Abstract
The heterogeneous and highly recombinogenic genus Haemophilus comprises several species, some of which are pathogenic to humans. All share an absolute requirement for blood-derived factors during growth. Certain species, such as the pathogen Haemophilus influenzae and the commensal Haemophilus haemolyticus , are thought to require both haemin (X-factor) and nicotinamide adenine dinucleotide (NAD, V-factor), whereas others, such as the informally classified ‘Haemophilus intermedius subsp. intermedius’, and Haemophilus parainfluenzae , only require V-factor. These differing growth requirements are commonly used for species differentiation, although a number of studies are now revealing issues with this approach. Here, we perform large-scale phylogenomics of 240 Haemophilus spp. genomes, including five ‘H. intermedius’ genomes generated in the current study, to reveal that strains of the ‘H. intermedius’ group are in fact haemin-independent H. haemolyticus (hiHh). Closer examination of these hiHh strains revealed that they encode an intact haemin biosynthesis pathway, unlike haemin-dependent H. haemolyticus and H. influenzae , which lack most haemin biosynthesis genes. Our results suggest that the common ancestor of modern-day H. haemolyticus and H. influenzae lost key haemin biosynthesis loci, likely as a consequence of specialized adaptation to otorhinolaryngeal and respiratory niches during their divergence from H. parainfluenzae . Genetic similarity analysis demonstrated that the haemin biosynthesis loci acquired in the hiHh lineage were likely laterally transferred from a H. parainfluenzae ancestor, and that this event probably occurred only once in hiHh. This study further challenges the validity of phenotypic methods for differentiating among Haemophilus species, and highlights the need for whole-genome sequencing for accurate characterization of species within this taxonomically challenging genus.
Keywords: Haemophilus haemolyticus, haemin-independent Haemophilus haemolyticus, 'Haemophilus intermedius', haemin biosynthesis, comparative genomics
Data Summary
Illumina NextSeq 500 whole-genome sequencing data generated from five ‘Haemophilus intermedius subsp. intermedius’ are available as 150 bp paired-end reads from the National Center for Biotechnology Information sequence read archive (SRA) under BioProject PRJNA509094. Whole-genome sequencing data for an additional 42 Haemophilus haemolyticus (including 6 haemin-independent strains), generated as part of previous genomic studies within our laboratory, have also been made available under BioProject PRJNA509094 as Illumina HiSeq 100 bp paired-end reads. Additionally, draft genome assemblies of the 11 haemin-independent H. haemolyticus are available from GenBank. The SRA and GenBank accession numbers are listed in Table S1 (available with the online version of this article). Accession numbers for publicly available Haemophilus influenzae , H. haemolyticus , Haemophilus parainfluenzae and Haemophilus spp. genomes used in this study are summarized in Table S1.
Impact Statement.
The human pathogen Haemophilus influenzae , and the closely related Haemophilus haemolyticus , a commensal of the human upper respiratory tract, require an exogenous source of the blood-derived factors haemin and nicotinamide adenine dinucleotide (NAD) for growth. Dependence on haemin and NAD is the primary phenotype used to discriminate these two species from other Haemophilus species, such as Haemophilus parainfluenzae , which requires only NAD supplementation for growth. Using comparative genomics, we assigned strains to a new lineage of H. haemolyticus that can synthesize haemin, a novel phenotype for this species. Herein termed haemin-independent H. haemolyticus (hiHh), members of this lineage harbour the complete set of the genes that encode a functional haemin biosynthesis pathway. We further demonstrated that members of the informal species ‘Haemophilus intermedius’ also reside in the hiHh lineage, resulting in a formal reclassification of this previously ‘fuzzy’ Haemophilus species. This work highlights the heterogeneous nature of Haemophilus genomes, and further demonstrates that accurate characterization of Haemophilus species cannot be achieved from phenotypic characteristics alone.
Introduction
The genus Haemophilus currently comprises 14 species that have been formally classified, 9 of which demonstrate host specificity for humans [1]. Additional informal Haemophilus species (e.g. ‘Haemophilus intermedius’) have also been described in the literature [2, 3]. All species have an absolute growth requirement for haemin (X-factor) and/or nicotinamide adenine dinucleotide (NAD, V-factor), both of which are derived from blood [1, 4]. The production of catalase, β-galactosidase, tryptophanase, urease, ornithine decarboxylase and haemolysis are additional phenotypic attributes used to characterize Haemophilus spp. [1]. However, such phenotypes can be variable both within and between species [1], resulting in ample opportunity for species misidentification.
Among the Haemophilus spp., Haemophilus influenzae is considered to be the most clinically relevant (especially for invasive disease), and much effort has been applied to its discrimination from other Haemophilus species. X- and V-factor dependence is the primary phenotypic method used to discriminate H. influenzae and Haemophilus haemolyticus from Haemophilus parainfluenzae in diagnostic and clinical trial settings [5, 6]. However, discrimination of H. influenzae from H. haemolyticus is more difficult. Both species occupy the same environmental niche, are thought to require both X- and V-factor for growth, and non-haemolytic H. haemolyticus strains can be morphologically indistinguishable from non-typeable H. influenzae [7]. Due to shared phenotypic characteristics between non-typeable H. influenzae and non-haemolytic H. haemolyticus , rapid (API NH; bioMeriéux) and automated biochemical differentiation [MALDI-TOF MS methods, such as VITEK 2 NH (bioMeriéux)] are also problematic, with false positive rates of up to 10 % [8–11] and species misidentification reported [11]. The close similarity of H. influenzae and H. haemolyticus also extends to a genetic level, with frequent recombination within and between these species [12, 13], particularly in non-typeable H. influenzae [14]. Hence, molecular discrimination of these species using PCR and fluorescence in situ hybridization (FISH) approaches have also been challenging [15, 16]. Thus, the availability of large-scale genomic data has been essential for correct phylogenetic placement of Haemophilus species and the identification of species-specific molecular targets to differentiate these two highly related but distinct species [17–21].
To further challenge our understanding of the characteristics used to delineate Haemophilus species, a haemin-synthesizing lineage of Haemophilus that is closely related to H. influenzae and H. haemolyticus , yet does not require haemin for growth, was identified in 1987 [2, 3]. Informally referred to as ‘Haemophilus intermedius subsp. intermedius’ or more simply, ‘Haemophilus intermedius’, these strains demonstrated similarity to H. influenzae using DNA–DNA hybridization [2]. However, in addition to only requiring V-factor for growth, their ability to ferment sucrose conflicted with key H. influenzae phenotypes [2]. In an attempt to delineate H. influenzae species boundaries, Nørskov-Lauritsen and colleagues further investigated difficult-to-classify Haemophilus spp., including the haemin-synthesizing ‘H. intermedius’ [3]. They observed that sucrose fermentation and haemin biosynthesis only ever occurred together, and phylogenetic relationships inferred from housekeeping gene and 16S rDNA sequences demonstrated that haemin-synthesizing strains fell outside the H. influenzae cluster, indicating that, whilst closely related, these strains were not H. influenzae . Further investigation of this unusual lineage showed the presence of chromosomally encoded haemin biosynthesis genes; however, these genes had no evidence of recent transfer from H. parainfluenzae , suggesting a more ancestral origin [3]. The evolutionary dynamics of this unusual H. influenzae -like, haemin-independent lineage has remained enigmatic.
To better understand the genetic relatedness of H. influenzae and H. haemolyticus near-neighbour species, six suspected H. parainfluenzae (which requires only V-factor for growth [1]) were genome sequenced. Comparative genomics demonstrated these isolates were highly genetically similar to H. haemolyticus , indicating that these isolates were related to the haemin-synthesizing ‘H. intermedius’. Here, we used comparative genomic analyses to reconstruct a phylogeny of 240 H . influenzae , H. haemolyticus and H. parainfluenzae strains to determine the phylogenomic placement of ‘H. intermedius’ among the established Haemophilus species clades. We subsequently investigated the genomes of 14 haemin-independent isolates previously identified as ‘H. intermedius’, H. haemolyticus or undefined Haemophilus spp. for the presence of haemin biosynthesis genes to genetically confirm the ability of these strains to grow in the absence of haemin, and to investigate the diversity and origin of these gene pathways in this unusual clade.
Methods
Haemophilus genomes
In total, 240 Haemophilus spp. genomes were examined in this study (Table S1). Forty-five H. haemolyticus (including six haemin-independent isolates) and three H. parainfluenzae genomes were generated as part of previous genomics studies within our laboratory [17, 18]. In the current study, we generated genome sequence data for five previously reported haemin-independent ‘H. intermedius’ strains (CCUG 11096, CCUG 15949, CCUG 30218, CCUG 31732, PN24 [3]; Table S1). DNA was extracted using a DNeasy blood and tissue kit (Qiagen) and diluted to 0.30 ng µl−1. DNA libraries were prepared from 1 ng genomic DNA on a Janus NGS Express robot (Perkin Elmer), using the Nextera XT DNA sample preparation kit in combination with the Nextera XT Index kit v2, set D (Illumina) according to the manufacturers' protocols. Dual-indexed paired-end 150 bp sequencing was performed on the Illumina NextSeq 500 using v2 chemistry on a medium flow cell (Illumina). We included publicly available genomes for an additional 152 H . influenzae , 12 H . haemolyticus , 21 H . parainfluenzae and 2 Haemophilus spp. (CCUG 66565 and F0629) [22–36] (Table S1). Previously incorrect [24] or incomplete species designations for 839_HINF, C1, F0397, 137_HINF, 159_HINF, 167_HINF, 614_HPAR and 841_HINF were changed based on our prior phylogenomic analyses [18], and the Haemophilus spp. strains CCUG 66565 and F0629 were reassigned to haemin-independent H. haemolyticus (hiHh) based on the phylogenomic analysis performed in the current study. In total, 64 H . haemolyticus genomes were used in this study, including 14 hiHh/‘H. intermedius’ isolates. Details for these hiHh/‘H. intermedius’ isolates are listed in Table 1.
Table 1.
hiHh isolates used in this study
|
Isolate |
Anatomical site |
Country of origin |
Year of isolation |
Haemin (X-factor) dependence* |
NAD (V-factor) dependence* |
Haemin biosynthesis pathway† |
Genome reference |
|---|---|---|---|---|---|---|---|
|
60819_B_Hi1 |
BAL |
Australia |
2010 |
− |
+ |
C5, PP-dependent, O2-independent |
[18] |
|
60824_B_Hi4 |
BAL |
Australia |
2010 |
− |
+ |
C5, PP-dependent, O2-independent |
[18] |
|
60971_B_Hi3 |
BAL |
Australia |
2012 |
− |
+ |
C5, PP-dependent, O2-independent |
[18] |
|
60982_B_Hi1 |
BAL |
Australia |
2012 |
− |
+ |
C5, PP-dependent, O2-independent |
[18] |
|
65117_B_Hi3 |
BAL |
Australia |
2011 |
− |
+ |
C5, PP-dependent, O2-independent |
[18] |
|
65151_B_Hi4 |
BAL |
Australia |
2011 |
− |
+ |
C5, PP-dependent, O2-independent |
[18] |
|
839_HINF |
BAL |
USA |
2013 |
Unknown |
Unknown |
C5, PP-dependent, O2-independent |
[24] |
|
CCUG 11096 |
Pleural fluid |
Sweden |
1981 |
− |
+ |
C5, PP-dependent, O2-independent |
[3] |
|
CCUG 15949 |
Eye |
Sweden |
1984 |
− |
+ |
C5, PP-dependent, O2-independent |
[3] |
|
CCUG 30218 |
Cerebrospinal fluid |
Sweden |
1992 |
− |
+ |
C5, PP-dependent, O2-independent |
[3] |
|
CCUG 31732 |
Ascitic fluid |
Sweden |
1993 |
− |
+ |
C5, PP-dependent, O2-independent |
[3] |
|
CCUG 66565 |
Sputum |
Sweden |
2014 |
Unknown |
Unknown |
C5, PP-dependent, O2-independent |
This study |
|
F0629 |
Oral cavity |
USA |
2015 |
Unknown |
Unknown |
C5, PP-dependent, O2-independent |
This study |
|
PN24 |
Urine |
Denmark |
2004 |
− |
+ |
C5, PP-dependent, O2-independent |
[3] |
Genome assemblies
Reference-assisted genome assemblies of previously unassembled Haemophilus genomes were generated with the Microbial Genome Assembler Pipeline (mgap) v0.0.1 (https://github.com/dsarov/MGAP---Microbial-Genome-Assembler-Pipeline) [37], which wraps Trimmomatic [38], Velvet [39], Gapfiller [40], abacas [41], image [42], sspace [43] and icorn2 [44], using default parameters. For assembling ‘H. intermedius’ genomes, the single-contig assembly of H. haemolyticus NCTC 10839 (GenBank accession no. LS483458.1) was used as the reference sequence. Species classification was based on phylogenomic grouping [17, 18, 36]. Species designation of apparent H. haemolyticus genomes (including the 14 hiHh/‘H. intermedius’) was confirmed by in silico detection of the H. haemolyticus molecular target hypD and the absence of the H. influenzae molecular target siaT [18]. Genome assemblies were annotated using Prokka v1.12-beta [45] with the --usegenus flag.
Phylogenetic analysis
To reconstruct a phylogeny of Haemophilus spp., sequence data for the 240 Haemophilus spp. genomes were mapped against the complete genome of H. influenzae 86–028 NP (GenBank accession no. CP000057.2) using SPANDx v3.2.1 [46], a genomics pipeline for comparative analysis of haploid genome datasets, which wraps Burrows-Wheeler Aligner [47], SAMtools [48], Picard Tools and Genome Analysis Tool Kit [49]. A H. haemolyticus phylogeny was also generated, where the 64 H . haemolyticus genomes were mapped to the merged, multi-contig assembly of the hiHh strain 60819_B_Hi1 (GenBank accession no. SDPA00000000) as the reference. Maximum parsimony phylogenomic trees were generated using paup v4.0a153 [50] and visualized using FigTree (http://tree.bio.ed.ac.uk/software/figtree/). Bootstrapping was performed in paup with 1000 replicates.
To confirm accurate phylogenetic placement of the 14 hiHh, ‘H. intermedius’ and Haemophilus spp. strains, the average nucleotide identity (ANI) with reference to the H. haemolyticus NCTC 10839, non-typeable H. influenzae 86–028 NP and H. parainfluenzae T3T1 genomes was calculated using fastANI [51] with default parameters.
Haemin biosynthesis pathway gene identification
Translated haemin biosynthesis pathway gene sequences (hemA, PARA_RS02505; hemL, PARA_RS08795; hemB, PARA_RS01040; hemC, PARA_RS02495; hemD, PARA_RS02500; hemE, PARA_RS04400; hemN, PARA_RS04215; hemG, PARA_RS02230; hemH, PARA_RS09990) were extracted from the T3T1 H. parainfluenzae genome (GenBank accession no. NC_015964.1), and queried against a database containing the 64 H . haemolyticus genomes using tBLASTn (blast+ v.2.2.29) [52]. Genome assembly annotations were manually reviewed to confirm the presence of haemin biosynthesis genes.
To optimize assembly of genetic regions harbouring haemin biosynthesis genes for downstream analysis, mgap assemblies were repeated using the hiHh 60819_B_Hi1 assembly as the scaffolding reference for the remaining 13 hiHh/‘H. intermedius’ strains, and 9 haemin-dependent H. haemolyticus (hdHh) strains. For each assembly, contigs were rearranged with car [53] using 60819_B_Hi1 as the reference prior to merging into a single contig. Genome assemblies were annotated using Prokka v1.12-beta [45]. Locally collinear block analyses of the annotated genome assemblies were performed using progressiveMAUVE v20150226 build 10 [54]. Genome alignments were visually assessed to determine whether nucleotide regions encoding haemin biosynthesis genes were syntenic. Artemis comparison tool (act) v13.0.0 [55] was used to visually represent the genetic architecture surrounding the haemin biosynthesis genes in H. haemolyticus . act plots were generated by comparing assembled whole genomes of representative ‘H. intermedius’ and H. haemolyticus strains to the 60819_B_Hi1 reference. To ensure that assembly scaffolding did not bias the results, the progressiveMAUVE analysis was repeated using mgap de novo assemblies (generated using default parameters).
Haemin biosynthesis pathway gene acquisition
In 2009, Nørskov-Lauritsen and colleagues showed that three haemin biosynthesis loci in ‘H. intermedius’ strains (hemB, hemE, hemN) appeared to be ancestral, with no evidence of recent lateral transfer from, for example, H. parainfluenzae [3]. To determine whether haemin biosynthesis pathway genes have evolved similarly to whole-genome evolution in hiHh/‘H. intermedius’, hiHh/‘H. intermedius’ Illumina data were aligned to a concatenated nucleotide sequence of haemin biosynthesis gene sequences extracted from the 60819_B_Hi1 assembly, or the merged, multi-contig assembly of 60819_B_Hi1, using SPANDx v.3.2.1. Maximum parsimony phylogenies were reconstructed from the orthologous SNP matrices and bootstrapped as described above. Phylogenies were compared by plotting a tanglegram in Dendroscope v.3.5.10 [56].
To confirm hiHh/‘H. intermedius’ haemin biosynthesis genes were not recently acquired from H. parainfluenzae , the concatenated nucleotide sequences of the H. parainfluenzae T3T1 haemin biosynthesis genes were used as the reference for a SPANDx alignment of the 14 hiHh/‘H. intermedius’ genomes and 24 H . parainfluenzae genomes. A maximum-likelihood phylogenetic tree was generated using RAxML [57].
To measure selective pressures on haemin biosynthesis gene maintenance, the ratio of non-synonymous to synonymous SNPs (dN/dS) in haemin biosynthesis genes was determined for the 14 hiHh/‘H. intermedius’ and 24 H . parainfluenzae strains. dN/dS ratios were calculated from multi-fasta files of hem gene sequences extracted from genome assemblies using slac [58] via the Datamonkey web application [59]. To compare, dN/dS ratios were also determined for the H. influenzae MLST genes adk, atpG, frdB, mdh, pgi and recA in the 14 hiHh/‘H. intermedius’ strains.
To determine the unique genetic content of the 14 hiHh/‘H. intermedius’ when compared with hdHh, a pangenome of the 64 H . haemolyticus genomes was generated using Roary v.3.12.0 [60], with an amino acid percentage identity cut-off of 85 %. A cut-off lower than the default (95 %) was used to reduce false classification of core genes shared by all H. haemolyticus as accessory genes due to potential sequence variation within the hiHh/‘H. intermedius’ genomes relative to hdHh. The pangenome was interrogated using plink v.1.07 [61] and the GeneratePLINK_Roary.sh script distributed within the SPANDx package [46] for the retrieval of coding sequences unique to either the 14 hiHh/‘H. intermedius’ or the 50 hdHh genomes. These unique genes were compared to the closed genome of H. parainfluenzae T3T1 using large-scale blast score ratio (ls-bsr, v1.00) [62] to ascertain their presence in this species. A blast score ratio (BSR) of ≥0.8 was considered indicative of potential acquisition due to recombination with H. parainfluenzae .
Results
Phylogenomics confirms that 'H. intermedius' is in fact hiHh
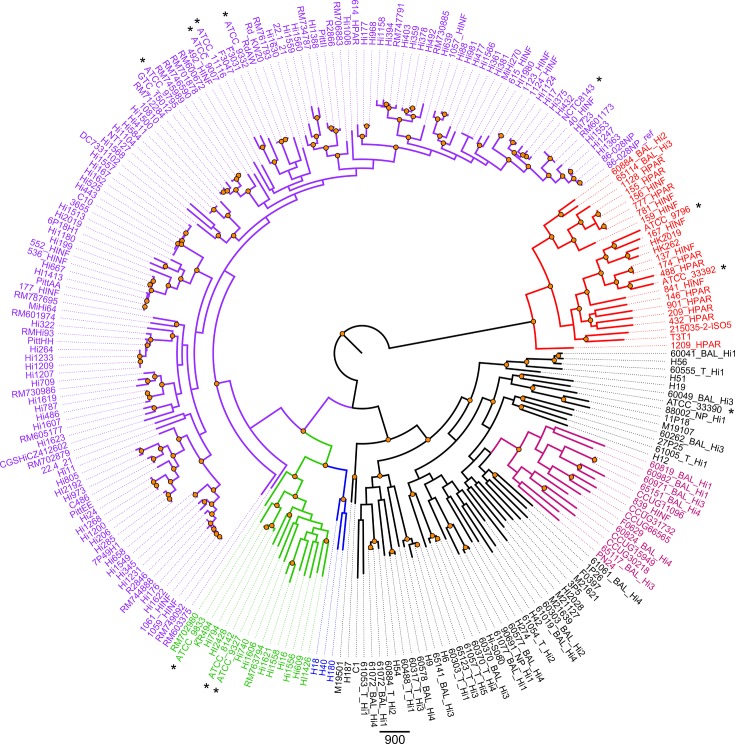
Phylogenomic reconstruction of 240 Haemophilus spp. genomes was carried out to determine the relatedness of the ‘H. intermedius’ isolates at the whole-genome level when compared with H. influenzae , H. haemolyticus and H. parainfluenzae strains. The six hiHh strains described in this study, 839_HINF [24], the Haemophilus sp. strains CCUG 66565 and F0629, and five previously assigned ‘H. intermedius’ (Tables 1and S1) all share a recent common ancestor and form a subclade within the H. haemolyticus clade (Fig. 1). Bootstrapping demonstrated that the hiHh subclade is 100 % supported (Fig. 1), and a maximum-likelihood phylogenomy verified the topology of the maximum parsimony phylogenomic reconstruction (Fig. S1). To confirm correct species association, the ANIs of the 64 genomes in the H. haemolyticus clade were calculated compared to NCTC 10839 H . haemolyticus , 86–028 NP non-typeable H. influenzae and T3T1 H. parainfluenzae genomes (Fig. S2). ‘H. intermedius’/hiHh genomes demonstrated the highest ANI to H. haemolyticus (92.95–93.44%), followed by H. influenzae (82.90–90.32%) and H. parainfluenzae (81.34–81.94%). Higher ANIs were observed for the hdHh genomes to H. haemolyticus (94.06–95.87%) and H. influenzae (91.21–92.85%), and a comparable ANI to H. parainfluenzae (81.02–82.37%) (Fig. S2). These results confirm that the informal ‘H. intermedius’ nomenclature should be renamed as hiHh to more accurately reflect its species designation, whilst differentiating this unusual clade from conventional haemin-dependent strains.
Fig. 1.
Phylogenomic analysis of 240 Haemophilus spp. to identify placement of hiHh and ‘H. intermedius’. A midpoint-rooted maximum parsimony tree was constructed using 30 345 orthologous, biallelic SNPs found among 152 H . influenzae (purple), including 16 clade I (green) [36] and three fucP-negative clade (blue) [17, 18] genomes, 64 H . haemolyticus (black) including 14 hiHh and ‘H. intermedius’ genomes (pink), and 24 H . parainfluenzae genomes (red). Consistency index=0.1482. Bootstrap values were inferred from 1000 replicates. Clades with >80 % support are annotated with a filled orange circle. Type strains are denoted with an asterisk. Bar, 900 bp.
A complete haemin biosynthesis pathway is present in hiHh
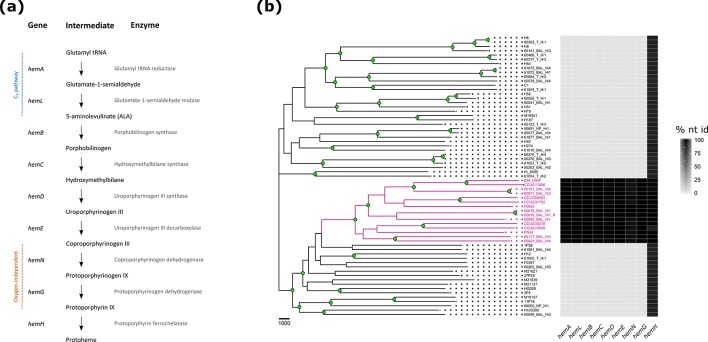
Consistent with their less fastidious growth requirements, genes encoding a functional haemin biosynthesis pathway were identified in the genomes of all 14 hiHh (Fig. 2). Based on their gene complement, these isolates utilize the C5 pathway of aminolevulinic acid (ALA) biosynthesis, as signified by genes encoding a Glu-tRNA reductase (hemA; PARA_RS02505 in H. parainfluenzae T3T1) and a glutamate-1-semialdehyde mutase (hemL; PARA_RS08795 in H. parainfluenzae ) [63]. The conversion of coproporphyrinogen III to protohaem is protoporphyrin-dependent, and occurs in an oxygen-independent manner in these isolates (Fig. 2) [63], consistent with H. parainfluenzae haemin biosynthesis.
Fig. 2.
The haemin biosynthesis pathway in hiHh. (a) The hiHh strains synthesize haemin by utilizing the C5 pathway for 5-aminolevulinic acid synthesis. Conversion of coproporphyrinogen III to protohaem occurs in a protoporphyrin-dependent, oxygen-independent manner. (b) Heatmap showing the percentage nucleotide identity of haemin biosynthesis genes when compared to reference gene sequences extracted from the assembled genome of hiHh 60819_BAL_Hi1. The heatmap is plotted against a H. haemolyticus midpoint-rooted, maximum parsimony tree, constructed using 153 468 orthologous, biallelic SNPs found amongst the 64 H . haemolyticus genomes. hiHh are shown in pink. Consistency index=0.2380. Bootstrap values were inferred from 1000 replicates. Nodes with >80 % support are annotated with a filled green circle. Bar, 1000 bp.
The hiHh genomes harboured two genes annotated as oxygen-independent coproporphyrinogen III oxidases (hemN). The hemN paralogues demonstrated <32 % amino acid identity within each of the hiHh genomes, indicating that they likely encode non-homologous isofunctional enzymes. However, further investigation of the hemN genes revealed that only one hemN was correctly annotated. The true hemN demonstrated 80 % amino acid identity to PARA_RS04215, which encodes an oxygen-independent coproporphyrinogen III oxidase in H. parainfluenzae T3T1. Further, a 67 % amino acid identity match to hemN of Escherichia coli (GenBank accession no. NC_000913.3) [64], and the occurrence of the two regions integral to HemN function (18GPRYTSYPTA27 and 306RNFQGYTT313) demonstrated that the true hiHh hemN gene encodes a functional coproporphyrinogen III oxidase [65].
The incorrectly annotated hemN gene demonstrated 87 % amino acid identity to the H. parainfluenzae T3T1 gene PARA_RS03220. This gene encodes the radical S-adenosyl methionine (SAM) family haem chaperone protein HemW, which is not part of the haemin biosynthesis pathway (Fig. 2a). The absence of the HemN functional regions and poor matching (29 % amino acid identity) to E. coli hemN is consistent with incorrect annotation of this gene [65].
The last gene in the protoporphyrin-dependent haemin-biosynthesis pathway, hemH (PARA_RS09990), encodes a protoporphyrin ferrochelatase that is ubiquitous in all H. influenzae [1] and H. haemolyticus genomes (Fig. 2b). hemH is likely a remnant of the original haemin biosynthesis pathway harboured by the H. influenzae / H. haemolyticus / H. parainfluenzae ancestor and, thus, is the only hem gene not reacquired by hiHh. An additional gene associated with the haemin biosynthesis pathway, hemX (PARA_RS02505), was also identified in all 64 H . haemolyticus genomes. Encoding a uroporphyrinogen III methyltransferase, hemX is required for the conversion of uroporphyrinogen III to precorrin-2, the substrate required for sirohaem synthesis [66]. hemX was also observed in all 24 H . parainfluenzae genomes examined in this study.
Each of the dispersed locations of haemin biosynthesis genes is syntenic across the 14 hiHh genomes
The progressiveMAUVE analysis demonstrated that the H. haemolyticus genomes consist of a very high number of predicted syntenic blocks, which are much smaller in size than the assembled contigs, and whose order is not very conserved. The haemin biosynthesis pathway genes are not found within a single operon on the hiHh chromosome; rather, the eight loci are located in seven distinct regions across the genome. The exception is the hemCD cluster (PARA_RS02495 and PARA_RS02500, respectively), which occurs in a ~5 kbp syntenic block in all 14 hiHh genomes, commencing with hemC (Fig. S3); all other core hem loci in the hiHh strains are found within individual syntenic blocks. In hdHh genomes, hemC and hemD are absent and the syntenic block instead commences with hemX (Fig. S3).
The hemA (~4.3 kbp), hemB (~2.7 kbp), hemE (~14.4 kbp) and hemG (~3.3–3.6 kbp) syntenic block structures are relatively well-conserved amongst the hiHh genomes (Figs S4, S5, S6 and S7), and these loci are absent in hdHh strains. hemN was the only haemin biosynthesis gene that did not reside in a syntenic block (Fig. S8). In the progressiveMAUVE analysis, the gene appears to be a composite of different syntenic fragments. For the hemH syntenic block (~12.7 kbp), three strains had additional genetic content between the putative esterase and putative flavin adenine dinucleotide (FAD)-linked oxidoreductase-encoding genes adjacent to hemH, contributing an additional 1.5 kbp (Fig. S9). Variability was also observed at both boundaries of the hemL syntenic block in the hiHh genomes (Fig. S10). Either hemL or the adjacent ata gene, encoding the Ata adhesin autotransporter, constitutes the boundary of the syntenic block in which hemL resides. At the opposite boundary, variability is observed after the manA gene, resulting in a syntenic block that ranges in size from ~9.6 to ~14 kbp. In the hdHh genomes, primarily both ata and hemL are absent from the syntenic block boundary; however, for one strain (60262_BAL_Hi3) only hemL was absent.
Using de novo assemblies, 4/7 hem syntenic blocks were predicted to be the same as determined using reference-assisted genome assemblies. Of the three remaining syntenic blocks (hemE, hemH and hemL), variation was observed in the form of a boundary shift at one end of each syntenic block, reducing their size to ~11.6, ~8.3 and ~2.8 kbp, respectively.
Haemin biosynthesis was likely acquired from a H. parainfluenzae ancestor in the early stages of hiHh divergent evolution
To identify the origin of the nine hem genes in hiHh using contemporary datasets, sequence data from hiHh strain 60819_B_Hi1 were first compared to the National Center for Biotechnology Information (NCBI) nr/nt database, which contained 290 closed or draft Haemophilus spp. genomes (on February 2018). The hem gene sequences were most similar to homologues in H. parainfluenzae [amino acid percentage identity scores ranging between 63 % (hemD) to 91 % (hemB)], consistent with this species being most closely related to H. haemolyticus and H. influenzae at the whole-genome level (Fig. 1, Table S2). Phylogenetic analysis of the concatenated hem nucleotide sequences from the 14 hiHh and 24 H . parainfluenzae genomes showed that all hiHh isolates clustered together and were distinct from the H. parainfluenzae strains (Fig. 3). Taken together, these results confirm the original findings of Nørskov-Lauritsen and colleagues [3] that the hiHh hem genes were not recently laterally acquired from H. parainfluenzae .
Fig. 3.
Maximum-likelihood phylogeny of haemin biosynthesis pathway genes in 14 hiHh (pink) and 24 H . parainfluenzae (black), constructed using 73 orthologous, biallelic SNPs, with reference to a concatenated nucleotide sequence of haemin biosynthesis genes from H. parainfluenzae T3T1 (GenBank accession no. NC_015964.1). Bar, nucleotide substitutions per site.
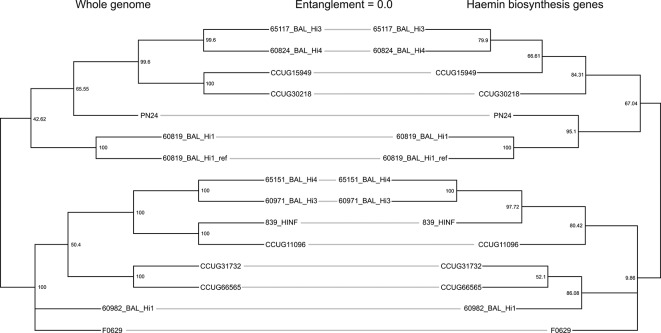
To determine whether hiHh hem evolution reflected whole-genome evolution, maximum parsimony phylogenies were reconstructed using SNPs identified from both datasets and compared (Fig. 4). Whilst not identical, the phylogenies did not demonstrate any entanglement, indicating that the hem genes likely did not evolve independently of the rest of the genome. The minor differences in tree topologies may be explained by selective pressure to maintain haemin biosynthesis. To investigate this, dN/dS ratios were calculated for each hem gene in both hiHh and H. parainfluenzae (Table S3). dN/dS scores ranged from 0.064 (hemB) to 0.215 (hemG) in hiHh, and 0.025 (hemL) to 0.175 (hemG) in H. parainfluenzae . Housekeeping gene dN/dS ratios were comparable to those calculated for the hem genes in the hiHh genomes, recA, 0.006; adk, 0.014; frdB, 0.025; mdh, 0.026; pgi, 0.051; and atpG, 0.692; demonstrating that the hem genes are under negative (purifying) selection in each of these populations, consistent with selective forces retaining the haemin biosynthesis capability in hiHh and H. parainfluenzae .
Fig. 4.
Evolution of the haemin biosynthesis pathway compared to whole-genome evolution in hiHh. Midpoint-rooted maximum parsimony trees of the 14 hiHh were constructed with reference to 60819_BAL_Hi1. Bootstrap values were inferred from 1000 replicates. The whole-genome phylogeny (left) was derived from 114 346 orthologous, biallelic, SNPs, using a merged, multi-contig 60819_BAL_Hi1 assembled genome as the reference. The haemin biosynthesis pathway phylogeny (right) was derived from 548 orthologous, biallelic, SNPs, with reference to a concatenated nucleotide sequence of haemin biosynthesis genes from 60819_BAL_Hi1. In the tanglegram plot, lines are used to connect the same taxa in both trees. The absence of entanglement does not reflect topological differences in the trees.
We next investigated the similarity of gene arrangements flanking the hem genes in H. parainfluenzae and hiHh to determine whether additional genetic content was shared during haemin biosynthesis acquisition in H. haemolyticus . Amongst the nine hem genes, four scenarios were observed with reference to the H. parainfluenzae T3T1 genome: (i) the entire syntenic block was present (hemCD; Fig. S3); (ii) the entire syntenic block plus additional neighbouring sequence (hemB, hemN and hemG; Figs S5, S8 and S7) was present; (iii) a fragment of the syntenic block was present (hemA, hemE and hemH; Figs S4, S6 and S9); and (iv) a fragment of the syntenic block plus additional neighbouring sequence (hemL; Fig. S10) was present. These observations indicate that, during haemin biosynthesis gene acquisition events, additional neighbouring coding sequences were likely also acquired, probably from the H. parainfluenzae ancestor.
Next, the pangenome of hiHh was examined to identify potential additional instances of recombination between the H. parainfluenzae and hiHh ancestors. Interrogation of a H. haemolyticus pangenome generated from 64 H . haemolyticus genomes identified 120 genes present in hiHh but absent in hdHh. ls-bsr comparisons of the 120 loci to the closed genome of H. parainfluenzae T3T1 demonstrated that 36/120 genes had a BSR ≥0.8 to orthologous coding sequences in H. parainfluenzae . Further pangenome interrogation identified 88 genes unique to hdHh, of which 35 had a BSR ≥0.8 to orthologous coding sequences in H. parainfluenzae . Collectively, this demonstrates that recombination between H. parainfluenzae and H. haemolyticus has likely occurred on multiple occasions, and is not limited to the haemin biosynthesis gene cluster.
Discussion
Haemin is required for a wide array of biological processes across all branches of life; therefore, it is not surprising that haemin biosynthesis is almost ubiquitous. For eubacteria, it is estimated that only ~13 % of species lack tetrapyrrole biosynthesis genes, the essential pathway for haemin synthesis [63]. Such organisms, including many Haemophilus species, have almost certainly lost the ability to synthesize haemin through evolutionary processes, most likely due to the abundance and availability of haemin in certain environmental niches, teamed with the capacity to acquire it exogenously.
In this study, we have identified an unusual H. haemolyticus lineage that synthesizes its own haemin, to which we have given the term hiHh to most accurately reflect its phenotypic and genotypic characteristics. Forming a well-supported clade within the H. haemolyticus lineage, hiHh taxa include isolates previously misclassified as either H. parainfluenzae , presumably due to colony morphology and growth factor requirements, or as the informally named ‘H. intermedius’ due to their high genetic similarity to H. influenzae and H. haemolyticus despite their unusual haemin independence [2]. ANI investigation of the hiHh taxa confirm their H. haemolyticus classification, despite ANI values lower than the accepted 95 % species cut-off (range: 92.95–93.44%). The observation that hdHh ANI values also straddled the recommended species cut-off indicates that a 95 % ANI cut-off is not appropriate for this species. It has previously been shown that members of Neisseria gonorrhoeae and Neisseria meningitidis can have ANI values <95 % [51], so whilst a 95 % ANI is appropriate for most bacterial species, it cannot be applied ubiquitously. Comparative genomics also confirmed that all hiHh examined in this study harboured a complement of genes encoding a functional haemin biosynthesis pathway. This pathway utilizes the C5 branch of aminolevulinic acid synthesis and the protoporphyrin-dependent branch of protohaem synthesis in an oxygen-independent manner [63], consistent with the haemin biosynthesis pathway in the near-neighbour H. parainfluenzae . The presence of this suite of genes, thus, confirms the haemin-independent phenotype observed in these hiHh strains.
Consistent with the lack of a complete set of hem genes in all H. influenzae and almost all H. haemolyticus strains characterized to date (Fig. 1), the ability to synthesize haemin was likely lost in H. influenzae and H. haemolyticus after divergence of this clade from the haemin-synthesizing H. parainfluenzae ancestor [1]. However, previous attempts to elucidate the genetic events associated with subsequent core hem gene pathway acquisition in hiHh have proven elusive due to limited nucleotide sequence data, resulting in insufficient evidence of recent lateral transfer from H. parainfluenzae based on nucleotide comparisons [3]. Using phylogenetic approaches, including phylogenomics, our findings point towards hem core gene acquisition early in the divergent evolution of hiHh from other H. haemolyticus clades, probably via lateral transfer from a H. parainfluenzae ancestor, rather than loss of these loci across several independent hdHh clades. This hypothesis is supported by several pieces of evidence. First, a tanglegram comparing SNP phylogenies of the concatenated hem genes versus the whole genome (Fig. 4) demonstrated that hem gene diversity reflects the genomic background of the hiHh strains, indicating long-term evolution of these genes in hiHh. The minor topological differences in the tanglegram are likely due to fewer characters in the hem only dataset. Second, tBLASTn analyses of the hem genes across all publicly available Haemophilus genomes failed to identify a close genetic relative, with closest matches to H. parainfluenzae (range: 63 to 91 % amino acid identity), ruling out recent lateral transfer from other genome-characterized Haemophilus species. Third, the universal presence of the core hem genes in hiHh strains suggests that these genes were acquired in the hiHh ancestor prior to evolutionary diversification. Finally, pan-genome analysis identified 120 genes shared amongst hiHh strains that were absent in hdHh, with 30 % of these genes demonstrating homology to those found in the H. parainfluenzae T3T1 genome. Two of these genes were co-located (scrB) or adjacent to (scrK) the hemA syntenic block (Fig. S4), which suggests their acquisition may have occurred at the same time as the hem genes.
The hiHh hem genes reside on seven discrete and chromosomally separated syntenic blocks, the architecture of which is principally conserved amongst hiHh genomes, and reflects hem gene arrangement in the H. parainfluenzae T3T1 genome. Dispersal of hem genes throughout the prokaryotic chromosome is thought to be more common than arrangement as an operon [67], although the latter has been observed for a small number of bacterial species [68–70]. At face value, the distinct chromosomal locations of the hem genes suggest they were acquired via multiple, independent events for each syntenic block. However, taxa harbouring partial haemin biosynthesis pathways were not observed in our dataset. Transduction was also considered; however, the absence of adjacent tRNA genes suggests this acquisition mechanism is impracticable (Figs S3, S4, S5, S6, S7, S8, S9 and S10). It was hypothesized that hiHh was the ancestral H. haemolyticus phenotype, yet the location of the hiHh most recent common ancestor within the H. haemolyticus phylogeny indicates that hem genes would need to have been lost multiple times during H. haemolyticus evolution. However, another hypothesis is that the hem loci were acquired during one, or perhaps two, rare but significant recombination events between the H. parainfluenzae ancestor and the hiHh ancestor that enabled the re-establishment of a functional haemin biosynthesis pathway in the hiHh lineage. This is supported by evidence that H. haemolyticus can readily recombine with other Haemophilus spp. [71, 72] and that recombination patterns in the closely related H. influenzae have recently been shown to involve multiple DNA blocks across the entire chromosome rather than affecting single regions only [73]. Thus, we propose that the core hem genes were acquired in hiHh through a recombination event with an ancestral H. parainfluenzae strain, with subsequent stable maintenance of the hem genes in this lineage.
The collection of hiHh isolates examined in this study spans 37 years across four countries in three continents (Table 1), demonstrating that hiHh is likely not a sporadic occurrence of a phenotypic variant. hiHh are likely more abundant than previously thought, but due to phenotypic misclassification as H. parainfluenzae or Haemophilus parahaemolyticus are prone to having been inaccurately documented and, thus, under-reported. Interestingly, Australian and North American hiHh isolates collected to date have all been cultured from bronchoalveolar lavage specimens, whereas the majority of the Swedish and Danish strains were cultured from infections at anatomical sites atypical for Haemophilus (Table 1). This differs from the standard ecological niche of hdHh, where it is a commensal of the human upper respiratory tract, sharing the same ecological niche as H. influenzae [74].
H. haemolyticus is generally considered to be a commensal [74], causing disease only on rare occasions [75], with such cases often associated with underlying chronic disease [76]. Therefore, the ability to synthesize haemin is potentially advantageous for H. haemolyticus , enabling niche expansion into environments where haemin is limited/absent [63]. Whilst not explored in this study, the potential role of hiHh in disease pathogenesis warrants exploration in order to understand its clinical relevance, and the importance of identifying hiHh in a diagnostic setting. Importantly, our study confirms that comparative genomics is currently the only method for accurately identifying hiHh strains, which involves detection of all nine hem genes in conjunction with the presence of hypD ( H. haemolyticus species-specific marker) and siaT absence ( H. influenzae species-specific marker) [18]. A move towards whole-genome sequencing classification of ‘fuzzy’ Haemophilus spp. will greatly aid in the unmasking of hiHh strains across a greater spectrum of patients and geographical regions.
In summary, this study has used comparative genomics to confirm a single, unusual clade of H. haemolyticus , the members of which are able to synthesize their own haemin. Our study also used various comparative genomic methods to identify the evolutionary origin for the haemin biosynthesis genes in hiHh, which it was not possible to elucidate using lower-resolution genotyping approaches. The ability to synthesize haemin conflicts with a key phenotype previously believed to be characteristic of H. haemolyticus , and provides further evidence that phenotypic tests are insufficient for accurately differentiating Haemophilus species. hiHh is a more accurate taxonomic classification for ‘H. intermedius’ [2, 3], and we propose that this terminology should now be used to describe H. haemolyticus strains that are haemin-independent. Finally, our approach demonstrates the utility and value of comparative genomics for accurate speciation of previously described ‘fuzzy’ or informal species classifications, particularly for highly recombinogenic organisms including Haemophilus species, which are readily confounded by lower-resolution genotyping and phenotyping approaches.
Data bibliography
1. Short-read sequence data for the Haemophilus haemolyticus strains sequenced as part of this and previous studies are available in the NCBI SRA under BioProject PRJNA509094, accession numbers are listed in Table S1 (2019).
2. The 11 haemin-independent H. haemolyticus draft genome assemblies generated as part of this study are available in GenBank, accession numbers are listed in Table S1 (2019).
3. Accession numbers for the publicly available Haemophilus influenzae, H. haemolyticus, Haemophilus parainfluenzae and Haemophilus spp. genomes used in this study are summarized in Table S1 (2019).
Supplementary Data
Funding information
This project was funded by the Channel 7 Children’s Research Foundation (award 151068), with support from the Australian National Health and Medical Research Council (NHMRC; award 1100310). Clinical specimens used in this project were partly funded by NHMRC awards 1023781 and 1042601. T.M.H. and H.C.S.-V. were supported by NHMRC Centre of Research Excellence in Respiratory Health of Aboriginal and Torres Strait Islander Children Fellowships (1079557); E.P.P. and D.S.S. were supported by Advance Queensland Fellowships (AQIRF0362018 and AQRF13016-17RD2).
Acknowledgements
The authors thank the Ear and Respiratory Health teams of the Menzies School of Health Research (Darwin, Australia) for specimen collection, and the families involved in the research studies. The authors are also grateful to Lea-Ann Kirkham for contributing isolates used within this study, and the Pasteurellaceae community for generously making their genome data publicly available.
Author contributions
The study was conceptualized by T.M.H., H.C.S.-V., E.P.P. and D.S.S. Funding was acquired by E.P.P. and H.C.S.-V., and resources were provided by E.P.P., N.N.-L., H.C.S.-V., J.B. and A.B.C. T.M.H. conducted the formal analysis, with methodology direction provided by E.P.P., D.S.S. and N.N.-L. T.M.H. wrote the original draft, and E.P.P., D.S.S., N.N.-L. and H.C.S.-V. critically reviewed and edited the manuscript. All authors reviewed and approved the final manuscript.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Footnotes
Abbreviations: ANI, average nucleotide identity; BSR, blast score ratio; dN/dS, non-synonymous SNPs/synonymous SNPs; hdHh, haemin-dependent Haemophilus haemolyticus; hiHh, haemin-independent Haemophilus haemolyticus; NAD, nicotinamide adenine dinucleotide; NCBI, National Center for Biotechnology Information; SRA, sequence read archive.
The sequences of the Haemophilus strains are available from the NCBI sequence read archive (SRA) under BioProject PRJNA509094, with accession numbers SRR8294000–SRR8294046.
All supporting data, code and protocols have been provided within the article or through supplementary data files. Ten supplementary figures and three supplementary tables are available with the online version of this article.
References
- 1.Nørskov-Lauritsen N. Classification, identification, and clinical significance of Haemophilus and Aggregatibacter species with host specificity for humans. Clin Microbiol Rev. 2014;27:214–240. doi: 10.1128/CMR.00103-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burbach S. Inaugural Dissertation. Philipps-Universität Marburg; Marburg, Germany: 1987. Reclassification of the genus Haemophilus Winslow, et al. 1917 based on nucleotide sequence homology. [Google Scholar]
- 3.Nørskov-Lauritsen N, Overballe MD, Kilian M. Delineation of the species Haemophilus influenzae by phenotype, multilocus sequence phylogeny, and detection of marker genes. J Bacteriol. 2009;191:822–831. doi: 10.1128/JB.00782-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thjötta T, Avery OT. Studies on bacterial nutrition: II. Growth accessory substances in the cultivation of hemophilic bacilli. J Exp Med. 1921;34:97–114. doi: 10.1084/jem.34.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wurzel DF, Marchant JM, Yerkovich ST, Upham JW, Petsky HL, et al. Protracted bacterial bronchitis in children: natural history and risk factors for bronchiectasis. Chest. 2016;150:1101–1108. doi: 10.1016/j.chest.2016.06.030. [DOI] [PubMed] [Google Scholar]
- 6.Goyal V, Grimwood K, Byrnes CA, Morris PS, Masters IB, et al. Amoxicillin–clavulanate versus azithromycin for respiratory exacerbations in children with bronchiectasis (BEST-2): a multicentre, double-blind, non-inferiority, randomised controlled trial. The Lancet. 2018;392:1197–1206. doi: 10.1016/S0140-6736(18)31723-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kilian M. A taxonomic study of the genus Haemophilus, with the proposal of a new species. J Gen Microbiol. 1976;93:9–62. doi: 10.1099/00221287-93-1-9. [DOI] [PubMed] [Google Scholar]
- 8.Munson EL, Doern GV. Comparison of three commercial test systems for biotyping Haemophilus influenzae and Haemophilus parainfluenzae . J Clin Microbiol. 2007;45:4051–4053. doi: 10.1128/JCM.01663-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barbé G, Babolat M, Boeufgras JM, Monget D, Freney J. Evaluation of API NH, a new 2-hour system for identification of Neisseria and Haemophilus species and Moraxella catarrhalis in a routine clinical laboratory. J Clin Microbiol. 1994;32:187–189. doi: 10.1128/jcm.32.1.187-189.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Valenza G, Ruoff C, Vogel U, Frosch M, Abele-Horn M. Microbiological evaluation of the new VITEK 2 Neisseria-Haemophilus identification card. J Clin Microbiol. 2007;45:3493–3497. doi: 10.1128/JCM.00953-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rennie RP, Brosnikoff C, Shokoples S, Reller LB, Mirrett S, et al. Multicenter evaluation of the new Vitek 2 Neisseria-Haemophilus identification card. J Clin Microbiol. 2008;46:2681–2685. doi: 10.1128/JCM.00449-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCrea KW, Xie J, LaCross N, Patel M, Mukundan D, et al. Relationships of nontypeable Haemophilus influenzae strains to hemolytic and nonhemolytic Haemophilus haemolyticus strains. J Clin Microbiol. 2008;46:406–416. doi: 10.1128/JCM.01832-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Witherden EA, Bajanca-Lavado MP, Tristram SG, Nunes A. Role of inter-species recombination of the ftsI gene in the dissemination of altered penicillin-binding-protein-3-mediated resistance in Haemophilus influenzae and Haemophilus haemolyticus . J Antimicrob Chemother. 2014;69:1501–1509. doi: 10.1093/jac/dku022. [DOI] [PubMed] [Google Scholar]
- 14.Connor TR, Corander J, Hanage WP. Population subdivision and the detection of recombination in non-typable Haemophilus influenzae . Microbiology. 2012;158:2958–2964. doi: 10.1099/mic.0.063073-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Binks MJ, Temple B, Kirkham LA, Wiertsema SP, Dunne EM, et al. Molecular surveillance of true nontypeable Haemophilus influenzae: an evaluation of PCR screening assays. PLoS One. 2012;7:e34083. doi: 10.1371/journal.pone.0034083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frickmann H, Christner M, Donat M, Berger A, Essig A, et al. Rapid discrimination of Haemophilus influenzae, H. parainfluenzae, and H. haemolyticus by fluorescence in situ hybridization (FISH) and two matrix-assisted laser-desorption-ionization time-of-flight mass spectrometry (MALDI-TOF-MS) platforms. PLoS One. 2013;8:e63222. doi: 10.1371/journal.pone.0063222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Price EP, Sarovich DS, Nosworthy E, Beissbarth J, Marsh RL, et al. Haemophilus influenzae: using comparative genomics to accurately identify a highly recombinogenic human pathogen. BMC Genomics. 2015;16:641. doi: 10.1186/s12864-015-1857-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Price EP, Harris TM, Spargo J, Nosworthy E, Beissbarth J, et al. Simultaneous identification of Haemophilus influenzae and Haemophilus haemolyticus using real-time PCR. Future Microbiol. 2017;12:585–593. doi: 10.2217/fmb-2016-0215. [DOI] [PubMed] [Google Scholar]
- 19.Pickering J, Binks MJ, Beissbarth J, Hare KM, Kirkham LAS, et al. A PCR-high-resolution melt assay for rapid differentiation of nontypeable Haemophilus influenzae and Haemophilus haemolyticus . J Clin Microbiol. 2014;52:663–667. doi: 10.1128/JCM.02191-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Latham R, Zhang B, Tristram S. Identifying Haemophilus haemolyticus and Haemophilus influenzae by SYBR Green real-time PCR. J Microbiol Methods. 2015;112:67–69. doi: 10.1016/j.mimet.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 21.Osman KL, Jefferies JMC, Woelk CH, Devos N, Pascal TG, et al. Patients with chronic obstructive pulmonary disease harbour a variation of Haemophilus species. Sci Rep. 2018;8:14734. doi: 10.1038/s41598-018-32973-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jordan IK, Conley AB, Antonov IV, Arthur RA, Cook ED, Cooper GP, et al. Genome sequences for five strains of the emerging pathogen Haemophilus haemolyticus . J Bacteriol. 2011;193:5879–5880. doi: 10.1128/JB.05863-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ormerod KL, George NM, Fraser JA, Wainwright C, Hugenholtz P. Comparative genomics of non-pseudomonal bacterial species colonising paediatric cystic fibrosis patients. PeerJ. 2015;3:e1223. doi: 10.7717/peerj.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roach DJ, Burton JN, Lee C, Stackhouse B, Butler-Wu SM, et al. A year of infection in the intensive care unit: prospective whole genome sequencing of bacterial clinical isolates reveals cryptic transmissions and novel microbiota. PLoS Genet. 2015;11:e1005413. doi: 10.1371/journal.pgen.1005413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Post DMB, Ketterer MR, Coffin JE, Reinders LM, Munson RS, et al. Comparative analyses of the lipooligosaccharides from nontypeable Haemophilus influenzae and Haemophilus haemolyticus show differences in sialic acid and phosphorylcholine modifications. Infect Immun. 2016;84:765–774. doi: 10.1128/IAI.01185-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang L, Xie J, Patel M, Bakhtyar A, Ehrlich GD, et al. Nontypeable Haemophilus influenzae genetic islands associated with chronic pulmonary infection. PLoS One. 2012;7:e44730. doi: 10.1371/journal.pone.0044730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hogg JS, Hu FZ, Janto B, Boissy R, Hayes J, et al. Characterization and modeling of the Haemophilus influenzae core and supragenomes based on the complete genomic sequences of Rd and 12 clinical nontypeable strains. Genome Biol. 2007;8:R103. doi: 10.1186/gb-2007-8-6-r103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harrison A, Dyer DW, Gillaspy A, Ray WC, Mungur R, et al. Genomic sequence of an otitis media isolate of nontypeable Haemophilus influenzae: comparative study with H. influenzae serotype D, strain KW20. J Bacteriol. 2005;187:4627–4636. doi: 10.1128/JB.187.13.4627-4636.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Strouts FR, Power P, Croucher NJ, Corton N, van Tonder A, et al. Lineage-specific virulence determinants of Haemophilus influenzae biogroup aegyptius. Emerg Infect Dis. 2012;18:449–457. doi: 10.3201/eid1803.110728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garmendia J, Viadas C, Calatayud L, Mell JC, Martí-Lliteras P, et al. Characterization of nontypable Haemophilus influenzae isolates recovered from adult patients with underlying chronic lung disease reveals genotypic and phenotypic traits associated with persistent infection. PLoS One. 2014;9:e97020. doi: 10.1371/journal.pone.0097020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mussa HJ, VanWagoner TM, Morton DJ, Seale TW, Whitby PW, et al. Draft genome sequences of eight nontypeable Haemophilus influenzae strains previously characterized using an electrophoretic typing scheme. Genome Announc. 2015;3:e01374-15. doi: 10.1128/genomeA.01374-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.VanWagoner TM, Morton DJ, Seale TW, Mussa HJ, Cole BK, et al. Draft genome sequences of six nontypeable Haemophilus influenzae strains that establish bacteremia in the infant rat model of invasive disease. Genome Announc. 2015;3:e00899-15. doi: 10.1128/genomeA.00899-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Giufrè M, De Chiara M, Censini S, Guidotti S, Torricelli G, et al. Whole-genome sequences of nonencapsulated Haemophilus influenzae strains isolated in Italy. Genome Announc. 2015;3:e00110-15. doi: 10.1128/genomeA.00110-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Su YC, Hörhold F, Singh B, Riesbeck K. Complete genome sequence of encapsulated Haemophilus influenzae type f KR494, an invasive isolate that caused necrotizing myositis. Genome Announc. 2013;1:e00470-13. doi: 10.1128/genomeA.00470-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Langen H, Takács B, Evers S, Berndt P, Lahm HW, et al. Two-dimensional map of the proteome of Haemophilus influenzae . Electrophoresis. 2000;21:411–429. doi: 10.1002/(SICI)1522-2683(20000101)21:2<411::AID-ELPS411>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 36.De Chiara M, Hood D, Muzzi A, Pickard DJ, Perkins T, et al. Genome sequencing of disease and carriage isolates of nontypeable Haemophilus influenzae identifies discrete population structure. Proc Natl Acad Sci USA. 2014;111:5439–5444. doi: 10.1073/pnas.1403353111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chapple SNJ, Sarovich DS, Holden MTG, Peacock SJ, Buller N, et al. Whole-genome sequencing of a quarter-century melioidosis outbreak in temperate Australia uncovers a region of low-prevalence endemicity. Microb Genom. 2016;2:e000067. doi: 10.1099/mgen.0.000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zerbino DR, Birney E. Velvet: algorithms for de novo short read assembly using de Bruijn graphs. Genome Res. 2008;18:821–829. doi: 10.1101/gr.074492.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boetzer M, Pirovano W. Toward almost closed genomes with GapFiller. Genome Biol. 2012;13:R56. doi: 10.1186/gb-2012-13-6-r56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Assefa S, Keane TM, Otto TD, Newbold C, Berriman M. ABACAS: algorithm-based automatic contiguation of assembled sequences. Bioinformatics. 2009;25:1968–1969. doi: 10.1093/bioinformatics/btp347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tsai IJ, Otto TD, Berriman M. Improving draft assemblies by iterative mapping and assembly of short reads to eliminate gaps. Genome Biol. 2010;11:R41. doi: 10.1186/gb-2010-11-4-r41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boetzer M, Henkel CV, Jansen HJ, Butler D, Pirovano W. Scaffolding pre-assembled contigs using SSPACE. Bioinformatics. 2011;27:578–579. doi: 10.1093/bioinformatics/btq683. [DOI] [PubMed] [Google Scholar]
- 44.Otto TD, Sanders M, Berriman M, Newbold C. Iterative correction of reference nucleotides (iCORN) using second generation sequencing technology. Bioinformatics. 2010;26:1704–1707. doi: 10.1093/bioinformatics/btq269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seemann T. Prokka: rapid prokaryotic genome annotation. Bioinformatics. 2014;30:2068–2069. doi: 10.1093/bioinformatics/btu153. [DOI] [PubMed] [Google Scholar]
- 46.Sarovich DS, Price EP. SPANDx: a genomics pipeline for comparative analysis of large haploid whole genome re-sequencing datasets. BMC Res Notes. 2014;7:618. doi: 10.1186/1756-0500-7-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, et al. The genome analysis toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010;20:1297–1303. doi: 10.1101/gr.107524.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Swofford DL. PAUP*: Phylogenetic Analysis Using Parsimony (*and Other Methods) version 4. Sunderland, MA: Sinauer Associates; 1998. [Google Scholar]
- 51.Jain C, Rodriguez-R LM, Phillippy AM, Konstantinidis KT, Aluru S. High throughput ANI analysis of 90K prokaryotic genomes reveals clear species boundaries. Nat Commun. 2018;9:5114. doi: 10.1038/s41467-018-07641-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Camacho C, Coulouris G, Avagyan V, Ma N, Papadopoulos J, et al. BLAST+: architecture and applications. BMC Bioinformatics. 2009;10:421. doi: 10.1186/1471-2105-10-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lu CL, Chen KT, Huang SY, Chiu HT. CAR: contig assembly of prokaryotic draft genomes using rearrangements. BMC Bioinformatics. 2014;15:381. doi: 10.1186/s12859-014-0381-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Darling AE, Mau B, Perna NT. progressiveMauve: multiple genome alignment with gene gain, loss and rearrangement. PLoS One. 2010;5:e11147. doi: 10.1371/journal.pone.0011147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Carver TJ, Rutherford KM, Berriman M, Rajandream MA, Barrell BG, et al. ACT: the Artemis comparison tool. Bioinformatics. 2005;21:3422–3423. doi: 10.1093/bioinformatics/bti553. [DOI] [PubMed] [Google Scholar]
- 56.Huson DH, Scornavacca C. Dendroscope 3: an interactive tool for rooted phylogenetic trees and networks. Syst Biol. 2012;61:1061–1067. doi: 10.1093/sysbio/sys062. [DOI] [PubMed] [Google Scholar]
- 57.Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30:1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kosakovsky Pond SL, Frost SDW. Not so different after all: a comparison of methods for detecting amino acid sites under selection. Mol Biol Evol. 2005;22:1208–1222. doi: 10.1093/molbev/msi105. [DOI] [PubMed] [Google Scholar]
- 59.Weaver S, Shank SD, Spielman SJ, Li M, Muse SV, et al. Datamonkey 2.0: a modern web application for characterizing selective and other evolutionary processes. Mol Biol Evol. 2018;35:773–777. doi: 10.1093/molbev/msx335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Page AJ, Cummins CA, Hunt M, Wong VK, Reuter S, et al. Roary: rapid large-scale prokaryote pan genome analysis. Bioinformatics. 2015;31:3691–3693. doi: 10.1093/bioinformatics/btv421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MAR, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sahl JW, Caporaso JG, Rasko DA, Keim P. The large-scale BLAST score ratio (LS-BSR) pipeline: a method to rapidly compare genetic content between bacterial genomes. PeerJ. 2014;2:e332. doi: 10.7717/peerj.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dailey HA, Dailey TA, Gerdes S, Jahn D, Jahn M, et al. Prokaryotic heme biosynthesis: multiple pathways to a common essential product. Microbiol Mol Biol Rev. 2017;81:e00048-16. doi: 10.1128/MMBR.00048-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Blattner FR, Plunkett G, Bloch CA, Perna NT, Burland V, et al. The complete genome sequence of Escherichia coli K-12. Science. 1997;277:1453–1462. doi: 10.1126/science.277.5331.1453. [DOI] [PubMed] [Google Scholar]
- 65.Dailey HA, Gerdes S, Dailey TA, Burch JS, Phillips JD. Noncanonical coproporphyrin-dependent bacterial heme biosynthesis pathway that does not use protoporphyrin. Proc Natl Acad Sci USA. 2015;112:2210–2215. doi: 10.1073/pnas.1416285112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Warren MJ, Stolowich NJ, Santander PJ, Roessner CA, Sowa BA, et al. Enzymatic synthesis of dihydrosirohydrochlorin (precorrin-2) and of a novel pyrrocorphin by uroporphyrinogen III methylase. FEBS Lett. 1990;261:76–80. doi: 10.1016/0014-5793(90)80640-5. [DOI] [PubMed] [Google Scholar]
- 67.Avissar YJ, Moberg PA. The common origins of the pigments of life-early steps of chlorophyll biosynthesis. Photosynth Res. 1995;44:221–242. doi: 10.1007/BF00048596. [DOI] [PubMed] [Google Scholar]
- 68.Hansson M, Rutberg L, Schröder I, Hederstedt L. The Bacillus subtilis hemAXCDBL gene cluster, which encodes enzymes of the biosynthetic pathway from glutamate to uroporphyrinogen III. J Bacteriol. 1991;173:2590–2599. doi: 10.1128/jb.173.8.2590-2599.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hansson M. Doctoral Thesis. Lund University; Lund, Sweden: 1994. Tetrapyrrole synthesis in Bacillus subtilis. [Google Scholar]
- 70.Moberg PA, Avissar YJ. A gene cluster in Chlorobium vibrioforme encoding the first enzymes of chlorophyll biosynthesis. Photosynth Res. 1994;41:253–259. doi: 10.1007/BF02184166. [DOI] [PubMed] [Google Scholar]
- 71.Takahata S, Ida T, Senju N, Sanbongi Y, Miyata A, et al. Horizontal gene transfer of ftsI, encoding penicillin-binding protein 3, in Haemophilus influenzae . Antimicrob Agents Chemother. 2007;51:1589–1595. doi: 10.1128/AAC.01545-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Søndergaard A, Witherden EA, Nørskov-Lauritsen N, Tristram SG. Interspecies transfer of the penicillin-binding protein 3-encoding gene ftsI between Haemophilus influenzae and Haemophilus haemolyticus can confer reduced susceptibility to β-lactam antimicrobial agents. Antimicrob Agents Chemother. 2015;59:4339–4342. doi: 10.1128/AAC.04854-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mell JC, Shumilina S, Hall IM, Redfield RJ. Transformation of natural genetic variation into Haemophilus influenzae genomes. PLoS Pathog. 2011;7:e1002151. doi: 10.1371/journal.ppat.1002151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Murphy TF, Brauer AL, Sethi S, Kilian M, Cai X, et al. Haemophilus haemolyticus: a human respiratory tract commensal to be distinguished from Haemophilus influenzae . J Infect Dis. 2007;195:81–89. doi: 10.1086/509824. [DOI] [PubMed] [Google Scholar]
- 75.Morton DJ, Hempel RJ, Whitby PW, Seale TW, Stull TL. An invasive Haemophilus haemolyticus isolate. J Clin Microbiol. 2012;50:1502–1503. doi: 10.1128/JCM.06688-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Anderson R, Wang X, Briere EC, Katz LS, Cohn AC, et al. Haemophilus haemolyticus isolates causing clinical disease. J Clin Microbiol. 2012;50:2462–2465. doi: 10.1128/JCM.06575-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.