Abstract
The amplified quenching of an anionic conjugated polymer, sulfonated poly(phenylene ethynylene) (PPE-SO3−), by a cationic quencher comprising a boronic acid functionalized benzyl viologen (p-BV2+), has been used to optically detect sugars. In the absence of sugar, a strong polymer/quencher interaction leads to superlinear quenching. In the presence of sugar at pH 7.4, the dicationic viologen derivative forms a neutral zwitterionic species, reducing its ability to complex with and quench the anionic polymer’s emission. Addition of sugar to the polymer/quencher system leads a large increase in the fluorescence intensity (up to 70-fold in one case). Spectral data, quenching parameters, and sugar titration curves are presented and discussed in terms of future development of glucose sensors.
For a decade, the development of synthetic chemosensors and sensors for the recognition and analysis of sugar has attracted much attention.1–6 Despite the progress that has been made, none of the developed technologies is robust enough for the development of in vivo sensors. Development of new glucose sensitive materials and new analytical approaches is still needed. Among analytical approaches, fluorescence spectroscopy is well-known for its promising potential as an analytical tool for in vivo medical analysis.
To date, the most promising chelator group used for the development of synthetic chemosensors for sugar is the boronic acid. Boronic acids form a covalent and reversible interaction with monosaccharides in water. The equilibrium is fast, and the affinity and selectivity with respect to different sugars can be enhanced.3,4,7 Several interesting fluorescence probes functionalized with boronic acids have been developed over the past decade. Mechanisms such as photoinduced electron transfer (PET),7 excited-state charge transfer (CT),8,9 and molecular rigidification,10 to name a few, have been investigated. Although the results obtained on these systems are promising, development of fluorescence probes is a complex process, optical responses are not easily predictable, and the mechanisms are limited to a few fluorophores.
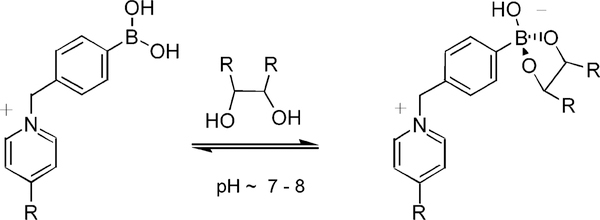
Development of sensor mechanisms that are independent of the nature of the fluorophore is needed. In this way, we used the change of the charge of the boronic acid group, which occurs concomitant with binding saccharides, to induce fluorescence intensity changes based on the disruption of a ion-pair interaction between a quencher and a fluorophore.11 The interaction of the boronic acids with diols is illustrated in Scheme 1.12 Phenylboronic acids are weak Lewis acids with pKa values ~9. Formation of a boronate ester via interaction with a diol increases the electrophilicity of the boron group, thereby reducing its pKa value to ~6. At pH values between 6.5 and 8.5, the boronic acid exists in its neutral form; however, in the presence of a sugar, it exists in its anionic form.
Scheme 1.
Interaction between Phenyl Boronic Acid and Sugar at near Neutral pH
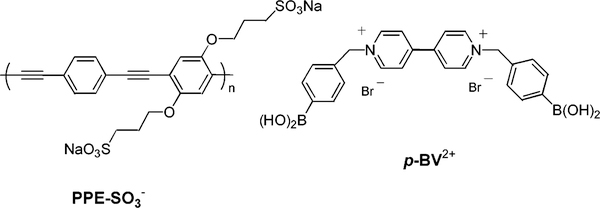
To take advantage of this change of charge, we used a cationic viologen derivative functionalized with two boronic acid groups, 4,4′-N,N′-bis(benzyl-4-boronic acid)-bipyridinium dibromide (p-BV2+, Figure 1).13 Bipyridinium derivatives are well-known for their propensity to act as electron acceptors and quench the emission of luminophores.11,14 Recently, the report of amplified quenching of anionic conjugated polymers by methyl viologen (MV2+) has attracted considerable interest.15,16 This system is very promising for the development of sensors and/or biosensors showing a significant intensity change.17 Interestingly, the ability of MV2+ to quench the emission of the polymer is strongly dependent on the charge of the quencher.18 In this communication, we report the amplified quenching effect of p-BV2+ on a water-soluble poly(phenylene ethynylene) (PPE-SO3−, Figure 1)19 and the application of this system to detect sugars in aqueous buffer solution.
Figure 1.
Molecular structures of the conjugated polymer and quencher.
Absorption and emission spectra of PPE-SO3− (2.5 × 10−6 M) in phosphate buffer solution (PBS) pH 7.4 (6 mM) show that the polymer exists in an aggregated state which is characterized by a broad and long-wavelength emission19 (see Supporting Information). Although the fluorescence quantum yield of the polymer is reduced by aggregation, the presence of the aggregates is not unfavorable because the quenching effect is amplified in the aggregates in comparison to the monomeric state.19
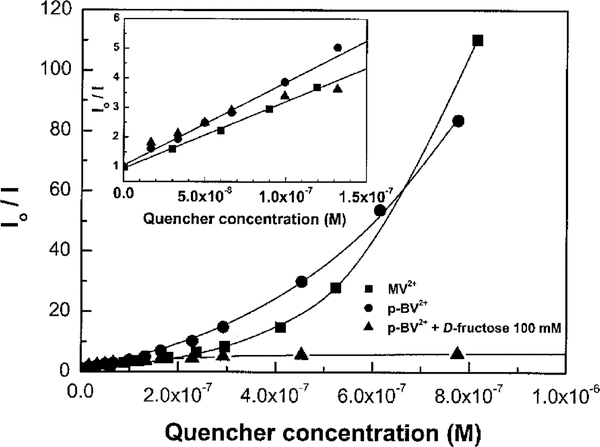
The presence of MV2+ and p-BV2+ induces a large decrease of the emission intensity of the polymer. This emission intensity decrease is accompanied by a red shift of the absorption band (Supporting Information). As previously described, the presence of the cationic quencher not only results in quenching of the fluorescence but also induces conformational changes and/or aggregation.19 Stern–Volmer plots for MV2+ and p-BV2+ in the absence and presence of D-fructose are displayed in Figure 2. At low quencher concentration, the plots are linear with Stern–Volmer constants of 2.3 × 107 M‒1 (MV2+) and 2.8 ×107 M‒1 (p-BV2+).The fact that the KSV values are similar for MV2+ and p-BV2+ indicates that the benzyl-4-boronic acid groups do not interfere with the electrostatic (ion-pairing) interaction between the cationic quencher and the anionic polymer. At higher quencher concentrations, the Stern–Volmer plots are curved upward (i.e., superlinear), which demonstrates that the ion-pairing interaction between the polymer and the quencher leads to static quenching with a large sphere of action.18 As shown in Figure 2, a significant reduction of the quenching efficiency of p-BV2+ is observed in the presence of D-fructose at higher quencher concentrations. Specifically, as the concentration of p-BV2+ increases beyond 200 nM, the fluorescence intensity reaches a “plateau”. The absence of the superlinear quenching behavior for the p-BV2+/PPE-SO3−system in the presence of D-fructose clearly demonstrates that the sugar–quencher interaction disrupts ion-pairing between the polymer and the quencher. It is believed that the reduced quenching efficiency in the presence of D-fructose is caused by the formation of a 2:1 boronate complex between the sugar and p-BV2+ (cf. Scheme 1) which is zwitterionic (and neutral).11
Figure 2.
Stern–Volmer plot of PPE-SO3−(2.5 × 10−6 M) quenching by bipyridinium derivatives, measured in PBS (6 mM) pH 7.4 (λex = 410 nm; λem = 535 nm). Insert: low quencher concentration profiles and linear Stern–Volmer fits.
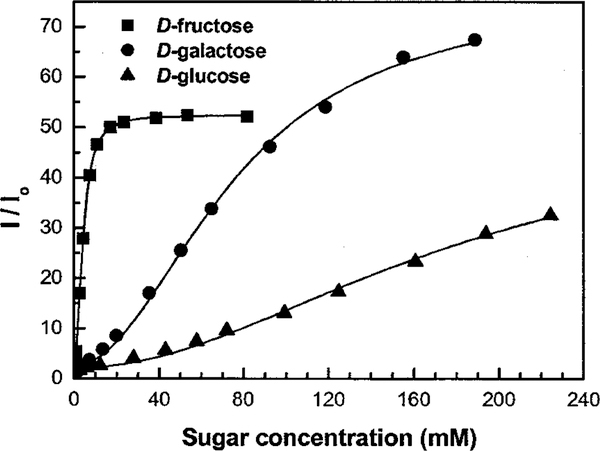
Figure 3 shows titration curves with three different sugars, where the concentrations of the polymer and p-BV2+ were fixed at 2.5 and 0.8 μM, respectively. In absence of sugar, the PPE-SO3−/p-BV2+ mixture is only weakly fluorescent due to the amplified quenching process. However, as the concentration of sugar increases, the amplified quenching is eliminated and a significant increase in the fluorescence intensity is observed (Supporting Information). With D-fructose, the fluorescence increase saturates at ca. 40 mM added sugar, and a 50-fold increase in intensity is observed. This represents the largest intensity change reported to date for a fluorescence-based sugar sensor system. From the titration curves, apparent 1:2 dissociation constants (KD) between the chelator group (p-BV2+) and sugar were calculated. Since these dissociation constants do not take into account the interaction between the quencher and the polymer, a more thorough analysis will be required to fully quantify the different equilbria involved in this system and to extract well-defined equilibrium constants for stepwise dissociation of the 2:1 sugar−p-BV2+ complexes. Nevertheless, the apparent dissociation constants follow the same order as observed for the corresponding benzene boronic acid complexes,12 i.e., D-fructose (2 × 10−5 M2) <D-galactose (6 × 10−3 M2) < D-glucose (3 × 10−2 M2). An unusual titration curve is observed for D-galactose, where the overall intensity change is larger than that observed for D-fructose, despite the fact that the D-galactose/p-BV2+ complex is less stable. More experiments are needed to understand this phenomenon, but it is likely that the effect arises due to a difference in the affinity of the sugar/pBV2+ complexes for PPE-SO3−.
Figure 3.
Titration curves against sugar for the PPE-SO3−(2.5 × 10−6 M)/p-BV2+ (8 × 10−7 M) system, measured in PBS (6 mM) pH 7.4 (λex ) 410 nm; λem = 535 nm).
Sugar sensor systems based on ion-pairing between a quencher (transducer) and a luminophore show significant promise. They are independent of the nature of the luminophore, allowing one to tune the wavelength and lifetime of the emission. In the present work we demonstrate that the use of a conjugated polyelectrolyte as the luminophore provides a means to fabricate sugar sensors with exceedingly high sensitivity. By comparison, a sugar sensor which relies upon a transducer that consists of a diboronic acid substituted viologen similar in structure to p-BV2+ with a monomeric pyrene sulfonate fluorophore exhibits a 5-fold increase in fluorescence intensity when titrated with D-fructose.11 Other sugar sensors which operate on mechanisms such as PET7 and CT6 also exhibit maximum intensity changes of 5-fold when titrated with sugars. We estimate that the amplified quenching process in the conjugated polymer system provides a 10-fold increase in sensitivity compared to the fluorescence-based sugar sensors that have been previously reported.
To develop the conjugated polyelectrolyte based sensors into a system that would be useful in vivo, it will be necessary to carefully explore the effect of the solution medium on the amplified quenching effect. For example, the ionic strength of the medium has a strong influence on the ion-pairing interaction between p-BV2+ and PPE-SO3− 20 and therefore will also influence the magnitude of the sensor response to sugars. Nevertheless, even if the amplified quenching effect is attenuated in solutions of higher ionic strength, the intensity change induced in the presence of sugar remains substantial, suggesting that the system will be useful for sugar detection in biological media. Further investigations seeking to optimize the response of the sensor in various homogeneous and heterogeneous media are currently underway and will be reported in a future publication.13
Supplementary Material
Acknowledgment.
J.R.L. acknowledges the Juvenile Diabetes Foundation International, 1–2000-546, and the NIH National Center for Research Resources, RR-08119. K.S.S. acknowledges the Army Research Office and the Defense Advanced Research Projects Agency, DAAD1900–1-002, for financial support of this work.
Footnotes
Supporting Information Available: Absorption and emission spectra of PPE-SO3- in absence and in the presence of p-BV2+. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- (1).James TD; Shinkai S In Host-Guest Chemistry, Topics in Current Chemistry; Vol. 218; Springer-Verlag: Berlin, 2002; pp 159200. [Google Scholar]
- (2).Hartley JH; James TD; Ward CJJ Chem. Soc., Perkin Trans. 1 2001, 19, 3155. [Google Scholar]
- (3).Eggert H; Fredericksen J; Morin C; Norrild JC J. Org. Chem. 1999, 64, 3846. [Google Scholar]
- (4).Yang W; He H; Drueckhammer DG Angew. Chem., Int. Ed. 2001, 40, 1714. [PubMed] [Google Scholar]
- (5).Gao S; Wang W; Wang B Bioorg. Chem. 2001, 29, 308. [DOI] [PubMed] [Google Scholar]
- (6).DiCesare N; Lakowicz JR Chem. Commun. 2001, 19, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).James TD; Sandanayake KRAS; Iguchi R; Shinkai SJ Am. Chem. Soc. 1995, 117, 8982. [Google Scholar]
- (8).Shinmori H; Takeuchi M; Shinkai S Tetrahedron 1995, 51, 1893. [Google Scholar]
- (9).DiCesare N; Lakowicz JR J. Phys. Chem. A 2001, 105, 6834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Takeuchi M; Mizuno T; Shinmori H; Nakashima M; Shinkai S Tetrahedron 1996, 52, 1195. [Google Scholar]
- (11).Camara JN; Suri JT; Cappuccio FE; Wessling RA; Singaram B Tetrahedron Lett. 2002, 43, 1139. [Google Scholar]
- (12).Lorand JP; Edwards JO J. Org. Chem. 1959, 24, 769. [Google Scholar]
- (13).DiCesare N; Lakowicz JR In preparation. [Google Scholar]
- (14).Serpone N In Photoinduced Electron Transfer; Fox MA, Chanon M, Eds.; Elsevier: Amsterdam, 1988; Vol. D, p 47. [Google Scholar]
- (15).Chen L; McBranch DW; Wang H-L; Helgeson R; Wudl F; Whitten DG Proc. Natl. Acad. Sci. U.S.A. 1999, 96, 12287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Heeger PS; Heeger AJ Proc. Natl. Acad. Sci. U.S.A. 1999, 96, 12219. [Google Scholar]
- (17).a) Swager TM Acc. Chem. Res. 1998, 31, 201. [Google Scholar]; (b) McQuade DT; Pullen AE; Swager TM Chem. Rev. 2000, 100, 2537. [DOI] [PubMed] [Google Scholar]; (c) Wosnick JH; Swager TM Curr. Opin. Chem. Biol. 2000, 4, 715. [DOI] [PubMed] [Google Scholar]
- (18).Wang D; Wang J; Moses D; Bazan GC; Heeger AJ Langmuir 2001, 17, 1262. [Google Scholar]
- (19).Tan C; Pinto MR; Schanze KS Chem. Commun. 2002, 5, 446. [DOI] [PubMed] [Google Scholar]
- (20).The Stern–Volmer constant of the p-BV2+/PPE-SO3− system decreases by approximately an order of magnitude from water (3.5 ×108 M−1) to PBS (6 mM) solution (2.8 × 107 M−1).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.