Abstract
Purpose
To determine the burden of retinal diseases and the degree of visual impairment associated with each disease, amongst Nigerians.
Patients and Methods
This was a hospital-based multicenter, prospective, cross-sectional, non-comparative study conducted from January to December 2018. Data was obtained from consecutive patients with a retinal diagnosis presenting at the general ophthalmic and specialty retina clinics in four hospitals (three public, and one private teaching eye department) in Nigeria. Biodata, visual acuity and refraction, intraocular pressure, findings on dilated retinal examination, diagnosis and systemic diseases were noted. Degree of monocular and bilateral visual loss associated with each diagnosed retinal disease was summarized and p value was calculated using chi-square test. P < 0.05 was considered significant.
Results
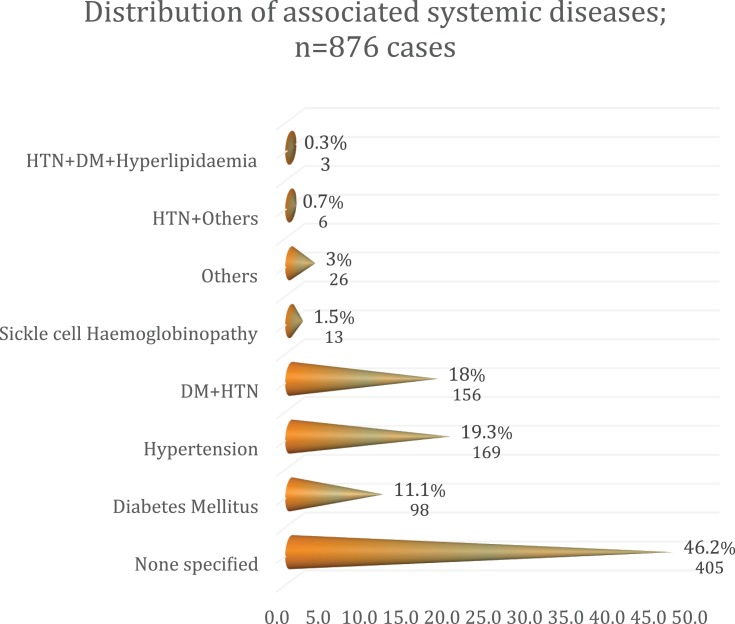
Eight hundred seventy-six of 8614 patients had a retinal diagnosis; establishing a hospital-based retinal disease prevalence of 9.8%. Male:female ratio was 1.1:1. The mean age of study patients was 49.97 (standard deviation 17.64 years). Mean symptom duration was 21.63 months (standard deviation 41.94). The mean intraocular pressure was 13.87 mmHg. Forty-three different retinal diseases were diagnosed. The most common was retinal complications of diabetes, i.e., diabetic retinopathy (DR) alone, diabetic macular edema (DME) alone and a combination of DR and DME, which accounted for 13.7%, 5.6% and 9.3%, respectively (contributed 28.6% of the entire diagnosis). This was followed by retinal detachment (RD), in 219 eyes (15.4%), dry age-related macular degeneration (AMD) in 124 eyes (8.7%). Nearly half of the eyes were blind or severely visually impaired. Blindness occurred in 34.1% of eyes; severe visual impairment in 8.2% of eyes and 29.7% had normal vision. There were 469 patients who had systemic diseases. The common systemic diseases were hypertension in 169 patients (19.3% of the total number of patients), hypertension and diabetes in 156 patients (18%), and diabetes alone in 98 patients (11.1%). Sickle cell disease was present in 1.5%.
Conclusion
There is need to invest in infrastructure, local training and development of systems for early detection and treatment of several retinal diseases in sub-Saharan Africa; DR and DME having the largest burden. Collaborative physician care and management of hypertension and diabetes could significantly reduce the burden of DR and DME.
Keywords: vitreoretinal diseases, sub-Saharan Africa, diabetic retinopathy, macular edema, retinal detachment
Introduction
Contrary to the previous notion and widespread belief, retinal diseases are not uncommon in Nigeria and sub-Saharan Africa (SSA) as has been demonstrated by research from the region.1 The Nigerian blindness survey which was conducted over a decade ago provided community-based data which showed that age-related macular degeneration (AMD) accounted for 3.9% of severe visual impairment and 1.8% of blindness, while other retinal diseases were responsible for 8.4% of severe visual impairment and 3.0% of blindness.2 There have been several reports on the spectrum of retinal diseases presenting in ophthalmic clinics in SSA, but the relatively small number of patients in each of this study has been a limitation; raising the need for a study with larger sample size.3–5
We have therefore conducted a study to provide more up-to-date information on the hospital-based burden of retinal diseases presenting to ophthalmic clinics in SSA, because of the multicenter nature of our study and the large sample size. Our aim was to determine amongst patients attending the retina subspecialty clinics and outpatient general ophthalmology clinics in four hospitals, the most common retinal diseases; the degree of vision loss in each of the diagnosed retinal disease and to provide information on the known co-existing systemic diseases seen amongst the patients.
Materials and Methods
We conducted a multicentre, prospective, cross sectional, non-comparative study.
This was done by the collaboration of four ophthalmic clinics that have a high traffic of ophthalmic patients. There was a retina specialist in each clinic, designated as the principal investigator (PI). The PI was responsible for the accuracy and timely reporting of data, which was collected from the participating clinics from January to December of 2018. The four clinics where located as follows: one in the southwest region, two in the south-south region and one in the north-central region of Nigeria. Ethical approval was obtained from the Jos University Teaching Hospital (JUTH). The study was conducted according to the tenets of the Helsinki declaration. All study participants gave a written informed consent and were given a choice not to participate if they chose not to. Consent from minors was obtained from parent or guardian who was required to give a written informed consent. All ages were included (there was no exclusion based on age).
Information was obtained prospectively from consecutive patients in attendance at the clinics. Each patient underwent a comprehensive eye examination including snellen visual acuity testing of each eye, refractive error assessment, intraocular pressure measurement, anterior segment examination using a slit lamp, dilated fundus biomicroscopy using a +90D or +78D lens, and +20D lens for binocular indirect ophthalmoscopy (BIO). Most of the diagnosis made was from clinical examination, however in some of the patients, it was necessary to perform ocular investigations including a digital B mode ultrasound, optical coherence tomography (OCT), fundus photography (FP) and fundus fluorescein angiography (FFA). The specific retinal diagnosis was written in the patient’s record after the consultation and in some cases after further ocular investigation had been done. This diagnosis was made by a competent consultant ophthalmologist and assumed to be the final diagnosis. In some cases, there was more than one retinal diagnosis in an eye. For instance, an eye could have a retino-choroidal scar as well as have a BRVO; each of the two diagnoses will be recorded separately during data reporting. Information on co-existing systemic disease could have been known previously, (in which case the patient volunteered the information), or it could have been diagnosed by the collaborating medical team after systemic workup.
Data from each of the four collaborating clinics were entered into an excel spreadsheet and transmitted at the end of each month to a central data collection point, where collation and analysis were done with IBM SPSS statistics version 22 (IBM Corp. Armonk, NY, USA). Study eyes in which diagnosis was inconclusive were removed from the data to be analysed. Age, symptom duration and intraocular pressure were summarized as mean and standard deviation. Age categories, retinal diagnoses, and associated systemic disease were summarized as frequency and percentage. Presenting visual acuity was summarized as frequency and percentages. Degree of monocular and bilateral vision loss associated with each retinal disease was summarized and p value calculated using chi-square. P < 0.05 was considered significant.
Definition of Terminologies
The study analyzed monocular visual acuity of affected eye and bilateral visual acuity for retinal conditions with a bilateral presentation.
Categorization of visual acuity was done using the ICD 10 categorization of visual impairment as indicated below
Near normal/mild visual impairment≥ 6/18
Moderate visual impairment6/24 to 6/60
Severe visual impairment< 6/60 to 3/60
Blindness< 3/60 to No perception of light
Legal blindness was defined as visual acuity less than 3/60 in the better eye.
Disorders with bilateral impact on vision were assessed as visual impairment in the better eye using the ICD 10 visual impairment categorization.
Results
A total of 876 patients presented with a retinal diagnosis from a pooled number of 8614 new patients seen in the 4 ophthalmic clinics over the one-year study period. This gives an estimated hospital prevalence of retinal diseases to be 9.8%. There were 455 males (52%) and 421 females (48%) giving a male: female ratio of 1.1:1. Forty-three (43) retinal diseases were diagnosed. The total number of eyes having a retina related diagnosis was 1374 eyes (686 right eyes and 688 left eyes), from which 1539 retinal diagnosis were made. Of the total number of patients, 394 patients (44.9%) had bilateral retinal conditions accounting for 788 eyes.
The age of participants ranged from infant to 95years with a mean age of 49.97 ±17.64 years. The age range 51–75 years accounted for majority (448, 51.1%) followed by 26–50 years (292, 33.3%) while ages 1–25 years and greater than 75 years accounted for the minorities (95, 10.9%) and (41, 4.7%), respectively.
The mean duration of symptoms was 21.63(SD 41.94) months. The mean intraocular pressure (IOP) was 13.8mmHg (SD 5.8) in the right eye and 13.9mmHg (SD 6.4) in the left eye.
A systemic disease was present in 53.8% of study patients. The commonest systemic association was hypertension alone (169 patients; 19.3%), followed by hypertension with diabetes (156 patients; 18%) then diabetes alone (98 patients; 11.1%). Sickle cell hemoglobinopathy was not a common systemic association since it was present in only 1.5% of the study participants. Details of systemic association can be seen in Figure 1, Table 1.
Figure 1.
Distribution of systemic diseases amongst the study participants
Table 1.
Systemic Diseases and Frequency
| Systemic Conditions | Frequency (%Age) |
|---|---|
| Hypertension | 169 (36.0) |
| Diabetes + Hypertension | 156 (33.3) |
| Diabetes mellitus | 98 (20.9) |
| Sickle cell hemoglobinopathy | 13 (2.8) |
| Hypertension + others | 6 (1.3) |
| Human Immunodeficiency Virus | 5 (1.1) |
| Renal failure | 4 (0.9) |
| Hypertension + Diabetes + Hyperlipidemia | 3 (0.6) |
| Osteoarthritis | 3 (0.6) |
| Asthma | 3 (0.6) |
| Trauma | 2 (0.4) |
| Leukemia | 2(0.4) |
| Pyelonephritis | 1(0.2) |
| Sarcoidosis | 1(0.2) |
| Rheumatology | 1(0.2) |
| Albinism | 1(0.2) |
| Post pre-eclampsia | 1(0.2) |
| Total | 469 (100) |
In general, monocular blindness was present in 454 of 1328 eyes (34.1%), while severe visual impairment was present in 110 eyes (8.2%). There were significant numbers also of moderate and milder degrees of visual impairment as shown in Table 2, which represents the distribution of visual impairment in eyes (combination of right and left eyes) recruited for the study.
Table 2.
Presenting Visual Acuity Categorization of Study Eyes (n=1328 Eyes). In 46 Eyes, Presenting Vision Could Not Be Determined
| Frequency | Percent (%) | |
|---|---|---|
| Mild visual impairment/Near normal | 395 | 29.7 |
| Moderate visual impairment | 369 | 27.9 |
| Severe visual impairment | 110 | 8.2 |
| Blindness | 454 | 34.1 |
| Total | 1328 | 100.0 |
The frequency of each retinal disease and distribution of monocular blindness and visual impairment is shown in Table 3. The most common retinal diagnosis is retinal complications of diabetes; i.e. diabetic retinopathy (DR) alone, diabetic macular edema (DME) alone and a combination of DR and DME accounting for 13.7%, 5.6% and 9.3%, respectively. Together, the three contributed 28.6% of the entire diagnosis made in this study. This was followed by retinal detachment, which was diagnosed in 219 eyes (15.4%), and dry AMD in 124 eyes (8.7%).
Table 3.
Shows the Diverse Retinal Diagnosis and a Categorization of the Degree of Visual Impairment
| Mild Visual Impairment/Near Normal Freq (%) | Moderate Visual Impairment Freq (%) | Severe Visual Impairment Freq (%) | Blindness Freq (%) | Total | |
|---|---|---|---|---|---|
| BRAO | 0 (0.0%) | 1 (50.0%) | 1 (50.0%) | 0 (0.0%) | 2 (100.0%) |
| BRVO | 7 (25.0%) | 8 (28.6%) | 1 (3.6%) | 12 (42.9%) | 28 (100.0%) |
| Choroidal rupture | 0 (0.0%) | 1 (33.3%) | 0 (0.0%) | 2 (66.7%) | 3 (100.0%) |
| CMO | 0 (0.0%) | 7 (63.6%) | 1 (9.1%) | 3 (27.3%) | 11 (100.0%) |
| CNVM | 2 (40.0%) | 2 (40.0%) | 1 (20.0%) | 0 (0.0%) | 5 (100.0%) |
| CRAO | 1 (14.3%) | 0 (0.0%) | 0 (0.0%) | 6 (85.7%) | 7 (100.0%) |
| CRVO | 4 (8.5%) | 7 (14.9%) | 5 (10.6%) | 31 (66.0%) | 47 (100.0%) |
| CSR | 0 (0.0%) | 10 (90.9%) | 0 (0.0%) | 1 (9.1%) | 11 (100.0%) |
| Diabetic maculopathy | 28 (35.0%) | 30 (37.5%) | 9 (11.2%) | 13 (16.3%) | 80 (100.0%) |
| Diabetic retinopathy | 77 (39.3%) | 49 (25.0%) | 10 (5.1%) | 60 (30.6%) | 196 (100.0%) |
| Diabetic retinopathy with maculopathy | 50 (37.9%) | 50 (37.9%) | 12 (9.1%) | 20 (15.1%) | 132 (100.0%) |
| Dry AMD | 53 (42.7%) | 40 (32.3%) | 7 (5.6%) | 24 (19.4%) | 124 (100.0%) |
| ERM | 7 (63.6%) | 2 (18.2%) | 1 (9.1%) | 1(9.1%) | 11 (100.0%) |
| HIV retinopathy | 0 (0.0%) | 1 (33.3%) | 0 (0.0%) | 2 (66.7%) | 3 (100.0%) |
| Hypertensive retinopathy | 43 (79.6%) | 5 (9.3%) | 2 (3.7%) | 4 (7.4%) | 54 (100.0%) |
| HRVO | 1 (20.0%) | 2 (40.0%) | 0 (0.0%) | 2 (40.0%) | 5 (100.0%) |
| PCV | 2 (11.1%) | 2 (11.1%) | 2 (11.1%) | 12 (66.7%) | 18 (100.0%) |
| Leukaemic retinopathy | 0 (0.0%) | 1 (25.0%) | 0 (0.0%) | 3 (75.0%) | 4 (100.0%) |
| Macular hole | 3 (11.1%) | 11 (40.7%) | 5 (18.5%) | 8 (29.6%) | 27 (100.0%) |
| Neuroretinitis | 0 (0.0%) | 1 (25.0%) | 0 (0.0%) | 3 (75.0%) | 4 (100.0%) |
| No view due to dense cataract | 0 (0.0%) | 0 (0.0%) | 3 (13.6%) | 19 (86.4%) | 22 (100.0%) |
| Other chorioretinitis | 5 (19.2%) | 8 (30.8%) | 1 (3.8%) | 12 (46.2%) | 26 (100.0%) |
| Retinal dystrophy | 3 (17.6%) | 6 (35.3%) | 6 (35.3%) | 2 (11.8%) | 17 (100.0%) |
| Pathological myopia | 2 (4.1%) | 11 (22.4%) | 10(20.4%) | 26 (53.1%) | 49 (100.0%) |
| PED | 2 (100.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 2 (100.0%) |
| PSCR | 5 (50.0%) | 1 (10.0%) | 3 (30.0%) | 1 (10.0%) | 10 (100.0%) |
| PVD | 25 (64.1%) | 6 (15.4%) | 2 (5.1%) | 6 (15.4%) | 39 (100.0%) |
| Retinoblastoma | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 2 (100.0%) | 2 (100.0%) |
| Retinal detachment | 11 (5.2%) | 36 (16.0%) | 18 (7.5%) | 154 (71.4%) | 219 (100.0%) |
| Peripheral Retinal degeneration | 20 (60.6%) | 7 (21.2%) | 1 (3.0%) | 5 (15.2%) | 33 (100.0%) |
| Retinal scar | 12 (27.9%) | 16(37.2%) | 6 (14.0%) | 9 (20.9%) | 43 (100.0%) |
| Retinitis sclopetaria | 0 (0.0%) | 0 (0.0%) | 1 (50.0%) | 1 (50.0%) | 2 (100.0%) |
| Retinal tear | 7 (70.0%) | 1 (10.0%) | 1 (10.0%) | 1 (10.0%) | 10 (100.0%) |
| ROP | 1 (50.0%) | 0 (0.0%) | 0 (0.0%) | 1 (50.0%) | 2 (100.0%) |
| Retinitis pigmentosa | 16 (28.6%) | 19 (33.9%) | 6 (10.7%) | 15 (26.8%) | 56 (100.0%) |
| SCR | 3 (42.9%) | 1 (14.3%) | 1 (14.3%) | 2 (28.6%) | 7 (100.0%) |
| Trauma | 5 (13.5%) | 12 (32.4%) | 0 (0.0%) | 20 (54.1%) | 37 (100.0%) |
| Uveitis | 7 (21.9%) | 9 (28.1%) | 5 (15.6%) | 11 (34.4%) | 32 (100.0%) |
| Valsalva retinopathy | 1 (50.0%) | 0 (0.0%) | 0 (0.0%) | 1 (50.0%) | 2 (100.0%) |
| Vasculitis | 1 (25.0%) | 3 (75.0%) | 0 (0.0%) | 0 (0.0%) | 4 (100.0%) |
| VMT | 0 (0.0%) | 6 (75.0%) | 1 (12.5%) | 1 (12.5%) | 8 (100.0%) |
| Wet AMD | 6 (18.8%) | 19 (59.4%) | 3 (9.4%) | 4 (12.5%) | 32 (100.0%) |
| Total | 1426 (100.0%) |
DR was present in a total of 328 (80.4%) of the 408 diabetic eyes in this series, while DME was present in 212 (52%) of the same number of diabetic eyes. DR was, therefore, more commonly diagnosed than DME. Monocular blindness and severe vision impairment were present in 23% and 7.6%, respectively, of all diabetic eyes; while 31.6% had moderate visual impairment.
Amongst those eyes diagnosed to have only DR, majority (77 eyes, 39.3%) had mild visual impairment/near normal vision, while 60 eyes (30.6%) were blind and 10 eyes (5.1%) had severe visual impairment. In DME only eyes, majority (30 eyes, 37.5%) had moderate visual impairment, 28 eyes (35.6%) had near normal/mild visual impairment while 13 eyes (16.3%) and 9 (11.2%) were blind or had severe visual impairment, respectively.
An assessment of the impact of DR and DME on bilateral vision showed that a majority, 107 patients (52.5%) had near normal/mild visual impairment in the better eye. We found that the combined prevalence of bilateral blindness from diabetes was 13.2%. Also, 12.5% of patients diagnosed to have only DME were bilaterally blind. The better eye in 40% of DME only eyes had severe to moderate visual impairment, while 47.5% were normal or had only partial impairment. Bilateral blindness was present in 18.4% of patients diagnosed to have only DR; 28.5% had severe or moderate visual impairment and 53.1% had normal or partial impairment in the better eye. In the combined DR and DME group, 6.1% had bilateral blindness, 39.4% were severely or moderately visually impaired while 54.5% had normal vision or partial impairment in the better eye. These findings are represented in Table 4 (P=0.193).
Table 4.
Visual Acuity in the Better Eye for Patients Diagnosed to Have Bilateral Diabetic Retinal Complications. (N= 204 Patients, p=0.193)
| Mild Visual Impairment/Near Normal Freq(%) | Moderate Visual Impairment Freq(%) | Severe Visual Impairment Freq(%) | Blindness Freq(%) | Total | |
|---|---|---|---|---|---|
| Diabetic maculopathy | 19 (47.5) | 14 (35.0) | 2 (5.0) | 5 (12.5) | 40 |
| Diabetic retinopathy | 52 (53.1) | 22 (22.4) | 6 (6.1) | 18 (18.4) | 98 |
| Diabetic retinopathy with maculopathy | 36 (54.5) | 24 (36.4) | 2 (3.0) | 4 (6.1) | 66 |
| Total | 107 | 60 | 10 | 27 | 204 |
Retinal detachment was the second commonest diagnosis occurring in 219 eyes (15.4%) (Table 3). The various types of RD encountered in the study include rhegmatogenous RD (RRD) which was the commonest and occurred in 159 eyes (72.6%), tractional RD (TRD) in 49 eyes (22.4%), exudative RD in 5eyes (2.3%) and combined mechanism RD in 6eyes (2.7%). Causes of TRD include PDR 28 (62.3%), PSCR 2 (4.4%) and in a number of cases the cause was unknown 15 (33.3%). One hundred and seventy-two eyes (78.9%) with RD presented with a visual acuity of 6/60 or less. A significant proportion of RD eyes (71.4%) were blind at presentation (Table 3). Thirty-two patients suffered bilateral RD of which 21 (65.6%) were bilaterally severely visually impaired or bilaterally blind, 9 patients (28.1%) had moderate visual impairment in the better eye and 6.3% had near normal/mild visual impairment in the better eye (Table 5).
Table 5.
Visual Acuity in the Better Eye for Patients Diagnosed to Have Bilateral Retinal Detachment (N= 32)
| VA Category | Total | |||||
|---|---|---|---|---|---|---|
| Blind (NLP) | Blind <3/60 to LP | Severe Visual Impairment <6/60 to 3/60 | Moderate Visual Impairment; <6/18 to 6/60 | Normal or Partial Impairment; >6/18 | ||
| Bilateral retinal detachment | 1 (3.1%) | 19 (59.4%) | 1 (3.1%) | 9 (28.1%) | 2 (6.3%) | 32 (100.0%) |
Dry AMD was present in 124 eyes (8.7%), while wet AMD occurred in 32 eyes (2.2%), confirming that dry AMD is more prevalent than wet AMD. The diagnosis of AMD was made mostly on the clinical finding of geographic atrophy, or in advanced stages of the disease, scarring in the macular area with associated drusenoid deposits. Some of the patients had FFA, fundus autofluorescence (FAF) and OCT to confirm the diagnosis, but a majority did not have this technology available to them. Twenty-four eyes accounting for 19.4% of dry AMD eyes were blind compared to 12.5% wet AMD as shown in Table 3. However, when evaluating eyes with visual acuity <6/60, wet AMD was almost equal to dry AMD, with wet AMD accounting for 21.9% (7 of 32 eyes) compared to 23.5% (31 of 124 eyes) in dry AMD.
PCV was diagnosed in 18 eyes (1.2%). The diagnosis of PCV was again made on mostly clinical examination. PCV was diagnosed if there was retinal and/or vitreous hemorrhage in the absence of drusenoid deposits. In some cases, there was a presence of the classic protruding orange red colored lesions seen on funduscopy. Some eyes had an FFA, OCT, and optical coherence tomography angiography (OCTA). Indocyanine green angiography (ICG)was not used in any of the clinics. Rate of blindness in PCV eyes was 66.6%, which is significantly higher than in both dry and wet AMD (Table 3). Also, 77.8% of eyes (14 of the 18 eyes) had a visual acuity < 6/60. PCV therefore, had a more debilitating impact on the vision, when compared to AMD.
VH was present in 80 eyes (5.2%). The commonest causes of VH were PCV, trauma, PDR, PSCR, PVD, RVO, retinal tear and valsalva retinopathy which was present in 22.5%, 15%, 8.8%, 5%, 5%, 3.75%, 1.25% and 1.25%, respectively, of VH eyes. In a majority of cases 30 eyes (37.5%), the cause of VH was unknown. In this case the VH obscures retina view, preventing detailed examination of the retina, to determine the diagnosis.
Retinitis pigmentosa (RP) was the most common retinal dystrophy, occurring in 56 eyes (3.6%). It was observed that 26.8% of RP eyes were blind, while 37.5% (21 of 56 eyes) had a vision < 6/60. Retinal vein occlusion (RVO) was the second most common retino vascular disease after retinal complications of diabetes. CRVO, BRVO and HRVO occurred in 3.3%, 2.0% and 0.4% of eyes, respectively. Blindness was present in 66% of CRVO eyes, 42.9% of BRVO eyes and 40% of HRVO eyes. Proliferative and non-proliferative sickle cell retinopathies were present in 17 eyes (1.2%).
The diagnosed diseases with the highest rates of mono ocular blindness are CRAO (85.7% of eyes), CRVO (76% of eyes), choroidal rupture (66.7% of eyes), RD (78% of eyes), and those eyes having a retinal pathology but no view of the fundus due to a cataract (86.4% of eyes), Table 3. The least common retinal diseases in this series include valsalva retinopathy, retinitis sclopetaria, CMV retinitis, ROP, retinoblastoma and BRAO, as shown in Table 3.
Discussion
There is growing interest in retina related causes of vision loss in SSA, partly because of the successes achieved in reducing the cataract backlog and addressing priority causes of vision loss such as trachoma and other causes of cornea blindness. This research provides real-life hospital-based frequencies of 43 retinal diseases. The fairly large number of patients and eyes, and the multicenter nature of the study are its strengths. However, the presence and visual impact of co-existing non-retina disease such as glaucoma and lens status was intentionally not taken into consideration. Such ocular diseases could have a profound effect on vision. Lack of uniformity or standardization of diagnosis and work up for the different diseases is another weakness. Despite these weaknesses, this study provides useful estimates that can be used for advocacy and planning for the care of retinal diseases in SSA. The reported prevalence of retinal diseases in this study is lower than the 12.5% reported by a similar study from Ethiopia.5
The lengthy duration of symptoms suggests that the clinical presentations of the retinal diseases are chronic manifestations and therefore result in a severe impact on the visual acuity. Emphasis should, therefore, be on health education and setting up programs to improve the health-seeking behavior of patients in SSA. This study reports a slightly higher number of male patients compared to females. This is common to the finding of Eze et al.3 It has been reported previously from the region, that males have more access to health care compared to females. All reports on retina diseases from SSA have consistently shown higher male numbers compared to females; this study simply agrees with this finding. The mean age of study participants is relatively young for retina diseases and is similar to, reports from southeastern Nigeria and Ethiopia that both report relatively young ages, in the fifth decade of life. This may be a reflection of the mostly younger age population known to occur in SSA.
This research confirms what has been previously reported about diabetes, that DR is a growing concern in SSA. Several studies from the region have reported DR as the commonest or a common finding in the retina clinics. The reported prevalence of diabetic retinopathy in Nigeria varies widely from 4.6% to 42.1%.6–11 The prevalence of any form of DR or DME from this study is within the reported range for Nigeria. What is interesting and being reported for the first time from SSA is the finding that DR alone is associated with more blindness than DME alone. Traditionally DME has been reported as the commonest cause of vision loss amongst diabetics, in research from other regions of the world. This finding may be related to patient delay in seeking eye care and the more advanced presentation of disease seen amongst Nigerian and African patients. Also, DR was more common (80.4%) compared with DME (52%). The reason for this finding is unclear, but may also be related to delay in presentation.
Kahloun et al found that 22.2% of diabetics in Tunisia were visually impaired, 4.4% legally blind and 17.8% partially sighted.12 Nwosu reported 18% bilateral blindness, 26% monocular blindness, 30% visual impairment in better eyes, and 20% bilateral visual impairment among DM patients in Southeast Nigeria.11 Our findings were slightly lower than the figures reported by Nwosu. It might be significant to note that, while his study was restricted to one eye clinic in one region of the country, our study represents findings from 4 clinics and from three regions of the country. This may account for the disparity. However, our findings and those from other researchers present evidence for setting up effective DR screening programs and treatment at the primary care level across the region to prevent diabetes-related blindness.
Retinal vein occlusion was the second commonest retino vascular disease. We found that CRVO was the commonest, followed by BRVO and then HRVO. This finding is similar to that reported by other studies from Nigeria that found CRVO to be the commonest presentation of RVO.13,14 This is at variance with findings of BRVO being more common, which has been reported by overseas researchers. A population-based study found the prevalence of CRVO to be lower than BRVO across all ethnic populations.15 The explanation for this difference could be that the Nigerian studies, were all hospital-based studies, and that the CRVO patients having more profound visual symptoms were likely to attend the eye clinics ahead of the BRVO patients, which were more likely to be less symptomatic compared to the CRVO patients.
Other retinovascular diseases worthy of note were hypertensive retinopathy (which has been previously reported on in the region),16,17 retinopathy of prematurity (an emerging retinal disease of increasing importance in the region),18–20 sickle cell retinopathy (proliferative and nonproliferative disease), and retinal artery occlusion. A significant number of these renovascular conditions are associated with systemic risk factors such as diabetes, hypertension, dyslipidemia and sickle cell hemoglobinopathy, which occurred as co-existing systemic disease in a significant number of study patients.
RD was the second commonest diagnosis. In the Ethiopian study, RD was the commonest retinal diagnosis and the commonest cause of both bilateral and monocular blindness.5 Majority of the RD in our study was RRD. A significant proportion of RD eyes (71.4%) were blind at presentation, while 30 (93.8%) of the 32 bilateral RD patients were blind or had severe visual impairment in the better eye. In SSA, therefore, RD is a significant concern and associated with severe vision loss and blindness. The situation is different in the more developed regions of the world. Some studies from SSA have reported on the burden of RD and outcome of its treatment.21–23 In one study RRD repair was the commonest indication (62%) out of a thousand vitrectomies performed in a single vitreoretinal unit in Nigeria.23 RRD was associated with a higher degree of PVR, which is a poor prognostic feature. PVR is a common occurrence in several African studies and efforts have to be made to change this since PVR is associated with a poor visual prognosis. Studies from SSA have reported various levels of success using scleral buckling technique for repair of RRD in the region.21,22 However, vitrectomy will be required for the more complex forms of RD including the advanced PVR RRD and TRD.23
AMD was a common retinal finding in studies from Nigeria.4,24 In one of the studies, AMD was the leading retinal disease.4 In this study, it is the leading macular degenerative disease; and amongst the three most commonly diagnosed retinal diseases. In the Nigerian national survey, AMD was reported to be responsible for 3.9% of severe visual impairment and 1.8% of blindness.2 AMD has also been reported as a leading presentation in the setting of low vision clinics and other population studies in the region.25,26 We found that dry and wet AMD occurred in a total of 156 eyes (10.9%). Dry AMD accounted for 79.5% of all AMD eyes. However, there was a slightly higher rate of mono ocular blindness and severe visual impairment in wet AMD (34%) compared to the dry AMD (30%), a reflection of the more visual damaging effect of wet AMD. It is likely that as the population in SSA ages, and with the adoption by several SSA communities of more western lifestyle, AMD will be diagnosed more often in the region and require greater attention as is already the trend in western nations, resulting in ever-increasing research into this disease.27 We need to be prepared for this in SSA by learning from our western colleagues and setting up early detection and treatment strategies.
VH was diagnosed in a significant number of eyes and the commonest cause of VH was PCV (18eyes; 22.5%). PCV represents 1.2% of all the diagnosis and had a higher rate of mono ocular blindness and severe visual impairment (77.8%) compared to AMD (24.4%). The significance of PCV in this study as the commonest known cause of VH ought to be borne in mind; since this is a new finding being reported from SSA.
Ten cases of PCV have been reported from Ibadan, with a significant number presenting with VH and requiring vitrectomy for treatment.28 PCV, therefore, should be a major concern because of its likelihood to present with VH and its severe visual damaging effect. Early diagnosis and treatment of PCV should be advocated. In a large proportion of VH eyes, the cause of VH could not be ascertained, because vitrectomy is required for removal of the VH or self-clearance is expected, before adequate viewing of the retina is possible to reveal the cause of VH and the diagnosis. This further strengthens the case for provision of vitreoretinal surgical services.
Retinitis pigmentosa (RP) was the most significant retinal dystrophy, seen in 56 eyes (3.9%). Over a third of the eyes (37.5%) presented blind or with severe visual impairment. RP is a disease of significance in SSA as several studies have reported significant numbers of patients presenting with blindness and severe vision loss.29–31 RP lends itself to research into ways of restoring vision in diseases with limited effect on the outer retina, but preserved inner retina. Considering the significant advances made in its treatment, it is likely that several patients in the region will benefit from such therapy including gene therapy in the near future.
Macular hole (MH) is worth mentioning since it occurred at the same rate as BRVO and very close to wet ARMD as shown in Table 3. There have been reports of MH surgery from the region.23,32 Though MHs have not been a concern in SSA before now, this may change in the future as the population also shifts from a predominantly younger age population to an older one, and as technology and skill become readily available to meet this need.
This study demonstrated that a significant proportion of patients suffered vision loss from retinal diseases. It also observed that diabetic-related conditions (diabetic retinopathy, diabetic maculopathy and diabetic retinopathy with maculopathy), retinal detachment, retinal vein occlusions (BRVO, CRVO and HRVO) and age-related macular degeneration (wet AMD and dry AMD) accounted for 60.5% of the total diagnosed retinal conditions.
There is need to develop effective state run DR screening programs and treatment of early stages of DR to reduce the incidence of more advanced vision damaging stages of DR encountered in this study.33 This ought to be discussed more often than is currently done at the primary, secondary and tertiary levels of eye care in SSA. The acquisition of diagnostic equipment, including affordable fundus photographs (FP), FFA, FAF, OCT, electrophysiology and possibly multimodal imaging units will greatly enhance the ability to make accurate diagnosis, plan treatment and monitor response to treatment or disease progression for a majority of the retinal diseases. Retinal laser photocoagulation and anti-vascular endothelial growth factor (VEGF) agents will enhance the treatment capabilities of many ophthalmic units in SSA to manage several retinovascular conditions. As has been shown by this study, vitreoretinal surgical services, and local training of vitreoretinal surgeons and support staff has become a priority.
To conclude, though modest efforts have been made in some parts of SSA by the provision of basic retinal imaging units, augmented with digital mobile handheld photographs using smartphones, OCTs and other technology, much more needs to be done in improving access to care, by early retina disease detection at the primary level of eye care and prompt referral to secondary and tertiary levels. Telemedicine could play an important role in achieving this using available cheap mobile photography technology. This ensures that retinal diseases such as DR, RD, RVO and many other retinal diseases are managed promptly for best anatomical and visual outcomes.
Abbreviations
AMD, Age-related macular degeneration; BRAO, Branch retinal artery occlusion; BRVO, Branch retinal vein occlusion; CMO, Cystoid macular oedema; CNVM, Choroidal neovascular membrane; CRAO, Central retinal artery occlusion; CRVO, Central retinal vein occlusion; CSR, Central serous retinopathy; DM, Diabetes mellitus; DR, Diabetic Retinopathy; DME, Diabetic macular edema; ERM, Epiretinal membrane; HRVO, Hemiretinal vein occlusion; PCV, Polypoidal choroidal vasculopathy; PDR, Proliferative diabetic retinopathy; PSCR, Proliferative sickle cell retinopathy; PVD, Posterior vitreous detachment; PVR, Proliferative vitreoretinopathy; PED, Pigment epithelial detachment; ROP, Retinopathy of prematurity; RD, Retinal detachment; SCR, Sickle cell retinopathy; VH, Vitreous hemorrhage; VMT, Vitreomacular traction syndrome.
Disclosure
None of the collaborating authors have any financial interest or other conflicts of interest to declare in this work.
References
- 1.Abiose A. Pattern of retinal diseases in Lagos. Ann Ophthalmol. 1979;11:1067–1072. [PubMed] [Google Scholar]
- 2.Abdull M, Sivasubramaniam S, Murthy GV, et al. Causes of blindness and visual impairment in Nigeria: the Nigeria national blindness and visual impairment survey. IOVS. 2009;50:4114–4120. [DOI] [PubMed] [Google Scholar]
- 3.Eze BI, Uche JN, Shiweobi JO. The burden and spectrum of vitreo-retinal diseases among ophthalmic outpatients in a resource-deficient tertiary eye care setting in South-eastern Nigeria. Middle East Afr J Ophthalmol. 2010;17:246–249. doi: 10.4103/0974-9233.65491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Uhumwangho OM, Itina EI. Retinal diseases in a tertiary hospital in Southern Nigeria. J West Afr Coll Surg. 2015;5:1–16. [PMC free article] [PubMed] [Google Scholar]
- 5.Teshome T, Melaku S, Bayu S. Pattern of retinal diseases at a teaching eye department, Addis Ababa, Ethiopia. Ethiop Med J. 2004;42:185–193. [PubMed] [Google Scholar]
- 6.Osuntokun BO. Diabetic retinopathy in Nigerians. A study of 758 patients. Br J Ophthalmol. 1969;53:652–663. doi: 10.1136/bjo.53.10.652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lawan A, Mohammed TB. Pattern of diabetic retinopathy in Kano, Nigeria. Ann Afr Med. 2012;11:75–79. doi: 10.4103/1596-3519.93528 [DOI] [PubMed] [Google Scholar]
- 8.Ashaye A, Arije A, Kuti M, et al. Retinopathy among type 2 diabetic patients seen at a tertiary hospital in Nigeria: a preliminary report. Clin Ophthalmol. 2008;2:103–108. doi: 10.2147/OPTH.S1532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Magulike NO, Chuka-Okosa CM, Oli JM. Diabetic eye disease in Enugu South-Eastern Nigeria – a preliminary report. Nig J Ophthalmol. 2003;11:30–33. [Google Scholar]
- 10.Ewuga RO, Adenuga OO, Wade PD, et al. Prevalence and risk factors for diabetic retinopathy in north-central Nigeria. Ghana Med J. 2018;52(4):215–221. doi: 10.4314/gmj.v52i4.8 [DOI] [Google Scholar]
- 11.Nwosu SN. Diabetic retinopathy in Nnewi, Nigeria. Nig J Ophthalmol. 2000;8:7–10. [Google Scholar]
- 12.Kahloun R, Jelliti B, Zaouali S, et al. Prevalence and causes of visual impairment in diabetic patients in Tunisia, North Africa. Eye (Lond). 2014;28:986–991. doi: 10.1038/eye.2014.131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fiebai B, Ejimadu CS, Komolafe RD. Incidence and risk factors for retinal vein occlusion at the university of Port Harcourt teaching hospital, Port Harcourt, Nigeria. Niger J Clin Pract. 2014;17:462–466. doi: 10.4103/1119-3077.134040 [DOI] [PubMed] [Google Scholar]
- 14.Uhumwangho OM, Oronsaye D. Retinal vein occlusion in Benin City, Nigeria. Niger J Surg. 2016;22:17–20. doi: 10.4103/1117-6806.169871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rogers S, McIntosh RL, Cheung N, Lim L, Wang JJ, Mitchell P. The prevalence of retinal vein occlusion: pooled data from population studies from the United States, Europe, Asia, and Australia. Ophthalmology. 2010;117:313–319. doi: 10.1016/j.ophtha.2009.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oluleye ST, Olusanya BA, Adeoye AM. Retinal vascular changes in hypertensive patients in Ibadan, sub-Saharan Africa. Int J Gen Med. 2016;9:285–290. doi: 10.2147/IJGM.S107241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Omotoso AB, Kolo PM, Olanrewaju TO, et al. Relationship between retinopathy and renal abnormalities in black hypertensive patients. Clin Hypertens. 2016;21(22):19. doi: 10.1186/s40885-016-0053-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang D, Duke R, Chan RP, et al. Retinopathy of prematurity in Africa: a systematic review. Ophthalmic Epidemiol. 2019;1:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Onyango O, Sitati S, Amolo L, et al. Retinopathy of prematurity in Kenya: prevalence and risk factors in a hospital with advanced neonatal care. Pan Afr Med J. 2018;29:152. doi: 10.11604/pamj.2018.29.152.14046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gilbert C, Malik ANJ, Nahar N. Epidemiology of ROP update - Africa is the new frontier. Semin Perinatol. 2019. doi: 10.1053/j.semperi.2019.05.002 [DOI] [PubMed] [Google Scholar]
- 21.Oluleye TS, Ibrahim O, Olusanya B. Scleral buckling for retinal detachment in Ibadan, Sub-Saharan Africa: anatomical and visual outcome. Clin Ophthalmol. 2013;7:1049–1052. doi: 10.2147/OPTH.S44407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nwosu SN, Akudinobi CU. Outcome of surgery for rhegmatogenous retinal detachment in a Nigerian eye hospital. Niger Postgrad Med J. 2014;21:315–318. [PubMed] [Google Scholar]
- 23.Okonkwo ON, Lewis K, Hassan AO, et al. Indications and outcomes of vitrectomy surgery in a series of 1000 black African eyes. BMJ Open Ophthalmol. 2019;4:e000083. doi: 10.1136/bmjophth-2017-000083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Onakpoya OH, Olateju SO, Ajayi IA. Retinal diseases in a tertiary hospital: the need for establishment of a vitreo-retinal care unit. J Natl Med Assoc. 2008;100:1286–1289. doi: 10.1016/S0027-9684(15)31506-6 [DOI] [PubMed] [Google Scholar]
- 25.Olusanya B, Onoja G, Ibraheem W, Bekibele C. Profile of patients presenting at a low vision clinic in a developing country. BMC Ophthalmol. 2012;30(12):31. doi: 10.1186/1471-2415-12-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fafowora OF, Osuntokun OO. Age-related eye disease in the elderly members of rural African community. East Afr Med J. 1997;74:435–437. [PubMed] [Google Scholar]
- 27.Ramin S, Soheilian M, Habibi G, et al. Age-related macular degeneration: a scientometric analysis. Med Hypothesis Discov Innov Ophthalmol. 2015;4:39–49. [PMC free article] [PubMed] [Google Scholar]
- 28.Oluleye TS, Babalola Y. Pattern of presentation of idiopathic polypoidal choroidal vasculopathy in Ibadan, Sub-Saharan Africa. Clin Ophthalmol. 2013;7:1373–1376. doi: 10.2147/OPTH.S47511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Onakpoya HO, Adeoti CO, Oluleye TS, et al. Clinical presentation and visual status of retinitis pigmentosa patients: a multicenter study in southwestern Nigeria. Clin Ophthalmol. 2016;10:1579–1583. doi: 10.2147/OPTH.S107890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ukponmwan CU, Atamah A. Retinitis pigmentosa in Benin, Nigeria. East Afr Med J. 2004;81:254–257. doi: 10.4314/eamj.v81i5.9169 [DOI] [PubMed] [Google Scholar]
- 31.Richard AI. Causes of blindness and low vision in Bayelsa State, Nigeria: a clinic based study. Nig Q J Hosp Med. 2010;20:125–128. [PubMed] [Google Scholar]
- 32.Okonkwo ON, Gyasi ME, Hassan AO, et al. Inverted internal limiting membrane technique maintains macular hole closure in retinal detachment following macular hole repair. Middle East Afr J Ophthalmol. 2018;25:167–169. doi: 10.4103/meajo.MEAJO_354_16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Momoh ZD, Agweye CT, Oguntolu V, Nkanga D. Diabetic retinopathy screening in Calabar, Nigeria: factors influencing referrals and uptake of screening service. Niger J Ophthalmol. 2017;25:118–122. [Google Scholar]