Abstract
Objectives:
In recent years, North America has witnessed a spike in the number of overdoses (OD) and OD-related deaths. The aim of this study was to assess spatial correlates of OD risk in Vancouver, Canada.
Methods:
Data utilized for this study was from three open and ongoing prospective cohorts of people who use drugs (PWUDs) in Vancouver, Canada. Logistic regression analyses with generalized linear mixed-effects models (GLMM) was used to examine correlates of residing in areas characterized by high OD rates. Mapping was used to examine areas showing OD clusters.
Results:
We included 1,336 PWUDs who resided in the downtown area. In multivariable analysis, higher availability of methadone clinics within walking distance, daily cocaine injectors and daily crack users had independent decreased odds of living within an OD cluster.
Conclusion:
This study found that higher availability of methadone clinics was associated with decreased odds of living within OD clusters.
Keywords: overdose, opioid, fentanyl, opioid agonist treatment
INTRODUCTION
Many settings throughout North America continue to experience unprecedented rates of morbidity and mortality associated with opioid overdoses (OD).1–3 In British Columbia, Canada, an approximately 300% increase in the number of OD deaths between 2014 and 2016 led to the declaration of a public health emergency in April 2016.4,5 The rapid increase in fatal overdoses has been attributed to the introduction of illicitly-manufactured fentanyl in the drug market, a highly potent substance which is difficult to detect and puts those who consume it at high risk of overdose and death.6 Similarly alarming trends have been identified in other jurisdictions in Canada the United States.7,8
A range of individual and contextual factors shape the OD risk of people who use illicit drugs (PWUD). These factors include substance use patterns and behaviors (e.g. type, frequency, and co-use), homelessness, recent incarceration and access to and availability of health and social services, including treatment for substance use disorders and harm reduction services.9–12 Although access to health services has been identified as one of the main factors shaping health outcomes among marginalized populations, to the best of our knowledge the relationship between spatial access to health services and its link to OD risk for PWUDs has been understudied. In particular, little is known about how spatial availability of addiction treatment services, like methadone maintenance clinics (MMT), shapes the PWUD risk of OD.
Methadone is the preferred method of treatment of opioid use disorders and is recommended by the Centers for Disease Control and Prevention13. Methadone treatment is associated with improved clinical and community outcomes, such as reductions in drug use, criminal behavior, and high-risk sexual behavior, which have been shown to influence OD mortality rates. Thus, the aim of this study was to investigate individual and contextual characteristics that correlate with OD risk in Vancouver, Canada, between 2013 and 2016. In particular, we focused on the spatial availability of treatment services for opioid use disorders (OUD, i.e., methadone maintenance clinics [MMT]), given the ongoing opioid-related overdose epidemic in Vancouver and many other North American jurisdictions, as well as the existence of evidence-based treatment.
Methods
Data and Setting
Data utilized for this study was derived from three ongoing open prospective cohorts of PWUD: the Vancouver Injection Drug Users Study (VIDUS), the AIDS Care Cohort to evaluate Exposure to Survival Services (ACCESS), and the At-Risk Youth Study (ARYS). Recruitment of participants, follow-up, and data collection were all performed through harmonized procedures. Recruitment took place in Vancouver’s Downtown East Side (DTES) and Downtown South areas, areas with high levels of illicit drug use, homelessness, and social marginalization. The recruitment process involved snowball sampling and extensive street outreach in those two neighborhoods. Eligibility criteria were similar for the three cohorts, including residence in the greater Vancouver area and use of illicit drugs other than or in addition to cannabis in the month prior to recruitment. In addition, each cohort has specific inclusion criteria: VIDUS consists of HIV-negative adults who had injected drugs in the month prior to enrolment; ACCESS of HIV-positive adults; and ARYS of street-involved youth (14-26 years).
Data is gathered following standard procedures, regardless of cohort affiliation, aimed at facilitating data merging and cross-cohort studies such as the present analysis. Specifically, after providing written informed consent, participants complete interviewer-administered questionnaires focusing on data related to socio-demographic characteristics, substance use patterns, health care access, and social/structural exposures, as well as undergo HIV/HCV testing at baseline, and every six months thereafter. For the present analysis, the study samples were restricted to participants who provided residential information for the period between May 2013 and December 2016, and lived in the following neighborhoods: Downtown, Downtown South, DTES, Strathcona, and Grandview Woodland (out of 24 possible neighborhoods in the City of Vancouver). The study focused on these areas because this is where most participants resided (comprising 72% of the cohort participants) and where most ODs have occurred in Vancouver according to our data. Participants receive CAD$ 30 at each study visit to compensate for their time and expertise. Further details concerning participants’ selection and study procedures can be gleaned from previously published studies.14,15 Ethical approval for the three cohort studies was granted by the University of British Columbia/Providence Health Care Research Ethics Board.
Outcome and Explanatory measures
The primary outcome of interest was whether participants resided in areas where high rates of self-reported non-fatal OD clusters were observed during the study period. These areas were defined and identified using the local Getis G cluster analysis method, described in the next paragraph. At each follow-up participant were asked if they experienced at least one OD in the past six months (yes vs. no). We decided to exclude ODs that had occurred at a supervised injection facility located in DTES (Insite), to avoid biasing the results given expected high clustering of ODs at this location, and because no fatal OD has ever occurred at Insite.16
Clusters of high OD occurrence were determined using the local Getis G function in ArcGIS 10.3.17 The local Getis G function enables the measurement of the concentration of high or low values within a defined area. Given a null hypothesis, meaning that no spatial clustering of ODs exists, the local Getis G allowed us to identify areas of high and low rates of OD occurrence within the geographical scope of our study.18 A local Getis G analysis was conducted in order to identify clusters of high rates of OD. Output from the local Getis G provided a likelihood value for each observation, with a 95% confidence, which indicated whether they were likely to be within a spatial cluster. We used that value as the outcome variable by determining if each participant resided within a cluster of high rates of OD (yes vs. no).
Our primary explanatory variable was a spatial measure assessing the number of MMT clinics within a 20-minute walking time from a participant’s place of residence. The location of clinics dispensing MMT during the study period and within the study area was drawn from publically available data, published by the provincial Opioid Agonist Treatment program.19
Geocoding of location data at the street address level was performed using Google Maps.20 Calculation of the number of MMT clinics within a 20 minute walking time from residence was performed using ESRI ArcGIS, specifically the network analyst function.17 The 20 minute walking time was chosen because it was previously shown to provide a good measure of neighborhood walkability21.
We also included additional explanatory variables, which we hypothesized might influence the relationship between OD risk and density of methadone clinics, including sociodemographic variables: age (per 10 years older); gender (male vs. non-male); ethnic background (Caucasian vs non-Caucasian); and highest level of education achieved (≥ high school diploma vs. < diploma). In addition, we looked at the following substance use variables: heroin injection (≥daily vs. <daily); cocaine injection (≥daily vs. <daily); prescription opioid injection (≥daily vs. <daily); crack use (≥daily vs. <daily); and medical or non-medical cannabis use (≥daily vs. <daily). The following variables pertaining to the engagement and access to substance use treatment and harm reduction services were also considered: proportion of injections at Insite (≥75% vs. < 75%); and enrollment in MMT (yes vs. no). Structural variables (variables that are related to societal structure) that were included in the analysis were: homelessness (yes vs. no); recent incarceration (yes vs. no); and sex work (yes vs. no). We also included HCV sero-status (positive vs. negative). Age, gender, ethnicity, and education level were time-fixed characteristics assessed at baseline. All the other variables were time-updated at each follow-up interview, and referred to the six-month period preceding each interview.
Analysis
First, we conducted a descriptive statistical analysis of the study sample based on whether the participants resided in areas showing a high occurrence of OD incidents. We used the Pearson’s Chi-squared test (or Fisher’s exact test for small cell counts) to evaluate categorical variables and the Mann-Whitney test to assess continuous variables.
To estimate the independent relationship between explanatory variables and residence in areas with high rates of OD, we conducted bivariable and multivariable generalized linear mixed-effects model (GLMM), with a logit-link function. This allowed us to analyze, over the course of multiple study visits, repeated assessments of the same participants. Only variables associated with the outcome in the bivariable analysis at significance level of p < 0.10 were considered for inclusion in the multivariable model. All statistical analyses were conducted in R 3.3.22
RESULTS
Between 2013 and 2016, 1,336 participants reported living in the study area at least once during the study period and were thus included in the analysis. These participants contributed 4,312 observations, a median of 3 (Interquartile Range (IQR): 2–5) per participant. Baseline characteristics of study participants stratified by residence in an area with high OD rates are presented in Table 1. Participants’ median age was 45 years (IQR 33–53), 870 (65%) self-reported male gender, and 735 (55%) self-reported Caucasian ancestry. The median number of MMT clinics within a 20 minutes walking time was 4 (IQR 2–4).
Table 1.
Baseline characteristics of 1,336 PWUD based on whether they resided within clusters of high rates of OD in the downtown area.
|
Within OD Cluster, n (%) |
||||
|---|---|---|---|---|
| Characteristic | Total, n (%) N=1,336 | Yes n = 252 | No n = 1,084 | p-value |
| Age (median, IQR) | 45 (33-53) | 45 (29-52) | 45 (34-53) | 0.035 |
| Gender | ||||
| male | 870 (65.1) | 194 (77.0) | 676 (62.4) | <0.001 |
| female | 466 (34.9) | 58 (23.0) | 408 (37.6) | |
| Ethnicity | ||||
| Caucasian | 735 (55.0) | 144 (57.1) | 591 (54.5) | 0.451 |
| Non-Caucasian | 601 (45.0) | 108 (42.9) | 132 (45.5) | |
| Education | ||||
| ≥ High school diploma | 604 (45.2) | 118 (46.8) | 486 (44.8) | 0.567 |
| < High school diploma | 732 (54.8) | 134 (53.2) | 598 (55.2) | |
| Homelessness | ||||
| Yes | 248 (18.6) | 49 (19.4) | 199 (18.4) | 0.689 |
| No | 1088 (81.4) | 203 (80.6) | 885 (81.6) | |
| HCV sero-status* | ||||
| Positive | 981 (73.4) | 160 (63.5) | 821 (75.7) | <0.001 |
| Negative | 355 (26.6) | 92 (36.5) | 263 (24.3) | |
| Proportion of injections at Insite* | ||||
| 100%-≥75% | 59 (4.4) | 6 (2.4) | 53 (4.9) | 0.081 |
| < 75% | 1277 (95.6) | 246 (97.6) | 1031 (95.1) | |
| Enrollment in MMT* | ||||
| Yes | 628 (47.0) | 94 (37.3) | 534 (49.3) | 0.001 |
| No | 708 (53.0) | 158 (62.7) | 550 (50.7) | |
| Recent incarceration* | ||||
| Yes | 92 (6.9) | 19 (7.5) | 73 (6.7) | 0.649 |
| No | 1244 (93.1) | 233 (92.5) | 1011 (93.3) | |
| Sex work* | ||||
| Yes | 139 (10.4) | 23 (9.1) | 116 (10.7) | 0.461 |
| No | 1197 (89.6) | 229 (90.9) | 968 (89.3) | |
| Heroin injection* | ||||
| ≥ daily | 267 (20.0) | 30 (11.9) | 237 (21.9) | <0.001 |
| < daily | 1069 (80.0) | 222 (88.1) | 847 (78.1) | |
| Cocaine injection* | ||||
| ≥ daily | 68 (5.1) | 5 (2.0) | 63 (5.8) | 0.013 |
| < daily | 1268 (94.9) | 247 (98.0) | 1021 (94.2) | |
| Prescription opioids injection* | ||||
| ≥ daily | 45 (3.4) | 8 (3.2) | 37 (3.4) | 0.850 |
| < daily | 1291 (96.6) | 244 (96.8) | 1047 (96.6) | |
| Crack use* | ||||
| ≥ daily | 160 (12.0) | 13 (5.2) | 147 (13.6) | <0.001 |
| < daily | 1176 (88.0) | 239 (94.8) | 937 (86.4) | |
| Cannabis use* | ||||
| ≥ daily | 360 (26.9) | 88 (34.9) | 272 (25.1) | 0.002 |
| < daily | 976 (73.1) | 164 (65.1) | 812 (74.9) | |
| Number of MMT clinics within 20 min walking distance (median, IQR) * | 4 (2-4) | 2 (2-2) | 2 (2-5) | <0.001 |
IQR = interquartile range
MMT=Methadone maintenance treatment
Refers to 6 months prior to the interview
During the study period, 256 (19%) participants experienced a total of 334 non-fatal ODs in the past six months. Of these, 30 ODs (9%, 20 participants) occurred at Insite, and were thus excluded from the present analysis. Of the 236 participants who experienced OD outside Insite, 69 participants experienced 83 ODs within the cluster (10.4%) during the study period, and the rest (173 participants, 221 events (6.2%)) outside a cluster. Within the OD cluster, 58 participants experienced a single OD during the study period (84%) and the rest experienced more than one OD, while outside of the OD cluster only 141 participants (81%) experienced a single OD and the rest experienced multiple ODs.
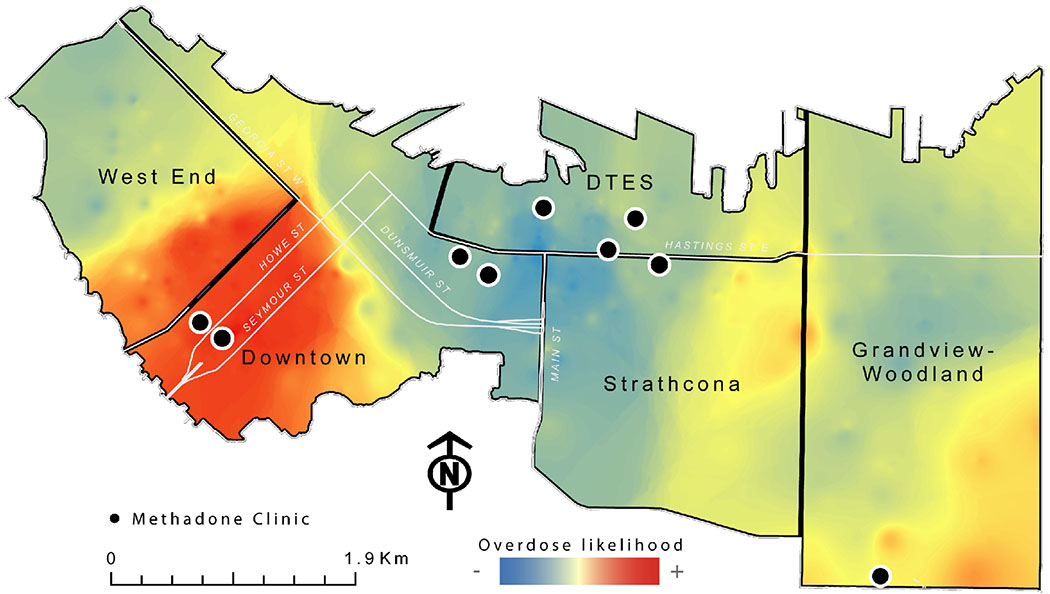
Figure 1 presents OD clusters located in the study area. As shown in this figure, there is a clear geographical clustering of high OD rates around the South Granville area, within the Downtown and West End neighborhoods. This area experienced an OD rate almost twice as high as all the other neighborhoods included in the study combined (Fig 1). Interestingly, only two clinics are located within the cluster of high OD rates (on the boundary between the Downtown and West End neighborhoods), while in the DTES neighborhood, an area where most PWUD reside, there are six MMT clinics and the OD rate is lower (depicted in light blue on the map).
Figure 1.
Geographic clusters of overdoses and Methadone Maintenance Treatment clinic location in Vancouver’s downtown area (2013-2016).
Table 2 shows the unadjusted and adjusted GLMM analyses of factors associated with residence within an OD cluster. The unadjusted model showed that daily prescription opioid was positively associated with living within an OD cluster (Odds Ratio [OR] = 2.62, 95% Confidence Interval [CI]: 1.12 – 6.12); while HCV-seropositivity (OR = 0.30, 95% CI: 0.11 - 0.83), daily cocaine injectors (OR = 0.02, 95% CI: 0 - 0.37), daily crack users (OR = 0.05, 95% CI: 0.01 - 0.24), and living in areas with high density of MMT clinics (OR = 0.33, 95% CI: 0.25 - 0.43) were negatively associated with residence in an OD cluster.
Table 2.
Bivariable and multivariable GLMM analysis of factors associated with residing within high OD clusters in the Vancouver downtown area (n=1,336, 4,312 events) between 2013-2016.
| Odds Ratio (OR) |
||
|---|---|---|
| Variable | Unadjusted OR (95% CI) | Adjusted OR (95% CI) |
| Number of MMT clinics within 20 min walking distance (per one clinic increase)* | 0.33 (0.25 - 0.43)† | 0.33 (0.25 - 0.43) |
| Age (per 10 years older) | 0.76 (0.50 - 1.16) | - |
| Male gender | 1.93 (0.63 - 5.98) | - |
| Caucasian ethnicity | 1.21 (0.44 - 3.28) | - |
| high school graduate | 1.1 (0.41 - 2.95) | - |
| Homeless | 0.51 (0.22 - 1.2) | - |
| HCV sero-positive* | 0.30 (0.11 - 0.83)† | - |
| All/most injections at Insite* | 1.46 (0.24 - 8.91) | - |
| Enrollment in MMT* | 0.99 (0.46 - 2.15) | - |
| Incarceration* | 1.05 (0.3 - 3.66) | - |
| Sex work* | 1.06 (0.33 - 3.42) | - |
| ≥ daily heroin injection* | 0.56 (0.22 - 1.40) | - |
| ≥ daily cocaine injection* | 0.02 (0 - 0.37)† | 0.02 (0.00 - 0.62) |
| ≥ daily prescription opioid injection* | 2.44 (0.55 - 10.91) | - |
| ≥ daily crack use* | 0.05 (0.01 - 0.24)† | 0.10 (0.02 - 0.46) |
| ≥ daily cannabis use* | 1.23 (0.6 - 2.53) | - |
MMT, Methadone Maintenance Treatment
Refers to 6 months prior to the interview
Significant at p <0.10 in the unadjusted analyses and included in the multivariable model.
In multivariable GLMM analysis, participants with higher availability of MMT clinics within walking distance (Adjusted Odds Ratio[AOR] = 0.33, 95% CI: 0.25–0.43), daily cocaine injectors (AOR = 0.02, 95% CI: 0.00–0.62), and daily crack users (AOR = 0.10, 95% CI: 0.02–0.46) had independent decreased odds of living within an OD cluster.
DISCUSSION
In this study, we observed a direct spatial relationship between lower density of MMT clinics and higher OD rates within Vancouver’s downtown area. Specifically, we found an inverse spatial relationship between availability of MMT clinics within walking distance and OD rates. A possible explanation is that walking is likely the primary mode of transportation for most PWUD living in the area, and therefore they are probably less likely to access MMT clinics that are not in close proximity to their place of residence, which in turn place them at increased risk of OD. Indeed, there is a well-established body of literature demonstrating the protective effect of MMT on OD risk and mortality among PWUD both in Vancouver and other settings. 23–25 Despite the lack of studies examining the spatial relationship between availability of addiction treatment and OD risk, this finding is similar to previously identified effects of a supervised injection site showing a 35% decrease in overdose rates within a radius of 500m around Insite after its opening in 2003.26 Collectively, these findings highlight the critical importance of spatial access to health services to improve health outcomes among PWUD. Therefore, in the context of the current OD epidemic in North America—in which opioids are the primary drivers of non-fatal and fatal ODs—these finding point to the urgent need to increase the accessibility of opioid agonist treatment (OAT).6,27 Among possible interventions, the integration of low-threshold OAT services within primary care services facilities may hold promise, as suggested by past research showing better geographic access to addiction treatment with this approach. 28,29Additional benefits of integrating services may include the ability to treat comorbidities, greater patient satisfaction, increased acceptance of treatment, and better health outcomes.30
Our analysis also found that frequent stimulant users (i.e., daily cocaine injectors, crack users) were less likely to live within areas with high OD rates. This finding may relate to the relatively less risk of overdose with stimulants compared to opioids (and fentanyl in particular). Recent anecdotal reports suggest that opioid users may be more likely to use (and potentially overdose) in groups due to concerns of their supply being contaminated with fentanyl, and thus having someone to assist with overdose management. Further qualitative research may help in better understanding this finding.
This study has strengths and limitations. Among its strengths, this study benefits from a combination of precise spatial information linked to sociodemographic, behavioural and social/structural data. The use of longitudinal data provided us with increased number of observations and therefore strengthens the accuracy of the results. The use of relatively current data, and therefore related to the ongoing crisis in opioid-related ODs, adds further relevance to the findings discussed. There are also some study weaknesses to be mentioned. First, most variables are derived from self-reported data. Thus, reporting bias and other individual factors may, as a result, influence their accuracy, however previous studies have supported the validity of self-reported data from PWUD.31,32 Second, causation cannot be clearly inferred, due to the observational nature of the study and the possible presence of unmeasured confounders. In fact, one might expect higher overdoses to occur near methadone clinics given that these areas may have a higher density of high risk drug users. Third, given that no publicly available information of facilities providing buprenorphine-based OAT existed at the moment, these were not included. However, most buprenorphine/naloxone is available through MMT clinics. Further, during the study period, buprenorphine-based OAT was not common in British Columbia, with more than 80% of individuals on OAT in the local health authority being on methadone maintenance treatment during the study period.33 Finally, the utilization of place of residence as a proxy for location of overdose may have resulted in the misallocation of some overdoses. That said, data from the provincial ministry of health indicates that 55.6% of OD fatalities occurred in private residences.34
PUBLIC HEALTH IMPLICATIONS
This study found a strong and negative association between higher availability of MMT clinics within walking distance and residence in areas with high overdose rates among PWUD in Vancouver’s downtown during the period of a generalized overdose crisis. In this context, findings from this study suggest that increased spatial availability of services providing opioid agonist treatment (e.g., methadone, buprenorphine/naloxone), through for example, integration with primary care services or other low-threshold community models, may play an integral role in facilitating access to these life-saving medications, and consequently in reducing rates of OD and OD related deaths.
What is already known on this subject?
Access to health and social services can reduce the risk of overdose among marginalized populations, however, the relationship between spatial access to these services and risk of overdose is understudied.
What this study adds?
This study shows a direct relationship between restricted access to methadone maintenance clinics and higher risk of overdose
Acknowledgments
The authors thank the study participants for their contributions to the research, as well as current and past researchers and staff.
Funding
This work was supported by the US National Institute on Drug Abuse (NIDA) at the US National Institutes of Health (NIH; U01-DA038886 and U01-DA021525). OA and MES are supported by Michael Smith Foundation for Health Research (MSFHR) and Canadian Institutes of Health Research (CIHR) post-doctoral fellowship awards. M-JM is supported in part by the NIH (R01-UA021525), a Scholar Award from MSFHR and a New Investigator award from CIHR. His institution has received a $1-million gift from NG Biomed Ltd., a private firm seeking a government license to produce medical cannabis, to support him. KH is supported by a CIHR New Investigator Award (MSH-141971) and a MSFHR Scholar Award.
REFERENCES
- 1.Jafari S, Buxton JA, Joe R. Rising Fentanyl-related Overdose Deaths in British Columbia. Canadian Journal of Addiction. 2015;6(1). [Google Scholar]
- 2.Marshall BD, Krieger MS, Yedinak JL, et al. Epidemiology of fentanyl-involved drug overdose deaths: A geospatial retrospective study in Rhode Island, USA. International Journal of Drug Policy. 2017. [DOI] [PubMed] [Google Scholar]
- 3.Peterson AB. Increases in fentanyl-related overdose deaths—Florida and Ohio, 2013–2015. MMWR. Morbidity and Mortality Weekly Report. 2016;65. [DOI] [PubMed] [Google Scholar]
- 4.Tyndall M Perspectives on the drug overdose crisis in BC. BRITISH COLUMBIA MEDICAL ASSOCIATION; 115-1665 W BROADWAY, VANCOUVER, BC V6J 5A4, CANADA; 2017. [Google Scholar]
- 5.BCCS. Fentanyl-detected illicit drug overdose deaths: January 1, 2012 to September 30, 2016. British Columbia Coroners Service: British Columbia Coroners Service;2016. [Google Scholar]
- 6.Sisco E, Verkouteren J, Staymates J, Lawrence J. Rapid detection of fentanyl, fentanyl analogues, and opioids for on-site or laboratory based drug seizure screening using thermal desorption DART-MS and ion mobility spectrometry. Forensic Chemistry. 2017;4:108–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gladden RM. Fentanyl law enforcement submissions and increases in synthetic opioid-involved overdose deaths—27 states, 2013–2014. MMWR. Morbidity and mortality weekly report. 2016;65. [DOI] [PubMed] [Google Scholar]
- 8.Ahamad K An urgent call to increase access to evidence-based opioid agonist therapy for prescription opioid use disorders. Canadian Medical Association. Journal. 2016;188(17/18):1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Milloy M-J, Milloy M-J, Wood E, et al. Incarceration experiences in a cohort of active injection drug users. Drug and alcohol review. 2008;27(6):693–699. [DOI] [PubMed] [Google Scholar]
- 10.Phillips M, Richardson L, Wood E, Nguyen P, Kerr T, DeBeck K. High-intensity drug use and health service access among street-involved youth in a Canadian setting. Substance use & misuse. 2015;50(14):1805–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carlson J, Sugano E, Millstein SG, Auerswald CL. Service utilization and the life cycle of youth homelessness. Journal of Adolescent Health. 2005;36(2):121. [DOI] [PubMed] [Google Scholar]
- 12.Hadland SE, Kerr T, Li K, Montaner JS, Wood E. Access to drug and alcohol treatment among a cohort of street-involved youth. Drug and alcohol dependence. 2009;101(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nielsen S, Larance B, Lintzeris N. Opioid agonist treatment for patients with dependence on prescription opioids. Jama. 2017;317(9):967–968. [DOI] [PubMed] [Google Scholar]
- 14.Strathdee SA, Palepu A, Cornelisse PA, et al. Barriers to use of free antiretroviral therapy in injection drug users. JAMA. 1998;280(6):547–549. [DOI] [PubMed] [Google Scholar]
- 15.Wood E, Hogg RS, Lima VD, et al. Highly active antiretroviral therapy and survival in HIV-infected injection drug users. Jama. 2008;300(5):550–554. [DOI] [PubMed] [Google Scholar]
- 16.VCH. Insite user statistics. 2016; http://www.vch.ca/public-health/harm-reduction/supervised-injection-sites/supervised-injection-user-statistics. Accessed 01/0/2017, 2017.
- 17.ArcGIS Desktop: Release 10. [computer program]. Redlands, CA: Environmental Systems Research Institute; 2012. [Google Scholar]
- 18.Getis A, Ord JK. The analysis of spatial association by use of distance statistics. Geographical analysis. 1992;24(3):189–206. [Google Scholar]
- 19.BCCSU. Mapping Treatment Providers. 2017; https://www.google.com/maps/d/u/0/viewer?ll=49.29861064596624%2C-122.95864102646487&z=11&mid=1LgEq4emHNvYX04CSw8uIIohGWI4, 2017.
- 20.Google. Google Maps. 2015; www.google.ca/maps, 2015.
- 21.Adams MA, Ryan S, Kerr J, et al. Validation of the Neighborhood Environment Walkability Scale (NEWS) items using geographic information systems. Journal of physical activity and health. 2009;6(s1):S113–S123. [DOI] [PubMed] [Google Scholar]
- 22.A language and environment for statistical computing. [computer program]. 2012.
- 23.Kerr T, Fairbairn N, Tyndall M, et al. Predictors of non-fatal overdose among a cohort of polysubstance-using injection drug users. Drug and alcohol dependence. 2007;87(1):39–45. [DOI] [PubMed] [Google Scholar]
- 24.Van Ameijden E, Langendam M, Coutinho R. Dose-effect relationship between overdose mortality and prescribed methadone dosage in low-threshold maintenance programs. Addictive Behaviors. 1999;24(4):559–563. [DOI] [PubMed] [Google Scholar]
- 25.Hall W, Strang J. Value for money in reducing opioid-related deaths. The Lancet Public Health. 2017;2(3):e124–e125. [DOI] [PubMed] [Google Scholar]
- 26.Marshall BD, Milloy MJ, Wood E, Montaner JS, Kerr T. Reduction in overdose mortality after the opening of North America’s first medically supervised safer injecting facility: a retrospective population-based study. The Lancet. 2011;377(9775):1429–1437. [DOI] [PubMed] [Google Scholar]
- 27.Cunningham SM, Haikal NA, Kraner JC. Fatal intoxication with acetyl fentanyl. Journal of forensic sciences. 2016;61(S1). [DOI] [PubMed] [Google Scholar]
- 28.MacGowan RJ, Swanson NM, Brackbill RM, Rugg DL, Barker T, Molde S. Retention in methadone maintenance treatment programs, Connecticut and Massachusetts, 1990–1993. Journal of psychoactive drugs. 1996;28(3):259–265. [DOI] [PubMed] [Google Scholar]
- 29.Novick DM, Joseph H, Salsitz EA, et al. Outcomes of treatment of socially rehabilitated methadone maintenance patients in physicians’ offices (medical maintenance). Journal of General Internal Medicine. 1994;9(3):127–130. [DOI] [PubMed] [Google Scholar]
- 30.Nolan S, Hayashi K, Milloy M-J, et al. The impact of low-threshold methadone maintenance treatment on mortality in a Canadian setting. Drug and alcohol dependence. 2015;156:57–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Irala J, Bigelow C, McCusker J, Hindin R, Zheng L. Reliability of self-reported human immunodeficiency virus risk behaviors in a residential drug treatment population. American Journal of Epidemiology. 1996;143(7):725–732. [DOI] [PubMed] [Google Scholar]
- 32.Langendam MW, Van Haastrecht H, Van Ameijden E. The validity of drug users’ self-reports in a non-treatment setting: prevalence and predictors of incorrect reporting methadone treatment modalities. International journal of Epidemiology. 1999;28(3):514–520. [DOI] [PubMed] [Google Scholar]
- 33.Health; BCMo. BC OPIOID SUBSTITUTION TREATMENT SYSTEM Performance Measures 2014/2015 - 2015/2016 http://www2.gov.bc.ca/assets/gov/health/about-bc-s-health-care-system/office-of-the-provincial-health-officer/reports-publications/special-reports/bc-ost-system-measures-14-15-and-15-16.pdf:gov.bc.ca;2017.
- 34.BCCDC. The BC Public Health Opioid Overdose Emergency. http://www.bccdc.ca: BCCDC;2017. [Google Scholar]