | (59.4–80.0) | 33pts > 1 year | no | no | 5yLC: 59.0%b 5yOS: 62.0%b |
ChSa Tumor volume <20 cc Non-recurrent tumour |
LC, local control; OS, overall survival; ChSa, chondrosarcoma; BS, brainstem; GTV, Gross tumor volume; CCJ, cranio-cervical junction; EUD, equivalent uniform dose; PT, proton therapy; fx, fraction; NR, not reported; OA, optic apparatus; pts, patients; FU, follow-up.
Hyperfractionated proton therapy
Entire cohort, including skull base and extra cranial tumours
Partial publication of cohort outcome in another paper
Chondrosarcoma only
For Progression-free survival
Median value
Protons should thus be the standard of care for sbC or sbChSa, as a dose escalation can be achieved with this treatment modality, maximizing the chances of cure for these challenging patients.
Pediatric brain tumours
Cancer affects more than 380’000 children aged 0–19 every year globally75 and is the leading cause of childhood death by disease in high-income countries (HIC). Nevertheless, cancer cure rates in HIC currently are near 80% and are on the rise thanks to new advances in medical treatments This leaves many childhood cancer survivors (CCS) with a potentially normal lifespan, during which maintaining both good health status and quality of life is of paramount importance.76 Treatment-related toxicity brings a significant morbidity burden on CCS, most of all for patients with primary brain cancers.77 Reasons for this are an increased sensitivity due to ongoing tissue growth and neuro-cognitive development, smaller anatomic dimensions bringing critical organs closer to treatment areas and a longer lifespan left to develop side-effects. The most significant toxicities associated with brain tumor irradiation are vascular complications such as radiation necrosis (RN) and Moya-Moya syndrome, impairment of neurocognitive development, including loss of IQ scores, visual, hearing or endocrine deficits as well as skin changes such as alopecia. In the case of CSI (Figure 1b), neck, thoracic, abdominal and pelvic organs can develop late sequelae of radiation therapy. As an example, vertebral body irradiation leads to decrease of adult height78–80 with a reported incidence rate of 3–26%.81 RN can result in numerous symptoms or deficits, depending on its location, such as seizures or motor impairment. Seven papers reported on RN induced by proton therapy in pediatric patients (Table 4). Sample sizes ranged between 17 and 313 patients, with ages between 19 months and 10 years. Time to RN ranged between 3 and 9 months. Median PT doses of 54 Gy RBE were used across all seven studies. Grade 3–4 RN ranged between 2 and 3.6% at 5 years.82–88 PT has demonstrated its ability to better spare uninvolved normal tissues including critical OARs compared to standard photon therapy. It has therefore become a widely accepted radiation modality for several childhood malignancies. Advantages of PT for the irradiation of brain tumours reside in a better sparing of healthy brain tissue and other OARs (Figure 2), not limited but including the cochlea, the pituitary gland, the hippocampus, the optic structures and the brainstem.89 For the spinal cord, posterior proton field arrangements (Figure 1b) allow for an excellent sparing of all organs anterior to the vertebral bodies as described above.90 Pediatric brain tumours, for which PT has been most commonly used, are craniopharyngiomas,91 ependymomas (Figure 2),92 germ cell tumours,93 low-grade gliomas,94 medulloblastomas and atypical teratoid/rhabdoid tumours (ATRT)95,96 (Table 5). Four retrospective studies, published with exclusively ependymoma patients, reported OS and PFS rates ranging from 84–100% to 76–80%.92,97–99 In medulloblastoma studies,100,102,103 sample sizes ranged between 15 and 59 patients, with ages between 2.9 and 6.6 years. The context of re-irradiation also makes a particularly strong case for the use of very conformal RT modalities such as PT, as treatment of recurrences can still lead to cure in some instances, such as for localized ependymoma relapses.101
Table 4.
Literature review of cerebral necrosis for proton therapy
| Author [ref] | Year | Tumor type | # pts | Median Age [years] |
Median Dose [GyRBE] (range) |
Time to RN [months] |
Toxicity | Prognostic Factors |
|---|---|---|---|---|---|---|---|---|
| Sabin et al.82 | 2013 | EP,CPC,PNET, MB, ATRT |
8/17 | 2.5 | 54 | 3.9 | 47% pseudoprogression | PT after chemotherapy |
| Indelicato et al.83 | 2014 | EP,CF,LGG, MB,PNET,PMRh | 11/313 | 5.9 | 54 | 3 | 3.8% Gr < 3@2y 2.1% Gr 3 @2y |
Age < 5y; PF; V55Gy, Dmax |
| McGovern et al.84 | 2014 | ATRT | 5/31 | 19 | Local PT 50.4 CSI 54 |
4 | 16% @2y Gr1-2 | Age < 5y; intensive Cht prior PT |
| Gunther et al.85 | 2015 | EP | 72 16/37 PT 6/35 IMRT |
4.4 PT 6.9 IMRT |
59.4 PT 54 IMRT |
3.8 PT 5.3 IMRT |
43%PT 17% IMRT 3PT persistent neurological deficits |
PTvsIMRT higher rate of imaging changes Age > 3 y BS |
| Giantsoudi et al.86 | 2016 | MB | 4/111 | 10.5 | 54 | 9 | 3.6%@5y 2.7%@5y (grade3+) |
WPF vs IF |
| Bojaxhiu et al.87 | 2018 | EP,CF,LGG, MB,PNET,other | 29/171 | 3.3 | 54 | 5 | 17%RN; 11%WMLs | Cht, EP, hydrocephalus |
| Gentile et al.88 | 2018 | MB,EP,ATRT | 5/216 | 6.6 | 54 | 8.5 | 2.0%@5y (grade ≥ 2) | Dmax < 55.8 Gy,V55 ≤ 6% |
GyRBE, Gray in relative biological effectiveness; ATRT, atypical teratoid rhabdoid tumor; CF, craniopharyngioma; CPC, choroid plexus carcinoma; CSI, craniospinal irradiation; Cht, chemotherapy; EP, ependymoma; IF, involved field; IMRT, intensity modulated radiation therapy; LGG, low grade glioma; MB, medulloblastoma; PF, posterior fossa; PMRh, parameningeal rhabdomyosarcoma; PNET, primitive neuroectodermal tumor; RN, radiation necrosis; WML, white matter lesion; WPF, whole posterior fossa; pts, patients.
Table 5.
Recent proton therapy studies for pediatric brain tumors
| Author [ref] | year | Tumor type | # pts | Median Age [years] |
Median Dose [GyRBE] (range) |
Median FU [months] (range) |
Outcome | Late Toxicity |
|---|---|---|---|---|---|---|---|---|
| De Amorim Bernstein et al.96 | 2013 | AT/RT | 10 | 2.3 | 50.4 (50.4–55.8); three pts CSI (18–23.4) |
27.3 (11.3–99.4) |
LC 100%, DC 80%, OS 90% | Endocrine G2 (2pts hypothyroidism, 3pts GH deficiency) |
| Mc Govern et al.84 | 2014 | AT/RT | 31 | 1.6 | 50.4 (9–54); 14 pts CSI (23.4–36) |
24 (3-53) |
Median OS 34.3mo, PFS 20.8mo | five pts imaging changes interpreted as RN |
| Weber et al.95 | 2015 | AT/RT | 15 | 1.4 | 54 all patients, no CSI | 33.4 (9.7–69.2) |
LF 20%, DBF 27%, SF 2%. 2y OS 64.6%, 2y PFS 66% | 2y tox free survival 90%. No decrease of QoL after PT |
| Bishop et al.91 | 2014 | Craniopharyngioma | 52 (21 PT) |
8.9 | 50.4 (50.4–54) | 59.6 (PT 33mo) |
3y OS 96%, nodular FFS 96%, cystic FFS 76%. Same outcome for PT and IMRT | Endocrine G2 77%. No difference between PT and IMRT |
| MacDonald et al.97 | 2013 | Ependymoma | 70 | 3.2 | 55.8 (50.4–60) | 46 (12–140.4) |
3y LC 83%, PFS 76%, OS 95% 5y LC 77%, DC 83% |
one pt hypothyroidism, two pts GH deficit, two pts hearing loss, two pts cavernoma. No drop in MI and OAS scores |
| Mizumoto et al.98 | 2015 | Ependymoma | 6 | 5 | 56.7 (50.4–61.2) | 24.5 (13-44) |
OS 100%, PFS 80% | one pt one-time seizure, one pt alopecia, no difficulty in daily life |
| Ares et al.92 | 2016 | Ependymoma | 50 | 2.6 | 59.4 (54–60) | 43.4 (8.5–113.7) |
5y LC 78%, OS 84% | 38% G1/2, two pts G3 deafness, one pt G5 brainstem necrosis |
| Sato et al.99 | 2017 | Ependymoma | 79 (41 PT) |
3.7 | 55.8 (50.4–59.4) | PT 31.2 (7.2–86.4) IMRT 58.8 (13.2–140.4) |
3y OS 81% IMRT vs 97% PT (p = .08), PFS 60% IMRT vs 82% PT (p = .0307), Recurrence 55% IMRT vs 17% PT (p = .005) | Vascular disorder G2 + 10% (6 RN, one stroke, one cavernoma) |
| MacDonald et al.93 | 2011 | Germ cell tumors | 22 | 11 | Total 44 (30.6–57.6) 1pt IF only seven pts WVRT 19.5–23.4 1pt WBRT 25.5 13 pts CSI 18.3–27 |
28 (13-97) |
LC 100%, PFS 95%, OS 100% | two pts hypothyroidism, 2pts GH deficit. No new NC or auditory deficit |
| Hug et al.94 | 2002 | Low grade glioma | 27 | 8.7 | 55.2 (50.4–63) | 39.6 (7.2–81.6) |
LF 22%, OS 85% | Moya-Moya one pt |
| Greenberger et al.78 | 2014 | Low grade glioma | 32 (nine mix PT and photons) |
11 | 52.2 (48.6–54) | 91.2 (38.4–218.4) |
6y PFS 89.7%, 8y PFS 82.8%, 8y OS 100% | Endocrine G2 > 80% at 10y (>40 GFy to pituitary and hypothalamus is RF), two pts G3 vasculopathy (Moya-Moya), age > 7y and hippocampus dose RF for NC decline, VA/VF decline four events, other visual tox nine events |
| Jimenez et al.100 | 2013 | Medulloblastoma / supratentorial PNET | 15 | 2.9 | Total 54 (39.6–54) CSI 21.6 (18–30.6) |
39 (3-102) |
3y LF 7.7%, OS 85.6% | Ototoxicity nine pts (2 G3), Endocrine G2 three pts, significant height loss, NS if GH deficiency pts excluded, no loss from baseline IQ |
| Eaton et al.101 | 2016 | Medulloblastoma | 88 (45 PT) | 6 | Total 54–55.8 CSI 23.4 (18–27) |
74.4 PT pts 84 photon pts |
6y RFS 78.8% PT vs 76.5% photon (p = .948). 6y OS 82% PT vs 87.6% photon (p = .285) | NR |
| Yock et al.102 | 2016 | Medulloblastoma | 59 | 6.6 | Total 54 CSI 23.4 (23.4–36) |
84 (62.4–98.4) |
3y PFS 83% 5y PFS 80%, OS 83% 7y PFS 75%, OS 81% |
Ototoxicity G3 + 12% at 3y and 16% at 5y and 7y, FSIQ decline by 1.5 point/y, Endocrine deficit 27%, 55 and 63% at 3, 5 and 7y, Cataract two pts, BS injury one pt, Stroke two pt |
NS, not statistically significative. WBRT, whole brain radiotherapy; AT/RT, atypical teratoid/rhabdoid tumor; CSI, craniospinal irradiation ; DBF, distant brain failure; DC, distant control; FFS, failure free survival;FSIQ, full scale intelligence quotient; FU, follow-up; G, toxicity grade;GH, growth hormone; GyRBE, Gray in relative biological effectiveness; IF, involved field;LC, local control; LF, local failure;MI, mean intelligence; NC, neurocognitive; NR, not reported; OAS, overall adaptive skills; OS, overall survival;PFS, progression free survival;RF, risk factor; RFS, recurrence free survival;RN, radiation necrosis; SF, spinal failure, PT: proton therapy;VA, visual acuity; VF, visual field; WVRT, whole ventricle radiotherapy; mo, months;pts, patients.
The administration of protons to children with brain tumors represent an unique opportunity to decrease the likelihood of late CNS toxicity by decreasing the integral dose to the brain, especially so in very young patients with tumors such as ATRTs, ependymomas or medulloblastomas.
Costs considerations/financial toxicity
Across HICs, cancer management costs are escalating, driven mainly by consumerism in health care, the demographic transition of a growing elderly population and by the delivery of costly new therapies. Although there is an association between high-spending health care systems and lower cancer mortality,104 it is questionable if the growth in cancer spending is sustainable in the long-time in high-income countries.
Proton therapy is an expensive anti cancer treatment, with a cost factor of approximately 2.5, when compared to modern RT techniques.105 This is certainly due to the considerable investment costs but also due to the high operation and maintenance costs. Ongoing technical developments may lead to cost reduction but it is not expected that a dramatic decrease in costs will be reached in the near future.106 As a result, there is an ongoing debate on the value of proton therapy and its cost-effectiveness (CE).107,108
As care for medical conditions, such as cancer, usually involves multiple disciplines and numerous interventions at different time-points, the true value cannot be determined by simply comparing costs of two treatment modalities. Consequently, CE analysis must also consider patient`s longtime outcome, toxicity and quality of life by tracking patient outcomes and costs longitudinally.109
Four publications on CE of proton therapy in brain tumours could be identified, of note all in pediatric cohorts (Table 6). All used Markov modelling110,112,113 and Monte Carlo simulations111 to compare proton vs photon therapy. All investigators have shown that proton therapy is cost-effective with regard to long-term risk of radiation side-effects. Three studies even demonstrated a cost saving effect of proton therapy.
Table 6.
Cost-effectiveness studies for proton vs photon therapy of brain tumors
| Author [ref] | year | Tumor type | Study design | Statistical Model Method |
Included Parameters |
Results |
|---|---|---|---|---|---|---|
| Lundkvist et al.110 | 2005 | Pediatric medulloblastoma | Comparison PBT vs IMRT | Markov cohort simulation model | Risk of hearing loss, IQ loss, GHD, hypothyroidism, osteoporosis, cardiac disease, fatal and nonfatal SMN |
Gain of QUALY of 0.68 per patient; Estimated cost difference (protons vs photons) per patient −23,646.5 EUR ICER of −34,622 EUR/QUALY →Cost effective →Cost saving |
| Mailhot Vega et al.111 | 2013 | Pediatric medulloblastoma | Comparison of PBT vs photon RT | Monte Carlo simulation | Risk of GHD, hearing loss, hypothyroidism, congestive heart failure coronary artery disease, ACTH deficiency, gonadotropin deficiency, SMN, death |
Gain of QUALY of 3.46; Total difference in costs (protons vs photons): - 32,579.1 Dollar ICER of −9,416 Dollar/QUALY →Cost effective →Cost saving |
| Hirano et al.112 | 2014 | Pediatric medulloblastoma | Comparison of PBT vs IMRT | Markov cohort simulation model | Risk of hearing loss due to cochlear dose for three different QoL measures (EQ-5D, HUI3, SF-6D) | Gain of QUALY between 0.98 and 1.82 and ICER of 11,773 and 21,716 Dollar/QUALY dependent on QoL measure used →Cost effective |
| Mailhot Vega et al.113 | 2015 | Pediatric CNS tumors | Comparison of PBT vs photon RT in hypothalamic dose sparing | Markov cohort simulation model | Risk of GHD | Hypothalamic proton doses between 5 and 25 Gy can be cost-effective, between 5 and 20 Gy even cost saving in some scenarios |
ACTH, adrenocorticotropic hormone; GHD, growth hormone deficiency; ICER, incremental cost-effectiveness ratio; IMRT, intensity modulated radiation therapy; IQ, intelligence quotient; PBT, proton beam therapy; QoL, quality of life; QUALY, quality adjusted life years; RT, radiation therapy; SMN, secondary malignant neoplasm.
These analyses are based on theoretical modelling concepts using assumptions, which remain questionable. Empirical comparative data establishing the clinical advantages and health economic appropriateness of proton compared to photon therapy is lacking but urgently needed. It is foreseen that costing data will be captured in the EORTC 1811 protocol/ParticleCare.
Most insurances reimburse the costs of proton therapy for brain tumours listed in this manuscript. Nevertheless, patients may experience expenses to cover costs for housing and traveling during 6–7 weeks treatment, special food and potentially lose wages. For some patients, these out of pocket payments can cause substantial financial distress that adversely affects a patient’s quality of life, treatment choice, treatment compliance, and treatment outcome.114 Treatment related financial distress can be just as toxic as the effects of chemotherapy or radiation and was therefore defined as a treatment related financial toxicity.115 Approximately 16% of patients undergoing proton therapy for brain tumours in Switzerland experience moderate to severe financial distress (unpublished own data). However, very limited evidence is available about the incidence of financial toxicity, its associated morbidity and its preventability in proton therapy.
Conclusions
The dose deposition advantage of PT for the treatment of brain tumours are instantly apparent when planning comparisons of proton vs photon are made. Evidence for PT in adult benign and low-grade tumours is however limited on retrospective analyses. The available data suggests that proton therapy achieves good local control in some high-grade tumours with acceptable toxicity and that the toxicity profile for low-grade tumours warrants prospective analyses. For skull-base, radio-resistant tumours, high-dose (i.e. >70 GyRBE) proton therapy, with non-stereotactic margins, have to be delivered to patients postoperatively. Delivering protons to children with brain tumours may increase the therapeutic ratio. In the era of EBM, high-quality data needs to be rapidly generated to justify the higher costing of this radiation modality, which can have substantial financial toxicity to the patients and their families.
Contributor Information
Damien C Weber, Email: damien.weber@psi.ch.
Pei S Lim, Email: peishuen.lim@psi.ch.
Sebastien Tran, Email: sebastien.tran@psi.ch.
Marc Walser, Email: marc.walser@psi.ch.
Alessandra Bolsi, Email: alessandra.bolsi@psi.ch.
Ulrike Kliebsch, Email: ulrike.kliebsch@psi.ch.
Jürgen Beer, Email: juergen.beer@psi.ch.
Barbara Bachtiary, Email: barbara.bachtiary@psi.ch.
Tony Lomax, Email: tony.lomax@psi.ch.
Alessia Pica, Email: alessia.pica@psi.ch.
REFERENCES
- 1.Palm RF, Oliver DE, Yang GQ, Abuodeh Y, Naghavi AO, Johnstone PAS. The role of dose escalation and proton therapy in perioperative or definitive treatment of chondrosarcoma and chordoma: an analysis of the National Cancer data base. Cancer 2019; 125: 642–51. doi: 10.1002/cncr.31958 [DOI] [PubMed] [Google Scholar]
- 2.Weber DC, Ares C, Villa S, Peerdeman SM, Renard L, Baumert BG, et al. Adjuvant postoperative high-dose radiotherapy for atypical and malignant meningioma: a phase-II parallel non-randomized and observation study (EORTC 22042-26042. Radiother Oncol 2018; 128: 260–5. doi: 10.1016/j.radonc.2018.06.018 [DOI] [PubMed] [Google Scholar]
- 3.Indelicato DJ, Rotondo RL, Uezono H, Sandler ES, Aldana PR, Ranalli NJ, et al. Outcomes following proton therapy for pediatric low-grade glioma. Int J Radiat Oncol Biol Phys 2019;. [DOI] [PubMed] [Google Scholar]
- 4.Sherman JC, Colvin MK, Mancuso SM, Batchelor TT, Oh KS, Loeffler JS, et al. Neurocognitive effects of proton radiation therapy in adults with low-grade glioma. J Neurooncol 2016; 126: 157–64. doi: 10.1007/s11060-015-1952-5 [DOI] [PubMed] [Google Scholar]
- 5.Lomax AJ. Charged particle therapy: the physics of interaction. Cancer J 2009; 15: 285–91. doi: 10.1097/PPO.0b013e3181af5cc7 [DOI] [PubMed] [Google Scholar]
- 6.Lomax A. Intensity modulation methods for proton radiotherapy. Phys Med Biol 1999; 44: 185–205. doi: 10.1088/0031-9155/44/1/014 [DOI] [PubMed] [Google Scholar]
- 7.Lambrecht M, Eekers DBP, Alapetite C, Burnet NG, Calugaru V, Coremans IEM, et al. Radiation dose constraints for organs at risk in neuro-oncology; the European particle therapy Network consensus. Radiother Oncol 2018; 128: 26–36. doi: 10.1016/j.radonc.2018.05.001 [DOI] [PubMed] [Google Scholar]
- 8.Vatner RE, Niemierko A, Misra M, Weyman EA, Goebel CP, Ebb DH, et al. Endocrine deficiency as a function of radiation dose to the hypothalamus and pituitary in pediatric and young adult patients with brain tumors. JCO 2018; 36: 2854–62. doi: 10.1200/JCO.2018.78.1492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zureick AH, Evans CL, Niemierko A, Grieco JA, Nichols AJ, Fullerton BC, et al. Left hippocampal dosimetry correlates with visual and verbal memory outcomes in survivors of pediatric brain tumors. Cancer 2018; 124: 2238–45. doi: 10.1002/cncr.31143 [DOI] [PubMed] [Google Scholar]
- 10.Adeberg S, Harrabi S, Bougatf N, Verma V, Windisch P, Bernhardt D, et al. Dosimetric comparison of proton radiation therapy, volumetric modulated Arc therapy, and three-dimensional conformal radiotherapy based on intracranial tumor location. Cancers 2018; 10: 401. doi: 10.3390/cancers10110401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Correia D, Terribilini D, Zepter S, Pica A, Bizzocchi N, Volken W, et al. Whole-ventricular irradiation for intracranial germ cell tumors: Dosimetric comparison of pencil beam scanned protons, intensity-modulated radiotherapy and volumetric-modulated Arc therapy. Clin Transl Radiat Oncol 2019; 15: 53–61. doi: 10.1016/j.ctro.2019.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.El-Fayech C, Haddy N, Allodji RS, Veres C, Diop F, Kahlouche A, et al. Cerebrovascular diseases in childhood cancer survivors: role of the radiation dose to Willis circle arteries. International Journal of Radiation Oncology*Biology*Physics 2017; 97: 278–86. doi: 10.1016/j.ijrobp.2016.10.015 [DOI] [PubMed] [Google Scholar]
- 13.Seravalli E, Bosman M, Lassen-Ramshad Y, Vestergaard A, Oldenburger F, Visser J, et al. Dosimetric comparison of five different techniques for craniospinal irradiation across 15 European centers: analysis on behalf of the SIOP-E-BTG (radiotherapy Working Group. Acta Oncologica 2018; 57: 1240–9. doi: 10.1080/0284186X.2018.1465588 [DOI] [PubMed] [Google Scholar]
- 14.Tamura M, Sakurai H, Mizumoto M, Kamizawa S, Murayama S, Yamashita H, et al. Lifetime attributable risk of radiation-induced secondary cancer from proton beam therapy compared with that of intensity-modulated X-ray therapy in randomly sampled pediatric cancer patients. J Radiat Res 2017; 58: 363–71. doi: 10.1093/jrr/rrw088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miralbell R, Lomax A, Cella L, Schneider U. Potential reduction of the incidence of radiation-induced second cancers by using proton beams in the treatment of pediatric tumors. International Journal of Radiation Oncology*Biology*Physics 2002; 54: 824–9. doi: 10.1016/S0360-3016(02)02982-6 [DOI] [PubMed] [Google Scholar]
- 16.Kahalley LS, Ris MD, Mahajan A, Okcu MF, Chintagumpala M, Paulino AC, et al. Prospective, longitudinal comparison of neurocognitive change in pediatric brain tumor patients treated with proton radiotherapy versus surgery only. Neuro Oncol 2019;07 Feb 2019. doi: 10.1093/neuonc/noz041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Verma V, Simone CB, Mishra MV. Quality of life and patient-reported outcomes following proton radiation therapy: a systematic review. J Natl Cancer Inst 2018; 110: 341–53. doi: 10.1093/jnci/djx208 [DOI] [PubMed] [Google Scholar]
- 18.McGowan SE, Albertini F, Thomas SJ, Lomax AJ. Defining robustness protocols: a method to include and evaluate robustness in clinical plans. Phys Med Biol 2015; 60: 2671–84. doi: 10.1088/0031-9155/60/7/2671 [DOI] [PubMed] [Google Scholar]
- 19.Unkelbach J, Alber M, Bangert M, Bokrantz R, Chan TCY, Deasy JO, et al. Robust radiotherapy planning. Phys Med Biol 2018; 63: 22TR02. doi: 10.1088/1361-6560/aae659 [DOI] [PubMed] [Google Scholar]
- 20.Paganetti H. Relative biological effectiveness (RBE) values for proton beam therapy. variations as a function of biological endpoint, dose, and linear energy transfer. Phys Med Biol 2014; 59: R419–R472. doi: 10.1088/0031-9155/59/22/R419 [DOI] [PubMed] [Google Scholar]
- 21.Lühr A, von Neubeck C, Krause M, Troost EGC. Relative biological effectiveness in proton beam therapy - Current knowledge and future challenges. Clin Transl Radiat Oncol 2018; 9: 35–41. doi: 10.1016/j.ctro.2018.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Unkelbach J, Botas P, Giantsoudi D, Gorissen BL, Paganetti H. Reoptimization of intensity modulated proton therapy plans based on linear energy transfer. International Journal of Radiation Oncology*Biology*Physics 2016; 96: 1097–106. doi: 10.1016/j.ijrobp.2016.08.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lühr A, von Neubeck C, Pawelke J, Seidlitz A, Peitzsch C, Bentzen SM, et al. "Radiobiology of Proton Therapy": Results of an international expert workshop. Radiother Oncol 2018; 128: 56–67. doi: 10.1016/j.radonc.2018.05.018 [DOI] [PubMed] [Google Scholar]
- 24.Peeler CR, Mirkovic D, Titt U, Blanchard P, Gunther JR, Mahajan A, et al. Clinical evidence of variable proton biological effectiveness in pediatric patients treated for ependymoma. Radiother Oncol 2016; 121: 395–401. doi: 10.1016/j.radonc.2016.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haas-Kogan D, Indelicato D, Paganetti H, Esiashvili N, Mahajan A, Yock T, et al. National Cancer Institute workshop on proton therapy for children: considerations regarding brainstem injury. International Journal of Radiation Oncology*Biology*Physics 2018; 101: 152–68. doi: 10.1016/j.ijrobp.2018.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fjæra LF, Li Z, Ytre-Hauge KS, Muren LP, Indelicato DJ, Lassen-Ramshad Y, et al. Linear energy transfer distributions in the brainstem depending on tumour location in intensity-modulated proton therapy of paediatric cancer. Acta Oncol 2017; 56: 763–8. doi: 10.1080/0284186X.2017.1314007 [DOI] [PubMed] [Google Scholar]
- 27.Grau C, Baumann M, Weber DC. Optimizing clinical research and generating prospective high-quality data in particle therapy in Europe: introducing the European particle therapy Network (EPTN. Radiother Oncol 2018; 128: 1–3. doi: 10.1016/j.radonc.2018.06.021 [DOI] [PubMed] [Google Scholar]
- 28.Cox JD. Impediments to comparative clinical trials with proton therapy. International Journal of Radiation Oncology*Biology*Physics 2016; 95: 4–8. doi: 10.1016/j.ijrobp.2015.09.036 [DOI] [PubMed] [Google Scholar]
- 29.Goitein M. Trials and tribulations in charged particle radiotherapy. Radiother Oncol 2010; 95: 23–31. doi: 10.1016/j.radonc.2009.06.012 [DOI] [PubMed] [Google Scholar]
- 30.Langendijk JA, Boersma LJ, Rasch CRN, van Vulpen M, Reitsma JB, van der Schaaf A, et al. Clinical trial strategies to compare protons with photons. Semin Radiat Oncol 2018; 28: 79–87. doi: 10.1016/j.semradonc.2017.11.008 [DOI] [PubMed] [Google Scholar]
- 31.Buckner JC, Shaw EG, Pugh SL, Chakravarti A, Gilbert MR, Barger GR, et al. Radiation plus procarbazine, CCNU, and vincristine in low-grade glioma. N Engl J Med 2016; 374: 1344–55. doi: 10.1056/NEJMoa1500925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Douw L, Klein M, Fagel SS, van den Heuvel J, Taphoorn MJ, Aaronson NK, et al. Cognitive and radiological effects of radiotherapy in patients with low-grade glioma: long-term follow-up. Lancet Neurol 2009; 8: 810–8. doi: 10.1016/S1474-4422(09)70204-2 [DOI] [PubMed] [Google Scholar]
- 33.Eekers DBP, Roelofs E, Cubillos-Mesías M, Niël C, Smeenk RJ, Hoeben A, et al. Intensity-modulated proton therapy decreases dose to organs at risk in low-grade glioma patients: results of a multicentric in silico ROCOCO trial. Acta Oncol 2019; 58: 57–65. doi: 10.1080/0284186X.2018.1529424 [DOI] [PubMed] [Google Scholar]
- 34.Shih HA, Sherman JC, Nachtigall LB, Colvin MK, Fullerton BC, Daartz J, et al. Proton therapy for low-grade gliomas: results from a prospective trial. Cancer 2015; 121: 1712–9. doi: 10.1002/cncr.29237 [DOI] [PubMed] [Google Scholar]
- 35.Hauswald H, Rieken S, Ecker S, Kessel KA, Herfarth K, Debus J, et al. First experiences in treatment of low-grade glioma grade I and II with proton therapy. Radiat Oncol 2012; 7: 189. doi: 10.1186/1748-717X-7-189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Badiyan SN, Ulmer S, Ahlhelm FJ, Fredh ASM, Kliebsch U, Calaminus G, et al. Clinical and radiologic outcomes in adults and children treated with Pencil-Beam scanning proton therapy for low-grade glioma. International Journal of Particle Therapy 2017; 3: 450–60. doi: 10.14338/IJPT-16-00031.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fitzek MM, Thornton AF, Harsh G, Rabinov JD, Munzenrider JE, Lev M, et al. Dose-escalation with proton/photon irradiation for Daumas-Duport lower-grade glioma: results of an institutional phase I/II trial. International Journal of Radiation Oncology*Biology*Physics 2001; 51: 131–7. doi: 10.1016/S0360-3016(01)01589-9 [DOI] [PubMed] [Google Scholar]
- 38.McDonald MW, Plankenhorn DA, McMullen KP, Henderson MA, Dropcho EJ, Shah MV, et al. Proton therapy for atypical meningiomas. J Neurooncol 2015; 123: 123–8. doi: 10.1007/s11060-015-1770-9 [DOI] [PubMed] [Google Scholar]
- 39.Halasz LM, Bussière MR, Dennis ER, Niemierko A, Chapman PH, Loeffler JS, et al. Proton stereotactic radiosurgery for the treatment of benign meningiomas. International Journal of Radiation Oncology*Biology*Physics 2011; 81: 1428–35. doi: 10.1016/j.ijrobp.2010.07.1991 [DOI] [PubMed] [Google Scholar]
- 40.Weber DC, Schneider R, Goitein G, Koch T, Ares C, Geismar JH, et al. Spot scanning-based proton therapy for intracranial meningioma: long-term results from the Paul Scherrer Institute. International Journal of Radiation Oncology*Biology*Physics 2012; 83: 865–71. doi: 10.1016/j.ijrobp.2011.08.027 [DOI] [PubMed] [Google Scholar]
- 41.Slater JD, Loredo LN, Chung A, Bush DA, Patyal B, Johnson WD, et al. Fractionated proton radiotherapy for benign cavernous sinus meningiomas. International Journal of Radiation Oncology*Biology*Physics 2012; 83: e633–7. doi: 10.1016/j.ijrobp.2012.01.079 [DOI] [PubMed] [Google Scholar]
- 42.Combs SE, Welzel T, Habermehl D, Rieken S, Dittmar J-O, Kessel K, et al. Prospective evaluation of early treatment outcome in patients with meningiomas treated with particle therapy based on target volume definition with MRI and 68Ga-DOTATOC-PET. Acta Oncol 2013; 52: 514–20. doi: 10.3109/0284186X.2013.762996 [DOI] [PubMed] [Google Scholar]
- 43.Vlachogiannis P, Gudjonsson O, Montelius A, Grusell E, Isacsson U, Nilsson K, et al. Hypofractionated high-energy proton-beam irradiation is an alternative treatment for WHO grade I meningiomas. Acta Neurochir 2017; 159: 2391–400. doi: 10.1007/s00701-017-3352-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Murray FR, Snider JW, Bolsi A, Lomax AJ, Walser M, Kliebsch U, et al. Long-term clinical outcomes of pencil beam scanning proton therapy for benign and non-benign intracranial meningiomas. International Journal of Radiation Oncology*Biology*Physics 2017; 99: 1190–8. doi: 10.1016/j.ijrobp.2017.08.005 [DOI] [PubMed] [Google Scholar]
- 45.El Shafie RA, Czech M, Kessel KA, Habermehl D, Weber D, Rieken S, et al. Clinical outcome after particle therapy for meningiomas of the skull base: toxicity and local control in patients treated with active rasterscanning. Radiat Oncol 2018; 13: 54. doi: 10.1186/s13014-018-1002-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smoll NR, Gautschi OP, Radovanovic I, Schaller K, Weber DC. Incidence and relative survival of chordomas: the standardized mortality ratio and the impact of chordomas on a population. Cancer 2013; 119: 2029–37. doi: 10.1002/cncr.28032 [DOI] [PubMed] [Google Scholar]
- 47.Pearlman AW, Friedman M. Radical radiation therapy of chordoma. American Journal of Roentgenology 1970; 108: 333–41. doi: 10.2214/ajr.108.2.333 [DOI] [PubMed] [Google Scholar]
- 48.Rich TA, Schiller A, Suit HD, Mankin HJ. Clinical and pathologic review of 48 cases of chordoma. Cancer 1985; 56: 182–7. doi: [DOI] [PubMed] [Google Scholar]
- 49.Debus J, Hug EB, Liebsch NJ, O'Farrel D, Finkelstein D, Efird J, et al. Brainstem tolerance to conformal radiotherapy of skull base tumors. International Journal of Radiation Oncology*Biology*Physics 1997; 39: 967–75. doi: 10.1016/S0360-3016(97)00364-7 [DOI] [PubMed] [Google Scholar]
- 50.Weber DC, Murray F, Combescure C, Calugaru V, Alapetite C, Albertini F, et al. Long term outcome of skull-base chondrosarcoma patients treated with high-dose proton therapy with or without conventional radiation therapy. Radiother Oncol 2018; 129: 520–6. doi: 10.1016/j.radonc.2018.06.040 [DOI] [PubMed] [Google Scholar]
- 51.Weber DC, Malyapa R, Albertini F, Bolsi A, Kliebsch U, Walser M, et al. Long term outcomes of patients with skull-base low-grade chondrosarcoma and chordoma patients treated with pencil beam scanning proton therapy. Radiother Oncol 2016; 120: 169–74. doi: 10.1016/j.radonc.2016.05.011 [DOI] [PubMed] [Google Scholar]
- 52.Hug EB, Loredo LN, Slater JD, DeVries A, Grove RI, Schaefer RA, et al. Proton radiation therapy for chordomas and chondrosarcomas of the skull base. J Neurosurg 1999; 91: 432–9. doi: 10.3171/jns.1999.91.3.0432 [DOI] [PubMed] [Google Scholar]
- 53.Fung V, Calugaru V, Bolle S, Mammar H, Alapetite C, Maingon P, et al. Proton beam therapy for skull base chordomas in 106 patients: a dose adaptive radiation protocol. Radiother Oncol 2018; 128: 198–202. doi: 10.1016/j.radonc.2017.12.017 [DOI] [PubMed] [Google Scholar]
- 54.Takagi M, Demizu Y, Nagano F, Terashima K, Fujii O, Jin D, et al. Treatment outcomes of proton or carbon ion therapy for skull base chordoma: a retrospective study. Radiat Oncol 2018; 13: 232. doi: 10.1186/s13014-018-1173-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Romero J, Cardenes H, la Torre A, Valcarcel F, Magallon R, Regueiro C, et al. Chordoma: results of radiation therapy in eighteen patients. Radiother Oncol 1993; 29: 27–32. doi: 10.1016/0167-8140(93)90169-9 [DOI] [PubMed] [Google Scholar]
- 56.Catton C, O'Sullivan B, Bell R, Laperriere N, Cummings B, Fornasier V, et al. Chordoma: long-term follow-up after radical photon irradiation. Radiother Oncol 1996; 41: 67–72. doi: 10.1016/S0167-8140(96)01805-1 [DOI] [PubMed] [Google Scholar]
- 57.Choy W, Terterov S, Ung N, Kaprealian T, Trang A, DeSalles A, et al. Adjuvant stereotactic radiosurgery and radiation therapy for the treatment of intracranial chordomas. J Neurol Surg B Skull Base 2016; 77: 038–46. doi: 10.1055/s-0035-1554907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zakaria WK, Hafez RF, Taha AN. Gamma knife management of skull base chordomas: is it a choice? Asian J Neurosurg 2018; 13: 1037–41. doi: 10.4103/ajns.AJNS_61_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zorlu F, Gultekin M, Cengiz M, Yildiz F, Akyol F, Gurkaynak M, et al. Fractionated stereotactic radiosurgery treatment results for skull base chordomas. Technol Cancer Res Treat 2014; 13: 11–19. doi: 10.7785/tcrt.2012.500354 [DOI] [PubMed] [Google Scholar]
- 60.Snider JW, Schneider RA, Poelma-Tap D, Stieb S, Murray FR, Placidi L, et al. Long-term outcomes and prognostic factors after Pencil-Beam scanning proton radiation therapy for spinal chordomas: a large, single-institution cohort. International Journal of Radiation Oncology*Biology*Physics 2018; 101: 226–33. doi: 10.1016/j.ijrobp.2018.01.060 [DOI] [PubMed] [Google Scholar]
- 61.Youn SH, Cho KH, Kim J-Y, Ha B, Lim YK, Jeong JH, et al. Clinical outcome of proton therapy for patients with chordomas. Radiat Oncol J 2018; 36: 182–91. doi: 10.3857/roj.2018.00164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hayashi Y, Mizumoto M, Akutsu H, Takano S, Matsumura A, Okumura T, et al. Hyperfractionated high-dose proton beam radiotherapy for clival chordomas after surgical removal. Br J Radiol 2016; 89: 20151051. doi: 10.1259/bjr.20151051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gatfield ER, Noble DJ, Barnett GC, Early NY, Hoole ACF, Kirkby NF, et al. Tumour volume and dose influence outcome after surgery and high-dose photon radiotherapy for chordoma and chondrosarcoma of the skull base and spine. Clin Oncol 2018; 30: 243–53. doi: 10.1016/j.clon.2018.01.002 [DOI] [PubMed] [Google Scholar]
- 64.Demizu Y, Mizumoto M, Onoe T, Nakamura N, Kikuchi Y, Shibata T, et al. Proton beam therapy for bone sarcomas of the skull base and spine: a retrospective nationwide multicenter study in Japan. Cancer Sci 2017; 108: 972–7. doi: 10.1111/cas.13192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Feuvret L, Bracci S, Calugaru V, Bolle S, Mammar H, De Marzi L, et al. Efficacy and safety of adjuvant proton therapy combined with surgery for chondrosarcoma of the skull base: a retrospective, population-based study. International Journal of Radiation Oncology*Biology*Physics 2016; 95: 312–21. doi: 10.1016/j.ijrobp.2015.12.016 [DOI] [PubMed] [Google Scholar]
- 66.Deraniyagala R, Yeung D, Mendenhall W, Li Z, Morris C, Mendenhall N, et al. Proton therapy for skull base chordomas: an outcome study from the University of Florida proton therapy Institute. J Neurol Surg B Skull Base 2014; 75: 053–7. doi: 10.1055/s-0033-1354579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yasuda M, Bresson D, Chibbaro S, Cornelius JF, Polivka M, Feuvret L, et al. Chordomas of the skull base and cervical spine: clinical outcomes associated with a multimodal surgical resection combined with proton-beam radiation in 40 patients. Neurosurg Rev 2012; 35: 171–83discussion 82-3. doi: 10.1007/s10143-011-0334-5 [DOI] [PubMed] [Google Scholar]
- 68.Fuji H, Nakasu Y, Ishida Y, Horiguchi S, Mitsuya K, Kashiwagi H, et al. Feasibility of proton beam therapy for chordoma and chondrosarcoma of the skull base. Skull Base 2011; 21: 201–6. doi: 10.1055/s-0031-1275636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Noël G, Habrand J-L, Mammar H, Pontvert D, Haie-Méder C, Hasboun D, et al. Combination of photon and proton radiation therapy for chordomas and chondrosarcomas of the skull base: the Centre de Protonthérapie D’Orsay experience. International Journal of Radiation Oncology*Biology*Physics 2001; 51: 392–8. doi: 10.1016/S0360-3016(01)01634-0 [DOI] [PubMed] [Google Scholar]
- 70.Terahara A, Niemierko A, Goitein M, Finkelstein D, Hug E, Liebsch N, et al. Analysis of the relationship between tumor dose inhomogeneity and local control in patients with skull base chordoma. International Journal of Radiation Oncology*Biology*Physics 1999; 45: 351–8. doi: 10.1016/S0360-3016(99)00146-7 [DOI] [PubMed] [Google Scholar]
- 71.Munzenrider JE, Liebsch NJ. Proton therapy for tumors of the skull base. Strahlenther Onkol 1999; 175 Suppl 2(Suppl 2): 57–63. doi: 10.1007/BF03038890 [DOI] [PubMed] [Google Scholar]
- 72.Austin-Seymour M, Munzenrider J, Goitein M, Verhey L, Urie M, Gentry R, et al. Fractionated proton radiation therapy of chordoma and low-grade chondrosarcoma of the base of the skull. J Neurosurg 1989; 70: 13–17. doi: 10.3171/jns.1989.70.1.0013 [DOI] [PubMed] [Google Scholar]
- 73.Berson AM, Castro JR, Petti P, Phillips TL, Gauger GE, Gutin P, et al. Charged particle irradiation of chordoma and chondrosarcoma of the base of skull and cervical spine: the Lawrence Berkeley laboratory experience. International Journal of Radiation Oncology*Biology*Physics 1988; 15: 559–65. doi: 10.1016/0360-3016(88)90295-7 [DOI] [PubMed] [Google Scholar]
- 74.Pehlivan B, Ares C, Lomax AJ, Stadelmann O, Goitein G, Timmermann B, et al. Temporal lobe toxicity analysis after proton radiation therapy for skull base tumors. International Journal of Radiation Oncology*Biology*Physics 2012; 83: 1432–40. doi: 10.1016/j.ijrobp.2011.10.042 [DOI] [PubMed] [Google Scholar]
- 75.Steliarova-Foucher E, Colombet M, Ries LAG, Moreno F, Dolya A, Bray F, et al. International incidence of childhood cancer, 2001-10: a population-based registry study. Lancet Oncol 2017; 18: 719–31. doi: 10.1016/S1470-2045(17)30186-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pui C-H, Pei D, Pappo AS, Howard SC, Cheng C, Sandlund JT, et al. Treatment outcomes in black and white children with cancer: results from the SEER database and ST Jude Children's Research Hospital, 1992 through 2007. JCO 2012; 30: 2005–12. doi: 10.1200/JCO.2011.40.8617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bhakta N, Liu Q, Ness KK, Baassiri M, Eissa H, Yeo F, et al. The cumulative burden of surviving childhood cancer: an initial report from the ST Jude lifetime cohort Study (SJLIFE. The Lancet 2017; 390: 2569–82. doi: 10.1016/S0140-6736(17)31610-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Greenberger BA, Pulsifer MB, Ebb DH, MacDonald SM, Jones RM, Butler WE, et al. Clinical outcomes and late Endocrine, neurocognitive, and visual profiles of proton radiation for pediatric low-grade gliomas. International Journal of Radiation Oncology*Biology*Physics 2014; 89: 1060–8. doi: 10.1016/j.ijrobp.2014.04.053 [DOI] [PubMed] [Google Scholar]
- 79.Moeller BJ, Chintagumpala M, Philip JJ, Grosshans DR, McAleer MF, Woo SY, et al. Low early ototoxicity rates for pediatric medulloblastoma patients treated with proton radiotherapy. Radiat Oncol 2011; 6: 58. doi: 10.1186/1748-717X-6-58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Krasin MJ, Constine LS, Friedman DL, Marks LB. Radiation-related treatment effects across the age spectrum: differences and similarities or what the old and young can learn from each other. Semin Radiat Oncol 2010; 20: 21–9. doi: 10.1016/j.semradonc.2009.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Plimpton SR, Stence N, Hemenway M, Hankinson TC, Foreman N, Liu AK. Cerebral radiation necrosis in pediatric patients. Pediatr Hematol Oncol 2015; 32: 78–83. doi: 10.3109/08880018.2013.791738 [DOI] [PubMed] [Google Scholar]
- 82.Sabin ND, Merchant TE, Harreld JH, Patay Z, Klimo P, Qaddoumi I, et al. Imaging changes in very young children with brain tumors treated with proton therapy and chemotherapy. AJNR Am J Neuroradiol 2013; 34: 446–50. doi: 10.3174/ajnr.A3219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Indelicato DJ, Flampouri S, Rotondo RL, Bradley JA, Morris CG, Aldana PR, et al. Incidence and dosimetric parameters of pediatric brainstem toxicity following proton therapy. Acta Oncol 2014; 53: 1298–304. doi: 10.3109/0284186X.2014.957414 [DOI] [PubMed] [Google Scholar]
- 84.McGovern SL, Okcu MF, Munsell MF, Kumbalasseriyil N, Grosshans DR, McAleer MF, et al. Outcomes and acute toxicities of proton therapy for pediatric atypical teratoid/rhabdoid tumor of the central nervous system. International Journal of Radiation Oncology*Biology*Physics 2014; 90: 1143–52. doi: 10.1016/j.ijrobp.2014.08.354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gunther JR, Sato M, Chintagumpala M, Ketonen L, Jones JY, Allen PK, et al. Imaging changes in pediatric intracranial ependymoma patients treated with proton beam radiation therapy compared to intensity modulated radiation therapy. International Journal of Radiation Oncology*Biology*Physics 2015; 93: 54–63. doi: 10.1016/j.ijrobp.2015.05.018 [DOI] [PubMed] [Google Scholar]
- 86.Giantsoudi D, Sethi RV, Yeap BY, Eaton BR, Ebb DH, Caruso PA, et al. Incidence of CNS injury for a cohort of 111 patients treated with proton therapy for medulloblastoma: let and RBE associations for areas of injury. International Journal of Radiation Oncology*Biology*Physics 2016; 95: 287–96. doi: 10.1016/j.ijrobp.2015.09.015 [DOI] [PubMed] [Google Scholar]
- 87.Bojaxhiu B, Ahlhelm F, Walser M, Placidi L, Kliebsch U, Mikroutsikos L, et al. Radiation Necrosis and White Matter Lesions in Pediatric Patients With Brain Tumors Treated With Pencil Beam Scanning Proton Therapy. International Journal of Radiation Oncology*Biology*Physics 2018; 100: 987–96. doi: 10.1016/j.ijrobp.2017.11.037 [DOI] [PubMed] [Google Scholar]
- 88.Gentile MS, Yeap BY, Paganetti H, Goebel CP, Gaudet DE, Gallotto SL, et al. Brainstem injury in pediatric patients with posterior fossa tumors treated with proton beam therapy and associated Dosimetric factors. International Journal of Radiation Oncology*Biology*Physics 2018; 100: 719–29. doi: 10.1016/j.ijrobp.2017.11.026 [DOI] [PubMed] [Google Scholar]
- 89.Merchant TE, Hua C-H, Shukla H, Ying X, Nill S, Oelfke U. Proton versus photon radiotherapy for common pediatric brain tumors: comparison of models of dose characteristics and their relationship to cognitive function. Pediatr Blood Cancer 2008; 51: 110–7. doi: 10.1002/pbc.21530 [DOI] [PubMed] [Google Scholar]
- 90.St. Clair WH, Adams JA, Bues M, Fullerton BC, La Shell S, Kooy HM, et al. Advantage of protons compared to conventional x-ray or IMRT in the treatment of a pediatric patient with medulloblastoma. International Journal of Radiation Oncology*Biology*Physics 2004; 58: 727–34. doi: 10.1016/S0360-3016(03)01574-8 [DOI] [PubMed] [Google Scholar]
- 91.Bishop AJ, Greenfield B, Mahajan A, Paulino AC, Okcu MF, Allen PK, et al. Proton beam therapy versus conformal photon radiation therapy for childhood craniopharyngioma: multi-institutional analysis of outcomes, cyst dynamics, and toxicity. International Journal of Radiation Oncology*Biology*Physics 2014; 90: 354–61. doi: 10.1016/j.ijrobp.2014.05.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ares C, Albertini F, Frei-Welte M, Bolsi A, Grotzer MA, Goitein G, et al. Pencil beam scanning proton therapy for pediatric intracranial ependymoma. J Neurooncol 2016; 128: 137–45. doi: 10.1007/s11060-016-2090-4 [DOI] [PubMed] [Google Scholar]
- 93.MacDonald SM, Trofimov A, Safai S, Adams J, Fullerton B, Ebb D, et al. Proton radiotherapy for pediatric central nervous system germ cell tumors: early clinical outcomes. International Journal of Radiation Oncology*Biology*Physics 2011; 79: 121–9. doi: 10.1016/j.ijrobp.2009.10.069 [DOI] [PubMed] [Google Scholar]
- 94.Hug EB, Muenter MW, Archambeau JO, DeVries A, Liwnicz B, Loredo LN, et al. Conformal proton radiation therapy for pediatric low-grade astrocytomas. Strahlenther Onkol 2002; 178: 10–17. doi: 10.1007/s00066-002-0874-2 [DOI] [PubMed] [Google Scholar]
- 95.Weber DC, Ares C, Malyapa R, Albertini F, Calaminus G, Kliebsch U, et al. Tumor control and QOL outcomes of very young children with atypical teratoid/rhabdoid tumor treated with focal only chemo-radiation therapy using pencil beam scanning proton therapy. J Neurooncol 2015; 121: 389–97. doi: 10.1007/s11060-014-1648-2 [DOI] [PubMed] [Google Scholar]
- 96.De Amorim Bernstein K, Sethi R, Trofimov A, Zeng C, Fullerton B, Yeap BY, et al. Early clinical outcomes using proton radiation for children with central nervous system atypical teratoid rhabdoid tumors. International Journal of Radiation Oncology*Biology*Physics 2013; 86: 114–20. doi: 10.1016/j.ijrobp.2012.12.004 [DOI] [PubMed] [Google Scholar]
- 97.MacDonald SM, Safai S, Trofimov A, Wolfgang J, Fullerton B, Yeap BY, et al. Proton radiotherapy for childhood ependymoma: initial clinical outcomes and dose comparisons. International Journal of Radiation Oncology*Biology*Physics 2008; 71: 979–86. doi: 10.1016/j.ijrobp.2007.11.065 [DOI] [PubMed] [Google Scholar]
- 98.Mizumoto M, Oshiro Y, Takizawa D, Fukushima T, Fukushima H, Yamamoto T, et al. Proton beam therapy for pediatric ependymoma. Pediatr Int 2015; 57: 567–71. doi: 10.1111/ped.12624 [DOI] [PubMed] [Google Scholar]
- 99.Sato M, Gunther JR, Mahajan A, Jo E, Paulino AC, Adesina AM, et al. Progression-free survival of children with localized ependymoma treated with intensity-modulated radiation therapy or proton-beam radiation therapy. Cancer 2017; 123: 2570–8. doi: 10.1002/cncr.30623 [DOI] [PubMed] [Google Scholar]
- 100.Jimenez RB, Sethi R, Depauw N, Pulsifer MB, Adams J, McBride SM, et al. Proton radiation therapy for pediatric medulloblastoma and supratentorial primitive neuroectodermal tumors: outcomes for very young children treated with upfront chemotherapy. International Journal of Radiation Oncology*Biology*Physics 2013; 87: 120–6. doi: 10.1016/j.ijrobp.2013.05.017 [DOI] [PubMed] [Google Scholar]
- 101.Eaton BR, Chowdhry V, Weaver K, Liu L, Ebb D, MacDonald SM, et al. Use of proton therapy for re-irradiation in pediatric intracranial ependymoma. Radiother Oncol 2015; 116: 301–8. doi: 10.1016/j.radonc.2015.07.023 [DOI] [PubMed] [Google Scholar]
- 102.Yock TI, Yeap BY, Ebb DH, Weyman E, Eaton BR, Sherry NA, et al. Long-term toxic effects of proton radiotherapy for paediatric medulloblastoma: a phase 2 single-arm study. Lancet Oncol 2016; 17: 287–98. doi: 10.1016/S1470-2045(15)00167-9 [DOI] [PubMed] [Google Scholar]
- 103.Eaton BR, Esiashvili N, Kim S, Patterson B, Weyman EA, Thornton LT, et al. Endocrine outcomes with proton and photon radiotherapy for standard risk medulloblastoma. Neuro Oncol 2016; 18: 881–7. doi: 10.1093/neuonc/nov302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Stevens W, Philipson TJ, Khan ZM, MacEwan JP, Linthicum MT, Goldman DP. Cancer mortality reductions were greatest among countries where cancer care spending rose the most, 1995–2007. Health Aff 2015; 34: 562–70. doi: 10.1377/hlthaff.2014.0634 [DOI] [PubMed] [Google Scholar]
- 105.Goitein M, Jermann M. The relative costs of proton and X-ray radiation therapy. Clin Oncol 2003; 15: S37–S50. doi: 10.1053/clon.2002.0174 [DOI] [PubMed] [Google Scholar]
- 106.Schippers JM, Lomax A, Garonna A, Parodi K. Can technological improvements reduce the cost of proton radiation therapy? Semin Radiat Oncol 2018; 28: 150–9. doi: 10.1016/j.semradonc.2017.11.007 [DOI] [PubMed] [Google Scholar]
- 107.Verma V, Mishra MV, Mehta MP. A systematic review of the cost and cost-effectiveness studies of proton radiotherapy. Cancer 2016; 122: 1483–501. doi: 10.1002/cncr.29882 [DOI] [PubMed] [Google Scholar]
- 108.Lievens Y, Pijls-Johannesma M. Health economic controversy and cost-effectiveness of proton therapy. Semin Radiat Oncol 2013; 23: 134–41. doi: 10.1016/j.semradonc.2012.11.005 [DOI] [PubMed] [Google Scholar]
- 109.Porter ME. What is value in health care? N Engl J Med 2010; 363: 2477–81. doi: 10.1056/NEJMp1011024 [DOI] [PubMed] [Google Scholar]
- 110.Lundkvist J, Ekman M, Ericsson SR, Jönsson B, Glimelius B. Cost-effectiveness of proton radiation in the treatment of childhood medulloblastoma. Cancer 2005; 103: 793–801. doi: 10.1002/cncr.20844 [DOI] [PubMed] [Google Scholar]
- 111.Mailhot Vega RB, Kim J, Bussière M, Hattangadi J, Hollander A, Michalski J, et al. Cost effectiveness of proton therapy compared with photon therapy in the management of pediatric medulloblastoma. Cancer 2013; 119: 4299–307. doi: 10.1002/cncr.28322 [DOI] [PubMed] [Google Scholar]
- 112.Hirano E, Fuji H, Onoe T, Kumar V, Shirato H, Kawabuchi K. Cost-effectiveness analysis of cochlear dose reduction by proton beam therapy for medulloblastoma in childhood. J Radiat Res 2014; 55: 320–7. doi: 10.1093/jrr/rrt112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mailhot Vega R, Kim J, Hollander A, Hattangadi-Gluth J, Michalski J, Tarbell NJ, et al. Cost effectiveness of proton versus photon radiation therapy with respect to the risk of growth hormone deficiency in children. Cancer 2015; 121: 1694–702. doi: 10.1002/cncr.29209 [DOI] [PubMed] [Google Scholar]
- 114.Zafar SY. Financial toxicity of cancer care: it's time to intervene. J Natl Cancer Inst 2016; 10811 12 2015. doi: 10.1093/jnci/djv370 [DOI] [PubMed] [Google Scholar]
- 115.Zafar SY, Peppercorn JM, Schrag D, Taylor DH, Goetzinger AM, Zhong X, et al. The financial toxicity of cancer treatment: a pilot study assessing out-of-pocket expenses and the insured cancer patient's experience. Oncologist 2013; 18: 381–90. doi: 10.1634/theoncologist.2012-0279 [DOI] [PMC free article] [PubMed] [Google Scholar]