Abstract
Radiation therapy is an essential component of treatment for locally advanced non-small cell lung cancer (NSCLC) but can be technically challenging because of the proximity of lung tumors to nearby critical organs or structures. The most effective strategy for reducing radiation-induced toxicity is to reduce unnecessary exposure of normal tissues by using advanced technology; examples from photon (X-ray) therapy have included three-dimensional conformal radiation therapy versus its predecessor, two-dimensional radiation therapy, and intensity-modulated photon radiation therapy versus its predecessor, three-dimensional conformal therapy. Using particle-beam therapy rather than photons offers the potential for further advantages because of the unique depth-dose characteristics of the particles, which can be exploited to allow still higher dose escalation to tumors with greater sparing of normal tissues, with the ultimate goal of improving local tumor control and survival while preserving quality of life by reducing treatment-related toxicity. However, the costs associated with particle therapy with protons are considerably higher than the current state of the art in photon technology, and evidence of clinical benefit from protons is increasingly being demanded to justify the higher financial burden on the healthcare system. Some such evidence is available from preclinical studies, from retrospective, single-institution clinical series, from analyses of national databases, and from single-arm prospective studies in addition to several ongoing randomized comparative trials. This review summarizes the rationale for and challenges of using proton therapy to treat thoracic cancers, reviews the current clinical experience, and suggests topics for future research.
Introduction
Locally advanced non-small cell lung cancer (NSCLC) is challenging to treat with radiation because of the proximity of the tumor to several organs at risk (OARs), including the esophagus, lung, heart, and bone marrow; other important structures or tissues include the brachial plexus, skin, and chest wall. In principle, the most effective strategy to reduce toxicity is to reduce unnecessary radiation exposure to the OARs by using advanced technology. Proton therapy offers the potential for substantial clinical advantages over conventional photon therapy because of its unique depth–dose characteristics, which can be exploited to significantly reduce normal tissue doses both proximal and distal to the target volume.1–5 These characteristics may in turn allow escalation of tumor doses with greater sparing of normal tissues, which presumably would improve local control and survival while at the same time reducing toxicity and improving quality of life.
However, proton therapy, like other forms of particle therapy, is significantly more costly than even the best available photon technology at the current state of the art, and evidence demonstrating clinical benefit after proton therapy is increasingly being demanded to justify the higher financial burden on the healthcare delivery system.6–9 Despite the high capital costs associated with charged particle therapy and the lack of evidence of clinical benefit in many types of cancer from direct comparisons, the increasing demand for improved technology in cancer treatment, particularly proton therapy, is evidenced by the numbers of facilities that have been built or are currently being built worldwide. Currently, a total of 76 particle therapy centers are operating worldwide, 25 in the United States alone, and many more are being planned (https://www.ptcog.ch/index.php/). By the end of 2017, about 200,000 patients will have been treated with charged particle therapy worldwide (https://www.ptcog.ch/index.php/). Along with the increased numbers of facilities and clinical use of particle therapy, knowledge of the uncertainties associated with particle therapy and methods to counteract these uncertainties in treatment planning and delivery is accumulating.
This review summarizes the rationale for and challenges of using charged particles to treat thoracic cancer, provides an update on clinical experience with protons for locally advanced lung cancer, and considers topics for future research.
Physical characteristics of charged particles
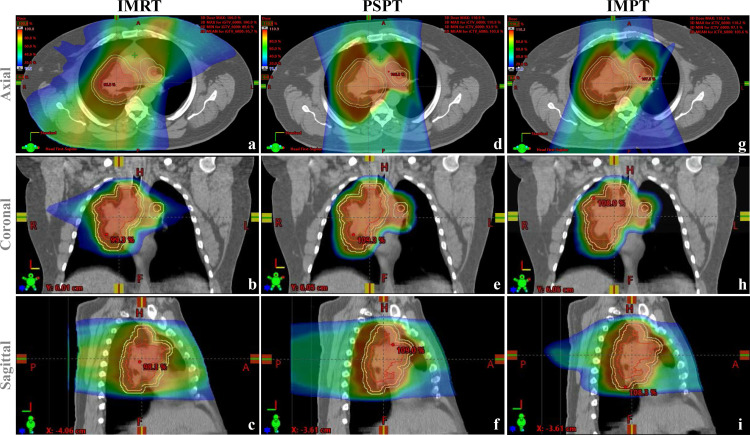
Mohan and Grosshans provided a thorough review of the physical characteristics of charged particles in 2017.10 Charged particles being used or explored for cancer radiotherapy at present include protons, carbon, and helium. All charged particles have similar depth-dose characteristics, termed the “Bragg peak.” When a “fast” charged particle moves through matter, it interacts with the electrons within atoms and causes ionization, depositing energy and dose along its path. The energy loss per unit path length is relatively constant until the particle reaches a peak (the Bragg peak), where energy is deposited at a depth that is a function of the energy and nature of the charged particle. No dose exits through the normal tissues beyond the Bragg peak. In passively scattering proton therapy (PSPT), the Bragg peak is spread out longitudinally and laterally to create the spread-out Bragg peak (SOBP) to provide a uniform dose to cover the entire volume of a target. Conformal coverage of the tumor is achieved by crafting range modulation wheels, compensators, and apertures specific for each patient. Pencil beam scanning proton therapy, on the other hand, uses magnetic scanning of thin beamlets of protons of a sequence of energies delivered from different directions to produce the desired pattern of dose distribution. The tumor is scanned layer by layer, one layer per energy, until the entire target has received the desired dose. Pencil beam scanning provides greater flexibility and control for ideal dose distribution and allows delivery of intensity-modulated proton treatment (IMPT). the most advanced form of proton therapy to date.10 Three sets of plans for a patient with locally advanced NSCLC to be treated with intensity-modulated (photon) radiation therapy (IMRT), PSPT, and IMPT are shown in Figure 1, illustrating the differences in dosimetric characteristics among these techniques.
Figure 1.
Comparative radiation dose distributions on plans for IMRT (panels a–c), PSPT (panels d–f), and IMPT (g–i) for a patient with locally advanced non-small cell lung cancer with a large right upper lung mass and involved contralateral hilar node. The red dose colorwash represents the high-dose distribution (100% isodose line); the blue dose colorwash depicts the low dose bath (10% isodose line); and the orange and green colorwashes represent intermediate dose. The GTV is contoured in red; the CTV in yellow; and the PTV in light blue. Both PSPT and IMPT are associated with significant reductions in the “low dose bath,” and IMPT provides better dose conformity to the target volumes than either PSPT or IMRT. CTV, clinical target volume; GTV, gross tumor volume; IMPT, intensity-modulated proton treatment; IMRT, intensity-modulated radiation therapy; PSPT, passively scattering proton therapy; PTV, planning target volume.
The biological interactions of ionizing radiation with tissues are related to the amount of energy transferred to those tissues over a specified path length (known as linear energy transfer, LET). For particles such as protons and helium, the LET is thought to be nearly equivalent to that of photons before it reaches the Bragg peak. Traditionally the relative biological effectiveness (RBE) of protons has been thought to be nearly equal to photons (RBE = 1.1). However, the RBE may increase as the function of the depth of penetration and is highest at the distal edge of the beam, i.e. at the Bragg peak, where all of the energy is transferred to tissue within a short distance of the peak.11,12 For heavier charged particles such as carbon, the density of ionization is still higher at the end of their range, resulting in higher RBEs of 1.5–3. Recent evidence has shown that RBE is not a constant but rather a complex, variable function of dose per fraction, LET, cell and tissue type, choice of end-point, and other factors.13,14
Currently, the most commonly used proton delivery technique involves placing the distal edge of the beam at the margin of the planning target volume. However, having a finite range in tissue and having a higher RBE at the distal edge of the beam could be potentially problematic, especially for thoracic cancers owing to movement of the tumor from motion of the lung and diaphragm, the potential for tumor target volumes to overlap critical organs such as the heart, lung, and esophagus, and tissue heterogeneity within the beam path. It is possible that OARs such as the heart act as the “stopping power” to create the Bragg peak, where the RBE is highest. In planning PSPT, tumor motion and tissue heterogeneity can be addressed by adding generous margins. These variables are assessed separately for each beam direction, and so some amount of dosimetric uncertainty is built in into the planning of each beam.15 However, adding generous internal and smearing margins may counteract the dosimetric advantage of the particle beam and cause more normal tissue injury at the distal edge of the field from the high RBE at the Bragg peak. In IMPT, by contrast, conformity of the proximal and lateral field is achieved by limiting the position of the spots to within the target region only. Dynamic apertures that can change shape layer by layer are being developed to address issues associated with unacceptably large spots in pencil beam scanning. In treatment planning, the position and intensities of a matrix of spots within the target volume for each scanned beam are determined by the treatment planning system to achieve the desired dose distribution.
Moreover, the IMPT dose distribution is more sensitive to uncertainties in set up and motion than PSPT. Of particular concern is the “interplay effect,” in which the pattern of dose deposition by the pencil scanning beam is affected by variations in soft tissue and air distance it encounters as a function of the respiratory cycle, i.e. the distance the beam has to travel to the target varies between inspiration and expiration. To account for this uncertainty, several motion management techniques have been implemented, including “respiratory gating,” in which the “beam-on” time is limited to certain periods in the patient’s respiratory cycle, as measured by highly sensitive infrared monitors; “free-breathing” with four-dimensional CT (4DCT), in which a maximum intensity projection is created with 4DCT that depicts a virtual target that encompasses the entirety of potential tumor positions during respiration; and “breath-hold,” in which a series of planning CT scans is obtained while the patient holds their breath at various times during the respiratory cycle to create a composite target, and treatment is also delivered while the patient holds their breath in a similar fashion. Sensitivity to uncertainties is also being addressed by the development of “robust optimization” techniques, which simultaneously consider numerous uncertainty scenarios and optimize beam intensities in the face of all of those scenarios.16,17
Current experience in using proton therapy for locally advanced non-small cell lung cancer
Locally advanced NSCLC is a potentially deadly disease that poses significant challenges for successful treatment. Radiotherapy combined with concurrent chemotherapy followed by adjuvant immunotherapy remains the mainstay of treatment for disease at this stage, for patients who can tolerate it.18–20 Although locally advanced NSCLC is quite likely to metastasize, many patients die of the consequences of uncontrolled intrathoracic tumors, and hence therapies that improve local control would be valuable in terms of extending survival.21 Thoracic radiation has become even more important in long-term survival for patients with NSCLC in the current era of immunotherapy. In the Phase I KEYNOTE-001 study, patients with locally advanced or metastatic NSCLC that expressed the programmed death ligand 1 (PDL1) were given the anti-programmed death-1 (PD1) antibody pembrolizumab as first-line or second-line therapy; the results of secondary analysis showed that radiotherapy given for palliation was associated with durable, positive effects on overall survival with tolerable safety.22 In the Phase III randomized PACIFIC trial, the anti-PDL1 antibody durvalumab was given as consolidation therapy for patients with Stage III NSCLC without disease progression after platinum-based chemoradiotherapy; that trial showed that consolidative durvalumab prolonged the median survival time from 28 months (for the consolidative placebo group) to 41 months.18 Radiation Therapy Oncology Group (RTOG) 0617 was a Phase III trial that compared overall survival after standard-dose (60 Gy in 6 weeks) or high-dose (74 Gy in 7.5 weeks) photon radiation, both given with concurrent carboplatin and paclitaxel, for patients with unresectable Stage III NSCLC.23 Interestingly, although the higher radiation dose was associated with poorer overall survival (median survival times of 28.7 months after 60 Gy vs 21.7 months after 74 Gy, p < 0.001), no difference was found between groups in cancer-related death rates (about 37% in both groups at 18 months). Multivariate analysis showed that the volume of the heart that received 35 Gy or more was an independent predictor of poor overall survival,23 suggesting that cardiac toxicity may have contributed to the early death of some of these patients. A secondary analysis of RTOG 0617 that compared the radiation techniques used (IMRT vs three-dimensional conformal radiation therapy, 3DCRT) showed that patients treated with IMRT had less lung and heart volume irradiated, and less toxicity, specifically radiation pneumonitis, although IMRT did not lead to an overall survival advantage.24 These findings are consistent with a report from Liao and colleagues25 that IMRT led to significant decreases in median irradiated volumes at several dose levels, including V15–V65 (p < 0.0001), compared with 3DCRT. However, V5, V10, and V70 were no different between the two techniques, suggesting that IMRT is of limited benefit in terms of minimizing lung exposure to low-dose radiation.26 The dosimetric characteristics of protons (their finite range in material and their depositing a sharply increased dose [the Bragg peak] at the target and none beyond it) make proton therapy an attractive alternative to IMRT, as protons provide the potential to further intensify the radiation dose without increasing toxicity to nearby normal tissues.
Outcomes after passively scattered proton beam therapy
Despite the technical challenges associated with using particle therapy for lung cancer, where tissue heterogeneity and motion can cause significant uncertainties in dose, proton beam therapy for lung cancer has been tested in clinical settings, although most of the results published to date have been from retrospective, single-institution serieses, from analyses of national databases, or from single-arm prospective studies.
Several retrospective studies have shown that proton therapy was associated with reduced lung, esophageal, and hematologic toxicity after concurrent chemoradiation relative to photon therapy, with acceptable rates of tumor control and survival.27 Another group studied patient-reported outcomes after 3DCRT, IMRT, or PSPT.28 In this prospective longitudinal observational study of 82 patients with unresectable primary or recurrent NSCLC, patients were asked to rate their symptoms by using the MD Anderson Symptom Inventory once a week for up to 12 weeks. Findings from that study revealed that despite the PSPT group receiving a significantly higher radiation target dose than the other two groups (p < 0.001), their symptoms (chiefly fatigue) were significantly less severe than for patients receiving IMRT or 3DCRT.28 A recent report of clinical outcomes among patients with locally advanced NSCLC prospectively treated with concurrent proton therapy [60–74 Gy (RBE)] and chemotherapy in a nonrandomized case-only observational study showed excellent median overall survival times of 40.4 months for those with Stage II NSCLC and 30.4 months for those with Stage III disease, with acceptable toxicity.29 A single-arm Phase II prospective study from the same institution in which concurrent chemotherapy was given with proton therapy to 74 Gy showed a median overall survival time of 26.5 months and a 5 year overall survival rate of 29%.30 A review of the National Cancer Data Base showed that of more than 243,800 patients who received radiation therapy for NSCLC, only 348 were treated with protons. Nevertheless, despite the imbalance in patient numbers between treatment groups, propensity-matched analysis showed that receipt of proton therapy was associated with better survival.31
The first randomized trial to directly compare IMRT with PSPT for NSCLC (NCT00915005) was reported by Liao and colleagues in 2018.32 The hypothesis for that trial was that PSPT exposes less lung tissue to radiation than IMRT, thereby reducing toxicity without compromising tumor control. Two primary end-points were evaluated, radiation pneumonitis and local failure. Eligible patients had Stage IIB-IV NSCLC (Stage IV eligible with single brain metastasis; or recurrent lung or mediastinal disease eligible after surgery) who were candidates for concurrent chemoradiation therapy. Pairs of treatment plans (for IMRT and PSPT) were created for each patient, and patients were eligible for randomization only if both plans satisfied the same prespecified dose–volume constraints for OARs at the same tumor dose. The results of this trial showed no association of PSPT with either end-point or with better dose–volume indices for the lung or esophagus, but PSPT was associated with better dose–volume indices for the heart. Compared with IMRT (n = 92), PSPT (n = 57) exposed less lung to doses of 5–10 Gy(RBE); more lung to ≥20 Gy (RBE); and less heart at all dose levels [5–80 Gy (RBE)]. The 12 month pneumonitis rate for all patients was 8.1% (6.5% for IMRT and 10.5% for PSPT), and the corresponding 12 month local failure rate was 10.7% (10.9% for IMRT and 10.5% for PSPT).32 In a secondary analysis of that trial, the incidence of grade ≥3 pneumonitis was significantly lower in both the IMRT and PSPT arms among patients who were enrolled after the trial mid-point (September 27, 2011) relative to those enrolled before that time. More importantly, the reduction in the incidence of pneumonitis was more pronounced in the PSPT arm (31.0% early vs 13.1% late, p = 0.027) than in the IMRT arm (21.1% early vs 18.2% late, p = 0.047), underscoring the possibility that a “learning curve” over the course of the trial may have affected this primary end-point.
In another secondary analysis of that trial,33 no differences in patterns of locoregional failure were noted between groups; indeed, marginal failure rates after PSPT were no different from those after IMRT despite the dose fall-off being much sharper with PSPT. The only independent predictor of marginal failure was having a T3 or T4 tumor. Another subanalysis of adaptive planning in the same study showed that 18% of patients required adaptive plans during treatment (12% for IMRT and 29% for PSPT), and that having a large tumor and receiving PSPT independently predicted the need for adaptive planning. Although the 5-year overall survival rate was poorer for those with large tumors (vs those with small tumors or large tumors without adaptive planning), the 5-year overall survival rate for patients with large tumors who received adaptive planning was similar to that for patients with small tumors. Finally, no differences were noted in local failure, marginal failure, or regional failure patterns with versus without adaptive planning, but having a response to chemoradiation (and therefore requiring adaptive planning) was associated with favorable survival.33
Yet in another secondary analysis of the same trial, receipt of PSPT was associated with higher uptake of fludeoxyglucose (FDG) by normal lung tissue on positron emission tomography (PET)compared with receipt of IMRT,34 which is consistent with the lack of reduction in pneumonitis in the PSPT group. Although mean lung dose was the only predictor of lung injury after IMRT, the volume receiving high-dose radiation was the only predictor of lung injury after PSPT.35 A voxel-based analysis of local dose differences in patients receiving IMRT vs PSPT in the same trial showed significant dose differences in the lower lungs and heart between patients with and without pneumonitis; notably, the anatomic regions significantly spared by PSPT did not match the clusters in which doses were correlated with pneumonitis.36 Another analysis of these trial data37 showed that the root-mean-squared dose (RMSD) to the lung was a better predictor of pneumonitis than was mean lung dose, and that the RMSD model predicted risk of pneumonitis equally well for IMRT and PSPT (in addition to 3DCRT). An important consequence of these findings is that delivery of higher doses to smaller volumes (vs lower doses to larger lung volumes) may increase the risk of pneumonitis, and thus RMSD seems to be particularly important for guiding the design of treatment plans, including those with protons, in which a higher-than-average volume of lung is exposed to the largest doses. Because RMSD has been found to be a superior predictor of pneumonitis in this and other studies,38 serious consideration should be given to clinical adoption of planning constraints based on RMSD to supplement the traditional constraints on mean lung dose and lung V20.37 Collectively, the knowledge gained from these secondary analyses emphasizes the importance of volume and spatial location of high-dose proton irradiation on the risk of pneumonitis and the relationship between pneumonitis and thoracic regional radiosensitivity, and underscores the importance of minimizing high-dose volumes through the use of highly conformal therapy. Specifically, the ability of IMRT to skirt normal structures, and the need for enlargement of the volume to be irradiated with protons to accommodate their inherent sensitivity to inter- and intra fractional uncertainties, may explain much of the lack of difference in primary end points in this trial. The trade-off between the superior conformity possible with protons and the need to enlarge the margins for PSPT could be resolved by improving proton therapy technology, e.g. by using IMPT rather than PSPT.
As alluded to earlier, another important contributor to overall survival after chemoradiation for NSCLC is the dose to the heart. Indeed, findings from RTOG 0617 identified heart V5 and V35 as being linked with overall survival. Xu and colleagues39 studied troponin levels as a marker of cardiac injury after chemoradiation; they found that cardiac troponin levels increased during thoracic radiation when the mean heart dose was 20 Gy or higher, but did not change if the mean heart dose was ≤2 Gy. They further found that an increase in troponin levels by a factor of 2 or more from the beginning of radiation was significantly associated with poor overall survival.39 All dosimetric studies to date comparing protons and photons have shown that proton therapy significantly reduces the volume of heart exposed at all dose levels.3,32,40 Findings from the Phase II randomized comparison trial also showed that PSPT led to significantly lower heart doses and reduced dose baths relative to IMRT.41 This reduction of unwanted irradiation of the heart may eventually translate to a survival benefit. This question is currently being addressed in another Phase III trial, RTOG 1308 (NCT01993810), which includes overall survival as an end-point after proton vs photon therapy.
Outcomes after intensity-modulated proton therapy
Pencil beam scanning proton therapy, the technology at the core of IMPT, represents an advancement over PSPT and at present is the most technologically advanced form of proton radiation therapy. With IMPT, conformal dose distributions are achieved by magnetic scanning of proton particles of different energies to cover the treatment volume layer by layer.42,43 However, as alluded to earlier, concerns have been expressed regarding the use of IMPT for treating lung cancer because of the uncertainties in proton RBE at the distal edge of the beam, the heterogeneity of tissues in the beam trajectory, and interplay effects between the motion of the scanning beam and lung aeration and diaphragmatic movement.44 Recent advances in robust optimization for treatment planning and in optimizing the spot delivery sequence allow IMPT to offer tighter margins and improved conformality of dose distributions relative to both PSPT and IMRT.10,45 Treatment of locally advanced NSCLC with IMPT is becoming more widely adopted as the numbers of proton therapy centers equipped with pencil beam scanning continue to grow worldwide.46
Promising outcomes have been reported from using IMPT with concurrent chemotherapy for locally advanced inoperable NSCLC.47 The median proton dose in that study was 67.3 CGE (range 59.4–78 CGE). IMPT was well tolerated, with no Grade 4–5 toxicity and an overall Grade 3 toxicity rate of 18%. The local control rate was 78.3% at 3 years, and the median overall survival time was 33.9 months. Interestingly, disease stage was not associated with overall survival, but cardiac dose (heart V40) was associated with poorer prognosis. These results compare favorably with results after PSPT in the randomized Phase II comparative trial, with corresponding rates of 3 year locoregional control of 55.8% after PSPT and 64.5% after IMPT. These results are even more encouraging given the difference in median gross tumor volumes between treatment comparison groups (70 cm3 PSPT vs 95.3 cm3 IMPT), suggesting that using IMPT for large, anatomically complex tumors seems to produce comparable if not better disease control compared with PSPT.32,47 The “dose painting” capability associated with intensity-modulated forms of radiation therapy has led clinicians to explore using dose escalation with simultaneously integrated boost (SIB) doses, for either IMRT or IMPT. In the Phase I portion of a Phase I/II trial,48 radiation doses were selectively escalated only to the SIB volume (the internal gross tumor volume + a 5 mm margin, to a total dose of 66–72 CGE given at 2.2–2.4 CGE/fraction), with the dose to the planning target volume (the internal gross tumor volume + an 8 mm margin for clinical target volume + 5 mm) kept at 60 CGE over 30 fractions. In an early report of 15 patients (6 given IMRT and 9 given IMPT) in that trial,48 the highest doses to the SIB were 72 Gy in the IMRT group and 78 CGE in the IMPT group. 9 patients (6 IMRT, 3 IMPT) received an SIB dose of 72 CGE, which translates to a biologically effective dose of 89.3 CGE, and 6 patients (all given IMPT) received an SIB dose of 78 CGE, for a biologically effective dose of 98.3 CGE. In terms of dose-limiting (grade ≥3) toxicity, 1 of the 9 patients given an SIB of 72 CGE developed esophagitis, and 2 of 6 patients given 78 CGE developed pneumonitis, one (Grade 3) at 3 months after treatment and the other (Grade 5, possibly related to treatment) at 2 months after treatment. At a median follow-up time of 25 months (range, 4.3–47.4 months), only one patient had developed a marginal recurrence.48 The SIB dose of 72 CGE was determined to be safe for use in the randomized Phase II portion of this trial, which is currently accruing patients (NCT01629498). Another important ongoing trial, RTOG 1308 (NCT01993810), is, as noted previously, a Phase III randomized trial comparing photons and protons for locally advanced NSCLC. Both PSPT and IMPT are allowed in this trial. Challenges in insurance coverage for proton therapy in general and for clinical trials in particular are leading to imbalances in patient numbers in each arm; indeed, one randomized clinical trial to compare stereotactic ablative (photon) radiation therapy with stereotactic ablative proton therapy had to be closed before the accrual goal was reached because of the high rate of insurance denial for trial coverage and the lack of volumetric image guidance (NCT01525446).
Future considerations
In the future, effective use of IMPT to improve outcomes for patients with locally advanced NSCLC depends on real-time volumetric image guidance, effective management of tumor and normal organ motion, and accurate modeling of particle–matter interactions and set-up uncertainties. Relative to photon therapy, interactions between proton particles and matter are more challenging to model, and they depend more strongly on heterogeneities in tissue density and motion. Uncertainties in treatment planning are more pronounced for IMPT than for PSPT, as the individual fields can generate significant dose gradients within the treatment volume. Many proton therapy treatment systems available today use simple analytical algorithms to calculate dose rather than Monte Carlo algorithms. However, these analytical algorithms can miscalculate dose to the target by as much as 31%, as compared with 12% with Monte Carlo algorithms.49 More and more centers are adapting Monte Carlo algorithms as a routine dose calculation algorithm.
The effective use of IMPT for locally advanced NSCLC also depends on a better understanding of the radiobiology of protons. Interested readers should refer to a comprehensive review on the topic of variable RBE by Willers and colleagues.50 Until recently, a generic RBE value of 1.1 has been used for preclinical research and clinical practice in proton therapy. However, the capacity for proton beams to cause biological damage was recently found to be substantially higher near the distal, high-LET region51–54 for tumors as well as normal tissues. This finding is supported by observations from laboratory studies demonstrating differential DNA damage along the beam path, with increased cell kill in the distal regions of the proton beams.14,52,53 Preclinical in vitro data also show that defects in certain DNA repair pathways in many types of cancer are associated with increased RBE.55–57 Even though clinical data on variable RBE are sparse at this time, early evidence is emerging. For example, Peeler and colleagues noted higher rates of change in MRI characteristics after proton therapy than after photon therapy in patients with ependymoma.58 Recalculations of the proton dose and LET distributions in these patients with Monte Carlo algorithms showed significant correlations between LET, dose, and regions of imaging change in these patients. These image changes may well indicate early radiation injury, and as such they could be used as a biomarker of different types of damage after different types of radiation. Underwood and colleagues also reported that changes in parenchymal lung density on CT images implied that the proton RBE for lung density is greater than 1.1.59 As noted earlier, higher uptake of fludeoxyglucose in the normal lung after radiation for lung cancer was noted for patients treated with protons than for those treated with photons in the randomized lung trial,25,34 and Monte Carlo calculation of these proton plans and assessment of correlations between the LET, dose, and regions of image changes are ongoing. On the other hand, no apparent increase was observed in brainstem necrosis in pediatric patients treated with proton therapy.60 In clinical practice with heavy ion therapy, biological effects models are routinely considered in the treatment planning process to limit high-LET deposition in critical normal tissues, and variable RBE is incorporated into every dosimetry model. The need to include variable RBE into clinical dosimetry for protons is increasingly being recognized, and laboratory, translational, and clinical research efforts to address this need are currently ongoing.
The effective use of IMPT for locally advanced NSCLC also depends on a better understanding of the immunomodulatory effect of protons. Cancer immunotherapy with immune checkpoint modulators such as anti-PD1 or anti-PDL1 agents is considered to be a breakthrough in the treatment of lung cancer. Radiotherapy can enhance the effect of immunotherapy by transforming tumors into an “in situ vaccine,” which immunotherapy then amplifies into a stronger systemic immune response via blood and lymphatic transport, overcoming the immunosuppressive characteristics of the tumor microenvironment.61 Both proton and photon irradiation of cultured cancer cell lines was shown to induce comparable upregulation of surface molecules involved in immune recognition (histocompatibility leukocyte antigen, intercellular adhesion molecule 1, and the tumor-associated antigens carcinoembryonic antigen and mucin 1). Proton radiation mediated the cell-surface expression of calreticulin, which increased tumor-cell sensitivity to killing by cytotoxic T lymphocytes. Protons also were found to upregulate calreticulin in cancer stem cells in a manner similar to that in non-cancer stem cells. These findings may shed light on the rationale for using proton therapy in combination with immunotherapy as first-line treatment, or as next-line therapy for patients in whom radiation therapy alone has failed, or for patients with limited treatment options.62 On the other hand, proton irradiation was found to cause more complex, incompletely repaired DNA damage, resulting in both acute and persistent increases in oxidative stress in the lungs in a lung cancer−susceptible mouse model (K-rasLA1),63 and mice treated with protons had increases in number and size of initiated and premalignant lesions and adenomas that were often infiltrated with inflammatory cells.63,64 Mice treated with protons also had shorter median survival times and increased rates of carcinoma relative to unirradiated controls and photon-treated mice. These findings suggest that exposure to proton irradiation enhances the progression of premalignant lesions to invasive carcinomas through persistent DNA damage, chronic oxidative stress, and immunosuppression.63,64 They further suggest that additional research to decipher the effect of protons on immunomodulation as well as their long-term effects will be critical for optimizing proton therapy.
Another line of investigation for both photon and proton therapy involves their effects on lymphocytes, especially CD8+ T cells, which are critical for the antitumor effects of radiation therapy given with immunotherapy.65 Lymphocytes are highly radiosensitive; their numbers start to decline as soon as the first fraction of radiation treatment, continue to decline until the end of the treatment, and begin to recover shortly after radiation is completed. The nadir lymphocyte count has been found to correlate with tumor volume and with the low dose bath (using lung V5 as a surrogate). Most interestingly, lymphocyte nadir is also highly correlated with progression-free survival and overall survival.66 This latter finding has been confirmed in several other types of cancer, including esophageal cancer, liver cancer, and small cell lung cancer, suggesting that lymphopenia may be a common factor that affects overall survival across different types of cancer.67–73 Proton therapy can significantly reduce the low-dose bath at all disease sites and therefore may be an effective way to avoid radiation-induced lymphopenia. Active research is ongoing to clarify the potential role of proton therapy in this regard.
Summary
Proton therapy has unique dosimetric characteristics in terms of Bragg peak, with no exit dose beyond the target. Clinically, proton therapy has shown great potential in treating a variety of types of cancer, and recent preclinical and clinical studies are beginning to shed light on the clinical implications of variability in RBE. Preclinical studies on the mechanisms by which protons modulate the immune system have provided a rationale for combining proton therapy with immunotherapy, although considerably more research is needed to understand both the short-term and the long-term effects of proton therapy. With the expansion in number of proton therapy centers worldwide comes a great need, and great opportunities, for basic scientists, clinical oncologists, and medical physicists to work together to translate the unique physical characteristics of proton therapy into effective clinical use, to the ultimate benefit of cancer patients.
Footnotes
Acknowledgment: The authors gratefully acknowledge the expertise of Christine F. Wogan, MS, ELS, of MD Anderson’s Division of Radiation Oncology in the development of this review.
Contributor Information
Olsi Gjyshi, Email: OGjyshi@mdanderson.org.
Zhongxing Liao, Email: zliao@mdanderson.org.
REFERENCES
- 1.Doyen J, Falk AT, Floquet V, Hérault J, Hannoun-Lévi J-M. Proton beams in cancer treatments: clinical outcomes and dosimetric comparisons with photon therapy. Cancer Treat Rev 2016; 43: 104–12. doi: 10.1016/j.ctrv.2015.12.007 [DOI] [PubMed] [Google Scholar]
- 2.Shiraishi Y, Xu C, Yang J, Komaki R, Lin SH. Dosimetric comparison to the heart and cardiac substructure in a large cohort of esophageal cancer patients treated with proton beam therapy or intensity-modulated radiation therapy. Radiother Oncol 2017; 125: 48–54. doi: 10.1016/j.radonc.2017.07.034 [DOI] [PubMed] [Google Scholar]
- 3.Chang JY, Zhang X, Wang X, Kang Y, Riley B, Bilton S, et al. Significant reduction of normal tissue dose by proton radiotherapy compared with three-dimensional conformal or intensity-modulated radiation therapy in stage I or stage III non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2006; 65: 1087–96. doi: 10.1016/j.ijrobp.2006.01.052 [DOI] [PubMed] [Google Scholar]
- 4.Fang P, Shiraishi Y, Verma V, Jiang W, Song J, Hobbs BP, et al. Lymphocyte-sparing effect of proton therapy in patients with esophageal cancer treated with definitive chemoradiation. International Journal of Particle Therapy 2017; 4: 23–32. doi: 10.14338/IJPT-17-00033.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Routman DM, Garant A, Lester SC, Day CN, Harmsen WS, Sanheuza CT, et al. A comparison of grade 4 lymphopenia with proton versus photon radiation therapy for esophageal cancer. Adv Radiat Oncol 2019; 4: 63–9. doi: 10.1016/j.adro.2018.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ning MS, Gomez DR, Shah AK, Kim CR, Palmer MB, Thaker NG, et al. The insurance approval process for proton radiation therapy: a significant barrier to patient care. Int J Radiat Oncol Biol Phys 2019; 104: 724–33. doi: 10.1016/j.ijrobp.2018.12.019 [DOI] [PubMed] [Google Scholar]
- 7.Bortfeld TR, Loeffler JS. Three ways to make proton therapy affordable. Nature 2017; 549: 451–3. doi: 10.1038/549451a [DOI] [PubMed] [Google Scholar]
- 8.Shah A, Ricci KI, Efstathiou JA. Beyond a moonshot: insurance coverage for proton therapy. Lancet Oncol 2016; 17: 559–61. doi: 10.1016/S1470-2045(16)00171-6 [DOI] [PubMed] [Google Scholar]
- 9.Johnstone PAS, Kerstiens J. Reconciling reimbursement for proton therapy. Int J Radiat Oncol Biol Phys 2016; 95: 9–10. doi: 10.1016/j.ijrobp.2015.09.037 [DOI] [PubMed] [Google Scholar]
- 10.Mohan R, Grosshans D. Proton therapy - Present and future. Adv Drug Deliv Rev 2017; 109: 26–44. doi: 10.1016/j.addr.2016.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paganetti H, Niemierko A, Ancukiewicz M, Gerweck LE, Goitein M, Loeffler JS, et al. Relative biological effectiveness (RBE) values for proton beam therapy. Int J Radiat Oncol Biol Phys 2002; 53: 407–21. doi: 10.1016/S0360-3016(02)02754-2 [DOI] [PubMed] [Google Scholar]
- 12.Gerweck LE, Kozin SV. Relative biological effectiveness of proton beams in clinical therapy. Radiother Oncol 1999; 50: 135–42. doi: 10.1016/S0167-8140(98)00092-9 [DOI] [PubMed] [Google Scholar]
- 13.Matney J, Park PC, Bluett J, Chen YP, Liu W, Court LE, et al. Effects of respiratory motion on passively scattered proton therapy versus intensity modulated photon therapy for stage III lung cancer: are proton plans more sensitive to breathing motion? Int J Radiat Oncol Biol Phys 2013; 87: 576–82. doi: 10.1016/j.ijrobp.2013.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guan F, Bronk L, Titt U, Lin SH, Mirkovic D, Kerr MD, et al. Spatial mapping of the biologic effectiveness of scanned particle beams: towards biologically optimized particle therapy. Sci Rep 2015; 5: 9850. doi: 10.1038/srep09850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moyers MF. Proton therapy : Van Dyk J, The Modern Technology of Radiation Oncology. A Compendium for Medical Physicists and Radiation Oncologists. Madison, WI: Medical Physics Publishing; 1999. 823–70. [Google Scholar]
- 16.Liu W, Zhang X, Li Y, Mohan R. Robust optimization of intensity modulated proton therapy. Med Phys 2012; 39: 1079–91. doi: 10.1118/1.3679340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu W, Liao Z, Schild SE, Liu Z, Li H, Li Y, et al. Impact of respiratory motion on worst-case scenario optimized intensity modulated proton therapy for lung cancers. Pract Radiat Oncol 2015; 5: e77–86. doi: 10.1016/j.prro.2014.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Overall survival with Durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med 2018; 379: 2342–50. doi: 10.1056/NEJMoa1809697 [DOI] [PubMed] [Google Scholar]
- 19.Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med 2017; 377: 1919–29. doi: 10.1056/NEJMoa1709937 [DOI] [PubMed] [Google Scholar]
- 20. National Comprehensive Cancer Network (NCCN) Guidelines Non-Small Cell Lung Cancer, Version 5; 2019. [Google Scholar]
- 21.Curran WJ, Paulus R, Langer CJ, Komaki R, Lee JS, Hauser S, et al. Sequential vs. concurrent chemoradiation for stage III non-small cell lung cancer: randomized phase III trial RTOG 9410. J Natl Cancer Inst 2011; 103: 1452–6010.1093/jnci/djr325.. doi: 10.1093/jnci/djr325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leighl NB, Hellmann MD, Hui R, Carcereny E, Felip E, Ahn M-J, et al. Pembrolizumab in patients with advanced non-small-cell lung cancer (KEYNOTE-001): 3-year results from an open-label, phase 1 study. Lancet Respir Med 2019; 7: 347–57. doi: 10.1016/S2213-2600(18)30500-9 [DOI] [PubMed] [Google Scholar]
- 23.Bradley JD, Paulus R, Komaki R, Masters G, Blumenschein G, Schild S, et al. Standard-Dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol 2015; 16: 187–99. doi: 10.1016/S1470-2045(14)71207-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chun SG, Hu C, Choy H, Komaki RU, Timmerman RD, Schild SE, et al. Impact of intensity-modulated radiation therapy technique for locally advanced Non–Small-Cell lung cancer: a secondary analysis of the NRG oncology RTOG 0617 randomized clinical trial. JCO 2017; 35: 56–62. doi: 10.1200/JCO.2016.69.1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liao ZX, Komaki RR, Thames HD, Liu HH, Tucker SL, Mohan R, et al. Influence of technologic advances on outcomes in patients with unresectable, locally advanced non-small-cell lung cancer receiving concomitant chemoradiotherapy. Int J Radiat Oncol Biol Phys 2010; 76: 775–81. doi: 10.1016/j.ijrobp.2009.02.032 [DOI] [PubMed] [Google Scholar]
- 26.Yom SS, Liao Z, Liu HH, Tucker SL, Hu C-S, Wei X, et al. Initial evaluation of treatment-related pneumonitis in advanced-stage non-small-cell lung cancer patients treated with concurrent chemotherapy and intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys 2007; 68: 94–102. doi: 10.1016/j.ijrobp.2006.12.031 [DOI] [PubMed] [Google Scholar]
- 27.Sejpal S, Komaki R, Tsao A, Chang JY, Liao Z, Wei X, et al. Early findings on toxicity of proton beam therapy with concurrent chemotherapy for nonsmall cell lung cancer. Cancer 2011; 117: 3004–13. doi: 10.1002/cncr.25848 [DOI] [PubMed] [Google Scholar]
- 28.Wang XS, Shi Q, Williams LA, Komaki R, Gomez DR, Lin SH, et al. Prospective study of patient-reported symptom burden in patients with non-small-cell lung cancer undergoing proton or photon chemoradiation therapy. J Pain Symptom Manage 2016; 51: 832–8. doi: 10.1016/j.jpainsymman.2015.12.316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nguyen Q-N, Ly NB, Komaki R, Levy LB, Gomez DR, Chang JY, et al. Long-Term outcomes after proton therapy, with concurrent chemotherapy, for stage II-III inoperable non-small cell lung cancer. Radiother Oncol 2015; 115: 367–72. doi: 10.1016/j.radonc.2015.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang JY, Verma V, Li M, Zhang W, Komaki R, Lu C, et al. Proton beam radiotherapy and concurrent chemotherapy for unresectable stage III non-small cell lung cancer: final results of a phase 2 study. JAMA Oncol 2017; 3: e172032: e172032. doi: 10.1001/jamaoncol.2017.2032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Higgins KA, O'Connell K, Liu Y, Gillespie TW, McDonald MW, Pillai RN, et al. Simone CB 2nd, Owonikoko tk, Belani CP, Khuri Fr, Curran WJ, Ramalingam SS, Behera M. National cancer database analysis of proton versus photon radiation therapy in non-small cell lung cancer. Int J Radiat Oncol Biol Phys 2017; 97: 128–37. [DOI] [PubMed] [Google Scholar]
- 32.Liao Z, Lee JJ, Komaki R, Gomez DR, O’Reilly MS, Fossella FV, et al. Bayesian adaptive randomization trial of passive scattering proton therapy and intensity-modulated photon radiotherapy for locally advanced Non–Small-Cell lung cancer. JCO 2018; 36: 1813–22. doi: 10.1200/JCO.2017.74.0720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang P, Xu T, Gomez DR, Deng W, Wei X, Elhalawani H, et al. Patterns of local-regional failure after intensity modulated radiation therapy or passive scattering proton therapy with concurrent chemotherapy for non-small cell lung cancer. Int J Radiat Oncol Biol Phys 2019; 103: 123–31. doi: 10.1016/j.ijrobp.2018.08.031 [DOI] [PubMed] [Google Scholar]
- 34.Yue J, McKeever M, Sio TT, Xu T, Huo J, Shi Q, et al. Association of lung fluorodeoxyglucose uptake with radiation pneumonitis after concurrent chemoradiation for non-small cell lung cancer. Clin Transl Radiat Oncol 2017; 4: 1–7. doi: 10.1016/j.ctro.2017.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shusharina N, Liao Z, Mohan R, Liu A, Niemierko A, Choi N, et al. Differences in lung injury after IMRT or proton therapy assessed by 18 FDG PET imaging. Radiother Oncol 2018; 128: 147–53. doi: 10.1016/j.radonc.2017.12.027 [DOI] [PubMed] [Google Scholar]
- 36.Palma G, Monti S, Xu T, Scifoni E, Yang P, Hahn SM, et al. Spatial dose patterns associated with radiation pneumonitis in a randomized trial comparing intensity-modulated photon therapy with passive scattering proton therapy for locally advanced non-small cell lung cancer. Int J Radiat Oncol Biol Phys 2019; 104: 1124–32. doi: 10.1016/j.ijrobp.2019.02.039 [DOI] [PubMed] [Google Scholar]
- 37.Tucker SL, Xu T, Paganetti H, Deist T, Verma V, Choi N, et al. Validation of effective dose as a better predictor of radiation pneumonitis risk than mean lung dose: secondary analysis of a randomized trial. Int J Radiat Oncol Biol Phys 2019; 103: 403–10. doi: 10.1016/j.ijrobp.2018.09.029 [DOI] [PubMed] [Google Scholar]
- 38.Tucker SL, Mohan R, Liengsawangwong R, Martel MK, Liao Z. Predicting pneumonitis risk: a dosimetric alternative to mean lung dose. Int J Radiat Oncol Biol Phys 2013; 85: 522–7. doi: 10.1016/j.ijrobp.2012.03.052 [DOI] [PubMed] [Google Scholar]
- 39.Xu T, Meng QH, Gomez DR, Levy LB, Komaki RU, Mohan R, et al. Serum troponin T levels are associated with radiation dose to heart during definitive chemoradiation therapy for non-small cell lung cancer. Int J Radiat Oncol Biol Phys 2015; 93(3S): E411–E412. doi: 10.1016/j.ijrobp.2015.07.1596 [DOI] [Google Scholar]
- 40.Ferris MJ, Martin KS, Switchenko JM, Kayode OA, Wolf J, Dang Q, et al. Sparing cardiac substructures with optimized volumetric modulated Arc therapy and intensity modulated proton therapy in thoracic radiation for locally advanced non-small cell lung cancer. Pract Radiat Oncol 2019; 8500: S1879–30125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Deist TM, Yang P, Oberije C, Allen PK, van Wijk Y, Gomez DR, et al. Dosimetric analysis of randomized proton and photon plans with respect to radiation toxicity: the importance of high dose regions in lung and esophagus. Int J Radiat Oncol Biol Phys 2017; 98: 246. doi: 10.1016/j.ijrobp.2017.01.187 [DOI] [Google Scholar]
- 42.St James S, Grassberger C, Lu H-M. Considerations when treating lung cancer with passive scatter or active scanning proton therapy. Transl Lung Cancer Res 2018; 7: 210–5. doi: 10.21037/tlcr.2018.04.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chang JY, Li H, Zhu XR, Liao Z, Zhao L, Liu A, et al. Clinical implementation of intensity modulated proton therapy for thoracic malignancies. Int J Radiat Oncol Biol Phys 2014; 90: 809–18. doi: 10.1016/j.ijrobp.2014.07.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liao Z, Simone CB. Particle therapy in non-small cell lung cancer. Transl Lung Cancer Res 2018; 7: 141–52. doi: 10.21037/tlcr.2018.04.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li H, Zhu XR, Zhang X. Reducing dose uncertainty for spot-scanning proton beam therapy of moving tumors by optimizing the spot delivery sequence. Int J Radiat Oncol Biol Phys 2015; 93: 547–56. doi: 10.1016/j.ijrobp.2015.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Waddle MR, Sio TT, Van Houten HK, Foote RL, Keole SR, Schild SE, et al. Photon and proton radiation therapy utilization in a population of more than 100 million commercially insured patients. Int J Radiat Oncol Biol Phys 2017; 99: 1078–82. doi: 10.1016/j.ijrobp.2017.07.042 [DOI] [PubMed] [Google Scholar]
- 47.Elhammali A, Blanchard P, Yoder A, Liao Z, Zhang X, Ronald Zhu X, et al. Clinical outcomes after intensity-modulated proton therapy with concurrent chemotherapy for inoperable non-small cell lung cancer. Radiother Oncol 2019; 136: 136–42. doi: 10.1016/j.radonc.2019.03.029 [DOI] [PubMed] [Google Scholar]
- 48.Jeter MD, Gomez D, Nguyen Q-N, Komaki R, Zhang X, Zhu X, et al. Simultaneous integrated boost for radiation dose escalation to the gross tumor volume with intensity modulated (photon) radiation therapy or intensity modulated proton therapy and concurrent chemotherapy for stage II to III non-small cell lung cancer: a phase 1 study. Int J Radiat Oncol Biol Phys 2018; 100: 730–7. doi: 10.1016/j.ijrobp.2017.10.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Taylor PA, Kry SF, Followill DS. Pencil Beam Algorithms Are Unsuitable for Proton Dose Calculations in Lung. Int J Radiat Oncol Biol Phys 2017; 99: 750–6. doi: 10.1016/j.ijrobp.2017.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Willers H, Allen A, Grosshans D, McMahon SJ, von Neubeck C, Wiese C, et al. Toward a variable RBE for proton beam therapy. Radiother Oncol 2018; 128: 68–75. doi: 10.1016/j.radonc.2018.05.019 [DOI] [PubMed] [Google Scholar]
- 51.Sørensen BS, Bassler N, Nielsen S, Horsman MR, Grzanka L, Spejlborg H, et al. Relative biological effectiveness (RBE) and distal edge effects of proton radiation on early damage in vivo. Acta Oncol 2017; 56: 1387–91. doi: 10.1080/0284186X.2017.1351621 [DOI] [PubMed] [Google Scholar]
- 52.Cuaron JJ, Chang C, Lovelock M, Higginson DS, Mah D, Cahlon O, et al. Exponential increase in relative biological effectiveness along distal edge of a proton Bragg peak as measured by deoxyribonucleic acid double-strand breaks. Int J Radiat Oncol Biol Phys 2016; 95: 62–9. doi: 10.1016/j.ijrobp.2016.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chaudhary P, Marshall TI, Currell FJ, Kacperek A, Schettino G, Prise KM. Variations in the processing of DNA double-strand breaks along 60-MeV therapeutic proton beams. Int J Radiat Oncol Biol Phys 2016; 95: 86–94. doi: 10.1016/j.ijrobp.2015.07.2279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Saager M, Peschke P, Brons S, Debus J, Karger CP. Determination of the proton RBE in the rat spinal cord: is there an increase towards the end of the spread-out Bragg peak? Radiother Oncol 2018; 128: 115–20. doi: 10.1016/j.radonc.2018.03.002 [DOI] [PubMed] [Google Scholar]
- 55.Zafar F, Seidler SB, Kronenberg A, Schild D, Wiese C. Homologous recombination contributes to the repair of DNA double-strand breaks induced by high-energy iron ions. Radiat Res 2010; 173: 27–39. doi: 10.1667/RR1910.1 [DOI] [PubMed] [Google Scholar]
- 56.Gerelchuluun A, Manabe E, Ishikawa T, Sun L, Itoh K, Sakae T, et al. The major DNA repair pathway after both proton and carbon-ion radiation is NHEJ, but the HR pathway is more relevant in carbon ions. Radiat Res 2015; 183: 345–56. doi: 10.1667/RR13904.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Takahashi A, Kubo M, Ma H, Nakagawa A, Yoshida Y, Isono M, et al. Nonhomologous end-joining repair plays a more important role than homologous recombination repair in defining radiosensitivity after exposure to high-LET radiation. Radiat Res 2014; 182: 338–44. doi: 10.1667/RR13782.1 [DOI] [PubMed] [Google Scholar]
- 58.Peeler CR, Mirkovic D, Titt U, Blanchard P, Gunther JR, Mahajan A, et al. Clinical evidence of variable proton biological effectiveness in pediatric patients treated for ependymoma. Radiotherapy and Oncology 2016; 121: 395–401. doi: 10.1016/j.radonc.2016.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Underwood TSA, Grassberger C, Bass R, MacDonald SM, Meyersohn NM, Yeap BY, et al. Asymptomatic late-phase radiographic changes among chest-wall patients are associated with a proton RBE exceeding 1.1. Int J Radiat Oncol Biol Phys 2018; 101: 809–19. doi: 10.1016/j.ijrobp.2018.03.037 [DOI] [PubMed] [Google Scholar]
- 60.Gentile MS, Yeap BY, Paganetti H, Goebel CP, Gaudet DE, Gallotto SL, et al. Brainstem injury in pediatric patients with posterior fossa tumors treated with proton beam therapy and associated dosimetric factors. Int J Radiat Oncol Biol Phys 2018; 100: 719–29. doi: 10.1016/j.ijrobp.2017.11.026 [DOI] [PubMed] [Google Scholar]
- 61.Formenti SC, Demaria S. Radiation therapy to convert the tumor into an in situ vaccine. Int J Radiat Oncol Biol Phys 2012; 84: 879–80. doi: 10.1016/j.ijrobp.2012.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gameiro SR, Malamas AS, Bernstein MB, Tsang KY, Vassantachart A, Sahoo N, et al. Tumor cells surviving exposure to proton or photon radiation share a common immunogenic modulation signature, rendering them more sensitive to T cell-mediated killing. Int J Radiat Oncol Biol Phys 2016; 95: 120–30. doi: 10.1016/j.ijrobp.2016.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Luitel K, Bozeman R, Kaisani A, Kim SB, Barron S, Richardson JA, et al. Proton radiation-induced cancer progression. Life Sci Space Res 2018; 19: 31–42. doi: 10.1016/j.lssr.2018.08.002 [DOI] [PubMed] [Google Scholar]
- 64.Kim SB, Bozeman RG, Kaisani A, Kim W, Zhang L, Richardson JA, et al. Radiation promotes colorectal cancer initiation and progression by inducing senescence-associated inflammatory responses. Oncogene 2016; 35: 3365–75. doi: 10.1038/onc.2015.395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Formenti SC, Demaria S. Combining radiotherapy and cancer immunotherapy: a paradigm shift. J Natl Cancer Inst 2013; 105: 256–65. doi: 10.1093/jnci/djs629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tang C, Liao Z, Gomez D, Levy L, Zhuang Y, Gebremichael RA, et al. Lymphopenia association with gross tumor volume and lung V5 and its effects on non-small cell lung cancer patient outcomes. Int J Radiat Oncol Biol Phys 2014; 89: 1084–91. doi: 10.1016/j.ijrobp.2014.04.025 [DOI] [PubMed] [Google Scholar]
- 67.Grossman SA, Ellsworth S, Campian J, Wild AT, Herman JM, Laheru D, et al. Survival in patients with severe lymphopenia following treatment with radiation and chemotherapy for newly diagnosed solid tumors. J Natl Compr Canc Netw 2015; 13: 1225–31. doi: 10.6004/jnccn.2015.0151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Byun HK, Kim N, Park S, Seong J. Acute severe lymphopenia by radiotherapy is associated with reduced overall survival in hepatocellular carcinoma. Strahlenther Onkol 2019; 18Epub ahead of print. doi: 10.1007/s00066-019-01462-5 [DOI] [PubMed] [Google Scholar]
- 69.Davuluri R, Jiang W, Fang P, Xu C, Komaki R, Gomez DR, et al. Lymphocyte nadir and esophageal cancer survival outcomes after chemoradiation therapy. Int J Radiat Oncol Biol Phys 2017; 99: 128–35. doi: 10.1016/j.ijrobp.2017.05.037 [DOI] [PubMed] [Google Scholar]
- 70.Wu ES, Oduyebo T, Cobb LP, Cholakian D, Kong X, Fader AN, et al. Lymphopenia and its association with survival in patients with locally advanced cervical cancer. Gynecol Oncol 2016; 140: 76–82. doi: 10.1016/j.ygyno.2015.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu L-T, Chen Q-Y, Tang L-Q, Guo S-S, Guo L, Mo H-Y, et al. The prognostic value of treatment-related lymphopenia in nasopharyngeal carcinoma patients. Cancer Res Treat 2018; 50: 19–29. doi: 10.4143/crt.2016.595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Campian JL, Sarai G, Ye X, Marur S, Grossman SA. Association between severe treatment-related lymphopenia and progression-free survival in patients with newly diagnosed squamous cell head and neck cancer. Head Neck 2014; 36: 1747–53. doi: 10.1002/hed.23535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wild AT, Ye X, Ellsworth SG, Smith JA, Narang AK, Garg T, et al. The association between chemoradiation-related lymphopenia and clinical outcomes in patients with locally advanced pancreatic adenocarcinoma. Am J Clin Oncol 2015; 38: 259–65. doi: 10.1097/COC.0b013e3182940ff9 [DOI] [PMC free article] [PubMed] [Google Scholar]