Abstract
Proton beam therapy is a highly conformal form of radiation therapy, which currently represents an important therapeutic component in multidisciplinary management in paediatric oncology. The precise adjustability of protons results in a reduction of radiation-related long-term side-effects and secondary malignancy induction, which is of particular importance for the quality of life. Proton irradiation has been shown to offer significant advantages over conventional photon-based radiotherapy, although the biological effectiveness of both irradiation modalities is comparable. This review evaluates current data from clinical and dosimetric studies on the treatment of tumours of the central nervous system, soft tissue and bone sarcomas of the head and neck region, paraspinal or pelvic region, and retinoblastoma. To date, the clinical results of irradiating childhood tumours with high-precision proton therapy are promising both with regard to tumour cure and the reduction of adverse events. Modern proton therapy techniques such as pencil beam scanning and intensity modulation are increasingly established modern facilities. However, further investigations with larger patient cohorts and longer follow-up periods are required, in order to be able to have clear evidence on clinical benefits.
Statement of search strategies used and sources of information
PubMed literature searches were carried out using the terms children, child, paediatric, infant, proton therapy, radiation therapy, supplemented with the appropriate terms of the different tumour types.
Introduction
More than 300,000 children worldwide are diagnosed with cancer every year.1 The most frequent paediatric cancers are leukaemias (31%), followed by brain tumours (about 24%) and lymphomas (about 11%). The survival rates have improved significantly up to 80% nowadays.2 Radiotherapy (RT) is recognized as an important therapeutic component in children with cancer and is frequently used in multimodal therapy strategies for solid tumours of the central nervous system (CNS), bone and soft tissue. In contrast, it is rarely used in leukaemia. In tumours such as lymphomas, nephroblastomas or neuroblastomas, RT is used according to risk grouping with regard to tumour stages, age, response to therapy and other parameters. For many patients, RT was deintensified over time establishing dose or volume reduction.3 In addition, the development of precise, highly conformal radiation techniques were able to further reduce dose to normal tissues and therefore the risk for therapy-related adverse events. Due to this evolution, RT is becoming better feasible in the treatment of paediatric cancer, even for very young patients, if needed. Proton beam therapy (PBT) is one of the most attractive tools to deliver local therapy with minimal dose distribution to the uninvolved tissue and with reduced integral dose. Although, numerous dosimetric comparisons demonstrate the superiority of irradiation with protons when compared to photons, the corresponding risk reduction by protons compared to photons has to be proved biologically and, most notably, clinically.4 Since the majority of published studies compare the outcome of PBT with historical photon two-dimensional and three-dimensional cohorts, comparisons with state-of-the art photon techniques, such as intensity modulated radiation therapy (IMRT), are performed predominatly in silico trials. Therefore, more clinical data and comparisons have to be collected in future in order to be able to draw reliable conclusions. This is a particular challenge, as randomized trials in children will not be carried out due to ethical.
PROTON BEAM THERAPY
The therapeutic potential of protons was first recognized in 1946 in a report by Wilson5 and as early as 1954, the first patient was irradiated with protons at the University of California.6 Due to the physical properties of protons, the beam ensures a maximal dose delivery to the tumour region while protecting the surrounding healthy tissue from low and medium dose. Protons are characterized by a steep dose fall-off distal to the target volume and medium dose load along the travel path. These characteristics result in a reduction of the dose burden and consequently a reduction of the risks for long-term side-effects as well as secondary malignancy induction. However, the biological effectiveness of protons is comparable to that of photons.7
PBT is considered as an innovative and conformal type of RT and has obtained increasing importance in oncology, especially for the treatment of childhood tumours as the immature, growing tissue of children makes them particularly vulnerable to radiation injury and the induction of secondary tumours8 (Figure 1). Due to its superior dose conformity and lower normal tissue dose, PBT offers the chance of decreasing acute and late radiation-related sequelae, thus allowing a better Quality of Life (QoL) for childhood cancer patients and survivors.
Figure 1. .
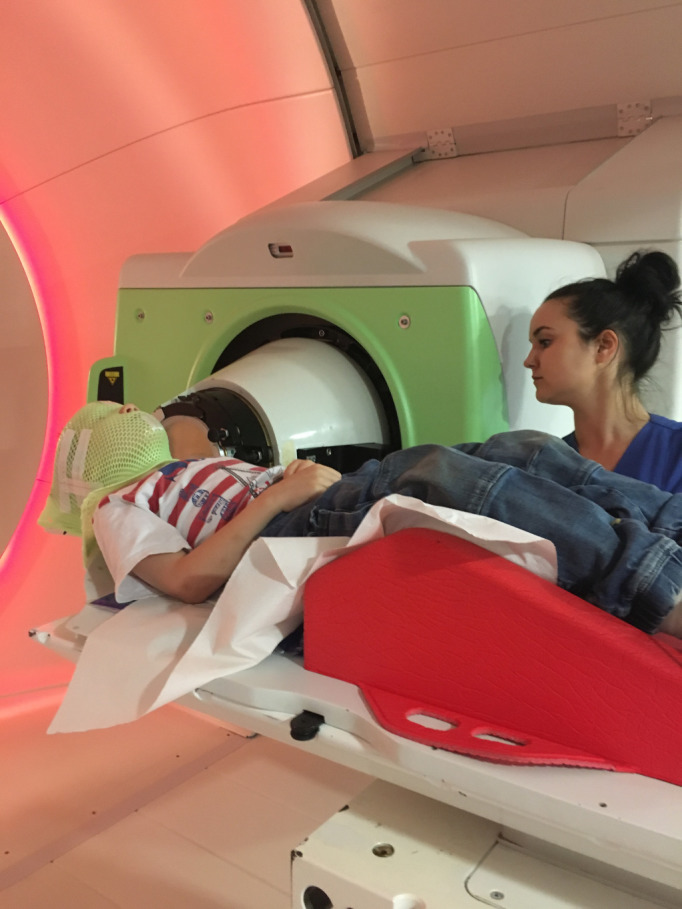
Positioning and immobilization of a child with an anaplastic ependymoma treated with pencil beam scanning proton therapy.
To date, more than 60 proton beam facilities are operational and even more are under construction or in planning status worldwide (http://ptcog.web.psi.ch/patient_statistics.html). Different PBT application modes are in use, such as passive scattering, uniform-scanning and pencil beam-scanning (PBS) techniques. For passively scattered protons, individually manufactured hardware devices (collimators and compensators) are needed to fit the beam to the target volume. PBS protons are essential for the application of intensity modulated PBT (IMPT), the most conformal proton modality particularly in larger and complex shaped volumes.
Paediatric PROTON BEAM THERAPY: CLINICAL EXPERIENCES
Due to the improved sparing of normal tissue, an increasing number of children are receiving PBT resulting in a growing body of literature and clinical experiences (Figure 2a). Paediatric patients with tumours of the CNS, such as ependymomas, medulloblastomas, craniopharyngiomas, germinomas and low-grade gliomas, comprise now a large proportion of patients receiving this treatment modality.9 Similarly, in non-CNS tumours, PBT is applied for a significant number of paediatric patients with soft tissue and bone sarcomas of head and neck (H&N) as well as paraspinal or pelvic regions, for retinoblastomas and neuroblastomas.10 Though clinical experience on PBT in paediatric oncology is now starting to emerge, clinical evidence is still scarce due to small cohorts and short follow-up times (Figure 2b). Potential advantages and previous experiences in PBT for the most common indications of childhood tumours are presented below.
Figure 2. .
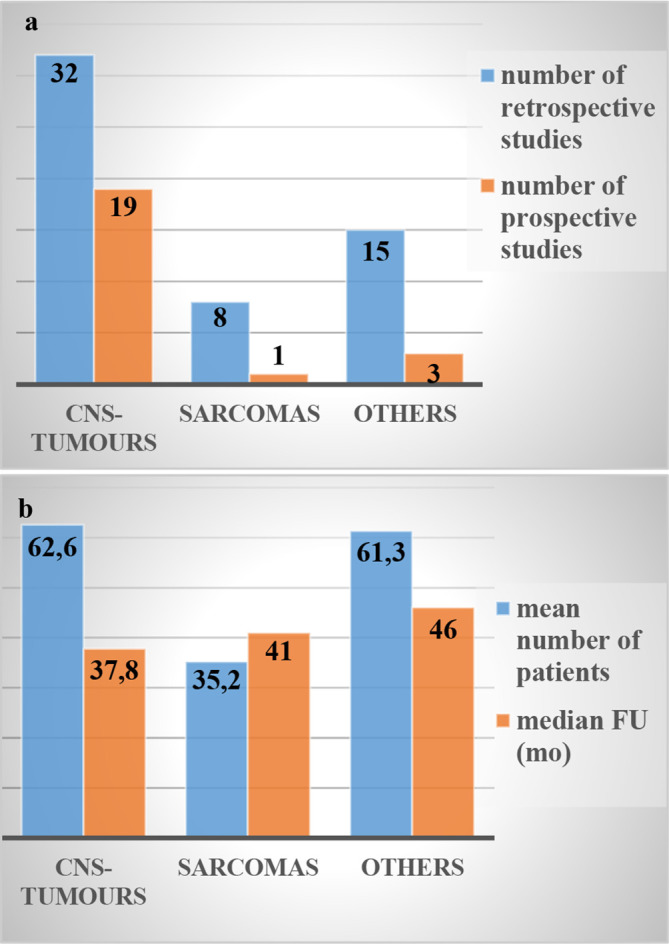
Evidence of paediatric PBT. All restrospective and prospective studies published in PubMed in the last ten years (2009–2019) on paediatric proton therapy are presented (a) with regard to mean number of patients and median FU period (b); FU (mo), follow up in months; CNS, central nervous system; FU, follow-up; PBT, proton beam therapy.
CNS—tumours
Treatment of brain neoplasms represents a particular challenge due to the sensitivity of various important critical anatomical structures (Figure 3). Late radiation-related adverse events can comprise neurological deficits, endocrine dysfunction, growth retardation, hearing impairment, vascular disorders, social and cognitive problems as well as secondary cancer incidence.8 Furthermore, an increased risk of cardiomyopathy or coronary vascular impairment arise when craniospinal irradiation (CSI) is performed.11 Several authors have reported a beneficial clinical outcome in terms of reduced acute toxicity and less late effects after PBT for embryonal tumours of the CNS, ependymomas, gliomas, craniopharyngiomas, germ cell tumours, meningiomas, pituitary adenomas and pineoblastomas.12–17
Figure 3. .
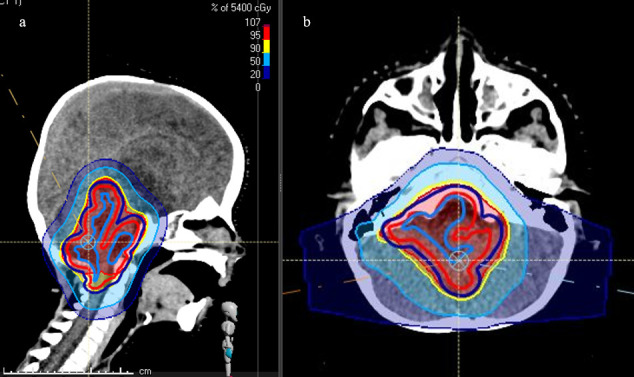
Pencil beam scanning proton therapy plan (sagittal (a) and axial (b) view) for a child with an ependymoma (RayStation®). Red area: covered by 95% isodose line, yellow: 90% isodose, light blue: 50% isodose, dark blue: 20% isodose.
Medulloblastomas
PBT has gained significant importance in the treatment of paediatric medulloblastomas (MB) because of the typically young age at presentation and the need for CSI due to frequent dissemination throughout the neuroaxis. A dosimetric comparisons with conventional irradiation techniques demonstrated that protons could not only eliminate the exit dose into chest, abdomen and pelvis but also reduced the dose to the brain and to critical CNS structures like the cochlea, the pituitary and the hypothalamus.18 Likewise, a dosimetric comparison of treatment plans for 18 MB patients revealed that CSI with protons reduced normal tissue dose compared to CSI with photons.19 However, both studies compare protons with outdated two-dimensional photon techniques. Modern photon techniques such as IMRT would achieve a more conformal coverage of the target volume. Still, compared to IMRT, PBT showed a measurable dose attenuation for non-target tissues outside the clinical target.20 Furthermore, the use of protons in CSI reduced the estimated risk of secondary cancer compared to conventional photons as revealed by comparative dose studies for paediatric MB patients.21,22 Even though, similar survival outcome and patterns of failure after proton- or photon-based RT in children with MB are described in various studies,23–25 others have confirmed the benefit of PBT clinical outcomes concerning long-term toxicity. Within a small cohort of 15 very young MB patients (<60 months) receiving chemotherapy followed by PBT, even including CSI in some cases, only 2 of 15 patients experienced ototoxicity and 3 of 15 required hormone replacement. Median CSI dose for this cohort was 21.6 Gy (relative biological effectiveness, (RBE) followed by a median boost dose of 54.0 Gy (RBE). At a medium follow-up (FU) of 39 months 13 patients were alive without evidence of disease recurrence.26 Low incidences of early high-grade ototoxicity were also observed in a cohort of 23 children with MB at a median age of 6 years (range, 3–16) who received PBT including CSI.27 Another prospective study of 59 paediatric patients with MB highlighted the benefit of protons on long-term toxicity for hearing, neuroendocrine and neurocognitive deficits compared to historical controls. The authors observed a lower decline of intelligence (IQ), no cardiac, pulmonary or gastrointestinal sequelae after PBT. Median FU was 7.0 years, median CSI dose applied was 23.4 Gy (RBE) and a median boost was 54.0 Gy (RBE).24 A multi-institutional cohort study on 77 children with standard risk MB reported a reduced risk of hypothyroidism, sex hormone deficiency and a reduced requirement for any hormone replacement therapy after irradiation with protons when compared to photons.28 Apart from the above presented data, which prove a benefit of PBT compared to RT with photons, comparable rates for CNS and brainstem injury were reported between proton and photon irradiation. In the report by Giantsoudi et al, 111 children with MB were treated with protons and the 5 year cumulative incidence of CNS injury was 3.6% at a median FU of 4.2 years, similar to historical data with photons.29 Likewise, in 84 children with MB, similar incidence of ototoxicity was reported when receiving PBT vs photon-RT.30
In summary, PBT for MB is considered as a chance to broaden the therapeutic window, especially with regard to young ages. Results on safety and efficacy are promising, particularly with regard to reducing late sequelae and the risk of secondary malignancies.
Ependymomas
Ependymomas are one of the most common tumours in children under 10 years of age, predominantly occurring in the brain. For paediatric ependymomas, best survival outcomes are reported after maximal surgical resection followed by focal cranial RT.31 A series of first clinical experiences in the treatment of paediatric intracranial and spine ependymomas with PBT indicated safety and effectiveness of postoperative PBT with low toxicities and similar disease control when compared to photon irradiation.32–38 In a cohort of 179 children with non-metastatic WHO Grade II/III intracranial ependymomas, 3 year local control (LC)-, progession free survival (PFS)- and overall survival (OS)-rates of 85%, 76% and 90%, respectively, were demonstrated with no unexpected toxicity.32 In a cohort of 79 children diagnosed with localized intracranial ependymomas treated with either IMRT or PBT, 3 year LC-, PFS- and OS-rates of 86%, 82% and 97%, respectively, were reported for proton irradiation and were comparable for both radiation modalities.33 50 paediatric ependymoma patients (WHO II–IV) treated post-operatively with PBT showed 5 year LC-, PFS- and OS-rates of 78%, 60% and 84%, respectively.34 Considering the high grade histology in 92% of patients and the number of patients with residual tumour ≥1.5 cc (18%), this outcome was still comparable to published reports of photon-treated cohorts. Furthermore, differences in the overall treatment like administration of additional chemotherapy have to be taken into account when evaluating reported outcome. Another study showed that PBT could reduce the dose to normal brain by 28–64% (median, 47%) compared to photon RT for paediatric WHO Grade II/III ependymomas. In their cohort of six patients with a median age of 5 years (range, 2–6), the median FU period was 24.5 months (range, 13–44) and the median radiation dose was 56.7 Gy (range, 50.4 to 61.2). At last FU all patients were alive with only one patient having experienced a local recurrence. All patients developed an Alopecia and mild dermatitis, but no severe toxicity was reported.35 The results of 70 paediatric patients with ependymomas suggested effective disease control using post-operative PBT with 3 year LC-, PFS- and OS-rates of 83%, 76% and 95%, respectively, few overall toxicities and no case of brainstem necrosis.36 In this cohort the median age at diagnosis was 38 months (range, 3 months–20 years) and the median PBT dose delivered was 55.8 Gy (range, 50.4–60.0) and the median FU time was 46 months (range, 12 months – 11.7 years). Normal intelligence was maintained and only a few patients developed growth hormone deficiency, hypothyroidism, or hearing loss after PBT.36
Since normal treatment effects observed in post-therapy imaging are difficult to distinguish from tumour recurrence, Gunther et al retrospectively evaluated imaging changes after post-operative PBT and IMRT in a cohort of 72 children with non-metastatic intracranial ependymomas. Imaging changes occurred in 6 IMRT and 16 PBT patients being symptomatic in 3 IMRT and 4 PBT patients, respectively. Grade 3 and 4 changes did occur in 4 and 2 patients in the PBT cohort only. One IMRT and one PBT patient each died due to disease progression and radiation necrosis, respectively. More aggressive treatment appears to be associated with an increased incidence of imaging changes. Not only age of diagnosis ≥3 years and time of radiation ≥3 years but also subtotal resection (STR) were associated with fewer imaging changes. Nevertheless, patients with imaging changes had a 4 year OS-rate of 90.4% compared to 82% in those without changes. The 4 year OS-rate of the individual PBT- and IMRT-cohorts were 87.5% and 78.8%, respectively, considering that the IMRT group had a higher percentage of STRs than the PBT group (20% vs 3%).39
More recently, re-irradiation has been established for local recurrences and was evaluated in several retrospective studies.40–42 One study demonstrated safety and efficacy of PBT for re-irradiation of intracranial ependymomas.43 CSI with protons in the case of disseminated disease recurrence has also been described with increased risk–benefit ratio.44 In summary, PBT is considered as a safe and effective treatment modality for paediatric ependymomas with low toxicities and similar disease control when compared to photon irradiation.
Low-grade gliomas
Low-grade gliomas (LGG) account for about 30%–40% of primary brain tumours in childhood.45 The median age of onset of the disease is 5–7 years. LGG grow slowly, displace locally and have an excellent prognosis for long-term survival. Surgery and RT are of major importance for local control, even if more recently systemic therapy was introduced to avoid or delay RT whenever possible in order to protect young children from significant late effects. PBT as a highly conformal RT technique was considered increasingly attractive for LGG in order to minimize dose burden in LGG patients. LGG is the fourth most common paediatric tumour to be treated with PBT. Although, dosimetric studies showed that PBT can reduce the low/intermediate radiation dose to uninvolved tissue in children with LGG20,46—clinical data on efficacy and toxicity remain limited.
In a prospective study, 174 children with non-metastatic LGG patients received PBT at a median age of 9 years (range, 2–21). The median FU was 4.4 years (range, 0.5–11.4) and 5 year LC-, PFS- and OS-rates of 85%, 84% and 92%, respectively, were described. Acute toxicities occurred to a small extent, since only 22 patients had nausea or vomiting, while 2 patients required corticosteroids. In term of late sequelae, an increase of 1% and 2% in the rate of visual deterioration and hearing loss, respectively, was observed after PBT, a positive result compared to those reported after photon therapy.47 Another study of 32 paediatric LGG patients demonstrated that PBT was effective and, depending on the tumour location, that it could spare dose to the temporal lobe, hippocampus and hypothalamic–pituitary–adrenal axis, correlating with less endocrine and neurocognitive complications. In this cohort the median age at diagnosis was 7.4 years (range, 0.8–20.4) and the median interval to radiation treatment was 2.1 years. The median FU time and radiation dose was 7.6 years (range, 3.2–18.2) and 52.2 Gy (RBE) (range, 48.6–54), respectively. 6 year and 8 year PFS-rates of 89.7% and 82.8%, respectively, were reported.48
Nevertheless, since pseudoprogression is a well-known phenomenon observed after RT in paediatric LGG patients, it should be considered when assessing the response to RT in LGG patients within the first year after RT. In a series of 83 paediatric LGG patients receiving IMRT (32) or PBT (51) with a median radiation dose of 50.4 Gy (RBE) (range, 45–59.4) significant rates of pseudoprogression were observed, particularly in the PBT cohort. However, 5 year LC-rates were 78% for the IMRT- and 90% for the PBT-cohort, with a trend toward improved LC with PBT (p = 0.099). The median FU was 5.6 years for the whole study cohort, with 6.1 and 4.2 years for the IMRT- and the PBT cohort, respectively.49
In summary, the presented data suggest the potential benefit of using PBT in the management of paediatric LGG. 5-year LC-rates were encouraging with only limited acute toxicity and reduced risk for endocrine and neurocognitive complication.
Sarcoma
Sarcomas are a heterogeneous group of malignancies, that originate from soft tissues (84%) or bones (14%).8 Although they can affect all age groups, they occur more frequently in the paediatric age group. In recent years, PBT was introduced into the multidisciplinary management in order to reduce the risk of side-effects (Figure 4). Though evidence is still scarce, published data on clinical outcomes in paediatric patients treated with PBT are encouraging.50–54 Mainly, sarcomas of the central axis, either in H&N region, spine or pelvis have been treated increasingly with PBT worldwide.
Figure 4. .
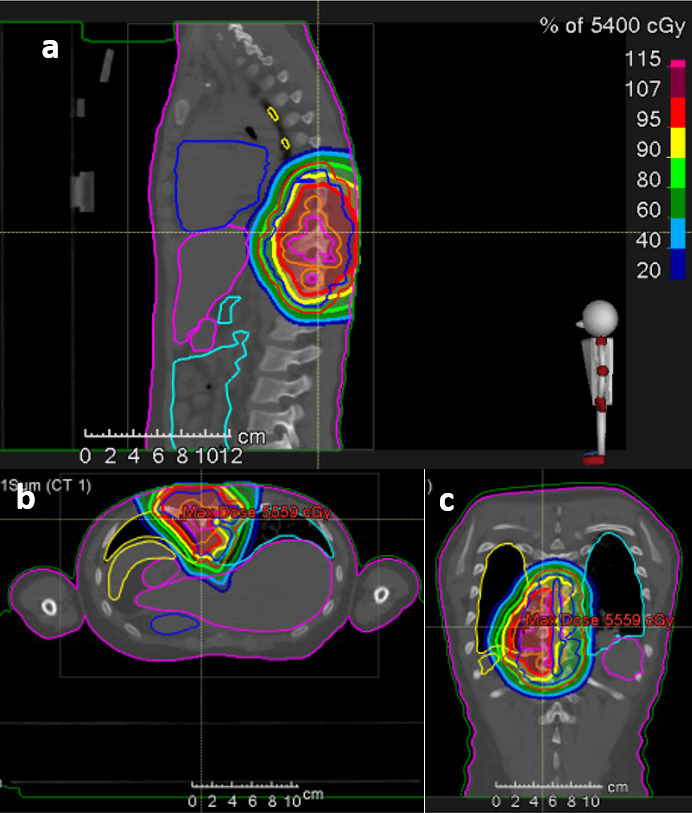
Pencil beam scanning proton therapy plan (sagittal (a), axial (b) and coronal (c) view) for a 11 year old child with an Ewing sarcoma of the thoracic (RayStation®). Red area: covered by 95% isodose line, yellow: 90% isodose, light green: 80% isodose, dark green: 60% isodose, light blue: 40% isodose, dark blue: 20% isodose.
Rhabdomyosarcomas
Rhabdomyosarcoma (RMS) is a highly malignant, locally invasive tumour and the most common soft-tissue sarcoma in childhood. The two most common histopathological subtypes, embryonal (75%) and alveolar (16%), differ in terms of age distribution, primary tumour sites, propensity for metastases and long-term outcome.55 RMS can occur all over the body, however, most common sites are H&N and genitourinary/pelvic.56 25% of the H&N–RMS show a parameningeal localization that rarely allows extensive surgery57 and recur early with relatively poor prognosis.50 RMS are treated in a multimodality approach, with RT playing an important role even in very young children. Local treatment strategies vary according to primary tumour type/site, age, histopathology and risk grouping. Local recurrence is the leading pattern of failure with relapse rates of 15%–37%.58 Advances in radiotherapeutic techniques enabled a reduction of late sequelae as endocrine deficits, facial hypoplasia, visual or orbital complications, hearing loss, neurocognitive deficits and bone retardation.57,59 In several studies on paediatric RMS at different sites60–62 PBT offered dosimetric advantages over photon-based IMRT with improved sparing to normal tissues and critical structures.
Clinical experiences in the treatment of paediatric RMS with PBT indicated safety and effectiveness with low acute toxicities and disease control comparable to photon irradiation.50,52,57–59,61,63,64 A retrospective observational study reported the clinical outcome of 55 children with a median age of 5 years (range 0–19) who received PBT for RMS in the H&N region (40), prostate (8) and others (7) with doses ranged from 36 to 60 Gy. The median FU time was 24.5 months (range, 1.5–32.3). 16% of the patients developed radiation-related acute toxicity of Grade ≥ 3, but recovered well after PBT. 87% of the patients experienced hematologic toxicities of Grade ≥ 3, but very likely not only related to PBT. On a short term, PBT achieved the same treatment effect as photon RT.59 In another cohort of 39 children with PM–RMS after a mean FU time of 41 months (range, 9–106), 5 year OS-rate of 73% with a crude failure rate of 23% was achieved. 89% of failures were local relapses. A delay of RT in PM–RMS seemed to compromise clinical outcome.50 Leiser et al reported that PBT was well tolerated with no treatment interruption or acute Grade >3 toxicity observed in a cohort of 83 children receiving PBT for embryonal (74) and alveolar (9) RMS at different sites. The mean FU time was 55.5 months, the 5 year PFS-rate was 78.5%. 16 children showed tumour recurrence or progression, 88% were in-field failures. Following univariate analysis, tumour site, Intergroup Rhabdomyosarcoma Studies Group Stage, Children’s Oncology Group-Risk group and tumour size were significant predictors of LC.63 A Phase II trial involving 57 patients with a median age of 3.5 years (range, 0.6–19.5), receiving a median proton radiation dose of 50.4 Gy (range, 36–50.4) and a mean FU time of 47 months (range, 14–102), described 5 year event-free survival (EFS-), OS- and LC-rates of 69%, 78% and 81%, respectively. 16 patients recurred, 10 of them locally.52 Although EFS-, OS- and LC-rates were similar to those observed in comparable photon studies, acute and late toxicity rates were favourable.52 Further clinical data demonstrated a reduction in late effects in a cohort of 17 patients with a median age of 3.4 years (range, 0.4–17.6) receiving PBT for PM-RMS. After a mean FU time of 5.0 years, in the 10 patients without recurrence, late effects related to PBT included reduced height velocity (3), endocrinopathies (2), mild facial hypoplasia (7), failure of permanent tooth eruption (3), dental caries (5), and chronic nasal/sinus congestion (2).57 Another study reported that PBT is well tolerated in a patient population of 7 children with bladder/prostate RMS treated with PBT at a median age of 30 months (range, 11–70) with doses ranging from 35 to 50.4 Gy. The mean FU time was 27 months (range, 10–90).61 To summarize current literature, PBT is widely used for paediatric RMS, particularly for parameningeal, paraspinal and pelvic sites. So far, acute and late toxicity was low and local control rates comparable to conventional radiation with photons be reported.
Ewing sarcoma
Ewing sarcomas (EWS) are aggressive, solid malignant tumours of childhood and adolescence with a peak incidence in the age group 10–15 years65. They predominantly occur in the bone, rarely in soft tissues. Most commonly affected site is the pelvis, followed by the long tubular bones of the femur, tibia and ribs. The multimodal treatment typically consists of an induction chemotherapy followed by local therapy by surgery with or without preoperative or adjuvant RT. If only biopsy is performed, definite RT can be a good option to achieve local control.66 After local therapy, chemotherapy is continued. Most recent data for EWS include results after photon-irradiation, reports on PBT in EWS are still limited.
One recent study examined the role of PBT for 15 paediatric EWS patients with a median age of 4 years (range, 1–14). PBT was delivered with a median dose of 55.8 Gy (range, 45.0–55.8). The 4 year OS- and EFS-rates were 94.6% and 84.8%, respectively, after a median FU of 52 months (range, 12–90). At start of PBT, either complete response (2) or partial response (13) was achieved. Generally, PBT was well-tolerated in this cohort with only few acute adverse effects.67 Furthermore, clinical outcomes of 38 children with EWS treated with PBS–PBT were published. The median age was 9.9 years (range, 0.4–38.9) and the median dose applied was 54.9 Gy (RBE) (range, 45.0–69.6). The estimated 5 year LC- and metastasis-free survival-rates were 81.5% and 76.4%, respectively. PBS protons were associated with a low prevalence of high-grade late toxicity.65 In a cohort of 343 paediatric patients with different tumour types including 30 EWS patients received PBT. The estimated 1-, 3-, and 5 year OS-rates were 88.6%, 73.1%, and 56.8%, respectively. In the EWS subgroup, toxicity ≥3 occurred in four patients. Grade III toxicities (gastic ulcer and myelitis) and Grade IV toxicities (myelitis and dysopia) occurred in two patients each.68 Another retrospective analysis investigated 30 children with EWS at different sites.66 The median age of the cohort was 10 years, the median RT dose applied was 54 Gy (RBE). After a median FU of 38.4 months, 3 year EFS-, LC- and OS-rates were 60%, 86%, and 89%, respectively. In summary, early results suggest that PBT for EWS is well-tolerated with encouraging tumour control rates.
Eye tumors
Treatment of eye tumours is one well-recognized domain of PBT. Historically, PBT was used mainly for uveal melanomas using passive scattering technique. It was demonstrated by various groups, that intensive local dose regimens were able to achieve high local control rates while preserving the eye and even vision for the majority of patients, despite the close proximity of critical structures to the target.69 Therefore, PBT was understood to be an attractive option to treat eye or orbit. In children, one major challenge is the treatment of retinoblastomas as it occurs in very young age.70 In addition, RB patients are prone to develop secondary malignancies due to genetic predisposition.70
Retinoblastoma
Retinoblastoma (RB) is the most common intraocular childhood malignancy with sporadic (60%) and inherited (40%) forms.70 RB occurs unilaterally in 60%–70% of cases and bilaterally in 30%–40% of cases.71 RT has a long history in the treatment of RB and was used with great success. However, RT was identified to increase the risk of secondary cancers particularly in children with hereditary RBs. And, RT will also have negative effect on the growth of the orbital soft tissue and bone. Therefore, it is currently performed only in second or third line after treatment failure or in very large tumours and high stages.72 PBT was expected to reduce the probability of radiation-induced late effects, particularly secondary sarcomas. So far, tumour control rates with PBT were similar to historical series.
In a study on six patients, three children with RB received PBT after primary chemotherapy. All three patients were enucleated after PBT due to tumour progression. Since radiation-related orbital and periorbital complications may occur, in all three cases a significant reduction of enophthalmia and orbital volume (OV) developed only after enucleation of the irradiated eye. Therefore, it is difficult to assess the long-term effects of PBT alone without considering the contribution of enucleation to OV loss..73 Recently, clinical outcomes of 49 children with RB treated with PBT were published. The median age at diagnosis in this cohort was 6 months and the median FU time was 8 years (range, 1–24). In 41 patients, tumour was bilateral and eleven of them underwent bilateral PBT with a median radiation dose of 44 Gy (RBE). Out of these 60 irradiated eyes from 41 patients, 11 eyes ultimately required enucleation. During FU, no patient died of disease, no patient developed either dissemination or an in-field second malignancy. Only one patient with hereditary disease developed an osteosarcoma of the femur 10 years after completion of RT.74 Recently, 12 additional paediatric RB patients were retrospectively studied by the group of Mouw et al. The median age at diagnosis in this cohort was 3 months (range, 1–20), the median PBT dose was 44 Gy (RBE) and the mean FU period was 12.9 years (range, 4.8–22.2). However, all patients had bilateral disease, but only two patients underwent bilateral PBT. In 3 of the 14 eyes treated with PBT enucleation was required due to disease progression. Seven of the non-enucleated eyes treated with PBT showed no or only mild visual impairment. None of the patients had abnormal reproductive hormone levels and all patients had normal sexual development.75 Another retrospective study of 86 RB patients receiving proton (55) or photon (31) RT, compared the risk of second malignancy between both RT modalities. The median age at treatment was 14.8 months (range, 0.9 months–12.1years) in the proton cohort and 10.0 months (range, 7 days–4.7 years) in the photon cohort, respectively. The median FU was 6.9 years (range, 12.4 months–24.4 years) for protons and 13.1 years (range, 17.1 months–23.9 years) for photons. In the proton cohort, one secondary malignancy (osteosarcoma of the distal femur) was diagnosed 9.0 years after RT. In the photon cohort, four in-field secondary malignancies (orbital sarcoma, maxillary sarcoma, temporary bone sarcoma and glioblastoma multiforme) developed 4.9–13.1 years after RT.76 Another study reported on three patients with sporadic RB (1, 4 and 5 years) which were managed with PBT due to failure of other treatments. PBT was effective in all three cases. Nevertheless, in two cases the tumour recurred after a few months, resulting in enucleation.77 In summary, PBT as part of multimodal therapy seems to offer a chance in RB patients to reduce the risk for enucleation, visual impairment and secondary malignancies.
Conclusion
To date, the clinical results for high-precision proton therapy are promising, both with regard to tumour control and to the reduction of adverse events. However, further investigations with larger patient cohorts and longer follow-up are required in order to define the role of proton beam therapy for late effects reduction. Still, it can be assumed that PBT will be used more and more frequently in children with cancer and will represent an essential component of the multidisciplinary care for children suffering from cancer in future. To date, activities are ongoing to launch international registries for children collecting data on photon and proton RT with respect to adverse events. This seems to be an important step forward in order to gain more knowledge on RT effects and benefits of modern RT technologies.
Footnotes
Acknowledgment: The authors would like to thank Theresa Steinmeier und Martina Stickan-Verfürth for their contributions to the manuscript.
Contributor Information
Heike Thomas, Email: heike.thomas@uk-essen.de.
Beate Timmermann, Email: beate.timmermann@uk-essen.de.
REFERENCES
- 1.Steliarova-Foucher E, Colombet M, Ries LAG, Moreno F, Dolya A, Bray F, et al. International incidence of childhood cancer, 2001-10: a population-based registry study. Lancet Oncol 2017; 18: 719–31. doi: 10.1016/S1470-2045(17)30186-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coura CF, Modesto PC. Impact of late radiation effects on cancer Survivor children: an integrative review. Einstein 2016; 14: 71–6. doi: 10.1590/S1679-45082015RW3102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Timmermann B, Schulze Schleithoff S. Moderne Strahlentherapie bei Krebserkrankungen Im Kindesalter. Onkologische Pflege 2018; 2: 30–5. [Google Scholar]
- 4.Leroy R, Benahmed N, Hulstaert F, Van Damme N, De Ruysscher D. Proton therapy in children: a systematic review of clinical effectiveness in 15 pediatric cancers. International Journal of radiation oncology, biology. Physics 2016; 95: 267–78. [DOI] [PubMed] [Google Scholar]
- 5.Wilson RR. Radiological use of fast protons. Radiology 1946; 47: 487–91. doi: 10.1148/47.5.487 [DOI] [PubMed] [Google Scholar]
- 6.Lawrence JH, Tobias CA, Born JL, McCOMBS RK, Roberts JE, Anger HO, et al. Pituitary irradiation with high-energy proton beams: a preliminary report. Cancer Res 1958; 18: 121–34. [PubMed] [Google Scholar]
- 7.Mohan R, Grosshans D. Proton therapy – present and future. Adv Drug Deliv Rev 2017; 109: 26–44. doi: 10.1016/j.addr.2016.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frisch S, Timmermann B. The evolving role of proton beam therapy for sarcomas. Clin Oncol 2017; 29: 500–6. doi: 10.1016/j.clon.2017.04.034 [DOI] [PubMed] [Google Scholar]
- 9.Odei B, Frandsen JE, Boothe D, Ermoian RP, Poppe MM. Patterns of care in proton radiation therapy for pediatric central nervous system malignancies. International Journal of radiation oncology, biology. Physics 2017; 97: 60–3. [DOI] [PubMed] [Google Scholar]
- 10.Rombi B, Vennarini S, Vinante L, Ravanelli D, Amichetti M. Proton radiotherapy for pediatric tumors: review of first clinical results. Ital J Pediatr 2014; 40: 74. doi: 10.1186/s13052-014-0074-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Armstrong GT. Long-Term survivors of childhood central nervous system malignancies: the experience of the childhood cancer Survivor study. Eur J Paediatr Neurol 2010; 14: 298–303. doi: 10.1016/j.ejpn.2009.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Indelicato DJ, Bradley JA, Sandler ES, Aldana PR, Sapp A, Gains JE, et al. Clinical outcomes following proton therapy for children with central nervous system tumors referred overseas. Pediatr Blood Cancer 2017; 64: e26654. doi: 10.1002/pbc.26654 [DOI] [PubMed] [Google Scholar]
- 13.Fukushima H, Fukushima T, Suzuki R, Iwabuchi A, Hidaka K, Masumoto K, et al. Co-Morbidity and quality of life in childhood cancer survivors treated with proton beam therapy. Pediatrics international : official journal of the Japan Pediatric Society 2017;. [DOI] [PubMed] [Google Scholar]
- 14.Yock TI, Bhat S, Szymonifka J, Yeap BY, Delahaye J, Donaldson SS, et al. Quality of life outcomes in proton and photon treated pediatric brain tumor survivors. Radiother Oncol 2014; 113: 89–94. doi: 10.1016/j.radonc.2014.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Indelicato DJ, Flampouri S, Rotondo RL, Bradley JA, Morris CG, Aldana PR, et al. Incidence and dosimetric parameters of pediatric brainstem toxicity following proton therapy. Acta Oncol 2014; 53: 1298–304. doi: 10.3109/0284186X.2014.957414 [DOI] [PubMed] [Google Scholar]
- 16.Combs SE, Kessel K, Habermehl D, Haberer T, Jäkel O, Debus J. Proton and carbon ion radiotherapy for primary brain tumors and tumors of the skull base. Acta Oncol 2013; 52: 1504–9. doi: 10.3109/0284186X.2013.818255 [DOI] [PubMed] [Google Scholar]
- 17.Suneja G, Poorvu PD, Hill-Kayser C, Lustig RA. Acute toxicity of proton beam radiation for pediatric central nervous system malignancies. Pediatr Blood Cancer 2013; 60: 1431–6. doi: 10.1002/pbc.24554 [DOI] [PubMed] [Google Scholar]
- 18.Zhang R, Howell RM, Taddei PJ, Giebeler A, Mahajan A, Newhauser WD. A comparative study on the risks of radiogenic second cancers and cardiac mortality in a set of pediatric medulloblastoma patients treated with photon or proton craniospinal irradiation. Radiotherapy and Oncology 2014; 113: 84–8. doi: 10.1016/j.radonc.2014.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Howell RM, Giebeler A, Koontz-Raisig W, Mahajan A, Etzel CJ, D'Amelio AM, et al. Comparison of therapeutic dosimetric data from passively scattered proton and photon craniospinal irradiations for medulloblastoma. Radiat Oncol 2012; 7: 116. doi: 10.1186/1748-717X-7-116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Armoogum KS, Thorp N. Dosimetric comparison and potential for improved clinical outcomes of paediatric CNS patients treated with protons or IMRT. Cancers 2015; 7: 706–22. doi: 10.3390/cancers7020706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stokkevåg CH, Engeseth G-M, Ytre-Hauge KS, Röhrich D, Odland OH, Muren LP, et al. Estimated risk of radiation-induced cancer following paediatric cranio-spinal irradiation with electron, photon and proton therapy. Acta Oncol 2014; 53: 1048–57. doi: 10.3109/0284186X.2014.928420 [DOI] [PubMed] [Google Scholar]
- 22.Zhang R, Howell RM, Giebeler A, Taddei PJ, Mahajan A, Newhauser WD. Comparison of risk of radiogenic second cancer following photon and proton craniospinal irradiation for a pediatric medulloblastoma patient. Phys Med Biol 2013; 58: 807–23. doi: 10.1088/0031-9155/58/4/807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eaton BR, Esiashvili N, Kim S, Weyman EA, Thornton LT, Mazewski C, et al. Clinical outcomes among children with standard-risk medulloblastoma treated with proton and photon radiation therapy: a comparison of disease control and overall survival. International Journal of radiation oncology, biology. Physics 2016; 94: 133–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yock TI, Yeap BY, Ebb DH, Weyman E, Eaton BR, Sherry NA, et al. Long-Term toxic effects of proton radiotherapy for paediatric medulloblastoma: a phase 2 single-arm study. Lancet Oncol 2016; 17: 287–98. doi: 10.1016/S1470-2045(15)00167-9 [DOI] [PubMed] [Google Scholar]
- 25.Sethi RV, Giantsoudi D, Raiford M, Malhi I, Niemierko A, Rapalino O, et al. Patterns of failure after proton therapy in medulloblastoma; linear energy transfer distributions and relative biological effectiveness associations for relapses. International Journal of radiation oncology, biology. Physics 2014; 88: 655–63. [DOI] [PubMed] [Google Scholar]
- 26.Jimenez RB, Sethi R, Depauw N, Pulsifer MB, Adams J, McBride SM, et al. Proton radiation therapy for pediatric medulloblastoma and supratentorial primitive neuroectodermal tumors: outcomes for very young children treated with upfront chemotherapy. International Journal of radiation oncology, biology. Physics 2013; 87: 120–6. [DOI] [PubMed] [Google Scholar]
- 27.Moeller BJ, Chintagumpala M, Philip JJ, Grosshans DR, McAleer MF, Woo SY, et al. Low early ototoxicity rates for pediatric medulloblastoma patients treated with proton radiotherapy. Radiat Oncol 2011; 6: 58. doi: 10.1186/1748-717X-6-58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eaton BR, Esiashvili N, Kim S, Patterson B, Weyman EA, Thornton LT, et al. Endocrine outcomes with proton and photon radiotherapy for standard risk medulloblastoma. Neuro Oncol 2016; 18: 881–7. doi: 10.1093/neuonc/nov302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Giantsoudi D, Sethi RV, Yeap BY, Eaton BR, Ebb DH, Caruso PA, et al. Incidence of CNS injury for a cohort of 111 patients treated with proton therapy for medulloblastoma: let and RBE associations for areas of injury. International Journal of radiation oncology, biology. Physics 2016; 95: 287–96. [DOI] [PubMed] [Google Scholar]
- 30.Paulino AC, Mahajan A, Ye R, Grosshans DR, Fatih Okcu M, Su J, et al. Ototoxicity and cochlear sparing in children with medulloblastoma: proton vs. photon radiotherapy. Radiother Oncol 2018; 128: 128–32. doi: 10.1016/j.radonc.2018.01.002 [DOI] [PubMed] [Google Scholar]
- 31.Thorp N, Gandola L. Management of ependymoma in children, adolescents and young adults. Clin Oncol 2019; 31: 162–70. doi: 10.1016/j.clon.2018.12.001 [DOI] [PubMed] [Google Scholar]
- 32.Indelicato DJ, Bradley JA, Rotondo RL, Nanda RH, Logie N, Sandler ES, et al. Outcomes following proton therapy for pediatric ependymoma. Acta Oncol 2018; 57: 644–8. doi: 10.1080/0284186X.2017.1413248 [DOI] [PubMed] [Google Scholar]
- 33.Sato M, Gunther JR, Mahajan A, Jo E, Paulino AC, Adesina AM, et al. Progression-Free survival of children with localized ependymoma treated with intensity-modulated radiation therapy or proton-beam radiation therapy. Cancer 2017; 123: 2570–8. doi: 10.1002/cncr.30623 [DOI] [PubMed] [Google Scholar]
- 34.Ares C, Albertini F, Frei-Welte M, Bolsi A, Grotzer MA, Goitein G, et al. Pencil beam scanning proton therapy for pediatric intracranial ependymoma. J Neurooncol 2016; 128: 137–45. doi: 10.1007/s11060-016-2090-4 [DOI] [PubMed] [Google Scholar]
- 35.Mizumoto M, Oshiro Y, Takizawa D, Fukushima T, Fukushima H, Yamamoto T, et al. Proton beam therapy for pediatric ependymoma. Pediatr Int 2015; 57: 567–71. doi: 10.1111/ped.12624 [DOI] [PubMed] [Google Scholar]
- 36.MacDonald SM, Sethi R, Lavally B, Yeap BY, Marcus KJ, Caruso P, et al. Proton radiotherapy for pediatric central nervous system ependymoma: clinical outcomes for 70 patients. Neuro Oncol 2013; 15: 1552–9. doi: 10.1093/neuonc/not121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Amsbaugh MJ, Grosshans DR, McAleer MF, Zhu R, Wages C, Crawford CN, et al. Proton therapy for spinal ependymomas: planning, acute toxicities, and preliminary outcomes. International Journal of radiation oncology, biology. Physics 2012; 83: 1419–24. [DOI] [PubMed] [Google Scholar]
- 38.MacDonald SM, Yock TI. Proton beam therapy following resection for childhood ependymoma. Child's Nervous System 2010; 26: 285–91. doi: 10.1007/s00381-009-1059-4 [DOI] [PubMed] [Google Scholar]
- 39.Gunther JR, Sato M, Chintagumpala M, Ketonen L, Jones JY, Allen PK, et al. Imaging changes in pediatric intracranial ependymoma patients treated with proton beam radiation therapy compared to intensity modulated radiation therapy. International Journal of radiation oncology, biology. Physics 2015; 93: 54–63. [DOI] [PubMed] [Google Scholar]
- 40.Merchant TE, Boop FA, Kun LE, Sanford RA. A retrospective study of surgery and reirradiation for recurrent ependymoma. International Journal of radiation oncology, biology. Physics 2008; 71: 87–97. [DOI] [PubMed] [Google Scholar]
- 41.Liu AK, Foreman NK, Gaspar LE, Trinidad E, Handler MH. Maximally safe resection followed by hypofractionated re-irradiation for locally recurrent ependymoma in children. Pediatr Blood Cancer 2009; 52: 804–7. doi: 10.1002/pbc.21982 [DOI] [PubMed] [Google Scholar]
- 42.Stauder MC, NI Laack N, Ahmed KA, Link MJ, Schomberg PJ, Pollock BE. Stereotactic radiosurgery for patients with recurrent intracranial ependymomas. J Neurooncol 2012; 108: 507–12. doi: 10.1007/s11060-012-0851-2 [DOI] [PubMed] [Google Scholar]
- 43.Eaton BR, Chowdhry V, Weaver K, Liu L, Ebb D, MacDonald SM, et al. Use of proton therapy for re-irradiation in pediatric intracranial ependymoma. Radiotherapy and Oncology 2015; 116: 301–8. doi: 10.1016/j.radonc.2015.07.023 [DOI] [PubMed] [Google Scholar]
- 44.Hill-Kayser C, Kirk M. Brainstem-sparing craniospinal irradiation delivered with pencil beam scanning proton therapy. Pediatr Blood Cancer 2015; 62: 718–20. doi: 10.1002/pbc.25378 [DOI] [PubMed] [Google Scholar]
- 45.Ajithkumar T, Taylor R, Kortmann RD. Radiotherapy in the management of paediatric low-grade gliomas. Clin Oncol 2019; 31: 151–61. doi: 10.1016/j.clon.2018.11.032 [DOI] [PubMed] [Google Scholar]
- 46.Harrabi SB, Bougatf N, Mohr A, Haberer T, Herfarth K, Combs SE, et al. Dosimetric advantages of proton therapy over conventional radiotherapy with photons in young patients and adults with low-grade glioma. Strahlentherapie und Onkologie 2016; 192: 759–69. doi: 10.1007/s00066-016-1005-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Indelicato DJ, Rotondo RL, Uezono H, Sandler ES, Aldana PR, Ranalli NJ, et al. Outcomes following proton therapy for pediatric low-grade glioma. International Journal of radiation oncology, biology. Physics 2019; 104: 149–56. [DOI] [PubMed] [Google Scholar]
- 48.Greenberger BA, Pulsifer MB, Ebb DH, MacDonald SM, Jones RM, Butler WE, et al. Clinical outcomes and late endocrine, neurocognitive, and visual profiles of proton radiation for pediatric low-grade gliomas. International Journal of radiation oncology, biology. Physics 2014; 89: 1060–8. [DOI] [PubMed] [Google Scholar]
- 49.Ludmir EB, Mahajan A, Paulino AC, Jones JY, Ketonen LM, Su JM, et al. Increased risk of pseudoprogression among pediatric low-grade glioma patients treated with proton versus photon radiotherapy. Neuro Oncol 2019; 21: 686–95. doi: 10.1093/neuonc/noz042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weber DC, Ares C, Albertini F, Frei-Welte M, Niggli FK, Schneider R, et al. Pencil beam scanning proton therapy for pediatric Parameningeal rhabdomyosarcomas: clinical outcome of patients treated at the Paul Scherrer Institute. Pediatr Blood Cancer 2016; 63: 1731–6. doi: 10.1002/pbc.25864 [DOI] [PubMed] [Google Scholar]
- 51.Rombi B, Ares C, Hug EB, Schneider R, Goitein G, Staab A, et al. Spot-scanning proton radiation therapy for pediatric chordoma and chondrosarcoma: clinical outcome of 26 patients treated at Paul scherrer Institute. International Journal of radiation oncology, biology. Physics 2013; 86: 578–84. [DOI] [PubMed] [Google Scholar]
- 52.Ladra MM, Szymonifka JD, Mahajan A, Friedmann AM, Yong Yeap B, Goebel CP, et al. Preliminary results of a phase II trial of proton radiotherapy for pediatric rhabdomyosarcoma. Journal of Clinical Oncology 2014; 32: 3762–70. doi: 10.1200/JCO.2014.56.1548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rieber JG, Kessel KA, Witt O, Behnisch W, Kulozik AE, Debus J, et al. Treatment tolerance of particle therapy in pediatric patients. Acta Oncol 2015; 54: 1049–55. doi: 10.3109/0284186X.2014.998273 [DOI] [PubMed] [Google Scholar]
- 54.Rutz HP, Weber DC, Goitein G, Ares C, Bolsi A, Lomax AJ, et al. Postoperative spot-scanning proton radiation therapy for chordoma and chondrosarcoma in children and adolescents: initial experience at Paul scherrer Institute. International Journal of radiation oncology, biology. Physics 2008; 71: 220–5. [DOI] [PubMed] [Google Scholar]
- 55.Malempati S, Hawkins DS. Rhabdomyosarcoma: review of the children's Oncology Group (COG) soft-tissue sarcoma Committee experience and rationale for current cog studies. Pediatr Blood Cancer 2012; 59: 5–10. doi: 10.1002/pbc.24118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Egas-Bejar D, Huh WW. Rhabdomyosarcoma in adolescent and young adult patients: current perspectives. Adolesc Health Med Ther 2014; 5: 115–25. doi: 10.2147/AHMT.S44582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Childs SK, Kozak KR, Friedmann AM, Yeap BY, Adams J, MacDonald SM, et al. Proton radiotherapy for parameningeal rhabdomyosarcoma: clinical outcomes and late effects. International Journal of radiation oncology, biology. Physics 2012; 82: 635–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vern-Gross TZ, Indelicato DJ, Bradley JA, Rotondo RL. Patterns of failure in pediatric rhabdomyosarcoma after proton therapy. International Journal of radiation oncology, biology. Physics 2016; 96: 1070–7. [DOI] [PubMed] [Google Scholar]
- 59.Mizumoto M, Murayama S, Akimoto T, Demizu Y, Fukushima T, Ishida Y, et al. Preliminary results of proton radiotherapy for pediatric rhabdomyosarcoma: a multi-institutional study in Japan. Cancer Med 2018; 7: 1870–4. doi: 10.1002/cam4.1464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ladra MM, Edgington SK, Mahajan A, Grosshans D, Szymonifka J, Khan F, et al. A dosimetric comparison of proton and intensity modulated radiation therapy in pediatric rhabdomyosarcoma patients enrolled on a prospective phase II proton study. Radiotherapy and Oncology 2014; 113: 77–83. doi: 10.1016/j.radonc.2014.08.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cotter SE, Herrup DA, Friedmann A, Macdonald SM, Pieretti RV, Robinson G, et al. Proton radiotherapy for pediatric bladder/prostate rhabdomyosarcoma: clinical outcomes and dosimetry compared to intensity-modulated radiation therapy. International Journal of radiation oncology, biology. Physics 2011; 81: 1367–73. [DOI] [PubMed] [Google Scholar]
- 62.Kozak KR, Adams J, Krejcarek SJ, Tarbell NJ, Yock TI. A dosimetric comparison of proton and intensity-modulated photon radiotherapy for pediatric parameningeal rhabdomyosarcomas. International Journal of radiation oncology, biology. Physics 2009; 74: 179–86. [DOI] [PubMed] [Google Scholar]
- 63.Leiser D, Calaminus G, Malyapa R, Bojaxhiu B, Albertini F, Kliebsch U, et al. Tumour control and quality of life in children with rhabdomyosarcoma treated with pencil beam scanning proton therapy. Radiother Oncol 2016; 120: 163–8. doi: 10.1016/j.radonc.2016.05.013 [DOI] [PubMed] [Google Scholar]
- 64.Fukushima H, Fukushima T, Sakai A, Suzuki R, Kobayashi C, Oshiro Y, et al. Tailor-Made treatment combined with proton beam therapy for children with genitourinary/pelvic rhabdomyosarcoma. Rep Pract Oncol Radiother 2015; 20: 217–22. doi: 10.1016/j.rpor.2014.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Weber DC, Murray FR, Correia D, Bolsi A, Frei-Welte M, Pica A, et al. Pencil beam scanned protons for the treatment of patients with Ewing sarcoma. Pediatr Blood Cancer 2017; 64: e26688. doi: 10.1002/pbc.26688 [DOI] [PubMed] [Google Scholar]
- 66.Rombi B, DeLaney TF, MacDonald SM, Huang MS, Ebb DH, Liebsch NJ, et al. Proton radiotherapy for pediatric Ewing's sarcoma: initial clinical outcomes. International Journal of radiation oncology, biology. Physics 2012; 82: 1142–8. [DOI] [PubMed] [Google Scholar]
- 67.Nakao T, Fukushima H, Fukushima T, Suzuki R, Hosaka S, Yamaki Y, et al. Interinstitutional patient transfers between rapid chemotherapy cycles were feasible to utilize proton beam therapy for pediatric Ewing sarcoma family of tumors. Reports of Practical Oncology & Radiotherapy 2018; 23: 442–50. doi: 10.1016/j.rpor.2018.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mizumoto M, Murayama S, Akimoto T, Demizu Y, Fukushima T, Ishida Y, et al. Proton beam therapy for pediatric malignancies: a retrospective observational multicenter study in Japan. Cancer Med 2016; 5: 1519–25. doi: 10.1002/cam4.743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mishra KK, Daftari IK. Proton therapy for the management of uveal melanoma and other ocular tumors. Chinese Clinical Oncology 2016; 5: 50. doi: 10.21037/cco.2016.07.06 [DOI] [PubMed] [Google Scholar]
- 70.Rao R, Honavar SG, Retinoblastoma HSG. Retinoblastoma. Indian J Pediatr 2017; 84: 937–44. doi: 10.1007/s12098-017-2395-0 [DOI] [PubMed] [Google Scholar]
- 71.Schefler AC, Kim RS. Recent advancements in the management of retinoblastoma and uveal melanoma. F1000. Research 2018; 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kim J-Y, Park Y. Treatment of retinoblastoma: the role of external beam radiotherapy. Yonsei Med J 2015; 56: 1478–91. doi: 10.3349/ymj.2015.56.6.1478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Choi YJ, Kim TW, Kim S, Choung H, Lee MJ, Kim N, et al. Effects on periocular tissues after proton beam radiation therapy for intraocular tumors. J Korean Med Sci 2018; 33: e120. doi: 10.3346/jkms.2018.33.e120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mouw KW, Sethi RV, Yeap BY, MacDonald SM, Chen YL, Tarbell NJ, et al. Proton radiation therapy for the treatment of retinoblastoma. International Journal of radiation oncology, biology. Physics 2014; 90: 863–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mouw KW, Yeap BY, Caruso P, Fay A, Misra M, Sethi RV, et al. Analysis of patient outcomes following proton radiation therapy for retinoblastoma. Advances in Radiation Oncology 2017; 2: 44–52. doi: 10.1016/j.adro.2016.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sethi RV, Shih HA, Yeap BY, Mouw KW, Petersen R, Kim DY, et al. Second nonocular tumors among survivors of retinoblastoma treated with contemporary photon and proton radiotherapy. Cancer 2014; 120: 126–33. doi: 10.1002/cncr.28387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chang JW, Yu YS, Kim JY, Shin DH, Choi J, Kim JH, et al. The clinical outcomes of proton beam radiation therapy for retinoblastomas that were resistant to chemotherapy and focal treatment. Korean Journal of Ophthalmology 2011; 25: 387–93. doi: 10.3341/kjo.2011.25.6.387 [DOI] [PMC free article] [PubMed] [Google Scholar]


