Abstract
Head and neck squamous cell carcinomas (HNSCC) preferentially spread to regional cervical tissues and lymph nodes. Here, we hypothesized that lymphotoxin-β (LTβ), receptor LTβR, and NF-κB-inducing kinase (NIK), promote the aberrant activation of alternative NF-κB2/RELB pathway and genes, that enhance migration and invasion of HNSCC. Genomic and expression alterations of the alternative NF-kB pathway were examined in 279 HNSCC tumors from The Cancer Genome Atlas (TCGA) and a panel of HNSCC lines. LTβR is amplified or overexpressed in HNSCC of the larynx or oral cavity, while LTβ, NIK, and RELB are overexpressed in cancers arising within lymphoid oropharyngeal and tonsillar sites. Similarly, subsets of HNSCC lines displayed overexpression of LTβR, NIK, and RELB proteins. Recombinant LTβ, and siRNA depletion of endogenous LTβR and NIK, modulated expression of LTβR, NIK and nuclear translocation of NF-κB2(p52)/RELB as well as functional NF-κB promoter reporter activity. Treatment with a NIK inhibitor (1,3[2H,4H]-Iso-Quinoline Dione) reduced the protein expression of NIK and NF-κB2(p52)/RELB, and blocked LTβ induced nuclear translocation of RELB. NIK and RELB siRNA knockdown or NIK inhibitor slowed HNSCC migration or invation in vitro. LTβ-induces expression of migration and metastasis related genes, including hepatocyte growth/scatter factor receptor MET. Knockdown of NIK or MET similarly inhibited the migration of HNSCC cell lines. This may help explain why HNSCC preferentially migrate to local lymph nodes, where LTβ is expressed. Our findings show that LTβ/LTβR promotes activation of the alternative NIK-NF-κB2/RELB pathway to enhance MET-mediated cell migration in HNSCC, which could be potential therapeutic targets in HNSCC.
Keywords: LTβ/LTβ receptor, NIK, RELB/NF-κB2, migration, HNSCC
1. Introduction
Head and neck squamous cell carcinoma (HNSCC) arises from epithelia of the upper aerodigestive tract, with an annual incidence of ~40,000 new cases, and affects over 300,000 survivors in the U.S. (1-3). Regional cervical spread and lymph node metastasis is a hallmark of HNSCC, and increased cell migration is an early and crucial event. Accordingly, many HNSCC tumors are first identified from lymph node metastasis, which is an important predictor of worse prognosis in HNSCC, decreasing survival by ~50% (4). Few investigations have addressed the molecular and biological mechanisms underlying cell migration of HNSCC. It is not very clear which factors and pathways promote migration of the primary tumor cells to lymph nodes, where the tumor cells gain growth and survival advantages.
Lymph nodes are secondary lymphoid organs, containing T and B lymphocytes and other immune cells. Many immune regulatory cytokines are produced in the lymph nodes, which nurture and modulate immune cell proliferation, maturation, migration, and defense functions. Among these, Lymphotoxin-β (LTβ) is one of the crucial cytokines produced by lymphocytes and lymphoid tissues (5-7). LTβ is an anchored transmembrane protein of 244 aa (18.5KDa), that can form soluble heterotrimers (LTα1β2, or LTα2β1), which are shed from the cell surface by proteolytic cleavage. LTβ heterotrimer LTα1β2 is capable of binding to receptor LTβR, which mediates signaling when internalized, and is then resynthesized (8). LTβ and LTβR play a critical role in controlling lymphoid organ development and facilitating efficient immune and inflammatory responses (6, 9-11). Interestingly, LTβR is implicated in the migration of T cells and neutrophils (12, 13), a feature deregulated in both hematopoietic and solid malignancies. Further, LTβR is not only expressed in lymphoid cells, but also in a wider range of normal tissues and tumor types, including many solid tumors (14, 15). However, the role of LTβ/LTβR mediated signaling and its involvement in HNSCC migration and other features of the malignant phenotype have not been characterized.
It has been well established that LTβ/LTβR signaling mediates activation of NF-κB inducing kinase (NIK), Inhibitor-κB Kinase-α (IKKα), and transcription factors NF-κB2/RELB in cells of the hematopoietic lineage and lymphoid system (16) (17). The NF-κB family contains five members, NF-κB1 (p105/p50) and NF-κB2 (p100/p52), RELA (p65), RELB, cREL. NF-κB1, RELA, are mainly involved in the canonical NF-κB pathways, while NF-κB2 and RELB participate in the non-canonical alternative NF-κB pathway (18, 19). Upon signaling from LTβ/LTβR, the kinase function of NIK is essential for IKKα induced NF-κB2/p100 processing to p52, which forms a complex with RELB that translocate to the nucleus to activate transcription of target genes (20, 21). Our laboratory previously identified the aberrant nuclear activation of canonical and alternative NF-κB/REL family members in HNSCC cells and tissues (22, 23). We and others previously documented that activation of NF-κB can modulate cancer-related inflammation and promote phenotypic alterations, such as cell proliferation, apoptosis evasion, therapeutic resistance, migration and metastasis (18, 22, 24, 25). However, the mechanism and role of LTβ and activation of the alternative NF-κB pathway in the malignant phenotype of HNSCC remain unknown.
Here, we hypothesized that LTβ/LTβR mediated signaling can activate alternative pathway and NF-κB2/RELB nuclear activation and promote migration of HNSCC. We discovered that LTβ and LTβR, NIK and RELB were overexpressed in subsets of HNSCC patient tissues and cell lines. In the HNSCC cell lines, LTβ induced activation of the alternative NF-κB pathway through NIK. NIK inhibitor or siRNA reduced protein expression of NIK, IKKα, RELB, and NF-κB2, and blocked RELB and NF-κB2 nuclear localization. Knockdown of NIK or RELB inhibited cell migration, and LTβ induced expression of genes implicated in metastasis and migration, including MET, which we previously showed promotes migration and metastasis of SCC (26). Our data revealed a novel role of LTβ mediated NIK activation of the alternative NF-κB pathway in expression of MET, which contributes to migration of HNSCC. Inhibition of migration by a NIK inhibitor provided evidence for the feasibility of therapeutic targeting of the alternative pathway. These signaling molecules involved in the alternative NF-κB pathway warrant further investigation as prognostic markers and therapeutic targets for HNSCC.
2. Materials and Methods
2.1. Cell lines and culture condition
A panel of 10 HNSCC cell lines was obtained from the University of Michigan squamous cell carcinoma (UM-SCC) series from Dr. T.E. Carey and cultured under standard growth conditions in MEM media with 10% FBS, penicillin and streptomycin (100 μg/mL) and 1% L-glutamine. Authentication of UM-SCC and other lines was done at the University of Michigan by DNA genotyping of alleles for 9 loci, D3S1358, D5S818, D7S820, D8S1179, D13S317, D18S51, D21S11, FGA, vWA, and the amelogenin locus in 2015, as described (27). These cell line stocks were used within 3 months of thawing and passage. UM-SCC 1 NF-κB Blazer reporter stable cell line, which contain stably expressed NF-κB transcription factor response elements upstream of the β-lactamase reporter gene, was established in our laboratory (28). This cell line is maintained in standard growth conditions supplemented with 10% FBS, penicillin and streptomycin (100 μg/mL), 1% NEAA, and Blasticidin (5μg/ml). All culture media, amino acids solutions, and antibiotics were purchased from Life Technology/Thermo Fisher Scientific. Cell DNA was sent for genotyping in 2008 and fall 2010 to compare and verify their unique origin from original stocks, as recently described (27). Human primary oral keratinocytes (HOK) from oral gingival mucosa were purchased from Science Cell Research laboratories and used as a control cell line. The cells were cultured in serum free Oral Keratinocyte Medium with supplements (Science Cell) for less than 4 passages.
2.2. Protein isolation and Western blot
The UM-SCC 46 cells were plated in 6 well plates at 3x105 cells per well or in 10 cm dishes at 1x106 cells per plate. Cells were treated with NIK inhibitor 1, 3[2H, 4H]-Isoquinolinedione (AK Scientific, Inc) (29, 30) for 24 hours starting from 500nM, 10μM and 25μM or with LTβ (LTα1β2, 100ng/ml, R&D Systems, CAT# 678-LY-010) stimulation for 4 hours and 24 hours. After 48 to 72 hours whole cells were harvested using NP40 lysis buffer (Life Science Technology) supplemented with Halt protease and phosphatase inhibitor cocktail (Thermo Fisher Scientific). Nuclear and cytoplasmic proteins were isolated using the Active Motif Kit (Active Motif). Proteins were quantitated by the Pierce BCA Protein Assay Kit (Life Technology). The equal amounts of lysates mixed with 5μl loading dye were run on NuPage® pre-cast 4-12% gradient Bis-Tris gel in MOPS SDS running buffer after heat denaturation. Samples were transferred for 6 minutes to nitrocellulose membranes using the Invitrogen iBlot system, according to the manufacturer’s protocol. Membranes were blocked for one hour in milk blocking buffer and incubated with primary antibodies overnight at 4°C. The antibodies were visualized with horseradish peroxidase-conjugated goat anti-mouse or anti-rabbit IgG (Santa Cruz Biotechnology) using enhanced chemiluminescence (Amersham and Thermo-scientific). Protein quantification was done by protein densitometry normalized to β-tubulin or actin with ImageJ 1.45k software. The following primary antibodies were used for Western blots: LTβR polyclonal antibody (Novus), NIK polyclonal antibody (Cell Signaling, Abcam), RELB monoclonal antibody (Cell Signaling), and NF-κB2/p52 monoclonal antibody (Millipore) and phospho-IKKα (Ser176) monoclonal antibody (Cell Signaling).
For testing NIK antibody’s specificity using the blocking peptide, UM-SCC 1 cell cytoplasmic lysates were prepared using a Nuclear Extraction Kit from Active Motif (Carlsbad, CA), and the proteins were quantitated using BCA assay. Duplicate samples of 15μg cytoplasmic proteins were loaded on Nu Page® pre-cast 4-12% gradient Bis-Tris gel after heat denaturation. After running, samples were transferred to Nitrocellulose membrane using the Invitrogen iBlot system according to the manufacturer’s protocol. Membrane was split in the middle to produce two identical blots. The NIK antibody (Abcam, ab19144, 0.5μg/ml) was pre-incubated with the blocking peptide (Abcam, ab8385 1μg/ml) or control buffer solution with agitation overnight at 4°C. Then the NIK antibody with or without blocking peptide were incubated with the membrane containing protein lysates overnight at 4°C, followed the standard Western blot protocol.
2.3. RNA isolation and qRT-PCR
For RNA isolation, cells were harvested using Trizol reagent (Life Technologies). The RNeasy Mini Kit from Qiagen (Santa Clarita, CA) was used for RNA isolation based on manufacturer's instructions. cDNA synthesis was performed on a GeneAmp® PCR system 9700 machine using the High-capacity cDNA Reverse Transcription Kit from Applied Biosciences (Foster City, CA) according to manufacturer’s instructions. Quantitative Real-time PCR was performed using TaqMan assay kit (Applied Biosystems). TaqMan Gene Expression RT-PCR primers includes LTβR, NIK, MET BIRC3, and SERPINE 1, were synthesized by Applied Biosystems. 30 ng of cDNA combined with primers and TaqMan Universal Master Mix (Applied Biosystems). The reactions were run in ViiA7 (AB Applied Biosystems). Relative gene expression was normalized to 18S endogenous control. Samples were assayed in triplicate and data are presented as the mean +/− standard deviation. Statistical Significance was determined using the student’s t-test and p values < 0.05 were considered statistically significant.
2.4. Gene knockdown by siRNA
siGENOME™ SMART pool siRNAs for LTβR,NIK and MET or non-targeting siRNA controls were purchased from IDT (Coralville IA, USA) and Dharmacon (Lafayette, CO, USA), and Ambion respectively. Individual siRNAs were tested and three duplexes of siRNAs with best knockdown efficiency and specificity for each target gene were selected. Cells were plated one day before transfection, and then transfected with transfection reagent alone as a control for nonspecific siRNA effects, or with 50 nM (Dharmacon) or 5 nM (IDT) of each siRNA, individually or in combination. Transfections were performed with Lipofectamine® 2000 or Lipofectamine® RNAiMAX transfection reagent, and Opti-MEM® reduced serum medium according to manufacturer’s instructions (Life Technologies). Cells were harvested at 48, 72, and 96 hours after transfection or with treatment of LTβ (100ng/ml) in respective wells 24hours before harvesting (31).
2.5. Reporter gene assay
UM-SCC 1 NF-κB Blazer reporter stable cell line was established by stable transfection of NF-κB reporter construct (gene blazer) and sorted in responding to TNF-α (28). Cells were plated in 96-well plates one day before transfection. β-lactamase reporter system (Life Technologies) was used to measure the LTβR and NIK knockdown effect on the NF-κB function by measuring the β-lactamase activity, which was recorded at 96 hours after siRNA transfection, with 24hours LTβ (100ng/ml) treatment before adding substrate (32). All measurements represent the mean of 6 replicates in each experimental condition.
2.6. Immunofluorescent microscopy
UM-SCC 46 cells were plated in Lab-TekR II chamber slide (Life Technologies) at 15,000 cells per well in 500μl complete media. Upon achieving 70 to 80% cell confluent, NIK inhibitor (1, 3[2H, 4H]-Isoquinolinedione) was added to individual wells followed by LTβ (100ng/ml) stimulation for another 4 or 12hours. Cells were then fixed using ice cold methanol for 15minutes, and permeabilized on ice (0.5% Triton X-100 and 0.05% SDS). Then cells were blocked on ice for 1 hour using blocking solution (0.1% Tween-20 and 3% BSA). Anti-RELB antibody (Santa Cruz) or Anti- NIK antibody (abcam) was added to each well at 1:100 dilution and incubated for 1 hour at room temperature. Cells were incubated with AF-594-linked IgG (1:1000 dilution) for 45 minutes in dark. The slides were mounted with DAPI VECTASHIELD mounting media, and were visualized on LSM 780 confocal microscope. Confocal images were analyzed using Zen 2012 SP1 software (black and blue editions).
2.7. Migration assay
UM-SCC 1 NF-κB Blazer reporter stable cells were seeded at 4x105 cells/well in 6-well plates and transfected with 100 nM siRNAs (Dharmacon) against NIK, RELB and MET alone, or in combination of NIK plus MET. Forty-eight hours later, scratches were made on the cell monolayers. For NIK inhibitor cells were treated for 24hours by adding NIK inhibitor at different concentrations after the cells reached 70–80% confluency. Wound closure was monitored at 0, 12, and 24 hours on an EVOS microscope (Life Technologies). Wound healing was quantitated by ImageJ 1.45k software (33) and plotted as a function of time.
2.8. Invasion Assay
QCM TM 24- Well kit (Fluorometric) ECM 554 used. UMSCC 1 cells that has been passaged 2-3 times and that are 80% confluent starved with serum free media for 24 h and then used for the invasion assay in presence and absence of FBS, LTB and inhibitor according to the protocol as directed in the Kit. Fluorescence measured in Synergy 2 fluorescence plate reader using 480/520 nm filter set at gain setting 65.
2.9. Statistical Analysis
Data were presented as mean ± standard deviation and significance was determined using the Student’s t-test p values of less than 0.05 were considered statistically significant.
3. Results
3.1. Overexpression and genetic alterations of LTα/β, LTβR, NIK (MAP3K14), and RELB in HNSCC tissues and cell lines
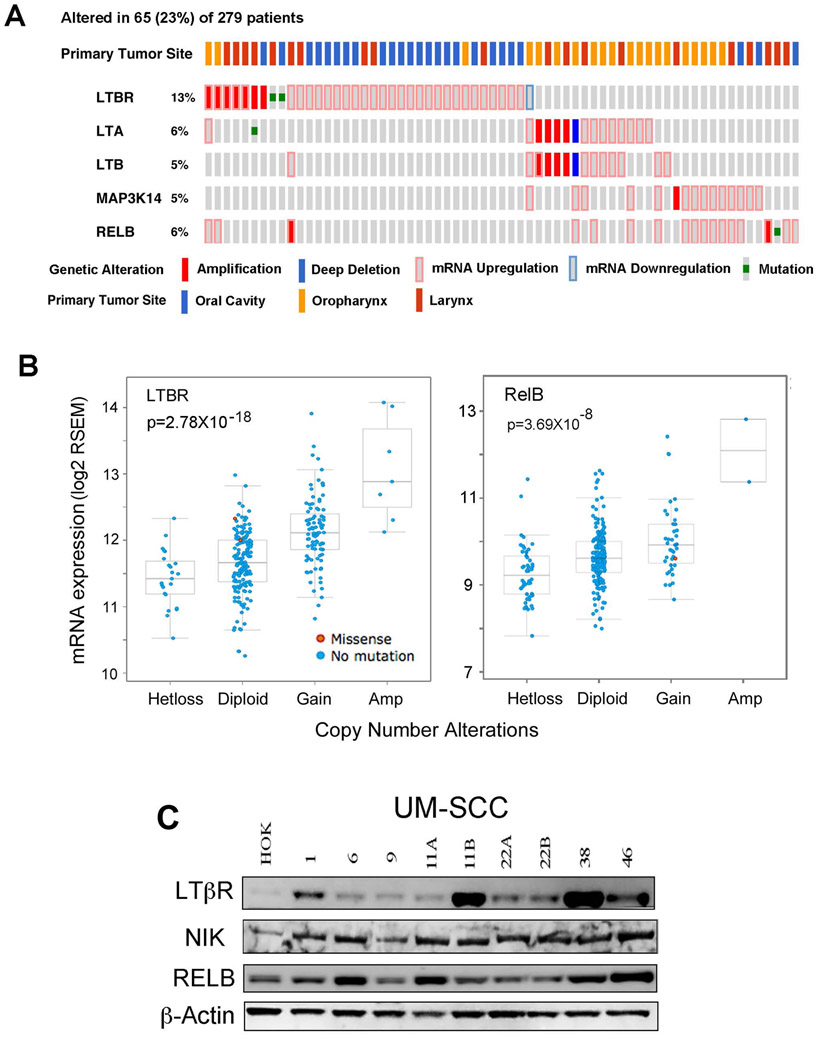
We previously showed that canonical and alternative NF-κB/REL subunits display aberrant nuclear activation in HNSCC tumors and cell lines (22, 23). However, the genomic status and expression of upstream LTβ/LTβR, NIK (MAP3K14) signal and RELB transactivating components of the alternative NF-κB pathway in HNSCC tumors has not been established. We explored if there are significant genomic and expression alterations of LTβ/LTβR, NIK, and RELB, using datasets from the HNSCC TCGA project comprising 279 human HNSCC cases (34). In Figure 1A, we present the oncoprint for individual HNSCC tumors (individual bars) with primary tumor sites labeled on the top, and those tumors displaying homozygous genetic and expression alterations of LTβR, LTα, LTβ, NIK, and RELB genes, below. Among 279 samples surveyed, 65 cases (23%) exhibited alterations in these molecules, mainly showing gene amplification and/or increased mRNA expression. The highest percentage of cases displayed alterations of LTβR (13%), with amplification and overexpression mainly seen in in HNSCC from larynx and oral cavity. Alternately, increased mRNA expression of LTα (6%) and LTβ (5%) lymphotoxin subunits, as well as NIK (5%) and RELB (6%) were often co-occurrent (p<0.05, Fisher’s exact test), and observed mainly in oropharyngeal /tonsillar cancers enriched for lymphoid tissues. Interestingly, patients carrying amplification and overexpression of LTβR are mutually exclusive from those bearing the alterations of other molecules involved in the alternative NF-κB pathway (p<0.0001, Fisher’s exact test, Figure 1A). Furthermore, the association between RNA expression and DNA copy number variation (CNV) for all of the above mentioned molecules was examined, and a highly significant correlation was found for CNV and expression of LTβR and RELB (Figure 1B). Thus, our data provide evidence for genomic alterations and/or increased expression affecting LTβR, LTα, LTβ, NIK, and/or RELB in HNSCC tumor tissues. We next surveyed the relative protein expression of alternative pathway signaling components LTβR, NIK and RELB in a panel of HNSCC (UM-SCC) cell lines and primary human oral keratinocytes (HOKs). Several UM-SCC cell lines showed increased LTβR, NIK, and/or RELB protein expression as compared to HOKs (Figure 1C, Supplemental Figure 1). Among these, two cell lines, UM-SCC 1 and 46, were selected for further studies.
Figure 1. The genetic and expression alterations of LTB/and LTBR, NIK (MAP3K14), and RELB in HNSCC tissues and cell lines.
Data of genetic and expression alterations were extracted from TCGA HNSCC project through cbioportal. (A) Oncoprint presents individual cases (each bar) with genetic and expression alterations of LTBR, LTA, LTB, MAP3K14, RELB. 65 (23%) cases with genetic and/or expression alterations were shown. Genetic and expression alterations are presented as solid red: homozygous amplification; solid blue, homozygous deletion; green: mutation; grey bar with blue frame: mRNA down-regulation compared with tumor mean; grey bar with pink frame: mRNA up-regulation compared with tumor mean. Primary tumor sites are indicated at the top, blue: oral cavity; orange: oropharynx; red: Larynx. % on the left represents the percentage of cases with alterations. (B) RNA expression and DNA copy number variation (CNV) were correlated and presented. The statistical correlation of RNA expression with CNV was examined and presented as p value. The X-axis showed DNA CNV, as Hetloss (shallow deletion), Diploid (normal), Gain (one copy gain), and AMP (amplification of two copies or higher). Y-axis is mRNA expression and presented as log2 RSEM (RNA-Seq by Expectation-Maximization). Mutation status, blue: no mutation; red: missense mutation. The relationship between CNV and RNA expression is tested by Pearson correlation. (C) Protein expression of LTβR, NIK and RELB in whole cell lysates from a panel of UM-SCC lines and HOK cells were detected by Western blot, using β-actin as a loading control.
3.2. LTβ and NIK promoted RELB and NF-κB2 protein nuclear translocation and induced NF-κB reporter activity
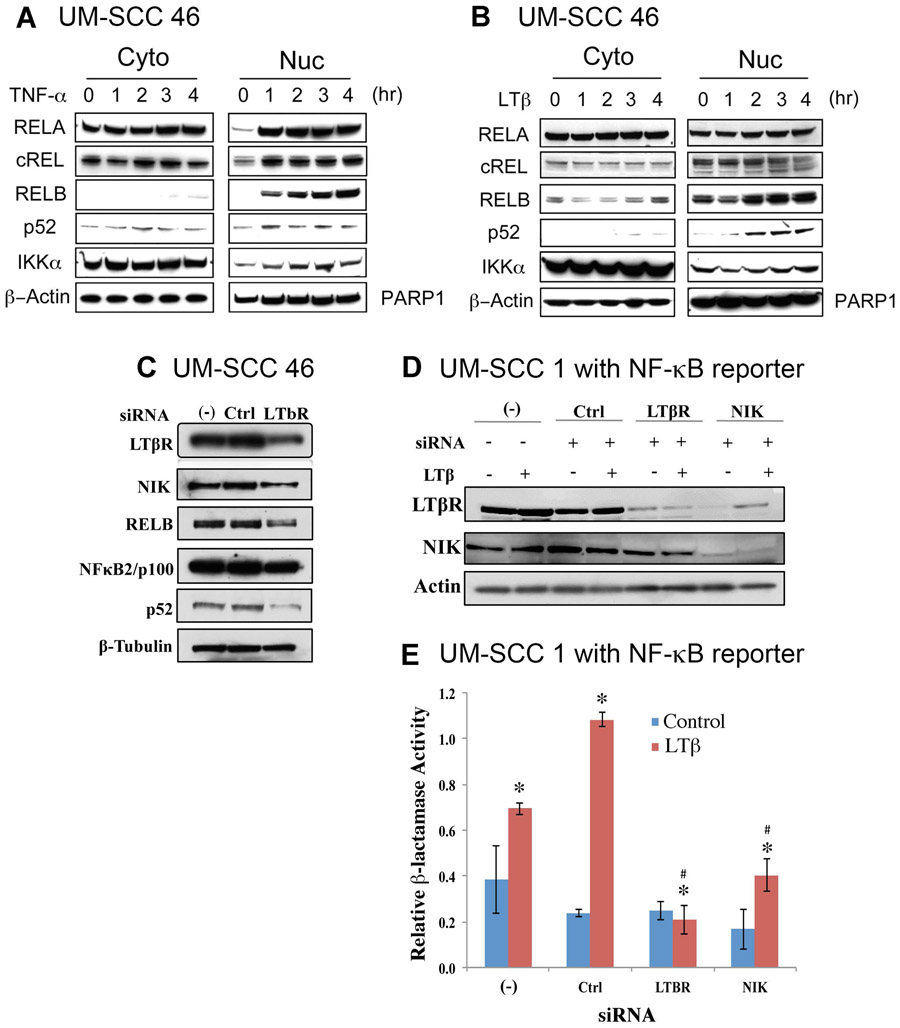
Next, we examined the effects of classical and alternative pathway ligands TNF-α and LTβ to stimulate NF-κB subunits in UM-SCC 46 cells, which displayed increased protein expression of LTβR, NIK and RELB (Figure 1C). TNF-α induced rapid and strong activation of canonical pathway subunits RELA or cREL within one hour, as well as slower induction of alternative pathway subunit RELB over 3-4 hours (Figure 2A, Supplemental Figure 2), consistent with prior reports that RELA can secondarily induce RELB expression and nuclear translocation respectively (22). In contrast, LTβ, which is a major upstream signal for the alternative NF-κB pathway in lymphoid tissues, did not strongly modulate canonical RELA/c-REL subunits, but selectively promoted a gradual increase in nuclear translocation of RELB and NF-κB2(p52) (Figure 2B, Supplemental Figure 3).
Figure 2. TNF-α and LTB stimulated and LTβR and NIK knocking down inhibited NF-κB proteins and function activity in HNSCC cell line.
The HNSCC cell line UM-SCC 46 was treated with TNFα (25ng/ml, A) or LTβ (100ng/ml, B), and the protein levels of NF-κB subunits were measured at different time points via Western blot. β-actin was used as a loading control for the cytoplasmic fraction, and PARP1 for the nuclear fraction. (C) Knockdown of LTβR modulated its target kinase NIK, RELB, NF-κB2/p100/p52 protein expression in UM-SCC-46 cells. Cells were transfected with LTβR siRNA, whole cell lysates were harvested 96h after transfection, and measured for LTβR, NIK, RELB and NF-κB2/p100/p52 by Western blot. β-tubulin was used as loading control. (D) Protein expression of LTβR and NIK after knockdown of LTBR and NIK by siRNAs in stable NF-κB Blazer Reporter UM-SCC-1 stable cell line. Whole cell lysates were harvested by sonication of samples from each well of the β-Lactamase assay plate. Western blot was performed and β-Actin was used as loading control. (E) Knockdown of LTBR and NIK by siRNAs affected NF-κB reporter function in NF-κB Blazer Reporter UM-SCC-1 stable cell line. Relative β-Lactamase units were measured after stimulation with LTβ (100ng/ml) for 24h before harvesting the cells at 96h. * indicates statistical significance induced by LTB treatment, and # indicates statistical significance after siRNA knockdown (p-value<0.05 by t-test). Data were calculated from triplicates of a representative experiment.
To study the functional effects of endogenous LTβR on NF-κB activation, we performed gene knockdown of LTβR by siRNA, and verified decreased LTβR mRNA between 48h-96h in the UM-SCC 46 cell line (Supplemental Figure 4A). We observed decreased LTBR protein expression by 96h after transfection (Figure 2C). Knockdown of LTβR decreased downstream alternative pathway NIK, RELB, and processed p52 proteins (Figure 2C, Supplemental Figure 5A). We next confirmed the effects of NIK siRNA knockdown, which significantly inhibited NIK mRNA between 48h-96h in UM-SCC 46 cells (Supplemental Figure 4B). To confirm the siRNA knockdown and LTB stimulation effects in a second HNSCC cell line, we selected UM-SCC 1 cells, which displayed increased expression of the alternative pathway components (Figure 1C), and for which we have established a stable NF-κB reporter cell line. We examined the effects of LTBR or NIK siRNA on their protein levels without or with LTβ stimulation in UM-SCC 1 cells. Knockdown LTBR by siRNA decreased LTBR, and NIK protein levels, when normalized for protein loading (Figure 2D, Supplemental Figure 5B). Knockdown of NIK protein was also associated with decreased LTBR protein expression (Figure 2D, Supplemental Figure 5B). In addition, NIK gene knockdown significantly decreased LTβ induced NIK mRNA expression (Supplemental Figure 4C), and RELB gene knockdown significantly decreased basal and LTβ induced RELB gene expression in UM-SCC 46 cells (Supplemental Figure 4D).
We next examined the effects of LTβ stimulation on functional NF-κB reporter activity after LTβR or NIK knockdown in the UM-SCC 1 NF-κB reporter line. LTβ treatment significantly increased the reporter activity in cells, and knockdown of LTβR or NIK reduced NF-κB reporter activity induced by LTβ stimulation (Figure 2E, Supplemental Figure 5C). Thus, we observed that LTβ modulates signals through alternative pathway proteins LTβR, NIK, RELB and NF-κB2 p52, and NF-κB reporter activity, in independent HNSCC cell lines.
3.3. NIK inhibitor (1, 3[2H, 4H]-Isoquinolinedione) decreased NIK protein and components of the alternative NF-κB pathway
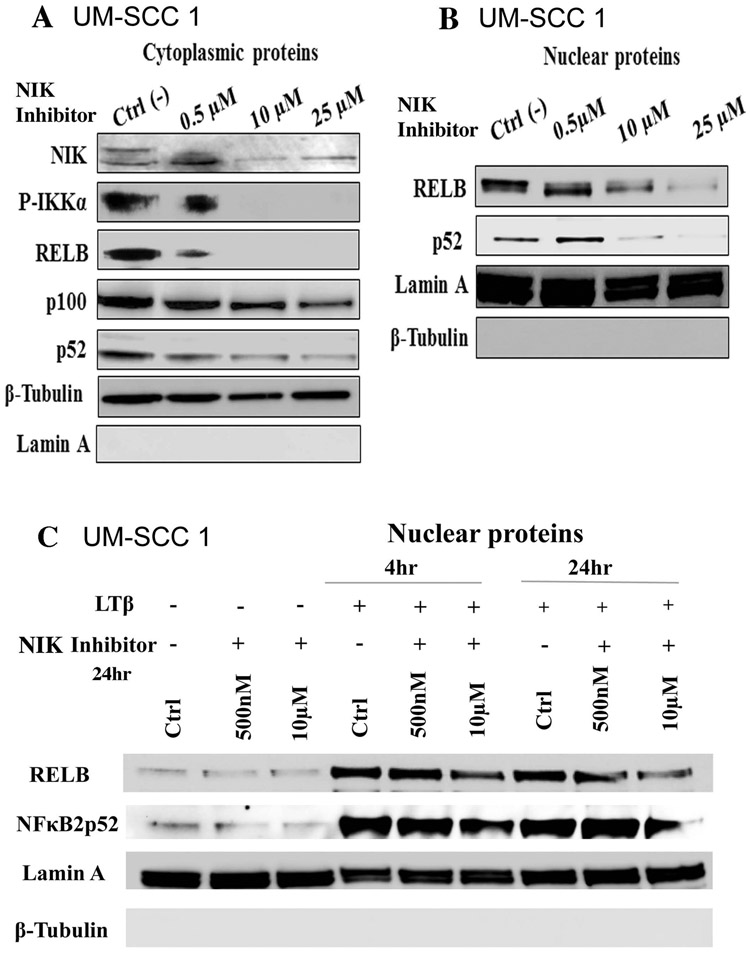
Next, we examined the effects of the NIK inhibitor (1,3[2H,4H]-Isoquinolinedione) over a range of concentrations on the downstream components of the alternative NF-κB pathway in UM-SCC 1 cells. We tested the NIK inhibitor in this cell line, because we have been successfully established cell migration assay and invasion assay using this adherent cell line. NIK inhibitor significantly inhibited NIK, p-IKKα, RELB, and NF-κB2/p100, and p52 protein expression in the cytoplasm in a dose dependent fashion (Figure 3A). After treatment, the NIK inhibitor partially reduced expression of all of the proteins examined at the dose of 0.5μM, and completely blocked p-IKKα and RELB protein expression at the doses of 10μM and 25μM (Figure 3A). In addition, significant reductions in nuclear protein expression of both RELB and NF-κB2/p52 were observed by the treatment of NIK inhibitor at different dosages (Figure 3B, Supplemental Figure 6A). As phosphorylation of NIK by IKKα has been reported as a doublet as observed in Figure 3A, we confirmed the NIK antibody specificity for both bands using a NIK-specific blocking peptide. A loss of the double bands of NIK protein was detected by the antibody after incubation with the blocking peptide (Supplemental Figure 6B). We next examined the effect of NIK inhibitor under LTβ stimulation for 4h and 24h (Figure 3C). LTβ stimulation increased both RELB and NF-κB2/p52 in nuclear fractions, while more abundant nuclear RELB and NF-κB2/p52 proteins remained when treated with both the NIK inhibitor and LTβ stimulation (Figure 3C). Our data shows that NIK inhibitor decreased the levels of NIK and downstream proteins involved in the alternative NF-κB pathway, while LTβ stimulation partially diminished the effects of the NIK inhibitor on RELB and NF-κB2/p52 nuclear protein. In these experiments, we used Lamin A as the protein loading control for the nuclear fraction, and did not observe significant effects of NIK inhibitor on Lamin A protein expression.
Figure 3. NIK Inhibitor (1, 3 [2H, 4H]-Isoquinolinedione) reduced expression and nuclear translocation of alternative NF-kB proteins.
The UM-SCC1 cells were treated with NIK inhibitor (1, 3 [2H, 4H]-Isoquinolinedione) at the concentration of 500nM, 10μM, 25μM for 24h. Cells were fractionated into cytoplasmic (A) and nuclear fractions (B) and analyzed by Western blot. Lamin A (Nuclear) and β-tubulin (cytoplasm) were used as loading controls. (C) Cells were treated with NIK inhibitor at 500nM, 10μM, for 24h, followed with LTβ (100ng/ml) treatment for 4h and 24h. RELB and NF-κB2/p52 were examined in the nuclear protein fractions. Lamin A and β-tubulin were used as loading controls.
3.4. NIK inhibitor decreased NIK and RELB expression and cytoplasmic/nuclear localization
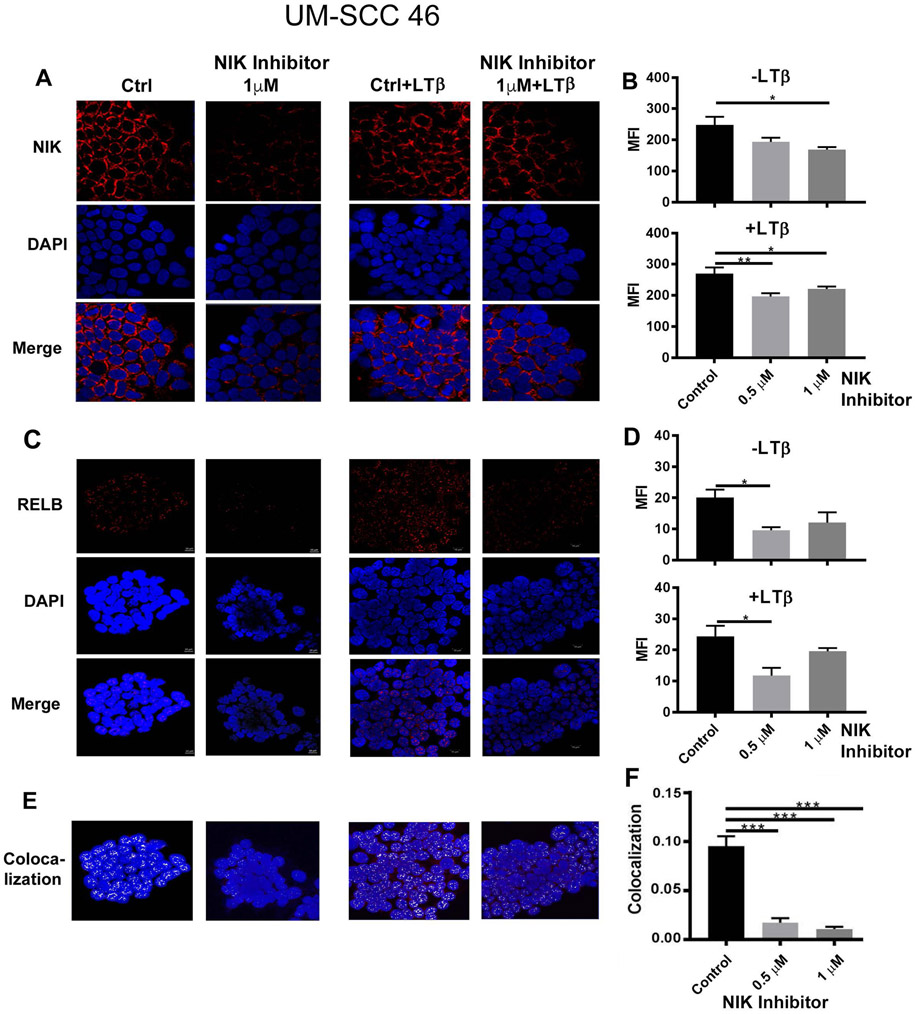
To further confirm the effects of the NIK inhibitor and LTβ on NIK and RELB expression and cytoplasmic/nuclear localization in another model, we treated UM-SCC 46 cells with the NIK inhibitor for 24 hours, or plus LTβ stimulation for 4 hours, and performed immunofluorescent staining using antibodies against NIK and RELB (Figure 4). The concentration range of the NIK inhibitor at 0.5 and 1μM in this assay was based on effects observed in the previous titration (Supplemental Figure 6A). 1μM NIK inhibitor decreased the diffuse staining pattern of NIK in the perinuclear cytoplasm by immunofluorescence (Figure 4A, left panels; 4B, upper panel), LTβ stimulation for 4 hours attenuated this inhibitory effect on NIK staining (Figure 4A, right panels, and 4B; lower panel), consistent with stabilization of NIK. RELB staining exhibited diffuse punctate nuclear staining pattern, while decreased RELB protein expression were observed when treated by NIK inhibitor at 0.5μM (Figure 4C, D). A strong inhibition of co-localized RELB staining with nuclear DAPI staining was observed, supporting an effective blockage of RELB nuclear localization and activation (Figure 4E, F). Together, these data show that the NIK inhibitor blocked NIK in the perinuclear cytoplasm and inhibited RELB nuclear localization, while LTβ stimulation enhanced NIK and partially reversed these effects.
Figure 4. NIK stability in perinuclear cytoplasm and RELB nuclear localization are inhibited by NIK inhibitor 1, 3[2H, 4H]-Isoquinolinedione.
(A) UM-SCC-46 cells were pre-treated with NIK inhibitor at 1μM for 24h (left panels), and then stimulated with LTB for 4h (100ng/ml, right panels). Cells were stained with anti-NIK antibody (red) and DAPI for nuclear staining (blue). The images were captured by immunofluorescence microscopy. (B) The quantifications of the images were presented as before (upper panel) or after treatment (lower panel) with different doses of NIK inhibitors (0.5 and 1μM). (C) The images of RELB immunostaining were presented, the left panels showed cells treated with NIK inhibitor but without LTB stimulation. The right panels showed cells treated with NIK inhibitor plus LTB stimulation. (D) Quantification of the images presented in panel C. Upper panel is from the cells treated with NIK inhibitor but without LTB treatment (left panels in C). The low panel is the quantification of the images from the right panel of C. RELB co-localization with DAPI nuclear staining (E) and quantification (F). The photography was taken by confocal microscopy with 63X. Statistical analysis: Student T-test, *p<0.05, **p<0.01, ***p<0.001.
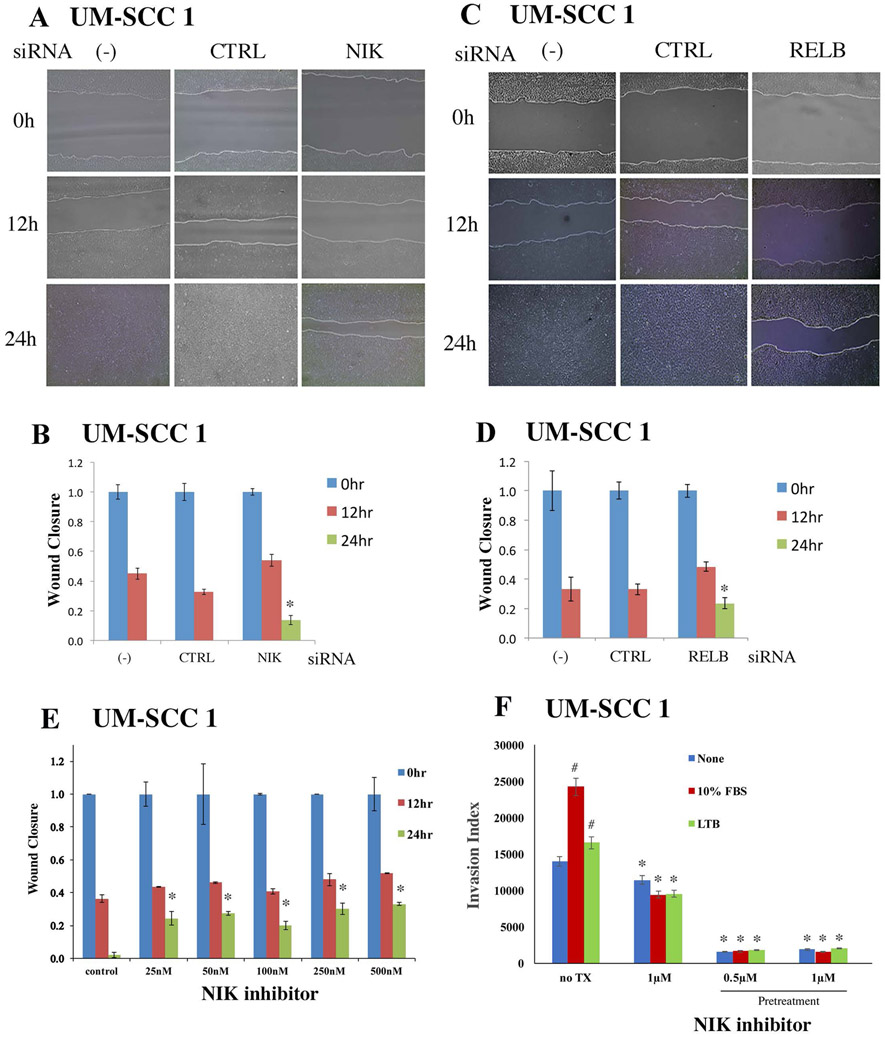
3.5. Knockdown of NIK and RELB inhibited cell migration in the HNSCC cells.
To explore the functional roles of alternative NIK signaling and RELB, we knocked down NIK or RELB individually, and examined their effects on cell migration as measured by wound closure in UM-SCC 1 cells. The assay examined cell migration as the wound closure for 12 and 24 hours after scratching a gap in the confluent monolayers. Knockdown of NIK (Figure 5A, B) or RELB (Figure 5 C, D) individually suppressed migration and closure. This effect was not attributable to anti-proliferative activity, as we did not observe significant effects on cell proliferation 24 hours after siRNA knockdown of LTBR and RELB, when measured by WST1 assay (Supplemental Figure 7A). We further analyzed cell migration affected by NIK inhibitor, and observed a dose dependent inhibitory effect on UM-SCC 1 cell migration (Figure 5E). The inhibitory effect on the cell migration was not due to the drug suppression of cell proliferation, as we have titrated the NIK inhibitor and showed there is no inhibitory effects on cell proliferation from day 1 through day 3 using the doses up to 20μM (Supplemental Figure 7B). Furthermore, we performed an invasion assay using UM-SCC 1 cells. While the cell migration/wound closure assay is to examine the collective cell migration in two dimensions, the invasion assay is using the transwell to analyze the ability of single cells to directionally respond to various chemo-attractants and migrate through a physical barrier and invade into a 3D matrix (matrigel). In this invasion assay, we observed that 10% FBS as the chemo-attractant, significantly increased cell invasion, while NIK inhibitor suppressed basal and FBS induced cell invasion (Figure 5F). Compared to FBS, LTβ alone only slightly enhanced invasion, indicating that serum may contain additional factor(s) that promote invasion. Thus, our data from NIK siRNA and inhibitor, as well as RELB siRNA, support the hypothesis that NIK and RELB activate the NF-κB alternative pathway and promote cell migration and invasion.
Figure 5. Knockdown of NIK or RELB by siRNAs or by NIK inhibitor inhibited migration of UM-SCC1 cells in vitro.
UM-SCC1 cells were transfected with siRNAs for NIK (A) or RELB (C) for 48h. Scratches were made on cell monolayers, and wound closure was monitored at 0, 12, and 24 h. (B, D) Quantification of wound healing from images was done using Image J program, and statistical significance was calculated. The distances between the wounds were measured, and significant differences by individual knockdowns were examined by t-test (*p <0.05). (E) UM-SCC1 cells were pretreated with the NIK inhibitor, and after 24h the cell monolayers were scratched and treated with the inhibitor. Wound closure was measured at 0h, 12h, and 24h from images using Image J program. * indicates a statistical significance when compared with controls (p-value <0.05 by t-test). (F) UM-SCC1 cells were starved in serum free media for 24hr, and plated in 100mm plates at 1x106cells per plate, with or without NIK inhibitor pretreatment for 4hr. The cells were subjected to invasion assay in EC matrix coated chamber in serum free media with chemoattractant in the outer wells, either adding 10% fetal bovine serum (FBS, red bar) or LTB (green bar). The invasion index was measured and calculated following the manufacturer’s protocol. # indicated a statistical significance of FBS or LTB induced invasion, and *indicates the statistical significance of NIK inhibitor decreased cell invasion compared to the corresponding controls (p-value <0.05 by t-test).
3.6. LTβ and NIK modulated expression of survival and metastasis genes, and knockdown of NIK and MET by siRNA inhibited cell migration
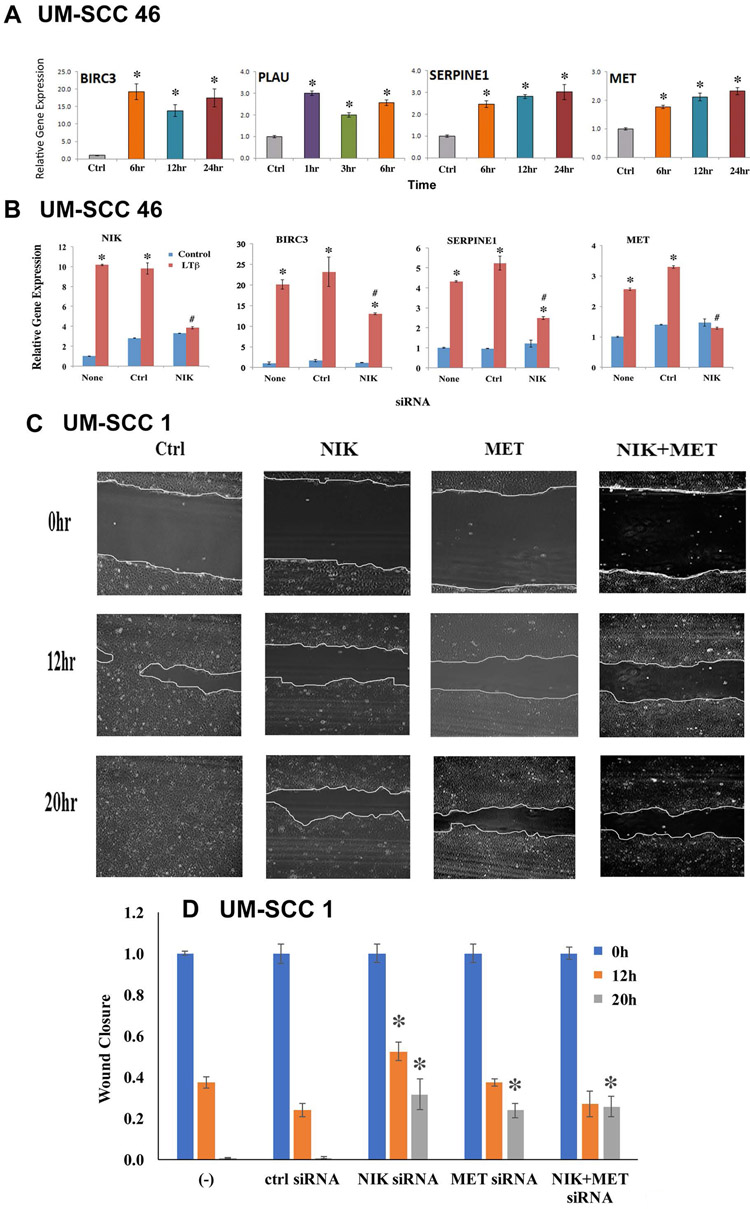
We previously identified NF-κB modulated genes that promote cell survival and migration in HNSCC (35-37). Of these, pilot oligonucleotide expression array screening for genes modulated by LTβ, identified BIRC3 (inhibitor of apoptosis 2, IAP2), PLAU and SERPINE1 proteinases, and MET receptor (38). However, how LTβ and NIK are involved in the regulation of expression of these genes has not been studied. Using quantitative RT-PCR, we confirmed the time course observed by expression profiling for these 4 genes in UM-SCC 46 cells (Figure 6A). Accordingly, LTβ treatment induced more than a 20-fold increase of BIRC3 expression by 6 hours. Induction of PLAU is relatively more rapid, peaking at one hour after treatment, while SERPINE1 and MET expression steadily increased between 6 hours and 24 hours.
Figure 6: LTβ, NIK, and MET modulated expression of survival and metastasis genes, and promoted cell migration.
(A) UM-SCC 46 cells were treated with LTβ (100ng/ml), and the relative mRNA expression of LTβ upregulated genes were examined by qRT-PCR at different time points. The data were compared with time 0 (no treatment), and calculated with three replicates as mean + SD. An asterisk indicates p-value <0.05 using t-test. (B) UM-SCC46 cells were transfected with 50nM NIK siRNA for 48h, mRNA expression of NIK, BIRC3, and SERPINE1, and MET were measured by qRT-PCR. Statistical significance differences were calculated with three replicates at *p ≤0.05 by t-test. (C) UM-SCC1 cells were transfected with NIK or MET siRNA alone, or in combination for 48h. Scratches were made on cell monolayers, wound closure was monitored at 0, 12 and 20h, and images were taken using EVOS microscope. (D) Quantification of wound healing with statistical significance. The distances between the wounds were measured, and significant differences were examined using image J. * indicates a statistical difference calculated with three replicates and shown with p-value <0.05 by t-test.
Since knockdown of NIK and RELB suppressed cell migration, we asked whether the activation of alternative NF-κB signaling molecules modulated gene expression involved in cell survival and migration. We showed that LTβ stimulation induced gene expression of NIK, BIRC3, SERPINE1, and MET in an independent experiment using UM-SCC 46 cells (Figure 6B), consistent with the observations in Figure 6A. Knockdown of NIK by siRNA completely blocked LTβ induced NIK and MET expression, while partially inhibiting LTβ induced SERPINE1 and BIRC3 gene expression (Figure 6B). Interestingly, MET is an hepatocyte growth factor receptor implicated in promoting cell migration, and we previously showed that MET overexpression promotes migration and scattering of murine SCC cells (26). In this experiment, we knocked down either NIK or MET alone, or in the combination. We found that knockdown of either gene individually significantly decreased cell migration in UM-SCC 1 cells, while the knockdown both genes together did not exhibit additive effects (Figure 6 C and D), consistent with MET being a target of the LTβ-NIK pathway. Together, our data shows that LTβ and NIK promote MET gene expression, and NIK and MET knockdown have similar inhibitory effects on cell migration.
4. Discussion
Most previous studies related to LTβ/LTβR signaling are in lymphocytes and lymphoid tissues, where LTβ/LTβR play critical roles in the communication between lymphocytes and stromal cells during lymphoid organogenesis (6, 7, 10), and in host defense for infectious, inflammatory, and autoimmune diseases (6, 11, 15, 18). Aberrant activation of LTβ/LTβR signaling and the effects on the malignant phenotype have not been well studied in cancers, especially in solid tumors. In this study, we provide evidence that genetic and expression alterations of signaling molecules involved in the alternative NF-κB pathways, including LTβ/LTβR, NIK and RELB, occur in HNSCC tissues and cell lines (Figure 1, 2). In HNSCC, LTβ/LTβR is an upstream modulatory signal for activation of NIK and alternative NF-κB subunits RELB and NF-κB2/p52 (Figure 2). Knockdown of LTBR and NIK by siRNA or a chemical NIK inhibitor diminished activation of the alternative NF-κB pathway (Figure 2-4, Supplemental 4-6), inhibited cell migration and invasion, and suppressed expression of the MET gene involved in cell migration (Figure 5, 6, Supplemental Figure 8). Our results revealed a novel mechanism whereby LTβ/LTβR signaling mediated activation of the alternative NF-κB pathway promotes migration and other mediators implicated in pathogenesis of HNSCC. The finding that LTβ activation of the alternative pathway promotes expression of MET and migration, could help explain why and how HNSCC preferentially spread to local lymph nodes, where LTβ is abundantly expressed by lymphoid cells (26, 39, 40).
In HNSCC tumor samples from the TCGA datasets, we observed tissue site-specific alterations of the signaling molecules involved in the alternative NF-κB pathway. LTβR genomic amplification at the chromosome 12p13.3 locus and/or its overexpression occurs in ~13% HNSCC cases, particularly those arising from larynx and oral cavity, which are mostly HPV (−). LTβR and RELB exhibited significant correlations between CNV and mRNA expression, which support a genetic basis as a contributing factor for the activation of alternative NF-κB signaling pathway in these HNSCC (Figure1B). In contrast, other molecules, such as LT ligands, NIK and RELB are amplified and/or overexpressed mainly in oropharyngeal tumors, which are enriched for HPV (+) HNSCC, and tonsil tissue containing lymphoid cells. Consistent with this, we observed aberrant nuclear immunostaining of NF-κB2/RELB in a series of predominantly oropharyngeal tumors (23). These tissues provide a microenvironment rich in lymphokines and cytokines, which could induce the overexpression of LT ligands, NIK and RELB genes. Interestingly, frequent LTβR gene amplification was also previously found in nasopharyngeal carcinoma (NPC), a distinct type of head and neck cancer associated with infection with Epstein-Barr virus (EBV) that was not represented in TCGA (41). NPC also often first presents clinically as swollen lymph nodes in the neck, and the tumors arise in a microenvironment heavily infiltrated with lymphoid cells. Together, these observations suggest that either amplified or overexpressed LTβR, as well as LT ligands, NIK and RELB, could contribute to aberrant activation of the alternative NF-κB signaling in different HNSCC subtypes.
Consistent with TCGA tumor tissue data, several HNSCC cell lines studied here exhibited increased protein expression of LTβR, NIK and RELB compared with HOK cells (Figure 1C). LTβR protein is profoundly increased in UM-SCC 1, 11B, 38, and 46 lines, which are derived from oral cavity or larynx, consistent with data in HNSCC tissues from TCGA datasets (Figure 1A, 1C). Furthermore, overexpression of NIK and RELB protein were observed in most of cell lines, indicating the broader downstream alterations affecting these HNSCC cell lines. We validated the significance of these results in two cell lines, using UM-SCC 46 and UM-SCC 1 cells in siRNA knockdown, NIK inhibitor, Western blot and/or reporter gene analyses. The results presented here are also consistent with our previous publications demonstrating the effects of LTβ and expression and function of IKKα and IKKβ functional mutants on alternative and classical pathway activation in UM-SCC 1 (22, 42), and LTβ and TRAF3 in HPV(+) line UM-SCC 47 (43). In those studies, IKKα and TRAF3 were also shown to modulate alternative pathway subunits as well as migration.
These tumor cell lines reflect similar alterations in expression of alternative pathway components observed in human HNSCC tissues, and provide in vitro models for mechanistic studies. As expected, TNF-α induced rapid and strong induction of the canonical pathway as well as expression of alternative NF-κB pathway components (Figure 2A, Supplemental Figure 2), as described in previous studies of other tumor cell lines by us and others (27, 36, 42, 44-46). This study provided direct evidence that LTβ/LTβR-NIK signaling mediates alternative NF-κB2/RELB activation. LTβ preferentially induced NF-κB2(p52)/RELB nuclear translocation in UM-SCC 46 cells (Figure 2B, Supplemental Figure 3). We previously treated UM-SCC 1 cells with LTβ, and demonstrated modulation of the alterative NF-κB pathway components IKKα and p100/p52 (22). In addition, we also studied the effects of LTβ in an HPV(+) HNSCC cell line, UM-SCC 47, and observed results consistent with those presented for HPV(−) line in Figure 1C (43). Knockdown of LTβR and NIK decreased NIK, RELB, NF-κB2/p100 and p52 protein expression, and suppressed the NF-κB reporter function by β-lactamase assay (Figure 2C-E). LTβ treatment stabilized NIK protein expression levels, and partially blocked the effects of NIK inhibitor on RELB and NF-κB2(p52) nuclear localization as shown by immunofluorescent staining (Figure 4). These data support the importance of LTβ signaling mediated activation of the alternative NF-κB pathway in HNSCC, as well as potential to counter the effects of NIK siRNA knockdown or a pharmacologic NIK inhibitor.
NIK has been reported to be the downstream kinase of LTβR in the alternative NF-κB pathway, and LTβ/LTβR binding enhances NIK stability (11, 47, 48). We demonstrated here that targeting of NIK either by siRNA or small molecule inhibitor 1, 3[2H, 4H]-Isoquinolinedione attenuates protein expression of NIK, p-IKKα, NF-κB2(p100/p52), and RELB as well as nuclear translocation of NF-κB2 and RELB (Figure 2C, D, and 3A, 4B). From the NIK structure (49, 50), it is known that the C-terminal domain has phosphorylation sites for IKKα (17, 21), consistent with the doublet observed in HNSCC by western in our study. The association of NIK with active IKKα mediates NF-κB2/p100 phosphorylation. Consistent with this, it appeared the NIK inhibitor beginning at a concentration of ~0.5 μM diminished IKKα and the slower migrating NIK band, followed by blocking NF-κB2/p52 and RELB heterodimer and nuclear translocation.
HNSCC often migrate to the lymph nodes, where high levels of LTβ may be produced and expressed locally. LTβ presence in the local lymph nodes and tumor microenvironment may attract and promote tumor cell migration and metastasis (6). The critical steps of tumor metastasis include cancer cell migration and invasion, which could be assayed in vitro by cell migration/wound closure assay and transwell invation assays. The cell migration assay, also known as wound healing assay, is an in vitro technique to examine the collective cell migration in two dimensions. In our experiment, the monolayer of HNSCC cells was scratched with a pipette tip and created a gap in the monolayer. The cells were migrated into the gap, remaining in contact during their coordinated movement. The cell migration was imaged over 24 hours using a light microscope. This experiment is testing the rate of wound closure, a measure of the speed of the collective motion of the cells. The migration of cancer cells is one important step related to cancer metastatic progression, where cancer cells must migrate to a distant site and form foci. The cell invasion is another critical step in cancer metastatic progression. The cancer cells must invade through extracellular matrix, intravasate into blood circulation, and attach to a distant site to form new cancer colonies. The invasion assay is using the transwell to analyze the ability of single cells to directionally respond to various chemo-attractants, migrate through a physical barrier, and invade into a 3D matrix. In this study, we obtained direct evidence that knockdown of NIK or RELB inhibited cell migration (Figure 5A-D). The modulation of cell migration by the alternate NF-κB and other pathways was supported by evidence that LTβ stimulation regulates expression of several genes implicated in cell migration, metastasis, and other features of the malignant phenotype. LTβ induced genes involved in cell survival (BIRC3/cIAP2), and cell migration and metastasis (PLAU, SERPINE1, MET) in HNSCC cells. Knockdown of NIK by siRNA completely inhibited LTβ induced NIK and MET expression, and partially blocked LTβ induced BIRC3/cIAP2 and SERPINE1 expression (Figure 6A, B). BIRC3/cIAP2 is a member of the inhibitor-of-apoptosis protein family, providing the anti-apoptosis signaling downstream of TNFR through TRAF1 and TRAF2 (51). PLAU/uPA converts plasminogen to plasmin, and stimulates cell migration via a PLAUR signaling complex containing TYK2 and PI3K (39, 40). SERPINE1 is involved in the extracellular matrix digestion, and could promote cell migration (52). We showed previously that MET, the receptor for hepatocyte growth factor/scatter factor, promotes cell migration and metastasis of SCC (26, 37). Knockdown of NIK or MET alone, and in combination, as well as treated with NIK inhibitor, directly inhibited cell migration (Figure 5 and 6). Supporting the contribution of other factors to migration, tumor cells cultured in 10% FBS, which contain EGF and HGF, more strongly induced invasion than LTβ alone (Figure 5). Together, these findings provide a missing link connecting LTβR and NIK mediated alternative NF-κB activation with MET/HGF expression and cell migration. Future studies in syngeneic murine models of metastatic SCC could enable dissection of the role of LTβ, LTBR and the alternative pathway using KO mice and cell lines. Our data could help explain the underlying mechanisms of early migration of HNSCC to the regional lymph nodes. Blocking activation of the alternative NF-κB pathway mediated through LTβ and NIK signaling could be a potential strategy for developing targeted therapy for local-regional HNSCC spread and metastasis. The recent development of specific NIK inhibitors (47) may increase the feasibility of in vivo studies in syngeneic preclinical models and human clinical trials.
Supplementary Material
Acknowledgements
This project is supported by NIDCD intramural projects Z01-DC-00016, 73, 74. We thank NIH Fellows Editorial Board and NIH library Editor Cindy Clark for their helpful suggestion in editing our manuscript. We thank Drs. Hong Yin (Feist-Weiller Cancer Center, LSUHSC-Shreveport) and Chiju Wei (Shantou University) for reading this manuscript and providing helpful suggestions. We like to express special thanks for technical supports from Sophie Carlson, and the caption service from Ms. Frances Freeman and Felicia Pickering, NIH Interpreters, Access Interpreting, Inc.
Footnotes
Conflict of Interest Statement: The authors have declared that no conflict of interest exists.
References:
- 1.Rothenberg SM, Ellisen LW. The molecular pathogenesis of head and neck squamous cell carcinoma. Journal of Clinical Investigation. 2012;122(6):1951–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016. Jan-Feb;66(1):7–30. PubMed PMID: 26742998. [DOI] [PubMed] [Google Scholar]
- 3.Leemans CR, Braakhuis BJ, Brakenhoff RH. The molecular biology of head and neck cancer. Nat Rev Cancer. 2011. January;11(1):9–22. PubMed PMID: 21160525. [DOI] [PubMed] [Google Scholar]
- 4.Shang Xie HX, Shan Xiaofeng, Liu Baozhong, Wang Kan, Cai* Zhigang. Clinicopathological and prognostic significance of survivin expression in patients with oral squamous cell carcinoma: evidence from a meta-analysis. PLOS One. 2015; 10(2):0116517. PubMed PMID: 25710884. Pubmed Central PMCID: PMC4339736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bauer J NS, Reisinger F, Zoller J, Yuan D,Heikenwalder M. Lymphotoxin, NF-kB, and cancer: the dark side of cytokines. Dig Dis. 2012;30(5):453–68. PubMed PMID: 23108301. [DOI] [PubMed] [Google Scholar]
- 6.Bjordahl RL, Steidl C,Gascoyne RD,Ware CF Lymphotoxin network pathways shape the tumor microenvironment. Curr Opin Immunol. 2013. April;25(2):222–9. PubMed PMID: 23339845. Pubmed Central PMCID: PMC3646954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Upadhyay V, Fu YX Lymphotoxin signalling in immune homeostasis and the control of microorganisms. Nat Rev Immunol. 2013. April;13(4):270–9. PubMed PMID: 23524463. Pubmed Central PMCID: PMC3900493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ganeff C, Remouchamps C,Boutaffala L,Benezech C,Galopin G,Vandepaer S et al. Induction of the alternative NF-kappaB pathway by lymphotoxin alphabeta (LTalphabeta) relieson internalization of LTbeta receptor. Mol Cell Biol. 2011. November;31(21):4319–34. PubMed PMID: 21896778. Pubmed Central PMCID: PMC3209329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dejardin E The alternative NF-kappaB pathway from biochemistry to biology: pitfalls and promises for future drug development. Biochem Pharmacol. 2006. October 30;72(9):1161–79. PubMed PMID: 16970925. [DOI] [PubMed] [Google Scholar]
- 10.Futterer Agnes MK, Luz Arne, Marie H, Kosco Vilois, Pfeffer Klaus. . The Lymphotoxin Beta Receptor Controls Organogenesis and Affinity Maturation in Peripheral Lymphoid Tissues. Immunity. 1998;9:59–70. [DOI] [PubMed] [Google Scholar]
- 11.Remouchamps C, Boutaffala L,Ganeff C,Dejardin E Biology and signal transduction pathways of the Lymphotoxin-alphabeta/LTbetaR system. Cytokine Growth Factor Rev. 2011. Oct-Dec;22(5–6):301–10. PubMed PMID: 22152226. [DOI] [PubMed] [Google Scholar]
- 12.Zhu M, Chin RK, Tumanov AV, Liu X, Fu YX. Lymphotoxin Receptor Is Required for the Migration and Selection of Autoreactive T Cells in Thymic Medulla. The Journal of Immunology. 2007; 179(12): 8069–75. [DOI] [PubMed] [Google Scholar]
- 13.Newman I WPC. Chemotactic activity of lymphotoxin and tumour necrosis factor alpha for human neutrophils. Immunology. 1989;66(2):318–20. [PMC free article] [PubMed] [Google Scholar]
- 14.De Trez C Lymphotoxin-beta receptor expression and its related signaling pathways govern dendritic cell homeostasis and function. Immunobiology. 2012. December;217(12): 1250–8. PubMed PMID: 22795648. [DOI] [PubMed] [Google Scholar]
- 15.Wolf MJ, Seleznik GM,Zeller N,Heikenwalder M The unexpected role of lymphotoxin beta receptor signaling in carcinogenesis: from lymphoid tissue formation to liver and prostate cancer development. Oncogene. 2010. September 09;29(36):5006–18. PubMed PMID: 20603617. [DOI] [PubMed] [Google Scholar]
- 16.Yamada T M T Yorita K Uchida D Matsushima A Iwamasa K et al. Abnormal Immune Function of Hemopoietic Cells from Alymphoplasia (aly) Mice, a Natural Strain with Mutant NF-B-Inducing Kinase. The Journal of Immunology. 2000;165(2):804–12. [DOI] [PubMed] [Google Scholar]
- 17.Sun SC. Non-canonical NF-kappaB signaling pathway. Cell Res. 2011. January;21(1):71–85. PubMed PMID: 21173796. Pubmed Central PMCID: PMC3193406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.VanWaes C Nuclear factor-kappaB in development, prevention, and therapy of cancer. Clin Cancer Res. 2007. February 15;13(4):1076–82. PubMed PMID: 17317814. [DOI] [PubMed] [Google Scholar]
- 19.Van Waes C, Yu M, Nottingham L, Karin M. Inhibitor-kappaB kinase in tumor promotion and suppression during progression of squamous cell carcinoma. Clin Cancer Res. 2007. September 01;13(17):4956–9. PubMed PMID: 17785544. [DOI] [PubMed] [Google Scholar]
- 20.Ling l, Cao Z, and Goeddel DV. NF-kB inducing kinase activates IKK alpha by phosphorylation of Ser-176. PNAS. 1998. 95:3792–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun S-C. The Non canonical NF-kB pathway. Immunological rReviews. 2012;246:125–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nottingham LK, Yan CH, Yang X, Si H, Coupar J, Bian Y, et al. Aberrant IKKalpha and IKKbeta cooperatively activate NF-kappaB and induce EGFR/AP1 signaling to promote survival and migration of head and neck cancer. Oncogene. 2014. February 27;33(9):1135–47. PubMed PMID: 23455325. Pubmed Central PMCID: PMC3926900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Allen C, Saigal K, Nottingham L, Arun P, Chen Z, Van Waes C. Bortezomib-induced apoptosis with limited clinical response is accompanied by inhibition of canonical but not alternative nuclear factor-{kappa}B subunits in head and neck cancer. Clin Cancer Res. 2008. July 01;14(13):4175–85. PubMed PMID: 18593997. [DOI] [PubMed] [Google Scholar]
- 24.DiDonato JA, Mercurio F, Michael Karin. NF-KB and the link between inflammation and cancer. Immunological Reviews. 2012; 246:: 379–400. [DOI] [PubMed] [Google Scholar]
- 25.Basseres DS, Baldwin AS. Nuclear factor-kappaB and inhibitor of kappaB kinase pathways in oncogenic initiation and progression. Oncogene. 2006. October 30;25(51):6817–30. PubMed PMID: 17072330. [DOI] [PubMed] [Google Scholar]
- 26.Dong G, Lee TL, Yeh NT, Geoghegan J, Van Waes C, Chen Z. Metastatic squamous cell carcinoma cells that overexpress c-Met exhibit enhanced angiogenesis factor expression, scattering and metastasis in response to hepatocyte growth factor. Oncogene. 2004. August 19;23(37):6199–208. PubMed PMID: 15221009. [DOI] [PubMed] [Google Scholar]
- 27.Brenner JC, Graham MP, Kumar B, Saunders LM, Kupfer R, Lyons RH, et al. Genotyping of 73 UM-SCC head and neck squamous cell carcinoma cell lines. Head Neck. 2010. April;32(4):417–26. PubMed PMID: 19760794. Pubmed Central PMCID: PMC3292176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Conti MA, Saleh AD, Brinster LR, Cheng H, Chen Z, Cornelius S, et al. Conditional deletion of nonmuscle myosin II-A in mouse tongue epithelium results in squamous cell carcinoma. Sci Rep. 2015. September 15;5:14068. PubMed PMID: 26369831. Pubmed Central PMCID: PMC4572924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ranuncolo SM, Pittaluga S, Evbuomwan MO, Jaffe ES, Lewis BA. Hodgkin lymphoma requires stabilized NIK and constitutive RelB expression for survival. Blood. 2012. November 1;120(18):3756–63. PubMed PMID: 22968463. Pubmed Central PMCID: PMC3488888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mortier J, Masereel B, Remouchamps C, Ganeff C, Piette J, Frederick R. NF-kappaB inducing kinase (NIK) inhibitors: identification of new scaffolds using virtual screening. Bioorg Med Chem Lett. 2010. August 1;20(15):4515–20. PubMed PMID: 20580552. [DOI] [PubMed] [Google Scholar]
- 31.Amarzguioui M Improved siRNA-mediated silencing in refractory adherent cell lines by detachment and transfection in suspension. Biotechniques. 2004;36(5):766–70. [DOI] [PubMed] [Google Scholar]
- 32.ZHANG JI-HU TDYC, and OLDENBURG KEVINR. A Simple Statistical Parameter for Use in Evaluation and Validation of High Throughput Screening Assays. JOURNAL OF BIOMOLECULAR SCREENING 1999; 4(2). [DOI] [PubMed] [Google Scholar]
- 33.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nature Methods. 2012;9(7):671–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cancer Genome Atlas N Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature. 2015. January 29;517(7536):576–82. PubMed PMID: 25631445. Pubmed Central PMCID: PMC4311405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eytan DF, Snow GE, Carlson S, Derakhshan A, Saleh A, Schiltz S, et al. SMAC Mimetic Birinapant plus Radiation Eradicates Human Head and Neck Cancers with Genomic Amplifications of Cell Death Genes FADD and BIRC2. Cancer Res. 2016. September 15;76(18):5442–54. PubMed PMID: 27469115. Pubmed Central PMCID: PMC5026594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Si H, Lu H, Yang X, Mattox A, Jang M, Bian Y, et al. TNF-alpha modulates genome-wide redistribution of DeltaNp63alpha/TAp73 and NF-kappaB cREL interactive binding on TP53 and AP-1 motifs to promote an oncogenic gene program in squamous cancer. Oncogene. 2016. November 03;35(44):5781–94. PubMed PMID: 27132513. Pubmed Central PMCID: PMC5093089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gang Dong ZC, Zhi-Yu Li, Ning T Yeh Caren Bancroft C, and Van Waes Carter. Hepatocyte growth factor/scatter factor-induced activation of MEK and P13K signal pathways contributes to expression of proangiogenic cytokines interleukin-8 and vascular endothelial growth factor in Head and Neck squamous cell carcinoma. Cancer res. 2001;61(15):5911–8. [PubMed] [Google Scholar]
- 38.Xinping Yang HC, Wang Ru, Jianhong Chen, Han Si, Anthony Saleh, Van Waes Carter, Chen Zhong. Genomic, RNAi Screening, and Functional Validation Reveals Cross Activation of Classical and Alternative NF-kB Pathways in Head and Neck Squamous Cell Carcinoma of Different HPV Status AACR 2017 meeting, Washington DC, 2017. 2017. [Google Scholar]
- 39.Kirsten L Elzer DAH, Mitchell I Chernin, and Josef F Novak. Differential Effects of Serine Proteases on the Migration of Normal and Tumor Cells: Implications for Tumor Microenvironment. Integrative Cancer Therapies. 2008. 7(4):282–94. [DOI] [PubMed] [Google Scholar]
- 40.Majumdar M, Tarui T, Shi B, Akakura N, Ruf W, Takada Y. Plasmin-induced migration requires signaling through protease-activated receptor 1 and integrin alpha(9)beta(1). J Biol Chem. 2004. September 03;279(36):37528–34. PubMed PMID: 15247268. [DOI] [PubMed] [Google Scholar]
- 41.Chou J, Lin YC, Kim J, You L, Xu Z, He B, et al. Nasopharyngeal carcinoma--review of the molecular mechanisms of tumorigenesis. Head Neck. 2008. July;30(7):946–63. PubMed PMID: 18446839. Pubmed Central PMCID: PMC3046044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang X, Lu H, Yan B, Romano RA, Bian Y, Friedman J, et al. DeltaNp63 versatilely regulates a Broad NF-kappaB gene program and promotes squamous epithelial proliferation, migration, and inflammation. Cancer Res. 2011. May 15;71(10):3688–700. PubMed PMID: 21576089. Pubmed Central PMCID: PMC3443863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang J, Chen T, Yang X, Cheng H, Spath SS, Clavijo PE, et al. Attenuated TRAF3 fosters alternative activation of NF-kappaB and reduced expression of anti-viral interferon, TP53, and RB to promote HPV-positive head and neck cancers. Cancer Res. 2018. June 19 PubMed PMID: 29921694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Druzgal CH, Chen Z, Yeh NT, Thomas GR, Ondrey FG, Duffey DC, et al. A pilot study of longitudinal serum cytokine and angiogenesis factor levels as markers of therapeutic response and survival in patients with head and neck squamous cell carcinoma. Head Neck. 2005. September;27(9):771–84. PubMed PMID: 15920746. [DOI] [PubMed] [Google Scholar]
- 45.Lu H, Yan C, Quan XX, Yang X, Zhang J, Bian Y, et al. CK2 phosphorylates and inhibits TAp73 tumor suppressor function to promote expression of cancer stem cell genes and phenotype in head and neck cancer. Neoplasia. 2014. October;16(10):789–800. PubMed PMID: 25379016. Pubmed Central PMCID: PMC4212254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Duffey DC, Crowl-Bancroft CV, Chen Z, Ondrey FG, Nejad-Sattari M, Dong G, et al. Inhibition of transcription factor nuclear factor-kappaB by a mutant inhibitor-kappaBalpha attenuates resistance of human head and neck squamous cell carcinoma to TNF-alpha caspase-mediated cell death. British journal of cancer. 2000. November;83(10):1367–74. PubMed PMID: 11044363. Pubmed Central PMCID: 2408789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liao G, Zhang M, Harhaj EW, Sun SC. Regulation of the NF-kappaB-inducing kinase by tumor necrosis factor receptor-associated factor 3-induced degradation. J Biol Chem. 2004. June 18;279(25):26243–50. PubMed PMID: 15084608. [DOI] [PubMed] [Google Scholar]
- 48.Uno M, Saitoh Y, Mochida K, Tsuruyama E, Kiyono T, Tmoto T, et al. NF-kappaB inducing kinase, a central signaling component of the non-canonical pathway of NF-kappaB, contributes to ovarian cancer progression. PLoS One. 2014;9(2):e88347. PubMed PMID: 24533079. Pubmed Central PMCID: PMC3922808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tao Z, Ghosh G. Understanding NIK regulation from its structure. Structure. 2012. October 10;20(10):1615–7. PubMed PMID: 23063006. [DOI] [PubMed] [Google Scholar]
- 50.Liu J, Sudom A, Min X, Cao Z, Gao X, Ayres M, et al. Structure of the nuclear factor kappaB-inducing kinase (NIK) kinase domain reveals a constitutively active conformation. J Biol Chem. 2012. August 10;287(33):27326–34. PubMed PMID: 22718757. Pubmed Central PMCID: PMC3431628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.de Almagro DV MC. BIRC3cIAP2 providing the anti-apoptosis signaling downstream of TNFR through TRAF1 and TRAF2. Exp Oncol 2012;34(3):200–11. [PubMed] [Google Scholar]
- 52.Simone TM, Higgins CE, Czekay RP, Law BK, Higgins SP, Archambeault J, et al. SERPINE1: A Molecular Switch in the Proliferation-Migration Dichotomy in Wound-"Activated" Keratinocytes. Adv Wound Care (New Rochelle). 2014. March 01;3(3):281–90. PubMed PMID: 24669362. Pubmed Central PMCID: PMC3955966. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.