Abstract
Differentiated thyroid cancer (DTC) is rare in children, but it still remains the most common endocrine malignancy in children. The aim of this study was to analyze treatment response to radioactive iodine (RAI) therapy, clinical outcomes, recurrences, survival analysis, and long-term follow-up. We retrospectively reviewed the medical records of 43 pediatric patients (≤17 years of age) with DTC diagnosis after thyroidectomy who were treated with RAI. The follow-up protocol consisted of detailed clinical examination, testing of thyroid function, determination of serum thyroglobulin (Tg), and anti-Tg antibodies, and neck ultrasonography application. Forty-three pediatric patients (34 females and 9 males) treated with RAI for DTC in our institute. The median follow-up period was 54 months. The histologic classification was papillary thyroid cancer in 41 patients and the remaining 2 patients had follicular thyroid cancer. After the long-term follow-up, complete remission, partial remission, and recurrent-persistent disease were observed in 37 patients, 3 patients, and 3 patients, respectively. Among the series, 1 death occurred due to multiple metastases. The mortality rate is 2.56%. Total thyroidectomy followed by RAI appears to be the most effective treatment for patients with pediatric DTC in terms of reducing the rate of relapse and improving surveillance for recurrent disease.
Keywords: Clinical outcome, long-term follow-up, pediatric differentiated thyroid carcinoma, radioactive iodine therapy, recurrence, survival analysis
INTRODUCTION
Differentiated thyroid cancer (DTC) is rare in children, constituting 0.5%–3.0% of all pediatric malignancies, but it still remains the most common endocrine malignancy in children. Papillary thyroid cancer (PTC) accounts for 90% or more of all childhood cases.[1,2,3,4]
Age is recognized as one of the most important prognostic factors for DTC. In patients younger than 18 years, DTC has been found to behave differently than in adults DTC is particularly rare in the first decade and more aggressive in ≤10 years children.[5,6] Children generally present with advanced disease at diagnosis. Extrathyroidal extension, tumor size, multicentricity, lymph node metastasis, and distant metastases at diagnosis, are more frequent and important risk factors in DTC.[2,7,8,9,10,11,12] Although DTC has high rates of local and distant recurrences, the outcome and prognosis are much better for pediatric patients with low mortality rate compared to the adults.[3,6,10,11,12] The goal of primary treatment of DTC is to eradicate disease and extend recurrence-free survival. Treatment of DTC consists of total or near total thyroidectomy with selective lymph node dissection (when involved) followed by remnant ablation with iodine-131 (I-131) in patients.[3,6,7,11] The aim of this study was to analyze treatment response to radioactive iodine (RAI) therapy, clinical outcomes, recurrences, survival analysis, and long-term follow-up data in pediatric DTC.
MATERIALS AND METHODS
We retrospectively reviewed the medical records of 43 pediatric patients (≤17 years of age) with differentiated thyroid carcinoma diagnosis after thyroidectomy who were treated with RAI and followed up in our clinic, between 1995 and 2015. Inclusion criteria were patients with well-differentiated thyroid carcinoma diagnosis who were diagnosed under 18-year-old, with minimum follow-up of 6 months. Exclusion criteria were patients older than 17 years, the presence of medullary thyroid carcinoma or anaplastic carcinoma. None of the patients had a history of the head-and-neck irradiation.
We analyzed the differences in the clinical variety between children ≤10 years and >10 years. The medical data were evaluated according to their age, gender, type of surgery, histopathology, RAI therapy, recurrences, long-term follow-up, survival analysis, and mortality rate.
All the patients had undergone total/subtotal thyroidectomy with or without cervical lymph node dissection.
Postoperative data on tumor characteristics included tumor size and type, tumor extension, multicentricity, extra thyroidal extension, lymph node metastasis, lymphatic invasion, soft tissue and vascular invasion.
Before the RAI treatment, neck ultrasonography (USG) and thyroid scintigraphy were applied to all patients. Based on guidelines of the American Thyroid Association (ATA) and of the European Thyroid Cancer Taskforce pretreatment serum concentration of free T4 (fT4), free T3 (fT3) thyroid-stimulating hormone (TSH), thyroglobulin (Tg), and anti-Tg antibodies (anti TgAb) were measured in order to reveal the presence or absence of residual thyroid tissue.
RAI therapy remnant ablation was performed within 4–6 weeks after the thyroid surgery, ranged from 30 to 200 mCi (mCi) (1.11–7.4 GigaBecquerel [GBq]) based on guidelines of the ATA and of the European Thyroid Cancer Taskforce.[11,13] Repeated RAI treatments were given to patients with evidence of local recurrence or distant metastases.
There are two main practices for RAI dosing, empiric fixed doses and individualized doses using either body surface area or weight. The empiric fixed doses are 30–100 mCi (1.11–3.7 GBq) for ablation, 150 mCi (5.55 GBq) for nodal involvement, and 150–200 mCi (5.55–7.4 GBq) for distant metastases. For children under or at the age of 10 years, doses were corrected using either body surface area or weight. The adult dose was then scaled down according to the pediatric patient's body weight by using the formula: Pediatric dose = Adult dose × body weight (kg)/70 (kg).[11,13] Older children (>10 years) were given empiric fixed doses.
All patients received thyroid-stimulating hormone suppression treatment with levothyroxine after the RAI therapy according to the ATA guidelines. Postablative I-131 whole body scan (WBS) was performed 5–7 days after radioiodine administration.
The follow-up protocol consisted of detailed clinical examination, testing of thyroid function (fT4, fT3, and TSH), determination of serum Tg and anti-TgAb, and neck USG application. Diagnostic WBS with I-131 (2–5 mCi) was performed 12 months after ablation therapy.
Intervals between examinations ranged from 3 to 6 months, depending on the estimated risk of disease recurrence. All patients in whom recurrence was suspected were confirmed using cytology and/or histology.
Response to treatment was evaluated with the following criteria:
Complete remission was achieved when the posttherapy whole-body I-131 scintigraphy and repeated diagnostic scans were negative for any remnants of the thyroid tissue or metastasis, and serum Tg levels were <2 ng/mL
Partial remission was considered when there were partial uptakes in the same areas on follow-up whole-body I-131 scintigraphy along with constantly high serum Tg levels
The recurrent-persistent disease was considered when there were new foci of radioiodine uptake on the whole-body I-131 scintigraphy, and/or the serum Tg levels were rising.
Statistical analysis
In the current study, statistical analysis was performed using IBM SPSS version 22.0 software (IBM Corp., Armonk, NY, USA). Compliance with the normal distribution of parameters was evaluated using Shapiro–Wilks test. Data were analyzed using descriptive statistical methods (mean, standard deviation, and frequency). Mann–Whitney U-test, and Chi-square test was used for the comparison of quantitative data between the two groups and Fisher's exact test, Chi-square test and Fisher Freeman Halton were used for the comparison of qualitative data. Logistics analysis was performed for multivariate analysis. The Kaplan–Meier method was used for survival analysis. The Log-Rank test was used to determine group differences in survival curves. The values of P < 0.05 were considered statistically significant with 95% of confidence interval (CI).
Ethics
The local ethics committee of Okmeydani Training and Research Hospital, located in Istanbul, Turkey approved the study (08.042014/188) and informed consent was obtained from all patients participating in this study.
RESULTS
In the current study, 43 patients (34 females, 9 males) treated with RAI for differentiated thyroid carcinoma in our institute. The age at diagnosis of DTC ranged from 3 to 17 years (mean age 14.7 ± 3.1 years) with female predominance (79%). The median follow-up period was 54 months (range 7–238 months). At diagnosis, 4 patients (9.3%) were 10 years of age or under and 39 patients (90.7%) over 10 years of age. There was no statistically significant difference at rates of recurrences in terms of age (P > 0.05). Family history of thyroid cancer was positive in 4 patients (9.3%), and none of the patients had a history of the head-and-neck irradiation. The clinical characteristics of all patients are summarized in Table 1.
Table 1.
Clinical and pathologic characteristics results and follow-up in pediatric differentiated thyroid cancer patients
| n (%) | |
|---|---|
| Sex | |
| Male | 9 (20.9) |
| Female | 34 (79.1) |
| Age | |
| ≤10 | 4 (9.3) |
| >10 | 39 (90.7) |
| Family story | 4 (9.3) |
| Surgery | |
| Total thyroidectomy | 41 (95.3) |
| Right lobe subtotal, left lobe total | 1 (2.3) |
| Bilateral subtotal | 1 (2.3) |
| Neck lymph node dissections | |
| Yes | 17 (39.5) |
| No | 26 (60.5) |
| Histopathology | |
| Papillary cancer | 41 (95.3) |
| Follicular cancer | 2 (4.7) |
| Histopathologic subtype | |
| Classic type | 23 (53.5) |
| Follicular variant | 13 (30.2) |
| Classic + follicular | 3 (7.0) |
| Diffuse sclerozan | 2 (4.7) |
| Multicentricity | |
| Yes | 23 (53.5) |
| No | 20 (46.5) |
| Lymph node metastasis | |
| Yes | 15 (34.9) |
| No | 28 (65.1) |
| Lymphatic invasion | |
| Yes | 24 (55.8) |
| No | 19 (44.2) |
| Vascular invasion | |
| Yes | 21 (48.8) |
| No | 22 (51.2) |
| Thyroid capsul invasion | |
| Yes | 24 (55.8) |
| No | 19 (44.2) |
| Distant metastasis | |
| Yes | 8 (18.6) |
| No | 35 (81.4) |
| RAI treatment | |
| 1 dose | 31 (72.1) |
| ≥1 doses | 12 (27.9) |
| Long-term follow-up results | |
| Recurrent-persistent disease | 3 (7.0) |
| Partial remission | 3 (7.0) |
| Complete remission | 37 (86.0) |
RAI: Radioactive iodine
In 41 of 43 patients (95.3%) total thyroidectomy was performed, one patient had bilateral subtotal thyroidectomy and 1 patient had right lobe total left lobe subtotal thyroidectomy. In 17 of patients (39.5%), neck lymph node dissections were performed.
The primary tumor size at initial surgery was minimum of 0.1 cm, and maximum of 5 cm (median size 2.13 ± 1.32 cm). In 35 patients (81.4%) primary tumor size was ≥1 cm, and in 8 patients (18.6%) it was <1 cm. There was no significant difference in tumor size among the recurrence and nonrecurrence groups (P > 0.05).
The histologic classification was PTC in 41 patients (95.3%) and the remaining 2 patients (4.7%) had follicular thyroid cancer (FTC). The histologic subtypes of PTC were classic type in 23 (53.5%), follicular variant in 15 (34.9%), diffuse sclerosis in 2 (4.7%), and classic and follicular variant in 3 patients (7%). There were no statistically significant difference rates at of recurrences in terms of histopathological subtypes (P > 0.05). Hurthle cell carcinoma or insular carcinoma was not found in our series.
Extrathyroidal extension was found in 24 patients (55.8%), multicentricity in 23 patients (53.5%), lymph node involvement in 15 patients (34.9%), lymphatic invasion in 24 patients (55.8%), and soft tissue and vascular invasion in 21 patients (48.8%). Recurrence rate was significantly influenced by tumor multicentricity (26.1%) (P < 0.05) and lymph node metastasis (33.3%) (P < 0.05). The risk of recurrence in patients with lymph node metastasis was 13.5 times more than patients without lymph node metastasis (odds ratio: 13.500; 95% CI: 1.400–130.191).
Regarding the TNM staging, 83.7% (36 patients) were TNM stage I and (7 patients) 16.3% stage II.
RAI treatment was administered for ablation of thyroid remnant in all of the patients after the surgery. RAI dose at ablation ranged from 30 to 200 mCi (1.11–7.4 GBq) and total RAI administered ranged from 30 to 850 mCi (1.11–31.4 GBq).
Thirty-one out of 43 patients (72.1%) were administered with a single dose of I-131, 12 patients (27.9%) underwent two or more dose of RAI treatment (between 2 and 4) for recurrence or distant metastasis. RAI treatment was repeated once in 7 patients, twice in 1 patient, three times in 1 patient, and four times in 3 patients. Total cumulative activities were 189.25 ± 177.02 mCi (6.99–6.5 GBq).
After the initial RAI ablation, WBS revealed thyroid remnants in all patients, cervical lymphadenopathy in 6 patients, lung metastasis in 4, bone metastasis (femur and sternum) in 2, and mediastinal lymphadenopathy in 1 patient.
Twenty-nine out of 37 patients had complete remission after the initial dose, 6 patients showed complete remissions in the second dose, one in third and one in forth dose.
After the last RAI treatment; recurrences were diagnosed in 9 patients (20.9%), including 1 recurrence in thyroid remnants, 1 neck lymph node metastasis, 2 neck lymph node metastasis and lung metastasis, 1 neck and mediastinal lymph node metastasis, 1 lung metastasis, 1 lung metastasis and recurrence in thyroid remnants, 1 multiple metastasis (lung, bone, and lymph node), 1 bone metastasis (sternum), and local invasion [Table 2].
Table 2.
Sites of carcinoma recurrences and follow-up
| n (9) | Evaluation of response to treatment | |
|---|---|---|
| Relapse in thyroid remnants | 1 | Partial remission |
| Neck lymph node metastasis | 1 | Complete remission |
| Neck lymph node metastasis and lung metastasis | 2 | Partial remission/recurrent-persistent disease |
| Neck and mediastinal lymph node metastasis | 1 | Partial remission |
| Lung metastasis | 1 | Complete remission |
| Lung metastasis and relapse in thyroid remnants | 1 | Complete remission |
| Multiple metastasis (lung and bone and lymph node) | 1 | Recurrent-persistent disease (Ex) |
| Bone metastasis (sternum) and local invasion | 1 | Recurrent-persistent disease |
After the long-term follow-up, among all studied patients, complete remission, partial remission, and recurrent-persistent disease were observed in 37 patients (86%), 3 patients (2M; 1F) (7%), and 3 patients (2M; 1F) (7%), respectively. Follow-up results are summarized in Table 3. Recurrence rate was significantly higher in males than females (P < 0.05). The risk of recurrence in males is 12.8 times more than females. (Odds ratio: 12,800; 95% CI: 1837–89206).
Table 3.
Long-term follow-up in children with differentiated thyroid cancer
| Recurrent-persistent disease (n=3), n (%) | Partial remission (n=3), n (%) | Complete remission (n=37), n (%) | |
|---|---|---|---|
| Histopathology | |||
| Follicular | 1 (33.3) | 0 (0) | 1 (2.7) |
| Papillary cancer | 2 (66.7) | 3 (100) | 36 (97.3) |
| Histopathologic subtype | |||
| Follicular | 1 (33.3) | 0 (0) | 14 (37.8) |
| Classic | 1 (33.3) | 3 (100) | 19 (51.4) |
| Classic and follicular | 1 (33.3) | 0 (0) | 2 (5.4) |
| Diffuse sclerozan | 0 (0) | 0 (0) | 2 (5.4) |
| Lymph node metastasis | 3 (100) | 2 (66.7) | 10 (27.0) |
| Lymphatic invasion | 3 (100) | 3 (100) | 18 (48.6) |
| Vascular invasion | 3 (100) | 3 (100) | 15 (40.5) |
| Thyroid capsul invasion | 3 (100) | 3 (100) | 18 (48.6) |
| Tumor size average±SD (median) | 3.3±2.14 (4) | 2.7±1.99 (1.6) | 1.99±1.19 (1.9) |
SD: Standard deviation
No long-term adverse effect (myelosuppression) or any other secondary malignant disease was documented in any of our patients.
The mortality rate in our series was 2.56%. Among our series, 1 death occurred. The patient was diagnosed with follicular Ca with a 5 cm tumor diameter with locoregional metastases extending beyond thyroid capsule invading soft-tissue DTC at the age of 14 years. Her TNM classification was T4aN1bM1 stage 2. She underwent total thyroidectomy and cervical lymph node dissection. She received radioiodine treatment five times with a mean cumulative dose of 700 mCi (25.9 GBq). The disease had an aggressive progression with high Tg levels. She died at the age of 21 due to disseminated pulmonary metastasis and bone metastasis.
There were 4 patients under or at 10 years of age. The youngest patient diagnosed was 3 years old. He underwent 5 surgical operations due to recurrence and received radioiodine treatment five times with mean cumulative dose of 400 mCi (14.8 GBq). The cervical and lung metastasis persisted during the follow-ups. In other 3 patients, under or at 10 years of age, complete remission is achieved by treated with a single dose (75%).
Five female patients (11.6% of all treated female patients) subsequently had healthy children, and no recurrence is seen during their follow-up.
In this study, 6 out of 43 patients (14%) were observed recurrences. The mean disease-free survival time based on recurrence was 184.91 ± 18.6 months [Figure 1].
Figure 1.
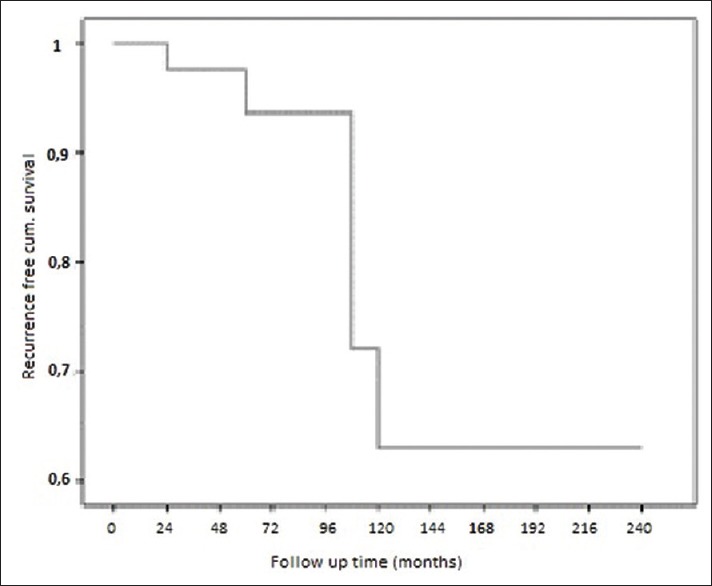
The mean disease-free survival time based on recurrence
In 2 out of 34 females (5.9%), 4 out of 9 males (44.4%) recurrences were observed in our study. The mean disease-free survival times in female and male groups were 212.86 ± 18.17 months, 102.28 ± 18.16 months, respectively. There were significantly higher in females than males in terms of the recurrence-free survival rate [P < 0.05, Figure 2].
Figure 2.
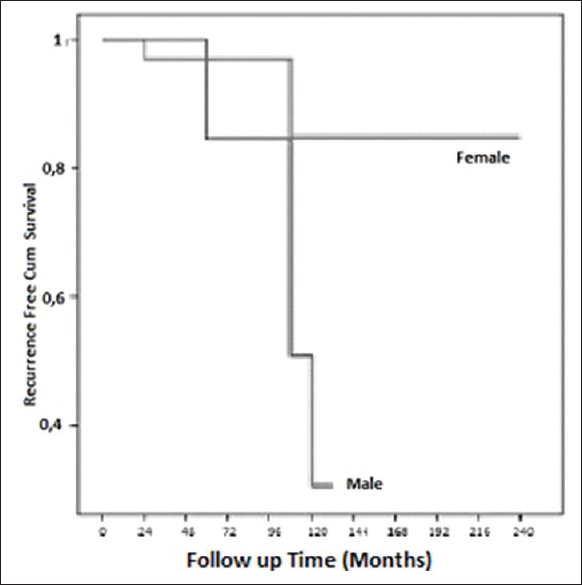
Disease free survival according to sex
In this study, a total of 4 children under the age of 10 is one of them (25%) recurrences were observed. In this study, a total of 39 boys over 10-year-old, 5 of them (12.8%) recurrences were observed. In 1 out of 4 patients under or at the age of 10 (25%), 5 out of 39 patients over the age of 10 (12.8%) recurrences were observed in our study. The mean disease-free survival times in under or at the age of 10 and over the age of 10 groups were 169.0 ± 48.79 months, 132.48 ± 8.16 months, respectively. When evaluated by the Log-Rank test, there was no significant difference between the two groups in terms of the recurrence-free survival rate [P > 0.05, Figure 3].
Figure 3.
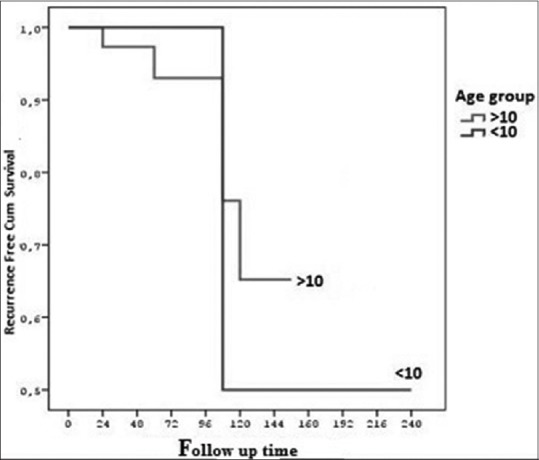
Disease free survival according to age
DISCUSSION
Childhood thyroid cancers are more often papillary and well differentiated. PTC is the most common histological type of thyroid carcinoma in pediatric patients, whereas FTC is uncommonly found.[5,11,14] In our series, PTCs and FTCs were found in 41 patients (95.3%) and in 2 patients (4.7%), respectively.
Age is the most important prognostic factor for DTC. The younger the patient is at initial diagnosis, the worse prognosis will be seen. Also, this rule is applicable for recurrence in children.[15,16]
In this study, the age at diagnosis of DTC ranged from 3 to 17 years (mean 14.7 ± 3.1 years) with female predominance (3.7:1) (79%). Contrary to the literature, there was no statistically significant difference at the rates of recurrences in terms of age (P > 0.05).
Papillary microcarcinoma, defined as a tumor <1 cm, is a rare diagnosis in childhood and in most studies accounts for <3% of sporadic PTC.[17,18] Zimmerman et al. described the incidence of microcarcinoma as 9%, whereas Park et al. revealed an incidence of 20% in their series,[12,19] which is concordant with the finding of an incidence of 18.6% (8 patients) in our study. Three patients (37.5%) had lymph node and lung metastasis. One patient had recurrence/persistence disease, whereas the other 2 achieved complete remission.
The smaller size of the thyroid gland tumor in children are usually multicentric and lead to earlier invasion of the thyroid capsule, regional lymph node involvement, extrathyroidal extension (ETE), and pulmonary metastasis.[11,12,17]
Previous studies have hypothesized that the tumor volumes tend to be relatively larger in children when compared to adults, probably attributed to smaller thyroid volume in children, thus higher chances of ETE and capsular invasion.[14,17] The common use of high-resolution USG contributed to the increased number of discovered small and microcarcinomas, which would not otherwise be discovered by palpation.
In our clinic in accordance with the ATA guideline recommendation 32(A), all of our patients underwent routine near-total or total thyroidectomy with postoperative RAI therapy.[11] This recommendation is based on data showing an increased incidence of bilateral and multifocal disease (30% and 65%, respectively), as well as an increased risk of recurrence.[1,6,9,10,11,20]
The aim of postsurgical RAI ablation is an effort to eliminate residual thyroid tissue in order to treat residual disease, such as metastatic or unresectable lesions, thus decrease the risks of thyroid cancer recurrence.[11] The second aim is to accurately apply whole-body 131-I scanning, used to detect distant sites of disease in otherwise asymptomatic patients.[21] In this study, patients over 10-year-old received empiric fixed doses in postsurgical RAI ablation, whereas patients under or at 10-year-old received individualized doses calculated by the above-mentioned formula.
Radioiodine therapy, although generally safe, has some potential side effects, classified as early and late side effects.[6] In the current study, short-term side effects of the radioiodine therapy were seen in our patients, but these side effects were well tolerated and temporary. Long-term adverse effect (myelosuppression) or any other secondary malignant disease was not documented in any of our patients.
The incidence of cervical lymph node involvement and distant metastasis has been reported as two-four times higher in children than in adults with DTC.[4,22] The range of prevalence reported in other pediatric series is in between 39% and 90%.[3,20,23,24,25] In our study, the incidence of lymph node involvement was found to be 39.5%, which is in the lower range of prevalence reported.
Mihailovic et al. revealed in their series, even with a 69% lymph node involvement in initial diagnosis, there was no correlation between lymph node metastasis at presentation and risk of recurrence.[3] In our study, the recurrence rate was significantly influenced by lymph node metastasis (33.3%) (P < 0.05).
Distant metastasis is seen in DTCs at presentation in children, the most common metastasis site being the lungs, with the incidence of 6%–33%.[8,12,14,19] Postoperative radioiodine evaluation immediately after primary thyroid disease surgery plays an important diagnostic role in pulmonary metastasis.[26] Pulmonary micrometastasis are generally diffuse and highly concentrate radioiodine, that is not apparent with chest radiographs or computerized tomography scanning but is apparent with RAI scans[24] and they respond well to radioiodine treatment.[27] In our study, after surgery, postablation RAI revealed 4 cases of lung metastasis (9.3%).
The distant metastasis outside the lungs is seen very rarely (20%).[7,24] In our study, distant metastasis was found in 2 bones and 1 mediastinal LAP excluding the lung metastasis.
Prior studies have shown that the recurrence rate ranges from 7.6% to 28%.[3,8,12,19,20,25] Larger tumor size (>3 cm), palpable cervical nodes, male sex, and multicentricity were suggested to be associated with an increased risk of recurrence.[3,20] Other studies reported that tumor size was not a significant risk factor for recurrence in children.[15,28,29] In our study, we found no correlation between tumor size and recurrence (P > 0.05). Golpanian et al., in agreement with prior studies, reported that lymph node involvement is associated with recurrence, whereas Mihailovic et al. with a 69% of lymph node involvement at presentation in their series, found no correlation between lymph node metastasis at presentation and recurrence.[1,3] In the current study, recurrence rate was significantly influenced by lymph node metastasis (33.3%) (P < 0.05).
Some authors have found that recurrent disease is frequent in multicentric PTC.[3,30] In the present study, tumor multicentricity significantly influenced the recurrence rate (26.1%) (P < 0.05), which was consistent with the literature.
Given the risk of recurrence many years after diagnosis, long-term follow-up is important for these patients.[31] Overall, thyroid cancer in children and adolescents has an excellent prognosis.[3] Demidchik et al. observed 5- and 10-year survival rate was 99.5% and 98.8%, respectively.[15] Parisi et al. reported overall survival of 98% at 10 years and overall survival of 95% at 20 years.[10] Hogan et al. stated 90% survival at 30 years, and Hay et al. detected 98% survival at 30–50 years after surgery.[6,32]
In this study; the mean disease-free survival time based on recurrence was (97.6%) at 15 years (7 month–20 years). The mean disease-free survival times in under or at the age of 10 and over the age of 10 groups were 14 and 11 years, respectively. When evaluated by the Log-Rank test, there were no significant differences between the two groups in terms of the recurrence-free survival rate (P > 0.05). There were significantly more females than males with recurrence-free survival rate (P < 0.05).
The disease-specific mortality for children with DTC is very low. Thus, it is unlikely that modification of current treatment protocols will further reduce the disease-specific mortality.[11,12] The mortality rates for children with DTC range from 0% to 5.3% and usually <3.0% in the majority of the reports.[9] In our series, 1 death occurred, due to disseminated pulmonary metastasis and bone metastasis. The mortality rate is 2.56%.
Limitations of the current study are related to its retrospective design. The patients treated with RAI in our clinic were analyzed after thyroidectomy. Therefore, the occurrence of surgical complications could not be evaluated. Another limitation, that may be controversial, is empiric fixed RAI doses given to patients over 10 years. Only for children under or at the age of 10, doses were corrected using either body surface area or weight by using the formula.
CONCLUSION
In the current study, there was no statistically significant difference between age, tumor diameter, and histopathology with recurrence, whereas there was a statistically significant difference between gender, multicentricity and lymph node metastasis with recurrence in pediatric DTC.
Total thyroidectomy followed by RAI appears to be the most effective treatment for patients with pediatric DTC in terms of reducing the rate of relapse and improving surveillance for recurrent disease. The use of RAI seems to be safe, with no adverse effects on subsequent fertility and pregnancy or secondary malignancy.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Golpanian S, Perez EA, Tashiro J, Lew JI, Sola JE, Hogan AR. Pediatric papillary thyroid carcinoma: Outcomes and survival predictors in 2504 surgical patients. Pediatr Surg Int. 2016;32:201–8. doi: 10.1007/s00383-015-3855-0. [DOI] [PubMed] [Google Scholar]
- 2.Lazar L, Lebenthal Y, Steinmetz A, Yackobovitch-Gavan M, Phillip M. Differentiated thyroid carcinoma in pediatric patients: Comparison of presentation and course between pre-pubertal children and adolescents. J Pediatr. 2009;154:708–14. doi: 10.1016/j.jpeds.2008.11.059. [DOI] [PubMed] [Google Scholar]
- 3.Mihailovic J, Nikoletic K, Srbovan D. Recurrent disease in juvenile differentiated thyroid carcinoma: Prognostic factors, treatments, and outcomes. J Nucl Med. 2014;55:710–7. doi: 10.2967/jnumed.113.130450. [DOI] [PubMed] [Google Scholar]
- 4.Kim SS, Kim SJ, Kim IJ, Kim BH, Jeon YK, Kim YK, et al. Comparison of clinical outcomes in differentiated thyroid carcinoma between children and young adult patients. Clin Nucl Med. 2012;37:850–3. doi: 10.1097/RLU.0b013e318262c5d6. [DOI] [PubMed] [Google Scholar]
- 5.Dottorini ME, Vignati A, Mazzucchelli L, Lomuscio G, Colombo L. Differentiated thyroid carcinoma in children and adolescents: A 37-year experience in 85 patients. J Nucl Med. 1997;38:669–75. [PubMed] [Google Scholar]
- 6.Hay ID, Gonzalez-Losada T, Reinalda MS, Honetschlager JA, Richards ML, Thompson GB, et al. Long-term outcome in 215 children and adolescents with papillary thyroid cancer treated during 1940 through 2008. World J Surg. 2010;34:1192–202. doi: 10.1007/s00268-009-0364-0. [DOI] [PubMed] [Google Scholar]
- 7.Vaisman F, Bulzico DA, Pessoa CH, Bordallo MA, Mendonça UB, Dias FL, et al. Prognostic factors of a good response to initial therapy in children and adolescents with differentiated thyroid cancer. Clinics (Sao Paulo) 2011;66:281–6. doi: 10.1590/S1807-59322011000200017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Enomoto Y, Enomoto K, Uchino S, Shibuya H, Watanabe S, Noguchi S. Clinical features, treatment, and long-term outcome of papillary thyroid cancer in children and adolescents without radiation exposure. World J Surg. 2012;36:1241–6. doi: 10.1007/s00268-012-1558-4. [DOI] [PubMed] [Google Scholar]
- 9.Wada N, Sugino K, Mimura T, Nagahama M, Kitagawa W, Shibuya H, et al. Pediatric differentiated thyroid carcinoma in stage I: Risk factor analysis for disease free survival. BMC Cancer. 2009;9:306. doi: 10.1186/1471-2407-9-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parisi MT, Eslamy H, Mankoff D. Management of differentiated thyroid cancer in children: Focus on the American Thyroid Association pediatric guidelines. Semin Nucl Med. 2016;46:147–64. doi: 10.1053/j.semnuclmed.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 11.Francis GL, Waguespack SG, Bauer AJ, Angelos P, Benvenga S, Cerutti JM, et al. Management guidelines for children with thyroid nodules and differentiated thyroid cancer. Thyroid. 2015;25:716–59. doi: 10.1089/thy.2014.0460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park S, Jeong JS, Ryu HR, Lee CR, Park JH, Kang SW, et al. Differentiated thyroid carcinoma of children and adolescents: 27-year experience in the Yonsei University health system. J Korean Med Sci. 2013;28:693–9. doi: 10.3346/jkms.2013.28.5.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luster M, Clarke SE, Dietlein M, Lassmann M, Lind P, Oyen WJ, et al. Guidelines for radioiodine therapy of differentiated thyroid cancer. Eur J Nucl Med Mol Imaging. 2008;35:1941–59. doi: 10.1007/s00259-008-0883-1. [DOI] [PubMed] [Google Scholar]
- 14.Bal CS, Padhy AK, Kumar A. Clinical features of differentiated thyroid carcinoma in children and adolescents from a Sub-Himalayan iodine-deficient endemic zone. Nucl Med Commun. 2001;22:881–7. doi: 10.1097/00006231-200108000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Demidchik YE, Demidchik EP, Reiners C, Biko J, Mine M, Saenko VA, et al. Comprehensive clinical assessment of 740 cases of surgically treated thyroid cancer in children of Belarus. Ann Surg. 2006;243:525–32. doi: 10.1097/01.sla.0000205977.74806.0b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Biko J, Reiners C, Kreissl MC, Verburg FA, Demidchik Y, Drozd V. Favourable course of disease after incomplete remission on (131)I therapy in children with pulmonary metastases of papillary thyroid carcinoma: 10 years follow-up. Eur J Nucl Med Mol Imaging. 2011;38:651–5. doi: 10.1007/s00259-010-1669-9. [DOI] [PubMed] [Google Scholar]
- 17.Jarzab B, Handkiewicz-Junak D. Differentiated thyroid cancer in children and adults: Same or distinct disease? Hormones (Athens) 2007;6:200–9. [PubMed] [Google Scholar]
- 18.Sakorafas GH, Giotakis J, Stafyla V. Papillary thyroid microcarcinoma: A surgical perspective. Cancer Treat Rev. 2005;31:423–38. doi: 10.1016/j.ctrv.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 19.Zimmerman D, Hay ID, Gough IR, Goellner JR, Ryan JJ, Grant CS, et al. Papillary thyroid carcinoma in children and adults: Long-term follow-up of 1039 patients conservatively treated at one institution during three decades. Surgery. 1988;104:1157–66. [PubMed] [Google Scholar]
- 20.Welch Dinauer CA, Tuttle RM, Robie DK, McClellan DR, Svec RL, Adair C, et al. Clinical features associated with metastasis and recurrence of differentiated thyroid cancer in children, adolescents and young adults. Clin Endocrinol (Oxf) 1998;49:619–28. doi: 10.1046/j.1365-2265.1998.00584.x. [DOI] [PubMed] [Google Scholar]
- 21.Rapkin L, Pashankar FD. Management of thyroid carcinoma in children and young adults. J Pediatr Hematol Oncol. 2012;34(Suppl 2):S39–46. doi: 10.1097/MPH.0b013e31824e37a6. [DOI] [PubMed] [Google Scholar]
- 22.Wang JT, Huang R, Kuang AR. Comparison of presentation and clinical outcome between children and young adults with differentiated thyroid cancer. Asian Pac J Cancer Prev. 2014;15:7271–5. doi: 10.7314/apjcp.2014.15.17.7271. [DOI] [PubMed] [Google Scholar]
- 23.Thompson GB, Hay ID. Current strategies for surgical management and adjuvant treatment of childhood papillary thyroid carcinoma. World J Surg. 2004;28:1187–98. doi: 10.1007/s00268-004-7605-z. [DOI] [PubMed] [Google Scholar]
- 24.Rivkees SA, Mazzaferri EL, Verburg FA, Reiners C, Luster M, Breuer CK, et al. The treatment of differentiated thyroid cancer in children: Emphasis on surgical approach and radioactive iodine therapy. Endocr Rev. 2011;32:798–826. doi: 10.1210/er.2011-0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klein Hesselink MS, Nies M, Bocca G, Brouwers AH, Burgerhof JG, van Dam EW, et al. Pediatric differentiated thyroid carcinoma in the Netherlands: A nationwide follow-up study. J Clin Endocrinol Metab. 2016;101:2031–9. doi: 10.1210/jc.2015-3290. [DOI] [PubMed] [Google Scholar]
- 26.Lazar L, Lebenthal Y, Segal K, Steinmetz A, Strenov Y, Cohen M, et al. Pediatric thyroid cancer: Postoperative classifications and response to initial therapy as prognostic factors. J Clin Endocrinol Metab. 2016;101:1970–9. doi: 10.1210/jc.2015-3960. [DOI] [PubMed] [Google Scholar]
- 27.Samuel AM, Rajashekharrao B, Shah DH. Pulmonary metastases in children and adolescents with well-differentiated thyroid cancer. J Nucl Med. 1998;39:1531–6. [PubMed] [Google Scholar]
- 28.Grigsby PW, Gal-Or A, Michalski JM, Doherty GM. Childhood and adolescent thyroid carcinoma. Cancer. 2002;95:724–9. doi: 10.1002/cncr.10725. [DOI] [PubMed] [Google Scholar]
- 29.Landau D, Vini L, A'Hern R, Harmer C. Thyroid cancer in children: The royal Marsden hospital experience. Eur J Cancer. 2000;36:214–20. doi: 10.1016/s0959-8049(99)00281-6. [DOI] [PubMed] [Google Scholar]
- 30.Clement SC, Kremer LC, Links TP, Mulder RL, Ronckers CM, van Eck-Smit BL, et al. Is outcome of differentiated thyroid carcinoma influenced by tumor stage at diagnosis? Cancer Treat Rev. 2015;41:9–16. doi: 10.1016/j.ctrv.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 31.Markovina S, Grigsby PW, Schwarz JK, DeWees T, Moley JF, Siegel BA, et al. Treatment approach, surveillance, and outcome of well-differentiated thyroid cancer in childhood and adolescence. Thyroid. 2014;24:1121–6. doi: 10.1089/thy.2013.0297. [DOI] [PubMed] [Google Scholar]
- 32.Hogan AR, Zhuge Y, Perez EA, Koniaris LG, Lew JI, Sola JE. Pediatric thyroid carcinoma: Incidence and outcomes in 1753 patients. J Surg Res. 2009;156:167–72. doi: 10.1016/j.jss.2009.03.098. [DOI] [PubMed] [Google Scholar]


