Abstract
Patients with squamous cell carcinoma of the oropharynx are generally treated with (chemo) radiation. Patients with oropharyngeal cancer have better survival than patients with squamous cell carcinoma of other head and neck subsites, especially when related to human papillomavirus. However, radiotherapy results in a substantial percentage of survivors suffering from significant treatment-related side-effects. Late radiation-induced side-effects are mostly irreversible and may even be progressive, and particularly xerostomia and dysphagia affect health-related quality of life. As the risk of radiation-induced side-effects highly depends on dose to healthy normal tissues, prevention of radiation-induced xerostomia and dysphagia and subsequent improvement of health-relatedquality of life can be obtained by applying proton therapy, which offers the opportunity to reduce the dose to both the salivary glands and anatomic structures involved in swallowing.
This review describes the results of the first cohort studies demonstrating that proton therapy results in lower dose levels in multiple organs at risk, which translates into reduced acute toxicity (i.e. up to 3 months after radiotherapy), while preserving tumour control. Next to reducing mucositis, tube feeding, xerostomia and distortion of the sense of taste, protons can improve general well-being by decreasing fatigue and nausea. Proton therapy results in decreased rates of tube feeding dependency and severe weight loss up to 1 year after radiotherapy, and may decrease the risk of radionecrosis of the mandible. Also, the model-based approach for selecting patients for proton therapy in the Netherlands is described in this review and future perspectives are discussed.
Introduction
Patients with squamous cell carcinoma of the head and neck can be curatively treated with surgery, radiotherapy and/or chemotherapy. For patients with squamous cell carcinoma of the oropharynx (OPC), the primary treatment strategy may consist of radiotherapy for Stage I-II disease and definitive chemoradiation for Stage III-IV disease. Survival in OPC patients, especially when related to human papillomavirus (HPV) is generally better than observed among those with squamous cell carcinoma of other head and neck subsites. In a retrospective analysis of patients included in the NRG Oncology RTOG 0129 trial, comparing concurrent chemoradiation with standard fractionation with accelerated chemoradiation, 63.8% of the patients with Stage III-IV oropharyngeal cancer had HPV-positive tumours.1 These patients had significantly better 3 year progression-free and overall survival rates compared to patients with HPV-negative tumours (73.7 vs 43.4%, and 82.4 vs 57.1% respectively). Locoregional failure was lower for HPV-positive tumours, but there was no difference in the occurrence of distant metastases.1 For HPV-positive patients, risk of death increases with each additional pack-years of tobacco smoking.1,2
Although survival in HPV-positive patients is favourable, radiotherapy or chemoradiation generally results in a substantial percentage of survivors suffering from treatment-related toxicities. Late radiation-induced side-effects are mostly irreversible and may even be progressive, and affect health-related quality of life (HRQoL).3 Langendijk et al evaluated the association between radiation-induced toxicity and HRQoL among patients treated for head and neck squamous cell carcinoma with radiotherapy alone or in combination with surgery and/or chemotherapy and showed that especially radiation-induced xerostomia and dysphagia significantly affected HRQoL.3
As the risk of radiation-induced toxicity heavily depends on the dose to healthy normal tissues, prevention of radiation-induced toxicity and subsequent improvement of HRQoL can be obtained by applying new radiation treatment strategies that can reduce the dose to the salivary glands and to anatomical structures involved in swallowing.3,4 Given the increasing incidence of HPV-associated head and neck cancer and the longer life expectancy of these patients, minimising the risk of acute and late radiation toxicity becomes increasingly important.
The use of more conformal radiotherapy techniques, such as intensity-modulated radiotherapy (IMRT), results in a significant reduction of the mean dose to the parotid glands and subsequently to less patient- and physician-rated xerostomia and ultimately better HRQoL.5,6
Another strategy to spare normal tissues to reduce late radiation toxicity could be the introduction of emerging radiation technologies, such as proton therapy, allowing for similar target dose coverage with lower dose levels to multiple organs at risk.
This review describes the advantages of the physical properties of protons compared to photons and the clinical benefit of treating patients with OPC with protons in terms of toxicity. Furthermore, the model-based approach for selecting patients for proton therapy in the Netherlands is described and future perspectives are discussed.
Physical properties and dosimetric advantages of protons
Protons continually interact with surrounding material, gradually losing more energy along their path until they stop entirely. This range is a function of the protons’ initial energy; by modulating the energy the protons can be made to stop at a desired depth in a patient. The dose deposited along the beam path remains relatively constant until reaching the end of range, whereby the dose peaks and then falls off to near-zero quite rapidly, a characteristic shape known as the Bragg peak. These two defining features of proton beams are the basis for proton therapy and its ability to reduce integral dose compared to photons.
Historically, head and neck cancer has not been a major indication for proton therapy due primarily to the technical difficulty in delivering dose to complex target volumes in a region with significant tissue heterogeneities. In recent years, however, important advances have been made that have facilitated a broader adoption of proton therapy for these indications. Spot scanning (or pencil beam scanning) technology can deliver thousands of narrow proton beams, each modulated for position, depth (energy) and monitor units (dose), shaping more conformal dose distributions using intensity-modulated proton therapy (IMPT) planning techniques. Changes in anatomy for example due to tumour shrinkage, oedema and filling of nasal cavities with mucus, may affect the dose delivered to both the tumour and organs at risk. However, robust optimisation and Monte Carlo dose calculation engines are effective tools to reduce most of the differences between the planned and delivered dose distribution due to patient set-up difficulty, proton range uncertainty and tissue heterogeneity.7 Nevertheless, weekly repeat CTs are still required during proton therapy to monitor anatomic changes and the influence on the dose distribution. If required, the radiation treatment plan can be adapted.
The biological damage of IMPT dose is generally considered to be higher than that of XRT dose for both tumours and healthy tissues. Thus, a constant relative biological effectiveness (RBE) of 1.1 is routinely used in clinical treatment planning as recommended by the International Commission on Radiation Units and Measurements.8 However, pre-clinical evidence demonstrated that the RBE of IMPT is not constant, but increases as protons lose more of their initial energy. This effect is most prominent at the end of the proton range, i.e. in the distal edge of the Bragg peak where the dose-weighted linear energy transfer (LETd) is highest.9 However, the spatial distribution of RBE in patients is unknown and cannot be measured directly. At present, limited data exist the extent this variable RBE translates into clinically relevant effects such as toxicity and locoregional tumour control.10–12
Several studies reported on proton therapy in OPC, but most of these studies do not report separately on HPV-positive OPC. We performed plan comparison studies in 91 patients with OPC, including 47 p16-negative patients and 44 p16-positive patients. A plan comparison was made as standard procedure to investigate if head and neck cancer patients qualify for proton therapy according to the model-based approach (see next paragraph).7,13 On average, in both the volumetric-modulated arc therapy (VMAT) and the IMPT plans, no differences were noted between p16-negative and p16-positive cases regarding the mean dose to the oral cavity, the pharyngeal constrictor superior, medius and inferior, the supraglottic and the parotid and submandibular glands. Therefore, we assume that results obtained in OPC in general of HPV-negative cases can be translated to the HPV-positive cases as well.
There are several studies showing that in OPC, using protons instead of photons results in dose reductions to various organs at risk, including to the parotid and submandibular glands, the swallowing structures, the oral cavity and central nervous system structures.14–18 In 10 patients with N0 OPC, treated with 70 Gy (RBE) to macroscopic tumour and 54 Gy (RBE) to the elective nodal areas, the mean dose to the parotid glands could be reduced to 16.8 Gy (RBE) with IMPT compared to 25.5 Gy with IMRT. Additionally, the mean dose values for the sublingual glands and the oral cavity were significantly lower with IMPT.18
In a cohort of 25 OPC patients, the mean dose to several organs at risk were significantly lower with IMPT than with IMRT, such as to the anterior and posterior oral cavity (8.3 vs 31 Gy, and 40.5 vs 54.3 Gy, respectively), to the inferior pharyngeal constrictor (32.8 vs 45.6 Gy), the middle pharyngeal constrictor (48.2 vs 57.0 Gy) and the esophagus (20.9 vs 33.6 Gy). Moreover, the dose to several central nervous system structures involved in nausea and vomiting were significantly lower in the IMPT plans.7
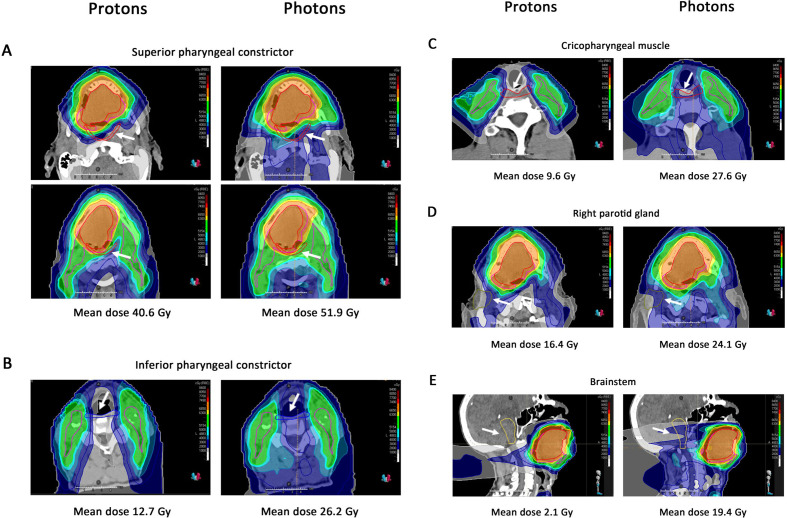
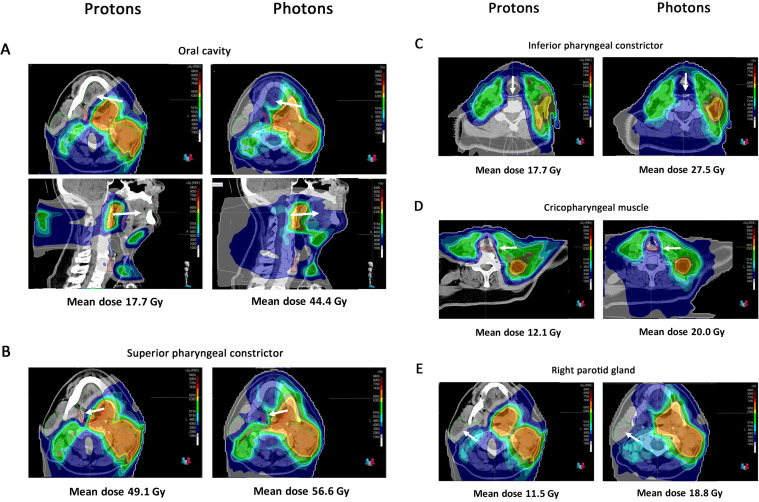
To illustrate this, Figures 1 and 2 show examples of dose distributions of photons and protons for two HPV-positive OPC patients from our centre. Compared to photons, protons resulted in much lower dose levels to multiple organs at risk simultaneously, including to the superior and inferior pharyngeal constrictor muscles (PCM), the cricopharyngeal muscle, the parotid glands, the oral cavity and the brainstem (Figures 1–2). Given that the risk of radiation-induced toxicity depends on dose to organs at risk, these lower dose levels are expected to translate into reduced treatment-related toxicity such as dysphagia and xerostomia, but also fatigue and nausea, which will be discussed further below.
Figure 1.
Proton and photon dose distribution of a patient with cT4N0M0 oropharyngeal cancer This 47-year-old patient presented with a cT4N0M0 oropharyngeal cancer (p16+/HPV+) involving the base of the tongue, mobile tongue and floor of the mouth. He was treated with chemoradiation: the primary tumour was treated up to 70 Gy in 35 fractions of 2 Gy; the elective neck Level I-IVa bilaterally was treated with 54.25 Gy in 35 fractions of 1.55 Gy. (A) Mean dose to the superior pharyngeal constrictor is 40.6 Gy for protons vs 51.9 Gy for photons. The superior pharyngeal constrictor is delineated in red, indicated by the white arrow. (B) Mean dose to the inferior pharyngeal constrictor is 12.7 Gy for protons vs 26.2 Gy for photons. The inferior pharyngeal constrictor is delineated in blue, indicated by the white arrow. (C) Mean dose to the cricopharyngeal muscle is 9.6 Gy for protons vs 27.6 Gy for photons. The cricopharyngeal muscle is delineated in red, indicated by the white arrow. (D) Mean dose to the right parotid gland is 16.4 Gy for protons vs 24.1 Gy for photons. The right parotid gland is delineated in olive and, indicated by the white arrow. (E) Mean dose to the brainstem is 2.1 Gy for protons vs 19.4 Gy for photons. The brainstem is delineated in yellow and is indicated with the white arrow. The red line represents the 70 Gy CTV and the pink line represents the 54.25 Gy CTV. CTV,clinical target volume; HPV, humanpapillomavirus.
Figure 2.
Proton and photon dose distribution of a patient with a cT1N3M0 oropharyngeal cancer This 66-year-old patient presented with a cT1N3M0 squamous cell carcinoma of the left tonsil (p16+/HPV+). He was treated with concurrent chemoradiation. The primary tumour on the left side and the pathological lymph nodes in Level II, III and IV in the left neck were treated with 35 fractions of 2 Gy to a total dose of 70 Gy. The elective neck Level II-IVa on the right side and Level I-V and Level VII on the left side were treated with 54.25 Gy in 35 fractions of 1.55 Gy. (A) Mean dose to the oral cavity is 17.7 Gy for protons vs 44.4 Gy for photons. The oral cavity is delineated in green and is indicated with the white arrow. (B) Mean dose to the superior pharyngeal constrictor is 49.1 Gy for protons vs 56.6 Gy for photons. The superior pharyngeal constrictor is delineated in red and is indicated with the white arrow. (C) Mean dose to the inferior pharyngeal constrictor is 17.7 Gy for protons vs 27.5 Gy for photons. The inferior pharyngeal constrictor is delineated in blue and is indicated with the white arrow. (D) Mean dose to the cricopharyngeal muscle is 12.1 Gy for protons vs 20.0 Gy for photons. The cricopharyngeal muscle is delineated in red and is indicated with the white arrow. (E) Mean dose to the right parotid gland is 11.5 Gy for protons vs 18.8 Gy for photons. The right parotid gland is delineated in olive and is indicated with the white arrow. The red line represents the 70 Gy CTV and the pink line represents the 54.25 Gy CTV. CTV,clinical target volume; HPV, humanpapillomavirus.
Clinical benefit of proton beam therapy in oropharyngeal cancer in terms of toxicity
At present, there are no data yet on the clinical benefit of protons based on randomised clinical trials (RCTs). However, the ongoing MD Anderson Cancer Center trial (NCT01893307) compares IMRT vs IMPT, both with concurrent chemotherapy, in Stage III-IV oropharyngeal cancer. The primary end point of this study is the rate and severity of late Grade 3–5 toxicity (www.clinicaltrials.gov). Second, a second RCT is emerging in the UK, i.e. the TORPEDdO-trial (TOxicity Reduction using Proton bEam therapy for Oropharyngeal cancer) which will compare IMRT and IMPT in locally advanced OPC treated with bilateral concurrent chemoradiation. In this study, a composite primary endpoint will be used including a patient-reported outcome measure (The University of Washington physical toxicity composite score and feeding tube dependence or severe weight loss 12 months after treatment.19
So far, the efficacy of protons to reduce radiation-induced toxicity has been investigated in some retrospective and prospective cohort studies. In this paragraph, we will summarise the most important clinical studies published so far.
In a retrospective study including 41 patients who underwent ipsilateral (post-operative) irradiation for major salivary gland cancer or cutaneous squamous cell carcinoma metastasis to the major salivary glands, proton therapy allowed better sparing of normal tissues and resulted in a significant reduction of acute treatment-related toxicity compared with IMRT.17 23 patients were treated with IMRT and 18 patients with proton therapy. Groups were well balanced regarding baseline, treatment and target volume characteristics. IMRT resulted higher maximum dose levels to the brainstem (median: 29.7 Gy for IMRT vs 0.6 Gy for proton therapy), higher maximum dose levels to the spinal cord (median: 36.3 vs 1.9 Gy) and higher dose levels to the oral cavity (mean: 20.6 vs 0.9 Gy) compared to proton therapy. These lower dose levels resulted in significantly lower acute toxicity rates among those treated with protons: Grade ≥2 acute dysgeusia (5.6 vs 65.2%), Grade ≥2 mucositis (16.7 vs 52.2%) and Grade ≥2 nausea (11.1 vs 56.5%). Moreover, patients treated with protons reported significantly lower rates of Grade ≥1 fatigue (38.9%) then those treated with IMRT (91.3%).20 This lower rate of fatigue might be explained by the lower dose to the brainstem (Figure 1E), as another study of head and neck cancer patients demonstrated that each Gy increase in maximum dose to the brainstem was associated with an increase of patient-reported acute fatigue during and 1 month after radiotherapy.21 No difference between IMPT and IMRT was noted with regard to 1 year locoregional control, freedom from distant metastases and overall survival. Longer follow-up and more patients are needed to determine whether the dose reductions obtained with protons also translates into lower rates of late toxicity, and to evaluate long-term locoregional tumour control. Although this study did not include patients with OPC patients, the results are relevant as patients with early stage OPC often receive unilateral irradiation as well, with comparable beam set-up.
The MD Anderson Cancer Center reported on a prospective cohort study which included 35 patients with OPC treated with IMPT and concurrent chemotherapy and 46 OPC patients treated with IMRT and a similar chemotherapy regimen. In the IMPT group, HPV status was negative in 5.7% of cases, positive in 74.3% and unknown in 20.0%, while in the IMRT group HPV-status was negative in 4.4%, positive in 13.0% and unknown in 82.6% respectively for the patients treated with IMRT. Patient-rated head and neck symptoms, including food taste, dry mouth, swallowing/chewing, fatigue, pain, appetite, mucus, sleep, drowsiness and distress were prospectively scored using the MDASI prior to and at several time points after completion of treatment. A significant reduction of gastrostomy tubes was observed during treatment with IMPT (20% for IMPT vs 48% for IMRT). Furthermore, taste changes during the subacute period and appetite during the subacute and chronic phase significantly favoured IMPT.18
Blanchard et al compared IMPT vs IMRT for OPC patients in a case matched analysis, including 50 IMPT and 100 IMRT patients.22 Patient weight, placement of a gastrostomy tube during or after radiation therapy, patient-rated fatigue and dry mouth were recorded. Locoregional control and overall and progression-free survival were similar between IMRT and IMPT. Moreover, no significant differences were noted in acute Grade 3 or higher dermatitis or mucositis. However, significantly lower rates of the composite endpoint grade ≥3 wt loss or gastrostomy tube feeding dependence were noted among patients treated with IMPT with an odds ratio of 0.44 at 3 months after radiotherapy (18.0% of patients treated with IMPT vs 34.0% for IMRT) and an odds ratio of 0.23 at 1 year after radiotherapy (8.0% of patients treated with IMPT vs 24.7% for IMRT). Furthermore, patient-reported grade ≥2 xerostomia at 3 months was significantly lower after IMPT with an odds ratio of 0.38 (42.0% with IMPT vs 61.2% with IMRT).19 These results indicate that the dose reductions obtained with IMPT results in significant reductions in xerostomia, tube feeding dependency and severe weight loss without compromising survival.
Radiation or chemoradiation with proton therapy (pencil beam scanning; n = 31) vs VMAT (n = 33) after transoral robotic surgery was compared in a cohort of 64 Stage I-IVA OPC patients. Patient-reported outcomes were prospectively collected up to 12 months after radiation.23 Both groups were similar in terms of age, site, stage, and dose delivered. Patients treated with proton therapy had significantly less dose to several normal structures involved in the production of saliva, including the contralateral parotid gland, ipsilateral and contralateral sublingual, ipsilateral and contralateral buccal, hard palate, tongue, and the oral cavity as a whole structure. This dose reduction translated into a clinical benefit in favour of proton therapy. While patients treated with proton therapy showed improvement in xerostomia from 3 to 12 months after radiation, VMAT patients reported stable or worsening xerostomia outcomes over this period. At 6 and 12 months after radiation, there was a clinically and statistically significant difference for xerostomia. Furthermore, dental problems were less for patients treated with proton therapy (3 and 6 months after radiation). Also, 12 months after radiation, more patients treated with VMAT complained of pain in the head and neck region. Physical and role function was better for OPC patients treated with proton therapy (6–12 months after radiation).
Zhang et al performed a retrospective analysis comparing mandibular dose levels and the rate of osteoradionecrosis in patients with OPC treated with IMRT (n = 534) or IMPT (n = 50).24 Minimum and mean mandibular doses were significantly lower for patients treated with IMPT. The V45-V70 were significantly associated with events of osteoradionecrosis and the volume of the mandible receiving various doses showed that percent volumes (V5-V70) were all significantly lower in the IMPT group compared to the IMRT group. This translated into a lower rate of osteoradionecrosis, which was 2.0% for IMPT vs 7.7% for IMRT.24
The results of these first cohort studies show that the lower dose levels to multiple organs at risk obtained with protons translate into reduced acute toxicity (i.e. up to 3 months after radiotherapy), while preserving tumour control. Next to lower rates of mucositis, tube feeding, xerostomia and distortion of the sense of taste, protons can improve general well-being by decreasing fatigue and nausea. Furthermore, proton therapy resulted in decreased rates of tube feeding dependency and severe weight loss up to 1 year after radiotherapy, and may decrease the risk of radionecrosis of the mandible. These results should be further validated in well-designed prospective studies either RCTs or model-based clinical evaluation studies.25 Furthermore, longer follow-up is required to establish the benefit of protons in terms of late toxicity, such as xerostomia and dysphagia.
Model-based approach for selecting patients with head and neck cancer for proton therapy
It should be noted that differences in dose distributions between photon and proton therapy do not necessarily imply a clinical benefit of protons for individual patients. To select patients who are most likely to benefit from protons in terms of reduction of toxicity, a stepwise approach has been introduced in the Netherlands, referred to as the model-based approach.7,23 This method uses Normal Tissue Complication Probability (NTCP) models, i.e. prediction models describing the relation between the dose delivered to organs at risk and the expected risk of a radiation-induced side-effect. In general, the estimated risk for a radiation-induced side-effect (the NTCP-value) will increase with increasing dose to specific organs at risk. As radiation-induced toxicity often depends on the radiation dose delivered to several organs at risk and also on independent other predictors, such as T-stage, weight loss, tumour location and concurrent chemotherapy, multivariable NTCP models are used to estimate the risk of radiation-induced toxicity based on differences in dose distribution in organs at risk.25,26 Multivariable NTCP models for xerostomia and dysphagia were developed and validated in head and neck cancer patients treated with photon radiotherapy.13 Next to some independent clinical predictors, the development of xerostomia is related to the dose to the parotid and submandibular glands. Swallowing problems correlate with dose to the oral cavity, and superior, middle and inferior pharyngeal constrictor muscles. When reliable multivariable NTCP models are available, both photon and proton radiation treatment plans can be optimised by decreasing the dose to these specific healthy tissue structures, without compromising the dose coverage of the therapeutic and prophylactic target volumes (model-based optimisation).10 By using individual planning comparative studies, in which dose distributions obtained with protons are compared with photon dose distribution, the potential benefit of proton therapy to further reduce toxicity can be assessed by integrating the results of the planning comparative study into NTCP models.7 In this way, the reduction in NTCP value for xerostomia and dysphagia by using protons instead of photons for each individual patient can be estimated in clinical practice, and the clinical benefit of proton therapy for an individual patient can be determined. This last step is required as not every change in dose to a specific organ at risk will result into a clinically relevant change in NTCP value.
The final number of patients who will be selected for proton therapy will depend on the threshold of NTCP value reduction that is accepted as clinically relevant. In the Netherlands, there is the consensus that a reduction of ≥10% for a Grade 2 radiation-induced toxicity and a reduction of ≥5% for a Grade 3 radiation-induced toxicity are clinically relevant.25 In case, there is a reduction of ≥15% for the summation of two Grade two complications (with a risk of ≥5% for each Grade two complication separately), or if there is a reduction of ≥7.5% for the summation of two Grade three complications (with a risk of ≥2.5% for each Grade three complication separately), proton therapy is also advised.
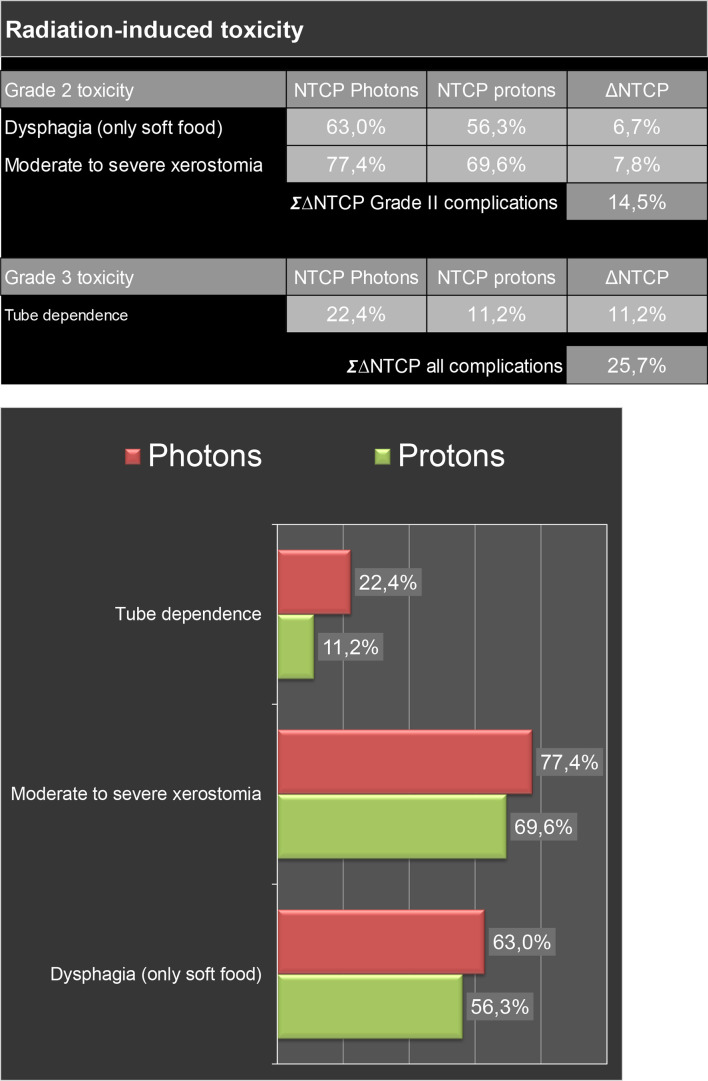
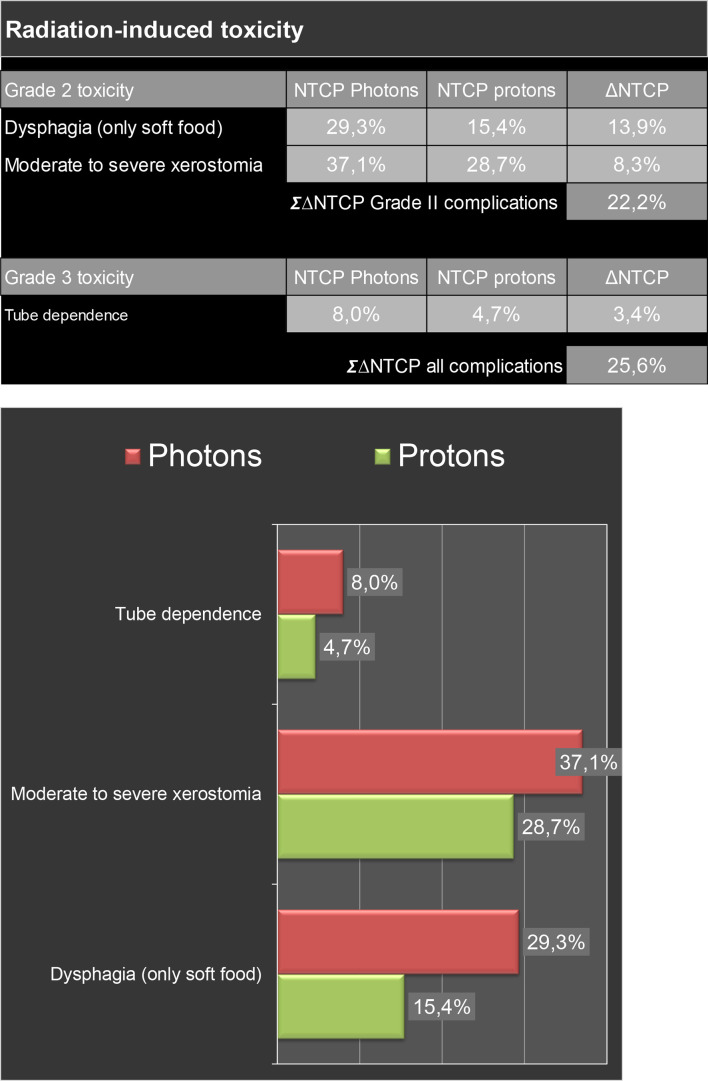
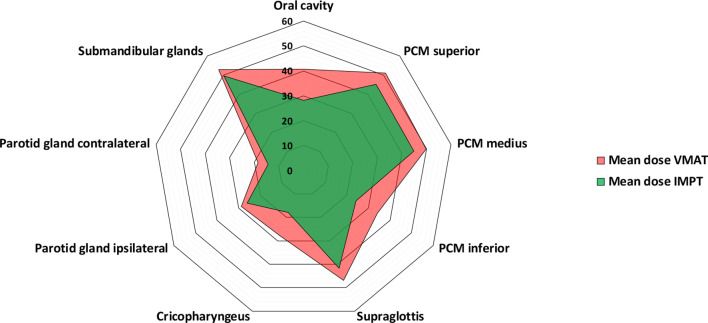
Since January 2018, the model-based selection procedure has been introduced in our centre. In Figures 3 and 4, estimated radiation-induced toxicity rates for two patients with OPC are shown for treatment with photon vs protons. Using the Dutch criteria for model-based selection, these patients qualified for proton therapy. Of the first 44 p16-positive cases included in this procedure, two patients did not qualify for a plan comparison as the NTCP-values of the VMAT-plans were already below the thresholds. In the 42 remaining patients, significant reductions were obtained using IMPT compared to VMAT in all organs-at-risk relevant for xerostomia and dysphagia (Figure 5). Eventually, 28 cases (67%) met the predefined selection criteria and thus qualified for proton therapy, of which 19 cases based on the ΔNTCP for dysphagia, 3 based on the ΔNTCP for xerostomia, 3 based on the ΔNTCP for tube feeding dependence and 3 based on the combination of the sum of ΔNTCP for dysphagia and xerostomia.
Figure 3.
Estimated radiation-induced toxicity rates of treatment with protons vs photons for a patient with cT4N0M0 oropharyngeal cancer Dose distribution of radiation with protons vs photons and dose to several organs at risk for this patient are shown in Figure 1. By using proton therapy, the lower dose to organs at risk translates into a decreased risk of tube dependency (11.2%). So, this patient was treated with proton therapy. Abbreviations: NTCP, normal tissue complication probability; ∑, sum; ∆, absolute difference.
Figure 4.
Estimated radiation-induced toxicity rates of treatment with protons vs photons for a patient with cT1N3M0 oropharyngeal cancer Dose distribution of radiation with protons vs photons and dose to several organs at risk for this patient are shown in Figure 2. By using proton therapy, the lower dose to organs at risk translates into a decreased risk of both Grade 2 and Grade 3 radiation-induced toxicity. So, this patient was treated with proton therapy. Abbreviations: NTCP, normal tissue complication probability; ∑, sum; ∆, absolute difference.
Figure 5.
Results of the plan comparison in 42 p16-positive OPC patients between VMAT (photons) and IMPT (protons). Both plans were optimised based on NTCP-models for moderate-to-severe patient-rated xerostomia (contralateral parotid gland), Grade ≥2 dysphagia (oral cavity and superior pharyngeal constrictor) and tube feeding dependence (superior and inferior pharyngeal constrictor, cricopharyngeal muscle and contralateral parotid gland). Abbreviations: IMPT, intensity-modulated proton therapy; PCM, pharyngeal constrictor muscle; VMAT, volumetric modulated arc therapy.
Model-based clinical evaluation
Next to selection of patients for proton therapy, the model-based approach can also be used for the clinical evaluation of proton therapy (i.e. model-based clinical evaluation). In a model-based clinical evaluation study, a plan comparison is made first in each individual patient. Then, the NTCP-profiles are calculated for both plans and a ∆NTCP-profile is then created by extracting the proton plan derived NTCP-profile from the photon plan derived NTCP-profile. Patients that qualify for protons according to the guidelines of model-based selection are then treated with proton therapy and the benefit of protons can then be determined by comparing the observed toxicity rates in the population of patients treated with protons with the mean NTCP-values derived from the photon plans.
Recently, Rwigema et al evaluated the clinical benefit of proton therapy in a retrospective analysis which included 30 OPC patients treated with IMPT.3 Toxicity outcome data were prospectively collected. In that study, mean NTCP-values for IMRT were 14.9 and 7.6% for Grade ≥2 dysphagia and Grade ≥3 dysphagia respectively, which were significantly lower for protons, i.e. 6.7 and 4.9% respectively, and were in line with observed rates of these two endpoints after proton therapy, i.e. 6.7 and 3.3%, respectively. For Grade ≥2 xerostomia, the mean NTCP-value for the photon plans was 18.6% which was significantly higher than the 4.7% obtained with the proton plans (p < 0.01) and in line with the observed Grade ≥2 xerostomia rate of 0% after proton therapy.
Conclusions and future perspectives
Due to the favourable prognosis and the rising incidence, the prevalence of HPV-positive OPC survivors at risk for late radiation-induced side-effects will rapidly increase. Therefore, minimising normal tissue radiation exposure to reduce acute and late radiation toxicity becomes increasingly important. Planning comparative studies show that proton therapy offers unique opportunities to decrease dose to virtually all organs at risk resulting in more favourable acute and late toxicity profile without compromising radiation dose delivered to the target volume. These results should be validated in well-designed clinical studies. By using model-based selection, patients that will benefit most from proton therapy can be identified.
Contributor Information
Tineke W.H. Meijer, Email: t.van.zon@umcg.nl.
Dan Scandurra, Email: d.scandurra@umcg.nl.
Johannes A. Langendijk, Email: j.a.langendijk@umcg.nl.
REFERENCES
- 1.Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tân PF, et al. . Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med Overseas Ed 2010; 363: 24–35. doi: 10.1056/NEJMoa0912217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gillison ML, Zhang Q, Jordan R, Xiao W, Westra WH, Trotti A, et al. . Tobacco smoking and increased risk of death and progression for patients with p16-positive and p16-negative oropharyngeal cancer. JCO 2012; 30: 2102–11. doi: 10.1200/JCO.2011.38.4099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Langendijk JA, Doornaert P, Verdonck-de Leeuw IM, Leemans CR, Aaronson NK, Slotman BJ. Impact of late treatment-related toxicity on quality of life among patients with head and neck cancer treated with radiotherapy. JCO 2008; 26: 3770–6. doi: 10.1200/JCO.2007.14.6647 [DOI] [PubMed] [Google Scholar]
- 4.Jellema AP, Slotman BJ, Doornaert P, Leemans CR, Langendijk JA. Impact of radiation-induced xerostomia on quality of life after primary radiotherapy among patients with head and neck cancer. Int J Radiat Oncol Biol Phys 2007; 69: 751–60. doi: 10.1016/j.ijrobp.2007.04.021 [DOI] [PubMed] [Google Scholar]
- 5.Gupta T, Agarwal J, Jain S, Phurailatpam R, Kannan S, Ghosh-Laskar S, et al. . Three-Dimensional conformal radiotherapy (3D-CRT) versus intensity modulated radiation therapy (IMRT) in squamous cell carcinoma of the head and neck: a randomized controlled trial. Radiother Oncol 2012; 104: 343–8. doi: 10.1016/j.radonc.2012.07.001 [DOI] [PubMed] [Google Scholar]
- 6.Vergeer MR, Doornaert PAH, Rietveld DHF, Leemans CR, Slotman BJ, Langendijk JA. Intensity-Modulated radiotherapy reduces radiation-induced morbidity and improves health-related quality of life: results of a nonrandomized prospective study using a standardized follow-up program. Int J Radiat Oncol Biol Phys 2009; 74: 1–8. doi: 10.1016/j.ijrobp.2008.07.059 [DOI] [PubMed] [Google Scholar]
- 7.Langendijk JA, Lambin P, De Ruysscher D, Widder J, Bos M, Verheij M. Selection of patients for radiotherapy with protons aiming at reduction of side effects: the model-based approach. Radiother Oncol 2013; 107: 267–73. doi: 10.1016/j.radonc.2013.05.007 [DOI] [PubMed] [Google Scholar]
- 8.ICRU Prescribing, recording, and reporting Proton-Beam. ICRU Report 78. Vol 7 2007.
- 9.Paganetti H, Niemierko A, Ancukiewicz M, Gerweck LE, Goitein M, Loeffler JS, et al. . Relative biological effectiveness (RBE) values for proton beam therapy. Int J Radiat Oncol Biol Phys 2002; 53: 407–21. doi: 10.1016/S0360-3016(02)02754-2 [DOI] [PubMed] [Google Scholar]
- 10.Harrabi S, Bauer J, Bahn E, Adeberg S, Haberer T, Alber M, et al. . Radiation-Induced brain injury after proton radiotherapy is linked to increased distal edge linear energy transfer (let) and anatomically variable radiation sensitivity. Int J Radiat Oncol Biol Phys 2019; 105: E99. doi: 10.1016/j.ijrobp.2019.06.2289 [DOI] [Google Scholar]
- 11.Eulitz J, Troost EGC, Raschke F, Schulz E, Lutz B, Dutz A, et al. . Predicting late magnetic resonance image changes in glioma patients after proton therapy. Acta Oncol 2019; 58: 1536–9. doi: 10.1080/0284186X.2019.1631477 [DOI] [PubMed] [Google Scholar]
- 12.Eulitz J, Lutz B, Wohlfahrt P, Dutz A, Enghardt W, Karpowitz C, et al. . A Monte Carlo based radiation response modelling framework to assess variability of clinical RBE in proton therapy. Phys Med Biol 2019; 64: 225020. doi: 10.1088/1361-6560/ab3841 [DOI] [PubMed] [Google Scholar]
- 13.Rwigema J-CM, Langendijk JA, Paul van der Laan H, Lukens JN, Swisher-McClure SD, Lin A. A model-based approach to predict short-term toxicity benefits with proton therapy for oropharyngeal cancer. Int J Radiat Oncol Biol Phys 2019; 104: 553–62. doi: 10.1016/j.ijrobp.2018.12.055 [DOI] [PubMed] [Google Scholar]
- 14.Apinorasethkul O, Kirk M, Teo K, Swisher-McClure S, Lukens JN, Lin A. Pencil beam scanning proton therapy vs rotational Arc radiation therapy: a treatment planning comparison for postoperative oropharyngeal cancer. Med Dosim 2017; 42: 7–11. doi: 10.1016/j.meddos.2016.09.004 [DOI] [PubMed] [Google Scholar]
- 15.Holliday EB, Kocak-Uzel E, Feng L, Thaker NG, Blanchard P, Rosenthal DI, et al. . Dosimetric advantages of intensity-modulated proton therapy for oropharyngeal cancer compared with intensity-modulated radiation: a case-matched control analysis. Med Dosim 2016; 41: 189–94. doi: 10.1016/j.meddos.2016.01.002 [DOI] [PubMed] [Google Scholar]
- 16.Kandula S, Zhu X, Garden AS, Gillin M, Rosenthal DI, Ang K-K, et al. . Spot-scanning beam proton therapy vs intensity-modulated radiation therapy for ipsilateral head and neck malignancies: a treatment planning comparison. Med Dosim 2013; 38: 390–4. doi: 10.1016/j.meddos.2013.05.001 [DOI] [PubMed] [Google Scholar]
- 17.van de Water TA, Bijl HP, Schilstra C, Pijls-Johannesma M, Langendijk JA. The potential benefit of radiotherapy with protons in head and neck cancer with respect to normal tissue sparing: a systematic review of literature. Oncologist 2011; 16: 366–77. doi: 10.1634/theoncologist.2010-0171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van de Water TA, Lomax AJ, Bijl HP, de Jong ME, Schilstra C, Hug EB, et al. . Potential benefits of scanned intensity-modulated proton therapy versus advanced photon therapy with regard to sparing of the salivary glands in oropharyngeal cancer. Int J Radiat Oncol Biol Phys 2011; 79: 1216–24. doi: 10.1016/j.ijrobp.2010.05.012 [DOI] [PubMed] [Google Scholar]
- 19.Price J, Hall E, West C, Thomson D. TORPEdO - A Phase III Trial of Intensity-modulated Proton Beam Therapy Versus Intensity-modulated Radiotherapy for Multi-toxicity Reduction in Oropharyngeal Cancer. Clin Oncol (R Coll Radiol) 2019; pii: S0936-6555: 30423–6. [DOI] [PubMed] [Google Scholar]
- 20.Romesser PB, Cahlon O, Scher E, Zhou Y, Berry SL, Rybkin A, et al. . Proton beam radiation therapy results in significantly reduced toxicity compared with intensity-modulated radiation therapy for head and neck tumors that require ipsilateral radiation. Radiother Oncol 2016; 118: 286–92. doi: 10.1016/j.radonc.2015.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sio TT, Lin H-K, Shi Q, Gunn GB, Cleeland CS, Lee JJ, et al. . Intensity modulated proton therapy versus intensity modulated photon radiation therapy for oropharyngeal cancer: first comparative results of patient-reported outcomes. Int J Radiat Oncol Biol Phys 2016; 95: 1107–14. doi: 10.1016/j.ijrobp.2016.02.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blanchard P, Garden AS, Gunn GB, Rosenthal DI, Morrison WH, Hernandez M, et al. . Intensity-modulated proton beam therapy (IMPT) versus intensity-modulated photon therapy (IMRT) for patients with oropharynx cancer - A case matched analysis. Radiother Oncol 2016; 120: 48–55. doi: 10.1016/j.radonc.2016.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sharma S, Zhou O, Thompson R, Gabriel P, Chalian A, Rassekh C, et al. . Quality of life of postoperative photon versus proton radiation therapy for oropharynx cancer. Int J Part Ther 2018; 5: 11–17. doi: 10.14338/IJPT-18-00032.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang W, Zhang X, Yang P, Blanchard P, Garden AS, Gunn B, et al. . Intensity-Modulated proton therapy and osteoradionecrosis in oropharyngeal cancer. Radiother Oncol 2017; 123: 401–5. doi: 10.1016/j.radonc.2017.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Langendijk JA, Boersma LJ, Rasch CRN, van Vulpen M, Reitsma JB, van der Schaaf A, et al. . Clinical trial strategies to compare protons with photons. Semin Radiat Oncol 2018; 28: 79–87. doi: 10.1016/j.semradonc.2017.11.008 [DOI] [PubMed] [Google Scholar]
- 26.Langendijk JA, Doornaert P, Rietveld DHF, Verdonck-de Leeuw IM, Leemans CR, Slotman BJ. A predictive model for swallowing dysfunction after curative radiotherapy in head and neck cancer. Radiother Oncol 2009; 90: 189–95. doi: 10.1016/j.radonc.2008.12.017 [DOI] [PubMed] [Google Scholar]