Abstract
Objective:
The Pediatric Proton/Photon Consortium Registry (PPCR) is a comprehensive data registry composed of pediatric patients treated with radiation. It was established to expedite outcomes-based research. The attributes which allow the PPCR to be a successful collaboration are reviewed.
Methods and materials:
Current eligibility criteria are radiotherapy patients < 22 years treated at one of the 15 US participating institutions. Detailed health and treatment data are collected about the disease presentation and treatment exposures, and annually thereafter, in REDCap (Research Electronic Data Capture). DICOM (Digital Imaging and Communications in Medicine) imaging and radiation plans are collected through MIM/MIMcloud. An optional patient-reported quality-of-life (PedsQL) study is administered at 10 sites.
Results:
Accrual started October 2012 with 2,775 participants enrolled as of 25 July 2019. Most patients, 62.0%, were treated for central nervous system (CNS) tumors, the most common of which are medulloblastoma (n = 349), ependymoma (n = 309), and glial/astrocytoma tumors (n = 279). The most common non-CNS diagnoses are rhabdomyosarcoma (n = 284), Ewing’s sarcoma (n = 153), and neuroblastoma (n = 130). While the majority of participants are US residents, 18.7% come from 36 other countries. Over 685 patients participate in the PedsQL study.
Conclusions:
The PPCR is a valuable research platform capable of answering countless research questions that will ultimately improve patient care. Centers outside of the USA are invited to participate directly or may engage with the PPCR to align data collection strategies to facilitate large-scale international research.
Advances in knowledge:
For investigators looking to carry out research in a large pediatric oncology cohort or interested in registry work, this paper provides an updated overview of the PPCR.
Introduction and objectives
The Pediatric Proton/Photon Consortium Registry (PPCR) is a consented registry currently comprised of 15 institutions that was established to expedite radiation outcomes-based research in pediatric oncology patients. It was established as a proton-specific registry, but it became apparent that including all radiation modalities would broadly enhance the scope of research possible. Therefore, in 2018, the PPCR protocol was amended to include photon patients and other forms of ionizing radiotherapy, such as particle therapies, permutations on photon-based treatments, and FLASH radiotherapy.1,2
Pediatric cancers are heterogeneous and comprise only 1% of all cancers, limiting the ability of single-institutions to amass clinical data and readily generate empirical results. Established cooperative groups, such as the Children’s Oncology Group or the International Society of Pediatric Oncology, are focused on addressing specific disease control-related questions often related to pharmacologic strategies and are limited in their ability to answer specific radiation treatment-related questions. Access to cooperative group data is resource-limited, even for members, and secondary analysis requests are often not readily accommodated. Furthermore, radiation plan access may be limited because the archival process can be incomplete or captured in paper format, which limits further analysis. Large cohort studies, such as the Childhood Cancer Survivor Study, address the question on how radiation contributes to late effects, but in radiation modalities that are no longer representative of modern radiation capabilities.3 The modern era of radiotherapy using intensity modulation or particle therapy holds much greater promise in reducing the late-effects typically seen.
The PPCR is the most comprehensive multi-institutional radiation-based pediatric patient registry in existence and is unique in its scope and depth.4 This data set brings the Quantitative Analysis of Normal Tissue Effects in the Clinic-like efforts in pediatrics to a much more robust level. Pediatric Normal Tissues Effects in the Clinic (PENTEC) is an ongoing effort to correlate dose to specific normal structures with late-effect outcomes in the pediatric population and establish quantitative evidence-based dose/volume/risk guidelines to inform radiation treatment planning.5 The PENTEC collaborative effort has found that previously published data is often heterogeneously reported or not specific enough to make dosimetric correlations. Through the collection of 3D treatment plans and late-effects data, the PPCR has the capability to be queried specifically for this purpose, which will further improve upon the PENTEC process and ultimately improve outcomes in pediatric cancer survivors.5
Radiation is a leading cause of late-effects in pediatric cancer survivors, and prior to the formation of the PPCR, there was no large-scale collaborative effort able to study the effects of proton therapy correlated with dosimetry.6 The PPCR puts radiotherapy in the greater context of clinical care through its extensive collection of data inclusive of chemotherapy exposure and surgery, and its capture of patient reported outcomes (PROs). Examination of radiotherapy and chemotherapy sequencing, chemotherapy effect on radiation tolerance, and in-depth dosimetric analyses in accordance with outcomes are just a few examples of topics that can be addressed leveraging the PPCR. With broad eligibility criteria, this collaborative multi-institutional effort can provide the sample sizes necessary to elucidate the impact of radiotherapy treatment on long-term outcomes in heterogeneously treated patients and capture the rarer tumors not studied in cooperative group settings. In the era of escalating healthcare expenditures, it has become critical for health insurers and governments to evaluate new technologies to decide whether to incorporate them into the healthcare armamentarium, and we have seen very promising radiotherapy technologies restricted due to a lack of data proving its benefits.7–9
Here, the consortium, its operations, recent changes, and an overview of the current PPCR cohort are reviewed. In addition to future directions, we also discuss characteristics of the Registry that have allowed it to flourish. It is our hope that data collection on pediatric oncology patients can be aligned internationally so we can learn maximally from every child we treat and accelerate progress in this most deserving patient population, and that this paper serves as a guide for other investigators with similar goals.
Methods and materials
Registry overview
The PPCR (formerly the Pediatric Proton Consortium Registry) was established in 2010 and began enrolling its first patient in October 2012.10,11 In October 2018, the Registry expanded to include all radiation-treated patients to enhance research capabilities and facilitate comparative effectiveness analysis. The PPCR coordinating center resides at Massachusetts General Hospital (MGH, Boston, MA, USA) and is comprised of five core individuals: principal investigator, project manager, biostatistician, and two clinical research coordinators. Table 1 lists the participating institutions with enrollment start date and participant total. Another three proton centers and four photon centers are in the process of joining. The PPCR is currently jointly funded by the NCI/MGH Federal Share of Proton Income research fund.
Table 1. .
Participant accrual by institution and date of first enrollment.
| Institution | Date of First Enrollment | Patients Enrolled |
|---|---|---|
| Massachusetts General Hospitala (Boston, MA) | Oct 2012 | 680 |
| Northwestern Medicine Chicago Proton Center (Chicago, IL) | Sep 2013 | 289 |
| University of Florida Health Proton Therapy Institute (Jacksonville, FL) | Nov 2013 | 563 |
| Washington University (St. Louis, MO) | Mar 2014 | 104 |
| MD Anderson Cancer Center (Houston, TX) | Jun 2014 | 427 |
| University of Pennsylvaniaa (Philadelphia, PA) | Jun 2014 | 88 |
| University of Washington (Seattle, WA) | Feb 2016 | 125 |
| ProCure Proton Therapy Centera (Somerset, NJ) | Jun 2016 | 84 |
| Mayo Clinica (Rochester, MN) | Jul 2016 | 128 |
| Oklahoma Proton Therapy Centera (Oklahoma City, OK) | Oct 2016 | 23 |
| Texas Center for Proton Therapya (Irving, TX) | Nov 2016 | 90 |
| Maryland Proton Therapy Centera (Baltimore, MD) | Apr 2017 | 55 |
| Cincinnati Children’s Hospital Medical Centera (Cincinnati, OH) | Oct 2017 | 114 |
| Provision Center for Proton Therapya (Knoxville, TN) | Mar 2019 | 5 |
| California Protons Cancer Therapy Centera (San Diego, CA) | Jul 2019b | 0b |
| TOTAL | 2775 |
Participating in optional patient-reported quality-of-life (QoL) study
Date site was first approved to enroll participants. No participants accrued at time of data analysis.
The primary objective of the PPCR is to accelerate radiation outcomes-based research to inform clinicians of the most promising and least toxic forms of treatment. Its secondary objective is to identify important trends in the administration and availability of these newer and promising treatments by tracking referral patterns and barriers to treatment such as lack of insurance coverage.
Enrollment/Eligibility
The PPCR is a consented registry. Any patient who receives radiation at a participating PPCR institution under 22 years of age at the time of radiotherapy start is eligible for enrollment and must consent to participate. Most patients are enrolled at some point during their primary treatment, although prior radiation treatment does not exclude them from being eligible. In October of 2018, we added the ability to consent patients who are seen in follow-up, and these patients comprise a separate retrospective cohort. This retrospective cohort will allow us to prospectively follow patients who were treated prior to the opening of the Registry at their institution, which will enhance the ability to describe late-effects and complications of treatment. Patients are eligible regardless of treatment modality, treatment time, tumor type, or concurrent treatment(s). Presently, each site follows their local institutional policies regarding consent/assent, including re-consenting participants when they reach the age of majority in the USA (18 years of age).12,13 PPCR qualifies as a minimal risk protocol which allows physicians, nurses, and clinical research coordinators to approach, consent, and enroll patients to optimize accrual. For eligible patients who do not consent, limited, non-identifying demographics and diagnosis information is collected to better understand if there is a bias as to who is not consenting.
Data infrastructure
Data are collected and managed in two Health Insurance Portability and Accountability Act (HIPAA)14-compliant internet-based electronic data capture (EDC) platforms: REDCap and MIM.15,16
REDCap
Treatment, diagnostic, clinical information, and PROs are collected and managed using REDCap.15,16 REDCap is an internationally available, secure, no-cost, web-based software platform for building and managing data capture and online surveys that is supported by the US National Institutes of Health (NIH). REDCap is compliant with health information privacy laws (including 21 CFR Part 11, FISMA, and HIPAA).14 The REDCap platform provides (1) an interface for validated data capture; (2) audit trails for tracking data manipulation and export procedures; and (3) automated export procedures for seamless data downloads. The PPCR clinical data is collected at baseline (pre-treatment), treatment, and follow-up timepoints (Table 2). Data are collected utilizing branching logic, such that only fields relevant to the previous answers are presented. Data may be extracted by manual download or in an automated fashion using built-in REDCap reporting features.
Table 2. .
Clinical data collected.
| Tests and Procedures | Pre-Treatment | Treatment | End of Treatment | Follow-up |
|---|---|---|---|---|
| Consent and Registration | X | |||
| Demographics | X | |||
| Primary Diagnosis | X | |||
| Baseline Health Inventory | X | |||
| Imaging | X | X | X | |
| Tumor-related Surgery | X | X | X (if applicable) | |
| Radiation | X | X | X (if applicable) | |
| Radiation Toxicity | X | X | ||
| Chemotherapy and Protocols | X | X (if applicable) | ||
| Disease Status | X | |||
| PedsQL Questionnaires | X | X | X | |
| Health Status Survey | X (MGH only) |
Baseline Health Status, Health deficits, medications, and specific testing such as neurocognitive, endocrine, and hearing. Symptoms are again captured at the end of treatment; Chemotherapy and Surgery, Tumor-related surgery, plus drug exposures and any applicable COG protocol information; Demographics, Contact information, insurance details, referring physicians/institution; Diagnosis and Staging, Details of diagnosis, relevant tumor markers, and cancer predisposition syndromes; Diagnostic Imaging, Imaging related to diagnosis/staging, any metastatic sites, and monitoring of pertinent sites at follow-up; Follow-up, Clinical/vital status, development of other malignancies, side effects/health events, education information, medications, and site-specific testing; PedsQL, Patient reported quality-of-life (QoL) questionnaire administered during first and last week of RT and then annually; Radiation Details, Dates and dosing details of radiation treatments (prior, current when enrolled on trial, and future, if applicable), and the current radiation plan is centrally archived.
MIM
De-identified radiation treatment plan and imaging studies are transmitted for storage through MIM with automation of data collection for dose-volume histogram (DVH) data for the target and normal structures. The PPCR utilizes MIM Software Inc.’s MIMcloud: a secure, internet-based DICOM and other file transmitting service.17 All files uploaded through MIMcloud are then stored on a server housed at and maintained by MGH. Final treatment plan files submitted are the composite treatment plan, a summation of the various phases of treatment, planning CT scan, target and normal contours, and dosimetry files. Treatment and follow-up DICOM imaging studies are also uploaded.
Accessing data
Consortium participants have the benefit of a platform for communication and quality-related evaluation of practices, as well as access to their own data for administrative purposes and to measure trends in patient referrals and care. Through REDCap tools and access rights, every institution has direct access to view, download, analyze, and report on their own patients without oversight from the PPCR coordinating center personnel. Tailored data exports of multi-institutional data are available to consortium members and outside investigators through a PPCR-specific “Request for Data” (RFD) form in REDCap. The form is processed by the data management team at MGH who queries the principal investigators at each site to determine whether they would like to opt in or out of the proposed study. Data is extracted from REDCap for those institutions who opted into the study, de-identified, and then distributed to the requesting investigator for analysis. This process typically takes 15–30 days from the original request.
Data monitoring
Monthly reports are run in REDCap to monitor validity of information and to generate Missing Fields Reports (MFRs) and Data Quality Reports for each PPCR institution. MFRs ensure that, at minimum, a core list of required critical fields have been filled in. Critical fields are a subset of the data fields judged to be the most important to understanding the patient’s diagnosis, treatment, and follow-up. It consists of approximately 38 fields (Supplemental appendix 1) and takes 10–15 min of data entry time per patient. Missing data is routinely filled in and outliers are re-reviewed within the medical record and addressed to ensure the data is correct and up-to-date at all participating sites.
Patient reported outcomes
In September 2015, the PPCR introduced the PedsQL PRO survey, which is an age-appropriate previously validated measure of health-related QoL (HRQoL) which accommodates both child and parent reporting.18–20 Ten of the 15 PPCR member institutions have implemented this optional study component. The PedsQL Core Module generates multiple, different summary, and subscores including total, physical, and psychosocial. Surveys are administered twice during radiation treatment and annually thereafter. REDCap’s functionality affords iPad or mobile phone use for patient interface, as well as emailing out a secure link to the survey in follow-up.
In addition to PedsQL administration, we are piloting a patient/parent-proxy reported health status survey at one of our PPCR sites to help alleviate the burden of follow-up data collection. Many radiation treatment centers are usually quaternary referral centers, and patients receive follow-up care back at their referring institution.13 Follow-up with the radiation treatment team can be highly variable, yet these long-term outcomes are of critical importance to evaluate the safety, efficacy, and toxicities of radiation treatment. The survey is designed to be completed in under 5 min on any electronic device. The survey questions include verifying or updating contact information, disease and health status, recent medical care, and social information including education or employment status. A survey non-response or an affirmative response to any health question that indicates a change in health status (i.e., new diagnosis or new medication) triggers an in-depth review of the patients’ medical records and soliciting of information from the referring hospital if they are not followed locally.
Results
As of 25 July 2019, 2775 participants have been enrolled. Overall accrual by institution is shown in Table 1 and Figure 1. Most enrolled patients comprise our prospective cohort (2715/2775, 97.8%). The PPCR cohort is 44.0% female and 69.7% Caucasian. Median age at the start of radiotherapy is 9.6 years (range:<1–21.9). While most patients treated at the PPCR sites are from the USA (Figure 2), 18.7% come from other countries. The most common countries that refer patients for treatment in the USA are Canada, England, Denmark, and Australia, but over 36 countries around the globe are represented (Figure 3). Demographic information is detailed in Table 3. CNS diagnoses comprise 62.0% (n = 1,682) of the cohort. The most common CNS diagnoses are medulloblastoma, ependymoma, and glial/astrocytoma tumors. The most common non-CNS diagnoses in the remaining 38% (n = 1,038) are rhabdomyosarcoma, Ewing’s sarcoma, neuroblastoma, and Hodgkin’s lymphoma. Primary diagnoses by CNS and non-CNS tumor type are illustrated by Figures 4 and 5, respectively. Details about radiation treatment are shown in Table 4. A total of 1194 plans have been sent to centralized storage on the PPCR MIM server.
Figure 1. .
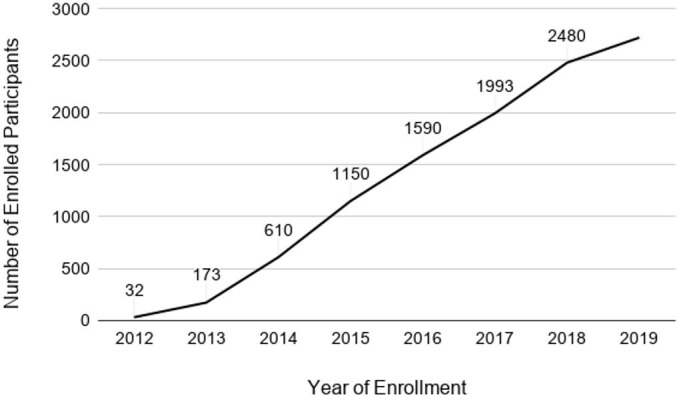
Total participant enrollment by year (n = 2775).
Figure 2. .
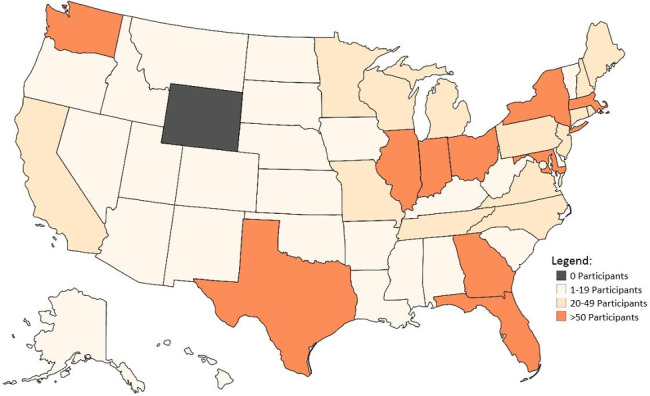
Participant residency by state in the USA.
Figure 3. .
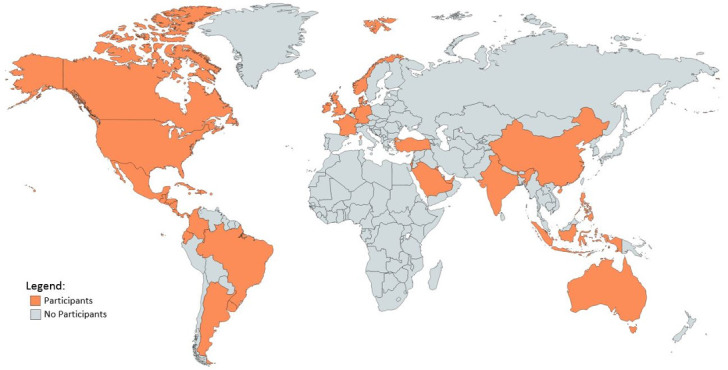
Participant residency by country.
Table 3. .
Participant demographics.
| All Patients n = 2720 (%)a | CNS n = 1686 (%)a | Non-CNS n = 1034 (%)a | |
|---|---|---|---|
| Age at RT Start | |||
| Median (range) in years | 9.6 (<1–21.9) | 9.4 (<1–21.9) | 10.0 (<1–21.9) |
| <5 Years | 586 (21.5) | 331 (19.6) | 255 (24.7) |
| >5 Years | 1704 (62.6) | 1085 (64.4) | 619 (59.9) |
| Gender | |||
| Male | 1524 (56.0) | 985 (58.4) | 539 (52.1) |
| Female | 1183 (43.5) | 696 (41.3) | 487 (47.1) |
| Race | |||
| White or Caucasian | 1897 (69.7) | 1182 (70.1) | 715 (69.1) |
| Black or African American | 187 (6.9) | 118 (7.0) | 69 (6.7) |
| Asian | 124 (4.6) | 83 (4.9) | 41 (4.0) |
| Arab/Middle Eastern | 31 (1.1) | 21 (1.3) | 10 (1.0) |
| Native American/Alaska Native | 10 (0.4) | 7 (0.4) | 3 (0.3) |
| Native Hawaiian or Other Pacific Islander | 9 (0.3) | 7 (0.4) | 2 (0.2) |
| Unknown or not reported | 295 (10.8) | 173 (10.3) | 122 (11.8) |
| Other | 132 (4.9) | 84 (5.0) | 48 (4.6) |
| Ethnicity | |||
| Hispanic or Latino | 291 (10.7) | 190 (11.3) | 101 (9.8) |
| Not Hispanic or Latino | 1898 (69.8) | 1183 (70.2) | 715 (69.1) |
| Unknown or not reported | 474 (17.4) | 281 (16.7) | 193 (18.7) |
| Residence | |||
| United States | 1892 (69.6) | 1201 (71.2) | 691 (66.9) |
| International | 507 (18.7) | 316 (18.7) | 191 (18.5) |
| Insurance | |||
| Covered | 1863 (68.5) | 1181 (70.0) | 682 (70.0) |
| Partial Coverage | 15 (<1) | 9 (<1) | 6 (<1) |
| Coverage Amount Unknown | 291 (10.7) | 167 (9.9) | 124 (12.0) |
| None | 67 (2.5) | 49 (2.9) | 18 (1.7) |
CNS, central nervous system; RT, radiation treatment.
Totals may not sum to 100% of cohort because of rounding, missing data fields, or data may not be inputted into the registry. A total of 2720 patients were enrolled at time of analysis. Unavailable data: Age 430/2720 (15.8%), gender 13/2720 (0.5%), race 79/2720 (2.9%), ethnicity 57/2720 (2.1%), insurance 484/2720 (17.8%), and residence 115/2720 (4.2%). Information on residency ineligible to be collected on 205 (7.5%) patients due to institutional IRB restrictions. Totals and percentages of race may exceed 100 due to multiple responses per patient.
Figure 4. .
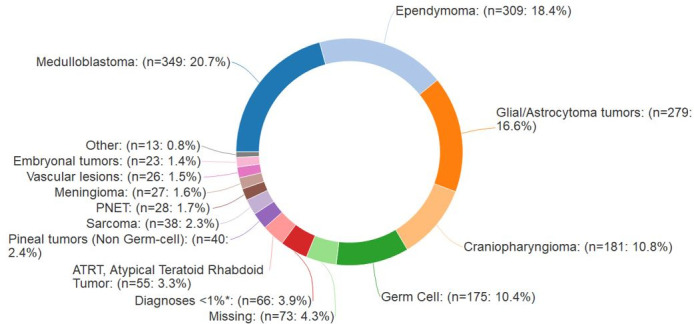
Intracranial and CNS diagnoses, n = 1682. CNS, central nervous system; NOC, not otherwise classified; PNET, primitive neuroectodermal tumor. *Diagnoses that individually compose <1% include: Neuroepithelial tumors, NOC (n = 10), Nerve sheath tumors (n = 14), Pituitary adenomas (n = 14), Choroid plexus tumors (n = 12), DNET (Dysembroyplastic neuroepithelial tumor) (n = 6), Neurocytoma (n = 5), Leukemia (n = 3), and Langerhan's histiocytosis (n = 2).
Figure 5. .
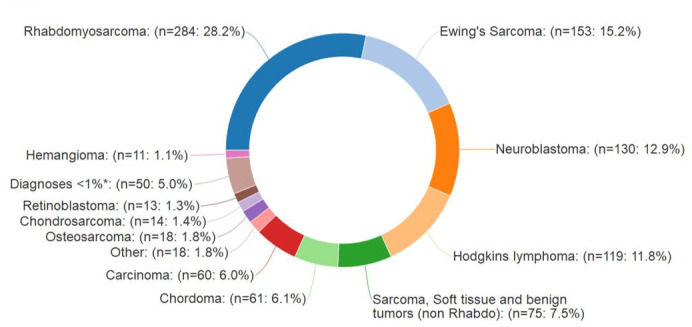
Non-CNS diagnoses, n = 1038. Non-CNS, non-central nervous system. *Diagnoses that individually compose <1% include: Ethesioneuroblastoma (n = 9), Non-Hodgkin's lymphoma (n = 9), Melanoma (n = 6), Germ cell (n = 5), Leukemia (n = 3), Paraganglioma/Pheochromocytoma (n = 4), Langerhan's Histiocytosis (n = 2), Pleomorphic adenoma (n = 2), Plasmacytoma (n = 1), and Giant cell tumor (n = 1).
Table 4. .
Radiation treatment details.
| Total n = 2290 (%)a | CNS n = 1416 (%)a | Non-CNS n = 874 (%)a | |
|---|---|---|---|
| RT Dose (Gy) median (range) | 54 (1.8–78) | 54 (6.3–72.1) | 50.4 (1.8–78) |
| Intent of RT | |||
| Curative | 2258 (98.6) | 1399 (98.8) | 859 (97.3) |
| Palliative | 30 (1.3) | 16 (1.1) | 14 (1.6) |
| Anesthesia During RT | |||
| Yes | 890 (38.9) | 569 (40.2) | 321 (36.7) |
| No | 1392 (60.8) | 840 (59.3) | 552 (63.2) |
| Source/Technique Received | |||
| Protons | 2271 (99.2) | 1405 (99.2) | 866 (99.1) |
| Photons | 154 (6.7) | 68 (4.8) | 86 (9.8) |
| Electrons | 6 (<1) | 3 (<1) | 3 (<1) |
| Mixed (Protons/Photons) | 141 (6.2) | 61 (4.3) | 80 (9.2) |
| Anatomical Region Treated | |||
| Craniospinal Irradiation (CSI) | 474 (20.7) | 457 (32.3) | 17 (2.0) |
| Whole Brain | 39 (1.7) | 37 (2.6) | 2 (<1) |
| Whole Ventricle | 121 (5.3) | 121 (8.6) | 0 (0) |
| Whole Posterior Fossa (PF) | 170 (7.4) | 169 (11.9) | 1 (<1) |
| Involved Field: Supratentorial | 692 (30.2) | 669 (47.3) | 23 (2.6) |
| Involved Field: Infratentorial | 261 (11.4) | 255 (18.0) | 6 (<1) |
| Involved Field: Spinal Region | 223 (9.7) | 142 (10.0) | 81 (9.3) |
| Whole Abdomen | 45 (2.0) | 0 (0) | 45 (5.2) |
| Whole Lung | 10 (<1) | 0 (0) | 10 (1.1) |
| Involved Field: Head and Neck | 472 (20.6) | 72 (5.1) | 400 (45.8) |
| Involved Field: Abdomen/Pelvis | 304 (13.3) | 40 (2.8) | 264 (30.2) |
| Involved Field: Thoracic | 171 (7.5) | 3 (<1) | 168 (19.2) |
| Involved Field: Extremity | 55 (2.4) | 2 (<1) | 53 (6.1) |
CNS, central nervous system; CSI, craniospinal irradiation; IF, involved field; IMPT, intensity modulated proton therapy; PF, posterior fossa; RT, radiation therapy.
Unavailable data: Anesthesia use 8/2290 (<1%).
Totals may not sum to 100% of the entire cohort due to rounding, missing data fields, or data not yet inputted into the registry. Totals and percentages of the source/technique received and area treated reflect occurrence within the registry and may exceed 100 due to multiple responses per patient.
At the time of this analysis, there were a total of 1,332 participants with a least one follow-up time point entered in the database. Of these, 94.9% (n = 1,265) were enrolled prior to June 2018 and due for annual follow-up. The median follow-up among these participants is 1.6 years (<1–16.2). At the time of analysis, 9.5% (n = 127) of participants died with 77 attributable to a primary tumor/malignancy and three to secondary tumors/malignancies. A total of 7.4% (n = 99) of participants have disease progression/recurrence/ transformation. Another 1.6% (n = 21) of participants have a new tumor identified. Detailed clinical and vital status at last follow-up is described in Table 5.
Table 5. .
Clinical and vital status at last follow-up.
| All patients n = 1332 (%)a | CNS n = 819 (%)a | Non-CNS n = 513 (%)a | |
|---|---|---|---|
| Vital Status at Latest Follow-up | |||
| NED/tumor controlled | 885 (66.4) | 550 (67.2) | 335 (65.3) |
| Alive with disease | 79 (5.9) | 50 (6.1) | 29 (5.7) |
| Disease progression/recurrence/ transformation | 99 (7.4) | 64 (7.8) | 35 (6.8) |
| Alive, disease status unknown | 76 (5.7) | 45 (5.5) | 31 (6.0) |
| Deceased | 106 (8.0) | 54 (6.6) | 52 (10.1) |
| New Tumor | |||
| Yes | 12 (<1) | 7 (<1) | 5 (<1) |
| No | 1320 (99.1) | 812 (99.1) | 508 (99.0) |
| Treatment Since Last Follow-Up | |||
| Yes | 212 (15.9) | 118 (14.4) | 94 (18.3) |
| No | 977 (73.3) | 618 (75.5) | 359 (70.0) |
| Unknown | 143 (10.7) | 83 (10.1) | 60 (11.7) |
| Imaging at Follow-Up | |||
| Yes | 1303 (97.8) | 800 (97.7) | 503 (98.1) |
| No | 5 (<1) | 3 (<1) | 2 (<1) |
| Missing | 24 (1.8) | 16 (2.0) | 8 (1.6) |
NED, No evidence of disease.
Total out of patients with at least one follow-up (n = 1332). Totals may not sum to 100% due to rounding, missing data fields, or data not yet inputted into the registry. Unavailable data: All patients 87/1332 (6.5%)
Discussion
The PPCR is a highly successful collaboration of investigators and is unique in the pediatric radiation oncology field. The PPCR provides a platform to learn from every patient and will help accelerate the pace of research on the costs and benefits of modern radiotherapy in the era of intensity modulated photon treatment and particle center treatment modalities. The heterogeneity of the patients enrolled and different practice patterns across the institutions can be evaluated for efficacy both in disease control and for other health outcome measures. This data set will be strengthened as other global radiation centers join our consortium or collect data in a similar manner that allows for high-powered, large scale studies with the potential to detect significant differences among treatment approaches. With over 2700 participants across the 15 participating institutions, the Registry is available as a resource for investigator-initiated research and for investigators wishing to partner with the PPCR to answer important questions in pediatric oncology.
Much of the success of the Registry to date is due to the commitment of the participating principal investigators and their departmental support. While no institution is currently funded to enroll patients and collect data, all are vested in the mission to learn from every patient and improve care and outcomes. Importantly, unlike other proton-based radiation oncology registries, there is no fee for members to join. The immediate benefit is members have instantaneous, direct access to their own institutional data, in addition to easy access to the larger multi-institutional data sets which can augment academic productivity and contribute meaningfully to the evolution of radiotherapy in the treatment of pediatric oncology patients.
Another success can be attributed to the use of a free, but robust database platform, REDCap, in which the US NIH has invested and continued to support. REDCap provides the PPCR management team direct unfettered access to its data and the freedom to manipulate and modify the database as medical disease specific paradigms evolve. New treatments and tumor biology can be and are regularly incorporated into the data collection platform. In contrast, database platforms managed by third party companies are unable to be nimbly adapted without undue cost. This has been a major obstacle for other radiation registries.
The Registry is partnered with the NCI, the MGH, as well as numerous industry sponsors which spreads out operations costs and brings together the appropriate people to govern it. MIM Software, Inc. offered support with their MIMcloud platform with an in-kind donation that allowed the collation and archiving of the radiation plans.17 This new system is highly efficient for the sites to use and allows DVH data to be exported and uploaded into the REDCap platform. The structure of the Registry is egalitarian with a steering committee comprised of the site investigators. Teleconference meetings are held quarterly, and in-person meetings are scheduled to coincide with major national or international meetings where pediatric radiation oncologists are likely to attend. Our external advisory board meets yearly and is comprised of four distinguished experts with leadership positions in cooperative groups, registries, and late-effects research.
The future direction of the PPCR includes potential expansion into other areas of promise, such as biological specimen banking to allow for interrogation of both tumor and normal tissue radiosensitivity.21,22 The PPCR can also be used for pairing with other efforts, such as radiomics. The NCI’s Radiation Epidemiology Branch plans a large-scale cohort study leveraging this data collection platform to evaluate secondary malignancies in both particle-related and photon-treated cohorts with data linkage to the Virtual Cancer Registries for second tumor and survival data. As the PPCR grows, we hope to attract more such partnerships which will help prove the value of this kind of dataset for years to come. We hope the PPCR will garner the interest of other investigators with similar goals of improving our cancer treatments and QoL of our childhood cancer survivors. We invite international partnerships in this pediatric oncology registry effort and look forward to accelerated learning on every one of our pediatric cancer patients.
Footnotes
Acknowledgements: The PPCR would like to thank MIM Software, Inc. for their gracious contributions to our research efforts.
Conflicts of Interest and Disclosures: Registry support received from the following companies: Ion Beam Applications (IBA, Neuve, Belgium), ProTom International, Inc (Flower Mound, Texas, USA), and Elekta (Stockholm, Stockholm County, Sweden).
Contributor Information
Miranda P. Lawell, Email: mlawell@mgh.harvard.edu.
Daniel J Indelicato, Email: dindelicato@floridaproton.org.
Arnold C Paulino, Email: APaulino@mdanderson.org.
William Hartsell, Email: William.Hartsell@nm.org.
Nadia N. Laack, Email: Laack.Nadia@mayo.edu.
Ralph P. Ermoian, Email: ralphpe@uw.edu.
John P. Perentesis, Email: John.Perentesis@cchmc.org.
Ralph Vatner, Email: vatnerrh@ucmail.uc.edu.
Stephanie Perkins, Email: sperkins@wustl.edu.
Victor S. Mangona, Email: Victor.Mangona@usoncology.com.
Christine E. Hill-Kayser, Email: hill@uphs.upenn.edu.
Suzanne L. Wolden, Email: woldens@MSKCC.ORG.
Young Kwok, Email: ykwok@umm.edu.
John Han-Chih Chang, Email: john.chang@okcproton.com.
J. Ben Wilkinson, Email: ben.wilkinson@provisionproton.com.
Iain MacEwan, Email: imacewan@ucsd.edu.
Andrew L. Chang, Email: andrewlchangmd@gmail.com.
Bree R. Eaton, Email: brupper@emory.edu.
Matthew M. Ladra, Email: mladra@jhmi.edu.
Sara L. Gallotto, Email: SGALLOTTO@mgh.harvard.edu.
Elizabeth A. Weyman, Email: eweyman@mgh.harvard.edu.
Benjamin V.M. Bajaj, Email: bbajaj@mgh.harvard.edu.
Beow Y. Yeap, Email: byeap@mgh.harvard.edu.
Amy Berrington de Gonzalez, Email: berringtona@mail.nih.gov.
Torunn I. Yock, Email: tyock@partners.org.
REFERENCES
- 1.Harrington KJ. Ultrahigh dose-rate radiotherapy: next steps for FLASH-RT. Clin Cancer Res 2019; 25: 3–5. doi: 10.1158/1078-0432.CCR-18-1796 [DOI] [PubMed] [Google Scholar]
- 2.Bourhis J, Montay-Gruel P, Gonçalves Jorge P, Bailat C, Petit B, Ollivier J, et al. Clinical translation of flash radiotherapy: why and how? Radiother Oncol 2019; 139: 11–17. doi: 10.1016/j.radonc.2019.04.008 [DOI] [PubMed] [Google Scholar]
- 3.Childhood Cancer Survivor Study: An Overview - National Cancer Institute 2007 [updated 01/10/2007 - 07:00. Available from: https://www.cancer.gov/types/childhood-cancers/ccss.
- 4.Berrington de Gonzalez A, Vikram B, Buchsbaum JC, de Vathaire F, Dörr W, Hass-Kogan D, et al. A clarion call for large-scale collaborative studies of pediatric proton therapy. Int J Radiat Oncol Biol Phys 2017; 98: 980–1. doi: 10.1016/j.ijrobp.2017.03.033 [DOI] [PubMed] [Google Scholar]
- 5.Constine LS, Ronckers CM, Hua C-H, Olch A, Kremer LCM, Jackson A, et al. Pediatric normal tissue effects in the clinic (PENTEC): an international collaboration to analyse normal tissue radiation Dose–Volume response relationships for paediatric cancer patients. Clin Oncol 2019; 31: 199–207. doi: 10.1016/j.clon.2019.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kasper HB, Raeke L, Indelicato DJ, et al. The pediatric proton Consortium registry: a multi-institutional collaboration in U. S. Proton Centers 2014;. [Google Scholar]
- 7.Yu NY, Sio TT, Mohindra P, Regine WF, Miller RC, Mahajan A, et al. The insurance approval process for proton beam therapy must change: prior authorization is crippling access to appropriate health care. Int J Radiat Oncol Biol Phys 2019; 104: 737–9. doi: 10.1016/j.ijrobp.2019.04.007 [DOI] [PubMed] [Google Scholar]
- 8.Ning MS, Gomez DR, Shah AK, Kim CR, Palmer MB, Thaker NG, et al. The insurance approval process for proton radiation therapy: a significant barrier to patient care. Int J Radiat Oncol Biol Phys 2019; 104: 724–33. doi: 10.1016/j.ijrobp.2018.12.019 [DOI] [PubMed] [Google Scholar]
- 9.Ojerholm E, Hill-Kayser CE. Insurance coverage decisions for pediatric proton therapy. Pediatr Blood Cancer 2018; 65: e26729. doi: 10.1002/pbc.26729 [DOI] [PubMed] [Google Scholar]
- 10.Yock T, Indelicato D, Hartsell W, Kasper HSH, Perkins S, Perkins S et al., (eds). Preliminary results from the pediatric proton consortium registry (PPCR): A collaboration of US proton centers to accelerate proton therapy research PEDIATRIC BLOOD & CANCER. NJ USA: WILEY-BLACKWELL 111 RIVER ST, HOBOKEN 07030-5774; 2014. [Google Scholar]
- 11.Hess CB, Indelicato DJ, Paulino AC, Hartsell WF, Hill-Kayser CE, Perkins SM, et al. An update from the pediatric proton Consortium registry. Front Oncol 2018; 8: 165. doi: 10.3389/fonc.2018.00165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baedorf Kassis S, Gallotto SL, Hess CB, Weyman E, Yock TI. Rethinking reconsent when minors reach adult age in minimal risk studies. Pediatr Blood Cancer 2018; 65: e26731. doi: 10.1002/pbc.26731 [DOI] [PubMed] [Google Scholar]
- 13.Lawell MP, Bajaj BVM, Gallotto SL, Hess CB, Patteson BE, Nartowicz JA, et al. Increased distance from a treating proton center is associated with diminished ability to follow patients enrolled on a multicenter radiation oncology registry. Radiother Oncol 2019; 134: 25–9. doi: 10.1016/j.radonc.2019.01.007 [DOI] [PubMed] [Google Scholar]
- 14.Health Insurance Portability and Accountability Act of 1996, Pub. L. No. 104-191 Stat. 1938 (2015-11-23, 2015).
- 15.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42: 377–81. doi: 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O'Neal L, et al. The REDCap Consortium: building an international community of software platform partners. J Biomed Inform 2019; 95: 103208. doi: 10.1016/j.jbi.2019.103208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.MIM Software [cited 1 July 2019]. Available from: https://www.mimsoftware.com/.
- 18.Varni JW, Burwinkle TM, Katz ER, Meeske K, Dickinson P. The PedsQL in pediatric cancer: reliability and validity of the pediatric quality of life inventory generic core scales, multidimensional fatigue scale, and cancer module. Cancer 2002; 94: 2090–106. doi: 10.1002/cncr.10428 [DOI] [PubMed] [Google Scholar]
- 19.Varni JW, Burwinkle TM, Seid M, Skarr D. The PedsQL 4.0 as a pediatric population health measure: feasibility, reliability, and validity. Ambul Pediatr 2003; 3: 329–41. doi: [DOI] [PubMed] [Google Scholar]
- 20.Varni JW, Limbers CA. The PedsQL multidimensional fatigue scale in young adults: feasibility, reliability and validity in a university student population. Qual Life Res 2008; 17: 105–14. doi: 10.1007/s11136-007-9282-5 [DOI] [PubMed] [Google Scholar]
- 21.Gillies RJ, Kinahan PE, Hricak H. Radiomics: images are more than pictures, they are data. Radiology 2016; 278: 563–77. doi: 10.1148/radiol.2015151169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hall WA, Bergom C, Thompson RF, Baschnagel AM, Vijayakumar S, Willers H, et al. Precision oncology and genomically guided radiation therapy: a report from the American Society for radiation Oncology/American association of physicists in Medicine/National cancer Institute precision medicine conference. Int J Radiat Oncol Biol Phys 2018; 101: 274–84. doi: 10.1016/j.ijrobp.2017.05.044 [DOI] [PubMed] [Google Scholar]


