Abstract
Proton arc therapy (PAT) has been proposed as a possible evolution for proton therapy. This commentary uses dosimetric and cancer risk evaluations from earlier studies to compare PAT with intensity modulated proton therapy. It is concluded that, although PAT may not produce better physical dose distributions than intensity modulated proton therapy, the radiobiological considerations associated with particular PAT techniques could offer the possibility of an increased therapeutic index.
Introduction
Current proton treatment methods usually seek to limit normal tissue doses by restricting the number of beams used.1 Therefore, since proton arc therapy (PAT) would introduce a non-negligible dose bath, the use of such a technique would appear to conflict with one of the key advantages offered by proton radiotherapy. However, there is a counterpoint in that considerations of range uncertainty2 and variations in relative biological effectiveness (RBE)3 could caution against the use of single or two-field static plans as a method to mitigate (or "spread") these uncertainties.
Physical aspects of PAT
There is a substantial literature comparing the dosimetric characteristics of PAT, intensity modulated proton therapy (IMPT) and volumetric arctherapy (VMAT) (intensity modulated radiotherapy, IMRT). This has considered PAT delivered with both, passive scattering (PS)4–6 and pencil beam scanning (PBS)6–13 delivery methods. In this brief commentary, we confine attention to PBS-based reports and discuss how they might provide some justification for PAT.
Conformity, homogeneity and integral dose
Table 1 summarizes the results of published dosimetric comparisons which involved a variety of anatomical sites and phantoms of different geometries. The evaluations involve three parameters: conformity index (CI), homogeneity index (HI) and integral body dose (IBD). (The definitions employed for each are not always the same in the publications considered; any differences are specified in the figure caption).
Table 1.
Summary of dosimetric comparisons between PAT and PS/PBS techniques from published literature
| Lung | Brain | Prostate | H&N | |||
|---|---|---|---|---|---|---|
| CI | Seco et al. | Ding et al. 2016 | Sanchez et al. | Sanchez et al. |
Ding et al. 2018 | Ding et al. 2016 |
| −1.25i (p = 0.003) |
−3.55i (N = 1) |
−0.16ii (N = 1) |
+0.01ii (N = 1) |
0.00i (p = 0.934) |
−0.02i (N = 1) |
|
| HI | +0.23iii (p < 0.001) |
−0.03iii (N = 1) |
+0.01 ii,iii (N = 1) |
0.00ii,iii (N = 1) |
0.00iii (p = 0.347) |
|
| IBD | −2%iv (p < 0.001) |
−14% (N = 1) |
−1.2%ii (N = 1) |
+3.4%ii (N = 1) |
−11% (p = 0.003) |
−7% (N = 1) |
CI, conformity index; HI, homogeneity index; IBD, integral body dose; PAT, proton arc therapy; PBS, pencil beam scanning; PS, passive scattering.
iCI is defined as the volume enclosed by 95%prescription isodose line divided by target volume.
iiCI is defined according to RTOG protocol17. Several methods to optimize the arc plans were presented. We select here the method “M1_R1”.
iiiHI is defined according to RTOG protocol14.
ivMean doses to ipsilateral lung are compared.
For CI and HI, results show difference between PAT plans and PS/PBS plans. Unless otherwise specified, the third row shows percentage difference between IBD to that obtained with PAT plans and PS/PBS plans, taking PAT plans as the percentage basis. When provided, level of significance is specified through p (for the null hypothesis of no significant improvement for PAT respect to PS/PBS techniques). Otherwise, the number of cases considered (N) is given. iCI is defined as the volume enclosed by 95% prescription isodoseline divided by target volume.
For lung treatments, CI improves with PAT, but the greatest improvement is reported from a single lung case.9 No significant differences are found for the HI index. For the other anatomical sites, no strong differences were found for HI and CI. Considering the variabilities in the methodologies, as well as the limited significance of the results, there is no strong evidence that PAT offers improved conformity or homogeneity. However, the dose imparted to organs at risk and body appears to be lower with PAT plans than with IMPT plans, the exception being the case in a brain phantom.15
Geometrical considerations in the design of PBS-PAT plans
The finite penetration depth of a pencil beam not only confers the benefits indicated above but also implies less normal tissue involvement.13 Since the lateral penumbra of a proton pencil beam increases with depth, reducing the sharp delineation between treatment volume and adjacent critical structure, then the benefit of PAT over VMAT could only be achieved by selecting beam angles that require less penetration depth. For PAT, this would suggest that the use of a larger number of smaller coplanar arcs may be more beneficial than the use of a single, wider arc. This is in line with Seco et al,6 who indicated that the increase in the number of fields to make a step-and-shoot plan decreases the CI. However, the use of narrower arcs to use lower energies must be balanced with the possible loss of conformity.
Robustness of PAT vs IMPT
A concern when using PAT (specially in its single energy version) is that the effect of the range uncertainty at every angle of the arc may induce hot or cold spots not accounted for and that they may be different from fraction to fraction. A working hypothesis would be that those hot or cold spots should be minimal because there is a counterbalancing effect on the uncertainty in between angles. Different authors have addressed the issues of range uncertainty and robustness in PAT. Seco et al6 reported that, based on the larger degrees of freedom, proton arc plans take advantage of the rotating beams to spread out the proton range uncertainty on the distal and proximal sides of the target. Similarly, Ding et al8,9 provide proof for this to be the case in complex patient geometries. Interestingly, single-energy layers are used to treat from a substantial number of angles of the lung case they show, meaning that the energy restriction, does not necessarily translate into loss of robustness. This might not necessarily be the case in other sites such as head and neck, where multiple layers per angle seem to be required to achieve dosimetric objectives and constrains. There is also the possibility of using distal gradient tracking,11 to control the steepness of the dose gradients to conform the target. To this date, no systematic study has been conducted with a sufficiently large patient pool to proof the statistical significance of the hypothesis stated above.
The consequences inferred from the above three sections are:
No strong evidence showing that PAT improves conformity or uniformity relative to IMPT.
Evidence that the normal tissue IBD is reduced with PAT.
Evidence that PAT may produce robust proton plans
Radiobiological aspects of PAT
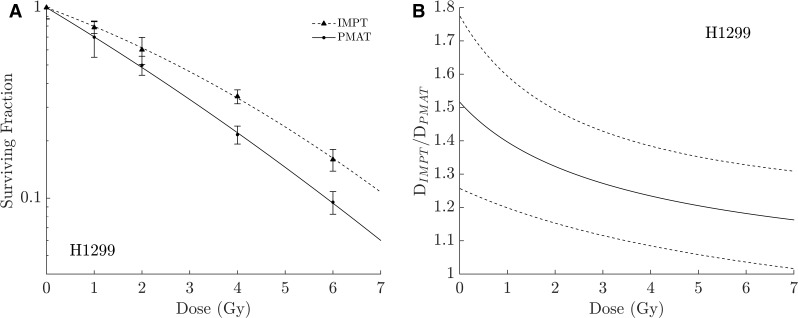
The main physical advantage of PAT resides in the reduction of the dose to organs at risk while keeping comparable conformity and uniformity, i.e. an improved therapeutic index (TI) is expected. But does this increase of the TI sufficiently justify the use of PAT? Could we further increase the TI with PAT by, not only reducing the normal tissue complication probability but simultaneously increasing the tumor control probability? This can be achieved by considering the radiobiological benefits accruing from an improved radiation quality associated with PAT. Fager et al,10 showed how increasing the number of IMPT fields can increase the linear energy transfer (LET) in the target without compromising the conformity or uniformity of the plan. This was achieved by using up to seven IMPT fields, with each field irradiating only the proximal segment of the target and stopping around its center, leading to a 12% decrease in dose whilst maintaining the same expected clinical outcome as the full target plans. As an evolution of this work, the use of monoenergetic PAT7 was proposed to allow simultaneous dose- and LET-painting of the target. In that proposed solution, the target is continuously irradiated with a rotating monoenergetic beam with all Bragg peaks sited within the target. Monoenergetic PAT has been previously proposed by different authors11,12 but none considered taking advantage of the increased LET values of proton beams for dose optimization purposes. Recently,16 RBE differences were examined when H1299 cells were exposed to the same dose delivered by IMPT or a monoenergetic PAT using a cylindrical solid water phantom exposed to 180° step-and-shoot PAT with 13 fields separated every 15° vs 3-field IMPT. Figure 1 shows how for these cells the monoenergetic PAT plan produces an RBE improvement of around 26% at typical clinical fraction sizes, explained by the fact that DNA damage from PAT is more complex and slower to repair than that from IMPT.16
Figure 1.
Left, Clonogenic assay showing a larger radiobiological effectiveness of PAT vs IMPT for H1299 cells. Right, relative dose of IMPT required to induce the same biological effect as PAT obtained from the clonogenic plots. Dashed lines represent the calculated 95% CI for this quantity. CI, confidence interval; IMPT, intensity modulated proton therapy; PAT, proton arc therapy.
The RBE improvement mentioned above is only for the specified cell line, and this cell line cannot be considered as representative to justify the potential radiobiological advantage of PAT vs IMPT. This advantage can only be justified based on in-vivo assays, and the example presented is shown to justify the need for further study. Therefore, in response to the questions above, this increase of effectiveness with PAT can be used to increase the TI in two different ways: by increasing the tumor control probability whilst maintaining the same fraction size as used with IMPT; alternatively, the fraction size could be reduced to match the effectiveness of the IMPT plan, which would imply a further reduction in the normal tissue complication probability. If an improved effectiveness of PAT is demonstrated clinically, it would represent a real opportunity to further expand the therapeutic opportunities offered by protons, which are currently somewhat limited.17
Technological challenges associated with the implementation of proton arc therapy
Proton arc dose calculation algorithms should incorporate the effects of rotation in their final solution. This rotational effect will be most noticeable at low dose rates, for large MU spots, fast rotational speeds or large field dimensions. In the simplest case of single layer control points, if any or a combination of these possibilities occur, the continuous motion of the gantry means that the spots contained in that layer are grouped in different gantry angles, effectively splitting that layer in sublayers throughout the delivery of the control point. The accurate incorporation of these effects into the calculation algorithm will be one of the elements required to ensure the agreement between calculated and delivered. A fast energy switching system will be essential for a fast delivery and to be able to use the largest portions of the arc to avoid a multiplicity of arcs to achieve conformity. Also, a small tolerance of the time description of the beam delivery system (hardware) will be required, which imply a much higher stability of beam current and beam positioning than currently offered. Ultimately, the speed of delivery of PAT will depend on the strategy of such delivery, which ultimately should be favored by the use of single energy beams15 and spot reduction schemes.18 Both of these schemes would in turn help with the radiobiological optimization mentioned above.
Conclusion
Potential clinical indications for PAT
While the dosimetric aspects of PAT require more investigation, this brief review does suggest a potential advantage of PAT over IMPT and which makes use of the proportionately higher LET that could be delivered to the target. From the above, the best current indication for PAT appears to be brain tumors, which normally require short proton ranges and with the symmetry of the head providing a greater region over which to arc the beam. This would allow use of single arcs in place of coplanar arcs, overall allowing the use of less degraded beams that would have the best spot characteristics. Less degraded beams additionally imply better beam quality (i.e. higher LETs than beams of longer range). Therefore, and despite being a seemingly retrograde application of proton therapy, the potential radiobiological advantages of PAT imply that more attention could be fruitfully devoted to researching this modality in more detail.
Footnotes
Funding: We acknowledge funding from Varian Medical Systems, Inc.
Contributor Information
Alejandro Carabe-Fernandez, Email: alexcarabe@gmail.com.
Alejandro Bertolet-Reina, Email: alejandro.bertolet@uphs.upenn.edu.
Ilias Karagounis, Email: iliask@mail.med.upenn.edu.
Kiet Huynh, Email: Huynh-kiet@cooperhealth.edu.
Roger G Dale, Email: r.dale@imperial.ac.uk.
REFERENCES
- 1.Paganetti H. Proton therapy physics. Series in Medical Physics and Biomedical Engineering 2012. [Google Scholar]
- 2.Lomax AJ. Intensity modulated proton therapy and its sensitivity to treatment uncertainties 1: the potential effects of calculational uncertainties. Phys Med Biol 2008; 53: 1027–42. doi: 10.1088/0031-9155/53/4/014 [DOI] [PubMed] [Google Scholar]
- 3.Paganetti H. Relative biological effectiveness (RBE) values for proton beam therapy. variations as a function of biological endpoint, dose, and linear energy transfer. Phys Med Biol 2014; 59: R419–72. doi: 10.1088/0031-9155/59/22/R419 [DOI] [PubMed] [Google Scholar]
- 4.Rah J-E, Kim G-Y, Oh DH, Kim TH, Kim JW, Kim DY, et al. A treatment planning study of proton Arc therapy for para-aortic lymph node tumors: dosimetric evaluation of conventional proton therapy, proton Arc therapy, and intensity modulated radiotherapy. Radiation Oncology 2016; 11: 1–10. doi: 10.1186/s13014-016-0717-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sandison GA, Papiez E, Bloch C, Morphis J. Phantom assessment of lung dose from proton Arc therapy. Int J Radiat Oncol Biol Phys 1997; 38: 891–7. doi: 10.1016/S0360-3016(97)00059-X [DOI] [PubMed] [Google Scholar]
- 6.Seco J, Gu G, Marcelos T, Kooy H, Willers H. Proton Arc reduces range uncertainty effects and improves conformality compared with photon volumetric modulated Arc therapy in stereotactic body radiation therapy for non-small cell lung cancer. Int J Radiat Oncol Biol Phys 2013; 87: 188–94. doi: 10.1016/j.ijrobp.2013.04.048 [DOI] [PubMed] [Google Scholar]
- 7.Carabe A. Development and delivery of biologically optimized treatment plans in proton radiotherapy. AAPM Annual Meeting 2014. [Google Scholar]
- 8.Ding X, Li X, Qin A, Zhou J, Yan D, Stevens C, et al. Have we reached proton beam therapy dosimetric limitations? – a novel robust, delivery-efficient and continuous spot-scanning proton Arc (SPARC) therapy is to improve the dosimetric outcome in treating prostate cancer. Acta Oncol 2018; 57: 435–7. doi: 10.1080/0284186X.2017.1358463 [DOI] [PubMed] [Google Scholar]
- 9.Ding X, Li X, Zhang JM, Kabolizadeh P, Stevens C, Yan D. Spot-Scanning proton Arc (SPARC) therapy: the first robust and Delivery-Efficient Spot-Scanning proton Arc therapy. Int J Radiat Oncol Biol Phys 2016; 96: 1107–16. doi: 10.1016/j.ijrobp.2016.08.049 [DOI] [PubMed] [Google Scholar]
- 10.Fager M, Toma-Dasu I, Kirk M, Dolney D, Diffenderfer ES, Vapiwala N, et al. Linear energy transfer painting with proton therapy: a means of reducing radiation doses with equivalent clinical effectiveness. Int J Radiat Oncol Biol Phys 2015; 91: 1057–64. doi: 10.1016/j.ijrobp.2014.12.049 [DOI] [PubMed] [Google Scholar]
- 11.Flynn RT, Barbee DL, Mackie TR, Jeraj R. Comparison of intensity modulated X-ray therapy and intensity modulated proton therapy for selective subvolume boosting: a phantom study. Phys Med Biol 2007; 52: 6073–91. doi: 10.1088/0031-9155/52/20/001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oelfke U, Bortfeld T. Intensity modulated radiotherapy with charged particle beams: studies of inverse treatment planning for rotation therapy. Med Phys 2000; 27: 1246–57. doi: 10.1118/1.599002 [DOI] [PubMed] [Google Scholar]
- 13.Sengbusch E, Pérez-Andújar A, DeLuca PM, Mackie TR. Maximum proton kinetic energy and patient-generated neutron fluence considerations in proton beam Arc delivery radiation therapy. Med Phys 2009; 36: 364–72. doi: 10.1118/1.3049787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shaw E, Kline R, Gillin M, Souhami L, Hirschfeld A, Dinapoli R, et al. Radiation therapy Oncology group: radiosurgery quality assurance guidelines. Int J Radiat Oncol Biol Phys 1993; 27: 1231–9. doi: 10.1016/0360-3016(93)90548-A [DOI] [PubMed] [Google Scholar]
- 15.Sanchez-Parcerisa D, Kirk M, Fager M, Burgdorf B, Stowe M, Solberg T, et al. Range optimization for mono- and bi-energetic proton modulated Arc therapy with pencil beam scanning. Phys Med Biol 2016; 61: N565–74. doi: 10.1088/0031-9155/61/21/N565 [DOI] [PubMed] [Google Scholar]
- 16.Carabe A, Karagounis IV, Huynh K, Bertolet A, Maity A, Dale RG. Radiobiological effectiveness difference of proton Arc beams versus conventional proton and photon beams. Physics in Medicine and Biology. (Submitted). [DOI] [PubMed] [Google Scholar]
- 17.ASTRO model policies. Proton beam therapy (PBT). 2014HP coding guidelines; ASTRO Model Policies. 2017. Available from: https://www.astro.org/uploadedFiles/Main_Site/Practice_Management/Reimbursement/AST RO%20PBT%20Model%20Policy%20FINAL.pdf.
- 18.van de Water S, Safai S, Schippers JM, Weber DC, Lomax AJ. Towards flash proton therapy: the impact of treatment planning and machine characteristics on achievable dose rates. Acta Oncol 2019; 58: 1463–9. doi: 10.1080/0284186X.2019.1627416 [DOI] [PubMed] [Google Scholar]