Abstract
Background
Alzheimer disease (AD) is a significant health issue for the elderly, and there are at present no clinically effective anti-AD agents. Prevention of Aβ-induced neurotoxicity is proposed as a possible modality for treatment of AD. miR-33 has been proven to promote Aβ secretion and impair Aβ clearance in neural cells. The present study assessed whether miR-33 is involved in AD pathology.
Material/Methods
miR-33 level was detected by qRT-PCR. The Akt/mTOR pathway was analyzed by Western blot analysis. Neuron inflammation and oxidative stress were measured using commercial detection kits. Flow cytometry and Western blot assay were conducted to assess cell apoptosis, and Western blot assay was used to assess synaptic protein levels.
Results
miR-33 expression level was markedly upregulated in SH-SY5Y cells treated with Aβ25–35. miR-33 knockdown suppressed inflammation, oxidative stress, and cell apoptosis. In addition, miR-33 knockdown improved synaptic plasticity, and the protective effect of miR-33 knockdown was discovered through suppressing activation of the Akt/mTOR signaling pathway.
Conclusions
Taken together, these findings suggest that miR-33 knockdown protects against Aβ25–35-induced inflammation, oxidative stress, apoptosis, and synaptic damage by suppressing activation of the Akt/mTOR pathway.
MeSH Keywords: Alzheimer Disease, Autophagy, Neurology
Background
Alzheimer disease (AD), characterized by memory loss and progressive cognitive dysfunction, is a common chronic neurodegenerative disease [1]. With the growing elderly population, the incidence of AD is increasing rapidly [2]. It is estimated that more than 47 million people worldwide have AD, making it a major global public health and social problem [3]. Thus, further in-depth study of AD is of great importance to medical research.
Senile plaques (SP), which are associated with excessive aggregation of amyloid-beta (Aβ) peptides, are the major manifestation of AD [4]. Aβ peptides accumulation is the primary driver of AD. It has been reported that aggregated and accumulated Aβ peptides can bring about hyperphosphorylation of tau protein and neurofibrillary tangles [5]. Evidence suggests that multiple factors, including apoptosis, inflammation, and oxidative stress, contribute to AD [6].
Autophagy is an essential degradation pathway in preventing protein misfolding or aggregation in mammalian cells [7]. Several studies have shown that defects in autophagy are major contributors to the etiology of AD [8]. Autophagy plays a vital role in generation and metabolism of Aβ, assembling of tau, and homeostasis and survival of neurons [7,9]. A substantial body of evidence demonstrates that the Akt/mTOR signaling pathway plays an important role in cell autophagy [10].
Synaptic plasticity is the basis for encoding and storing information in learning and memory. Alterations of synaptic plasticity can be observed in AD [11]. It is now widely accepted that neuronal autophagy is particularly important for synapse plasticity [12]. Normal function of autophagy is essential for the maintenance of local axon homeostasis under stress conditions [13]. The loss of synapse-related proteins, including PSD, NMDA receptor, and synapsin-1, is related to the severity of dementia in AD [14]. Pin1 is a peptidyl-prolyl isomerase, and the loss of Pin1 activity can result in aberrant synaptic structure and exacerbate synaptic degeneration [15].
MicroRNAs (miRNAs) are a class of non-coding RNAs with 18–22 nucleotides [16]. miRNAs play versatile biological functions through targeting the 3′-untranslated region (3′UTR) of their target mRNAs [17]. Recently, miRNAs have emerged as new potential therapeutic targets for neurodegenerative diseases [18]. Evidence supports that miR-33 is involved in many kinds of diseases [19]. Work by Kim et al. suggests that overexpression of miR-33 can promote Aβ secretion and impair Aβ clearance in neural cells, representing a potential therapeutic target for AD [20]. A study by Ouimet et al. found that miR-33 can inhibit apoptotic cell clearance through an autophagy-dependent mechanism [21]. Moreover, the miR-33-AMPK axis can lead to BNIP3-dependent autophagy through the AKT/mTOR signal pathway [22].
Thus, the present study investigated whether miR-33 can affect apoptosis of neural cells and synaptic plasticity through the AKT/mTOR signal pathway.
Material and Methods
Cell culture
The human neuroblastoma cell line SH-SH-SY5Y was obtained from ATCC and maintained in DMEM basal culture medium supplemented with 10% FBS and 1% penicillin/streptomycin in a 5% CO2 incubator at 37°C. To study the role of miR-33 in neurotoxicity, SH-SH-SY5Y cells (80~90% confluence) were pretreated with Aβ25–35 at a concentration of 20 μM/L for 24 h and then transfected with miR-33 NC or miR-33 siRNA using Lipofactamine2000 (Invitrogen, Carlsbad, CA, U.S.A.).
qRT-PCR
QRT-PCR was performed to assess miR-33 mRNA level after treatment or transfection. The experiments were conducted according to routine protocols. Total RNA from SH-SH-SY5Y cells were extracted using TRIzol® reagent (Invitrogen, USA) and equal amounts RNA were transcribed into cDNA using a cDNA synthesis kit (Takara, Japan). qPCR was carried out using a SYBR Premix Ex Taq kit (Takara, Japan) in a Step One Plus™ real-time PCR system (Applied Biosystems, Foster City, CA). The PCR was performed under the following reaction conditions: pre-denaturation at 95°C for 30 s, 35 cycles of 95°C for 10 s, and 60°C for 60 s. The 2−ΔΔCt method was used to calculate the relative expression of each gene, with GAPDH as an internal control. Each experiment was performed in triplicate and each value represents the average of 3 independent experiments.
Western blot assay
Total cellular proteins were harvested using RIPA lysis buffer (Beyotime, CN) supplemented with the proteinase inhibitor and phosphatase inhibitor, and the soluble protein concentration was quantified with a BCA assay kit (Beyotime, CN). Equal protein samples were separated by polyacrylamide gel electrophoresis, then the proteins were transferred onto PVDF membranes. The membranes were treated with the following primary antibodies overnight at 4°C: p-AKT, AKT, p-mTOR, mTOR, beclin1, Atg5, P62, Bcl-2, Bax, cleaved caspase3, caspase3, and GAPDH. On the next day, PVDF membranes were treated with HRP-conjugated secondary antibodies. The target proteins were normalized to GAPDH in an enhanced chemiluminescence system.
Immunofluorescence analysis
Immunofluorescence staining was performed to evaluate autophagy in SH-SH-SY5Y cells. After different treatments, cells were fixed with 4% paraformaldehyde. Then, the cells were permeabilized with 0.2% Triton X-100 and blocked with 1% BSA for 20 min at room temperature. The cells were then incubated overnight with anti-LC3II primary antibody at 4°C. After rinsing 3 times with PBS, corresponding secondary antibody was used for incubation. DAPI was used to stain nuclei. Cells were observed and photographed under a confocal microscope.
Enzyme-linked immunosorbent assay
Briefly, SH-SH-SY5Y cells at a density of 1×105 were subjected to the indicated treatments. Untreated cells were used as the control. The concentrations of the inflammatory cytokines TNF-a, IL-1β, and IL-6 in cell supernatant collected from each group were detected using an Enzyme-Linked Immunosorbent Assay kit (R&D Systems, USA) following the manufacturer’s instructions. Absorbance at 450 nm was recorded in a microplate reader (Thermo Fisher Scientific, USA). ELISA was repeated 3 times.
Determination of intracellular ROS level
An ROS Assay Kit (Beyotime, China) was used to detect the ROS levels. After being washed with D-Hank’s solution, cells at the density of 5×104 cells/ml were then incubated with DCFH-DA for 30 min at 37°C. Afterwards, cells were washed again with D-Hank’s solution and then analyzed using flow cytometry.
Measurement of MDA, LDH, and SOD levels
Cells were collected and lysed. The supernatant was collected by centrifugation at 3000 rpm for 15 min at 4°C. The MDA levels and the activities of LDH and SOD were determined using corresponding commercial detection kits (Nanjing JianCheng Institute of Biological Engineering, China) following the manufacturer’s recommendations.
Apoptosis analysis assay
The annexin-V fluorescein isothiocyanate (FITC) apoptosis detection kit (Beyotime Inst., China) was used to detect cell apoptosis following the manufacturer’s instructions. After indicated treatments, cells were harvested and re-suspended in cold binding buffer. Cells were double-stained with annexin-V FITC (10 μL) and propidium iodide (PI) (5 μL) for 15 min in the dark following the manufacturer’s protocols. Subsequently, cell apoptosis was analyzed using a flow cytometer.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 7 (GraphPad Software, La Jolla, CA). Each group underwent at least 3 independent experiments, and all data are represented as the mean±SD. The comparisons among multiple groups were analyzed using one-way ANOVA followed by Tukey’s post hoc test. A statistically significant difference was defined as p<0.05.
Results
miR-33 is upregulated in Aβ25–35-treated SH-SY5Y cells
To investigate the effect of miR-33 in AD, qRT-PCR assay was first used to assess miR-33 expression. Data in Figure 1 revealed that the miR-33 level was markedly increased in Aβ25–35-treated SH-SY5Y cells.
Figure 1.

miR-33 is upregulated in Aβ25–35-induced SH-SY5Y cells. miR-33 mRNA level was determined using qRT-PCR assay. *** P<0.001 vs. control group.
miR-33 knockdown promotes autophagy by suppressing Akt/mTOR pathway
To test the effects of miR-33 on the Akt/mTOR pathway, we transfected SH-SY5Y cells with miR-33 inhibitor and analyzed the Akt/mTOR pathway by Western blot assay. As shown in Figure 2A, SH-SY5Y cells were transfected with miR-33 inhibitor and showed significantly decreased miR-33 mRNA expression compared with the control group. The phosphorylation levels of AKT/mTOR were visibly increased in Aβ25–35-treated SH-SY5Y cells. However, miR-33 inhibitor down-regulated p-AKT/mTOR expression (Figure 2B, 2C). As expected, silencing miR-33 inhibited p62 and promoted Atg5 and Beclin1 expression, while the level of autophagy in the Aβ25–35+miR-33 inhibitor group was reversed after treatment with 3-MA (autophagy inhibitor) (Figure 2D). The LC3II level was also measured by immunofluorescence assay. LC3II levels were significantly increased by miR-33 inhibitor at Aβ25–35-stimulated conditions Figure 2E, and this effect was abolished by 3-MA.
Figure 2.
miR-33 knockdown promotes autophagy by suppressing Akt/mTOR pathway. (A) miR-33 level was determined using qRT-PCR assay. (B, C) The levels of Akt, p-Akt, mTOR, and p-mTOR protein were measured by Western blot assay. (D) Atg5, Beclin1, and p62 protein levels were measured by Western blot assay. (E) Immunofluorescence staining revealed the expression of LC3II. * P<0.05, *** P<0.001 vs. control group, # P<0.05, ## P<0.01 vs. Aβ25–35 group.
miR-33 knockdown inhibits inflammation and oxidative stress
To investigate the role of miR-33 in neuron inflammation and oxidative stress during AD, miR-33 inhibitor was used to evaluate the effect of miR-33 knockdown on inflammation and oxidative stress. Inflammation and oxidative stress were significantly enhanced by Aβ25–35 (Figure 3A, 3B). Moreover, downregulation of miR-33 reduced Aβ25–35 induced inflammation and oxidative stress. However, the cell inflammation and oxidative stress were evidently enhanced in the Aβ25–35+miR-33 inhibitor+3-MA group compared with the Aβ25–35+miR-33 inhibitor group. Taken together, these findings indicate that downregulation of miR-33 attenuates Aβ25–35-induced neuronal inflammation and oxidative stress by inhibiting the Akt/mTOR pathway.
Figure 3.
miR-33 knockdown inhibits inflammation and oxidative stress. (A) TNF-α, IL-1β, and IL-6 protein levels were measured by ELISA. (B) ROS was assessed by DCFH-DA staining; Changes in MDA content and SOD and LDH activities were measured by commercial detection kits. * P<0.05, ** P<0.01, *** P<0.001 vs. control group, ## P<0.01, ### P<0.001 vs. Aβ25–35 group.
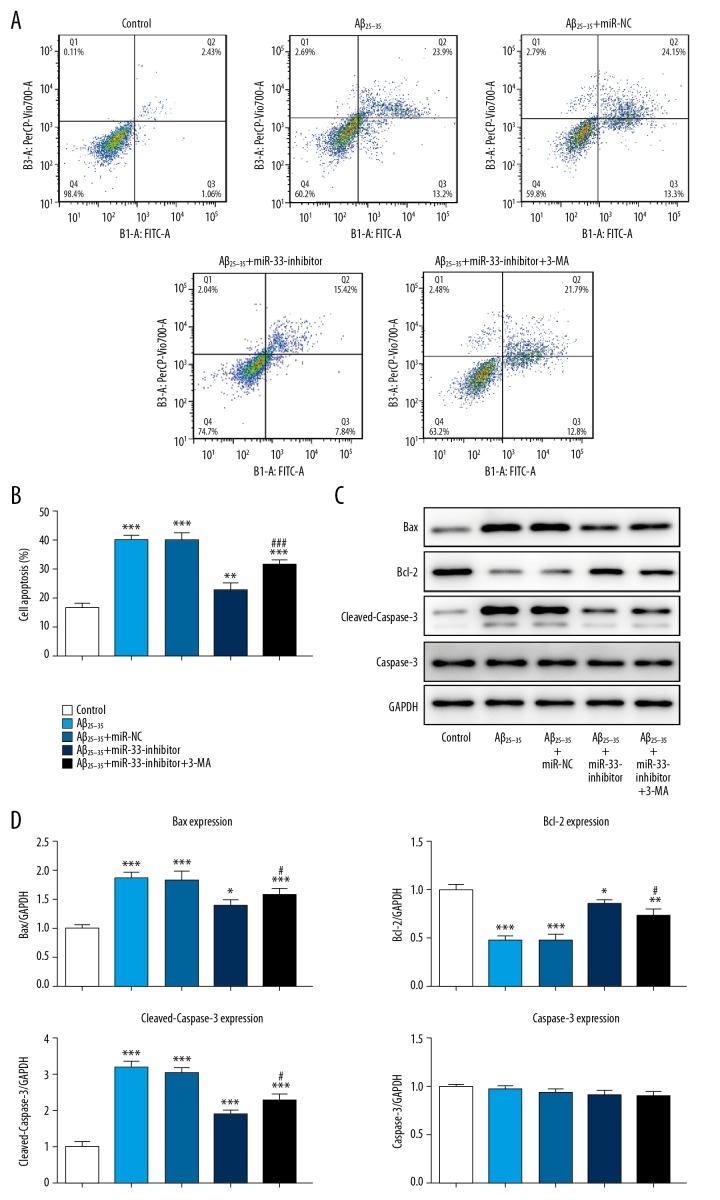
miR-33 knockdown suppresses cell apoptosis
To analyze the contribution of miR-33 knockdown to apoptosis, flow cytometry and Western blot assay were conducted. The results demonstrated that, in comparison with control group, Aβ25–35 obviously promoted SH-SY5Y cell apoptosis, and silencing miR-33 suppressed apoptosis (Figure 4A, 4B). Furthermore, compared with the Aβ25–35 group, miR-33 inhibitor drastically promoted Bcl-2 and inhibited Bax and cleaved caspase 3 expression (Figure 4C, 4D). On the contrary, the effect of miR-33 knockdown on cell apoptosis was strikingly reversed by 3-MA treatment.
Figure 4.
miR-33 knockdown suppresses cell apoptosis. (A, B) Cell apoptosis was determined using flow cytometry assay. (C, D) Bcl-2, Bax, cleaved caspase 3, and caspase 3 protein levels were measured by Western blot. * P<0.05, ** P<0.01, *** P<0.001 vs. control group, # P<0.05, vs. Aβ25–35 group.
miR-33 knockdown improves synaptic plasticity by activating autophagy
To clarify whether downregulation of miR-33 was correlated with the improvement in synaptic plasticity, we investigated synaptic protein levels by Western blot assay. PSD95, Pin1, NR2A, and NMDAR1 levels were decreased by Aβ25–35 in SH-SY5Y cells (Figure 5). However, miR-33 interference increased the PSD95, Pin1, NR2A, and NMDAR1 levels. In addition, the result showed that 3-MA treatment inhibited the increase of synaptic protein expression.
Figure 5.
miR-33 knockdown improves synaptic plasticity by activating autophagy. PSD95, Pin1, NR2A, and NMDAR1 levels were detected by Western blot. * P<0.05, ** P<0.01, *** P<0.001 vs. control group, # P<0.05, vs. Aβ25–35 group.
Discussion
With the growth of the elderly population, the incidence rate of AD is increasing year by year [23]. There is a great need for novel strategies for early diagnosis and treatment of AD [24]. miRNAs can potentially serve as biomarkers and therapeutic targets for many psycho-neurologic disorders [25]. A previous study showed that miR-33 was a potential therapeutic target [20], but the mechanism is unclear.
At present, it is well established that Aβ has toxic effects on neurons in AD [26]. Aβ25–35 is a shorter toxic fragment with high cytotoxicity and enhanced neurotoxicity, and it has been widely used to establish AD models in vitro [27]. A recent study proved that miR-33 regulates secretion and clearance of Aβ in neural cells [20]. Similarly, we also found high expression of miR-33 in an Aβ25–35-induced AD model in vitro. Autophagy is a lysosome degradation process that can remove and repurpose damaged cytoplasmic contents and aggregated proteins to maintain cell homeostasis [7]. A report showed that mTOR reduced Aβ levels in neurons and improved Aβ-induced cognitive deficits by regulating autophagy [28], indicating that the Akt/mTOR signal pathway might play a neuroprotective role in AD progression. The present findings suggest that miR-33 interference strikingly inhibits activation of the Akt/mTOR signal pathway and upregulates autophagy. Oxidative stress, inflammation, and the endogenous caspase apoptosis pathway have been implicated in Aβ neurotoxicity [6,26]. In this study, we uncovered the role of miR-33 in apoptosis, inflammation, and oxidative stress of neurons. The results indicated that downregulation of miR-33 caused a decrease in oxidative stress, inflammation, and apoptosis, while these trends were reversed by treatment with 3-MA (an autophagy inhibitor). Recently, synaptic plasticity deficits have emerged as the main cause of memory impairment in AD. Accumulated Aβ protein results in synapse deterioration [11]. The present study found that the expression of PSD95, Pin1, NR1, and NR2A in the Aβ25–35 group was significantly decreased, and miR-33 interference caused a significant increase in synapse-related proteins through regulating autophagy.
Conclusions
miR-33 interference can attenuate Aβ-induced neuronal injury, possibly via regulating autophagy through the Akt/mTOR signal pathway. Our study suggests miR-33 as a potential clinical research focus and a novel strategy for AD therapy.
Footnotes
Source of support: Popularization and Application Project of Research Fund of Sichuan Provincial Health Commission (grant no. 16PJ427)
Availability of data and materials
The analyzed datasets generated during the present study are available from the corresponding author on reasonable request.
Conflict of interest
None.
References
- 1.Knight EM, Martins IV, Gümüsgöz S, et al. High-fat diet-induced memory impairment in triple-transgenic Alzheimer’s disease (3×TgAD) mice is independent of changes in amyloid and tau pathology. Neurobiol Aging. 2014;35:1821–32. doi: 10.1016/j.neurobiolaging.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feldman HH, Estabrooks CA. The Canadian dementia challenge: Ensuring optimal care and services for those at risk or with dementia throughout the country. Can J Public Health. 2017;108:e95–97. doi: 10.17269/CJPH.108.5852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sereia AL, de Oliveira MT, Baranoski A, et al. In vitro evaluation of the protective effects of plant extracts against amyloid-beta peptide-induced toxicity in human neuroblastoma SH-SH-SY5Y cells. PLoS One. 2019;14:e0212089. doi: 10.1371/journal.pone.0212089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ji S, Li S, Zhao X, et al. Protective role of phenylethanoid glycosides, Torenoside B and Savatiside A, in Alzheimer’s disease. Exp Ther Med. 2019;17:3755–67. doi: 10.3892/etm.2019.7355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kalra J, Khan A. Reducing Aβ load and tau phosphorylation: Emerging perspective for treating Alzheimer’s disease. Eur J Pharmacol. 2015;764:571–81. doi: 10.1016/j.ejphar.2015.07.043. [DOI] [PubMed] [Google Scholar]
- 6.Li LX, Liu MY, Jiang X, et al. Metformin inhibits Aβ25–35-induced apoptotic cell death in SH-SH-SY5Y cells. Basic Clin Pharmacol Toxicol. 2019;125:439–49. doi: 10.1111/bcpt.13279. [DOI] [PubMed] [Google Scholar]
- 7.Li Q, Liu Y, Sun M. Autophagy and Alzheimer’s Disease. Cell Mol Neurobiol. 2017;37:377–88. doi: 10.1007/s10571-016-0386-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caccamo A, Ferreira E, Branca C, Oddo S. p62 improves AD-like pathology by increasing autophagy. Mol Psychiatry. 2017;22:865–73. doi: 10.1038/mp.2016.139. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9.Liu J, Li L. Targeting autophagy for the treatment of Alzheimer’s disease: Challenges and opportunities. Front Mol Neurosci. 2019;12:203. doi: 10.3389/fnmol.2019.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xue R, Meng Q, Lu D, et al. Mitofusin2 induces cell autophagy of pancreatic cancer through Inhibiting the PI3K/Akt/mTOR signaling pathway. Oxid Med Cell Longev. 2018;2018 doi: 10.1155/2018/2798070. 2798070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jackson J, Jambrina E, Li J, et al. Targeting the synapse in Alzheimer’s disease. Front Neurosci. 2019;13:735. doi: 10.3389/fnins.2019.00735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hernandez D, Torres CA, Setlik W, et al. Regulation of presynaptic neurotransmission by macroautophagy. Neuron. 2012;74:277–84. doi: 10.1016/j.neuron.2012.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yue Z, Wang QJ, Komatsu M. Neuronal autophagy: Going the distance to the axon. Autophagy. 2008;4:94–96. doi: 10.4161/auto.5202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu N, Li AD, Ji LL, et al. miR-132 regulates the expression of synaptic proteins in APP/PS1 transgenic mice through C1q. Eur J Histochem. 2019;63(2) doi: 10.4081/ejh.2019.3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu L, Ren Z, Chow FE, et al. Pathological role of peptidyl-prolyl isomerase Pin1 in the disruption of synaptic plasticity in Alzheimer’s disease. Neural Plast. 2017;2017 doi: 10.1155/2017/3270725. 3270725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu Q, Yuan X, Bai J, et al. MicroRNA-181a protects against pericyte apoptosis via directly targeting FOXO1: Implication for ameliorated cognitive deficits in APP/PS1 mice. Aging (Albany NY) 2019;11:6120–33. doi: 10.18632/aging.102171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bartel DP. Metazoan MicroRNAs. Cell. 2018;173:20–51. doi: 10.1016/j.cell.2018.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takousis P, Sadlon A, Schulz J, et al. Differential expression of microRNAs in Alzheimer’s disease brain, blood, and cerebrospinal fluid. Alzheimers Dement. 2019;15:1468–77. doi: 10.1016/j.jalz.2019.06.4952. [DOI] [PubMed] [Google Scholar]
- 19.Alrob OA, Khatib S, Naser SA. MicroRNAs 33, 122, and 208: potential novel targets in the treatment of obesity, diabetes, and heart-related diseases. J Physiol Biochem. 2017;73:307–14. doi: 10.1007/s13105-016-0543-z. [DOI] [PubMed] [Google Scholar]
- 20.Kim J, Yoon H, Horie T, et al. microRNA-33 regulates ApoE lipidation and Amyloid-β metabolism in the brain. J Neurosci. 2015;35:14717–26. doi: 10.1523/JNEUROSCI.2053-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ouimet M, Ediriweera H, Afonso MS, et al. microRNA-33 regulates macrophage autophagy in atherosclerosis. Arterioscler Thromb Vasc Biol. 2017;37:1058–67. doi: 10.1161/ATVBAHA.116.308916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen M, Li X, Fan R, et al. Cadmium induces BNIP3-dependent autophagy in chicken spleen by modulating miR-33-AMPK axis. Chemosphere. 2018;194:396–402. doi: 10.1016/j.chemosphere.2017.12.026. [DOI] [PubMed] [Google Scholar]
- 23.Bhardwaj D, Mitra C, Narasimhulu CA, et al. Alzheimer’s disease-current status and future directions. J Med Food. 2017;20:1141–51. doi: 10.1089/jmf.2017.0093. [DOI] [PubMed] [Google Scholar]
- 24.Willén K, Sroka A, Takahashi RH, Gouras GK. Heterogeneous association of Alzheimer’s disease-linked Amyloid-β and Amyloid-β protein precursor with synapses. J Alzheimers Dis. 2017;60:511–24. doi: 10.3233/JAD-170262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’Connor RM, Gururajan A, Dinan TG, et al. All roads lead to the miRNome: miRNAs have a central role in the molecular pathophysiology of psychiatric disorders. Trends Pharmacol Sci. 2016;37:1029–44. doi: 10.1016/j.tips.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 26.Wang Z, Xiong L, Wang G, et al. Insulin-like growth factor-1 protects SH-SH-SY5Y cells against β-amyloid-induced apoptosis via the PI3K/Akt-Nrf2 pathway. Exp Gerontol. 2017;87(Pt A):23–32. doi: 10.1016/j.exger.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 27.Kim JH. Brain-derived neurotrophic factor exerts neuroprotective actions against amyloid β-induced apoptosis in neuroblastoma cells. Exp Ther Med. 2014;8:1891–95. doi: 10.3892/etm.2014.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spilman P, Podlutskaya N, Hart MJ, et al. Inhibition of mTOR by rapamycin abolishes cognitive deficits and reduces amyloid-beta levels in a mouse model of Alzheimer’s disease. PLoS One. 2010;5:e9979. doi: 10.1371/journal.pone.0009979. [DOI] [PMC free article] [PubMed] [Google Scholar]