Abstract
Re-irradiation can offer a potentially curative solution in case of progression after initial therapy; however, a second course of radiotherapy can be associated with an increased risk of severe side-effects. Particle therapy with protons and especially carbon ions spares surrounding tissue better than most photon techniques, thus it is of high potential for re-irradiation. Irradiation of tumors of the brain, head and neck and skull base involves several delicate risk organs, e.g. optic system, brainstem, salivary gland or swallowing muscles. Adequate local control rates with tolerable side-effects have been described for several tumors of these locations as meningioma, adenoid cystic carcinoma, chordoma or chondrosarcoma and head and neck tumors. High life time doses nonetheless lead to a different scope of side-effects, e.g. an enhanced rate of carotid blow outs has been reported. This review summarizes the current data on particle irradiation of the aforementioned locations and malignancies.
Introduction
In case of tumor relapse or progression of solid tumors after initial radiotherapy treatment options are surgery and re-irradiation as potentially curative local treatment or systemic therapy e.g. chemotherapy which is in many cases a palliative option. As the local treatment options are generally limited, there are only few situations where long-term control, tantamount to cure, can be achieved and in many cases the treatment intent is palliative. Due to this complexity, all treatment decisions have to be obtained interdisciplinary and should consider patients specific characteristics. Still, in selected cases, re-irradiation can be indicated and offers good chances to reach local control. The previously prescribed dose, the dose distribution and vicinity to surrounding organs at risk as well as the time between primary and re-irradiation frame the possibilities and limitations of re-irradiation. Performing re-irradiation, one has to keep dose to surrounding pre-irradiated tissue as low as possible, however the chances of local control are dose-dependent. Prescribing high doses is albeit delicate especially in the head and neck location because of the very common close location of sensitive organs at risk such as brain stem, spinal cord or optic nerves. Depending on the accuracy and precision of the available treatment technique, the risk of severe side-effects might be intolerably high. Thus, generally only high-precision techniques qualify for re-irradiation. The characteristics of particle therapy promise a superior risk-benefit-profile as they offer excellent sparing of the normal tissues outside the treatment target volume. This review summarizes the data on re-irradiation with protons and carbon ions in the most delicate locations of the brain, head and neck and skull base (Tables 1 and 2). Proton re-irradiation has been previously reviewed by others.10
Table 1.
Treatment overview and results of particle re-irradiation for meningioma, ependymoma, adenoid cystic carcinoma, chordoma/ chondrosarcoma and head and neck sarcoma
| Reference | N | Tumor entity | Previous RT: RT modality, Median dose |
Re-RT specifics: RT modality, Median dose |
Median time to local progression | Median Follow-up | Clinical Outcome | Toxicity |
|---|---|---|---|---|---|---|---|---|
| El Shafie et al.1 | 42 | Meningeoma WHO I: 10 WHO II: 25 WHO II: 6 Unknown: 1 |
IMRT (n = 16): 52.9 Gy(12.1–62.4 Gy) 3DCRT(n = 16): 54 Gy (50,5 Gy-55,8Gy) SRS (n = 7): 12,1 Gy (12–17 Gy) FSRT (n = 1): 58,8 Gy |
Protons n = 8 Carbon ions n = 34 51 Gy (RBE) 15–60 Gy (RBE) |
15.3 m | 49.7 m | 1y/ 2y PFS: 71%/56.5%, 1y/2y OS: 89.6%/ 71.4% |
No grade IV or V toxicity. CNS necrosis: 2.4% Grade I 4.8% Grade II |
| Eaton et al.2 | 20 (14) | Ependymoma | Protons 85% photons 15% 55.8 Gy (RBE)(52.5–59.4) |
Protons: 50.4 Gy (RBE)(35–55.8) |
23.9 m | 37.8 m | 3y PFS 28.1% 3y OS 78.6% |
CNS necrosis: 21.4% Grade II |
| Jensen et al.3 | 52 | ACC | 66 Gy/ Gy (RBE) (20-115) | Carbon ions 92.3%, Carbon ion and photon IMRT 7,7%: 51 Gy (RBE)/ 63 Gy BED and cumulative dose of 128 Gy BED [67–182 Gy] |
61 m | 14 m | 1y LC 70.3% 1y DC 72.6% |
CNS necrosis: 15.4% Grade I 3.8% Grade III ICA hemorrhage: 3.8% Grade IV 5.8% osteoradionecrosis |
| Uhl et al.4 | 25 | Chordoma/ chondro- sarcoma | Particles 56%: 60 Gy (RBE)(42-72) Photons 44%: 66 Gy (38–72.5) |
Carbon ions: 51 Gy (RBE) [45–60 Gy (RBE)]/ 63.8 Gy BED (α/β = 2) [56.2 Gy −75 Gy] |
84 m | 14 m | 2y LPFS 79.3% | One toxicity >Grade II: Grade III osteoradionecrosis CNS necrosis: 20% Grade I |
| Yang et al.5 | 11 | Head and neck sarcoma | 68 Gy (13–78) | Carbon ions: 60 Gy (RBE) |
30.6 m | 13.1 m | 1y LPFS 74.6%, 1y OS 86.5% |
One Grade IV treatment associated bleeding during treatment. Second deadly hemorrhage. after treatment |
RT: Radiotherapy, IMRT: Intensity modulated radiotherapy, 3DCRT: three-dimensional conformal radiation therapy, SRS: Stereotactic radiosurgery, FSRT: Fractionated stereotactic radiotherapy, RBE: relative biological effectiveness, BED: Biologically effective dose, CNS: Central nervous system, ICA: Internal carotid artery, m: months, y: years, PFS: Progression-free survival, OS: Overall survival, LC: local control, DC: distant control, LPFS: Local progression-free survival, ACC: Adenoid cystic carcinoma
Table 2.
Treatment overview and results of particle re-irradiation for head and neck cancer
| Reference | N | Tumor entity | Previous RT: RT modality, Median dose |
Re-RT specifics: RT modality, Median dose |
Median time to local progression | Median Follow-up | Clinical Outcome | Toxicity |
|---|---|---|---|---|---|---|---|---|
| Romesser et al.6 | 92 | HNC 56.5% SCC |
61.4 Gy (RBE)(IQR 54.0–69.9) | Protons: 60.6 Gy (50.0–66.1) |
34.4 m | 13.3 m | Locoregional failure: 25.1% 1y OS: 65.2% 1y distant PFS: 84% |
Acute toxicity Grade III: mucositis (9.9%), dysphagia (9.1%), esophagitis (9.1%), dermatitis (3.3%) Late toxicity Grade III: skin toxicity (8.7%), dysphagia (7.1%) 9.1% reactive gastrostomy tube Two cases of Grade V hemorrhage |
| Phan et al.7 | 60 | HNC 66.67% SCC |
60 Gy (30-70) | Protons: Definitive RT 66 Gy (50-70) Adjuvant RT 61.5 Gy (50-70) |
47.1 m | 13.6 m/ 20.3m* | 50 patients finished RT*: 1y LRC 80.8% 1y PFS 60.1% 1y OS 81.3% |
30% Grade III acute toxicity 20% Grade III late toxicity 10% reactive feeding tubes Two patients with potential Grade V toxicity |
| Held et al.8 | 229 | HNC 54% ACC 26% SCC |
Photons/ or photons and carbon ions: 67.4 Gy (36.5–84) (EQD2) |
Carbon ions: 51 Gy (RBE) (36-66) |
46.8 m | 28.5 m | 1y LC 60% Median local PFS: 24.2 m Median OS: 26.1 m 1 year OS: 72% |
Acute toxicity Grade III: 3.1% Late toxicity Grade III: 14.5% |
| Hu et al.9 | 75 | NPC | IMRT 96%: 70 Gy (66–75.75) |
Carbon ions: 57.6 Gy (RBE) (50-66) |
29 m | 15.4 m | 1y local recurrence-free survival: 86.6% 1y PFS: 82.2% 1y OS: 98.1% |
Late toxicity Grade III or IV: 9.3% mucosal necrosis 1.3% xerostomia 1.3% temporal lobe necrosis |
RT: Radiotherapy, IMRT: Intensity modulated radiotherapy, 3DCRT: three-dimensional conformal radiation therapy, SRS: Stereotactic radiosurgery, FSRT: Fractionated stereotactic radiotherapy, RBE: relative biological effectiveness, BED: Biologically effective dose, CNS: Central nervous system, ICA: Internal carotid artery, m: months, y: years, PFS: Progression-free survival, OS: Overall survival, LC: local control, DC: distant control, LPFS: Local progression-free survival, ACC: Adenoid cystic carcinoma, SCC: Squamous cell carcinoma, HNC: Head and neck cancer, NPC: Nasopharynx cancer, IQR: interquartile range, EQD2: equivalent dose in 2 Gy fractions
Differences in protons and carbon ions
Particle therapy offers a superior dose distribution with decreased integral dose to the surrounding healthy tissue compared to most modern photon techniques. Protons and carbon ions are the only two clinically used charged particles so far, but work is already underway to implement helium ions as an alternative to protons with additional beneficial features. Due to the complexity and the costs of particle therapy facilities accessibility progresses only slowly. Around 80 proton facilities and 13 carbon ion facilities are in operation worldwide (Particle Therapy Co-Operative Group, PTCOG, September 2019) and the numbers are constantly rising. The physical and biological characteristics of particle beams are unique. The inverse depth-dose profile is characteristic, the energy deposition in the entrance channel is very low until the end of the range where the dose increase and fall-off is extremely steep and results in the so-called Bragg peak. There is virtually no exit dose beyond the target for both protons and carbons ions, for carbon ions there is a small dose tail based on nuclear fragmentation which can be compensated by the arrangement of beams during treatment planning. It is worth noting that the lateral beam widening (penumbra) of carbon ions is significantly smaller than the penumbra of protons at the same range by the factor of approximately 3.5. The biological characteristics of particle therapy are based on the energy loss in tissue and the linear energy transfer of carbon ions is higher than of protons, thus is the biological effectiveness. While a fixed relative biological effectiveness (RBE) of 1.1 is commonly used in most planning systems for protons, the RBE of carbon ions varies based on different parameters and has to be calculated by complex biological models (e.g., the local effect modal), typically it is enhanced by the factor of 2–5. Different possibilities of beam application lead to varying doses to normal tissue with active beam delivery being superior over passive scattering.11–13
Brain tumors: meningioma and glioma
Radiation therapy has been firmly established in the treatment of intracranial meningioma of the skull base. Complex anatomy, involvement of vital structures such as cranial nerves, blood vessels and the cavernous sinus, is common and limits neurosurgical accessibility and complete resection. Different treatment techniques such as stereotactic radiosurgery (SRS), fractionated stereotactic radiotherapy, intensity modulated radiotherapy and proton therapy yield excellent long-term control rates.14–17 Nonetheless, local progression can occur and in this case treatment options are generally limited. Depending on the time interval since primary treatment and previous dose distribution to the surrounding organs at risk, re-irradiation can be offered in selected cases. El Shafie et al published the results of 42 patients treated with protons (n = 8) and carbon ions (n = 34) for recurrent intracranial meningioma after previous irradiation. Treatment was performed safely without interruptions and no grade IV or V toxicities were observed. In total, three patients developed radiation necrosis, two required surgery (grade III) and one was treated by corticosteroid administration (grade I). Differing fractional schemes were applied depending on the foregoing treatment and aiming for doses upward of 50 Gy (RBE) for WHO grade I and 54 Gy (RBE) for WHO grades II to III meningeomas. The median cumulative dose was 51 Gy (RBE) (range 15–60 Gy (RBE)), four patients received a bimodal treatment with a carbon ion boost and a photon base plan; 15–17 fractions of 3 Gy (RBE) carbon ion up to the total dose of 45–51 Gy (RBE) was the most commonest prescribed scheme. Proton therapy was applied in smaller doses per fraction of 1.8 or 2.0 Gy (RBE). The progression-free survival (PFS) after 12 months accounted for 71% and 56.5% after 24 months, and the overall survival (OS) was 89.6 and 71.4%, respectively. Histology impacted PFS significantly, for high WHO grades II/III, the median PFS was 25.7 months while median PFS was not reached for WHO grade I tumors.1 High precision techniques are required for irradiation next to critical organs at risk; particle therapy thus is not only an effective and tolerable option but rather frequently also the only treatment option.
Proton re-irradiation has been furthermore investigated in the treatment of pediatric ependymoma, one of the most common pediatric brain malignancies. In a heterogeneous cohort of 20 patients, 70% received involved field radiotherapy with protons for local failure with a median dose of 50.4 Gy (RBE). The 3-year OS and PFS were 78.6 and 28.1% with a median follow-up of 37.8 months. Three out of 14 patients experienced grade II treatment-associated changes locally (radiation necrosis); the time for development of symptomatic changes ranged from 1.9 to 31.9 months. The patients were treated successfully by oral medication.2
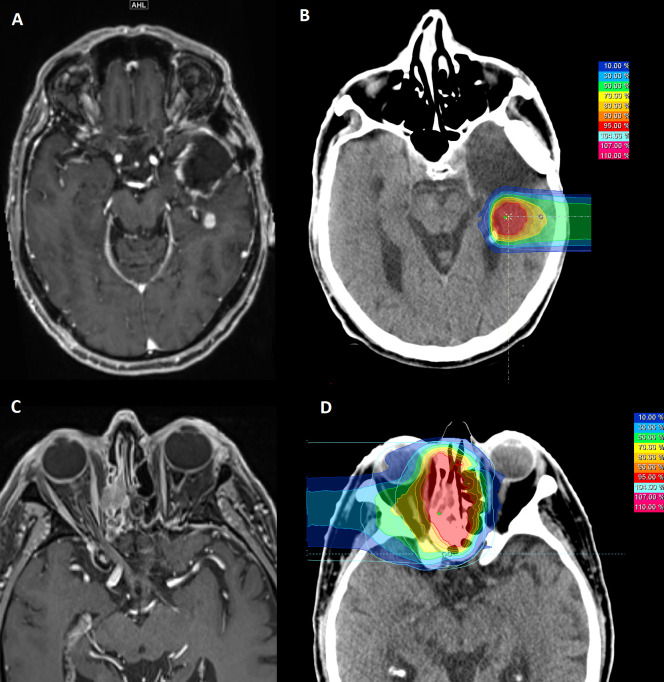
Glioblastoma WHO grade IV is the most common and aggressive brain tumor in adult patients. After initial standard of care surgery and/or chemoradiotherapy (Stupp protocol 18) recurrence commonly occurs and carries a poor prognosis. Response to treatment is heterogeneous and survival data depend on prognostic factors as tumor volume, age, performance status, possibility of resection and time between primary diagnosis and initial treatment and current progression.19 In case of recurrence, treatment options include surgery if feasible, second course of radiotherapy or chemotherapy e.g. with alkylating nitrosurea (Lomustine, CCNU) or re-exposition to temozolomide. Small volume re-irradiation can be performed with carbon ions, e.g. with 45 Gy (RBE) in 15 fractions 20,21 safely and efficiently (unpublished clinical experience from Heidelberg Ion-Beam Therapy Center, Figure 1 A, B). However, the final results of the according CINDERELLA trial are still pending.21 Comparing to the currently published data by the Italian Association of Radiation Oncology (AIRO) on recurrent glioma, carbon ion therapy might have benefits. In the current work, experience of 300 patients who received photon re-irradiation for recurrent glioma with a median biological effective dose (BED10) of 43 Gy was reported. The median OS was 9.7 months, the OS at 1 and 2 years were 41 and 17.7%. High delivered total doses (BED10 >43 Gy) were positively associated with patient’s survival (p = 0.04). Performing re-irradiation with carbon ions allows prescribing doses up to 58.5 Gy BED10.22 As a logical consequence of the AIRO results, dose escalation seems desirable, however reliable evidence is urgently needed.
Figure 1.
MRI (A, C) and according dose-distribution (B, D) for re-irradiation of recurrent tumors: (A, B) 54-year-old patient with glioblastoma WHO °IV, re-irradiation with carbon ions up to 45 Gy (RBE) in 15 fractions, (C, D) 84-year-old patient with recurrent adenoid cystic carcinoma of the paranasal sinuses; re-irradiation was performed with carbon ions up to 51 Gy (RBE) in 17 fractions
Rare malignancies: Adenoid cystic carcinoma, chordoma and chondrosarcoma
Adenoic cystic carcinoma (ACC) is a rare salivary gland malignancy arising in the minor salivary gland of the paranasal sinuses and major salivary glands as the parotid and submandibular gland among others. Due to its perivascular and perineural spread infiltration of the skull base along the cranial foramina is quite common, especially in case of recurrence. Generally local relapse in head and neck cancer is a major therapeutic challenge. In ACC, the situation is often even more complex. Primary complete resection is often not possible due to characteristic infiltrative growth, and due to radioresistance high initial doses are applied after incomplete resection. Consequently, treatment options are limited. In case of a relapse, surgery as a first choice for treatment is not feasible in most cases. Re-irradiation with photons has been used only in a limited amount of cases as the potential for severe side-effects was considered to be intolerably high in the past. Neutrons have been quite promising regarding control rates,23 but the effects were overshadowed by considerable side-effects.24 Re-irradiation can lead to long-term control, but local control is dose-dependent. Jensen et al. published in 2015 the results of 52 patients who received re-irradiation with carbon ions. The median age was 55 years and the follow-up was 14 months (1–39 months), only four patients were treated after R1 resection and 43 for inoperable local relapse, 92.3% of patients were treated with carbon ion only, the rest with photon IMRT and a carbon ion boost. With a median dose of 51 Gy (RBE)/63 Gy BED (α/β = 2) and cumulative dose of 128 Gy BED (67–182 Gy) a local and distant control at 1 year of 70.3 and 72.6% was achieved, respectively. Despite the high cumulative dose, no higher grade acute reactions (> grade II, NCI CTC v.4) were observed. Higher grade late toxicity was rare including CNS necrosis requiring surgery (grade III, 3.8%), tissue necrosis in the nasopharynx consequently leading to carotid artery hemorrhage (grade IV, 3.8%) and osteoradionecrosis (5.8%).3 Based on this experience, patients with recurrent pre-irradiated ACC are typically treated up to a dose of 51 Gy (RBE) in 17 fractions of 3 Gy (RBE) at the Heidelberg Ion-Beam Therapy Center (Figure 1 B,D).
Chordoma and chrondrosarcoma are rare malignant bone tumors, which frequently involve the skull base. They arise from remnants of the notochord and cartilage, respectively. In only rare cases, metastatic disease is prevalent, thus sufficient local control is crucial. A microscopically complete resection is an exception due to the complexity of the anatomy of the skull base. Function preserving surgery followed by particle beam radiotherapy is nowadays the treatment of choice with excellent local control rates.25–27 Still there are a number of patients with relapse of skull base chordoma or chondrosarcoma. The inability to spare neighboring organs at risk sufficiently with conventional treatment techniques and the great potential of severe side-effects hampered a second course of radiotherapy in the past. Uhl et al published 25 cases of patients who were irradiated with carbon ions from January 2010 to October 2012 (n = 5 for skull base chondrosarcoma and n = 20 for chordoma). In this retrospective study, the clinical target volume (CTV) included the visible tumor mass on MRI with a 3 mm safety margin and the planning target volume included the CTV with a 3 mm margin. Exceeding tissue tolerance was avoided and the median prescribed dose was 51 Gy (RBE) [45–60 Gy (RBE)]/ 63.8 Gy BED (56.2 Gy −75 Gy) for a α/β = 2, given in five to six fractions per week with a single dose of 3 Gy (RBE). Two patients received a third course of radiotherapy. With a median follow-up time of 14 months (2–30), the estimated 2-year local progression free survival (LPFS) was 79.3%. Only one case of toxicity higher than grade II was observed, which was osteoradionecrosis requiring surgery (grade III). In general, re-irradiation of recurrent skull base chordoma and chondrosarcoma was shown to be safe and feasible.4 Similar data were provided by the Shanghai Proton and Heavy Ion Center. In a heterogeneous cohort of 19 patients with locally recurrent or radiation-induced second primary sarcoma of the head and neck in total, 11 patients were treated with carbon ion radiotherapy after previous radiotherapy. With a median follow-up of 13.1 months and a median dose of 60 Gy (RBE), the actuarial 12 months LPFS was 74.6% and overall survival (OS) was 86.5% for all 19 patients. Two patients hat a grade IV bleeding during treatment, including one rupture of an optic artery aneurysm which was not attributed to the disease or treatment. The second patient had a treatment-related bleeding during treatment and died of second hemorrhage 3.5 months after the treatment.5
Head and neck tumors
In the multimodal treatment of head and neck cancer, a significant portion of patients requires radiation therapy. Despite the advances in treatment techniques, some patients develop a local recurrence; the management of this scenario remains challenging and locoregional failure is the most common cause of death after definitive treatment. Additionally, metachronous tumors in previously irradiated volumes can occur. Besides surgery, re-irradiation is the only potentially curative treatment option, especially in locally advanced tumors. However, many patients are not offered re-irradiation and are referred to palliative chemotherapy based on concerns of severe side-effects. Although re-irradiation of recurrent tumors of the head and neck is one typical indication for proton therapy reported data is limited (Table 2). Romesser et al reported on 92 patients treated i by proton beam re-irradiation. With a median follow-up of 13.3 months among surviving patients and a median dose of 60.6 Gy (RBE), the incidence of locoregional failure of 25.1% was observed. The actuarial 12-month OS rate was 65.2% and the 12-month freedom from distant metastasis rate was 84.0%. The cohort showed a heterogeneous initial tumor site but 56.5% of patients had squamous cell carcinoma histology. Comparing to historical photon IMRT cohorts, the rate of acute grade III dermatitis and mucositis (3 and 10%, respectively) as well as the rate of reactive gastrostomy tubes (9.1%) was favorably lower. In total two patients developed grade V bleeding caused by significant ulceration and necrosis in the absence of active tumor on PET-CT.6 The multicentric in silico ROCOCO trial compared intensity modulated proton and ion therapy with volumetric modulated arc therapy (VMAT) and depicted the dosimetric background for these clinical findings. The mean dose of all investigated organs at risk (OAR’s, n = 22) was significantly reduced by ion therapy and for 15 of 22 OAR’s by proton therapy. The maximum dose to 2% of volume (D2) for brainstem and spinal cord was significantly reduced by both particle types compared to VMAT.28 Phan et al reported the results of 60 patients who received proton beam re-irradiation up to a median dose of 66 Gy. With a median follow-up of 20.3 months for 50 patients who completed re-irradiation, the local control rates in 1 year and 2 years were 80.8 and 72.8% and the 1 year and 2 year OS rates were 81.3 and 69.0%, respectively. A greater portion of patients had squamous cell histology (n = 40). The rate of acute grade III toxicity was 30% and late grade III toxicity 20%. In total feeding tubes were required by 22% of patients, 10.1% had a reactive feeding tube. Two patients had potential grade V treatment-related toxicity, both experienced osteoradionecrosis (hyoid bone and clivus).7 Re-irradiation of retropharyngeal lymph node metastases was reported for 19 patients who have been treated by conventionally fractionated IMRT or proton therapy (n = 4) in 58% of cases and by stereotactic single or hypofractionated radiotherapy in 42% of cases. The 1-year local control, 1-year locoregional control, OS and PFS were 100%, 94%, 92 and 92%, respectively. Only patients treated with IMRT experienced acute grade III toxicity (16%) and there was no late toxicity grade III. It is worth noting that the rate of systemic therapy was 74%.29 The patients treated at Heidelberg Ion-Beam Therapy Center between 2010 and 2017 by carbon ion re-irradiation for recurrent head and neck cancer (n = 229) have been investigated by Held et al.8In total 54% of primary tumors were adenoid cystic carcinoma and 26% squamous cell carcinoma. The median interval between primary and re-irradiation was 3.9 years, the median dose 51 Gy (RBE) in 17 fractions. The median cumulative lifetime dose after the re-irradiation accounted for 132.8 Gy (range 88.8–155.0 Gy). With a median local PFS of 24.2 months and median OS 26.1 months, the rate of late toxicity grade III was 14.5% and included the portion of visual or hearing impairments that were discussed and accepted by the patient in favor of local control when the tumor was infiltrating the optic system or the inner ear. Comparing to previously published data, cumulative rates of late toxicity grade III at 2 years after photon re-irradiation of approximately 30% carbon ion strikes as more favorable.30 Further encouraging results were reported by Hu et al for nasopharyngeal carcinoma (n = 75) with similar toxicity rates. Patients received 50–66 Gy (RBE), the 1 year OS and PFS were 98.1% and 82.2, respectively.9 The ongoing NCT03217188 trial compares a conventionally fractionated full dose re-irradiation up to 70 Gy (RBE) in 2 Gy (RBE) fractions with a palliative hypofractionated regimen called Quad-Shot for patients with recurrent head and neck cancer. Further urgently needed studies are currently in planning and aim to provide additional evidence and establish the role of particle re-irradiation for head and neck cancer.
Differences in side effects: Carotid blowout and CNS necrosis
Carotid blowout (CB), a rupture of the carotid artery or its main branches, is a severe complication which is seldom seen in primary radiation therapy and increases in case of re-irradiation. CB is a consequence of loss of soft tissue surrounding the carotid artery or pathologic alterations of the carotid wall itself. In a cohort of 96 patients treated in National Center of Oncological Hadrontherapy (CNAO) (18% treated with protons and 82% with carbon ions), an actuarial CB rate of 2.7% was observed; interestingly two patients treated with protons experienced fatal orinasal bleeding and no CB was observed in the carbon ion group. The median prescribed cumulative lifetime dose was 120 Gy (RBE) [32–197 Gy (RBE)]. Cumulative EQD2 exceeding 120 Gy (RBE) to the carotid artery was avoided in contrast to the approach published by Jensen et al and might explain the lower complication rate in the carbon ion cohort. On the other hand this apparent difference might be contributed to the small study cohorts.31 Whether restricting the carotids to a maximum dose reduces the rate of carotid blow out or imperils local control by under-dosing parts of the tumor volume remains to be answered.
Regarding the recovery of tolerance in re-irradiation of tumors of the CNS or in close vicinity, a recover capacity of approximately 40% for the brain stem and spinal cord can be assumed in cases when an interval of more than 1 year lies between radiation and re-irradiation.3,4 If the optic nerve or chiasm is involved in the tumor, the potential risks of visual loss have to be discussed with the patient as compression and subclinical damage can reduce the tolerance dose. More detailed information on patient selection and BED calculations for retreatment and recovery of neural tolerance was published previously by others.32Temporal lobe reactions (TLR) are often observed in particle re-irradiation of the skull base. Those are defined as visible contrast medium (CM) enhancement at approximately MRI within the high dose volume and are usually accompanied by a surrounding T2W enhancement. These areas can be reversible spontaneously but also evolve into CNS necrosis.33 The correct diagnosis remains difficult, until now no imaging modality allows differentiating from tumor progression exactly. The investigation of brain tissue specimen allows a definite diagnosis, however only high-grade CNS necroses require surgery as first treatment and typically glucocorticoids are the initial treatment option of symptomatic lesions whereas asymptomatic grade I reactions can be observed primarily. In the cohort of Jensen et al, the rate of grade I reactions was 15.4% and 3.8% of patients underwent surgery (CNS necrosis grade III).3 Uhl et al observed asymptomatic TLR (grade I) in 20% of patients.4 In a cohort of 217 patients treated with carbon ion re-radiotherapy for recurrent head and neck cancer, Held et al observed a rate of 16.6% (n = 36) for radiation-induced CNS necrosis with a medium follow-up time of 25.3 months, the majority of these findings were asymptomatic (n = 17) and located in the temporal lobe (83.4%). The median time for the occurrence of grades I, II and III lesions was 9.2, 10.2 and 16.6 months, respectively. Radiographic response, defined as 25% reduction of T2-abnormality, was observed in 16%, 29.4 and 80% of rades I, II and III CNS-necrosis.34 One possible explanation for these reactions of the brain tissue is a blood–brain barrier breakdown after irradiation which leads to extracellular edema. Consequently, patients can experience neurological symptoms e.g. headaches, seizures or vertigo. Schlampp et al. analyzed patients treated at the GSI Darmstadt (Germany) with carbon ions for skull base chordoma and chondrosarcoma. In a cohort of 59 patients, 10 developed a TLR after a medium time of 1.2 years with a median size of CM enhancement of 2.1 cm³; only two patients developed according neurological symptoms. CM enhancements manifested in areas of the temporal lobe directly adjacent to high dose volumes and at least partially included in PTV2, which was the boost plan PTV. One year after their appearance, all TLR’s showed no further growth and six diminished in size. The biological equivalent tolerance doses for the 5 and 50% probability of TLR were 68.8 3.3 Gy (RBE) and 87.3 2.8 Gy (RBE), respectively.35 Particle therapy especially with carbon ions allows dose escalation in close vicinity to vulnerable organs as the temporal lobe and leads to dose concepts with high cumulative doses which cannot be realized by any other technique to this extent. One has to assume that the risk for TLR and brain necrosis increases with a rising cumulative dose in irradiation and especially re-irradiation. In cases where steroidal treatment is not sufficient, low-dose bevacizumab appears to be a promising agent which can effectively improve radiographic and clinical response.36
Conclusion
Treatment of recurrent disease is challenging in most situations. In the head and neck, brain and skull base complex anatomy limits the possibilities of local treatment. High-precision techniques as proton or carbon ion are very promising for re-irradiation. Due to their steep dose gradients, they consequently allow dose escalation and sparing of neighboring healthy tissue. Particularly, carbon ion therapy offers superior physical and biological characteristics which can be utilized for re-irradiation. The level of evidence for particle re-irradiation summarized here is generally still quite low as there are mainly small retrospectively reported cohorts available. To date the majority of patients requiring re-irradiation will be treated by photon radiotherapy, as due to the complexity and the associated costs there are only a few carbon ion facilities in the world and also the access to proton facilities is limited to just a small number of patients. Still particle therapy compares favorably to the reported historic photon cohorts, thus research of these new treatment techniques for re-irradiation will continue. Considerations on cost-effectiveness include not only the direct treatment costs but also the costs of further progression and treatment37,38 and particle re-irradiation might prove to be expensive but cost-effective in future, if it will be possible to add substantial evidence to the suggested improved control rates and less severe side-effects. Currently, treatment appears to be feasible and safe; the majority of patients finish treatment without major interruptions or a high rate of higher grade acute toxicities. Most reports have short-term follow-up only, thus the extent of late toxicities might be underestimated. A special scope of side-effects arises with re-irradiation, a higher risk of radiation necrosis and particularly carotid blow-out has to be assumed. Further shared experience from the particles centers and favorably investigations in form of prospective studies are necessary in order to establish particle therapy as the superior modality for potentially curative salvage irradiation in an otherwise desperate situation.
Contributor Information
Katharina Seidensaal, Email: katharina.seidensaal@med.uni-heidelberg.de.
Semi Ben Harrabi, Email: semi.harrabi@med.uni-heidelberg.de.
Matthias Uhl, Email: matthias.uhl@med.uni-heidelberg.de.
REFERENCES
- 1.El Shafie RA, Czech M, Kessel KA, Habermehl D, Weber D, Rieken S, et al. . Evaluation of particle radiotherapy for the re-irradiation of recurrent intracranial meningioma. Radiat Oncol 2018; 13: 86. doi: 10.1186/s13014-018-1026-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eaton BR, Chowdhry V, Weaver K, Liu L, Ebb D, MacDonald SM, et al. . Use of proton therapy for re-irradiation in pediatric intracranial ependymoma. Radiother Oncol 2015; 116: 301–8. doi: 10.1016/j.radonc.2015.07.023 [DOI] [PubMed] [Google Scholar]
- 3.Jensen AD, Poulakis M, Nikoghosyan AV, Chaudhri N, Uhl M, Münter MW, et al. . Re-Irradiation of adenoid cystic carcinoma: analysis and evaluation of outcome in 52 consecutive patients treated with raster-scanned carbon ion therapy. Radiother Oncol 2015; 114: 182–8. doi: 10.1016/j.radonc.2015.01.002 [DOI] [PubMed] [Google Scholar]
- 4.Uhl M, Welzel T, Oelmann J, Habl G, Hauswald H, Jensen A, et al. . Active raster scanning with carbon ions: reirradiation in patients with recurrent skull base chordomas and chondrosarcomas. Strahlenther Onkol 2014; 190: 686–91. doi: 10.1007/s00066-014-0608-2 [DOI] [PubMed] [Google Scholar]
- 5.Yang J, Gao J, Wu X, Hu J, Hu W, Kong L, et al. . Salvage carbon ion radiation therapy for locally recurrent or radiation-induced second primary sarcoma of the head and neck. J Cancer 2018; 9: 2215–23. doi: 10.7150/jca.24313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Romesser PB, Cahlon O, Scher ED, Hug EB, Sine K, DeSelm C, et al. . Proton beam reirradiation for recurrent head and neck cancer: multi-institutional report on feasibility and early outcomes. Int J Radiat Oncol Biol Phys 2016; 95: 386–95. doi: 10.1016/j.ijrobp.2016.02.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Phan J, Sio TT, Nguyen TP, Takiar V, Gunn GB, Garden AS, et al. . Reirradiation of head and neck cancers with proton therapy: outcomes and analyses. Int J Radiat Oncol Biol Phys 2016; 96: 30–41. doi: 10.1016/j.ijrobp.2016.03.053 [DOI] [PubMed] [Google Scholar]
- 8.Held T, et al. Carbon ion reirradiation for recurrent head and neck cancer: a single-institutional experience. Int J Radiat Oncol Biol Phys 2019;. [DOI] [PubMed] [Google Scholar]
- 9.Hu J, Bao C, Gao J, Guan X, Hu W, Yang J, et al. . Salvage treatment using carbon ion radiation in patients with locoregionally recurrent nasopharyngeal carcinoma: initial results. Cancer 2018; 124: 2427–37. doi: 10.1002/cncr.31318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verma V, Rwigema J-CM, Malyapa RS, Regine WF, Simone CB. Systematic assessment of clinical outcomes and toxicities of proton radiotherapy for reirradiation. Radiother Oncol 2017; 125: 21–30. doi: 10.1016/j.radonc.2017.08.005 [DOI] [PubMed] [Google Scholar]
- 11.Weber U, Kraft G. Comparison of carbon ions versus protons. Cancer J 2009; 15: 325–32. doi: 10.1097/PPO.0b013e3181b01935 [DOI] [PubMed] [Google Scholar]
- 12.Schardt D, Elsässer T, Schulz-Ertner D. Heavy-Ion tumor therapy: physical and radiobiological benefits. Rev Mod Phys 2010; 82: 383–425. doi: 10.1103/RevModPhys.82.383 [DOI] [Google Scholar]
- 13.Uhl M, Herfarth K, Debus J. Comparing the use of protons and carbon ions for treatment. The Cancer Journal 2014; 20: 433–9. doi: 10.1097/PPO.0000000000000078 [DOI] [PubMed] [Google Scholar]
- 14.El Shafie RA, Czech M, Kessel KA, Habermehl D, Weber D, Rieken S, et al. . Clinical outcome after particle therapy for meningiomas of the skull base: toxicity and local control in patients treated with active rasterscanning. Radiat Oncol 2018; 13: 54. doi: 10.1186/s13014-018-1002-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sanford NN, Yeap BY, Larvie M, Daartz J, Munzenrider JE, Liebsch NJ, et al. . Prospective, randomized study of radiation dose escalation with combined Proton-Photon therapy for benign meningiomas. Int J Radiat Oncol Biol Phys 2017; 99: 787–96. doi: 10.1016/j.ijrobp.2017.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murray FR, Snider JW, Bolsi A, Lomax AJ, Walser M, Kliebsch U, et al. . Long-Term clinical outcomes of pencil beam scanning proton therapy for benign and non-benign intracranial meningiomas. Int J Radiat Oncol Biol Phys 2017; 99: 1190–8. doi: 10.1016/j.ijrobp.2017.08.005 [DOI] [PubMed] [Google Scholar]
- 17.Santacroce A, Walier M, Régis J, Liščák R, Motti E, Lindquist C, et al. . Long-Term tumor control of benign intracranial meningiomas after radiosurgery in a series of 4565 patients. Neurosurgery 2012; 70: 32–9. doi: 10.1227/NEU.0b013e31822d408a [DOI] [PubMed] [Google Scholar]
- 18.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJB, et al. . Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005; 352: 987–96. doi: 10.1056/NEJMoa043330 [DOI] [PubMed] [Google Scholar]
- 19.Combs SE, Niyazi M, Adeberg S, Bougatf N, Kaul D, Fleischmann DF, et al. . Re-Irradiation of recurrent gliomas: pooled analysis and validation of an established prognostic score-report of the radiation Oncology Group (ROG) of the German cancer Consortium (DKTK. Cancer Med 2018; 7: 1742–9. doi: 10.1002/cam4.1425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rieken S, Habermehl D, Haberer T, Jaekel O, Debus J, Combs SE, et al. . Proton and carbon ion radiotherapy for primary brain tumors delivered with active raster scanning at the Heidelberg ion therapy center (HIT): early treatment results and study concepts. Radiat Oncol 2012; 7: 41. doi: 10.1186/1748-717X-7-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Combs SE, Burkholder I, Edler L, Rieken S, Habermehl D, Jäkel O, et al. . Randomised phase I/II study to evaluate carbon ion radiotherapy versus fractionated stereotactic radiotherapy in patients with recurrent or progressive gliomas: the Cinderella trial. BMC Cancer 2010; 10: 533. doi: 10.1186/1471-2407-10-533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Navarria P, Minniti G, Clerici E, Tomatis S, Pinzi V, Ciammella P, et al. . Re-Irradiation for recurrent glioma: outcome evaluation, toxicity and prognostic factors assessment. A multicenter study of the radiation oncology Italian association (AIRO. J Neurooncol 2019; 142: 59–67. doi: 10.1007/s11060-018-03059-x [DOI] [PubMed] [Google Scholar]
- 23.Douglas JG, Koh W-jin, Austin-Seymour M, Laramore GE. Treatment of salivary gland neoplasms with fast neutron radiotherapy. Arch Otolaryngol Head Neck Surg 2003; 129: 944–8. doi: 10.1001/archotol.129.9.944 [DOI] [PubMed] [Google Scholar]
- 24.Huber PE, Debus J, Latz D, Zierhut D, Bischof M, Wannenmacher M, et al. . Radiotherapy for advanced adenoid cystic carcinoma: neutrons, photons or mixed beam? Radiother Oncol 2001; 59: 161–7. doi: 10.1016/S0167-8140(00)00273-5 [DOI] [PubMed] [Google Scholar]
- 25.Uhl M, Mattke M, Welzel T, Oelmann J, Habl G, Jensen AD, et al. . High control rate in patients with chondrosarcoma of the skull base after carbon ion therapy: first report of long-term results. Cancer 2014; 120: 1579–85. doi: 10.1002/cncr.28606 [DOI] [PubMed] [Google Scholar]
- 26.Uhl M, Mattke M, Welzel T, Roeder F, Oelmann J, Habl G, et al. . Highly effective treatment of skull base chordoma with carbon ion irradiation using a raster scan technique in 155 patients: first long-term results. Cancer 2014; 120: 3410–7. doi: 10.1002/cncr.28877 [DOI] [PubMed] [Google Scholar]
- 27.Mattke M, Vogt K, Bougatf N, Welzel T, Oelmann-Avendano J, Hauswald H, et al. . High control rates of proton- and carbon-ion-beam treatment with intensity-modulated active raster scanning in 101 patients with skull base chondrosarcoma at the Heidelberg ion beam therapy center. Cancer 2018; 124: 2036–44. doi: 10.1002/cncr.31298 [DOI] [PubMed] [Google Scholar]
- 28.Eekers DBP, Roelofs E, Jelen U, Kirk M, Granzier M, Ammazzalorso F, et al. . Benefit of particle therapy in re-irradiation of head and neck patients. Results of a multicentric in silico ROCOCO trial. Radiotherapy and Oncology 2016; 121: 387–94. doi: 10.1016/j.radonc.2016.08.020 [DOI] [PubMed] [Google Scholar]
- 29.Pollard C, Nguyen TP, Ng SP, Frank SJ, Garden AS, Gunn GB, et al. . Clinical outcomes after local field conformal reirradiation of patients with retropharyngeal nodal metastasis. Head Neck 2017; 39: 2079–87. doi: 10.1002/hed.24872 [DOI] [PubMed] [Google Scholar]
- 30.Takiar V, Garden AS, Ma D, Morrison WH, Edson M, Zafereo ME, et al. . Reirradiation of head and neck cancers with intensity modulated radiation therapy: outcomes and analyses. Int J Radiat Oncol Biol Phys 2016; 95: 1117–31. doi: 10.1016/j.ijrobp.2016.03.015 [DOI] [PubMed] [Google Scholar]
- 31.Dale JE, Molinelli S, Ciurlia E, Ciocca M, Bonora M, Vitolo V, et al. . Risk of carotid blowout after reirradiation with particle therapy. Adv Radiat Oncol 2017; 2: 465–74. doi: 10.1016/j.adro.2017.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jones B, Grant W. Retreatment of central nervous system tumours. Clin Oncol 2014; 26: 407–18. doi: 10.1016/j.clon.2014.04.027 [DOI] [PubMed] [Google Scholar]
- 33.Kishimoto R, Mizoe J-etsu, Komatsu S, Kandatsu S, Obata T, Tsujii H, et al. . Mr imaging of brain injury induced by carbon ion radiotherapy for head and neck tumors. Magn Reson Med Sci 2005; 4: 159–64. doi: 10.2463/mrms.4.159 [DOI] [PubMed] [Google Scholar]
- 34.Held T, Akbaba S, Lang K, Harrabi S, Bernhardt D, Freudlsperger C, et al. . Clinical management of Blood–Brain barrier disruptions after active Raster-Scanned carbon ion Re-Radiotherapy in patients with recurrent head-and-neck cancer. Cancers 2019; 11: 383. doi: 10.3390/cancers11030383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schlampp I, Karger CP, Jäkel O, Scholz M, Didinger B, Nikoghosyan A, et al. . Temporal lobe reactions after radiotherapy with carbon ions: incidence and estimation of the relative biological effectiveness by the local effect model. Int J Radiat Oncol Biol Phys 2011; 80: 815–23. doi: 10.1016/j.ijrobp.2010.03.001 [DOI] [PubMed] [Google Scholar]
- 36.Delishaj D, Ursino S, Pasqualetti F, Cristaudo A, Cosottini M, Fabrini MG, et al. . Bevacizumab for the treatment of radiation-induced cerebral necrosis: a systematic review of the literature. J Clin Med Res 2017; 9: 273–80. doi: 10.14740/jocmr2936e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sprave T, Verma V, Sterzing F, Bruckner T, Hees K, Land B, et al. . Cost-Effectiveness of carbon ion radiation therapy for skull base chordoma utilizing long-term (10-year) outcome data. Anticancer Res 2018; 38: 4853–8. doi: 10.21873/anticanres.12797 [DOI] [PubMed] [Google Scholar]
- 38.Jäkel O, Land B, Combs SE, Schulz-Ertner D, Debus J. On the cost-effectiveness of carbon ion radiation therapy for skull base chordoma. Radiother Oncol 2007; 83: 133–8. doi: 10.1016/j.radonc.2007.03.010 [DOI] [PubMed] [Google Scholar]